Abstract
The mRNA- and DNA-based “genetic” COVID-19 vaccines can induce a broad range of adverse events (AEs), with statistics showing significant variation depending on the timing and data analysis methods used. Focusing only on lipid-nanoparticle-enclosed mRNA (mRNA-LNP) vaccines, this review traces the evolution of statistical conclusions on the prevalence and incidence of AEs associated with these vaccines, from initial underestimation of atypical, severe toxicities to recent claims suggesting the possible contribution of COVID-19 vaccinations to the excess deaths observed in many countries over the past few years. Among hundreds of different AEs listed in Pfizer’s pharmacovigilance survey, the present analysis categorizes the main symptoms according to organ systems, with nearly all of them being affected. Using data from the US Vaccine Adverse Event Reporting System and a global vaccination dataset, a comparison of the prevalence and incidence rates of AEs induced by genetic versus flu vaccines revealed an average 26-fold increase in AEs with the use of genetic vaccines. The difference is especially pronounced in the case of severe ‘Brighton-listed’ AEs, which are also observed in COVID-19 and post-COVID conditions. Among these, the increases in incidence rates relative to flu vaccines, given as x-fold rises, were 636x, 530x, 220x, 231x, 155x, 90x, and 133x for myocarditis, thrombosis, death, myocardial infarction, tachycardia, dyspnea, and hypertension, respectively. This review delineates the concept that genetic vaccines can be regarded as prophylactic immuno-gene therapies and that the observed chronic disabling AEs might be categorized as iatrogenic orphan diseases. It also examines the unique vaccine characteristics that could be causally related to abnormal immune responses, which potentially lead to adverse events and complications. These new insights may contribute to improving the safety of this platform technology and assessing the risk/benefit balance of various products.
1. Introduction
mRNA-based vaccines, Pfizer-BioNTech’s BNT162b2 (Comirnaty) and Moderna’s mRNA-1273 (Spikevax), became the most widely used preventive measure against the SARS-CoV-2 virus during the COVID-19 pandemic. Through the blending of nanotechnology with genetic engineering, this innovative approach introduced a novel class of medical intervention with promising applications beyond vaccination [1,2,3].
However, like any groundbreaking technology, this innovation has brought along challenges, such as the unexpected rise of a broad range of adverse events (AEs) and complications. This was not a major issue until the gravity and lethality of the COVID-19 pandemic made the overwhelming benefit of vaccination over the risk of the disease unambiguous. Later on, however, as a result of the success of global immunization campaigns and gradual attenuation of the pathogenicity of new virus variants, the World Health Organization declared (in May 2023) that COVID-19 was no longer a global public health emergency. At this point, the risk/benefit ratio should have been reevaluated in light of the increasing number of severe, disabling AEs [4,5]. Nevertheless, sustaining COVID-19 immunity through repeated vaccinations remains the prevailing suggestion of guidelines, as demonstrated by the US FDA’s recent approval of updated mRNA vaccines for adults and children, including ’emergency use’ authorization for infants aged 6 months and older [6]. The AEs and complications associated with these vaccines remain unresolved, necessitating a reassessment of the risk/benefit ratio of mRNA vaccinations. A better understanding of the issue’s scope and the mechanisms driving these AEs would be instrumental in this assessment. This review seeks to offer such an analysis.
Although the AE profile of DNA-based genetic vaccines, such as AstraZeneca’s Vaxzevria and Johnson & Johnson/Janssen’s Jcovden, may in some regards be even worse than that of the mRNA vaccines, these vaccines have been withdrawn from the market and are therefore not included in the present review.
2. The “Special Interest” Symptoms of Post-Vaccination Syndrome and Public Reaction
The collection of AEs caused by the COVID-19 mRNA vaccines has been called “post-vaccination syndrome” [7,8,9,10], and some of these symptoms overlap with those of COVID-19 and post-COVID, referred to as “symptoms of special interest” or “Brighton case” symptoms, a compilation of AEs by the “Brighton Collaboration”, an international network of experts in drug and vaccine safety [11,12,13,14]. Over the past few years, post-vaccination syndrome has attracted widespread public and scientific attention. Concerns have been raised that these AEs and complications may have played a role in the excess mortality recorded in various Western nations in recent years [15,16]. Physician coalitions have called for a moratorium, legal actions for compensation have been filed, and political debates have reached major institutions such as the British Parliament and the U.S. Congress. Additionally, public hearings and media discussions continue to showcase insights from experts, vaccine-injured individuals, and concerned public figures. Analysis of the literature in PubMed [17], using the search engine End-Note (and search terms “COVID-19”, “mRNA vaccines”, and “adverse events”) returned nearly 1500 articles (January, 2025) focusing on AEs and challenging the universal claim that these vaccines are “safe”. The latter statement is based on the low incidence rate of AEs in the 0.03–0.5 % range (see later), defined as the number of AE reactors related to the overall number of vaccine recipients in a certain time-window. Indeed, the above incidence range counts as low by pharmacotherapy standards, where higher AE rates are generally accepted. However, vaccines differ in this regard, as AEs in a large population of healthy individuals are less acceptable than in patients receiving pharmacotherapy for existing illnesses. Additionally, the global scale of these vaccinations has led to a very high prevalence of AEs, i.e., the total number of affected people in a certain time, imposing a significant burden on society. For these reasons, the accurate quantification of vaccine-induced AEs is critical to assessing their risk/benefit ratio. Unfortunately, in the case of COVID-19 vaccines, the AE statistics vary significantly based on the time, data collection, and analysis methods.
3. The Unique Features of mRNA Vaccines and Their Adverse Effects
The mRNA in the Comirnaty and Spikevax codes for de novo, in loco antigen synthesis in immune cells represents a revolutionary innovation in vaccine technology. Its advantages include the simplification, acceleration, and cost-reduction of vaccine production [18]. This efficiency facilitates a quick response to viral mutations and allows for the possibility of delivering multiple antigens at once, enabling combined vaccines against multiple viral strains.
Table 1 shows an organ system-classified list of COVID-19 adverse events of special interest (AESIs), which is used to identify those special vaccine-induced severe AEs that resemble COVID-19, rather than those caused by traditional vaccines. The spectrum of AEs is uniquely broad and includes rare symptoms and diseases that are atypical for most other vaccines, drugs, or even toxic agents, but which are common in infection with SARS-CoV-2. This points to one or more very fundamental mechanisms of interference with multiple biological processes that are also seen in COVID-19 and post-COVID-19 syndrome. Obviously, the occasional manifestation of AEs must depend on individual genetic and epigenetic factors, just as the rise and spectrum of symptoms in acute and chronic (long) COVID-19.

