PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation
Abstract
1. Introduction
2. Obstacles and Strategies for Optimal PROTAC Delivery
3. Delivery Systems for PROTACs
3.1. Polymeric Nanoparticle-Based Delivery Systems
3.2. Emulsion-Based Delivery Systems
3.3. Solid Dispersion-Based Delivery Systems
3.4. Lipid Nanoparticle-Bassed Delivery Systems
3.5. Liposome-Based Delivery Systems
3.6. Exosome-Based Delivery Systems
4. Summary
5. Discussion and Future Perspective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACNPs | Antibody-conjugated nanoparticles |
| AP-NLC | ARV-825-loaded PEGylated NLCs |
| API | Active pharmaceutical ingredient |
| AR | Androgen receptor |
| ARV-SNEP | ARV-825-loaded self-nanoemulsifying preconcentrate |
| ASDs | Amorphous solid dispersion |
| BBB | Blood–brain barrier |
| CME | Camel milk-derived exosome |
| CRBN | Cereblon |
| DS-PLGA | Disulfide-linked poly (lactic-co-glycolic acid) |
| EPR | Enhanced permeability and retention |
| ER | Estrogen receptor |
| GALARV | Galactose-decorated nanoliposomal formulation |
| GSH | Glutathione |
| HCC | Hepatocellular carcinoma |
| LLCM | Lung cancer cell membrane |
| LNP | Lipid nanoparticles |
| MPRO | Folate-PEG-PROTAC micelles |
| MSPM | Mixed-shell polymeric micelle |
| MZ1-NPs | MZ1-loaded polymeric nanoparticles |
| nChap | Nanochaperone-based |
| NLC | Nanostructured lipid carrier |
| NSCLC | Non-small cell lung cancer |
| PCL | Polycaprolactone |
| PDSA | Poly(disulfide amide) |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PEO-PPO-PEO | Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) |
| PGDAT | Self-assembling PROTAC nanoparticle |
| PLA | Polylactide |
| PLGA | Poly(lactic-co-glycolic acid) |
| POI | Protein of interest |
| PROTAC | Proteolysis targeting chimeras |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| RCNprotac | X-ray radiation responsive PROTAC nanomicelle |
| ROS | Reactive oxygen species |
| SNEDDS | Self-nanoemulsifying drug delivery system |
| SP | Substance P |
| TAMs | Tumor-associated macrophages |
| TNBC | Triple-negative breast cancer |
| UPS | Ubiquitin–proteosome system |
| VHL | Von Hippel–Lindau |
References
- Spradlin, J.N.; Zhang, E.; Nomura, D.K. Reimagining Druggability Using Chemoproteomic Platforms. Acc. Chem. Res. 2021, 54, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, J.; Zhong, K.; Tong, A.; Jia, D. Targeted protein degradation: Mechanisms, strategies and application. Signal Transduct. Target. Ther. 2022, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, H.; Yang, Y.; He, M.; Wu, Y.; Song, Y.; Tong, Y.; Rao, Y. PROTACs: Great opportunities for academia and industry. Signal Transduct. Target. Ther. 2019, 4, 64. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Graham, H. The mechanism of action and clinical value of PROTACs: A graphical review. Cell Signal 2022, 99, 110446. [Google Scholar] [CrossRef]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Xiao, M.; Zhao, J.; Wang, Q.; Liu, J.; Ma, L. Recent Advances of Degradation Technologies Based on PROTAC Mechanism. Biomolecules 2022, 12, 1257. [Google Scholar] [CrossRef]
- Li, K.; Crews, C.M. PROTACs: Past, present and future. Chem. Soc. Rev. 2022, 51, 5214–5236. [Google Scholar] [CrossRef]
- Ge, J.; Li, S.; Weng, G.; Wang, H.; Fang, M.; Sun, H.; Deng, Y.; Hsieh, C.-Y.; Li, D.; Hou, T. PROTAC-DB 3.0: An updated database of PROTACs with extended pharmacokinetic parameters. Nucleic Acids Res. 2024, 53, D1510–D1515. [Google Scholar] [CrossRef]
- Weng, G.; Cai, X.; Cao, D.; Du, H.; Shen, C.; Deng, Y.; He, Q.; Yang, B.; Li, D.; Hou, T. PROTAC-DB 2.0: An updated database of PROTACs. Nucleic Acids Res. 2023, 51, D1367–D1372. [Google Scholar] [CrossRef]
