The Effect of Gut Microbiome Perturbation on the Bioavailability of Glycyrrhizic Acid in Rats
Abstract
1. Introduction
2. Materials and Methods
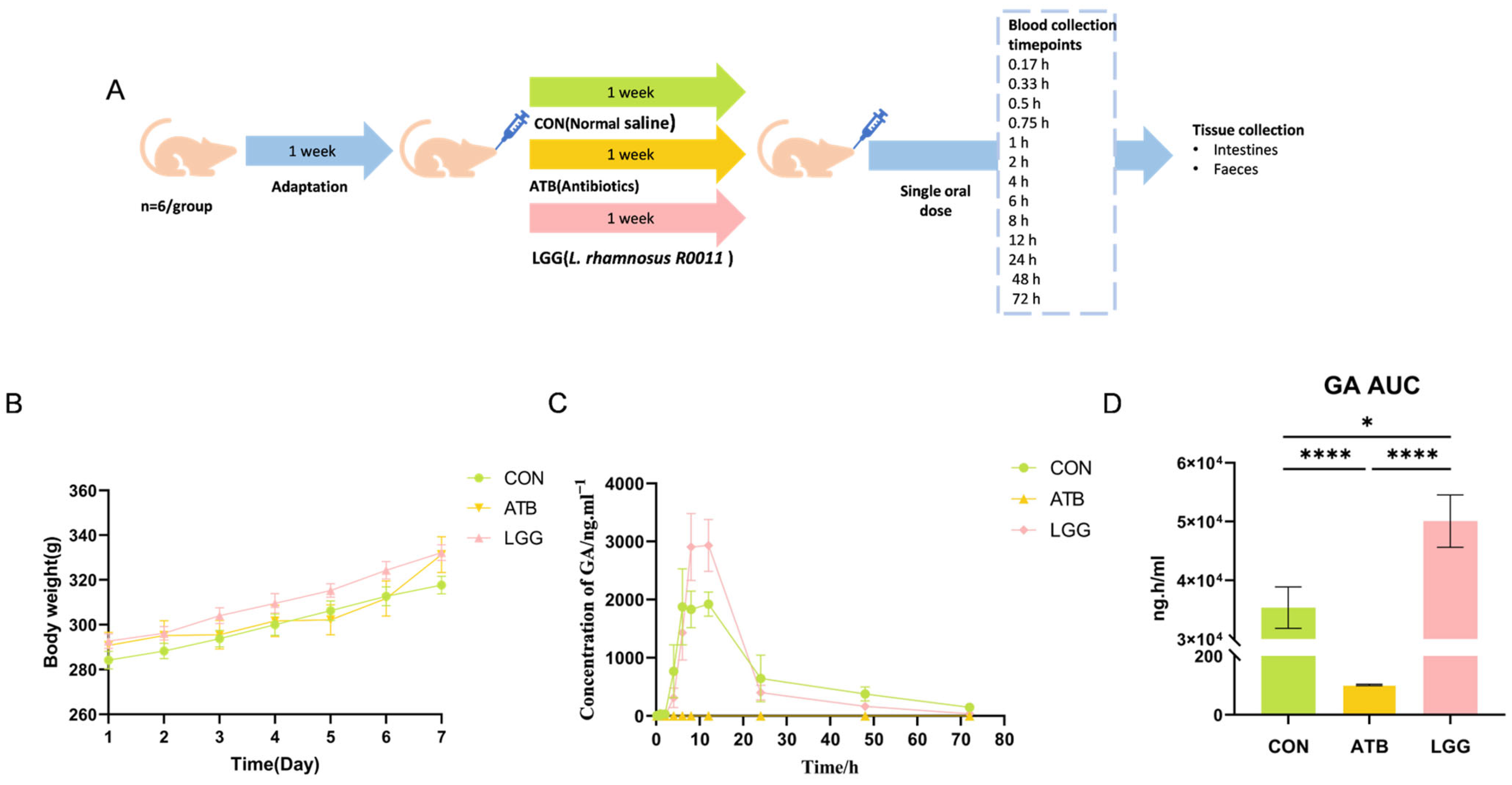
2.1. Animals
2.2. Antibiotic and R0011 Treatment
2.3. Sample Collection
2.4. Cell Culture
2.5. Preparation of Fecal Water
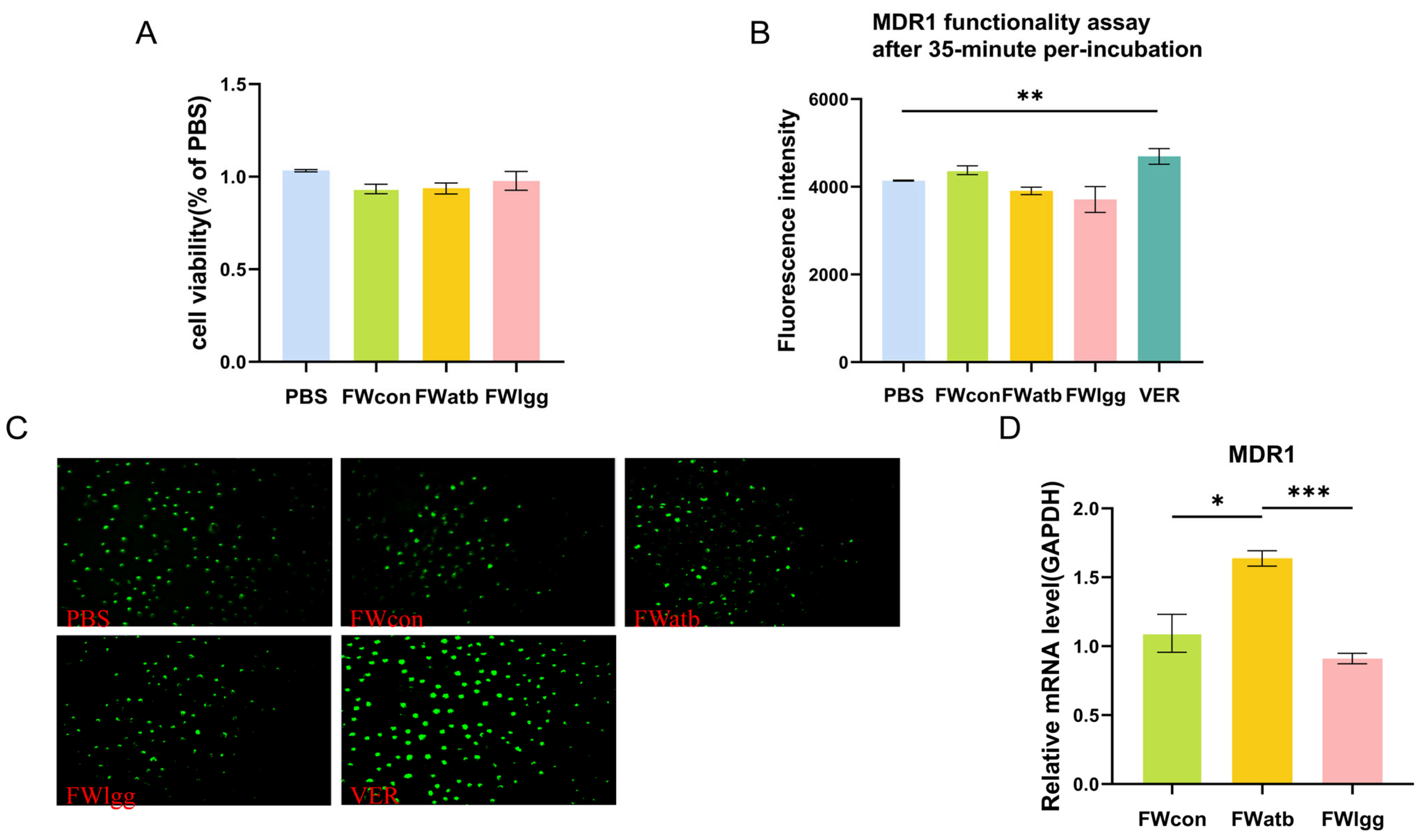
2.6. FW Cytotoxicity Determination
2.7. Co-Incubation of Cells and FW
2.8. Determination of MDR1 Function in Cells
2.9. Real-Time Fluorescence Quantitative PCR
2.10. Pharmacokinetic Analysis of GA
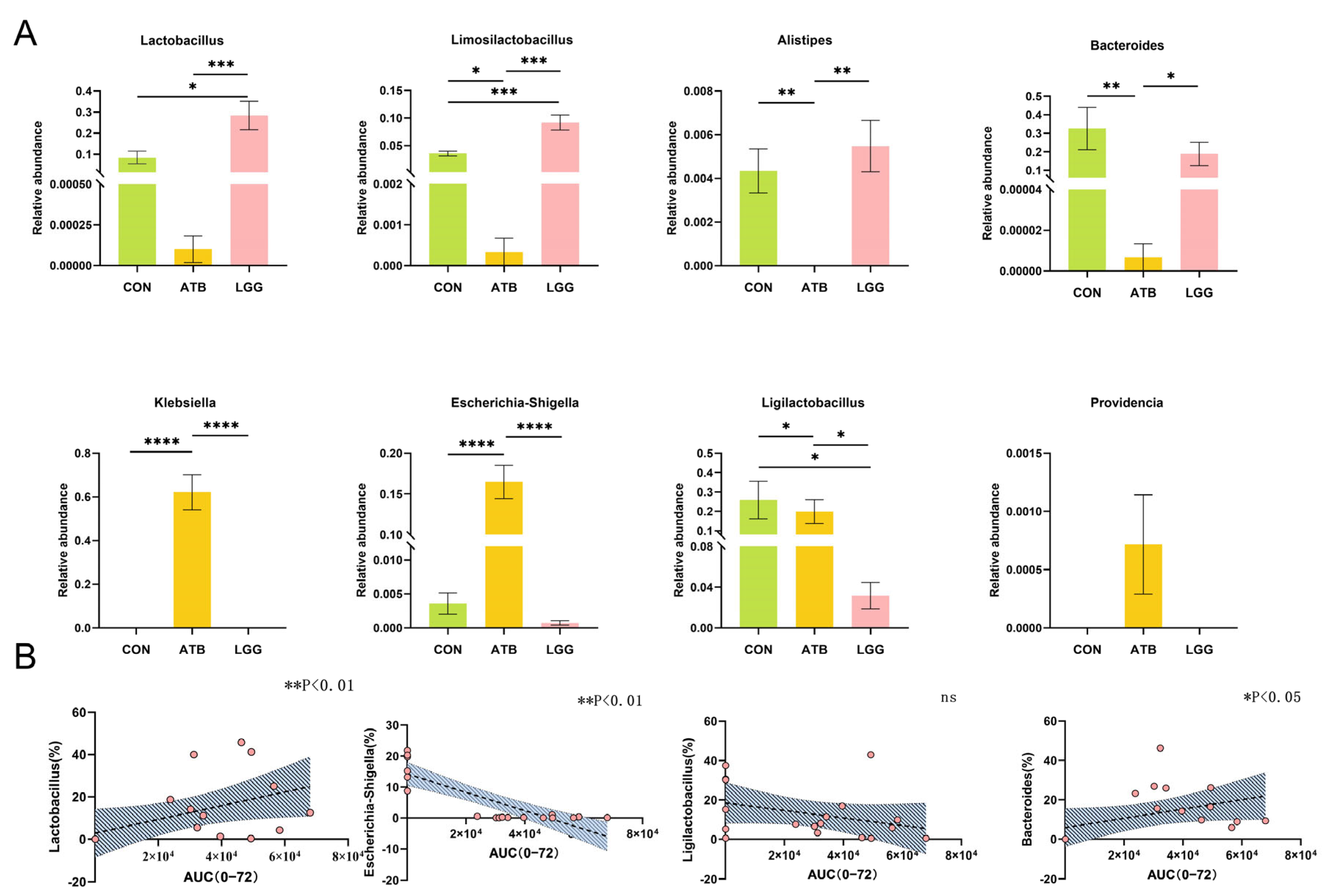
2.11. Intestinal Flora Analysis
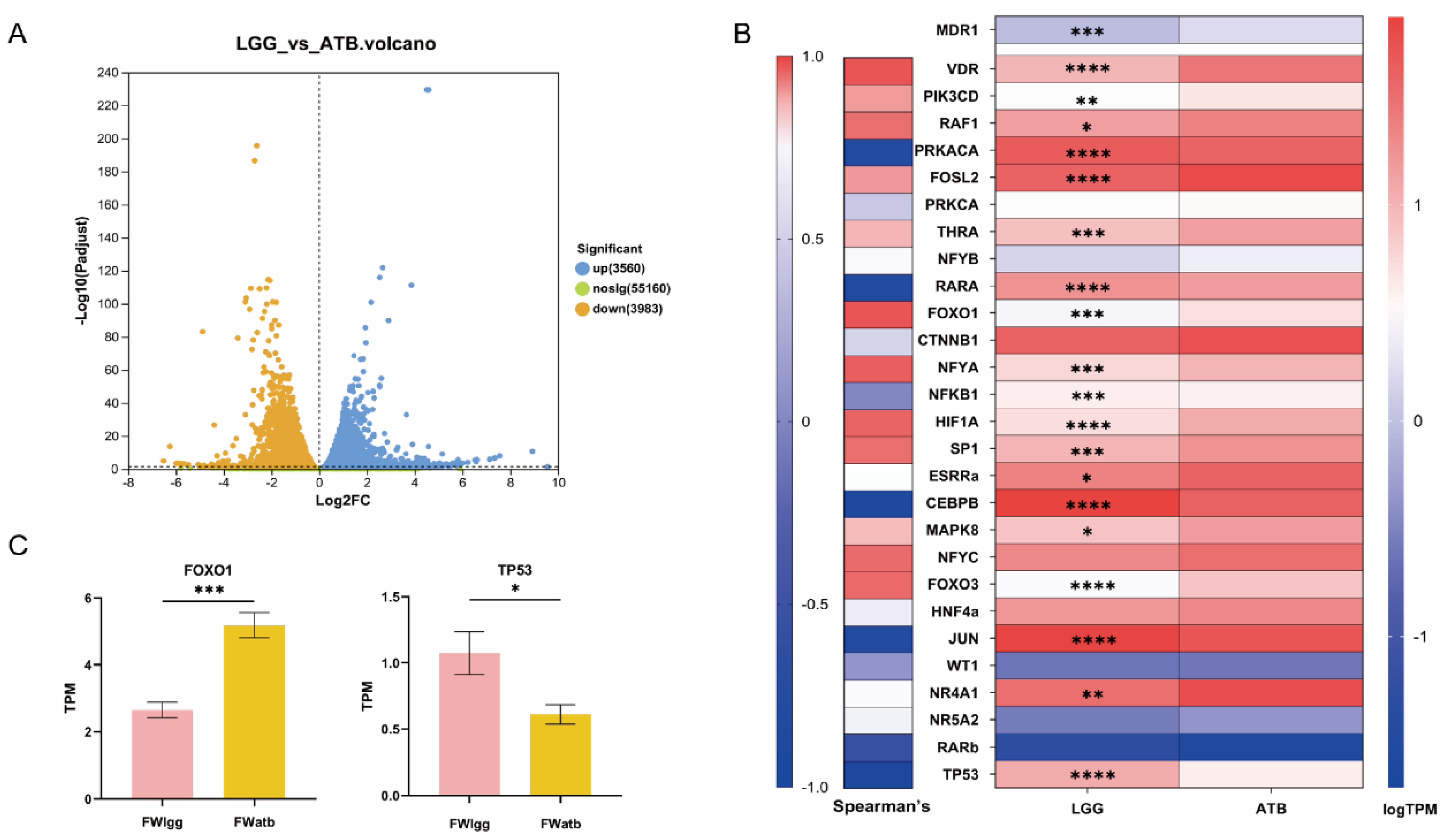
2.12. Cellular Eukaryotic Reference Transcriptome Analysis
2.13. Statistical Analysis
3. Results
3.1. Antibiotics and R0011 Differentially Impact the Metabolism of GL
3.2. Antibiotic and R0011 Preconditioning-Altered Intestinal Flora
3.3. The AUC Value of GA Correlated with the Change in Gut Microbiome Composition
3.4. The Expression of MDR1 in the Ileum Decreased Under R0011 Intervention
3.5. Gut Microbial Metabolites Affect MDR1 Expression but There Is No Direct Impact on Function
3.6. Gut Microbial Metabolites Regulate MDR1 Expression by Transcriptional Regulators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamath, S.; Stringer, A.M.; Prestidge, C.A.; Joyce, P. Targeting the gut microbiome to control drug pharmacomicrobiomics: The next frontier in oral drug delivery. Expert Opin. Drug Deliv. 2023, 20, 1315–1331. [Google Scholar] [CrossRef] [PubMed]