Table 1.
List of COVID-19 adverse events of special interest (AESIs) *.
4. Prevalence and Incidence of Adverse Events Caused by mRNA-LNP Vaccines: Inconsistent Statistics
Clinical trials conducted before approval, large-scale post-marketing safety surveillance, prospective multicenter studies, and AE monitoring systems have yielded significantly different statistics on the AEs caused by Comirnaty.
In the initial phase II/III randomized clinical trial studying the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals (ClinicalTrials.gov ID: NCT04368728) 21,720 and 21,728 subjects were vaccinated with Comirnaty or placebo, respectively. Polack et al. reported no significant difference between the vaccine and placebo groups in the incidence of mild, common side effects of vaccinations. The observed severe AEs were claimed to have a “low incidence” in both groups that were similar to those caused by other viral vaccines [19]. This was the pivotal study leading to the emergency use authorization of Comirnaty. However, a secondary analysis of the same data by Fraiman et al., counting the Brighton-listed AEs [12], found a 36% higher risk of severe AEs in the vaccine group compared to placebo. As it turned out, the selection of AEs for statistical analysis was limited only to the mild symptoms in the Polack et al. study [19], while the reanalysis focused on severe, Brighton-case AEs. The statistics in the latter study showed 18 (1.2–34.9, 95% CI) serious AEs over placebo in 10,000 participants, corresponding to 1 person displaying a severe vaccine-induced AE in about 556 participants (0.18%) [12]. The ratio of “special interest” AEs among all serious AEs was ~56% [12].
Three months after the global rollout of Comirnaty, Pfizer-BioNTech’s originally confidential, now publicly accessible, post-authorization safety report [20] gave an account of 42,086 AE case reports on 158,893 events out of 126,212,580 vaccine doses in 56 countries. This means that 0.13% and 0.03% AE incidences were related to events or reactors, respectively, or one reactor for every ~3000 vaccine recipients. Reactions were observed mainly in the 31–50-year-old age range and three -times more often in women than man, and full recovery ensued in 47%. The rest recovered with sequalae or did not recover within 3 months. The report listed 2.9% fatality among the reactors (1223 deaths), implying ~0.001% of overall vaccinations resulted in fatality, or one death in about one hundred thousand (103,200) vaccinations. However, the relationship between vaccination and reported death is uncertain.
Beyond the fact that 53% of the reactive individuals recovered with sequalae or did not recover within 3 months, what is astonishing in this report is the approximately 1,100 different words or terms for AEs used in the appended nine-page cumulative list of AEs [20]. Among many unique, unprecedented AEs, the list contained ~220 different types of conditions including the syllable “itis” (implying inflammation) and/or the word “autoimmune” (which is an inflammatory process). Thus, about 20% of all AEs involves inflammation.. Nevertheless, the surveys’ summary aligned with the conclusion of the Phase II-III study [19], claiming that “the data do not reveal any novel safety concerns or risks requiring label changes”.
The statement on safety was reinforced in an international 6-month efficacy and safety study involving 21,926 recipients of Comirnaty and an equal number of participants who received placebo [21]. This study reported a 16.3% occurrence of “any event” over placebo, which is about 125 times higher than the AE event incidence in Pfizer’s 3-month safety surveillance (0.13%) [20], and the 0.51% of severe reactions was about 100-fold higher than that in the 3-month safety surveillance study [20]. Nevertheless, again, “no new safety signals were identified during the longer follow-up period” [21].
The next set of comprehensive statistics, provided by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), were based on continuous monitoring through various reporting systems, including the Vaccine Adverse Event Reporting System (VAERS) [22,23]. Unlike clinical trials wherein the documentation of AEs is part of the study, the VAERS entries are volunteer reports by healthcare providers and patients. The first 6-month post-marketing analysis of the AEs attributed to Comirnaty and Spikevax, based on the VAERS data [24,25], gave a 0.11% AE reactor incidence rate (out of 298,792,852 doses), near four-fold higher than the corresponding value in the 3-month Pfizer survey (0.03%) [20].
One reason for the above ambiguities regarding the occurrence of mRNA-LNP-induced AEs is inconsistency in data collection. A 2010 study by the U.S. Department of Health and Human Services estimated that fewer than 1% of vaccine AEs and only 1–13% of serious events are reported to VAERS [26]. The reporting process appears to be complex, and not all AEs are documented, especially if they are mild or if the individual does not associate them with vaccination.. Despite these limitations, for comparing AEs associated with COVID-19 vaccines with those associated with other vaccines, such as flu vaccines, VAERS appeared to be the most suitable data source.
5. Comparison of mRNA-LNP and Flu Vaccines
In addition to the prevalence and incidence of AEs, which reflect on the clinical impact of side effects, another key question regarding vaccine safety is how the risk of AEs compares to that of other vaccines, especially those that are also offered to a large population. In the case of COVID-19 mRNA vaccines, seasonal flu vaccines may serve as the best reference since they are also administered to millions and target an airborne virus, like SARS-CoV-2. Table 2 compares the prevalence and incidence of AEs associated with Comirnaty, Spikevax, and Jcovden (Janssen), a DNA-containing vaccine developed by Johnson & Johnson, with those associated with flu vaccines. The latter data were obtained by aggregating the AEs of 12 flu vaccines listed in VAERS (see legend to Table 2).

Table 2.
VAERS-reported adverse events associated with genetic (mRNA and DNA) COVID-19 vaccines and 12 flu vaccines combined, from December 2020 to May 2023.
It is seen in the table that the incidence rate of AEs caused by the analyzed COVID-19 vaccines over 2.5 years was 25–26 times higher than that of flu vaccines during the same time. Considering only the DNA-based vaccine, Jcovden, the relative risk of experiencing an AE compared to the flu vaccine is 54-fold higher. This also means that the DNA vaccine caused ~2-fold more AEs than the mRNA vaccines. A comparison of Comirnaty and Spikevax suggested 57% more reactions in the case of Spikevax. The substantial difference between the flu vaccine and the 2 mRNA vaccines and the relative similarity between Comirnaty and Spikevax in causing AEs provide clear indication that it is the mRNA-LNP technology, rather than any other special features of the 2 mRNA vaccines, that accounts for the increased risk of AEs. On the other hand, the 20- and 32-fold increase in relative risk calculated for Comirnaty vs. Spikevax shows comparably increased toxicities, for which that of Spikevax is somewhat higher than that of Comirnaty.
