Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with increasing incidence and mortality rates, highlighting the urgent need for early diagnosis and treatment. However, early diagnosis of PDAC is extremely challenging due to the atypical early symptoms or the absence of noticeable symptoms. As a result, many patients are diagnosed with local metastasis, and even patients who are eligible for surgical resection have a high postoperative recurrence rate. Consequently, chemotherapy remains the primary treatment for PDAC. However, the unique biological characteristics of PDAC not only promote tumor progression and metastasis but also often lead to chemoresistance, a significant barrier to successful treatment. Recently, nanomaterials have garnered significant attention as promising materials for diagnosing and treating PDAC, showing great potential in cancer therapy, imaging, and drug delivery. Novel targeted nanomedicines, which encapsulate chemotherapy drugs and gene therapy products, offer significant advantages in overcoming resistance. These nanomedicines not only provide innovative solutions to the limitations of conventional chemotherapy but also improve the selectivity for cancer cells to enhance therapeutic outcomes. Current research is focused on the development of advanced nanomedicines, such as liposomes, nanotubes, and polymer-lipid hybrid systems, aimed at making chemotherapy more effective and longer lasting. This review provides a detailed overview of various nanomedicines utilized in the diagnosis and treatment of PDAC and outlines future directions for their development and key breakthroughs.
1. Introduction
1.1. Introduction of Nanomedicines
Recently, the rising incidence and mortality rates of cancer have posed significant challenges to global public health, making its diagnosis and treatment critical issues. Nanomedicine (NPs) has been applied in cancer diagnosis and treatment due to its advantages, including strong targeting capability, high drug stability, and the ability to load a variety of materials. Compared to traditional drugs, NPs are smaller in size and enable specific cancer biomarker detection in a simple and non-invasive manner, offering multiple advantages in early tumor diagnosis due to their enhanced sensitivity. Additionally, due to the enhanced permeability and retention (EPR) effect, NPs preferentially accumulate in tumor tissues, improving their diagnostic and therapeutic efficacy and specificity [1,2]. Moreover, by loading biomolecules, such as DNA, RNA, peptides, or antibody fragments, onto the surface of NPs, they can be selectively internalized in certain tumor cells through specific or overexpressed receptors while minimizing toxicity to normal cells [3]. Therefore, more advanced drugs targeting different types of NPs (such as liposomes, micelles, polymeric nanoparticles, and inorganic nanoparticles) are currently under development and being applied in the diagnosis and treatment of PDAC. In the following sections, we introduce different NPs that have been applied in the diagnosis and treatment of PDAC.
1.2. Challenges in Diagnosis and Treatment of PDAC
In recent years, cancer, the second leading cause of death in the United States, has attracted global attention for its diagnosis and treatment [4]. Pancreatic cancer is a highly aggressive malignancy and the fourth leading cause of cancer-related deaths. More than 90% of pancreatic cancers are PDAC, which has a five-year survival rate of less than 10% [5]. Due to its insidious onset, subtle symptoms, and lack of specific biomarkers, early diagnosis is often challenging. The clinically used marker carbohydrate antigen 19-9 (CA19-9) has a sensitivity of 76.1%; however, it lacks tumor specificity, as elevated levels can also be observed in various malignancies and other conditions (such as liver damage, bile duct obstruction, and pancreatitis) [6,7,8]. Additionally, imaging techniques, like computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), are often utilized in the diagnosis of PDAC. CT and MRI are commonly used imaging modalities, and their sensitivity is correlated with tumor size. For tumors smaller than 20 mm in diameter, the first-line imaging modality, CT, has limited sensitivity and may not offer significant clinical assistance in the early diagnosis of PDAC [9]. Therefore, identifying more specific and sensitive tumor markers or imaging techniques has become a key focus of current research.