- Viglioli, M.; Rizzo, S.M.; Alessandri, G.; Fontana, F.; Milani, C.; Turroni, F.; Mancabelli, L.; Croci, N.; Rivara, S.; Vacondio, F.; et al. Investigating drug-gut microbiota interactions: Reductive and hydrolytic metabolism of oral glucocorticoids by in vitro artificial gut microbiota. Int. J. Pharm. 2024, 665, 124663. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e965. [Google Scholar] [CrossRef]
- Degraeve, A.L.; Haufroid, V.; Loriot, A.; Gatto, L.; Andries, V.; Vereecke, L.; Elens, L.; Bindels, L.B. Gut microbiome modulates tacrolimus pharmacokinetics through the transcriptional regulation of ABCB1. Microbiome 2023, 11, 138. [Google Scholar] [CrossRef]
- Subramanian, V.S.; Sabui, S.; Moradi, H.; Marchant, J.S.; Said, H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 556–565. [Google Scholar] [CrossRef]
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183. [Google Scholar] [CrossRef]
- Xie, Q.S.; Zhang, J.X.; Liu, M.; Liu, P.H.; Wang, Z.J.; Zhu, L.; Jiang, L.; Jin, M.M.; Liu, X.N.; Liu, L.; et al. Short-chain fatty acids exert opposite effects on the expression and function of p-glycoprotein and breast cancer resistance protein in rat intestine. Acta Pharmacol. Sin. 2021, 42, 470–481. [Google Scholar]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab. Dispos. 2014, 42, 1508–1513. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, M.S.; Jeong, J.J.; Lim, S.M.; Kim, I.S.; Yoo, H.H.; Kim, D.H. Effect of Probiotics on Pharmacokinetics of Orally Administered Acetaminophen in Mice. Drug Metab. Dispos. 2018, 46, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [PubMed]
- Li, W.X.; Lu, Y.F.; Wang, F.; Ai, B.; Jin, S.B.; Li, S.; Xu, G.H.; Jin, C.H. Application of 18β-glycyrrhetinic acid in the structural modification of natural products: A review. Mol. Divers. 2024, 29, 739–781. [Google Scholar] [PubMed]
- Shen, C.; Zhu, J.; Song, J.; Wang, J.; Shen, B.; Yuan, H.; Li, X. Formulation of pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 2020, 46, 1100–1107. [Google Scholar]
- Yuan, T.; Wang, J.; Chen, L.; Shan, J.; Di, L. Lactobacillus murinus Improved the Bioavailability of Orally Administered Glycyrrhizic Acid in Rats. Front. Microbiol. 2020, 11, 597. [Google Scholar]
- Wang, Q.; Lv, L.; Jiang, H.; Wang, K.; Yan, R.; Li, Y.; Ye, J.; Wu, J.; Wang, Q.; Bian, X.; et al. Lactobacillus helveticus R0052 alleviates liver injury by modulating gut microbiome and metabolome in D-galactosamine-treated rats. Appl. Microbiol. Biotechnol. 2019, 103, 9673–9686. [Google Scholar]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar]
- Bobin-Dubigeon, C.; Bard, J.M.; Luu, T.H.; Le Vacon, F.; Nazih, H. Basolateral Secretion from Caco-2 Cells Pretreated with Fecal Waters from Breast Cancer Patients Affects MCF7 Cell Viability. Nutrients 2020, 13, 31. [Google Scholar] [CrossRef]