Statistics on individual AEs: Using the flu vaccines as a comparator, Table 3 shows the incidence rates of 12 Brighton-case AEs caused by the mRNA and flu vaccines in order of decreasing prevalence.

Table 3.
VAERS data on the prevalence and incidence of selected flu and COVID-19 mRNA vaccine-induced AEs in the US from December 2020 to May 2023.
As in the case of all AEs combined (Table 2), the mRNA-LNP vaccine-induced incidence rates of all 12 distinct AEs were massively higher than those after flu vaccination, with heart disease and thrombosis having the highest, roughly a ~1200- and ~500-fold increased risk, respectively. These data also show that the incidence rates of different AEs substantially vary within the 6–200 AEs/M range.
The vaccine-related fatal outcomes reported to VAERS (Table 3) suggest approximately one death per 33,000 vaccine recipients, or an incidence rate of ~0.003%, which is close to the ~0.001% reported in Comirnaty’s 3-month post-market surveillance [20]. These ratios, taken together with the immunization statistics (a total of 4600 M Comirnaty and 817 M Spikevax doses distributed across the world through 2024), imply death cases approximately in the 55,000–160,000 range worldwide. However, as mentioned, the death reports are ambiguous as they could be coincidental, usually associated with a comorbidity. A further useful piece of information is that the percent of severe AEs relative to all AEs is in the ~4 and ~18% range [24,28,29], although in the 3-month post-market surveillance [20] it reached or exceeded 40–50% for many AEs.
Figure 1 shows rough estimates of the prevalence of mRNA vaccine-induced different AEs only in the USA and Europe during the COVID-19 pandemic, based on dose statistics and VAERS-based incidence rates in the USA (italicized as AE/M in column 5 of Table 3). The increasing order of bars visualizes the conclusion, that because of the larger number of vaccinations in Europe, the prevalence of different symptoms is also higher in Europe. It is also notable in the figure that after fever, rash and dyspnea were the most frequent AEs, whose coincidental occurrence is typical of liposome and other nanoparticle-induced complement (C) activation [30,31,32], as discussed below in detail.
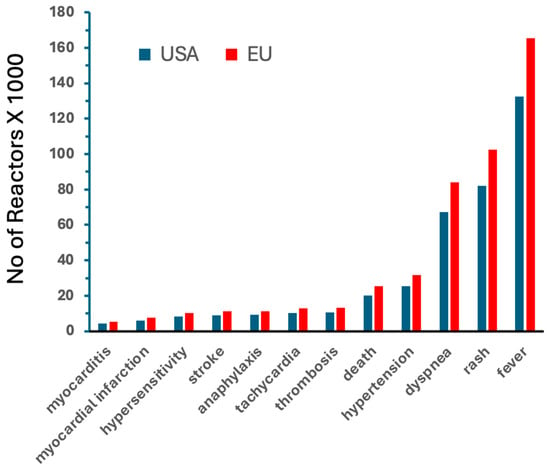
Figure 1.
Rough estimates of the prevalence of mRNA vaccine-induced AEs in the USA and Europe during the COVID-19 pandemic, between December 2020 and May 2023. The calculations of the total number of AE reactors were based on the incidence rates for mRNA vaccines shown in Table 3 (obtained from the VAERS), which were multiplied by the number of Comirnaty + Spikevax mRNA doses injected during this period, obtained from the Our World in Data public database [27].
6. Complement Activation as a Possible Contributor to Acute AEs
Most attention paid to vaccine AEs is focused on the inflammatory and autoimmune complications that affect the heart, nerve, and coagulation systems, overlooking the fact noted in Figure 1 that the -runner-up symptoms in the AE prevalence list are rash, and dyspnea. These may be due to pseudoallergy that results from complement activation by the LNPs.. Thus, the association of skin symptoms (e.g., rash, urticaria) with cardiopulmonary distress, which manifests as dyspnea, hyper- and hypotension, tachycardia, bradycardia, or arrhythmia, suggests the involvement of complement activation [30,31,32]. Two other AEs that could also be related to complement activation are thrombosis and thrombocytopenia, since anaphylatoxins and the terminal complement complex activate the endothelial cells and platelets [33,34,35]. Further support for the involvement of complement activation in the observed acute vaccine reactions is the fact that the “AEs of special interest”, associated with mRNA vaccines, share similarities with the inflammatory symptoms of COVID-19, wherein intense complement activation plays a key pathogenic role [36,37,38,39,40]. Furthermore, C3, the central molecule in the complement activation cascade, is a ubiquitous innate mediator of inflammatory responses undergoing activation in most systemic inflammatory processes [41]. Nevertheless, the strongest and most direct evidence for complement activation playing a role in the observed acute AEs is that Comirnaty is a potent complement activator, as shown in pigs [42], human serum in vitro [43], and pig blood in vivo [44].
As for the mechanism of complement activation, we observed the involvement of the alternative pathway in human serum [43], while, in sera containing high anti-PEG antibody titers, complement activation via the classical pathway also becomes prominent [44]. As pointed out earlier, virtually all components of Comirnaty have the capability to activate complement, including the ionizable lipid (ALC-0315), DSPC, cholesterol, and the spike protein [32].
In conclusion, the most immediate and frequent inflammatory symptoms associated with mRNA vaccines may be due, in a great part, to complement activation. The produced anaphylatoxins, in addition to causing anaphylactic reactivity [45], also “spark the flame in early autoimmunity” [46,47]. The hypersensitivity reaction caused by complement activation, called complement activation-related pseudoallergy (CARPA) [30,31,32], can be life threatening in people with high anti-PEG antibody level and/or severe allergy [46]. Hence, genetic predisposition to allergic and/or autoimmune reactions, such as atopic constitution, represents a risk factor for vaccine-induced acute hypersensitivity reactions, anaphylaxis, or autoimmunity. Importantly, these reactions can be prevented or attenuated by complement inhibitors [41,48,49].
Because of the resemblance to pathogenic viruses, complement activation by the vaccine nanoparticles, an inherent feature of LNPs [32], is biologically rationalizable. Thus, beyond the vaccines, other present and future products of the mRNA-LNP technology may have to face the complement activation problem. Figure 2 outlines the reaction sequence by which vaccine-induced complement activation may cause the discussed AEs.