- Han, X.; Sun, Y. Strategies for the discovery of oral PROTAC degraders aimed at cancer therapy. Cell Rep. Phys. Sci. 2022, 3, 101062. [Google Scholar] [CrossRef]
- Sincere, N.I.; Anand, K.; Ashique, S.; Yang, J.; You, C. PROTACs: Emerging Targeted Protein Degradation Approaches for Advanced Druggable Strategies. Molecules 2023, 28, 4014. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Targeted protein degraders crowd into the clinic. Nat. Rev. Drug Discov. 2021, 20, 247–250. [Google Scholar] [CrossRef]
- Moon, Y.; Jeon, S.I.; Shim, M.K.; Kim, K. Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy. Pharmaceutics 2023, 15, 411. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Chen, Y.; Tandon, I.; Heelan, W.; Wang, Y.; Tang, W.; Hu, Q. Proteolysis-targeting chimera (PROTAC) delivery system: Advancing protein degraders towards clinical translation. Chem. Soc. Rev. 2022, 51, 5330–5350. [Google Scholar] [CrossRef]
- Fan, L.; Tong, W.; Wei, A.; Mu, X. Progress of proteolysis-targeting chimeras (PROTACs) delivery system in tumor treatment. Int. J. Biol. Macromol. 2024, 275, 133680. [Google Scholar] [CrossRef]
- An, S.; Fu, L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. eBioMedicine 2018, 36, 553–562. [Google Scholar] [CrossRef]
- Kumbhar, P.; Kolekar, K.; Kamble, V.; Umeyor, C.E.; Disouza, J.; Patravale, V.B. Future of Trends in the Design and Development of PROTAC. In PROTAC-Mediated Protein Degradation: A Paradigm Shift in Cancer Therapeutics; Nandave, M., Jain, P., Eds.; Springer Nature: Singapore, 2024; pp. 117–134. [Google Scholar]
- Pei, H.; Peng, Y.; Zhao, Q.; Chen, Y. Small molecule PROTACs: An emerging technology for targeted therapy in drug discovery. RSC Adv. 2019, 9, 16967–16976. [Google Scholar] [CrossRef]
- Cecchini, C.; Pannilunghi, S.; Tardy, S.; Scapozza, L. From Conception to Development: Investigating PROTACs Features for Improved Cell Permeability and Successful Protein Degradation. Front. Chem. 2021, 9, 672267. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Wang, S.; Liu, L.; Feng, Z.; Zhang, H.; Gong, Q.; Sun, Y.; Guo, Y.; Li, R. Current strategies for improving limitations of proteolysis targeting chimeras. Chin. Chem. Lett. 2023, 34, 107927. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Chen, W.; Wu, Y.; Xing, D. New-generation advanced PROTACs as potential therapeutic agents in cancer therapy. Mol. Cancer 2024, 23, 110. [Google Scholar] [CrossRef]
- Abeje, Y.E.; Wieske, L.H.E.; Poongavanam, V.; Maassen, S.; Atilaw, Y.; Cromm, P.; Lehmann, L.; Erdelyi, M.; Meibom, D.; Kihlberg, J. Impact of Linker Composition on VHL PROTAC Cell Permeability. J. Med. Chem. 2025, 68, 638–657. [Google Scholar] [CrossRef]
- Ciulli, A.; Trainor, N. A beginner’s guide to PROTACs and targeted protein degradation. Biochemist 2021, 43, 74–79. [Google Scholar] [CrossRef]
- Nalawansha, D.A.; Crews, C.M. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem. Biol. 2020, 27, 998–1014. [Google Scholar] [CrossRef]
- Benowitz, A.B.; Scott-Stevens, P.T.; Harling, J.D. Challenges and opportunities for in vivo PROTAC delivery. Future Med. Chem. 2022, 14, 119–121. [Google Scholar] [CrossRef]
- Yang, W.; Saboo, S.; Zhou, L.; Askin, S.; Bak, A. Early evaluation of opportunities in oral delivery of PROTACs to overcome their molecular challenges. Drug Discov. Today 2024, 29, 103865. [Google Scholar] [CrossRef]
