Traditional Chinese Medicine-Loaded Hydrogels: An Emerging Strategy for the Treatment of Bone Infections
Abstract
1. Introduction
2. Classification and Advantages of Hydrogels
2.1. Classification of Hydrogels
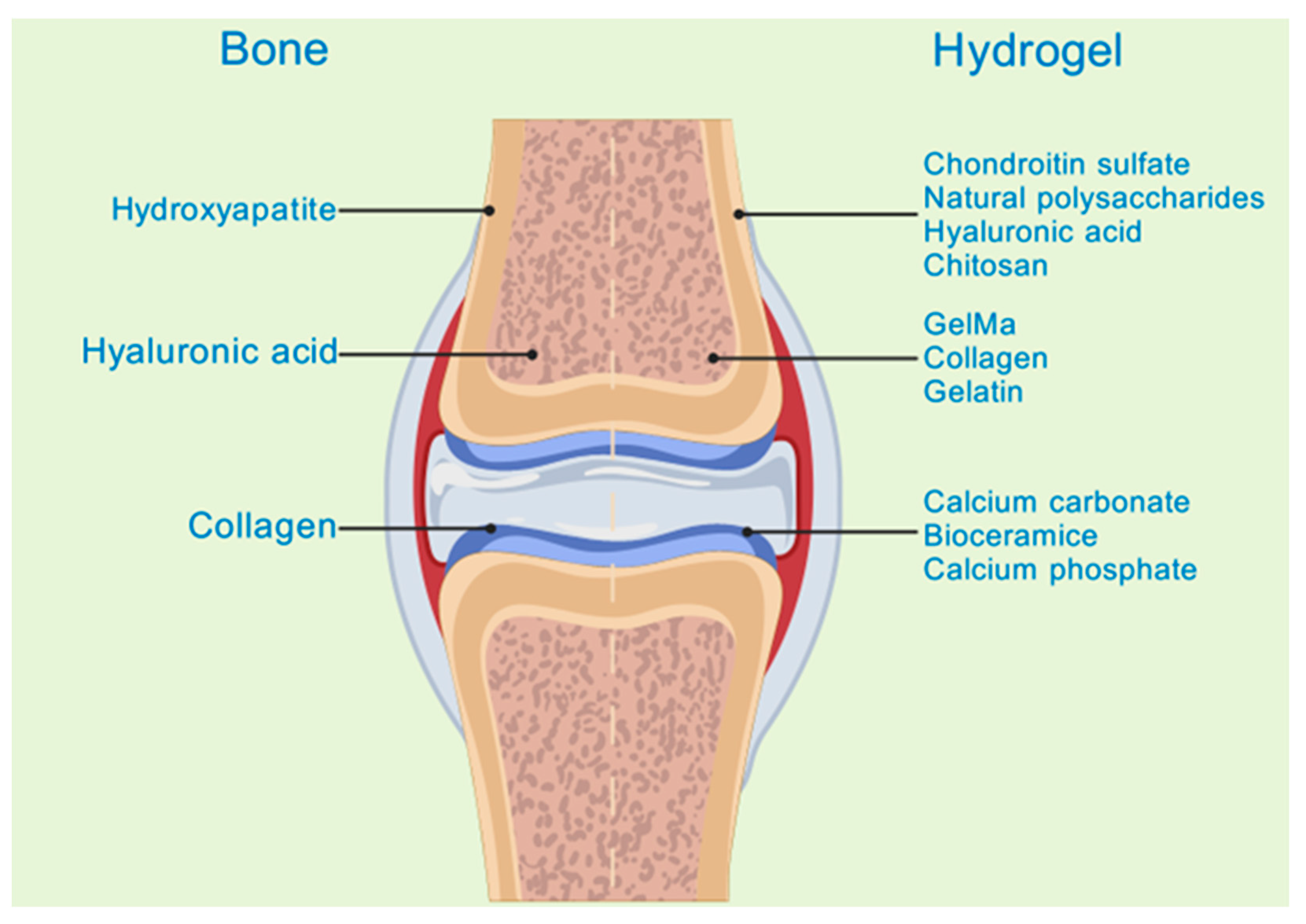
2.2. Advantages of Treating Bone Infections with Hydrogels Loaded with TCM
2.2.1. Improving Drug Stability and Bioavailability
2.2.2. Achieving Targeted Drug Delivery and Controlled Release
2.2.3. Synergistically Enhancing the Therapeutic Effect
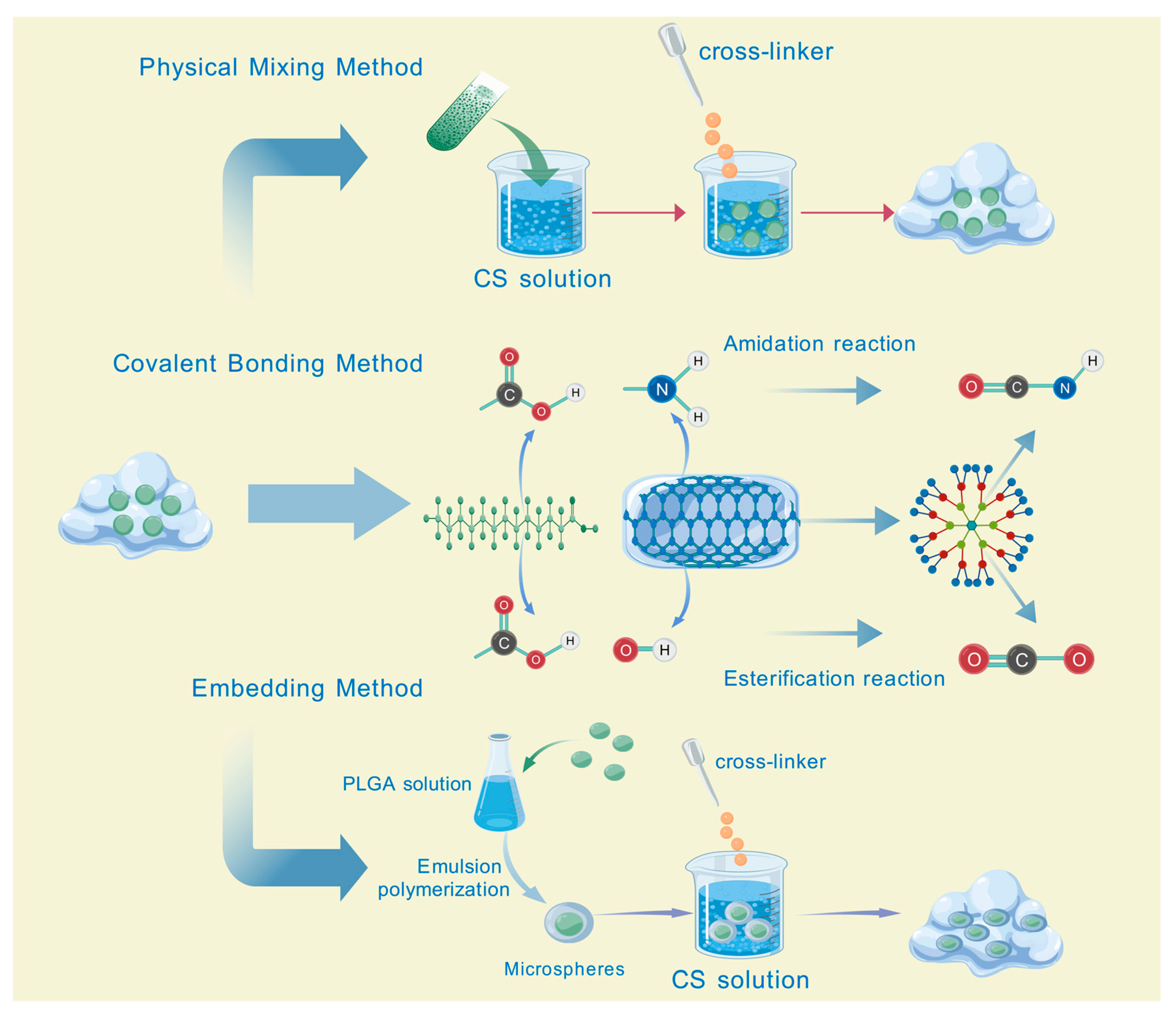
2.3. Preparation Methods of Hydrogels Loaded with TCM
2.4. The Release Mechanisms of Traditional Chinese Medicine from Hydrogels
2.5. The Stimuli for the Gel-Sol Transition of Hydrogels
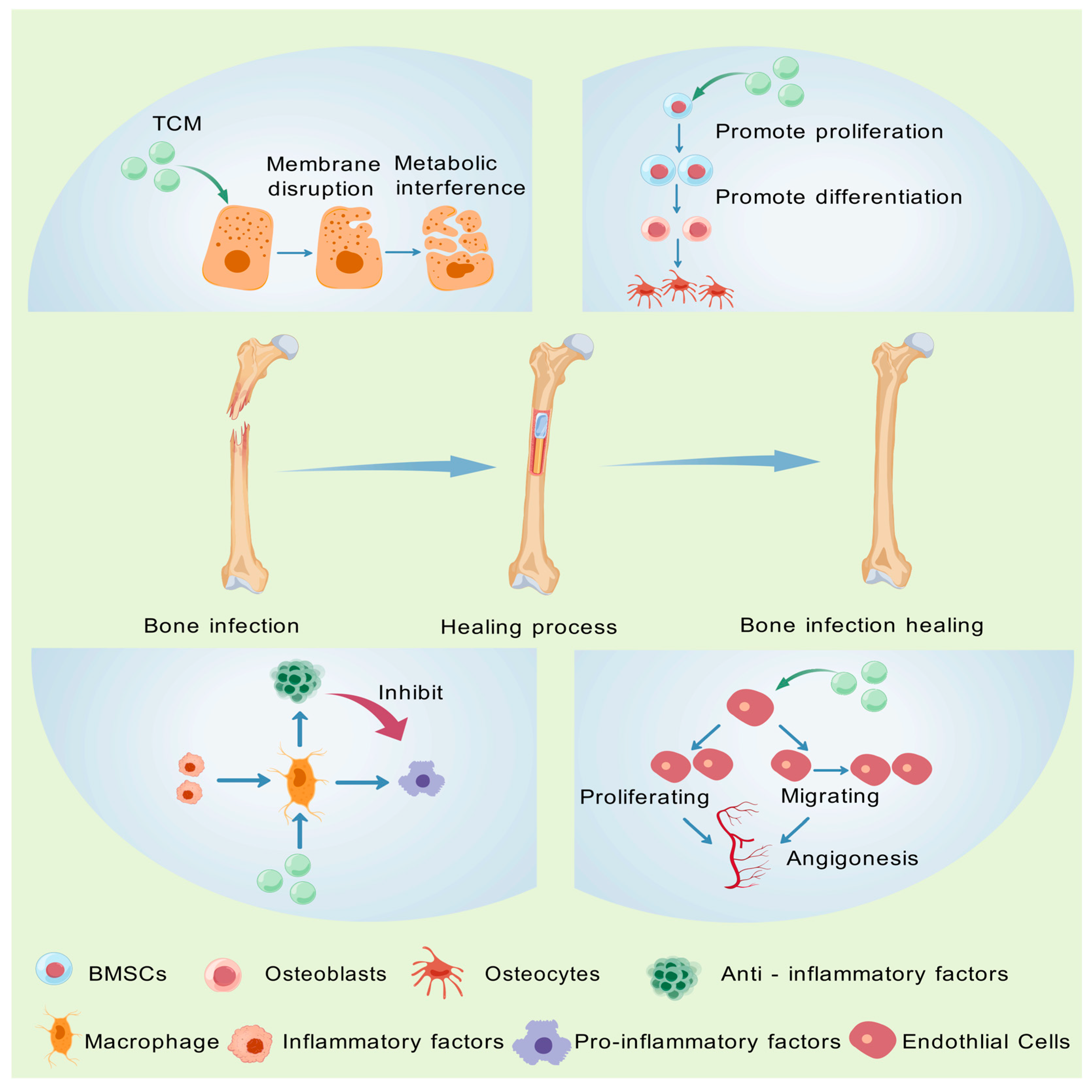
3. Mechanisms of TCM in the Treatment of Bone Infections
3.1. Antibacterial Activity
3.2. Promotion of Osteoblast Proliferation
3.3. Anti-Inflammation
3.4. Angiogenesis Promotion
3.5. Promotion of Nerve Repair
4. Challenges and Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barroco, C.; Meyssonnier, V.; Suva, D.; Pham, T.T. Management of bone and joint infections: Update. Rev. Med. Suisse 2023, 19, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.d.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Alsoof, D.; Anderson, G.; McDonald, C.L.; Basques, B.; Kuris, E.; Daniels, A.H. Diagnosis and Management of Vertebral Compression Fracture. Am. J. Med. 2022, 135, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, F.; Wang, H.; Xu, X.; Xu, S.; Zhang, C.; Zhang, Y.; Lu, M.; Zhang, Y.; Zhou, M.; et al. Systemic antibiotics increase microbiota pathogenicity and oral bone loss. Int. J. Oral Sci. 2023, 15, 4. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Fu, Z.; Liu, Y.; Man, Z.; Shi, C.; Tang, C.; Chen, C.; Chai, Q.; Yang, Z.; et al. Surficial nano-deposition locoregionally yielding bactericidal super CAR-macrophages expedites periprosthetic osseointegration. Sci. Adv. 2023, 9, eadg3365. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Li, D.; Li, X.; Liu, Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Wu, J.L.; Ma, J.C.; Wang, H.; Wen, J.; Huang, S.; Lee, M.; Bai, X.; Cui, Z.K. Intrinsic antibacterial and osteoinductive sterosomes promote infected bone healing. J. Control. Release 2023, 354, 713–725. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Du, M.D.; He, K.Y.; Fan, S.Q.; Li, J.Y.; Liu, J.F.; Lei, Z.Q.; Qin, G. The Mechanism by Which Cyperus rotundus Ameliorates Osteoarthritis: A Work Based on Network Pharmacology. J. Inflamm. Res. 2024, 17, 7893–7912. [Google Scholar] [CrossRef]
- Gao, Z.-R.; Feng, Y.-Z.; Zhao, Y.-Q.; Zhao, J.; Zhou, Y.-H.; Ye, Q.; Chen, Y.; Tan, L.; Zhang, S.-H.; Feng, Y.; et al. Traditional Chinese medicine promotes bone regeneration in bone tissue engineering. Chin. Med. 2022, 17, 86. [Google Scholar] [CrossRef]
- Yang, R.; Yan, L.; Xu, T.; Zhang, K.; Lu, X.; Xie, C.; Fu, W. Injectable bioadhesive hydrogel as a local nanomedicine depot for targeted regulation of inflammation and ferroptosis in rheumatoid arthritis. Biomaterials 2024, 311, 122706. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.M.; Gan, Y.C.; Qiu, X.Z.; Gao, Z.C.; Wang, H.; Chen, S.X.; Xiong, Y.; Liu, G.H.; Lin, S.E.; et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: New biosynthesis methods, recent advances, and emerging applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Xu, C.; Fu, H.; Ploch, M.; D’Souza, S.; Lustig, S.; Long, X.; Hong, Y.; Dai, G. 3D Bioprinting Highly Elastic PEG-PCL-DA Hydrogel for Soft Tissue Fabrication and Biomechanical Stimulation. Adv. Funct. Mater. 2024, 34, 2313942. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, W.; Zhang, C.; Li, X.; Guo, X.; Wang, Y.; Yuan, Y.; Jiang, B.; Jin, Y. Multi-Solvent-Induced Gradient Aggregation Rendered Superstrong, Tough, Stretchable, and Fatigue-Resistant Lignin-Based Supramolecular Hydrogels. Adv. Funct. Mater. 2024, 35, 2417206. [Google Scholar] [CrossRef]