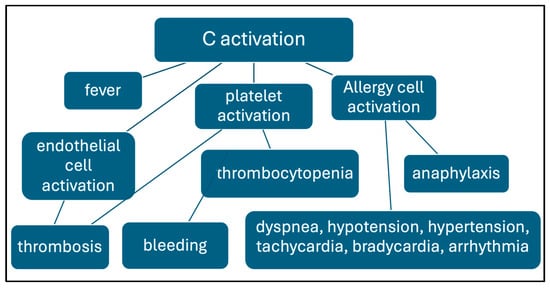
Figure 2.
Reaction sequence of complement-mediated AEs caused by mRNA vaccines. The connections between the framed processes and symptoms suggest causal relationships.
7. Regulatory Classification of mRNA Vaccines
Despite the wide acceptance of the use of “mRNA vaccines”, the regulatory classification of Comirnaty and Spikevax is ambiguous. The proposed classification terms include “biotechnological medicines”, “biological products/drugs/medicines”, and “gene-based vaccines”. Some authors even argue that these formulations are not even vaccines but representations of gene therapy, as they exert the transfection of genetic material (nucleic acid) to change genetic information. However, it may not be considered as gene therapy on the grounds that the DNA does not change, at least in theory, and that the therapeutic goal of vaccination is not genetic correction. A possible alternative to all above terminologies is immuno-gene therapy, a term that has previously been applied in cancer immunotherapy [50]. It expresses that gene translation is used to modify an immune phenomenon, namely, to accelerate the processing and presentation of an antigen. Its implications for public health and regulatory standards lie in the more accurate definition of the intervention.
8. The Orphan Disease Proposition for Categorizing Persistent and/or Disabling Vaccine-Induced Chronic AEs
An orphan disease is a rare medical condition that affects a small percentage of the population and therefore lacks sufficient research, treatment options, and financial incentives for drug development [51,52,53,54]. These diseases are usually genetic disorders, such as Huntington’s disease, cystic fibrosis, Duchenne muscular dystrophy, and certain rare cancers.
Despite the higher incidence of vaccine-related AEs compared to flu vaccines (Table 2 and Table 3), Figure 1 shows that the cumulative number of mRNA vaccine-induced chronic, potentially persistent, debilitating AEs (myocarditis, myocardial infarction, stroke, thrombosis), as of May 2023, remained below about 10,000 in the U.S., which is well below the threshold of 200,000 patients used to define the upper limit for orphan disease categorization in the U.S. [53]. These vaccine injuries, in addition to being orphan diseases, could also be identified as “iatrogenic”, acknowledging that, unlike the genetic orphan diseases, they are unintended consequences of a medical intervention. The significance of this distinction lies in the fact that many countries have implemented special policies for orphan diseases that ensure dedicated research funding and the development of specialized treatments [52,53,54]. The U.S. Orphan Drug Act of 1983 [55] provides a relevant example of initiatives aimed at addressing the needs of patients with rare diseases.
9. The European Experience: Paul-Ehrlich-Institute Statistics
The COVID-19 vaccine-induced AEs are closely monitored in Europe as well. In Germany, the Paul Ehrlich Institute (PEI), a participant in the WHO-led Vaccine Safety Net project, serves as a primary source of statistics on genetic vaccine-induced AEs [28]. According to the PEI, the incidence rate of severe AEs (of special interest) associated with mRNA vaccines was approximately 0.2 per 1000 doses, or 0.02% [28]. For comparison, the corresponding values from various U.S. statistics mentioned earlier in this review were 0.03% [20], 0.13% (VAERS, Table 2), 0.18% [12], and 0.5% [21].
Table 4 presents the incidence rates of AEs caused by mRNA- and DNA-containing vaccines, published by PEI [28]. While the list and rank of symptoms differ from the US data (Table 3), cardiopulmonary distress (e.g., dyspnea, arrhythmia) is among the top on the AE list with both types of vaccines. As discussed above, these symptoms can be linked to activation of the complement system, providing additional support for the causal role of complement activation in the lead AEs of genetic vaccines.

Table 4.
The incidence rates of “special interest” AEs* caused by two mRNA and two DNA-containing genetic vaccines in Germany.
Figure 3 compares the incidence rates of DNA vaccine-induced AEs and those associated with mRNA vaccines. The bars with different colors represent the combined AE/M values for the 2 mRNA (Comirnaty and Spikevax) and 2 DNA (Vaxzevria and Jcovden) vaccines, and the secondary y-axis (dotted line) quantitates the ratio of AE incidence rates between the two vaccine types. Except for myocarditis and pericarditis, these rates were at least twice as high for the DNA vaccines compared to the mRNA vaccines. Notably, DNA vaccines showed a 10–13-fold higher incidence of thrombocytopenia and cerebral thrombosis, which aligns with their suspension and eventual withdrawal due to thrombotic thrombocytopenia and severe cerebral events, particularly sinus thrombosis. As previously noted, complement activation plays a key pathogenic role in these hemostatic abnormalities [33,35], which is in keeping with the fact that adenoviruses -used in DNA vaccines-, are particularly strong activators of the complement system [56].
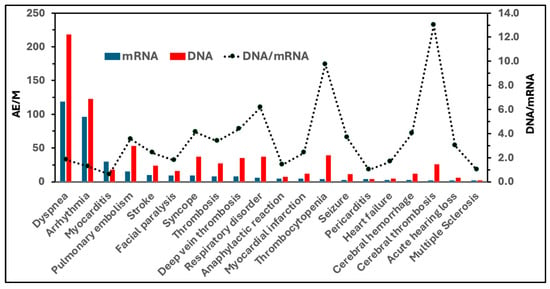
Figure 3.
Incidence rates of “special interest” AEs caused by mRNA and DNA-based genetic vaccines in Germany (visual representation of data from Table 4). Blue bars represent the summed incidences (AEs/M) associated with mRNA vaccines (Comirnaty and Spikevax), while red bars represent those associated with DNA vaccines (Jcovden and Vaxzevria). The dotted line (right y-axis) shows the DNA/mRNA ratios, indicating the fold-increase in the incidence of each AE observed with DNA vaccines compared to mRNA vaccines.
10. Potential Plausible Causes of Adverse Events Inherent to the mRNA-LNP Platform
It seems likely that the root cause of vaccine-induced AEs and complications may be the same as the main concern with gene therapy, namely unintended immune processes, off-target effects, and unforeseen toxicities [57]. It was mentioned that complement activation is an inherent property of mRNA-LNPs; however, the idea map in Figure 4 suggests many more features of mRNA vaccines that are different from those of traditional vaccines, which may be linked to AEs.