Since the difficulty of early diagnosis, many patients are diagnosed with local metastasis, and less than 20% of patients are eligible for surgical resection, with a postoperative recurrence rate as high as 71% [7]. Therefore, chemotherapy remains the primary treatment modality for PDAC. However, the inherent chemoresistance of PDAC and physical barriers to drug delivery significantly contribute to chemotherapy resistance. Several hereditary or acquired gene mutations, like KRAS, TP53, and transforming growth factor beta (TGF-β), play an important role in this unfavorable prognosis. Additionally, a defining feature of PDAC is its dense tumor stroma, which can constitute over 90% of the total tumor volume and plays a major role in tumor progression and chemoresistance. The stroma microenvironment comprises cancer-associated fibroblasts (CAFs), pancreatic stellate cells (PSCs), endothelial cells, and immune and endocrine cells [10]. The fibrotic barrier increases interstitial pressure and reduces micro-vessel density, thereby impeding drug penetration. Tortuous and low-permeable vessels lead to the uneven distribution of medicines in tumor tissues, and the tight junctions of vascular endothelial cells also lead to a decrease in drug permeability in tumor tissues [11]. Moreover, stromal components such as CAFs promote tumor progression by secreting pro-tumor cytokines. Additionally, this persistent tumor escapes immune detection through the secretion of immunosuppressive factors, the expression of immune checkpoints, and the creation of low T lymphocytes [12]. In summary, the tumor microenvironment (TME) in PDAC—characterized by high pressure, hypoxia, acidic pH, immune evasion, and a fibrous stroma—promotes rapid tumor proliferation, metastasis, and invasion, leading to poor prognoses associated with conventional therapies, leading to obstacles in the delivery of drugs and necessitating the need for further research to address these challenges.
2. The Diagnosis of PDAC Based on Nanomedicine
As PDAC is a highly malignant tumor, there are no specific tumor biomarkers or imaging techniques for its diagnosis. In recent years, with the advancement of nanotechnology, targeted biomarkers, and nanomedicines, including those used as imaging agents, have shown considerable success in the diagnosis of PDAC (Figure 1). In this section, we discuss the different nanomedicines applied in laboratory detection and imaging techniques.
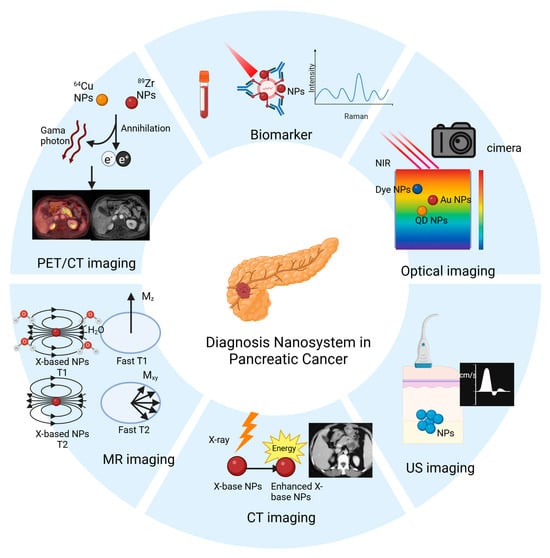
Figure 1.
Applications of nanomaterials in the diagnosis of PDAC, including nanomaterial-based in vitro detection techniques, and nanomaterial-enabled in vivo imaging techniques (e.g., optical imaging, ultrasound (US) imaging, CT imaging, MR imaging, and PET/CT imaging).
2.1. Biomarkers Detection Based on Nanomedicine
As an important method for the early diagnosis of cancer, laboratory detection remians at the forefront of development. In this section, we introduce nanomedicines based on different biomarkers for sensitive and specific diagnosis of pancreatic cancer (Table 1).

Table 1.
Nanomaterials used for laboratory tests.
2.1.1. Detection of Biomarkers in Body Fluids
Body fluids are readily accessible biological materials that are essential for disease diagnosis. Various nanomedicines have been developed to enhance the sensitivity and specificity of PDAC diagnosis. CA19-9, a commonly used blood biomarker for PDAC diagnosis, exhibits limited sensitivity. To improve detection accuracy, Huang et al. used gold nanoparticles (AuNPs)@PThi nanomedicine. When immobilized on a a glassy carbon electrode, it functions as a sensitive redox probe for the electrochemical detection of CA19-9. This approach enabled ultrasensitive detection across a concentration range of 6.5–520 U/mL, with a calculated detection limit of 0.26 U/mL [13].
In addition to CA19-9, other blood protein markers, such as carcinoembryonic antigen (CEA), are commonly used to diagnose PDAC. For instance, Liu et al. developed an enzyme-labeled AuNP probe. By encapsulating AuNPs with antibodies, single-stranded DNA (ssDNA), and horseradish peroxidase (HRP), the nanoparticles achieved sensitive and specific detection of CEA with a detection limit of 12 ng/L in human serum, which is approximately 130 times more sensitive than conventional ELISA [14]. Similarly, Krasnoslobodtsev et al. utilized AuNPs as a potential biomarker for detecting overexpressed mucin MUC4 in the blood of patients with PDAC through the surface-enhanced Raman scattering (SERS) effect, enabling the highly sensitive detection of a single AuNP [15].
