Abstract
Background/Objectives: Tacrolimus dosing traditionally relies on therapeutic drug monitoring in whole blood, while assessment in plasma may better reflect its effect and reveal overdosing impacting health-related quality of life (HRQoL). Methods: In this cross-sectional study, 898 kidney transplant recipients (KTRs) who were at least 12 months post-transplantation were included. Plasma and whole blood tacrolimus concentrations were compared using Passing–Bablok regression analyses and Bland–Altman plots. Furthermore, the relationship with daily tacrolimus dose and with HRQoL (mental component summary (MCS), physical component summary (PCS)) was explored using linear regression by comparing standardized coefficients. Lastly, mediation analyses explored the effect of various tacrolimus-related side effects on the association between tacrolimus concentrations and HRQoL. Results: Comparison of the methods revealed a constant bias and a slight proportional bias between whole blood and plasma tacrolimus concentrations. The Bland–Altman plots indicated poor agreement with a statistically significant ratio difference (p < 0.001). Both whole blood and plasma concentrations were significantly associated with daily tacrolimus dose (both p < 0.001). Compared to whole blood tacrolimus concentrations, plasma tacrolimus concentration showed a strong negative association with worse HRQoL (PCS: st. β = −0.12, p = 0.01; MCS: st. β = −0.14, p < 0.001). The associations between plasma tacrolimus concentrations and HRQoL were mediated by fatigue severity (proportion mediated on PCS: 67.8%, MCS: 59.5%) and reduced kidney function (proportion mediated on PCS: 16.7%, MCS: 12.9%). Conclusions: In conclusion, compared with whole blood tacrolimus concentrations, plasma tacrolimus concentrations exhibited a negative association with HRQoL in KTRs. Consequently, therapeutic drug monitoring using plasma tacrolimus concentrations may reduce the occurrence of tacrolimus-related toxicity.
1. Introduction
Tacrolimus has been the cornerstone of immunosuppression after kidney transplantation since the 1990s and has played a leading role in improving the 5-year survival rates in kidney transplant recipients (KTRs) [1]. At the same time, tacrolimus is known for its wide range of adverse events, in particular, nephrotoxicity, neurotoxicity, and the development of post-transplantation diabetes mellitus (PTDM) and gastrointestinal toxicity [2]. As a result, the use of tacrolimus can have a tremendous impact on patients’ morbidity and health-related quality of life (HRQoL) [3].
Tacrolimus binds extensively to erythrocytes and circulating plasma proteins including albumin and α1-acid glycoprotein, leaving only 0.3–2% as the free active fraction in plasma [4]. The narrow therapeutic window and large variety of side effects, together with the high inter- and intra-patient pharmacokinetic variability of tacrolimus, require therapeutic drug monitoring (TDM) [2]. Due to limitations of the analytical techniques available at the time of introduction of tacrolimus as an immunosuppressive drug, assessment of plasma concentrations was not yet feasible. Thus, TDM has historically relied on the assessment of whole blood concentrations [5]. During the last decade, the relationship between whole blood tacrolimus concentrations and outcomes has been studied extensively, along with refinement of the therapeutic range for whole blood tacrolimus TDM to minimize adverse effects and improve effectiveness [5]. However, the use of whole blood trough levels for TDM is currently debated since the relationship with clinical outcomes is not always apparent [5,6]. From a clinical perspective, determining either the free effective fraction of tacrolimus or the concentration within its active compartment, i.e., within T-cells, seems to be preferable [7,8]. However, determining the free fraction of tacrolimus or intracellular concentrations remains methodologically challenging and is not easily standardized [9,10,11,12]. Due to advances in analytical methodology, a possible solution is now to determine plasma tacrolimus levels, which linearly correlate with unbound tacrolimus plasma concentrations [4].
Therefore, the current study investigated whether plasma tacrolimus levels exhibited a stronger association with HRQoL than whole blood tacrolimus levels in KTRs. First, we compared plasma and whole blood tacrolimus levels and daily tacrolimus doses. Second, we compared the relationship between plasma and whole blood tacrolimus levels and HRQoL and explored the mediating effect of various tacrolimus-related side effects.
2. Materials and Methods
2.1. Patient Population and Study Design
The present study falls within the scope of the TransplantLines Biobank and Cohort Study at the University Medical Center Groningen (UMCG), the Netherlands (ClinicalTrials.gov identifier: NCT03272841) [13]. Participants include new transplant candidates, previously transplanted patients who received their graft prior to the start of TransplantLines, and (potential) living kidney or liver donors. All participants provided written informed consent prior to their inclusion. The study was approved by the local Medical Ethical Committee (METc 2014/077). All procedures were in accordance with the Declaration of Helsinki and the Declaration of Istanbul. The manuscript was prepared following the Strengthening the Reporting of Observation Studies in Epidemiology (STROBE) guidelines [14]. For the current study, we included data of KTRs with a functional graft for at least 12 months post-transplant and documented tacrolimus use. Subjects were excluded if blood samples were hemolytic or if peak levels of tacrolimus were determined.
For included subjects, demographic and clinical data were extracted from medical records. Clinical examinations were performed during a study visit at the outpatient clinic. On the day of inclusion, blood samples were obtained after an overnight fasting period of 8 to 12 h and before morning dosage of tacrolimus.
Most transplant recipients received tapering triple-immunosuppressive therapy consisting of prednisolone, mycophenolate mofetil, and tacrolimus. Tacrolimus dosing was individualized through TDM to achieve whole blood trough concentrations of 4–6 ng/mL from six months or longer after transplantation.
2.2. Tacrolimus Analyses
Prior to the study visit, 10 mL EDTA blood samples were collected for both whole blood tacrolimus monitoring and tacrolimus plasma analyses. Beforehand, patients were instructed to administer tacrolimus in case of a once-daily dosing regimen at 10:00 a.m. and for a twice-daily dosing regimen at 10:00 a.m. and 10:00 p.m. Blood sampling was generally performed at ≈8:00 a.m., which was considered to be ≈10 h or ≈22 h after the last drug administration. Whole blood samples were analyzed within 4 h and were processed to plasma within the same time frame by centrifugation at 1300× g for 10 min at room temperature (20 °C). Plasma samples were subsequently stored at −80 °C until analysis, which was performed within 3 freeze–thaw cycles. The lower limit of quantification (LLOQ) of the method was 0.05 µg/L, while for some patients the tacrolimus plasma levels were below this threshold, but tacrolimus could still be detected. To avoid exclusion of these patients and since measurements below the LLOQ are unreliable, the imputation method of 1/2 of the LLOQ was chosen. Thus, plasma concentrations were set at 0.025 µg/L [15]. All tacrolimus analyses were performed at the Laboratory of Clinical Pharmacy and Pharmacology at the UMCG using a TSQ Quantiva Mass Spectrometer with Vanquish UHPLC system, both from Thermo Fisher Scientific (Waltham, MA, USA), using a validated high-throughput LC-MS/MS method [16,17,18]. Zijp et al. published the precision data for the used method [18].
2.3. Health-Related Quality of Life
To assess HRQoL, participants completed the 36-item Short-Form Health Survey (SF-36) [19]. The SF-36 can be divided into eight subdomains: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, energy/fatigue or vitality, social functioning, role limitations due to personal or emotional problems, and general mental health [20]. All scales range from 0 to 100, with higher scores indicating higher levels of functioning or well-being.
These subdomains can be aggregated into two summary scores: the physical component summary (PCS) and the mental component summary (MCS). The component summary scores were calculated using a standardized three-step procedure: all eight subscale scores were standardized using a linear z-score transformation using normative data from the 1998 US general population [21]. Next, the z-scores were multiplied by the subscale factor score coefficients derived from the oblique factor rotation, which allows for correlation of the physical and mental health constructs, and were subsequently summed. Finally, t-scores were calculated by multiplying the obtained PCS and MCS sums by 10 and adding 50 to the product, to yield a mean of 50 and a standard deviation of 10 for the US norm population [22].
2.4. Tacrolimus-Related Side Effects
The following section outlines the data collection methods and procedures used to assess the various tacrolimus-related side effects. To assess fatigue, participants completed the Checklist Individual Strength 20-revised (CIS20-R), which was developed as a self-reported multidimensional instrument to assess qualitatively distinct and relevant aspects of fatigue [23]. For the analyses in the current study, the subscale fatigue severity was used, which ranges from 8 to 56, with higher scores indicating a higher level of fatigue. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), a validated 19-item questionnaire that assesses seven components of sleep quality [24]. Duration of sleep, sleep disturbances, sleep latency, daytime dysfunction due to sleepiness, sleep efficiency, overall sleep quality, and sleep medication use were scored and combined into a final score ranging from 0 to 21, with higher scores indicating poorer sleep quality. Recently, it has been shown that KTRs suffer from dysbiosis, a disruption in the balance of the gut microbiome, and that immunosuppressive drugs are a driving factor [25]. Therefore, the Shannon Diversity Index, a measure of the overall diversity of the gut microbiome, which is often decreased in dysbiosis, was included in the current study as a tacrolimus-related patient outcome [26]. The Shannon Diversity Index was calculated on species abundance data obtained from previously performed fecal metagenomics. A detailed method section can be found in Swarte et al. [25]. Another side effect of tacrolimus is diarrhea, which is highly underreported in regular questionnaires in transplant patients [27]. Diarrhea was assessed by measuring stool water content, of which detailed methods are described in Douwes et al. [27]. As a representor for kidney function, the estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula, which is validated and differentiated by sex and plasma creatinine levels [28]. Creatinine analyses were performed using a Roche Cobas 8000 platform (Roche, Mannheim, Germany). Creatinine measurements were calibrated to the isotope-dilution mass spectrometry reference method. PTDM was defined as diabetes that developed in the post-transplant period, excluding transient post-transplant hyperglycemia that may occur in the early post-transplant period [29,30,31]. Since all participants in the current study were enrolled at least 12 months after transplantation, diabetes was defined as a fasting plasma glucose higher than or equal to 7.0 mmol/L, HbA1c above or equal to 48 mmol/mol, or the use of antidiabetic medication [29]. Analyses of fasting blood glucose were performed using a Roche Cobas 8000 platform (Roche, Mannheim, Germany), and HbA1c levels were determined using a Tosoh G8 (ion exchange HPLC) (Tosoh/Sysmex Europe, Norderstedt, Germany). Information on alopecia was obtained from the updated and validated 59-item Modified Transplant Symptom Occurrence and Symptom Distress Scale (MTSOSD-59R), in which subjects rated, amongst other symptoms, the occurrence of alopecia [32]. Tremor was assessed using a Dutch translation of the Fahn–Tolosa–Marin Tremor Rating Scale part C (TRS-C), which includes eight questions assessing patient-perceived tremor occurrence during activities of daily living [33]. Scores are summed to a maximum of 32, with higher scores indicating more severe tremor symptoms. The Fahn–Tolosa–Marin scale has been shown to be a valid tool for assessing tremor in clinical practice; for logistical reasons, only part C was evaluated in patients in the current study [34].
2.5. Statistical Analyses
Continuous variables with a normal distribution were described as mean ± standard deviation or as median [interquartile range] if the distribution was skewed. Histograms and Q-Q plots were used to assess the distribution of continuous variables. Categorical variables were presented as frequencies with percentages.
To compare the methods of tacrolimus whole blood concentrations with tacrolimus plasma concentrations and to investigate any linear relationship between the methods, Passing–Bablok regression analyses were performed. The Passing–Bablok regression method is a robust, non-parametric method, which is not sensitive to non-normally distributed errors and outliers in the data, making it a suitable approach for comparing measurements from different analytical methods allowing to determine any systematic bias between them [35]. Furthermore, to evaluate the agreement between whole blood tacrolimus concentrations and plasma tacrolimus concentrations, Bland–Altman absolute difference plots and Bland–Altman ratio difference plots were produced (the ratio was calculated as plasma tacrolimus/whole blood tacrolimus). For these analyses, scaled values of plasma and whole blood tacrolimus concentrations were calculated by dividing the respective concentration by the standard deviation of plasma or whole blood tacrolimus of KTRs. Assumptions of normality and homoscedasticity of the residuals were checked with visual evaluation of the Q-Q plot and residual plots, respectively [36]. We followed the guidelines from the European Medicines Agency (EMA), which state that any two methods can be considered to be in agreement if their difference is within ±20% around the mean difference for >67% of the samples [37].
To assess the relation between whole blood and plasma tacrolimus concentrations and the respective daily tacrolimus doses, linear regression analyses were performed. To prevent collinearity, in two separate regression models either whole blood or plasma tacrolimus concentration was tested as an independent variable. The residuals of the linear regression models were plotted and checked for normal distribution. Subsequently, to assess whether plasma tacrolimus concentration better reflect the daily tacrolimus dose than whole blood tacrolimus concentration, we tested whether the standardized regression coefficient of the former was significantly larger than that of the latter using bootstrapping with 1000 replications. The proportion of replications for which the standardized regression coefficient of plasma tacrolimus concentration was smaller than or equal to the standardized regression coefficient of whole blood tacrolimus concentration, determined the significance of this test.
To investigate the association of whole blood and plasma tacrolimus concentrations with the primary outcome PCS and MCS of HRQoL, the same methodology as described above was followed. We tested for effect modification by age and sex. Additionally, sensitivity analyses with adjustment of whole blood tacrolimus levels for hematocrit were performed since it has been shown that patients with low hematocrit exhibit a larger difference between whole blood and unbound tacrolimus concentrations [4]. The hematocrit-corrected whole blood tacrolimus model was compared with the crude model with plasma tacrolimus concentration. Furthermore, sensitivity analyses were added that assessed the intra-patient variability (IPV) of plasma tacrolimus concentrations and its association with the PCS and MCS of HRQoL. IPV was calculated by dividing the standard deviation by the mean of two repeated measurements of plasma tacrolimus concentrations per patient and multiplying by 100. This provided a coefficient of variability (COV) per patient. No repeated measurements of HRQoL were available for this cohort.
To unravel possible mediators for the association found between plasma tacrolimus and HRQoL, mediation analyses were performed with the following tacrolimus-related side effects as possible mediators: fatigue, sleep quality, gut microbiome dysbiosis, diarrhea, kidney function, PTDM, alopecia, and tremor. First, assumptions for mediation analyses were checked, which included assessing the significant associations between the potential mediator and plasma tacrolimus concentration and the association between the potential mediator and HRQoL controlling for plasma tacrolimus concentration. Mediation analyses were then performed according to the method of Preacher and Hayes [38,39]. The mediated proportion was calculated as the ratio of the indirect effect and total effect of plasma tacrolimus on HRQoL. It indicates the extent to which the mediating variable explains the total effect.
SPSS version 28 for Windows (IBM, Armonk, NY, USA) and R Statistical Software version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses. p values ≤ 0.05 were considered statistically significant. Due to logistical constraints inherent in conducting a large cohort study, some data points were missing for certain KTRs (as outlined in Section 3.1). For each analysis, only participants with complete data relevant to that specific analysis were included, resulting in varying sample sizes reported for each analysis.
3. Results
3.1. Study Population
In the current study, 898 KTRs were included, with a mean age of 55.1 ± 13.8 years (Table 1). One subject was excluded due to hemolysis of the blood sample, and 25 subjects were excluded because of measurement of tacrolimus peak levels (Figure 1).