- Zhong, Z.; Quiñones-Pérez, M.; Dai, Z.; Juarez, V.M.; Bhatia, E.; Carlson, C.R.; Shah, S.B.; Patel, A.; Fang, Z.; Hu, T.; et al. Human immune organoids to decode B cell response in healthy donors and patients with lymphoma. Nat. Mater. 2025, 24, 297–311. [Google Scholar] [CrossRef]
- Long, J.; Zhou, G.; Yu, X.; Xu, J.; Hu, L.; Pranovich, A.; Yong, Q.; Xie, Z.H.; Xu, C. Harnessing chemical functionality of xylan hemicellulose towards carbohydrate polymer-based pH/magnetic dual-responsive nanocomposite hydrogel for drug delivery. Carbohydr. Polym. 2024, 343, 122461. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, J.; Zhu, H.; Liu, S.; Jia, L.; Liu, W.; Wang, Q.; Wang, S.; Liu, W.; Zhou, J.; et al. Extrusion bioprinting of elastin-containing bioactive double-network tough hydrogels for complex elastic tissue regeneration. Aggregate 2024, 5, e477. [Google Scholar] [CrossRef]
- Gorodzha, S.; Douglas, T.E.L.; Samal, S.K.; Detsch, R.; Cholewa-Kowalska, K.; Braeckmans, K.; Boccaccini, A.R.; Skirtach, A.G.; Weinhardt, V.; Baumbach, T.; et al. High-resolution synchrotron X-ray analysis of bioglass-enriched hydrogels. J. Biomed. Mater. Res. Part A 2016, 104, 1194–1201. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Kirkpatrick, B.E.; Hach, G.K.; Nelson, B.R.; Skillin, N.P.; Lee, J.S.; Hibbard, L.P.; Dhand, A.P.; Grotheer, H.S.; Miksch, C.E.; Salazar, V.; et al. Photochemical Control of Network Topology in PEG Hydrogels. Adv. Mater. 2024, 36, 2409603. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Nanoclay-Composite Hydrogels for Bone Tissue Engineering. Gels 2024, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Y.; Liu, Z.; Liu, K.; Xiang, X. Balancing stretchability and conductivity: Carbon nanotube layer-enhanced non-ionic conductive hydrogels with a sandwich structure. Chem. Eng. J. 2024, 500, 156641. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Liu, Y.; Zhu, L.; Gan, X.; Jiang, S.; Zhang, X.; Dilixiati, M.; Bai, M.; Zeng, J.; Shi, S.; et al. Recent Advances in Polysaccharides Derived from the Genus Panax: Preparation Strategies, Structural Profiles, Functional Properties and Structure-Activity Relationships. J. Agric. Food Chem. 2024, 72, 26074–26097. [Google Scholar] [CrossRef]
- Xin, C.; Cheng, Z.; Liu, W.; Li, W.; Zhu, H. The antibacterial and hemostatic activity of Gastrodia elata polysaccharide-based hydrogel embedded with drug-carrying microspheres accelerates diabetic wound healing. Chem. Eng. J. 2024, 492, 152403. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Zhan, W.; Wang, Y.; Sun, X.; Zhang, Y.; Liu, X.; Han, B.; Bai, Y.; Shen, L.; et al. Sustained Release of Neuroprotective Drugs Curcumin and Edaravone from Supramolecular Hydrogel for Ischemic Stroke Treatment. Adv. Funct. Mater. 2023, 33, 2303930. [Google Scholar] [CrossRef]
- He, X.; He, S.; Xiang, G.; Deng, L.; Zhang, H.; Wang, Y.; Li, J.; Lu, H. Precise Lubrication and Protection of Cartilage Damage by Targeting Hydrogel Microsphere. Adv. Mater. 2024, 36, 2405943. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Liu, T.; Yu, X.; Bai, Y.; Zhang, Y.; Chen, X. Nanocomposite hydrogel with NIR/magnet/enzyme multiple responsiveness to accurately manipulate local drugs for on-demand tumor therapy. Biomaterials 2020, 262, 120357. [Google Scholar] [CrossRef]