In addition to tumor-specific proteins, alterations in nucleic acid expression levels can serve as predictive markers for tumor occurrence. Compared to non-malignant cells, microRNAs (miRs) such as miR-10b, miR-21, miR-155, and miR-196a were significantly upregulated, while miR-31 and miR-357 were significantly downregulated in PDAC cells, facilitating the development of diagnostic tools [24]. Leveraging this, Joshi et al. developed an AuNP with a localized surface plasmon resonance (LSPR) sensor to detect miR levels in the plasma of patients with PDAC. This method allows for the simultaneous measurement of multiple miRs while differentiating PDAC from chronic pancreatitis patients [16]. Furthermore, Xu and Liao group utilized an α-hemolysin (αHL) single nanopore to detect miR-21 and distinguish complex molecular signals of miR-21, miR-155, and miR-196a, providing new PDAC diagnosis insights [17].
Extracellular vesicles (EVs), a heterogeneous group of cell membrane structures associated with tumor progression and metastasis, are potential biomarkers [25]. Rodrigues et al. developed a semiconductor nanomaterial-based rapid fluorescence immunoassay to normalize EV biomarker levels to EV abundance. This approach enabled the detection of the expression of two pancreatic cancer biomarkers, live erythropoietin-producing hepatocellular A 2 (EphA2), and epithelial cell adhesion molecule (EpCAM) [18].
Lewis et al. developed an alternating current (AC) electrokinetic microarray chip capable of directly identifying and quantifying Evs in whole blood, serum, or plasma. The biomarkers glypican-1 and CD63 could distinguish 20 PDAC patient samples from 11 healthy controls with 99% sensitivity and 82% specificity [19]. Similarly, Li et al. designed a polydopamine (PDA)-modified slide that encapsulated antibodies targeting exosome surface proteins (anti-macrophage migration inhibitory factor (MIF),anti-Glypican1 (GPC1), anti-CD63, or anti-epidermal growth factor receptor (EGFR)). By capturing exosomes from samples and forming a “chip-exosome-PDA encapsulated antibody-reporter-Ag (shell)–Au (core) multilayer (PEARL) label” complex, this system enables ultra-sensitive detection of metastatic and non-metastatic PDAC through Raman spectroscopy, differentiation stage I-II from stage III tumors without histopathological examination [20].
Urine, an easily obtainable and storable body fluid, also offers the potential for PDAC detection. Liu et al. investigated a nanobiotinylated liposome based on isothermal amplification (LAMP) to detect urinary Regenerating Family Member 1 Alpha (REG1A), a protein biomarker for PDAC. Compared with polymerase chain reaction (PCR), LAMP is simpler, faster, and more specific, with a detection limit of 1 fg/mL [21].
2.1.2. Detection of Biomarkers in Cells and Tissues
In addition to body fluids, abnormally expressed antibodies or proteins in tumor cells and tissues are commonly used for early tumor detection. Optical nanoprobes have emerged as promising diagnostic tools. For example, Gisela et al. developed a sensor for CA19-9 detection by immobilizing anti-CA19-9 antibodies on screen-printed interdigitated electrodes (SPIDEs). The device achieved a CA19-9 detection limit of 0.12 U/mL [22].
To detect another PDAC biomarker, claudin-4, Hwang et al. constructed nanoprobes based on modified apoferritin (mAFTN) and functionalized CdSe (ZnS) quantum dots (QDs). This nanomedicine showed fluorescence detection sensitivity 27 times higher than that of conventional organic fluorophores and six times higher than that of individual QDs [26].
Eck et al. synthesized AuNPs with heterobifunctional polyethylene glycol (PEG) and covalently linked them to a F19 monoclonal antibody targeting fibroblast activation protein (FAP), which is highly expressed in PDAC-reactive stromal fibroblasts. The resulting conjugates successfully labeled tumor stroma in approximately 5 µm thick excised PDAC tissue slices and were imaged using dark-field microscopy at around 560 nm resonance scattering [23].
