Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Three-Dimensional (3D) Modeling and Printing of Bone and Cartilage Grafts
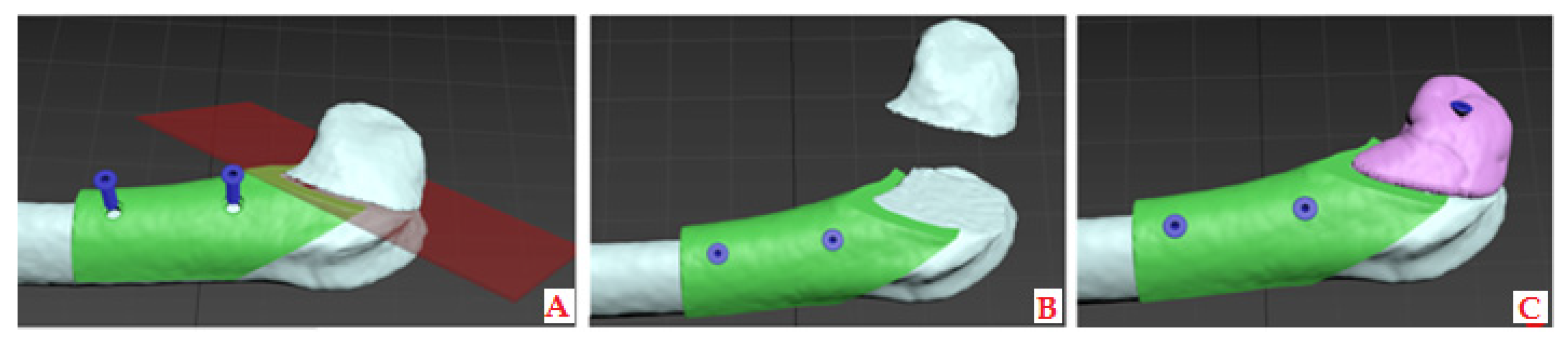
2.2.1. CBCT Data to Printable 3D Model Transformation
2.2.2. Modeling and 3D Printing of the Constructs with the Desired Porosity
2.3. In Vitro Study on SCAPs
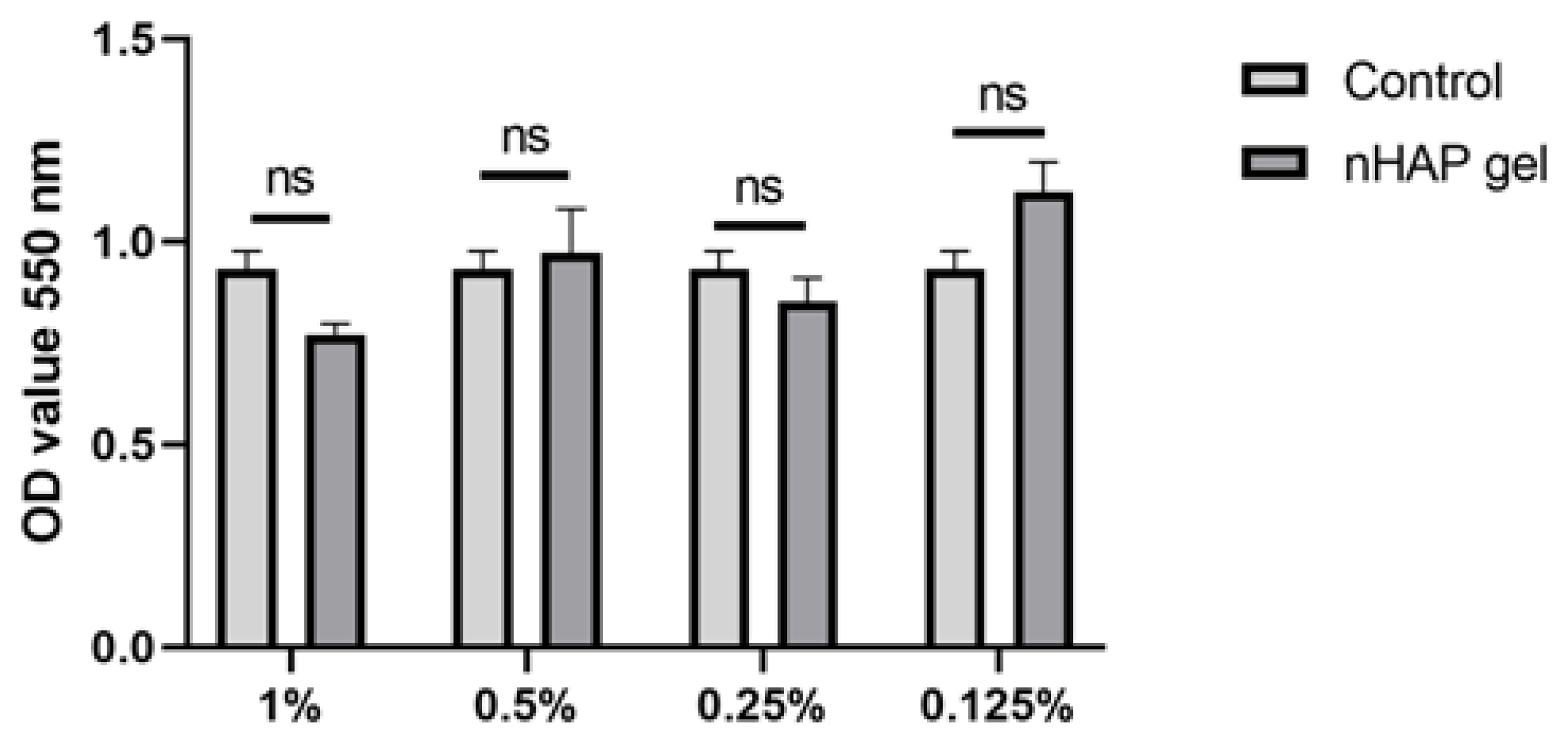
2.3.1. Mitochondrial Activity
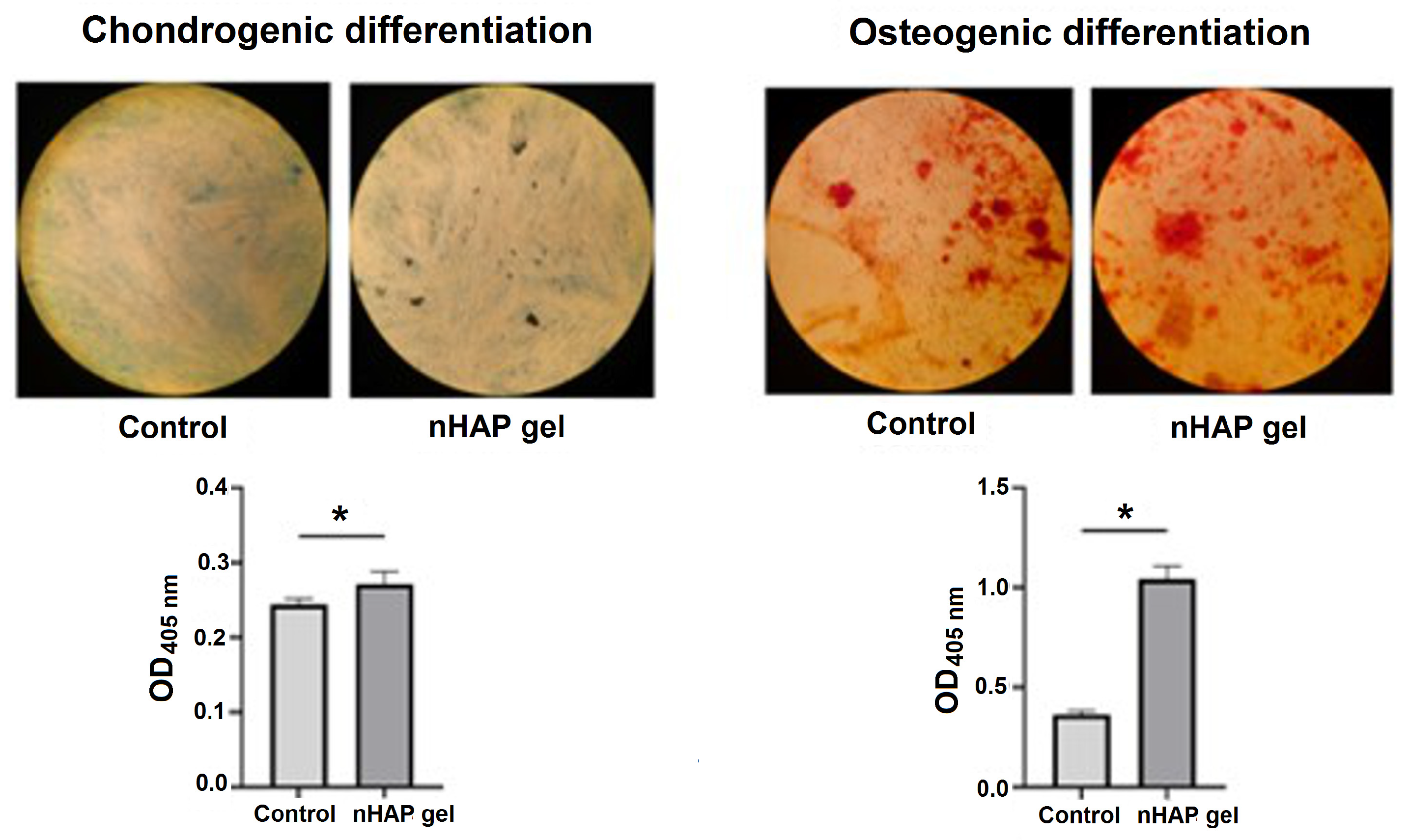
2.3.2. Osteogenic and Chondrogenic Differentiation
2.4. In Vivo Customized Bone Model Implantation in Rabbit Joint
2.4.1. Histological and Histomorphometric Analysis
2.4.2. qPCR Analysis of Tissue Samples
RNA Isolation and Reverse Transcription
Gene Expression Analysis of Osteogenic and Chondrogenic Differentiation
2.5. Statistical Analysis
3. Results
3.1. In Vitro Results on SCAP Cells
3.1.1. Mitochondrial Activity
3.1.2. Osteogenic and Chondrogenic Differentiation
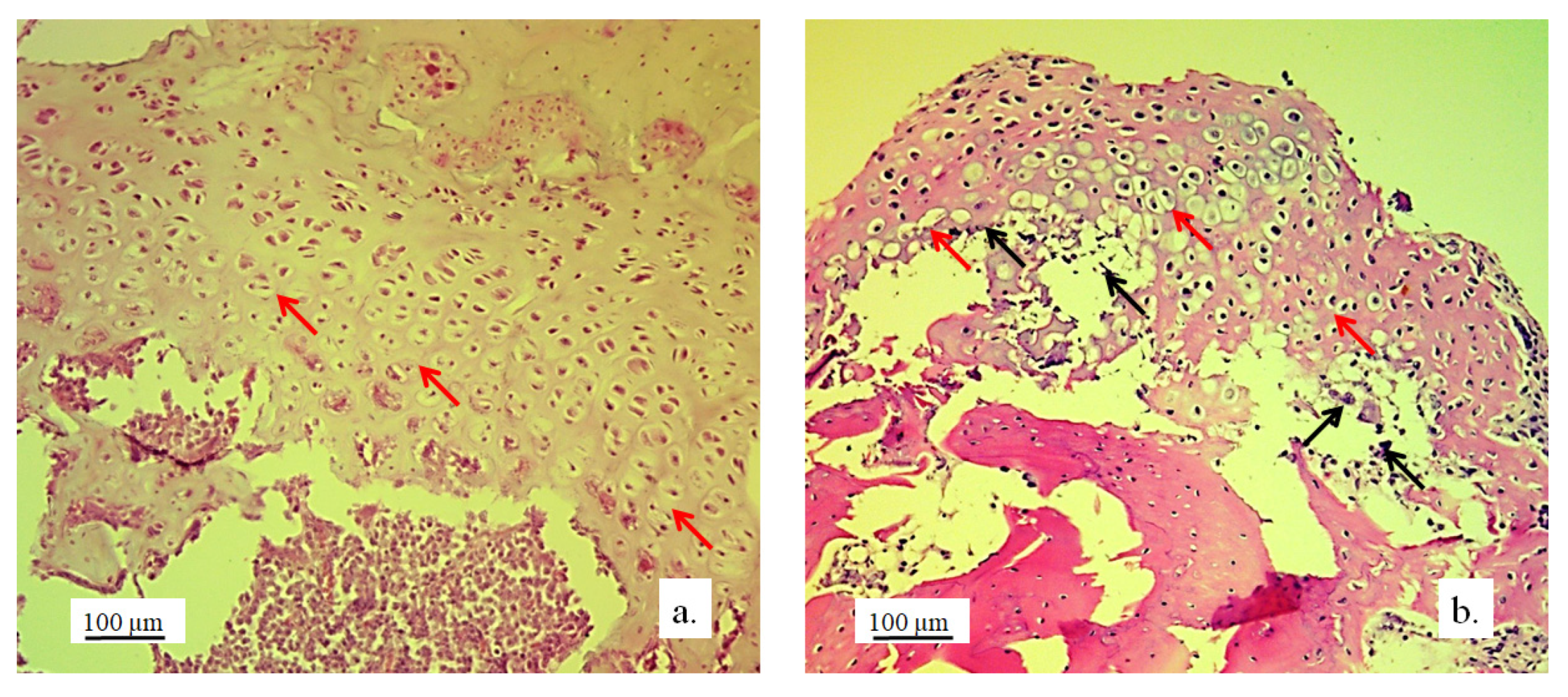
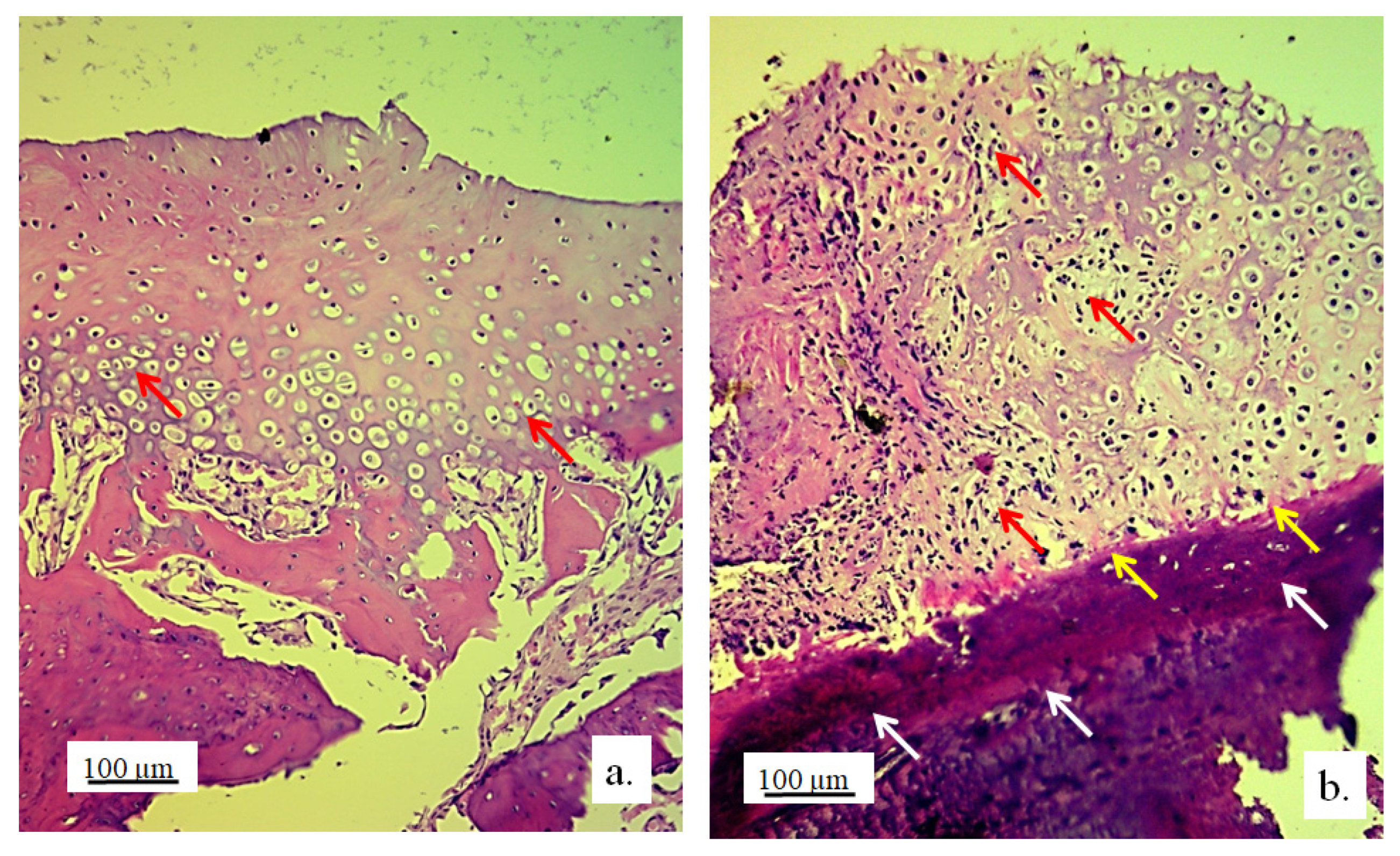
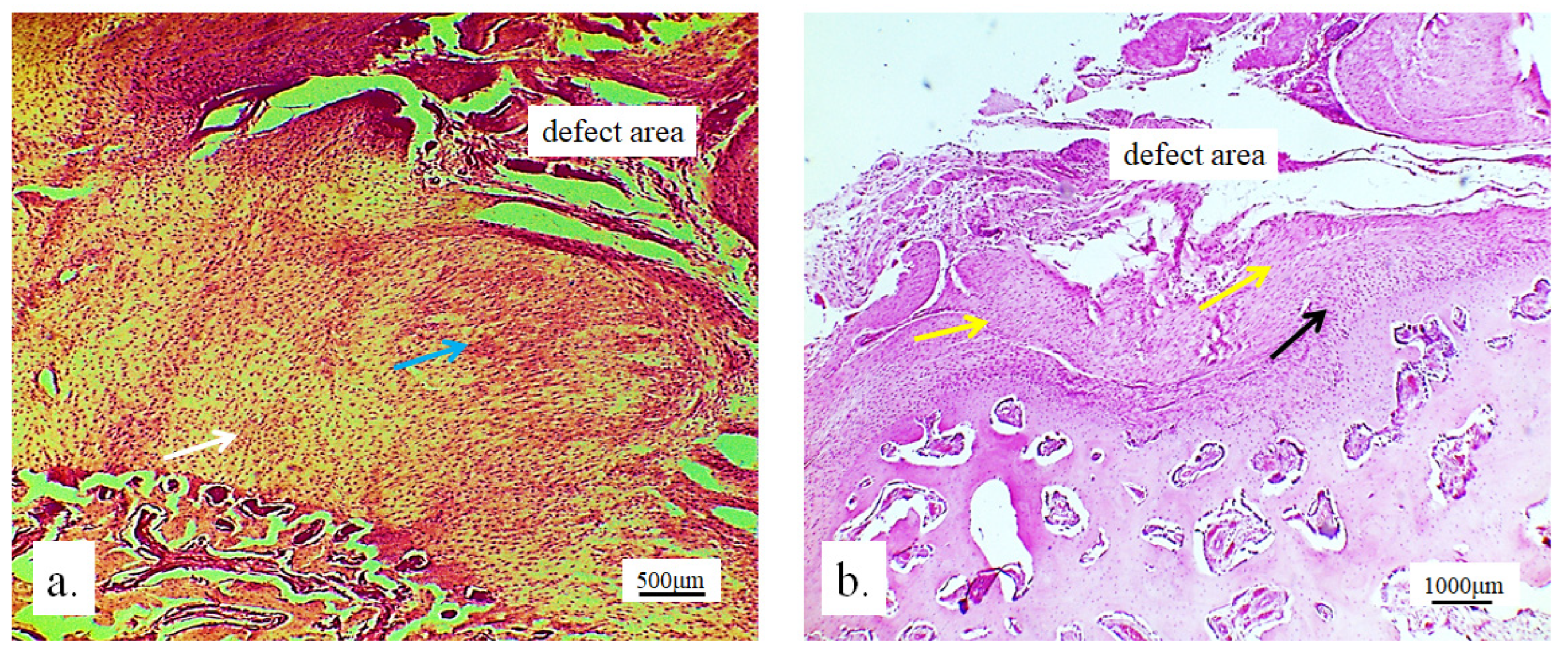