- O’ Donovan, D.H.; De Fusco, C.; Kuhnke, L.; Reichel, A. Trends in Molecular Properties, Bioavailability, and Permeability across the Bayer Compound Collection. J. Med. Chem. 2023, 66, 2347–2360. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Hessa, T.; Sharma, A.; Mariappan, M.; Eshleman, H.D.; Gutierrez, E.; Hegde, R.S. Protein targeting and degradation are coupled for elimination of mislocalized proteins. Nature 2011, 475, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.; Lim, C.; Hoang, N.H.; Joo, H.; Lee, J.W.; Kim, D.-w.; Lee, E.S.; Youn, Y.S.; Kim, J.O.; Oh, K.T. Nanomedicines for oral administration based on diverse nanoplatform. J. Pharm. Investig. 2016, 46, 351–362. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Yang, K.; Yu, G.; Yang, Z.; Yue, L.; Zhang, X.; Sun, C.; Wei, J.; Rao, L.; Chen, X.; Wang, R. Supramolecular Polymerization-Induced Nanoassemblies for Self-Augmented Cascade Chemotherapy and Chemodynamic Therapy of Tumor. Angew. Chem. Int. Ed. 2021, 60, 17570–17578. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Grzelczak, M.; Altantzis, T.; Goris, B.; Pérez-Juste, J.; Bals, S.; Van Tendeloo, G.; Donaldson, S.H., Jr.; Chmelka, B.F.; Israelachvili, J.N.; et al. Hydrophobic Interactions Modulate Self-Assembly of Nanoparticles. ACS Nano 2012, 6, 11059–11065. [Google Scholar] [CrossRef]
- Tao, J.; Yi, C.; Dong, W.; Zhang, Y.; He, H.; Yang, Y.; Ye, S.; Wu, Q.; Shen, X.; Yang, F.; et al. Competitive hydrogen bonding and electrostatic interactions-mediated alternating nanoparticles copolymerization. Nano Res. 2025, 18, 94907086. [Google Scholar] [CrossRef]
- Droumaguet, B.L.; Grande, D. Diblock and Triblock Copolymers as Nanostructured Precursors to Functional Nanoporous Materials: From Design to Application. ACS Appl. Mater. Interfaces 2023, 15, 58023–58040. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: An update. J. Pharm. Investig. 2018, 48, 61–75. [Google Scholar] [CrossRef]
- Hoang, N.H.; Lim, C.; Sim, T.; Oh, K.T. Triblock copolymers for nano-sized drug delivery systems. J. Pharm. Investig. 2017, 47, 27–35. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Parmar, V.S.; Malhotra, S.; Chhillar, A.K. Biodegradable diblock copolymeric PEG-PCL nanoparticles: Synthesis, characterization and applications as anticancer drug delivery agents. Polymer 2020, 207, 122901. [Google Scholar] [CrossRef]
- Locatelli, E.; Comes Franchini, M. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1316. [Google Scholar] [CrossRef]
- Saraswat, A.; Patki, M.; Fu, Y.; Barot, S.; Dukhande, V.V.; Patel, K. Nanoformulation of PROteolysis TArgeting Chimera targeting ’undruggable’ c-Myc for the treatment of pancreatic cancer. Nanomedicine 2020, 15, 1761–1777. [Google Scholar] [CrossRef]
- Yang, T.; Hu, Y.; Miao, J.; Chen, J.; Liu, J.; Cheng, Y.; Gao, X. A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm. Sin. B 2022, 12, 2658–2671. [Google Scholar] [CrossRef]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef]
- Mohamed, S.; Parayath, N.N.; Taurin, S.; Greish, K. Polymeric nano-micelles: Versatile platform for targeted delivery in cancer. Ther. Deliv. 2014, 5, 1101–1121. [Google Scholar] [CrossRef]
- Aw, M.S.; Kurian, M.; Losic, D. Polymeric Micelles for Multidrug Delivery and Combination Therapy. Chem. Eur. J. 2013, 19, 12586–12601. [Google Scholar] [CrossRef]
- Shrestha, B.; Tang, L.; Romero, G. Nanoparticles-Mediated Combination Therapies for Cancer Treatment. Adv. Ther. 2019, 2, 1900076. [Google Scholar] [CrossRef]
- Choi, J.Y.; Thapa, R.K.; Yong, C.S.; Kim, J.O. Nanoparticle-based combination drug delivery systems for synergistic cancer treatment. J. Pharm. Investig. 2016, 46, 325–339. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.D.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled Delivery of BET-PROTACs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, F.; Zhang, Y.; Xu, M.; Xu, L.; Liu, Y.; Ma, R.; Shi, L. Synergizing CXCL9 with BRD4-PROTAC Using Nanochaperone Boosts Robust T Cell-Dependent Antitumor Immune Responses for Cancer Immunotherapy. Adv. Funct. Mater. 2024, 34, 2314203. [Google Scholar] [CrossRef]