2.2. Imaging Examination Based on Nanomedicine
In addition to the laboratory detection of tumor biomarkers, radiological techniques are crucial for the early diagnosis of PDAC. In contrast, conventional imaging methods detect tumors only when they exceed 1 cm in diameter, with results highly relying on factors such as the operator’s experience and the patient’s condition [23]. To overcome these limitations, the development of nanomedicine-based imaging approaches has become a promising research direction aimed at enhancing diagnostic sensitivity and specificity.
When integrated with imaging modalities such as near-infrared (NIR) fluorescence, MRI, CT, PET, US imaging, and photoacoustic imaging (PAI), nanoplatforms offer prolonged circulation times and improve tumor targeting through active or passive NPs modification (Table 2). These systems play a prominent role in drug delivery monitoring, treatment response assessment, and image-guided therapy [27].

Table 2.
Nanomaterials for imaging examinations.
2.2.1. NIR Imaging
NIR fluorescence imaging, operating in the range of 700–900 nm, offers superior contrast and deeper tissue penetration compared to visible light, and has attracted much attention for the early diagnosis of PDAC [35]. The combination of nanomedicines with NIR dyes has emerged as a promising research avenue.
For instance, Li et al. developed silica nanoparticles (bMSN@Cy7.5-FA NPs) by conjugating the NIR dye Cy 7.5 with folic acid (FA), which can be used for in vivo tumor visualization with peak fluorescence intensity within 12 h, providing an effective platform for early PDAC diagnosis [28]. Similarly, the croconaine (CR) dye has shown potential for expanding NIR applications. Dong et al. constructed a photothermal molecular delivery system (CR@E8-EVs) by conjugating Cadherin 17 (CDH17) nanobodies with Evs. This system exhibits strong NIR absorption, excellent photothermal properties, good biocompatibility, and remarkable active tumor-targeting capability [29].
Han et al. combined GEM with the NIR dye IR780 to form human serum albumin (HSA)-GEM/IR780 complexes, which showed enhanced tumor accumulation and retention, with detectable fluorescence signals persisting up to 72 h post-injection. This extended retention is promising, since most cyanine NIR probes exhibit rapid degradation in vivo [36]. One plausible explanation for this phenomenon is defective lymphatic drainage in tumor tissues, which reduces the clearance of nanomedicines and prolongs their local retention [37].
Targeting the overexpressed cholecystokinin B (CCK-B) receptor in PDAC, AP1153 shows high specificity and affinity (15 pM), nearly three orders of magnitude greater than that of its peptide ligand, gastrin [30]. Building on this, Abraham et al. conjugated AP1153 with indocyanine green (ICG) to form AP1153-ICG-NJs, achieving peak fluorescence at 18 h post-injection for highly specific early PDAC detection [38]. Likewise, Clawson et al. synthesized amorphous calcium phosphate silica nanoparticles (CPSNP) that covalently coupled ICG with AP1153, achieving tumor fluorescence peaks between 15 and 18 h without stimulating PDAC cell proliferation, further facilitating early PDAC diagnosis [30].
2.2.2. CT Imaging
Enhanced CT imaging is a conventional adjunctive tool for the early diagnosis of PDAC. However, the short circulation half-life of iodine-based contrast agents necessitates precise control of the injection speed and timing during the examination. In contrast, stable nanoparticles ranging from 10 to 500 nm exhibit prolonged blood circulation and can accumulate at tumor sites through active targeting, offering distinct advantages for early PDAC detection [39,40].
Inorganic nanomaterials, including gold (Au), bismuth (Bi), and platinum (Pt), are considered potential alternatives for contrast enhancement. Trono et al. designed AuNPs for PDAC imaging, showing preferential uptake by PDAC cells at an optimal diameter of 20 nm. In addition to metallic nanoparticles, Xu et al. employed bismuth subcarbonate nanotubes (BNTs) for tumor-targeted imaging. BNTs not only provide superior contrast compared to conventional contrast agents but also exhibit therapeutic potential by suppressing tumor volume through combined radiotherapy and chemotherapy [41].
2.2.3. MR Imaging
MRI is commonly used for clinical diagnosis, especially for detecting small cystic lesions. However, its sensitivity in identifying PDAC remains limited, hindering early diagnosis. To address this issue, Holbrook et al. designed a Gd(III) contrast agent (Lip-Gd@AuNPs) that can accumulate in pancreatic tissue, providing significant signal enhancement. By allowing clear detection of the pancreas with a contrast ratio exceeding 35:1, this method contributes to the early diagnosis of PDAC [32].