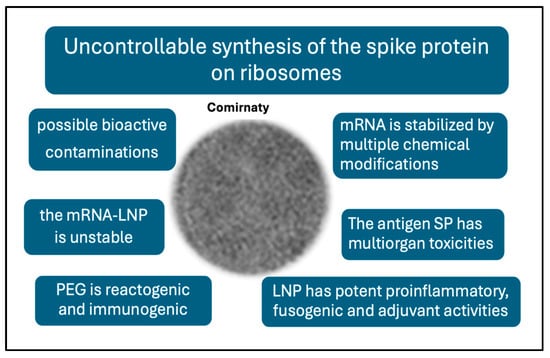
Figure 4.
Unique features of the mRNA vaccines that are not characteristic of traditional vaccines and may be linked to AEs.
These differences include the following [58]: (i) The highly controlled, multistep pathway of antigen processing and presentation in natural immunogenicity is replaced in mRNA vaccines by uncontrollable ribosomal synthesis of the antigen. This results in a diversification of SP processing and presentation in unnatural ways [58]. (ii) Nucleoside and other chemical modifications increase the stability of the synthetic mRNA, and, hence, the efficacy of SP translation and its in vivo stability [59,60]. (iii) The prefusion-stabilized antigen SP has diverse, multiorgan toxicities [61,62,63]. Its rapid entry into the bloodstream and whole-body tissue distribution [64,65] can cause autoimmune reactions and a variety of cell and tissue damages. (iv) The LNP is a robust proinflammatory agent [66,67] and multiorgan mRNA transfectant [68,69] which underlies systemic inflammation and autoimmune phenomena. (iv) The stabilizer polymer (polyethylene glycol, PEG) on the vaccine nanoparticle surface has anaphylactic reactogenicity and immunogenicity, implying possible anti-vaccine immune reactivity [44,46]. (v) the vaccine nanoparticles are unstable in water [70]. (vi) The injectable vaccine may contain residual DNA fragments, [71], odd molecular assemblies, and inorganic metals and complexes [72]. The above deviations from traditional vaccines offer plausible explanations for the AEs of mRNA vaccines [58], which may be perceived as collateral damage from the otherwise successful fight against COVID-19.
Figure 4 presents a spider map of possible adverse vaccine features, a comprehensive theory on the root causes of AEs. The concept is expanded into a flowchart of potential cause–effect relationships among the reactions and symptoms (Figure 5), wherein the suggested steps may occur independently or simultaneously and may act in additive or synergistic ways. Furthermore, the emergence and severity of AEs are influenced by genetic and epigenetic factors, as well as various external and internal conditions.
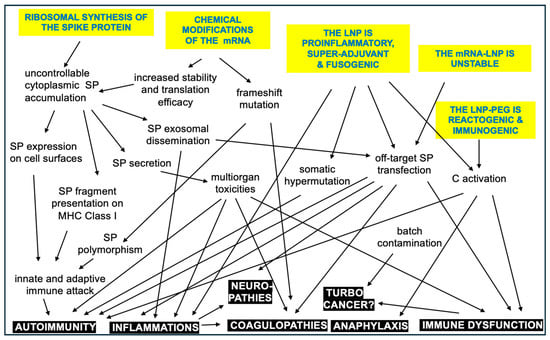
Figure 5.
Hypothetic immune processes and phenomena contributing to the AEs caused by mRNA vaccines. The arrows point from cause to potential effect in the intertwined reaction cascade. The inherent vaccine properties are itemized with blue in yellow background, and the adverse events or complications are white in black. It needs to be strongly emphasized that this diagram is NOT a reaction scheme of the normal operation of the vaccine in non-reactive people [73].
11. Outlook
The rare occurrence of severe AEs during mass vaccinations is not unprecedented. A notable example is the 1976 swine flu pandemic in the U.S., where an increase in the incidence of AEs (e.g., Guillain–Barré syndrome) and complications resulted in approximately 30 deaths after 43 M vaccinations, ultimately leading to the suspension of the vaccine [74]. This example underscores that the term “safe” is relative and depends on varying criteria across different times and contexts.
At present, the mainstream scientific literature and public health institutions hold the mRNA-LNP vaccines safe with a high benefit/risk ratio. Accordingly, this technology platform is garnering unprecedented interest and investment. The FDA has recently approved Moderna’s second mRNA vaccine, mRESVIA (mRNA-1345), against respiratory syncytial virus [75,76], and over 300 new mRNA-LNP-based drugs are in development across dozens of companies. Novel mRNA vaccines targeting influenza, Zika virus, HIV, cytomegalovirus, and cancer are undergoing clinical trials, and a great number of preclinical studies suggest the potential utility of mRNA-LNPs as anticancer immunotherapies and multivalent vaccines [77,78,79]. However, the AEs discussed in this review present a biological barrier to the success of the mRNA-LNP technology. Therefore, as the field advances, it is crucial to develop a deeper understanding of the immune mechanisms underlying the AEs and complications potentially caused by genetic vaccines and other mRNA-LNP-based products.
Funding
This research was funded by the European Union Horizon 2020 project 825828 (Expert) and Hungarian 2022-1.2.5-TÉT-IPARI-KR-2022-00009.
Acknowledgments
The help of Miklos Szebeni in performing the VAERS analysis is highly appreciated.
Conflicts of Interest
The author is affiliated, among others, with an immune toxicology provider SME, SeroScience LLC.
References
- Sahin, U.; Kariko, K.; Tureci, O. Mrna-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [PubMed]
- Kariko, K.; Whitehead, K.; van der Meel, R. What Does the Success of Mrna Vaccines Tell Us About the Future of Biological Therapeutics? Cell Syst. 2021, 12, 757–758. [Google Scholar] [PubMed]
- Cullis, P.R.; Felgner, P.L. The 60-Year Evolution of Lipid Nanoparticles for Nucleic Acid Delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar]
- Igyarto, B.Z.; Jacobsen, S.; Ndeupen, S. Future Considerations for the Mrna-Lipid Nanoparticle Vaccine Platform. Curr. Opin. Virol. 2021, 48, 65–72. [Google Scholar]
- DeSantis, R.; Ladapo, J.A. Health Alert on Mrna COVID-19 Vaccine Safety. 2023. Available online: https://www.floridahealth.gov/_documents/newsroom/press-releases/2023/02/20230215-updated-health-alert.pdf (accessed on 15 February 2023).