Table 1.
Baseline characteristics of study population.
Figure 1.
Flowchart.
3.2. Comparison of Tacrolimus Concentrations in Whole Blood and Plasma
Among KTRs, different tacrolimus formulations were prescribed: 573 KTRs (63.8%) used Prograf, 224 KTRs (24.9%) used Advagraf, 89 KTRs (9.9%) used Envarsus, and for 12 KTRs (1.3%), the formulation was unknown. The mean whole blood tacrolimus concentration was 5.7 ± 1.9 µg/L (range: 1.0–16.3) and mean plasma tacrolimus concentration was 0.13 ± 0.07 µg/L (range: 0.03–0.65). In total, 35 KTRs (4.3%) had a plasma tacrolimus concentration below 0.05 μg/L. The median plasma tacrolimus concentration of KTRs with BLOQ levels was 0.04 [0.03–0.05] μg/L (the actual measurement was unavailable for 1 KTR of the 35 KTRs with values BLOQ). When we repeated all the statistical analyses, with these BLOQ values included rather than the values set at 0.025 μg/L, all the results remained materially unchanged.
We compared tacrolimus measured in whole blood and plasma using Passing–Bablok regression analyses and Bland–Altman plots (Table 2 and Figure 2). The scaled whole blood tacrolimus versus plasma tacrolimus concentration showed a constant bias and a slight proportional bias. Bland–Altman analyses showed no absolute difference between scaled whole blood tacrolimus and scaled plasma tacrolimus concentrations, but a statistically significant ratio difference was observed (p < 0.001, Table 2 and Figure 3). In total, 36% of the paired concentrations were within ±20% of the mean ratio, not meeting the predefined minimum of >67% [37]. These results indicate poor agreement and therefore suggest that plasma and whole blood tacrolimus concentrations cannot be interpreted interchangeably.