- Hu, X.; He, J.; Qiao, L.; Wang, C.; Wang, Y.; Yu, R.; Xu, W.; Wang, F.; Yang, S.; Zhang, X.; et al. Multifunctional Dual Network Hydrogel Loaded with Novel Tea Polyphenol Magnesium Nanoparticles Accelerates Wound Repair of MRSA Infected Diabetes. Adv. Funct. Mater. 2024, 34, 2312140. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Qi, D.; Wang, N.; Cheng, Y.; Zhao, Y.; Meng, L.; Yue, X.; She, P.; Gao, H. Application of Porous Polyetheretherketone Scaffold/Vancomycin-Loaded Thermosensitive Hydrogel Composites for Antibacterial Therapy in Bone Repair. Macromol. Biosci. 2022, 22, 2200114. [Google Scholar] [CrossRef]
- Motasadizadeh, H.; Tavakoli, M.; Damoogh, S.; Mottaghitalab, F.; Gholami, M.; Atyabi, F.; Farokhi, M.; Dinarvand, R. Dual drug delivery system of teicoplanin and phenamil based on pH-sensitive silk fibroin/sodium alginate hydrogel scaffold for treating chronic bone infection. Biomater. Adv. 2022, 139, 213032. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dong, X.; Wang, Y.; Wu, X.; Dai, H. Dopamine-modified chitosan hydrogel for spinal cord injury. Carbohydr. Polym. 2022, 298, 120047. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Gan, K.; Lian, H.; Yang, T.; Huang, J.; Chen, J.; Su, Y.; Zhao, J.; Xu, J.; Liu, Q. Periplogenin attenuates LPS-mediated inflammatory osteolysis through the suppression of osteoclastogenesis via reducing the NF-κB and MAPK signaling pathways. Cell Death Discov. 2024, 10, 86. [Google Scholar] [CrossRef]
- An, X.; Zhou, Q.; Sheng, S.; Deng, A.; Liu, H.; Wang, X.; Zhang, Q.; Jing, Y.; Xu, K.; He, C.; et al. Enhanced Chondrogenic Potential and Osteoarthritis Treatment Using Cyaonoside A-Induced MSC Delivered via a Hyaluronic Acid-Based Hydrogel System. Aging Dis. 2025, 17, 1. [Google Scholar] [CrossRef]
- Stamenkovska, M.; Hadzi-Petrushev, N.; Nikodinovski, A.; Gagov, H.; Atanasova-Panchevska, N.; Mitrokhin, V.; Kamkin, A.; Mladenov, M. Application of curcumine and its derivatives in the treatment of cardiovascular diseases: A review. Int. J. Food Prop. 2021, 24, 1510–1528. [Google Scholar] [CrossRef]
- Sun, Z.; Ding, Y.; Wang, Z.; Luo, H.; Feng, Q.; Cao, X. Ros-responsive hydrogel with size-dependent sequential release effects for anti-bacterial and anti-inflammation in diabetic wound healing. Chem. Eng. J. 2024, 493, 152511. [Google Scholar] [CrossRef]
- Sun, Q.; Hou, Y.; Chu, Z.; Wei, Q. Soft overcomes the hard: Flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioact. Mater. 2022, 10, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, T.-Y.; Yang, H.-C.; Ding, M.-H.; Chen, L.-J.; Yu, S.-Y.; Meng, X.-S.; Jin, J.-J.; Sun, S.-Z.; Zhang, J.; et al. Design and Fabrication of Viscoelastic Hydrogels as Extracellular Matrix Mimicry for Cell Engineering. Chem Bio Eng. 2024, 1, 916–933. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Li, X.; Qiu, Y.; Yi, Y.; Wei, Y.; Wang, Y. All-Natural Injectable Antibacterial Hydrogel Enabled by Chitosan and Borneol. Biomacromolecules 2024, 25, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Bock, N.; Dargaville, B.L.; Hutmacher, D.W. Deciphering the Molecular Mechanism of Water Interaction with Gelatin Methacryloyl Hydrogels: Role of Ionic Strength, pH, Drug Loading and Hydrogel Network Characteristics. Biomedicines 2021, 9, 574. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, H.; Qiu, J.; Wang, S.; Ouyang, L.; Qiao, Y.; Liu, X. Mechanical Force Induced Self-Assembly of Chinese Herbal Hydrogel with Synergistic Effects of Antibacterial Activity and Immune Regulation for Wound Healing. Small 2022, 18, 2201766. [Google Scholar] [CrossRef]
- FitzSimons, T.M.; Anslyn, E.V.; Rosales, A.M. Effect of pH on the Properties of Hydrogels Cross-Linked via Dynamic Thia-Michael Addition Bonds. ACS Polym. Au 2022, 2, 129–136. [Google Scholar] [CrossRef]
- Li, N.; Zhang, G.; Liu, Y.; Sun, L.; Zhao, X.; Ding, L.; Liu, Y.; Wang, M.; Ren, X. A Natural Self-Assembled Gel-Sponge with Hierarchical Porous Structure for Rapid Hemostasis and Antibacterial. Adv. Healthc. Mater. 2023, 12, e2301465. [Google Scholar] [CrossRef]