- FDA. Fda Approves and Authorizes Updated Mrna COVID-19 Vaccines to Better Protect Against Currently Circulating Variants. FDA New Release. 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-and-authorizes-updated-mrna-covid-19-vaccines-better-protect-against-currently (accessed on 15 February 2023).
- Oueijan, R.I.; Hill, O.R.; Ahiawodzi, P.D.; Fasinu, P.S.; Thompson, D.K. Rare Heterogeneous Adverse Events Associated with mRNA-Based COVID-19 Vaccines: A Systematic Review. Medicines 2022, 9, 43. [Google Scholar]
- Krumholz, H.M.; Wu, Y.; Sawano, M.; Shah, R.; Zhou, T.; Arun, A.S.; Khosla, P.; Kaleem, S.; Vashist, A.; Bhattacharjee, B.; et al. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences after COVID-19 Immunization. medRxiv 2023. medRxiv:2023.11.09.23298266. [Google Scholar] [CrossRef]
- Palmer, M.; Bhakdi, S.; Hooker, B.; Holland, M.; DesBois, M.; Rasnick, D.; Fitts, C.A. Mrna Vaccine Toxicity; D4ce.Org. 2023. Available online: https://doctors4covidethics.org/mrna-vaccine-toxicity/ (accessed on 23 March 2025).
- Shrestha, Y.; Venkataraman, R. The Prevalence of Post-COVID-19 Vaccination Syndrome and Quality of Life among COVID-19-Vaccinated Individuals. Vacunas 2024, 25, 7–18. [Google Scholar]
- Law, B. Priority List of COVID-19 Adverse Events of Special Interest: Quarterly Update December 2020. Safety Platform for Emergency Vaccines (SPEAC). 2021. Available online: https://brightoncollaboration.org/wp-content/uploads/2023/08/SO2_D2.1.2_V1.2_COVID-19_AESI-update_V1.3-1.pdf (accessed on 23 March 2025).
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious Adverse Events of Special Interest Following mRNA COVID-19 Vaccination in Randomized Trials in Adults. Vaccine 2022, 40, 5798–5805. [Google Scholar]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 Vaccines and Adverse Events of Special Interest: A Multinational Global Vaccine Data Network (Gvdn) Cohort Study of 99 Million Vaccinated Individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar]
- Levitan, B.; Hadler, S.C.; Hurst, W.; Izurieta, H.S.; Smith, E.R.; Baker, N.L.; Bauchau, V.; Chandler, R.; Chen, R.T.; Craig, D.; et al. The Brighton Collaboration Standardized Module for Vaccine Benefit-Risk Assessment. Vaccine 2024, 42, 972–986. [Google Scholar]
- Mostert, S.; Hoogland, M.; Huibers, M.; Kaspers, G. Excess Mortality across Countries in the Western World since the COVID-19 Pandemic: ‘Our World in Data’ Estimates of January 2020 to December 2022. BMJ Public Health 2024, 2, e000282. [Google Scholar] [CrossRef]
- Rancourt, D.G.; Hickey, J.; Linard, C. Spatiotemporal Variation of Excess All-Cause Mortality in the World (125 Countries) During the COVID Period 2020-2023 Regarding Socio-Economic Factors and Public-Health and Medical Interventions. Correlation. 2024. Available online: https://correlation-canada.org/covid-excess-mortality-125-countries/ (accessed on 19 July 2024).
- Wang, S.; Zhang, K.; Du, J. Pubmed Captures More Fine-Grained Bibliographic Data on Scientific Commentary Than Web of Science: A Comparative Analysis. BMJ Health Care Inf. Inform. 2024, 31, e101017. [Google Scholar]
- Du, P.; Li, N.; Tang, S.; Zhou, Z.; Liu, Z.; Wang, T.; Li, J.; Zeng, S.; Chen, J. Development and Evaluation of Vaccination Strategies for Addressing the Continuous Evolution SARS-CoV-2 Based on Recombinant Trimeric Protein Technology: Potential for Cross-Neutralizing Activity and Broad Coronavirus Response. Heliyon 2024, 10, e34492. [Google Scholar] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the Bnt162b2 Mrna COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [PubMed]
- Worldwide Safety. Cumulative Analysis of Post-Authorization Adverse Event Reports of Pf-07302048 (Bnt162b2) Received through 28-Feb-2021. 2021. Available online: https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf?fbclid=IwAR2tWI7DKw0cc2lj8 (accessed on 23 March 2025).
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the Bnt162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar]
- VAERS. Vaers Ids. 2023. Available online: https://vaers.hhs.gov/data/datasets.html (accessed on 23 March 2025).
- OpenVAERS. Vaers COVID Vaccine Adverse Event Reports. 2024. Available online: https://openvaers.com/covid-data (accessed on 23 March 2025).
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of Mrna Vaccines Administered During the Initial 6 Months of the Us COVID-19 Vaccination Programme: An Observational Study of Reports to the Vaccine Adverse Event Reporting System and V-Safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar]
- Wikipedia. Timeline of the COVID-19 Pandemic in the United States. Wikipedia. 2025. Available online: https://en.wikipedia.org/wiki/Timeline_of_the_COVID-19_pandemic_in_the_United_States_%282021%29?utm_source=chatgpt.com (accessed on 23 March 2025).
- Ross, L.; Klompas, M. Electronic Support for Public Health–Vaccine Adverse Event Reporting System (Esp:Vaers). In Grant. Final. Report: Grant ID: R18 HS 017045. 2010. Available online: https://www.corruptedsystem.com/uploads/r18hs017045-lazarus-final-report-2011.pdf (accessed on 23 March 2025).
- Our World in Data. 2024. Available online: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer?country=European+Union~HUN~USA (accessed on 23 March 2025).
- Institute, Paul Erlich. Safety Report. 2022. Available online: https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/safety-reports/safety-report-27-december-2020-31-march-2022.pdf?__blob=publicationFile&v=8 (accessed on 23 March 2025).