Table 2.
Comparison between scaled whole blood and plasma tacrolimus concentrations.
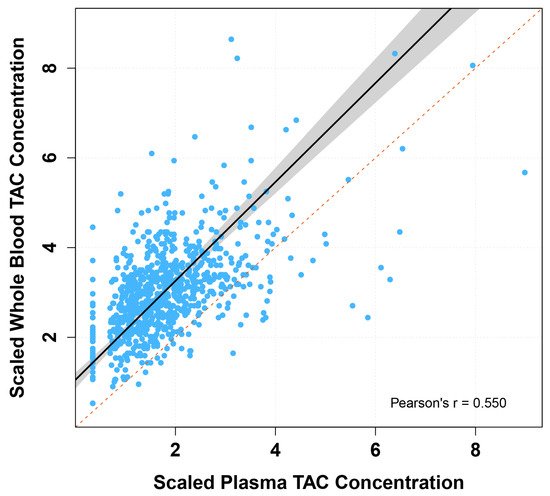
Figure 2.
Passing–Bablok regression fit of scaled whole blood versus plasma tacrolimus concentrations. TAC: tacrolimus. Passing–Bablok fit of scaled tacrolimus plasma concentration versus scaled tacrolimus whole blood concentration (solid black line), with 95% confidence interval (grey area) and line of identity (dotted red line).
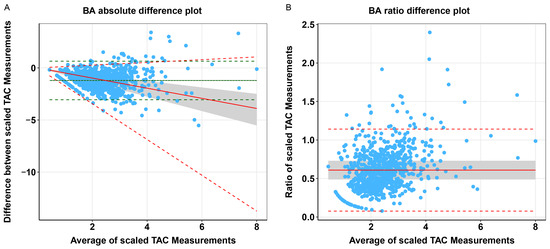
Figure 3.
Comparison of scaled whole blood vs. plasma tacrolimus concentrations. BA: Bland–Altman, TAC: tacrolimus. (A) Bland–Altman absolute difference plot of scaled tacrolimus whole blood vs. scaled plasma concentrations in KTRs, with mean difference (solid green line) and upper and lower limits of agreement (dashed green lines) based on the standard Bland–Altman formulae, with difference as a linear function of the mean (dotted grey line), predicted difference corrected for heteroscedasticity using the ratio of the data (solid red line), and the corresponding upper and lower limits of agreement (dashed red lines). (B) Bland–Altman ratio difference plot of scaled tacrolimus whole blood vs. scaled plasma concentrations in KTRs, with mean difference (solid red line) and upper and lower limits of agreement (dashed red lines). N.B. The grey area represents the ±20% limits around the mean difference between scaled tacrolimus whole blood and plasma concentrations.
3.3. Tacrolimus Concentrations and Dose Associations
The associations of whole blood and plasma tacrolimus concentrations with the tacrolimus dose were statistically significant (both p < 0.001), see Figure 4. When comparing the plasma tacrolimus model with the whole blood tacrolimus model, no statistically significant difference was found between the bootstrap standardized regression coefficients (Table 3).
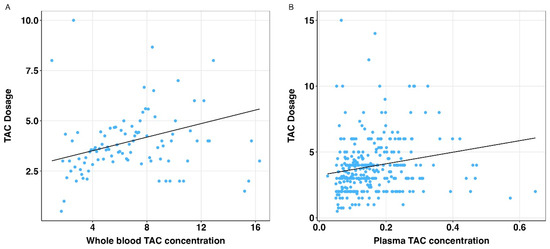
Figure 4.
Relation of whole blood and plasma tacrolimus concentrations and daily tacrolimus dose. TAC: tacrolimus. (A) Scatterplot with regression line (solid black line) for association between whole blood TAC concentrations and daily TAC dosage. (B) Scatterplot with regression line (solid black line) for association between plasma TAC concentrations and daily TAC dosage.

Table 3.
Association of whole blood and plasma TAC concentrations with daily tacrolimus dose.
3.4. Assessment of Tacrolimus Concentrations and Health-Related Quality of Life
To test the hypothesis that plasma tacrolimus concentrations exhibit a stronger negative association with HRQoL than whole blood concentrations, the standardized regression coefficients of the whole blood and plasma tacrolimus concentrations were compared using a bootstrap procedure.
As shown in Table 4, higher plasma tacrolimus concentrations were statistically significantly associated with lower PCS and MCS (p = 0.003, p < 0.001, respectively); however, no statistically significant association was found between whole blood tacrolimus concentrations and PCS and MCS. When comparing the plasma tacrolimus model with the whole blood tacrolimus model, the bootstrapped standardized coefficients for the associations of plasma tacrolimus with the PCS and MCS were significantly lower than those for whole blood tacrolimus (PCS: st. β = −0.12 vs. −0.03, p = 0.01; MCS; st. β = −0.14 vs. 0.001, p < 0.001). No effect modification by age or sex was found (see Appendix A, Table A1).