- Wu, J.; Xie, X.; Zheng, Z.; Li, G.; Wang, X.; Wang, Y. Effect of pH on polyethylene glycol (PEG)-induced silk microsphere formation for drug delivery. Mater. Sci. Eng. C 2017, 80, 549–557. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Q.; Lin, S.; Li, J. Water: The soul of hydrogels. Progress Mater. Sci. 2025, 148, 101378. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Ahmadi, M. Degradable hydrogels: Design mechanisms and versatile applications. Mater. Today Sustain. 2023, 23, 100468. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhou, X.S.; Fang, J. Synthesis and characterization of temperature sensitive hemicellulose-based hydrogels. Carbohydr. Polym. 2011, 86, 1113–1117. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.-C. In Situ Synthesis of Magnetic Field-Responsive Hemicellulose Hydrogels for Drug Delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Tsujii, T.; Kawada-Matsuo, M.; Migita, H.; Ohta, K.; Oogai, Y.; Yamasaki, Y.; Komatsuzawa, H. Antibacterial activity of phellodendron bark against Streptococcus mutans. Microbiol. Immunol. 2020, 64, 424–434. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, F.; Jiang, M.; Lin, M.; Dai, Y.; Wang, W.; Xiong, Z.; Liu, H.; Xu, M.; Wang, L. Berberine inhibits the tarO gene to impact MRSA cell wall synthesis. Sci. Rep. 2025, 15, 6927. [Google Scholar] [CrossRef] [PubMed]
- Soltani, L.; Ghaneialvar, H.; Abbasi, N.; Bayat, P.; Nazari, M. Chitosan/alginate scaffold enhanced with Berberis vulgaris extract for osteocyte differentiation of ovine fetal stem cells. Cell Biochem. Funct. 2024, 42, e3924. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, Y.; Su, W.; Wu, G.; Li, P. A gelatin-chitosan-based film containing berberine hydrochloride/polypyrrole that promotes infectious wound healing through antibacterial and antioxidant properties, and electrical conductivity. Int. J. Biol. Macromol. 2025, 305, 141228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Hao, P.Y.; Jiang, S.Q.; Zhang, W.H.; Ren, L.J.; Zheng, H.J.; Chen, Y.W.; Chen, J.L.; Park, H.J. Preparation and antibacterial activity of chitosan grafted cyclodextrin hydrogel loaded berberine hydrochloride using dual gelling agent. J. Mol. Struct. 2024, 1295, 136709. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Li, T.; Li, W.; Guo, X.; Tan, T.; Xiang, C.; Ouyang, Z. Unraveling the potential mechanisms of the anti-osteoporotic effects of the Achyranthes bidentata-Dipsacus asper herb pair: A network pharmacology and experimental study. Front. Pharmacol. 2023, 14, 1242194. [Google Scholar] [CrossRef]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef]
- Poon, C.C.; Au-Yeung, C.; Wong, K.Y.; Chan, Z.; Zhou, L.P.; Li, G.; Wang, Y.; Zhang, Y.; Wong, M.S. Icariin promotes cell adhesion for osteogenesis in bone marrow stromal cells via binding to integrin α5β1. Phytomedicine 2024, 133, 155887. [Google Scholar] [CrossRef]
- Liu, H.; Jiao, Y.; Forouzanfar, T.; Wu, G.; Guo, R.; Lin, H. High-strength double-network silk fibroin based hydrogel loaded with Icariin and BMSCs to inhibit osteoclasts and promote osteogenic differentiation to enhance bone repair. Biomater. Adv. 2024, 160, 213856. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Shang, X.; Chen, L.; Shi, X.; Xu, W.; Fu, S.; He, Q.; Liang, Q.; Ma, J.; et al. Sequential delivery of IL-10 and icariin using nanoparticle/hydrogel hybrid system for prompting bone defect repair. Mater. Today Bio 2024, 29, 101374. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, P.; Zhao, Y.; Fang, X.; Wu, Z.; Qi, X. Bone mesenchymal stem cell-derived exosomes involved co-delivery and synergism effect with icariin via mussel-inspired multifunctional hydrogel for cartilage protection. Asian J. Pharm. Sci. 2023, 18, 100799. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qin, K.; Wang, B.; Liu, B.; Yu, W.; Li, Z.; Zhao, D. Crocin promotes osteogenesis differentiation of bone marrow mesenchymal stem cells. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 680–688. [Google Scholar] [CrossRef]