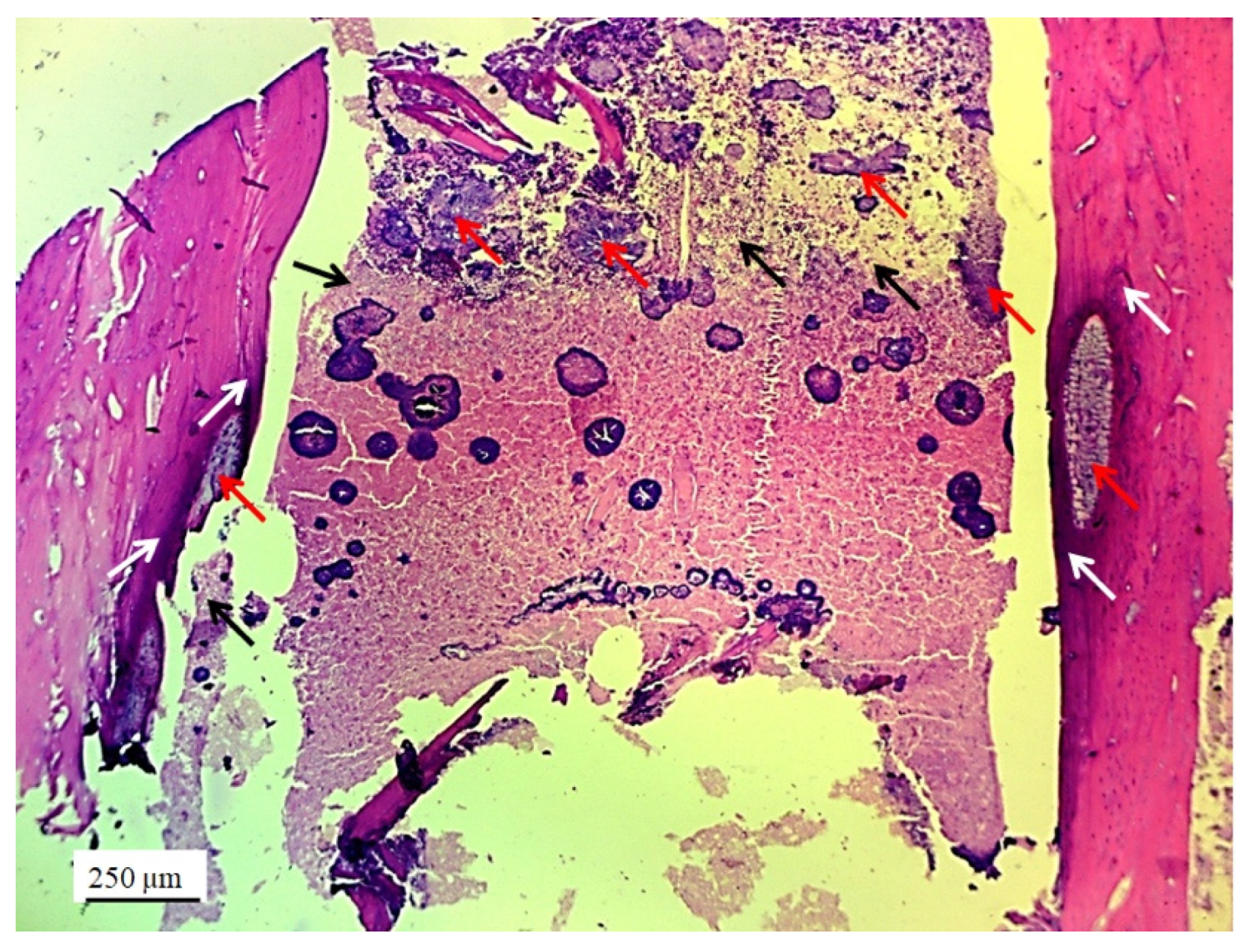
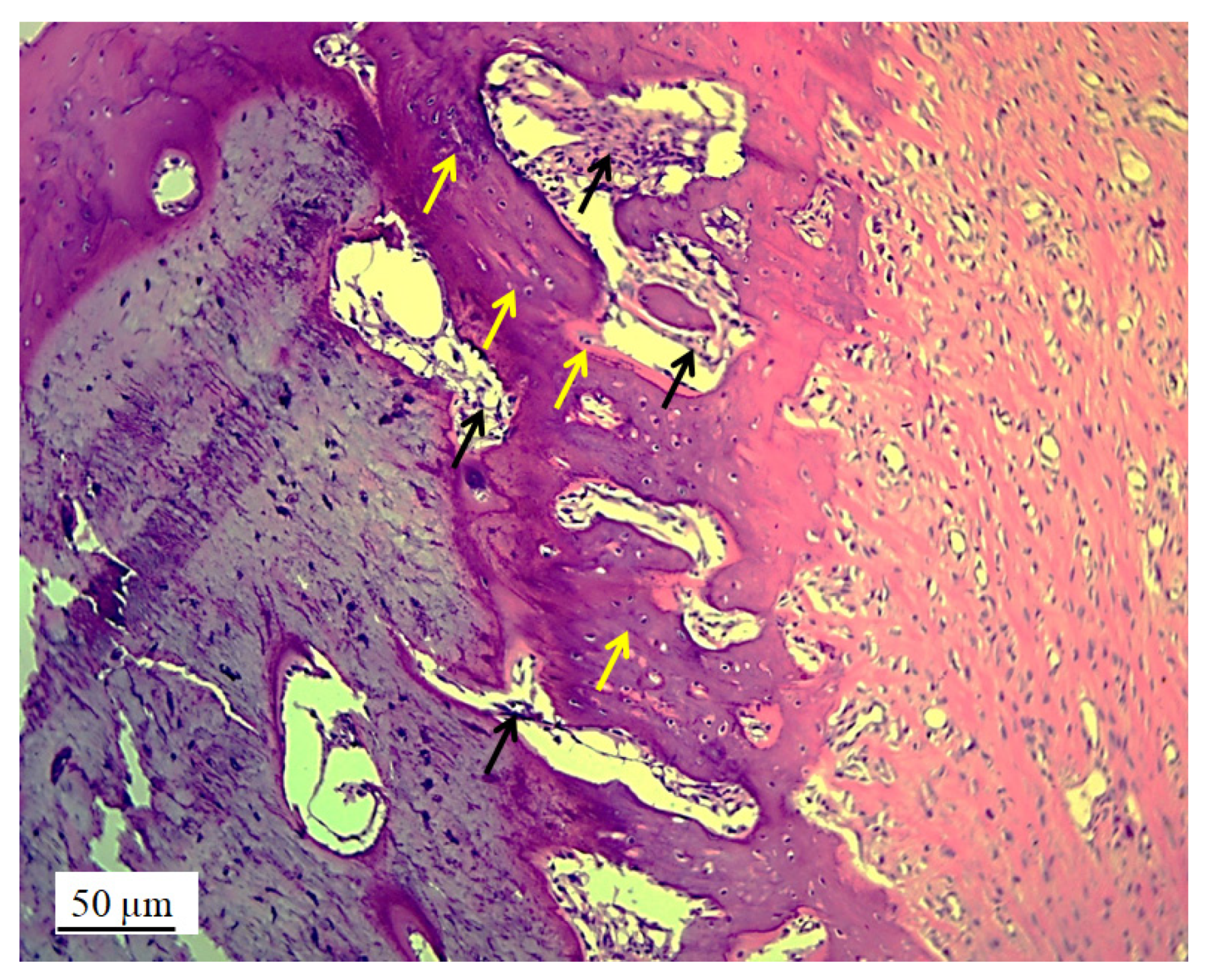
3.2. Stereological and Histological Findings
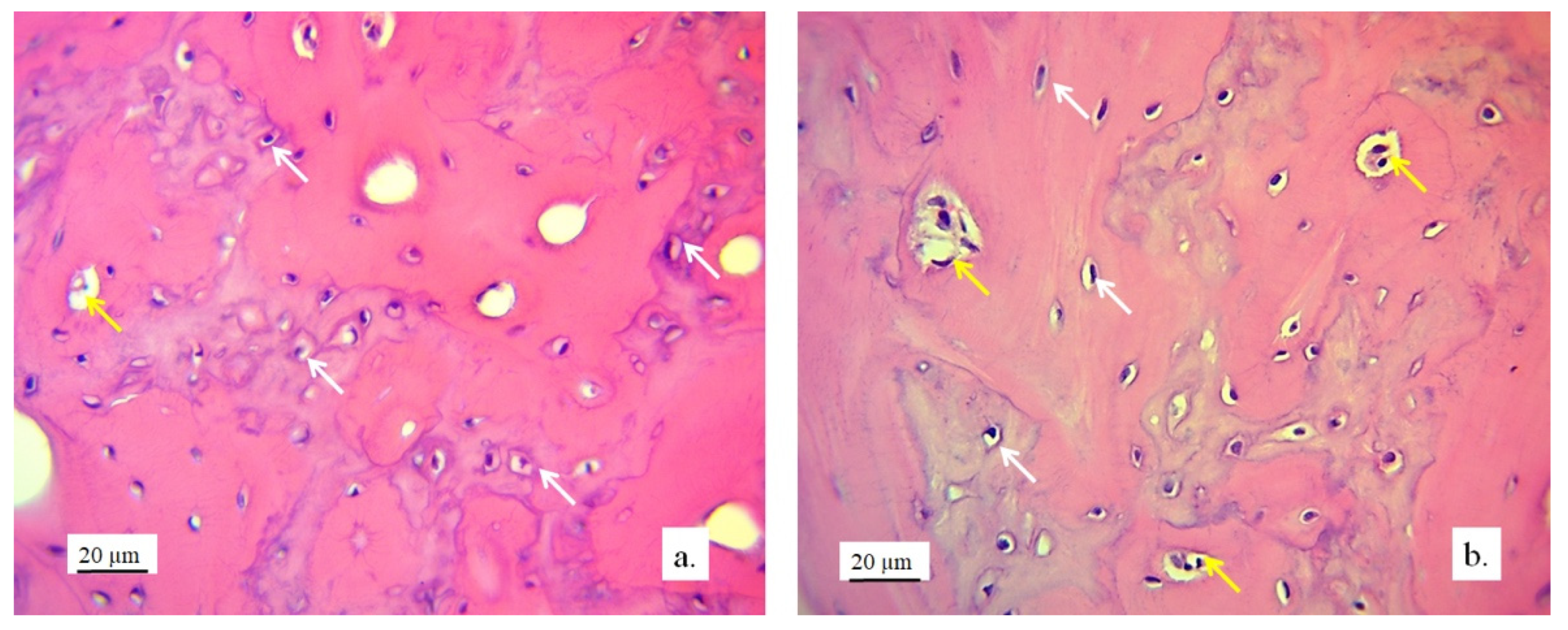
3.2.1. Hyaline Cartilage in the Rabbit Joint Region
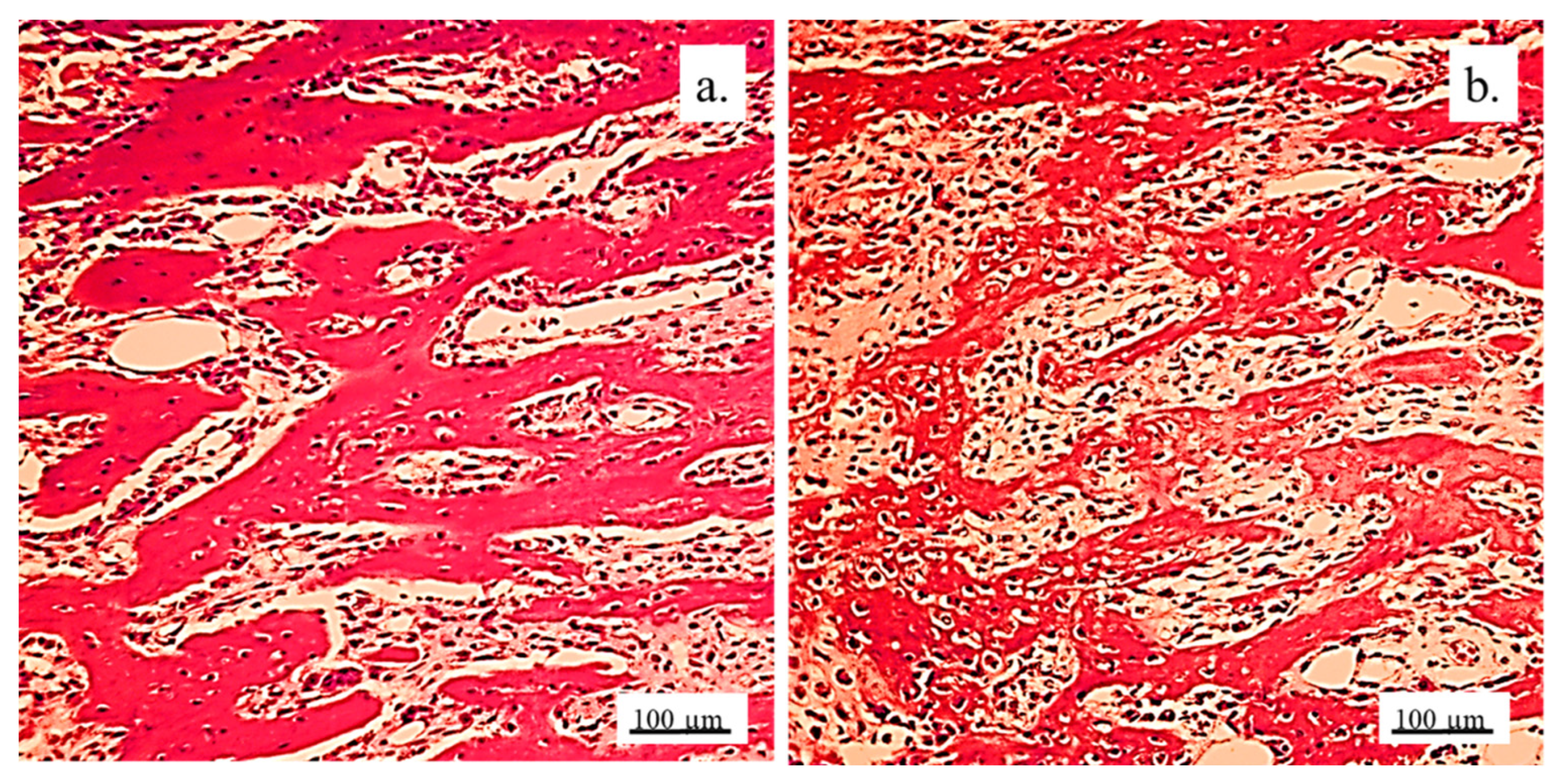
3.2.2. Bone Tissue in the Rabbit Joint Defect Region (Sub-Cartilage Region)
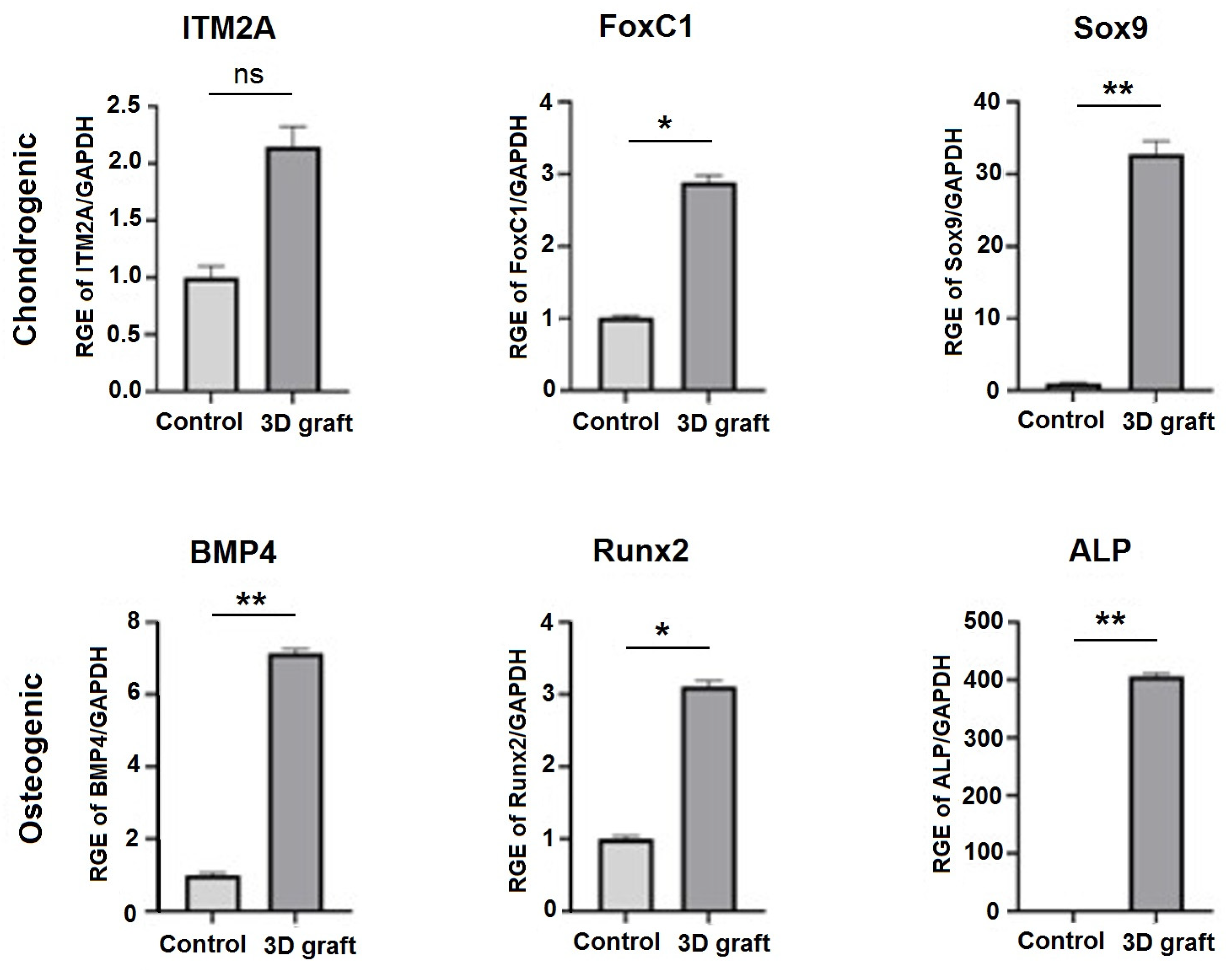
3.3. qPCR Analysis of Osteogenic and Chondrogenic Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| nHAP | nanohydroxyapatite |
| PLGA | Poly(lactide-co-glycolide) |
| SCAPs | stem cells from apical papilla |
| ALBO-OS | name of material based on nHAP and PLGA |
| BMP2 | bone morphogenetic protein 2 |
| PEVA | polyethylene vinyl acetate |
| PEVV | polyethylene vinyl versatate |
| SHMP | sodium hexametaphosphate |
| HEC | hydroxyethyl cellulose |
| SH | sodium hyaluronate |
| CH | chondroitin sulfate |
| CBCT | cone beam computed tomography |
| STL | Standard Tessellation Language |
| RKA | randomized Kruskal’s maze-generating algorithm |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetetrazolium bromide |