- Yano, K.; Shimizu, S.; Tomono, T.; Ogihara, T. Gastrointestinal Hormone Cholecystokinin Increases P-Glycoprotein Membrane Localization and Transport Activity in Caco-2 Cells. J. Pharm. Sci. 2017, 106, 2650–2656. [Google Scholar]
- Yuan, Z.; Shi, X.; Qiu, Y.; Jia, T.; Yuan, X.; Zou, Y.; Liu, C.; Yu, H.; Yuan, Y.; He, X.; et al. Reversal of P-gp-mediated multidrug resistance in colon cancer by cinobufagin. Oncol. Rep. 2017, 37, 1815–1825. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.E.; Dente, M.J.; Lei, X.; Sallis, B.F.; Loew, E.B.; Meza-Segura, M.; Fitzgerald, K.A.; McCormick, B.A. Microbial Metabolites Orchestrate a Distinct Multi-Tiered Regulatory Network in the Intestinal Epithelium That Directs P-Glycoprotein Expression. mBio 2022, 13, e01993-22. [Google Scholar]
- Cai, J.; Auster, A.; Cho, S.; Lai, Z. Dissecting the human gut microbiome to better decipher drug liability: A once-forgotten organ takes center stage. J. Adv. Res. 2023, 52, 171–201. [Google Scholar]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar]
- Kang, M.; Kang, M.; Yoo, J.; Lee, J.; Lee, S.; Yun, B.; Song, M.; Kim, J.M.; Kim, H.W.; Yang, J.; et al. Dietary supplementation with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by modulating the gut microbiota and metabolites in dexamethasone-induced models. Food Funct. 2024, 15, 4936–4953. [Google Scholar]
- Ferrer, M.; Martins dos Santos, V.A.; Ott, S.J.; Moya, A. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut Microbes 2014, 5, 64–70. [Google Scholar]