- He, Y.; Ju, Y.; Hu, Y.; Wang, B.; Che, S.; Jian, Y.; Zhuo, W.; Fu, X.; Cheng, Y.; Zheng, S.; et al. Brd4 proteolysis-targeting chimera nanoparticles sensitized colorectal cancer chemotherapy. J. Control. Release 2023, 354, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yu, J.; Hao, T.; Wang, W.; Wei, M.; Li, G. Advances in Polymeric Micelles: Responsive and Targeting Approaches for Cancer Immunotherapy in the Tumor Microenvironment. Pharmaceutics 2023, 15, 2622. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, X.; Zhang, X. Recent advances in stimuli-responsive polymeric micelles via click chemistry. Polym. Chem. 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, X.; Lei, S.; Cheng, W.; Lai, Y.; Li, M.; Yang, L.; Liu, P.; Chen, X.-h.; Huang, M.; et al. A region-confined PROTAC nanoplatform for spatiotemporally tunable protein degradation and enhanced cancer therapy. Nat. Commun. 2024, 15, 6608. [Google Scholar] [CrossRef]
- Liu, H.-J.; Chen, W.; Wu, G.; Zhou, J.; Liu, C.; Tang, Z.; Huang, X.; Gao, J.; Xiao, Y.; Kong, N.; et al. Glutathione-Scavenging Nanoparticle-Mediated PROTACs Delivery for Targeted Protein Degradation and Amplified Antitumor Effects. Adv. Sci. 2023, 10, 2207439. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Peng, R.; Chen, S.; Shen, A.; Zhao, L.; Tang, W.; Wang, X.-H.; Li, Z.-Y.; Zha, Z.-G.; Yi, M.; et al. Versatile Nano-PROTAC-Induced Epigenetic Reader Degradation for Efficient Lung Cancer Therapy. Adv. Sci. 2022, 9, 2202039. [Google Scholar] [CrossRef]
- Guan, X.; Xu, X.; Tao, Y.; Deng, X.; He, L.; Lin, Z.; Chang, J.; Huang, J.; Zhou, D.; Yu, X.; et al. Dual targeting and bioresponsive nano-PROTAC induced precise and effective lung cancer therapy. J. Nanobiotechnol. 2024, 22, 692. [Google Scholar] [CrossRef]
- Ma, J.; Fang, L.; Sun, Z.; Li, M.; Fan, T.; Xiang, G.; Ma, X. Folate-PEG-PROTAC Micelles for Enhancing Tumor-Specific Targeting Proteolysis In Vivo. Adv. Healthc. Mater. 2024, 13, 2400109. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yun, Y.; Li, C.; Ruan, Y.; Muraoka, O.; Xie, W.; Sun, X. Radiation responsive PROTAC nanoparticles for tumor-specific proteolysis enhanced radiotherapy. J. Mater. Chem. B 2024, 12, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral lipid-based drug delivery systems—An overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef]
- Sripriya, R.; Raja, K.M.; Santhosh, G.; Chandrasekaran, M.; Noel, M. The effect of structure of oil phase, surfactant and co-surfactant on the physicochemical and electrochemical properties of bicontinuous microemulsion. J. Colloid Interface Sci. 2007, 314, 712–717. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Taher, S.; Al-Kinani, K.; Hammoudi, Z.; Ghareeb, M. Co-surfactant effect of polyethylene glycol 400 on microemulsion using BCS class II model drug. J. Adv. Pharm. Educ. Res. 2022, 12, 63–69. [Google Scholar] [CrossRef]
- Lim, C.; Lee, D.; Kim, M.; Lee, S.; Shin, Y.; Ramsey, J.D.; Choi, H.-G.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Development of a sorafenib-loaded solid self-nanoemulsifying drug delivery system: Formulation optimization and characterization of enhanced properties. J. Drug Deliv. Sci. Technol. 2023, 82, 104374. [Google Scholar] [CrossRef]
- Mudassir, J.; Raza, A.; Khan, M.A.; Hameed, H.; Shazly, G.A.; Irfan, A.; Rana, S.J.; Abbas, K.; Arshad, M.S.; Muhammad, S.; et al. Design and Evaluation of Hydrophobic Ion Paired Insulin Loaded Self Micro-Emulsifying Drug Delivery System for Oral Delivery. Pharmaceutics 2023, 15, 1973. [Google Scholar] [CrossRef]