| DMSO | dimethyl sulfoxide |
| RNA | Ribonucleic acid |
| DNA | Deoxyribonucleic acid |
| qPCR | quantitative polymerase chain reaction |
| BMP4 | bone morphogenic protein 4 |
| RUNX2 | runt-related transcription factor 2 |
| ITM2A | integral membrane protein 2A |
| FOXC1 | forkhead box C1 |
| SOX9 | SRY-box transcription factor 9 |
| GAPDH | Gene glyceraldehyde-3-phosphate dehydrogenase |
| NCR | Nucleocytoplasmic ratio |
| ALP | alkaline phosphatase |
Appendix A
Custom 3D Printer Fabrication

References
- Yang, C.; Wu, H.; Chen, S.; Kang, G. Three-Dimensional Bioglass-Collagen-Phosphatidylserine Scaffolds Designed with Functionally Graded Structure and Mechanical Features. Biomed. Eng. Biomed. Tech. 2018, 63, 255–259. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Liu, M.; Chen, Y.; Cao, Y.; Yu, X. Chitosan-Collagen/Organomontmorillonite Scaffold for Bone Tissue Engineering. Front. Mater. Sci. 2015, 9, 405–412. [Google Scholar] [CrossRef]
- Liu, L.; Shi, G.; Cui, Y.; Li, H.; Li, Z.; Zeng, Q.; Guo, Y. Individual Construction of Freeform-Fabricated Polycaprolactone Scaffolds for Osteogenesis. Biomed. Eng. Biomed. Tech. 2017, 62, 467–479. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Wang, J. Effect of Steroidal Saponins-Loaded Nano-Bioglass/Phosphatidylserine/Collagen Bone Substitute on Bone Healing. Biomed. Eng. Biomed. Tech. 2017, 62, 487–491. [Google Scholar] [CrossRef]
- Lee, K.G.; Lee, K.S.; Kang, Y.J.; Hwang, J.H.; Lee, S.H.; Park, S.H.; Park, Y.; Cho, Y.S.; Lee, B.K. Rabbit Calvarial Defect Model for Customized 3D-Printed Bone Grafts. Tissue Eng. Part C Methods 2018, 24, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Fatimi, A.; Magdalena Musuc, A.; Paiva Santos, A.C.; Paszkiewicz, S.; Igwe Idumah, C.; Singh, S.; Varma, R.S.; Berrada, M. Nano-Hydroxyapatite (nHAp) Scaffolds for Bone Regeneration: Preparation, Characterization and Biological Applications. J. Drug Deliv. Sci. Technol. 2024, 95, 105601. [Google Scholar] [CrossRef]