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodes-Guirao, L. A Global Database of COVID-19 Vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [PubMed]
- Szebeni, J.; Storm, G.; Ljubimova, J.Y.; Castells, M.; Phillips, E.J.; Turjeman, K.; Barenholz, Y.; Crommelin, D.J.A.; Dobrovolskaia, M.A. Applying Lessons Learned from Nanomedicines to Understand Rare Hypersensitivity Reactions to mRNA-Based SARS-CoV-2 Vaccines. Nat. Nanotechnol. 2022, 17, 337–346. [Google Scholar]
- Wolfram, R. Links between Complement Activation and Thrombosis. Blood 2019, 134 (Suppl. S1), SCI-40. [Google Scholar]
- Luo, S.; Hu, D.; Wang, M.; Zipfel, P.F.; Hu, Y. Complement in Hemolysis- and Thrombosis- Related Diseases. Front. Immunol. 2020, 11, 1212. [Google Scholar]
- Rawish, E.; Sauter, M.; Sauter, R.; Nording, H.; Langer, H.F. Complement, Inflammation and Thrombosis. Br. J. Pharmacol. 2021, 178, 2892–2904. [Google Scholar]
- Alosaimi, B.; Mubarak, A.; Hamed, M.E.; Almutairi, A.Z.; Alrashed, A.A.; AlJuryyan, A.; Enani, M.; Alenzi, F.Q.; Alturaiki, W. Complement Anaphylatoxins and Inflammatory Cytokines as Prognostic Markers for COVID-19 Severity and in-Hospital Mortality. Front. Immunol. 2021, 12, 668725. [Google Scholar]
- Lim, E.H.T.; van Amstel, R.B.E.; de Boer, V.V.; van Vught, L.A.; de Bruin, S.; Brouwer, M.C.; Vlaar, A.P.J.; van de Beek, D. Complement Activation in COVID-19 and Targeted Therapeutic Options: A Scoping Review. Blood Rev. 2023, 57, 100995. [Google Scholar]
- Siggins, M.K.; Davies, K.; Fellows, R.; Thwaites, R.S.; Baillie, J.K.; Semple, M.G.; Openshaw, P.J.M.; Zelek, W.M.; Harris, C.L.; Morgan, B.P.; et al. Alternative Pathway Dysregulation in Tissues Drives Sustained Complement Activation and Predicts Outcome across the Disease Course in COVID-19. Immunology 2023, 168, 473–492. [Google Scholar]
- Meroni, P.L.; Croci, S.; Lonati, P.A.; Pregnolato, F.; Spaggiari, L.; Besutti, G.; Bonacini, M.; Ferrigno, I.; Rossi, A.; Hetland, G.; et al. Complement Activation Predicts Negative Outcomes in COVID-19: The Experience from Northen Italian Patients. Autoimmun. Rev. 2023, 22, 103232. [Google Scholar] [PubMed]
- Ellsworth, C.R.; Chen, Z.; Xiao, M.T.; Qian, C.; Wang, C.; Khatun, M.S.; Liu, S.; Islamuddin, M.; Maness, N.J.; Halperin, J.A.; et al. Enhanced Complement Activation and Mac Formation Accelerates Severe COVID-19. Cell Mol. Life Sci. 2024, 81, 405. [Google Scholar]
- Mastellos, D.C.; Ricklin, D.; Hajishengallis, E.; Hajishengallis, G.; Lambris, J.D. Complement Therapeutics in Inflammatory Diseases: Promising Drug Candidates for C3-Targeted Intervention. Mol. Oral. Microbiol. 2016, 31, 3–17. [Google Scholar]
- Dezsi, L.; Meszaros, T.; Kozma, G.; H-Velkei, M.; Olah, C.Z.; Szabo, M.; Patko, Z.; Fulop, T.; Hennies, M.; Szebeni, M.; et al. A Naturally Hypersensitive Porcine Model May Help Understand the Mechanism of COVID-19 Mrna Vaccine-Induced Rare (Pseudo) Allergic Reactions: Complement Activation as a Possible Contributing Factor. Geroscience 2022, 44, 597–618. [Google Scholar]
- Bakos, T.; Meszaros, T.; Kozma, G.T.; Berenyi, P.; Facsko, R.; Farkas, H.; Dezsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef] [PubMed]
- Barta, B.A.; Radovits, T.; Dobos, A.B.; Kozma, G.T.; Meszaros, T.; Berenyi, P.; Facsko, R.; Fulop, T.; Merkely, B.; Szebeni, J. Comirnaty-Induced Cardiopulmonary Distress and Other Symptoms of Complement-Mediated Pseudo-Anaphylaxis in a Hyperimmune Pig Model: Causal Role of Anti-Peg Antibodies. Vaccine X 2024, 19, 100497. [Google Scholar] [PubMed]
- Szebeni, J.; Baranyi, L.; Savay, S.; Bodo, M.; Milosevits, J.; Alving, C.R.; Bunger, R. Complement Activation-Related Cardiac Anaphylaxis in Pigs: Role of C5a Anaphylatoxin and Adenosine in Liposome-Induced Abnormalities in ECG and Heart Function. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1050-8. [Google Scholar] [PubMed]
- Kozma, G.T.; Meszaros, T.; Berenyi, P.; Facsko, R.; Patko, Z.; Olah, C.Z.; Nagy, A.; Fulop, T.G.; Glatter, K.A.; Radovits, T.; et al. Role of Anti-Polyethylene Glycol (PEG) Antibodies in the Allergic Reactions to PEG-Containing COVID-19 Vaccines: Evidence for Immunogenicity of PEG. Vaccine 2023, 41, 4561–4570. [Google Scholar]
- Schanzenbacher, J.; Kohl, J.; Karsten, C.M. Anaphylatoxins Spark the Flame in Early Autoimmunity. Front. Immunol. 2022, 13, 958392. [Google Scholar]
- Ricklin, D.; Mastellos, D.C.; Lambris, J.D. Therapeutic Targeting of the Complement System. Nat. Rev. Drug Discov. 2019, 9, 10.1038. [Google Scholar]
- Kolev, M.; Kolu, N.; Yeh, M.; Parikh, A.; Deschatelets, P. The Future of Complement Therapeutics. Explor. Immunol. 2024, 4, 577–615. [Google Scholar]
- Kyte, J.A.; Gaudernack, G. Immuno-Gene Therapy of Cancer with Tumour-Mrna Transfected Dendritic Cells. Cancer Immunol. Immunother. 2006, 55, 1432–1442. [Google Scholar]
- Daniel, M.G.; Pawlik, T.M.; Fader, A.N.; Esnaola, N.F.; Makary, M.A. The Orphan Drug Act: Restoring the Mission to Rare Diseases. Am. J. Clin. Oncol. 2016, 39, 210–213. [Google Scholar]
- Gabay, M. The Orphan Drug Act: An Appropriate Approval Pathway for Treatments of Rare Diseases? Hosp. Pharm. 2019, 54, 283–284. [Google Scholar]
- Schouten, A. KEi Briefing Note 2020:4 Selected Government Definitions of Orphan or Rare Diseases. Knowledge Ecology International. Revised . 3 November 2020. Available online: https://www.keionline.org/wp-content/uploads/KEI-Briefing-Note-2020-4-Defining-Rare-Diseases.pdf?utm_source=chatgpt.com (accessed on 23 March 2025).