- Duan, Y.; Su, Y.-T.; Ren, J.; Zhou, Q.; Tang, M.; Li, J.; Li, S.-X. Kidney tonifying traditional Chinese medicine: Potential implications for the prevention and treatment of osteoporosis. Front. Pharmacol. 2023, 13, 1063899. [Google Scholar] [CrossRef]
- Adamopoulos, I.E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018, 30, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Linghu, K.; Cui, W.; Li, T.; Tuo, Y.; Wang, D.; Pan, H.; Zhang, T.; Lin, L.; Yu, H.; Hu, X.; et al. Small molecule α-methylene-γ-butyrolactone, an evolutionarily conserved moiety in sesquiterpene lactones, ameliorates arthritic phenotype via interference DNA binding activity of NF-κB. Acta Pharm. Sin. B 2024, 14, 3561–3575. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, H.; Zhu, Z.; Ling, Z.; Zeng, F.; Wang, Y.; Wang, J. Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules 2023, 28, 1873. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Ma, G.; Li, M.; Wu, H.; Zhang, Y.; Zhang, C.; Ma, Z.; Huang, L. Targeted delivery of triptolide by dendritic cell-derived exosomes for colitis and rheumatoid arthritis therapy in murine models. Br. J. Pharmacol. 2023, 180, 330–346. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Qi, S.; Wang, Y.; Dai, L.; Nie, W.; Lin, M.; He, H.; Ye, N.; Fu, C.; et al. “Dual sensitive supramolecular curcumin nanoparticles” in “advanced yeast particles” mediate macrophage reprogramming, ROS scavenging and inflammation resolution for ulcerative colitis treatment. J. Nanobiotechnol. 2023, 21, 321. [Google Scholar] [CrossRef]
- Kalantarnia, F.; Maleki, S.; Shamloo, A.; Akbarnataj, K.; Tavoosi, S.N. A thermo-responsive chitosan-based injectable hydrogel for delivery of curcumin-loaded polycaprolactone microspheres to articular cartilage: In-vitro and in-vivo assessments. Carbohydr. Polym. Technol. Appl. 2025, 9, 100678. [Google Scholar] [CrossRef]
- Wu, X.; Meng, F.; Lin, Z.; Sun, Y.; Li, H.; Zhang, S.; Bian, B.; Su, W.; Wang, X.; Liu, H.; et al. A tissue regeneration-promoting hydrogel dressing incorporating zirconium MOF loaded with curcumin for multi-modal healing of bacterial-infected wounds. Mater. Today Chem. 2025, 44, 102568. [Google Scholar] [CrossRef]
- Lotfi, M.-S.; Sheibani, M.; Jafari-Sabet, M. Quercetin-based biomaterials for enhanced bone regeneration and tissue engineering. Tissue Cell 2024, 91, 102626. [Google Scholar] [CrossRef]
- Ding, P.; Lu, Y.; Zhao, C.; Guo, W.; Nie, L. Preparation of injectable self-healing hydrogels using carboxymethyl chitosan and oxidized dextran with incorporating quercetin-loaded PF127 micelles for wound healing. Mater. Today Commun. 2024, 41, 110463. [Google Scholar] [CrossRef]
- Wang, Q.; An, J.; Xia, Q.; Pan, D.; Du, L.; He, J.; Sun, Y.; Wang, Y.; Cao, J.; Zhou, C. Insights into the fabrication and antibacterial effect of fibrinogen hydrolysate-carrageenan loading apigenin and quercetin composite hydrogels. Int. J. Biol. Macromol. 2024, 279, 135517. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, B.; Yu, R.; Lu, X.; Li, C.; Zhang, H.; Yang, F.; Zhao, R.; Bao, W.; Yin, X.; et al. ETV2 regulating PHD2-HIF-1α axis controls metabolism reprogramming promotes vascularized bone regeneration. Bioact. Mater. 2024, 37, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Cao, L.; Li, W.; Ye, Y.C.; Shi, Z.X.; Wang, Z.R.; Sun, L.X.; Wang, J.W.; Jia, L.T.; et al. Tanshinone IIA and Astragaloside IV promote the angiogenesis of mesenchymal stem cell-derived endothelial cell-like cells via upregulation of Cx37, Cx40 and Cx43. Exp. Ther. Med. 2018, 15, 1847–1854. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, S.; Zhao, Y.; Sun, Y.; Xu, Z.; Yuan, B.; Chen, X. Tanshinone IIA attenuates osteoclastogenesis in ovariectomized mice by inactivating NF-kB and Akt signaling pathways. Am. J. Transl. Res. 2018, 10, 1457–1468. [Google Scholar]