- Paraš, S.; Trišić, D.; Mitrović Ajtić, O.; Prokić, B.; Drobne, D.; Živković, S.; Jokanović, V. Toxicological Profile of Nanostructured Bone Substitute Based on Hydroxyapatite and Poly(Lactide-Co-Glycolide) after Subchronic Oral Exposure of Rats. Nanomaterials 2020, 10, 918. [Google Scholar] [CrossRef]
- Mitić, D.; Čarkić, J.; Jaćimović, J.; Lazarević, M.; Jakšić Karišik, M.; Toljić, B.; Milašin, J. The Impact of Nano-Hydroxyapatite Scaffold Enrichment on Bone Regeneration In Vivo—A Systematic Review. Biomimetics 2024, 9, 386. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Jokanović, M.; Milosavljević, P.; Sopta, J. In Vivo Investigation of ALBO-OS Scaffold Based on Hydroxyapatite and PLGA. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Soldatović, I.; Antonijević, D.; Milosavljević, P.; Sjerobabin, N.; Sopta, J. Extraordinary Biological Properties of a New Calcium Hydroxyapatite/Poly(Lactide-Co-Glycolide)-Based Scaffold Confirmed by in Vivo Investigation. Biomed. Eng. Biomed. Tech. 2017, 62, 295–306. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.J.; Zhu, X.D.; Vaidya, S.; Xiang, Z.; Fan, Y.J.; Zhang, X.D. Enhanced Repair of a Critical-Sized Segmental Bone Defect in Rabbit Femur by Surface Microstructured Porous Titanium. J. Mater. Sci. Mater. Med. 2014, 25, 1747–1756. [Google Scholar] [CrossRef]
- Fassbender, M.; Minkwitz, S.; Thiele, M.; Wildemann, B. Efficacy of Two Different Demineralised Bone Matrix Grafts to Promote Bone Healing in a Critical-Size-Defect: A Radiological, Histological and Histomorphometric Study in Rat Femurs. Int. Orthop. (SICOT) 2014, 38, 1963–1969. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, P.; Wang, Z.; Lyu, Z.; Wu, H. Tissue-Engineered Composite Scaffold of Poly(Lactide-Co-Glycolide) and Hydroxyapatite Nanoparticles Seeded with Autologous Mesenchymal Stem Cells for Bone Regeneration. J. Zhejiang Univ. Sci. B 2017, 18, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Chinta, M.L.; Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Parcha, S.R. Assessment of Properties, Applications and Limitations of Scaffolds Based on Cellulose and Its Derivatives for Cartilage Tissue Engineering: A Review. Int. J. Biol. Macromol. 2021, 175, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Miralles, G.; Baudoin, R.; Dumas, D.; Baptiste, D.; Hubert, P.; Stoltz, J.F.; Dellacherie, E.; Mainard, D.; Netter, P.; Payan, E. Sodium Alginate Sponges with or without Sodium Hyaluronate: In Vitro Engineering of Cartilage. J. Biomed. Mater. Res. 2001, 57, 268–278. [Google Scholar] [CrossRef]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.-W. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar] [CrossRef]
- Micic, M.; Antonijevic, D.; Milutinovic-Smiljanic, S.; Trisic, D.; Colovic, B.; Kosanovic, D.; Prokic, B.; Vasic, J.; Zivkovic, S.; Milasin, J.; et al. Developing a Novel Resorptive Hydroxyapatite-Based Bone Substitute for over-Critical Size Defect Reconstruction: Physicochemical and Biological Characterization and Proof of Concept in Segmental Rabbit’s Ulna Reconstruction. Biomed. Eng. Biomed. Tech. 2020, 65, 491–505. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 5 March 2025).