- Tran, P.; Park, J.-S. Recent trends of self-emulsifying drug delivery system for enhancing the oral bioavailability of poorly water-soluble drugs. J. Pharm. Investig. 2021, 51, 439–463. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. Int. Sch. Res. Not. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.; Fu, Y.; Patel, K. BRD4 PROTAC as a novel therapeutic approach for the treatment of vemurafenib resistant melanoma: Preformulation studies, formulation development and in vitro evaluation. Eur. J. Pharm. Sci. 2019, 138, 105039. [Google Scholar] [CrossRef]
- Saraswat, A.; Vartak, R.; Hegazy, R.; Fu, Y.; Rao, T.J.R.; Billack, B.; Patel, K. Oral lipid nanocomplex of BRD4 PROteolysis TArgeting Chimera and vemurafenib for drug-resistant malignant melanoma. Biomed. Pharmacother. 2023, 168, 115754. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- He, Y.; Ho, C. Amorphous Solid Dispersions: Utilization and Challenges in Drug Discovery and Development. J. Pharm. Sci. 2015, 104, 3237–3258. [Google Scholar] [CrossRef]
- Van Duong, T.; Van den Mooter, G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part II: Amorphous carriers. Expert Opin. Drug Deliv. 2016, 13, 1681–1694. [Google Scholar] [CrossRef]
- Arpagaus, C. PLA/PLGA nanoparticles prepared by nano spray drying. J. Pharm. Investig. 2019, 49, 405–426. [Google Scholar] [CrossRef]
- Bapat, P.; Paul, S.; Tseng, Y.-C.; Taylor, L.S. Interplay of Drug–Polymer Interactions and Release Performance for HPMCAS-Based Amorphous Solid Dispersions. Mol. Pharm. 2024, 21, 1466–1478. [Google Scholar] [CrossRef]
- Pöstges, F.; Kayser, K.; Appelhaus, J.; Monschke, M.; Gütschow, M.; Steinebach, C.; Wagner, K.G. Solubility Enhanced Formulation Approaches to Overcome Oral Delivery Obstacles of PROTACs. Pharmaceutics 2023, 15, 156. [Google Scholar] [CrossRef] [PubMed]
- Mareczek, L.; Mueller, L.K.; Halstenberg, L.; Geiger, T.M.; Walz, M.; Zheng, M.; Hausch, F. Use of Poly(vinyl alcohol) in Spray-Dried Dispersions: Enhancing Solubility and Stability of Proteolysis Targeting Chimeras. Pharmaceutics 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.; Harms, M.; Mäder, K. ASDs of PROTACs: Spray-dried solid dispersions as enabling formulations. Int. J. Pharm. 2024, 650, 123725. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Gupta, B.; Kim, J.O. Recent progress in cancer immunotherapy approaches based on nanoparticle delivery devices. J. Pharm. Investig. 2021, 51, 399–412. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.S.; Alqabbani, A.A.; Aldayel, A.M. Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef]
- Lee, M.-K. Clinical usefulness of liposomal formulations in cancer therapy: Lessons from the experiences of doxorubicin. J. Pharm. Investig. 2019, 49, 203–214. [Google Scholar] [CrossRef]
- Liu, Y.; Bravo, K.M.C.; Liu, J. Targeted liposomal drug delivery: A nanoscience and biophysical perspective. Nanoscale Horiz. 2021, 6, 78–94. [Google Scholar] [CrossRef]
- Hegde, M.M.; Prabhu, S.; Mutalik, S.; Chatterjee, A.; Goda, J.S.; Satish Rao, B.S. Multifunctional lipidic nanocarriers for effective therapy of glioblastoma: Recent advances in stimuli-responsive, receptor and subcellular targeted approaches. J. Pharm. Investig. 2022, 52, 49–74. [Google Scholar] [CrossRef]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2021, 51, 35–51. [Google Scholar] [CrossRef]
- Vartak, R.; Saraswat, A.; Yang, Y.; Chen, Z.S.; Patel, K. Susceptibility of Lung Carcinoma Cells to Nanostructured Lipid Carrier of ARV-825, a BRD4 Degrading Proteolysis Targeting Chimera. Pharm. Res. 2022, 39, 2745–2759. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, M.; Ma, F.; Yang, L.; Glass, Z.; Xu, Q. Enhanced protein degradation by intracellular delivery of pre-fused PROTACs using lipid-like nanoparticles. J. Control. Release 2021, 330, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, A.; Vemana, H.P.; Dukhande, V.; Patel, K. Novel gene therapy for drug-resistant melanoma: Synergistic combination of PTEN plasmid and BRD4 PROTAC-loaded lipid nanocarriers. Mol. Ther. Nucleic Acids 2024, 35, 102292. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Noh, G.; Keum, T.; Bashyal, S.; Seo, J.-E.; Shrawani, L.; Kim, J.H.; Lee, S. Recent progress in hydrophobic ion-pairing and lipid-based drug delivery systems for enhanced oral delivery of biopharmaceuticals. J. Pharm. Investig. 2022, 52, 75–93. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.; Rogers, K.; Delehanty, J. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Lee, M.-K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.; Pu, C.; Cui, J.; Huang, K.; Wang, H.; Zhao, Y. Nanoliposomal Bcl-xL proteolysis-targeting chimera enhances anti-cancer effects on cervical and breast cancer without on-target toxicities. Adv. Compos. Hybrid Mater. 2023, 6, 78. [Google Scholar] [CrossRef]
- Saraswat, A.; Vemana, H.P.; Dukhande, V.V.; Patel, K. Galactose-decorated liver tumor-specific nanoliposomes incorporating selective BRD4-targeted PROTAC for hepatocellular carcinoma therapy. Heliyon 2022, 8, e08702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, Y.; Wang, J.; Gu, M.; Wang, Y.; Zhang, X.; Zhang, Y.; Yu, W.; Liu, Y.; Yuan, W.-E.; et al. Co-delivery of PROTAC and siRNA via novel liposomes for the treatment of malignant tumors. J. Colloid Interface Sci. 2025, 678, 896–907. [Google Scholar] [CrossRef]
- Fu, Y.; Rathod, D.; Patel, K. Protein kinase C inhibitor anchored BRD4 PROTAC PEGylated nanoliposomes for the treatment of vemurafenib-resistant melanoma. Exp. Cell Res. 2020, 396, 112275. [Google Scholar] [CrossRef]
- Fu, Y.; Saraswat, A.; Wei, Z.; Agrawal, M.Y.; Dukhande, V.V.; Reznik, S.E.; Patel, K. Development of Dual ARV-825 and Nintedanib-Loaded PEGylated Nano-Liposomes for Synergistic Efficacy in Vemurafnib-Resistant Melanoma. Pharmaceutics 2021, 13, 1005. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Cui, B.; Yan, Q.; Qiu, C.; Zhu, Z.; Wen, L.; Chen, W. Liposomes-mediated enhanced antitumor effect of docetaxel with BRD4-PROTAC as synergist for breast cancer chemotherapy/immunotherapy. Int. J. Pharm. 2025, 668, 124973. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Ghosh, A.; Alexiou, A.; Thorat, N.D. Exosome-mediated PROTAC delivery for treatment of RNA viral infections and zoonosis. Drug Discov. Today 2024, 29, 104044. [Google Scholar] [CrossRef]
- Mukerjee, N.; Maitra, S.; Ghosh, A.; Subramaniyan, V.; Sharma, R. Exosome-mediated PROTACs delivery to target viral infections. Drug Dev. Res. 2023, 84, 1031–1036. [Google Scholar] [CrossRef]
- Nathani, A.; Aare, M.; Sun, L.; Bagde, A.; Li, Y.; Rishi, A.; Singh, M. Unlocking the Potential of Camel Milk-Derived Exosomes as Novel Delivery Systems: Enhanced Bioavailability of ARV-825 PROTAC for Cancer Therapy. Pharmaceutics 2024, 16, 1070. [Google Scholar] [CrossRef]
- Aparna, T.N.; Kumar, R.; Ali, S.R.; Patel, D.J.; Julekha, K.; Begum, T.; Bala, J.; Kumar, P. Silica Nanoparticles: A Promising Vehicle for Anti-Cancer Drugs Delivery. AAPS PharmSciTech 2025, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Shabnum, S.S.; Siranjeevi, R.; Raj, C.K.; Nivetha, P.; Benazir, K. A Comprehensive Review on Recent Progress in Carbon Nanotubes for Biomedical Application. Environ. Qual. Manag. 2025, 34, e70040. [Google Scholar] [CrossRef]
- Henning, N.J.; Boike, L.; Spradlin, J.N.; Ward, C.C.; Liu, G.; Zhang, E.; Belcher, B.P.; Brittain, S.M.; Hesse, M.J.; Dovala, D.; et al. Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 2022, 18, 412–421. [Google Scholar] [CrossRef]