Table 4.
Association of whole blood and plasma TAC concentrations with HRQoL.
In sensitivity analyses, whole blood tacrolimus adjusted for hematocrit was not statistically significantly associated with PCS or MCS. When comparing the crude plasma tacrolimus model with the adjusted whole blood tacrolimus model, the results of the primary analysis were confirmed (see Appendix A, Table A2).
In additional sensitivity analyses, IPV was assessed in a subset of the study cohort. In 162 KTRs, tacrolimus plasma concentrations were measured at 12 months and 24 months after transplantation. There was no significant difference in the plasma concentration of tacrolimus at 12 and 24 months after transplantation (Wilcoxon signed-rank test, V = 8734, p = 0.20; see Appendix B, Figure A1A). In total, 71 KTRs had a COV > 30%, which is considered a high IPV (see Appendix B, Figure A1B) [40]. Next, we analyzed whether COV was associated with HRQoL by correlating COV with PCS and MCS. COV was not significantly associated with the PCS or MCS (r = 0.01, p = 0.88 and r = 0.04, p = 0.59, respectively, see Appendix B, Figure A1C,D).
3.5. Mediation Effects on Health-Related Quality of Life
To identify potential mediators of the statistically significant association found between plasma tacrolimus and the PCS and MCS of HRQoL, mediation analyses were performed.
Assessing the relationship between plasma tacrolimus concentration and the potential mediators revealed a statistically significant association with fatigue severity (st. β = 0.11, p = 0.01), gut microbiome dysbiosis (st. β = −0.14, p = 0.002), and kidney function (st. β = −0.14, p < 0.001). No association was found between plasma tacrolimus concentration and sleep quality, diarrhea, PTDM, alopecia, or tremor.
As shown in Figure 5, fatigue severity was found to be a partial mediator of the effect of plasma tacrolimus on the PCS (proportion mediated: 67.8%) and the MCS (proportion mediated: 59.5%), without significant direct effect. Furthermore, lower kidney function was found to be a partial mediator between plasma tacrolimus and the PCS (proportion mediated: 16.7%) and the MCS (proportion mediated: 12.9%). Importantly, the direct effect between kidney function and the PCS (st. β = −0.11; p = 0.03) and the MCS (st. β = −0.12, p = 0.004) remained statistically significant. Sleep quality, gut microbiome dysbiosis, diarrhea, PTDM, alopecia, and tremor did not function as mediators between plasma tacrolimus and the PCS and MCS of HRQoL. The detailed results can be found in Appendix C.
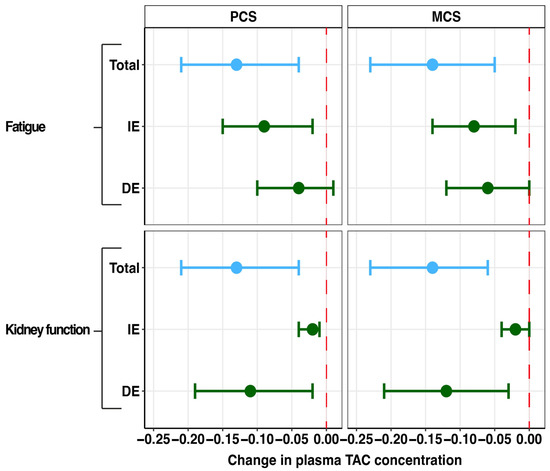
Figure 5.
Forest plot of mediation effects of tacrolimus side effects on the associations of plasma tacrolimus with physical and mental component scores. PCS: physical component summary, MCS: mental component summary, DE: direct effect, IE: indirect effect, TAC: tacrolimus. Data are presented as point estimates (linear regression beta coefficients) and 95% confidence intervals (beta coefficient ±1.96 (standard error [beta coefficient]).
4. Discussion
In the current study, plasma tacrolimus levels were associated with worse HRQoL, whereas whole blood tacrolimus levels did not show a statistically significant association. We found that fatigue and reduced kidney function mediated part of the associations between plasma tacrolimus concentrations and HRQoL. Additionally, we demonstrated that plasma and whole blood tacrolimus levels cannot be used interchangeably due to bias and poor agreement between these two methods in KTRs. The daily administered tacrolimus dose was strongly associated with tacrolimus concentrations, measured in either whole blood or plasma.
When comparing the method of determining plasma tacrolimus levels to whole blood tacrolimus levels, Passing–Bablok regression and Bland–Altman ratio difference plots showed that plasma and whole blood tacrolimus cannot be used interchangeably. We demonstrated that high whole blood tacrolimus levels do not necessarily reflect high plasma tacrolimus levels. This conclusion is supported by the findings of Sikma et al., who showed a non-linear relationship between whole blood and unbound tacrolimus plasma concentrations in recipients early after thoracic organ transplantation, especially for higher whole blood concentrations [4]. Interestingly, both whole blood and plasma tacrolimus concentrations were significantly associated with daily tacrolimus dose; however, the association between particularly higher whole blood tacrolimus concentrations and dose seems less evident (see Figure 4). In clinical practice, this means that TDM based on whole blood tacrolimus concentration, especially at higher levels, seems less reliable. In line with this, it was shown that patients with a low hematocrit were likely to have lower whole blood tacrolimus concentrations while the unbound concentration or plasma concentration remained around the population mean [4,41]. This phenomenon could be explained by saturation of the binding receptors for FK506 (tacrolimus) on erythrocytes which are the immunophilins FKBP12 and FKBP13 [25]. Consequently, in clinical practice, dose adjustment based on whole blood TDM in patients with low hematocrit levels would most likely lead to overdosing and consequently increase the risk of tacrolimus-related toxicity. Another interesting observation is that CYP3A4/5 genetic polymorphisms are associated with variations in the absorption and clearance of tacrolimus during intestinal and hepatic metabolism, resulting in changes to the exposure and starting dose of tacrolimus [42,43]. Since information on these genetic polymorphisms is lacking in the current study cohort, it remains unclear whether these variations are equally detectable in plasma tacrolimus levels. Interestingly, CYP3A5 genetic polymorphisms have so far not been shown to influence IPV of whole blood tacrolimus [44]. Future studies should investigate whether the IPV of plasma tacrolimus concentrations is affected by these genetic polymorphisms.
In the current study, we found, compared to whole-blood tacrolimus levels, a statistically significant association of plasma tacrolimus levels with lower levels of PCS and MCS (HRQoL) in KTRs. Considering the pharmacokinetics of tacrolimus, our findings confirm the important role of the free fraction of tacrolimus as the effective part, which was previously shown to be linearly related to plasma tacrolimus concentrations [4]. Measuring plasma tacrolimus concentrations might more accurately reflect toxicologically relevant concentrations of the drug and may therefore better correlate with clinical outcomes such as HRQoL. Interestingly, several studies have suggested the use of hematocrit-corrected whole blood concentrations in clinical practice, considering the bio-analytical challenges of determining plasma tacrolimus concentrations due to the susceptibility to hemolysis [4,45]. However, as shown in a sensitivity analysis of the current study, plasma tacrolimus concentrations were statistically significantly associated with worse PCS and MCS of HRQoL, whereas hematocrit-corrected whole blood levels did not exhibit any association with HRQoL (see Appendix A, Table A2). Previous studies have shown that many adverse effects of tacrolimus are dose-related, i.e., nephrotoxicity, neurotoxicity, glucose metabolism disturbances, and gastrointestinal disturbances [46]. In line with existing literature, we showed that fatigue and reduced kidney function partially mediated the relationship between plasma tacrolimus concentrations and HRQoL in KTRs. To summarize, our data may suggest the superiority of measuring plasma tacrolimus concentrations for TDM in minimizing post-transplant side effects. However, future research is needed to investigate the relationship between plasma tacrolimus concentrations and the main therapeutic goals of preventing graft rejection and ensuring graft survival.
This study is a first step in investigating whether the above-described non-linear relationship between whole blood and plasma tacrolimus levels is reflected in tacrolimus-related toxicity as expressed by HRQoL. Important to note is that several medications commonly used among KTRs, including antifungal agents and calcium channel blockers, can affect tacrolimus concentrations, which should be considered when interpreting the results [47]. Medication use in this study population is displayed in Appendix D (Table A13).
A limitation of the current study was that the cross-sectional study design did not allow the analysis of causal relationships between tacrolimus levels and quality of life; longitudinal studies are required to assess the effect of the treatment. Tacrolimus concentrations are known to vary widely between and within patients, and a single trough concentration is limited in estimating the area under the blood concentration–time curve (AUC), which reflects total drug exposure. However, trough concentrations are used in common practice and have been correlated with clinical outcomes, where the patients in this cohort are at least 12 months after transplantation and therefore regarded as relatively stable [48,49]. For the subanalysis of IPV, it is important to note that we had a relatively low number of patients with repeated measurements at two time points. For future research, analyzing IPV for plasma tacrolimus concentrations and transplantation outcomes, including HRQoL and rejection, with a larger patient cohort and more frequent plasma tacrolimus measurements post-transplantation would be of great interest. To confirm the clinical significance of the findings, future studies should seek to confirm the potential superiority of plasma tacrolimus concentrations for TDM in a prospective study comparing patient outcomes of groups whose TDM is either based on whole blood or plasma tacrolimus concentrations, where blood is sampled on multiple time points after drug administration.
5. Conclusions
Plasma tacrolimus concentrations were statistically significantly associated with worse HRQoL in KTRs, contrary to the current clinical standard of measuring whole blood tacrolimus levels. This suggests that TDM with plasma tacrolimus concentrations may offer an opportunity to reduce the occurrence of tacrolimus-related toxicity.
Author Contributions
Conceptualization, S.N., J.C.S., T.J.K., T.R.Z., D.J.T. and S.J.L.B.; methodology, S.N., J.C.S., I.M.N., H.R.M. and J.R.B.; formal analysis, S.N., J.C.S. and I.M.N.; investigation, S.N., J.C.S., T.J.K., M.v.L. and N.L.R.; data curation, T.J.K.; writing—original draft preparation, S.N. and J.C.S.; writing—review and editing, T.J.K., I.M.N., T.R.Z., H.R.M., M.v.L., N.L.R., J.R.B., R.K.W., G.D., S.P.B., D.J.T. and S.J.L.B.; visualization, S.N., J.C.S. and I.M.N.; supervision, H.R.M., R.K.W., G.D., S.P.B., D.J.T. and S.J.L.B.; project administration, T.J.K. and S.J.L.B.; funding acquisition, S.J.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The TransplantLines Biobank and Cohort study was supported by grants from Astellas BV (project code: TransplantLines Biobank and Cohort study), Chiesi Pharmaceuticals BV (project code: PA-SP/PRJ-2020-9136), and NWO/TTW via a partnership program with DSM, Animal Nutrition and Health, The Netherlands (project code: 14939). The project was co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of so-called PPP-allowances, made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships (project code: PPP-2019-032 and PPP-2022-015). The funders had no role in the study design, data collection, analysis, reporting, or the decision to submit for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the University Medical Center Groningen (METc 2014/077; date: 25 August 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the sensitivity of the data and the restrictions from the informed consent but are available from the corresponding author on reasonable request.
Acknowledgments
This study was performed using the infrastructure and data provided by the TransplantLines Biobank and Cohort Study, which is registered at ClinicalTrials.gov under the identifier NCT03272841. We would like to thank J. Roggeveld, laboratory technician at the UMCG, who was involved in the tacrolimus analyses. We would also like to thank Shuyan Zhang for her valuable comments and contributions to the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CI | Confidence Interval |
| CIS | Checklist Individual Strength |
| HRQoL | Health-Related Quality of Life |
| IPV | Intra-patient variability |
| KTR | Kidney Transplant Recipient |
| LOA | Limits of Agreement |
| MCS | Mental Component Summary |
| MTSOSD-59R | 59-item Modified Transplant Symptom Occurrence and Symptom Distress Scale |
| PCS | Physical Component Summary |
| PTDM | Post-transplantation Diabetes Mellitus |
| SD | Standard Deviation |
| SF-36 | 36-item Short-Form Health Survey |
| TAC | Tacrolimus |
| TDM | Therapeutic Drug Monitoring |
| UMCG | University Medical Center Groningen |
Appendix A