- Cussotto, S.; Walsh, J.; Golubeva, A.V.; Zhdanov, A.V.; Strain, C.R.; Fouhy, F.; Stanton, C.; Dinan, T.G.; Hyland, N.P.; Clarke, G.; et al. The gut microbiome influences the bioavailability of olanzapine in rats. EBioMedicine 2021, 66, 103307. [Google Scholar]
- Chen, M.L.; Huang, X.; Wang, H.; Hegner, C.; Liu, Y.; Shang, J.; Eliason, A.; Diao, H.; Park, H.; Frey, B.; et al. CAR directs T cell adaptation to bile acids in the small intestine. Nature 2021, 593, 147–151. [Google Scholar]
- Shavva, V.S.; Babina, A.V.; Nekrasova, E.V.; Lisunov, A.V.; Dizhe, E.B.; Oleinikova, G.N.; Orlov, S.V. Insulin Downregulates the Expression of ATP-binding Cassette Transporter A-I in Human Hepatoma Cell Line HepG2 in a FOXO1 and LXR Dependent Manner. Cell Biochem. Biophys. 2023, 81, 151–160. [Google Scholar]
- Wang, Z.; Li, Y.; Mao, R.; Zhang, Y.; Wen, J.; Liu, Q.; Liu, Y.; Zhang, T. DNAJB8 in small extracellular vesicles promotes Oxaliplatin resistance through TP53/MDR1 pathway in colon cancer. Cell Death Dis. 2022, 13, 151. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| MDR1 | GAGCCCATCCTGTTTGACTG | TGTCTCCCACTCTGGTGTTG |
| MRP2 | TTCTGGATCCTCTCGGTCTTATG | ATCTGGAAACCGTAGGAGACGAA |
| BCRP | CAATGGGATCATGAAACCTG | GAGGCTGATGAATGGAGAA |
| ZO-1 | CTGATGGTGTTCTGCCAAATTC | GTCGCAAACCCACACTATCT |
| Occludin | CCATCTGACTATGCGGAAAGAG | TACCAGAGGCGGTGACTTAT |
| Human Genes MDR1 | CACCACTGGAGCATTGACTACC | TTGCCAACCATAGATGAAGGAT |