- Kabir, M.; Sun, N.; Hu, X.; Martin, T.C.; Yi, J.; Zhong, Y.; Xiong, Y.; Kaniskan, H.Ü.; Gu, W.; Parsons, R.; et al. Acetylation Targeting Chimera Enables Acetylation of the Tumor Suppressor p53. J. Am. Chem. Soc. 2023, 145, 14932–14944. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Hu, Z.; An, E.; Okeke, I.; Zheng, S.; Luo, X.; Gong, A.; Jaime-Figueroa, S.; Crews, C.M. Modulation of Phosphoprotein Activity by Phosphorylation Targeting Chimeras (PhosTACs). ACS Chem. Biol. 2021, 16, 2808–2815. [Google Scholar] [CrossRef]
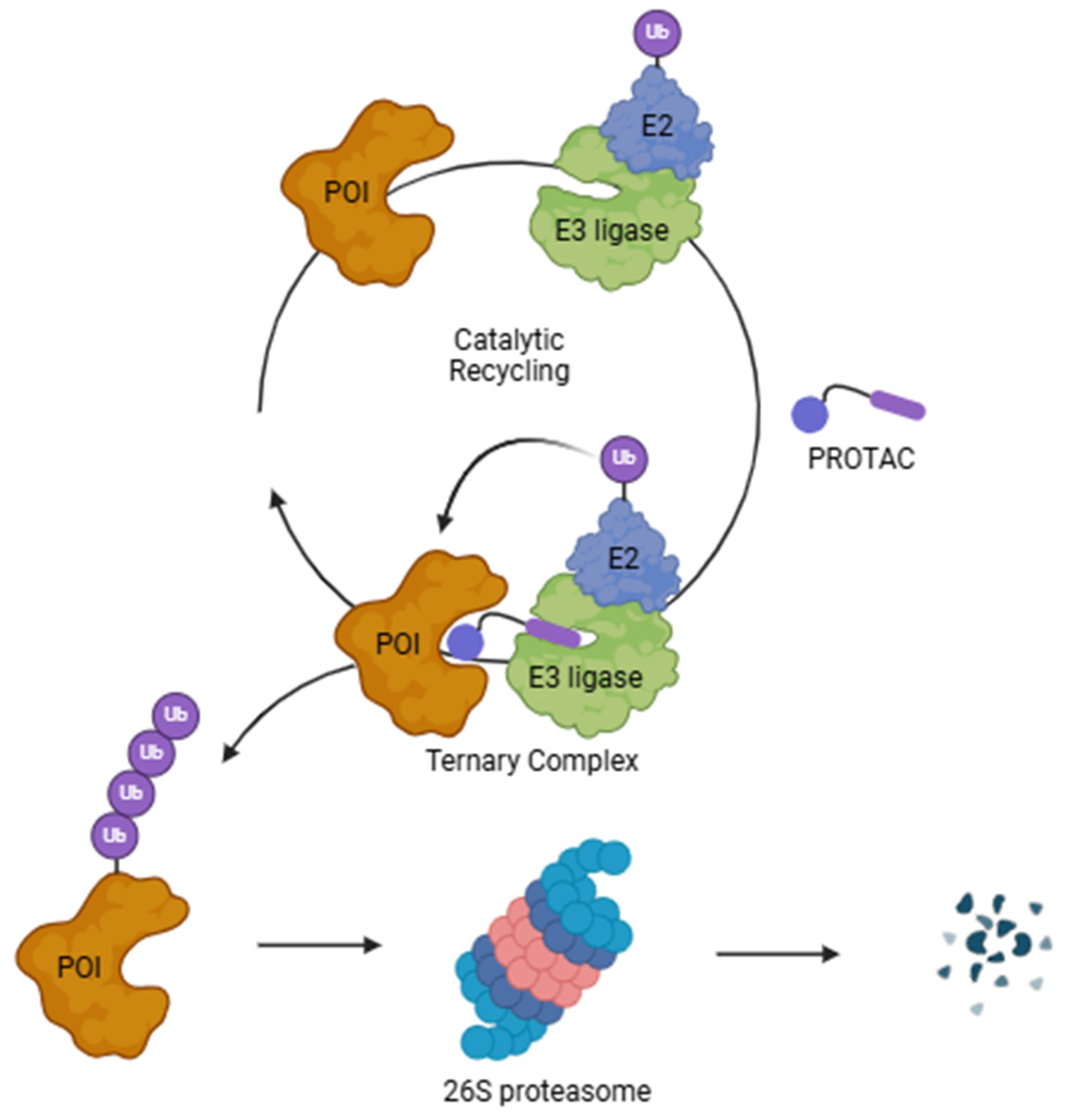



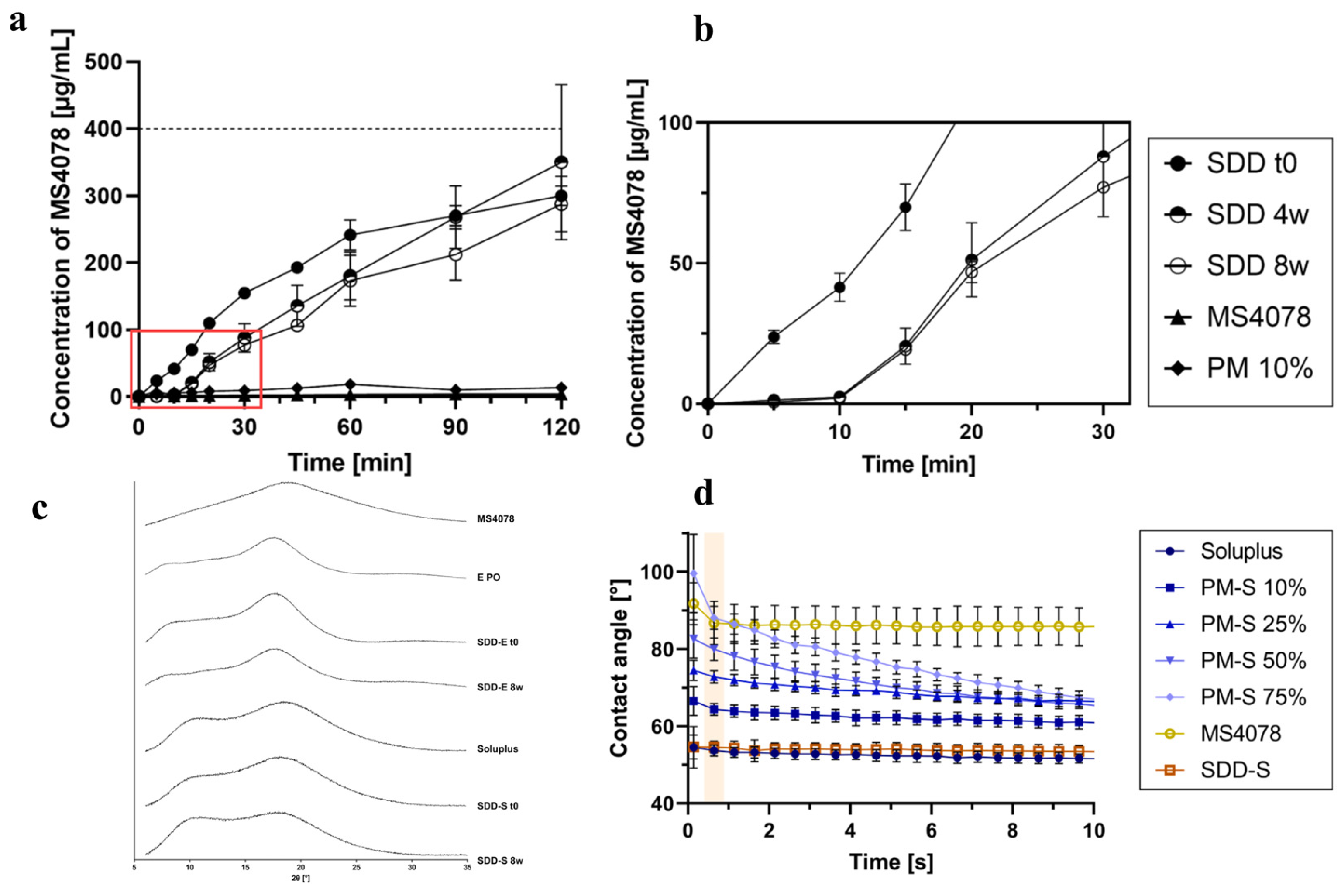

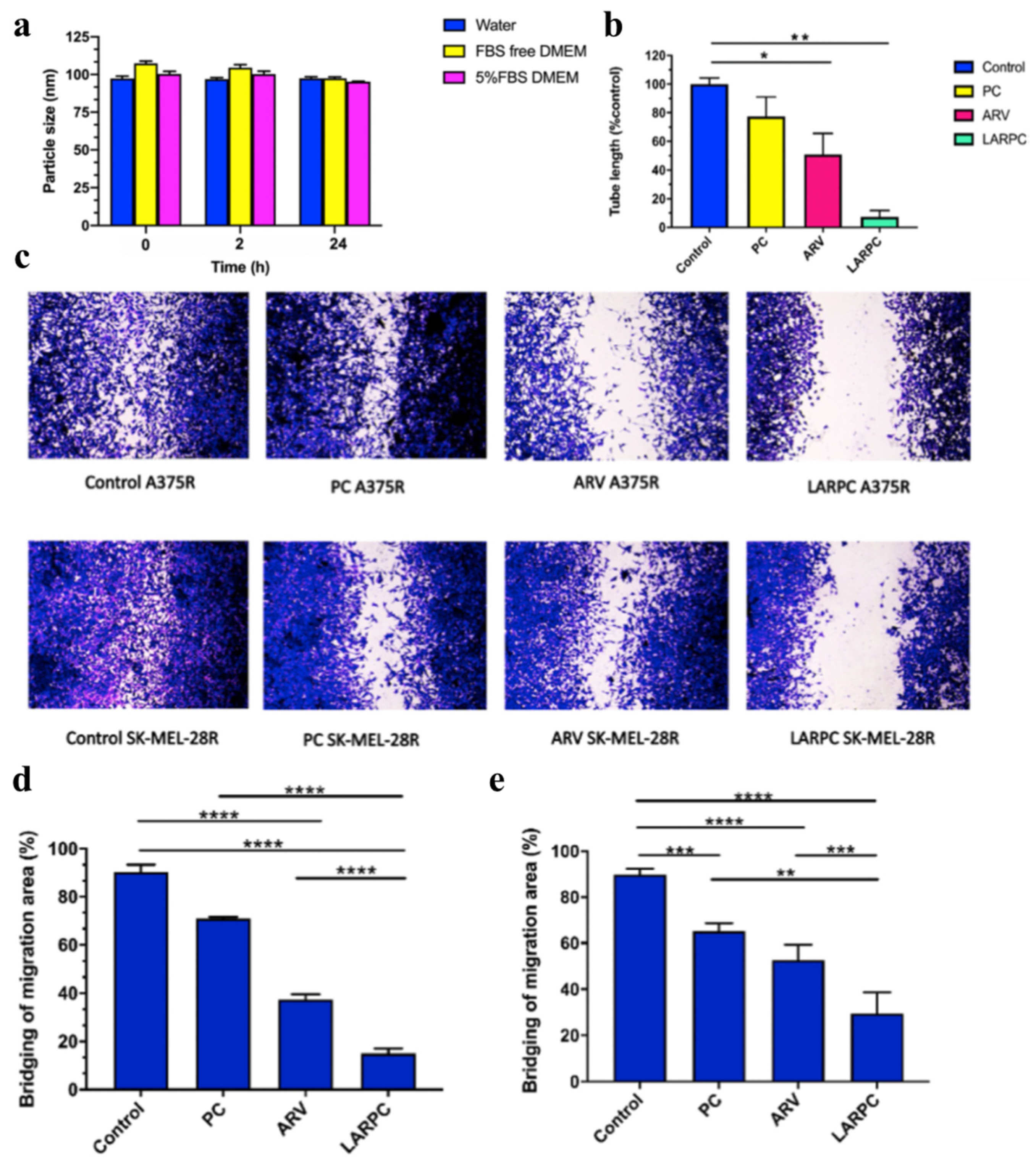
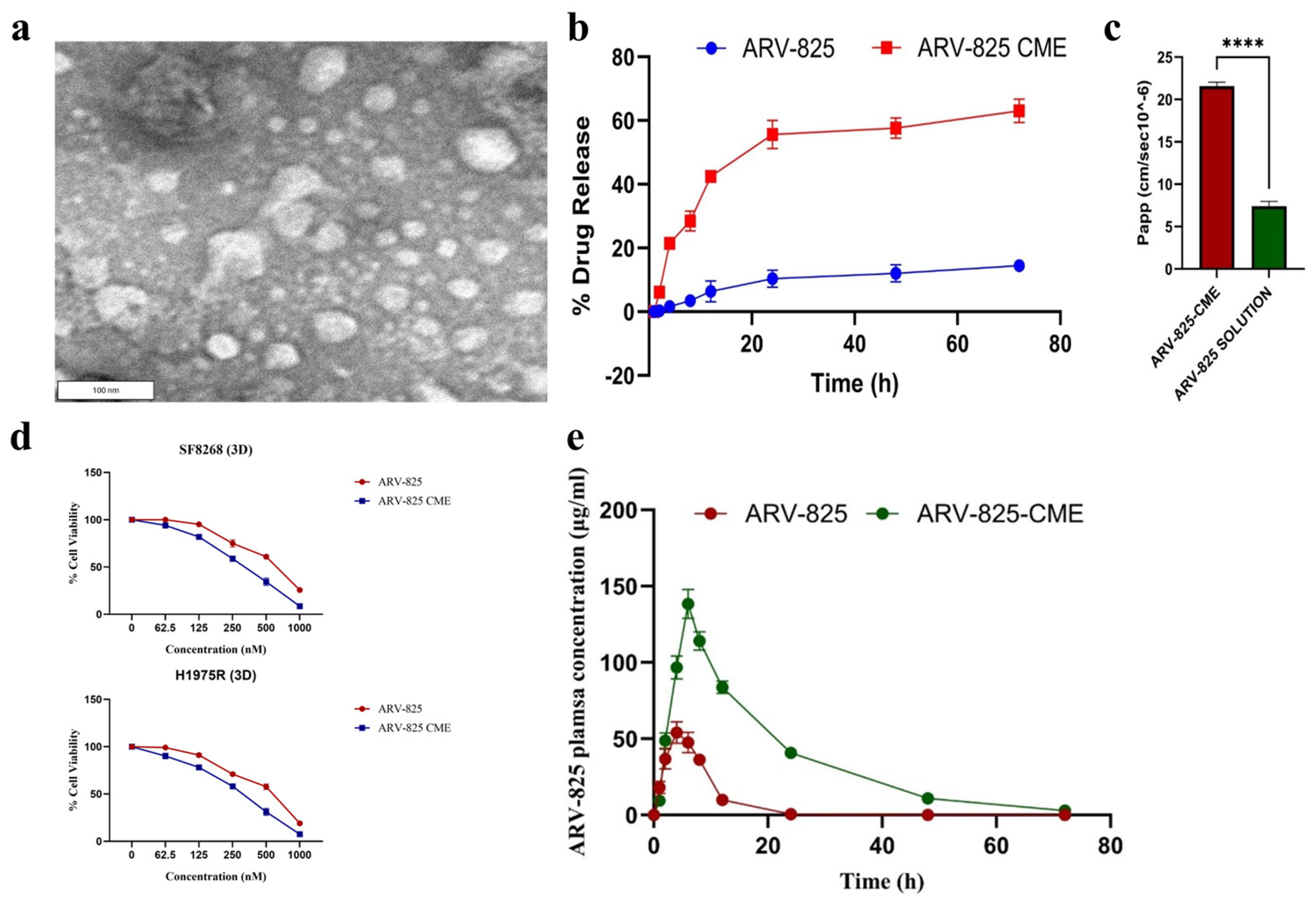
| PROTAC | POI | E3 Ligase | Clinical Phase | ROA | Diseases | Target Type | Clinical Trial Number |
|---|---|---|---|---|---|---|---|
| ARV-110 | AR | CRBN | Phase II/III | Oral | Prostate cancer | Nuclear receptor | NCT03888612 |
| ARV-471 | ER | CRBN | Phase III | Oral | Breast cancer | Nuclear receptor | NCT04072952 |
| ARV-766 | AR | - | Phase I/II | Oral | Prostate cancer | Nuclear receptor | NCT05067140 |
| AC682 | ER | CRBN | Phase I | Oral | Breast cancer | Nuclear receptor | NCT05080842 |
| CC-94676 | AR | CRBN | Phase I | Oral | Prostate cancer | Nuclear receptor | NCT04428788 |
| DT2216 | Bcl-xL | VHL | Phase I | I.V | Liquid and solid tumors | Anti-apoptotic protein | NCT04886622 |
| FHD-609 | BRD9 | CRBN | Phase I | I.V | Synovial sarcoma | Nuclear protein | NCT04965753 |
| KT-333 | STAT3 | - | Phase I | I.V | Liquid and solid tumors | Nuclear protein | NCT05225584 |
| KT-413 | IRAK4 | CRBN | Phase I | I.V | DLBCL (MYD88-mutant) | Serine/threonine kinase | NCT05233033 |
| KT-474 | IRAK4 | CRBN | Phase I | Oral | Autoimmune diseases | Serine/threonine kinase | NCT04772885 |
| NX-2127 | BTK | CRBN | Phase I | Oral | B cell malignancies | Non-receptor tyrosine kinase | NCT04830137 |
| NX-5948 | BTK | CRBN | Phase I | Oral | B cell malignancies and autoimmune diseases | Non-receptor tyrosine kinase | NCT05131022 |
| CFT8634 | BRD9 | CRBN | Phase I/II | Oral | Synovial sarcoma | Nuclear protein | NCT05355573 |
| CFT1946 | BRAF-V600X | CRBN | Phase I/II | Oral | Solid tumors | Serine/threonine kinase | NCT05668585 |
| CFT8919 | EGFR-L858R | CRBN | Phase I | Oral | Non-small cell lung cancer (NSCLC) | Receptor tyrosine kinase | NCT06641609 |
| CG001419 | TRK | CRBN | Phase I | Oral | Cancer and other indications | Receptor tyrosine kinase | NCT06636500 |
| PROTAC | POI | E3 Ligase | Diseases/Cell Lines | Particle Size (nm) | Zeta Potential (mV) | Delivery System | Improvements | Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ARV-825 | BRD4 | - | Pancreatic cancer | 89.63 ± 16.39 | - | Polymeric nanoparticle | Prolonged half-life, enhanced cell permeability | - | [45] |
| ARV-825 | BRD4 | - | Glioma | 26.3 ± 0.7 | −13.3 ± 8.0 | Polymeric nanoparticle | Penetrates BBB, increased stability, and reduced toxicity | Slow drug release (26.68% at 96 h) | [46] |
| MZ1 | BRD4 | - | HER2-positive breast cancer | 114 ± 2.3 | 31.8 ± 0.5 | Polymeric nanoparticle | Targeted delivery | - | [52] |
| dBET6 | BRD4 | CRBN | CD8+ T cells | - | - | Polymeric nanoparticle | Minimized toxicity and enhanced stability | No formulation stability data | [53] |
| ARV-825 | BRD4 | CRBN | Colorectal cancer | 59.31 | −0.64 | Polymeric nanoparticle | Enhanced cell permeability and EPR effects | Drug release depends on redox-responsive release | [54] |
| ARV-771 | BRD4 | - | TNBC cells | Polymeric nanoparticle | Cell permeability enhancement | - | [57] | ||
| ARV-771 | BRD4 | VHL | HeLa and B16F10 cells | 118 | −32.1 | Polymeric nanoparticle | Improved solubility and intracellular delivery | Unstable particle size, low reproducibility | [58] |
| dBET6 | BRD4 | CRBN | Lung cancer | 229.71 ± 72.1 | −29.0 | Stimuli-responsive and NPs | Targeted delivery | Particle size is over 200 nm, high dependency on pH and GSH level | [59] |