Table A1.
Testing for potential effect modification.
Table A1.
Testing for potential effect modification.
| Coef β (95% CI) | St. β | p-Value | ||
|---|---|---|---|---|
| Model 1: PCS ~ Whole blood TAC | Interaction term Whole blood TAC × Age | 0.02 (−0.01–0.06) | 0.004 | 0.18 |
| Interaction term Whole blood TAC × Sex | 0.56 (−0.31–1.43) | 0.11 | 0.21 | |
| Model 2: PCS ~ Plasma TAC | Interaction term Plasma TAC × Age | −0.24 (−1.24–0.76) | −0.002 | 0.64 |
| Interaction term Plasma TAC × Sex | 10.18 (−12.42–32.79) | 0.07 | 0.38 | |
| Model 3: MCS ~ Whole blood TAC | Interaction term Whole blood TAC × Age | 0.02 (−0.002–0.06) | 0.01 | 0.07 |
| Interaction term Whole blood TAC × Sex | 0.39 (−0.35–1.13) | 0.09 | 0.30 | |
| Model 4: MCS ~ Plasma TAC | Interaction term Plasma TAC × Age | −0.21 (−1.06–0.64) | −0.002 | 0.63 |
| Interaction term Plasma TAC × Sex | 5.86 (−13.27–24.99) | 0.05 | 0.55 |
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Linear regression analyses, testing for effect modification by age or sex through adding interaction term to the model.

Table A2.
Association of whole blood and plasma TAC concentrations with HRQoL (with adjustment for hematocrit).
Table A2.
Association of whole blood and plasma TAC concentrations with HRQoL (with adjustment for hematocrit).
| Whole Blood TAC (Adjusted Model *) | Plasma TAC (Crude Model) | p-Value 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Coef β (95% CI) | St. β | p-Value | N | Coef β (95% CI) | St. β | p-Value | ||
| PCS | 614 | −0.21 (−0.62–0.21) | −0.04 | 0.33 | 593 | −16.94 (−27.97–5.90) | −0.12 | 0.003 | 0.01 |
| MCS | 614 | −0.05 (−0.41–0.30) | −0.01 | 0.76 | 593 | −16.01 (−25.34–6.68) | −0.14 | <0.001 | <0.001 |
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Outcome variable: PCS or MCS, HRQoL. Linear regression analyses, normal distribution of residuals was checked. 1 Testing proportional difference of bootstrap standardized β (1000 replications) * Adjusted for hematocrit.
Appendix B
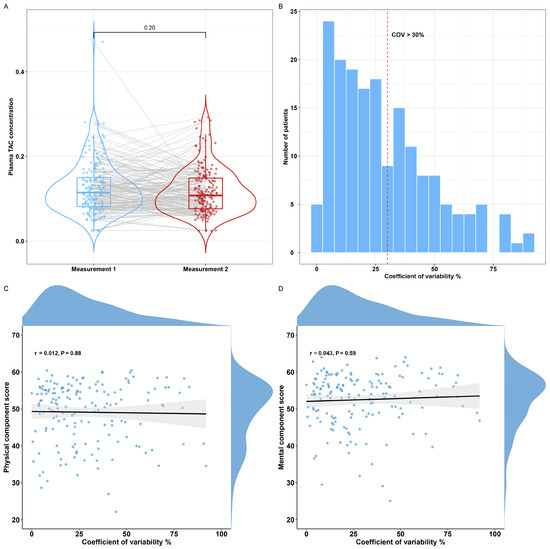
Figure A1.
Overview of the intra-patient variability for plasma tacrolimus. (A) Boxplot depicting plasma concentrations of tacrolimus for repeated measurements of 162 patients. (B) Histogram depicting the coefficient of variability for repeated plasma tacrolimus measurements with a COV > 30% indicating a high intra-patient variability (red dotted line). (C,D) Correlation plot for the coefficient of variability and the PCS and MCS. PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus, COV: coefficient of variability.
Appendix C
In the following, the results of the mediation analyses of the various tacrolimus-related side effects and their effect on the association between plasma tacrolimus concentrations and the PCS and MCS of HRQoL are summarized.
Figure A2.
Illustration of potential mediating pathway.
Appendix C.1. Potential Mediator Fatigue Severity
Fatigue severity fulfilled the assumptions for mediation analyses (see Table A3). Fatigue severity was found to be a partial mediator of the effect of plasma tacrolimus on the PCS (proportion mediated: 67.8%) and the MCS (proportion mediated: 59.5%), without significant direct effect (see Table A4).

Table A3.
Association between fatigue severity and plasma tacrolimus (checking assumptions).
Table A3.
Association between fatigue severity and plasma tacrolimus (checking assumptions).
| Outcome: Fatigue Severity | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | 20.09 (5.69; 34.49) | 0.11 | 0.01 |
| Outcome: PCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −5.67 (−12.98; 1.64) | −0.04 | 0.13 |
| Fatigue severity, CIS20-R | −0.54 (−0.58; −0.50) | −0.004 | <0.001 |
| Outcome: MCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −6.58 (−13.09; −0.07) | −0.06 | 0.05 |
| Fatigue severity, CIS20-R | −0.44 (−0.47; −0.41) | −0.004 | <0.001 |
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Linear regression analyses, normal distribution of residuals was checked.