- Chen, W.; Xu, Y.; Li, H.; Dai, Y.; Zhou, G.; Zhou, Z.; Xia, H.; Liu, H. Tanshinone IIA Delivery Silk Fibroin Scaffolds Significantly Enhance Articular Cartilage Defect Repairing via Promoting Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 21470–21480. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Chen, M.; Deng, H.; Tian, G.; Guo, W.; Yi, G.; Guo, Q.; Chen, Z.; Liu, S. STS loaded PCL-MECM based hydrogel hybrid scaffolds promote meniscal regeneration via modulating macrophage phenotype polarization. Biomater. Sci. 2023, 11, 2759–2774. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q.; Zhou, S.; Xu, S.; Yao, K. Tetramethylpyrazine: A review on its mechanisms and functions. Biomed. Pharmacother. 2022, 150, 113005. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, L.; Wang, N.; Huang, P.; Chang, D.; Zhou, X.; Seto, S.; Li, D.; Hou, J. Hydroxysafflor Yellow A promotes angiogenesis of brain microvascular endothelial cells from ischemia/reperfusion injury via glycolysis pathway in vitro. J. Stroke Cerebrovasc. Dis. 2025, 34, 108107. [Google Scholar] [CrossRef]
- Sun, W.; Ye, B.; Chen, S.; Zeng, L.; Lu, H.; Wan, Y.; Gao, Q.; Chen, K.; Qu, Y.; Wu, B.; et al. Neuro–bone tissue engineering: Emerging mechanisms, potential strategies, and current challenges. Bone Res. 2023, 11, 65. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Wang, Y.; Zhang, H.; Qin, Q.; Xu, Z.; Liu, S.; Wang, X.; Qu, Y.; Liu, Y.; et al. GDNF promotes the proliferation and osteogenic differentiation of jaw bone marrow mesenchymal stem cells via the Nr4a1/PI3K/Akt pathway. Cell. Signal. 2023, 108, 110721. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, D.; Zhang, D.; Zhang, W.; Liu, C.; Cheng, H.; Song, Y.; Tan, Q. Ginsenoside Re Promotes Nerve Regeneration by Facilitating the Proliferation, Differentiation and Migration of Schwann Cells via the ERK- and JNK-Dependent Pathway in Rat Model of Sciatic Nerve Crush Injury. Cell. Mol. Neurobiol. 2015, 35, 827–840. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yao, Y.; Ren, Y.; Lin, J.; Hu, J.; Zheng, M.; Song, X.; Zhao, T.; Chen, Y.Y.; et al. β-Elemene Enhances GAP-43 Expression and Neurite Outgrowth by Inhibiting RhoA Kinase Activation in Rats with Spinal Cord Injury. Neuroscience 2018, 383, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, J.; Yuan, Z.; Pi, W.; Huang, X.; Lin, X.; Zhang, Y.; Lei, H.; Wang, P. Natural Carrier-Free Binary Small Molecule Self-Assembled Hydrogel Synergize Antibacterial Effects and Promote Wound Healing by Inhibiting Virulence Factors and Alleviating the Inflammatory Response. Small 2023, 19, 2205528. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Barati, A.; Moradi, S.; Arjomandzadegan, M. CMC-based hydrogels loaded with Hypericum perforatum nanoemulsion for potential wound dressing applications. J. Bioact. Compat. Polym. 2022, 37, 316–331. [Google Scholar] [CrossRef]
- Md, S.; Abdullah, S.; Alhakamy, N.A.; Shaik, R.A.; Ansari, A.R.; Riadi, Y.; Ahmad, J.; Ali, R.; Gorain, B.; Karim, S. Sustained-release ginseng/sodium alginate nano hydrogel formulation, characterization, and in vivo assessment to facilitate wound healing. J. Drug Deliv. Sci. Technol. 2022, 74, 103565. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.-X.; Yao, Y.-T.; Zhou, T.-J.; Jiang, H.-L.; Li, C.-j. Injectable rhein-assisted crosslinked hydrogel for efficient local osteosarcoma chemotherapy. Int. J. Pharm. 2023, 634, 122637. [Google Scholar] [CrossRef]