| Pharmacokinetic Parameters | CON | ATB | LGG |
|---|---|---|---|
| Tmax/h | 8.67 ± 2.73 | 2.33 ± 4.75 * | 10.67 ± 2.07 |
| Cmax/ng·mL−1 | 3119.67 ± 1611.8 | 3.17 ± 2.84 *** | 3670.93 ± 1038.73 |
| AUC(0−t)/ng·mL−1·h | 35,345.7 ± 8638.0 | 101.91 ± 5.90 *** | 50,070.3 ± 10,933.0 * |
| MRT(0−t)/h | 17.89 ± 6.12 | 35.4 ± 0.990 *** | 16.07 ± 3.95 |
| CL (mL−1·h−1·kg) | 1191.41 ± 524.99 | 87,419.27 ± 64,090.95 *** | 1123.34 ± 348.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Li, H.; Zhang, Z.; Zang, Y.; Jiang, S.; Yuan, T. The Effect of Gut Microbiome Perturbation on the Bioavailability of Glycyrrhizic Acid in Rats. Pharmaceutics 2025, 17, 457. https://doi.org/10.3390/pharmaceutics17040457
Shi T, Li H, Zhang Z, Zang Y, Jiang S, Yuan T. The Effect of Gut Microbiome Perturbation on the Bioavailability of Glycyrrhizic Acid in Rats. Pharmaceutics. 2025; 17(4):457. https://doi.org/10.3390/pharmaceutics17040457
Chicago/Turabian StyleShi, Tiantian, Huifang Li, Zihao Zhang, Yuying Zang, Shu Jiang, and Tianjie Yuan. 2025. "The Effect of Gut Microbiome Perturbation on the Bioavailability of Glycyrrhizic Acid in Rats" Pharmaceutics 17, no. 4: 457. https://doi.org/10.3390/pharmaceutics17040457
APA StyleShi, T., Li, H., Zhang, Z., Zang, Y., Jiang, S., & Yuan, T. (2025). The Effect of Gut Microbiome Perturbation on the Bioavailability of Glycyrrhizic Acid in Rats. Pharmaceutics, 17(4), 457. https://doi.org/10.3390/pharmaceutics17040457