Table A4.
Mediation analyses for the effects of fatigue on the suggested causal effects of tacrolimus on HRQoL.
Table A4.
Mediation analyses for the effects of fatigue on the suggested causal effects of tacrolimus on HRQoL.
| Multivariable Model | |||||
|---|---|---|---|---|---|
| Effect | B (95% CI) | p-Value | Proportion Mediated | p-Value | |
| PCS of HRQoL | Indirect effect | −0.09 (−0.15; −0.02) | 0.01 | 67.8% | 0.01 |
| Direct effect | −0.04 (−0.10; 0.01) | 0.14 | |||
| Total effect | −0.13 (−0.21; −0.04) | 0.01 | |||
| MCS of HRQoL | Indirect effect | −0.08 (−0.14; −0.02) | 0.01 | 59.5% | 0.02 |
| Direct effect | −0.06 (−0.12; 0.00) | 0.05 | |||
| Total effect | −0.14 (−0.23; −0.05) | 0.002 | |||
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Potential mediator: Fatigue severity (CIS20-R). Regression coefficients (B) are presented alongside 95% confidence intervals. Mediation analyses were performed as proposed by Preacher and Hayes [38] using 1000 bootstrapped samples. The size of the mediated effect is calculated as the indirect effect divided by the total effect multiplied by 100%.
Appendix C.2. Potential Mediator Sleep Quality
Plasma tacrolimus was not significantly associated with sleep quality, as assessed by the PSQI (see Table A5). Therefore, no further mediation analyses were performed.

Table A5.
Association between sleep quality and plasma tacrolimus (checking assumptions).
Table A5.
Association between sleep quality and plasma tacrolimus (checking assumptions).
| Outcome: Sleep Quality | |||
|---|---|---|---|
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | 2.42 (−1.43; 6.28) | 0.05 | 0.21 |
TAC: tacrolimus. Linear regression analyses, normal distribution of residuals was checked.
Appendix C.3. Potential Mediator Gut Microbiome Outcomes
Plasma tacrolimus was significantly associated with the Shannon Diversity Index (st. β = −0.14; p = 0.002); however, no significant association between the Shannon Diversity Index and PCS and MCS was found (see Table A6). Therefore, no further mediation analyses were performed.
Plasma tacrolimus was not significantly associated with diarrhea, as assessed by the water percentage in stool samples (see Table A7). Therefore, no further mediation analyses were performed.

Table A6.
Association between Shannon Diversity Index and plasma tacrolimus (checking assumptions).
Table A6.
Association between Shannon Diversity Index and plasma tacrolimus (checking assumptions).
| Outcome: Shannon Diversity Index | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −1.11 (−1.80; −0.41) | −0.14 | 0.002 |
| Outcome: PCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −13.17 (−26.55; 0.21) | −0.10 | 0.05 |
| Shannon Diversity Index | 0.22 (−1.53; 1.97) | 0.002 | 0.80 |
| Outcome: MCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −11.90 (−23.15; −0.64) | −0.10 | 0.04 |
| Shannon Diversity Index | 0.27 (−1.20; 1.74) | 0.002 | 0.72 |
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Linear regression analyses, normal distribution of residuals was checked.

Table A7.
Association between diarrhea and plasma tacrolimus (checking assumptions).
Table A7.
Association between diarrhea and plasma tacrolimus (checking assumptions).
| Outcome: Diarrhea | |||
|---|---|---|---|
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −2.63 (−13.35; 8.09) | −0.03 | 0.63 |
TAC: tacrolimus. Linear regression analyses, normal distribution of residuals was checked.
Appendix C.4. Potential Mediator Kidney Function
Kidney function assessed as eGFR fulfilled the assumptions for mediation analyses (see Table A8). Lower kidney function was found to be a partial mediator of the effect of plasma tacrolimus on the PCS (proportion mediated: 16.7%) and the MCS (proportion mediated: 12.9%). The direct effect between kidney function and the PCS (st. β = −0.11; p = 0.03) and the MCS (st. β = −0.12, p = 0.004) remained statistically significant (see Table A9).

Table A8.
Association between kidney function (eGFR) and plasma tacrolimus (checking assumptions).
Table A8.
Association between kidney function (eGFR) and plasma tacrolimus (checking assumptions).
| Outcome: eGFR | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −35.59 (−53.55; −17.62) | −0.14 | <0.001 |
| Outcome: PCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −13.82 (−24.91; −2.73) | −0.10 | 0.02 |
| Kidney function, eGFR | 0.07 (0.03; 0.11) | 0.001 | <0.001 |
| Outcome: MCS | |||
| B (95% CI) | St. β | p-Value | |
| Plasma TAC | −13.76 (−23.17; −4.37) | −0.12 | 0.004 |
| Kidney function, eGFR | 0.05 (0.02; 0.09) | 0.0004 | 0.01 |
eGFR: estimated glomerular filtration rate, PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Linear regression analyses, normal distribution of residuals was checked.

Table A9.
Mediation analyses for the effects of kidney function on the suggested causal effects of tacrolimus on HRQoL.
Table A9.
Mediation analyses for the effects of kidney function on the suggested causal effects of tacrolimus on HRQoL.
| Multivariable Model | |||||
|---|---|---|---|---|---|
| Effect | B (95% CI) | p-Value | Proportion Mediated | p-Value | |
| PCS of HRQoL | Indirect effect | −0.02 (−0.04; −0.01) | 0.002 | 16.7% | 0.01 |
| Direct effect | −0.11 (−0.19; −0.02) | 0.03 | |||
| Total effect | −0.13 (−0.21; −0.04) | 0.004 | |||
| MCS of HRQoL | Indirect effect | −0.02 (−0.04; 0.00) | 0.02 | 12.9% | 0.02 |
| Direct effect | −0.12 (−0.21; −0.03) | 0.004 | |||
| Total effect | −0.14 (−0.23; −0.06) | <0.001 | |||
PCS: physical component summary, MCS: mental component summary, TAC: tacrolimus. Potential mediator: Kidney function (eGFR). Regression coefficients (B) are presented alongside 95% confidence intervals. Mediation analyses were performed as proposed by Preacher and Hayes [38] using 1000 bootstrapped samples. The size of the mediated effect is calculated as the indirect effect divided by the total effect multiplied by 100%.
Appendix C.5. Potential Mediator Post-Transplantation Diabetes Mellitus
Plasma tacrolimus was not significantly associated with the binary outcome post-transplantation diabetes mellitus (see Table A10). Therefore, no further mediation analyses were performed.

Table A10.
Association between post-transplantation diabetes mellitus and plasma tacrolimus (checking assumptions).
Table A10.
Association between post-transplantation diabetes mellitus and plasma tacrolimus (checking assumptions).
| Outcome: PTDM | ||
|---|---|---|
| OR (95% CI) | p-Value | |
| Plasma TAC | 1.11 (0.93; 1.31) | 0.23 |
PTDM: post-transplantation diabetes mellitus, TAC: tacrolimus. Binomial logistic regression analysis.
Appendix C.6. Potential Mediator Alopecia
Plasma tacrolimus was not significantly associated with the binary outcome alopecia, as assessed as part of the MTSOSD-59R (see Table A11). Therefore, no further mediation analyses were performed.

Table A11.
Association between alopecia and plasma tacrolimus (checking assumptions).
Table A11.
Association between alopecia and plasma tacrolimus (checking assumptions).
| Outcome: Alopecia | ||
|---|---|---|
| OR (95% CI) | p-Value | |
| Plasma TAC | 1.09 (0.93; 1.28) | 0.31 |
TAC: tacrolimus. Binomial logistic regression analysis.
Appendix C.7. Potential Mediator Tremor
Plasma tacrolimus was not significantly associated with tremor, as assessed by the TRS-C (see Table A12). Therefore, no further mediation analyses were performed.