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111096. [Google Scholar] [CrossRef]
- Chi, J.; Sun, L.; Cai, L.; Fan, L.; Shao, C.; Shang, L.; Zhao, Y. Chinese herb microneedle patch for wound healing. Bioact. Mater. 2021, 6, 3507–3514. [Google Scholar] [CrossRef]
- Jakfar, S.; Lin, T.C.; Chen, Z.Y.; Yang, I.H.; Gani, B.A.; Ningsih, D.S.; Kusuma, H.; Chang, C.T.; Lin, F.H. A Polysaccharide Isolated from the Herb Bletilla striata Combined with Methylcellulose to Form a Hydrogel via Self-Assembly as a Wound Dressing. Int. J. Mol. Sci. 2022, 23, 12019. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, S.; Tian, M.; Yang, P.; Ding, Y.; Wang, Y.; Duan, G.; Zhang, D.; Chen, B.; Tan, Q. Facile preparation of a novel nanoemulsion based hyaluronic acid hydrogel loading with Poria cocos triterpenoids extract for wound dressing. Int. J. Biol. Macromol. 2023, 226, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, Y.; Li, Z.; Xu, M.; Lu, Y.; Li, X. Puerarin-containing rhein-crosslinked tyramine-modified hyaluronic acid hydrogel for antibacterial and anti-inflammatory wound dressings. Int. J. Biol. Macromol. 2024, 271, 132527. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.; Gao, B.; Dang, Z.; Yang, S.; Chung, C.-H.; Yu, X.; Zhou, X.; Lin, Z.; Cheang, L.H.; et al. Development of a multi-functional naringin-loaded bioglass/carboxymethyl chitosan/silk fibroin porous scaffold for hemostasis and critical size bone regeneration. Int. J. Biol. Macromol. 2025, 290, 138888. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Zhou, Z.; Fang, B.; Chen, Z.; Huang, Y.; Hu, Y.; Liu, H. Berberine oleanolic acid complex salt grafted hyaluronic acid/silk fibroin (BOA-g-HA/SF) composite scaffold promotes cartilage tissue regeneration under IL-1β caused stress. Int. J. Biol. Macromol. 2023, 250, 126104. [Google Scholar] [CrossRef]
- Guo, B.; Qiao, F.; Liao, Y.; Song, L.; He, J. Triptolide laden reduced graphene oxide transdermal hydrogel to manage knee arthritis: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2021, 32, 1288–1300. [Google Scholar] [CrossRef]
- Xia, H.; Jin, H.; Cheng, Y.; Cheng, Z.; Xu, Y. The Controlled Release and Anti-Inflammatory Activity of a Tetramethylpyrazine-Loaded Thermosensitive Poloxamer Hydrogel. Pharm. Res. 2019, 36, 52. [Google Scholar] [CrossRef]
| TCM | Molecular Formula | Intermolecular Forces | Function | Ref. |
|---|---|---|---|---|
| Baicalin |  | Baicalin contains active functional groups such as hydroxyl and carboxyl groups. It can combine with the corresponding groups on the polymer chains of hydrogels through covalent bonds, such as ester bonds. | Antibacterial and anti-inflammatory, promoting wound healing. | [95] |
| Hypericin |  | Hypericin molecules possess polar groups, such as hydroxyl groups. These groups can form hydrogen bonds with hydrophilic groups in hydrogels, such as carboxyl and hydroxyl groups. | Antibacterial, anti-inflammatory, and reduction in scar formation. | [96] |
| Ginsenoside | 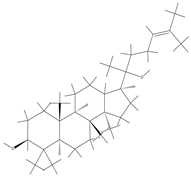 | Ginsenoside molecules contain functional groups like hydroxyl and carboxyl groups, which can bind to the active groups on hydrogel polymer chains via covalent bonds. | Anti-inflammatory, antioxidant, antibacterial, and promoting angiogenesis. | [97] |
| Rhein | 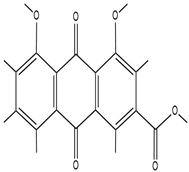 | Rhein molecules have certain hydrophobicity and planar structure. The three-dimensional network structure of hydrogels has pores and hydrophobic regions, which can adsorb rhein inside through physical interactions such as van der Waals forces. | Induce the differentiation of bone marrow mesenchymal stem cells (BMSCs) into osteoblasts. | [98] |