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, W.; Lin, D.; Yu, Y.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. Bioinspired Trimodal Macro/Micro/Nano-Porous Scaffolds Loading rhBMP-2 for Complete Regeneration of Critical Size Bone Defect. Acta Biomater. 2016, 32, 309–323. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, C.Y.; Liu, Y.; Li, J.; Wang, Y.X.; Zheng, S.G. RUNX2 Regulates Osteoblast Differentiation via the BMP4 Signaling Pathway. J. Dent. Res. 2022, 101, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Comparative Study on the Role of Gelatin, Chitosan and Their Combination as Tissue Engineered Scaffolds on Healing and Regeneration of Critical Sized Bone Defects: An in Vivo Study. J. Mater. Sci. Mater. Med. 2016, 27, 155. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lv, K.; Zhang, W.; Zhang, X.; Jiang, X.; Zhang, F. The Healing of Critical-Size Calvarial Bone Defects in Rat with rhPDGF-BB, BMSCs, and β-TCP Scaffolds. J. Mater. Sci. Mater. Med. 2012, 23, 1073–1084. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Zhao, D.; Jiang, W.; Du, Z.; Li, Q.; Jiang, C.; Han, D. Three Dimensional Printed Polylactic Acid-Hydroxyapatite Composite Scaffolds for Prefabricating Vascularized Tissue Engineered Bone: An in Vivo Bioreactor Model. Sci. Rep. 2017, 7, 15255. [Google Scholar] [CrossRef]
- Temple, J.P.; Yeager, K.; Bhumiratana, S.; Vunjak-Novakovic, G.; Grayson, W.L. Bioreactor Cultivation of Anatomically Shaped Human Bone Grafts. In Biomimetics and Stem Cells; Vunjak-Novakovic, G., Turksen, K., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2013; Volume 1202, pp. 57–78. [Google Scholar] [CrossRef]
- Nezhurina, E.K.; Karalkin, P.A.; Komlev, V.S.; Sviridova, I.K.; Kirsanova, V.A.; Akhmedova, S.A.; Shanskiy, Y.D.; Fedotov, A.Y.; Barinov, S.M.; Sergeeva, N.S. Physicochemical and Osteoplastic Characteristics of 3D Printed Bone Grafts Based on Synthetic Calcium Phosphates and Natural Polymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012047. [Google Scholar] [CrossRef]
- Hettwer, W.; Horstmann, P.F.; Bischoff, S.; Güllmar, D.; Reichenbach, J.R.; Poh, P.S.P.; Van Griensven, M.; Gras, F.; Diefenbeck, M. Establishment and Effects of Allograft and Synthetic Bone Graft Substitute Treatment of a Critical Size Metaphyseal Bone Defect Model in the Sheep Femur. APMIS 2019, 127, 53–63. [Google Scholar] [CrossRef]
- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, K.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schütz, M.; et al. Tissue Engineering and Regenerative Approaches to Improving the Healing of Large Bone Defects. eCM 2016, 32, 87–110. [Google Scholar] [CrossRef]
- Liang, J.; Liu, P.; Yang, X.; Liu, L.; Zhang, Y.; Wang, Q.; Zhao, H. Biomaterial-Based Scaffolds in Promotion of Cartilage Regeneration: Recent Advances and Emerging Applications. J. Orthop. Transl. 2023, 41, 54–62. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the Many Facets of Its Regulation in the Chondrocyte Lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Yagi, H. Regulation of Cartilage Development and Diseases by Transcription Factors. J. Bone Metab. 2017, 24, 147. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Börger, M.; Hennig, T.; Winter, A.; Kasten, P.; Richter, W. Enhanced ITM2A Expression Inhibits Chondrogenic Differentiation of Mesenchymal Stem Cells. Differentiation 2009, 78, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic Mineralized Hierarchical Hybrid Scaffolds Based on in Situ Synthesis of Nano-Hydroxyapatite/Chitosan/Chondroitin Sulfate/Hyaluronic Acid for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]





| BMP4 | Rv | 5′ GGG TGA GTG GAT GG AAC 3′ |
| Fw | 5′ CTC GAT GAG TAT GAT AAG GTG GTA 3′ | |
| RUNX2 | Rv | 5′ GTC TCG GTG GCT GGT AGT GA 3′ |
| Fw | 5′ ACA AAC AAC CAC AGA ACC ACA AGT 3′ | |
| ALP | Rv | 5′ ATG GCA GTG AAG GGC TTC TT 3′ |
| Fw | 5′ CCA CGT CTT CAC ATT TGG TG 3′ | |
| ITM2A | Rv | 5′ GTCCTGCCAAGATGAATGAA 3′ |
| Fw | 5′ AGGATCTCCTCTTGCAGTC 3′ | |
| FOXC1 | Rv | 5′ CGGGGCTCTCGATCTTGGGCA 3′ |
| Fw | 5′ AAGACCGAGAACGGTACGTG 3′ | |
| SOX9 | Rv | 5′ CTC TTT TGC ACC CCT CCC ATT 3′ |
| Fw | 5′ GAC TTC ACA TGT CCC AGC ACT A 3′ | |
| GAPDH | Rv | 5′ CCC TGT TGC TGT AGC CAA ATT CGT 3′ |
| Fw | 5′ TCA TGA CCA CAG TCC ATG CCA TCA 3′ |
| Parameters | Control | 3D Graft |
|---|---|---|
| Volume density of chondrocytes (mm0) | 0.362 ± 0.044 * | 0.295 ± 0.051 |
| Volume density of cartilage matrix (mm0) | 0.638 ± 0.076 | 0.705 ± 0.079 |
| Number of chondrocytes | 24,805.1 ± 3007.4 *** | 17,545.6 ± 2107.4 |
| Numerical density of chondrocytes (mm−3) | 3664.4 ± 417.8 ** | 2931.4 ± 386.2 |
| Surface area of chondrocytes (μm2) | 238.5 ± 22.6 | 305.7 ± 28.6 *** |
| Surface area of chondrocytes’ nuclei (μm2) | 75.3 ± 9.9 | 98.6 ± 10.2 *** |
| Surface area of lacunae of cartilage (μm2) | 136.5 ± 12.4 | 199.4 ± 16.5 **** |
| NCR of chondrocytes | 0.323 ± 0.021 | 0.402 ± 0.033 *** |
| Volume density of osteocytes (mm0) | 0.266 ± 0.019 ** | 0.225 ± 0.017 |
| Volume density of bone matrix (mm0) | 0.560 ± 0.044 *** | 0.447 ± 0.041 |
| Volume density of blood vessels in bone tissue (mm0) | 0.174 ± 0.015 | 0.328 ± 0.024 **** |
| Number of osteocytes | 28574.2 ± 2314.6 **** | 21759.1 ± 2219.4 |
| Numerical density of osteocytes (mm−3) | 3015.6 ± 353.3 * | 2554.5 ± 311.1 |
| Surface area of osteocytes (μm2) | 172.6 ± 15.4 *** | 139.3 ± 13.2 |
| Surface area of osteocytes nuclei (μm2) | 69.4 ± 8.4 | 60.5 ± 8.2 |
| Nucleocytoplasmic ratio (NCR) of osteocytes | 0.325 ± 0.031 | 0.409 ± 0.038 *** |
| Diameter of blood vessels (µm) | 55.5 ± 6.6 | 68.4 ± 7.2 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraš, S.; Petrović, B.; Mitić, D.; Lazarević, M.; Popović Bajić, M.; Živković, M.; Mićić, M.; Biočanin, V.; Živković, S.; Jokanović, V. Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering. Pharmaceutics 2025, 17, 489. https://doi.org/10.3390/pharmaceutics17040489
Paraš S, Petrović B, Mitić D, Lazarević M, Popović Bajić M, Živković M, Mićić M, Biočanin V, Živković S, Jokanović V. Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering. Pharmaceutics. 2025; 17(4):489. https://doi.org/10.3390/pharmaceutics17040489
Chicago/Turabian StyleParaš, Smiljana, Božana Petrović, Dijana Mitić, Miloš Lazarević, Marijana Popović Bajić, Marija Živković, Milutin Mićić, Vladimir Biočanin, Slavoljub Živković, and Vukoman Jokanović. 2025. "Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering" Pharmaceutics 17, no. 4: 489. https://doi.org/10.3390/pharmaceutics17040489
APA StyleParaš, S., Petrović, B., Mitić, D., Lazarević, M., Popović Bajić, M., Živković, M., Mićić, M., Biočanin, V., Živković, S., & Jokanović, V. (2025). Three-Dimensional-Printed Bone Grafts for Simultaneous Bone and Cartilage Regeneration: A Promising Approach to Osteochondral Tissue Engineering. Pharmaceutics, 17(4), 489. https://doi.org/10.3390/pharmaceutics17040489