Table A12.
Association between tremor and plasma tacrolimus (checking assumptions).
Table A12.
Association between tremor and plasma tacrolimus (checking assumptions).
| Outcome: Tremor | ||
|---|---|---|
| IRR (95% CI) | p-Value | |
| Plasma TAC | 1.17 (0.86; 1.59) | 0.31 |
TAC: tacrolimus. Negative binomial regression due to Poisson distribution with overdispersion of outcome variable tremor.
Appendix D

Table A13.
Medication use.
Table A13.
Medication use.
| N (%) | |
| Other immunosuppressants | |
| Prednisolone | 873 (97.3) |
| Mycophenolate mofetil | 718 (80.0) |
| Azathioprine | 51 (5.7) |
| MTOR inhibitors (everolimus or sirolimus) | 42 (4.7) |
| Antihypertensives | |
| Beta-blockers | 538 (60.0) |
| Calcium channel blockers | 407 (45.4) |
| Alpha blockers | 136 (15.2) |
| Diuretics | 213 (23.8) |
| Angiotensin-converting enzyme inhibitors | 173 (19.3) |
| Angiotensin II receptor blockers | 111 (12.4) |
| Lipid-lowering treatment | |
| Statins | 508 (56.6) |
| Gemfibrozil | 50 (5.6) |
| Ezetimibe | 16 (1.8) |
| Antidiabetic Medication | |
| Metformin | 100 (11.2) |
| Sulfonylureas | 53 (5.9) |
| SGLT2 inhibitors | 3 (0.3) |
| DPP4 inhibitors | 10 (1.1) |
| GLP-1 receptor agonists | 5 (0.6) |
| Insulin | 121 (13.5) |
| Anticoagulants | |
| Acetylsalicylic acid | 188 (21.0) |
| Dipyridamole | 5 (0.6) |
| Clopidogrel | 48 (5.4) |
| Apixaban | 12 (1.3) |
| Non-vitamin K oral anticoagulants | 12 (2.0) |
| Vitamin K antagonists | 105 (11.7) |
| Gastroprotective agents | |
| Proton pump inhibitors | 661 (73.7) |
| H2-receptor antagonists | 32 (3.6) |
| Antacids | 11 (1.2) |
| Mucosal protective agents | 3 (0.3) |
| Antibiotics | |
| Sulfonamides | 35 (3.9) |
| Fluoroquinolones | 14 (1.6) |
| Macrolides | 10 (1.1) |
| Penicillin | 2 (0.2) |
| Antifungal agents | |
| Imidazoles | 16 (1.8) |
| Antidepressant Medication | |
| Tricyclic antidepressants | 22 (2.5) |
| Selective serotonin reuptake inhibitors | 29 (3.2) |
| Sleep medication | |
| Benzodiazepines | 65 (7.3) |
| Melatonin agonists | 3 (0.3) |
References
- Sikma, M.A.; van Maarseveen, E.M.; van de Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Donker, D.W.; Kesecioglu, J.; Meulenbelt, J. Pharmacokinetics and Toxicity of Tacrolimus Early after Heart and Lung Transplantation. Am. J. Transplant. 2015, 15, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, H.E.; Darley, D.R.; Brett, J.; Day, R.O.; Carland, J.E. Identifying the association between tacrolimus exposure and toxicity in heart and lung transplant recipients: A systematic review. Transplant. Rev. 2021, 35, 100610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hemmelder, M.H.; Bos, W.J.W.; Snoep, J.D.; de Vries, A.P.J.; Dekker, F.W.; Meuleman, Y. Mapping health-related quality of life after kidney transplantation by group comparisons: A systematic review. Nephrol. Dial. Transplant. 2021, 36, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Sikma, M.A.; Van Maarseveen, E.M.; Hunault, C.C.; Moreno, J.M.; Van de Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Grutters, J.C.; Kesecioglu, J.; De Lange, D.W.; et al. Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation. Clin. Pharmacokinet. 2020, 59, 771–780. [Google Scholar] [CrossRef]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Bouamar, R.; Shuker, N.; Hesselink, D.A.; Weimar, W.; Ekberg, H.; Kaplan, B.; Bernasconi, C.; van Gelder, T. Tacrolimus Predose Concentrations Do Not Predict the Risk of Acute Rejection After Renal Transplantation: A Pooled Analysis From Three Randomized-Controlled Clinical Trials. Am. J. Transplant. 2013, 13, 1253–1261. [Google Scholar] [CrossRef]
- Udomkarnjananun, S.; Schagen, M.R.; Volarević, H.; van de Velde, D.; Dieterich, M.; Matic, M.; Baan, C.C.; Reinders, M.E.J.; de Winter, B.C.M.; Hesselink, D.A. Prediction of the Intra-T Lymphocyte Tacrolimus Concentration after Kidney Transplantation with Population Pharmacokinetic Modeling. Clin. Pharmacol. Ther. 2025, 117, 162–173. [Google Scholar] [CrossRef]
- Lemaitre, F.; Blanchet, B.; Latournerie, M.; Antignac, M.; Houssel-Debry, P.; Verdier, M.-C.; Dermu, M.; Camus, C.; Le Priol, J.; Roussel, M.; et al. Pharmacokinetics and pharmacodynamics of tacrolimus in liver transplant recipients: Inside the white blood cells. Clin. Biochem. 2015, 48, 406–411. [Google Scholar] [CrossRef]
- Lemaitre, F.; Vethe, N.T.; D’Avolio, A.; Tron, C.; Robertsen, I.; De Winter, B.; Denicolo, A.; Koch, B.C.P.; Venkataramanan, R.; Van Gelder, T.; et al. Measuring Intracellular Concentrations of Calcineurin Inhibitors: Expert Consensus from the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Expert Panel. Ther. Drug Monit. 2020, 42, 665–670. [Google Scholar] [CrossRef]
- Han, S.S.; Yang, S.H.; Kim, M.C.; Cho, J.-Y.; Min, S.-I.; Lee, J.P.; Kim, D.K.; Ha, J.; Kim, Y.S. Monitoring the intracellular tacrolimus concentration in kidney transplant recipients with stable graft function. PLoS ONE 2016, 11, e0153491. [Google Scholar] [CrossRef]
- Stienstra, N.A.; Sikma, M.A.; Van Dapperen, A.L.; De Lange, D.W.; Van Maarseveen, E.M. Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC-MS/MS. Ther. Drug Monit. 2016, 38, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Udomkarnjananun, S.; Eiamsitrakoon, T.; de Winter, B.C.M.; van Gelder, T.; Hesselink, D.A. Should we abandon therapeutic drug monitoring of tacrolimus in whole blood and move to intracellular concentration measurements? Br. J. Clin. Pharmacol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and design of TransplantLines: A prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Hecht, M.; Veigure, R.; Couchman, L.; Barker, C.I.S.; Standing, J.F.; Takkis, K.; Evard, H.; Johnston, A.; Herodes, K.; Leito, I.; et al. Utilization of data below the analytical limit of quantitation in pharmacokinetic analysis and modeling: Promoting interdisciplinary debate. Bioanalysis 2018, 10, 1229–1248. [Google Scholar] [CrossRef]
- Koster, R.A.; Dijkers, E.C.F.; Uges, D.R.A. Robust, high-throughput LC-MS/MS method for therapeutic drug monitoring of cyclosporine, tacrolimus, everolimus, and sirolimus in whole blood. Ther. Drug Monit. 2009, 31, 116–125. [Google Scholar] [CrossRef]
- Zijp, T.R.; van Hateren, K.; Kuiper, H.; Jongedijk, E.M.; Touw, D.J. Ultra-high throughput dual channel liquid chromatography with tandem mass spectrometry for quantification of four immunosuppressants in whole blood for therapeutic drug monitoring. J. Chromatogr. A 2023, 1702, 464086. [Google Scholar] [CrossRef]
- Zijp, T.R.; Knobbe, T.J.; van Hateren, K.; Roggeveld, J.; Blokzijl, H.; Gan, C.T.; Bakker, S.J.; Jongedijk, E.M.; TransplantLines Investigators; Touw, D.J. Expeditious quantification of plasma tacrolimus with liquid chromatography tandem mass spectrometry in solid organ transplantation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1222, 123709. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Muller, M.; Cohen, P.D.; Essink-Bot, M.-L.; Fekkes, M.; Sanderman, R.; Sprangers, M.A.; Velde, A.t.; Verrips, E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J. Clin. Epidemiol. 1998, 51, 1055–1068. [Google Scholar] [CrossRef]