| Epigallocatechin gallate |  | Polar groups such as hydroxyl and carboxyl groups in hydrogels can form numerous hydrogen bonds with the phenolic hydroxyl groups of EGCG. Additionally, the benzene ring structure of EGCG may also interact with the aromatic-structured parts in hydrogels through π–π stacking. | Anti-inflammation, anti-oxidation, and promotion of cartilage regeneration. | [99] |
| Asiatic acid |  | Asiatic acid molecules contain polar groups such as hydroxyl groups, which can form hydrogen bonds with hydrophilic groups in hydrogels. Meanwhile, its partial carbon-chain structure is hydrophobic and can interact hydrophobically with the hydrophobic regions of hydrogels. | Antioxidant, anti-inflammatory, and antibacterial. | [100] |
| Bletilla striata polysaccharide | Not single component | \ | Antibacterial, antioxidant, and promote angiogenesis. | [101] |
| Poria cocos triterpenes extract | Not single component | \ | Anti-inflammatory, promote the polarization of M2 macrophages and angiogenesis. | [102] |
| Puerarin | 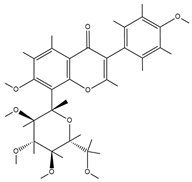 | Puerarin molecules contain multiple polar groups such as hydroxyl groups. Hydrogels are also rich in hydrophilic groups like hydroxyl and amino groups. They can interact with each other through hydrogen bonds. | Antibacterial, anti-inflammatory, and promotion of cell migration and proliferation. | [103] |
| Naringin |  | Naringin molecules contain multiple polar groups such as hydroxyl groups. There are also a large number of hydrophilic groups like hydroxyl and carboxyl groups in hydrogels. They can combine with each other through hydrogen bonds. | Antibacterial, hemostatic, promoting bone regeneration, and facilitating neurovascularization. | [104] |
| Oleanolic acid | 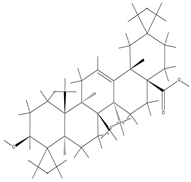 | Oleanolic acid has certain hydrophobicity. If there are hydrophobic regions in hydrogels, they can bind to oleanolic acid through hydrophobic interactions. | Anti-inflammation and promotion of cartilage regeneration. | [105] |
| Triptolide |  | Triptolide has certain hydrophobicity. If hydrogels contain hydrophobic regions or segments, they can bind to triptolide through hydrophobic interactions. | Anti-inflammatory, promotes the proliferation and differentiation of articular chondrocytes. | [106] |
| Tetramethylpyrazine |  | Tetramethylpyrazine itself has relatively weak polarity. However, if there are a large number of strongly polar groups such as hydroxyl and carboxyl groups in hydrogels, and the nitrogen atoms in tetramethylpyrazine molecules can act as hydrogen-bond acceptors, weak hydrogen bonds can form between them and the hydroxyl donors in hydrogels. | Anti-inflammatory, improves local blood circulation, and promotes injury repair. | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Yue, Y.; Hu, H.; Lv, S. Traditional Chinese Medicine-Loaded Hydrogels: An Emerging Strategy for the Treatment of Bone Infections. Pharmaceutics 2025, 17, 514. https://doi.org/10.3390/pharmaceutics17040514
Jin X, Yue Y, Hu H, Lv S. Traditional Chinese Medicine-Loaded Hydrogels: An Emerging Strategy for the Treatment of Bone Infections. Pharmaceutics. 2025; 17(4):514. https://doi.org/10.3390/pharmaceutics17040514
Chicago/Turabian StyleJin, Xueyi, Yujie Yue, Huaanzi Hu, and Songwei Lv. 2025. "Traditional Chinese Medicine-Loaded Hydrogels: An Emerging Strategy for the Treatment of Bone Infections" Pharmaceutics 17, no. 4: 514. https://doi.org/10.3390/pharmaceutics17040514
APA StyleJin, X., Yue, Y., Hu, H., & Lv, S. (2025). Traditional Chinese Medicine-Loaded Hydrogels: An Emerging Strategy for the Treatment of Bone Infections. Pharmaceutics, 17(4), 514. https://doi.org/10.3390/pharmaceutics17040514