- Fermaglich, L.J.; Miller, K.L. A Comprehensive Study of the Rare Diseases and Conditions Targeted by Orphan Drug Designations and Approvals over the Forty Years of the Orphan Drug Act. Orphanet J. Rare Dis. 2023, 18, 163. [Google Scholar] [PubMed]
- Sanders, T.I. The Orphan Drug Act. Prog. Clin. Biol. Res. 1983, 127, 207–215. [Google Scholar] [PubMed]
- Tian, J.; Xu, Z.; Smith, J.S.; Hofherr, S.E.; Barry, M.A.; Byrnes, A.P. Adenovirus Activates Complement by Distinctly Different Mechanisms in Vitro and in Vivo: Indirect Complement Activation by Virions in Vivo. J. Virol. 2009, 83, 5648–5658. [Google Scholar] [PubMed]
- Radmer, A.; Bodurtha, J. Prospects, Realities, and Safety Concerns of Gene Therapy. Va. Med. Q. 1992, 119, 98–100. [Google Scholar]
- Szebeni, J. The Unique Features and Collateral Immune Effects of Mrna-Based COVID-19 Vaccines: Potential Plausible Causes of Adverse Events and Complications. Preprints. 2025. Available online: https://www.preprints.org/manuscript/202501.1462/v1 (accessed on 23 March 2025).
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the Optimal Mrna for Therapy: Hplc Purification Eliminates Immune Activation and Improves Translation of Nucleoside-Modified, Protein-Encoding Mrna. Nucleic Acids Res. 2011, 39, e142. [Google Scholar]
- Moghaddar, M.; Radman, R.; Macreadie, I. Severity, Pathogenicity and Transmissibility of Delta and Lambda Variants of SARS-CoV-2, Toxicity of Spike Protein and Possibilities for Future Prevention of COVID-19. Microorganisms 2021, 9, 2167. [Google Scholar] [CrossRef]
- Schwartz, L.; Aparicio-Alonso, M.; Henry, M.; Radman, M.; Attal, R.; Bakkar, A. Toxicity of the Spike Protein of COVID-19 Is a Redox Shift Phenomenon: A Novel Therapeutic Approach. Free Radic. Biol. Med. 2023, 206, 106–110. [Google Scholar]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-Lasting, Biochemically Modified mRNA, and Its Frameshifted Recombinant Spike Proteins in Human Tissues and Circulation after COVID-19 Vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified mRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control Release 2015, 217, 345–351. [Google Scholar]
- Pfizer Australia Pty Ltd. Nonclinical Evaluation Report: Bnt162b2 [Mrna] COVID-19 Vaccine (Comirnatytm). 2021. Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf (accessed on 23 March 2025).
- Sharma, P.; Hoorn, D.; Aitha, A.; Breier, D.; Peer, D. The Immunostimulatory Nature of Mrna Lipid Nanoparticles. Adv. Drug Deliv. Rev. 2024, 205, 115175. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.; Rossi, R.; Sechi, F.; Buonocore, D.; Sorrenti, M.; Perteghella, S.; Peviani, M.; Bonferoni, M.C. Effect of Lipid Nanoparticle Physico-Chemical Properties and Composition on Their Interaction with the Immune System. Pharmaceutics 2024, 16, 1521. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar]
- Ferraresso, F.; Badior, K.; Seadler, M.; Zhang, Y.; Wietrzny, A.; Cau, M.F.; Haugen, A.; Rodriguez, G.G.; Dyer, M.R.; Cullis, P.R.; et al. Protein Is Expressed in All Major Organs after Intravenous Infusion of Mrna-Lipid Nanoparticles in Swine. Mol. Ther. Methods Clin. Dev. 2024, 32, 101314. [Google Scholar] [PubMed]
- Szebeni, J.; Kiss, B.; Bozo, T.; Turjeman, K.; Levi-Kalisman, Y.; Barenholz, Y.; Kellermayer, M. Insights into the Structure of Comirnaty COVID-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA-Lipid Complexes. ACS Nano 2023, 17, 13147–13157. [Google Scholar]
- König, B.; Kirchner, J.O. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 Mrna Vaccine Comirnaty®. Methods Protoc. 2024, 7, 41. [Google Scholar] [CrossRef]
- Diblasi, L.; Monteverde, M.; Nonis, D.; Sangorrín, M. At Least 55 Undeclared Chemical Elements Found in COVID-19 Vaccines from Astrazeneca, Cansino, Moderna, Pfizer, Sinopharm and Sputnik V, with Precise Icp-Ms. Int. J. Vaccine Theory Pract. Res. 2024, 3, 1367–1393. [Google Scholar]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate Immune Mechanisms of Mrna Vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar]
- Fineberg, H.V. Swine Flu of 1976: Lessons from the Past. An Interview with Dr Harvey V Fineberg. Bull World Health Organ 2009, 87, 414–415. [Google Scholar]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Efficacy and Safety of an Mrna-Based Rsv Pref Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- ModernaTX, Inc. Prescribing Information: Respiratory Syncytial Virus Vaccine) Injectable Suspension, for Intramuscular Use. 2024. Available online: https://www.fda.gov/media/179005/download?attachment=&utm_source=chatgpt.com (accessed on 23 March 2025).
- Wang, Y.S.; Kumari, M.; Chen, G.H.; Hong, M.H.; Yuan, J.P.; Tsai, J.L.; Wu, H.C. Mrna-Based Vaccines and Therapeutics: An in-Depth Survey of Current and Upcoming Clinical Applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar]
- Ladak, R.J.; He, A.J.; Huang, Y.H.; Ding, Y. The Current Landscape of Mrna Vaccines against Viruses and Cancer-a Mini Review. Front. Immunol. 2022, 13, 885371. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer Mrna Vaccines: Clinical Advances and Future Opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).