- Laucis, N.C.; Hays, R.D.; Bhattacharyya, T. Scoring the SF-36 in orthopaedics: A brief guide. J. Bone Jt. Surg—Am. Vol. 2014, 97, 1628–1634. [Google Scholar] [CrossRef]
- Taft, C.; Karlsson, J.; Sullivan, M. Do SF-36 Summary Component Scores Accurately Summarize Subscale Scores ? Qual. Life Res. 2001, 10, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Farivar, S.S.; Cunningham, W.E.; Hays, R.D. Correlated physical and mental health summary scores for the SF-36 and SF-12 Health Survey, V.1. Health Qual. Life Outcomes 2007, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Worm-Smeitink, M.; Gielissen, M.; Bloot, L.; van Laarhoven, H.; van Engelen, B.; van Riel, P.; Bleijenberg, G.; Nikolaus, S.; Knoop, H. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J.; Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Swarte, J.C.; Li, Y.; Hu, S.; Björk, J.R.; Gacesa, R.; Vila, A.V.; Douwes, R.M.; Collij, V.; Kurilshikov, A.; Post, A.; et al. Gut microbiome dysbiosis is associated with increased mortality after solid organ transplantation. Sci. Transl. Med. 2022, 14, eabn7566. [Google Scholar] [CrossRef]
- Reese, A.T.; Dunn, R.R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. mBio 2018, 9, e01294-18. [Google Scholar] [CrossRef]
- Douwes, R.M.; Swarte, J.C.; Post, A.; Annema, C.; Harmsen, H.J.M.; Bakker, S.J.L. Discrepancy between self-perceived mycophenolic acid-associated diarrhea and stool water content after kidney transplantation. Clin. Transplant. 2021, 35, e14321. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) Creatinine Equation: More Accurate GFR Estimates, Lower CKD Prevalence Estimates, and Better Risk Predictions. Am. J. Kidney Dis. 2010, 55, 622–627. [Google Scholar] [CrossRef]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef]
- Werzowa, J.; Hecking, M.; Haidinger, M.; Döller, D.; Sharif, A.; Tura, A.; Säemann, M.D. The Diagnosis of Posttransplantation Diabetes Mellitus: Meeting the Challenges. Curr. Diabetes Rep. 2015, 15, 27. [Google Scholar] [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Dobbels, F.; Moons, P.; Abraham, I.; Larsen, C.P.; Dupont, L.; De Geest, S. Measuring symptom experience of side-effects of immunosuppressive drugs: The Modified Transplant Symptom Occurence and Distress Scale. Transpl. Int. 2008, 21, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Tolosa, E.; Marin, C. Clinical Rating Scale for Tremor. In Parkinson’s Disease and Movement Disorders, 2nd ed.; Urban & Schwarzenberg: Munich, Germany, 1993; pp. 225–234. [Google Scholar]
- Elble, R.; Bain, P.; João Forjaz, M.; Haubenberger, D.; Testa, C.; Goetz, C.G.; Leentjens, A.F.G.; Martinez-Martin, P.; Traon, A.P.; Post, B.; et al. Task force report: Scales for screening and evaluating tremor: Critique and recommendations. Mov. Disord. 2013, 28, 1793–1800. [Google Scholar] [CrossRef]
- Bilic-Zulle, L. Comparison of methods: Passing and Bablok regression. Biochem. Med. 2011, 21, 49–52. [Google Scholar] [CrossRef]
- Haghayegh, S.; Kang, H.A.; Khoshnevis, S.; Smolensky, M.H.; Smolensky, M.H.; Diller, K.R. A comprehensive guideline for Bland-Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol. Meas. 2020, 41, 055012. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; EMA Guidance Document; EMA: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Preacher, K.J.; Hayes, A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. Comput. 2004, 36, 717–731. [Google Scholar] [CrossRef]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Piburn, K.H.; Sigurjonsdottir, V.K.; Indridason, O.S.; Maestretti, L.; Patton, M.V.; McGrath, A.; Palsson, R.; Gallo, A.; Chaudhuri, A.; Grimm, P.C. Patterns in Tacrolimus Variability and Association with De Novo Donor-Specific Antibody Formation in Pediatric Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2022, 17, 1194–1203. [Google Scholar] [CrossRef]
- Koomen, J.V.; Knobbe, T.J.; Zijp, T.R.; Kremer, D.; Gan, C.T.; Verschuuren, E.A.; Bakker, S.J.; Touw, D.J.; Colin, P.J. A Joint Pharmacokinetic Model for the Simultaneous Description of Plasma and Whole Blood Tacrolimus Concentrations in Kidney and Lung Transplant Recipients. Clin. Pharmacokinet. 2023, 62, 1117–1128. [Google Scholar] [CrossRef]
- Mohammed Ali, Z.; Meertens, M.; Fernández, B.; Fontova, P.; Vidal-Alabró, A.; Rigo-Bonnin, R.; Melilli, E.; Cruzado, J.M.; Grinyó, J.M.; Colom, H.; et al. CYP3A5*3 and CYP3A4*22 Cluster Polymorphism Effects on LCP-Tac Tacrolimus Exposure: Population Pharmacokinetic Approach. Pharmaceutics 2023, 15, 2699. [Google Scholar] [CrossRef]
- Pallet, N.; Jannot, A.-S.; El Bahri, M.; Etienne, I.; Buchler, M.; de Ligny, B.H.; Choukroun, G.; Colosio, C.; Thierry, A.; Vigneau, C.; et al. Kidney transplant recipients carrying the CYP3A4*22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am. J. Transplant. 2015, 15, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Nuchjumroon, A.; Vadcharavivad, S.; Singhan, W.; Poosoonthornsri, M.; Chancharoenthana, W.; Udomkarnjananun, S.; Townamchai, N.; Avihingsanon, Y.; Praditpornsilpa, K.; Eiam-Ong, S. Comparison of Tacrolimus Intra-Patient Variability during 6–12 Months after Kidney Transplantation between CYP3A5 Expressers and Nonexpressers. J. Clin. Med. 2022, 11, 6320. [Google Scholar] [CrossRef]
- Schijvens, A.M.; van Hesteren, F.H.S.; Cornelissen, E.A.M.; Bootsma-Robroeks, C.M.H.H.T.; Brüggemann, R.J.M.; Burger, D.M.; de Wildt, S.N.; Schreuder, M.F.; ter Heine, R. The potential impact of hematocrit correction on evaluation of tacrolimus target exposure in pediatric kidney transplant patients. Pediatr. Nephrol. 2019, 34, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Campagne, O.; Mager, D.E.; Brazeau, D.; Venuto, R.C.; Tornatore, K.M. The impact of tacrolimus exposure on extrarenal adverse effects in adult renal transplant recipients. Br. J. Clin. Pharmacol. 2019, 85, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, R.; Stocker, S.L.; Hennig, S.; Day, R.O.; Carland, J.E. Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin. Pharmacokinet. 2020, 59, 1357–1392. [Google Scholar] [CrossRef]
- Albilal, S.; Shawaqfeh, M.S.; Albusaysi, S.; Fetyani, L.; Alnashmi, F.; Alshehri, S.D.; Albekairy, N.A.; Akhulaif, A.; Alzahrani, L.; Alwuhayde, M.; et al. Tacrolimus Trough Level Variation and Its Correlation to Clinical Outcomes and Consequences in Solid Organ Transplantation. Transpl. Res. Risk Manag. 2023, 15, 1–11. [Google Scholar] [CrossRef]
- Gaynor, J.J.; Ciancio, G.; Guerra, G.; Sageshima, J.; Roth, D.; Goldstein, M.J.; Chen, L.; Kupin, W.; Mattiazzi, A.; Tueros, L.; et al. Lower tacrolimus trough levels are associated with subsequently higher acute rejection risk during the first 12 months after kidney transplantation. Transpl. Int. 2016, 29, 216–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).