| dBET6 | BRD4 | - | Lung cancer | - | - | Stimuli-responsive and NPs | High drug-loading capacity, improved stability | - | [60] |
| MS39 | EGFR | VHL | HCC-827 and PC-9 cells | 202 ± 1.7 | −7.1 ± 0.12 | Self-assembled NPs | Increased stability and cell permeability | Reproducibility issue | [61] |
| MZ1 | BRD4 | VHL | - | 141.80 ± 5.66 | - | Stimuli-responsive delivery | Enhanced permeability and EPR effect | Long and pricey development process | [62] |
| ARV-825 | BRD4 | CRBN | Vemurafenib-resistant melanoma cells | 45.02 | -3.78 | SNEDDS | Enhanced solubility | Rapid precipitation, high concentration of DMA, stability relies on the selection and balance of excipients | [75] |
| ARV-825 | BRD4 | - | Vemurafenib-resistant melanoma cells | - | - | Emulsion | Enhanced solubility | - | [76] |
| ARCC-4 | AR | VHL | - | - | - | ASDs | Enhanced solubility and stability | Low drug loading, high dependency on polymer and its concentration | [82] |
| ARV-110 and SelDeg51 | - | - | - | - | - | ASDs | Enhanced solubility and stability | Long-term stability issues, low drug loading, pH-dependent dissolution profiles | [83] |
| MS4078 | - | - | - | - | - | ASDs | Enhanced solubility and stability | Long-term stability issues | [84] |
| ARV-825 | BRD4 | CRBN | NSCLC | 56.33 ± 0.42 | −21 ± 1.24 | LNPs | Improved solubility, stability, and intracellular delivery | Long-term stability issues | [93] |
| ARV-771 | BRD4 | VHL | HeLa cells | - | - | LNPs | Cell permeability enhancement | Low encapsulation efficiency | [94] |
| ARV-825 | BRD4 | - | BRAFi-resistant melanoma cells | 100 | - | LNPs | Lowered dosing and improved safety | - | [95] |
| DT2216 | Bcl-xL | - | Cervical and breast cancer | ~100 | - | Liposome | Good bioavailability in cells, reduced off-target and side effect | systemic toxicity concerns due to long-term released (up to 120 h) | [103] |
| ARV-825 | BRD4 | CRBN | Vemurafenib-resistant melanoma cells | 93.83 ± 10.05 | −27.30 | Liposome | Improved solubility and stability | Low apoptotic effect (<50%) | [104] |
| DT2216 | Bcl-xL | - | - | 200–300 | - | Liposome | Improved solubility | Low encapsulation efficiency, formulation stability | [105] |
| ARV-825 | BRD4 | - | Vemurafenib-resistant melanoma cells | 105.25 ± 2.76 | 26.6 | Liposome | Enhanced stability and minimized side effects | - | [106] |
| ARV-825 | BRD4 | - | Vemurafenib-resistant melanoma cells | 111.1 ± 6.55 | 13.9 ± 6.62 | Liposome | Improved cell permeability and stability | Long-term stability issues | [107] |
| ARV-825 | BRD4 | - | Breast cancer | ~115.7 | −17.1 | Liposome | Increased solubility, reduced systemic toxicity | - | [108] |
| ARV-825 | - | - | - | 136.8 ± 1.94 | - | Exosome | Improved cellular uptake and cell permeability | Entrapment efficiency below 50% | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syahputra, E.W.; Lee, H.; Cho, H.; Park, H.J.; Park, K.-S.; Hwang, D. PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics 2025, 17, 501. https://doi.org/10.3390/pharmaceutics17040501
Syahputra EW, Lee H, Cho H, Park HJ, Park K-S, Hwang D. PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics. 2025; 17(4):501. https://doi.org/10.3390/pharmaceutics17040501
Chicago/Turabian StyleSyahputra, Endry Wahyu, Hyunji Lee, Hyukjun Cho, Hyun Jin Park, Kwang-Su Park, and Duhyeong Hwang. 2025. "PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation" Pharmaceutics 17, no. 4: 501. https://doi.org/10.3390/pharmaceutics17040501
APA StyleSyahputra, E. W., Lee, H., Cho, H., Park, H. J., Park, K.-S., & Hwang, D. (2025). PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics, 17(4), 501. https://doi.org/10.3390/pharmaceutics17040501