Biocompatible superparamagnetic iron oxide nanoparticles (SPIONs) have also been explored as MRI contrast agents, offering reduced toxicological risk and avoiding nephrogenic systemic fibrosis (NSF) [42]. He et al. developed a chemokine receptor 4 (CXCR4)-targeted ultrasmall superparamagnetic iron oxide (CXCR4-USPIO) for PDAC imaging. Their findings showed that the T2 enhancement ratio and ΔR2 values could semi-quantitatively assess CXCR4 expression in four pancreatic cancer cell lines (AsPC-1, BxPC-3, CFPAC-1, and PANC-1), potentially serving as prognostic indicators [33].
In another study, Zhou et al. designed ultrasmall superparamagnetic iron oxide nanoparticles (USPIO-NPs) that could serve as MRI contrast agents or be cross-linked with NIR dyes to achieve dual MR/NIR imaging [43]. Wang et al. designed superparamagnetic iron oxide NPs (Dex-g-PCL/SPIO nanoparticles) targeting enolase 1 (ENO1), a protein upregulated in PDAC. Both in vitro and in vivo MRI studies have demonstrated enhanced detection efficiency for PDAC [34].
2.2.4. PET Imaging
PET/CT, as an emerging diagnostic method, is increasingly used for tumor diagnosis and metastasis monitoring due to its high sensitivity and specificity. However, conventional contrast agents like 18F-fluorodeoxyglucose (18F) exhibit limited sensitivity for PDAC detection and are prone to false-positive results under inflammatory and hyperglycemic conditions, reducing their suitability for early diagnosis [44]. In contrast, some nanoparticle-based contrast agents not only minimize false-positive results but also offer enhanced specificity for PDAC, making them a promising tool for early diagnosis.
For example, Chakrabarti et al. designed a hybrid nanoprobe ([64Cu]KRAS-IGF1) based on the commonly mutated gene, Kirsten rat sarcoma viral oncogene homolog (KRAS), in PDAC. After four hours post-injection, the PDAC signal intensity was 8.6 ± 1.4 times more intense than that of the contralateral muscle [45]. Additionally, Reiner et al. constructed nanoparticles ([89Zr]-NRep) by conjugating Doxil with zirconium-89, which accumulate in tumor tissues regardless of size and enable precise quantitative measurement of Doxil distribution within the tumors [46].
3. The Treatment of PDAC Based on Nanomedicine
The robust desmoplastic stroma and immunosuppressive TME of PDAC hinder effective drug delivery, leading to chemoresistance. Nanomedicines, due to their small size and EPR effect, can be combined with therapeutic molecules to achieve effective accumulation at the tumor site while reducing toxicity to surrounding cells. Many advanced drugs targeting different types of NPs (such as liposomes, micelles, polymeric nanoparticles, and inorganic nanoparticles) are currently under development and are being applied in the treatment of PDAC (Figure 2).
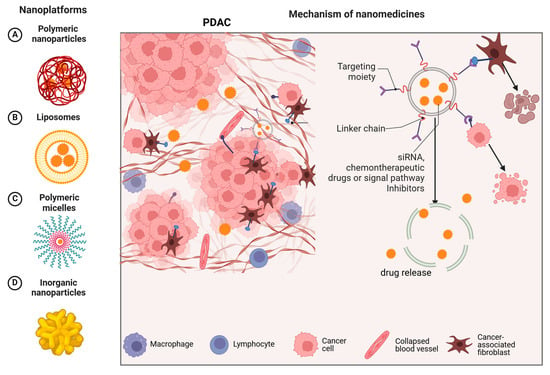
Figure 2.
The left side A, B, C, and D show different types of nanomedicine delivery systems. The right side explains how these nanoplatforms operate, emphasizing the targeting moiety, linker chain, and therapeutic components (such as siRNA, chemotherapeutic drugs, or signal pathway inhibitors) that work together to enhance the treatment efficacy.
In this section, we introduce four types of nanomedicines for the treatment of PDAC (Table 3). Each category of nanomedicine is discussed in detail concerning chemotherapy, immunotherapy, targeted therapy, and other relevant approaches.

Table 3.
Nanomedicines for the treatment of PDAC.
3.1. Polymeric Nanoparticle
Polymeric nanoparticles are particles with a size ranging from 1 to 1000 nm, which can encapsulate, embed, or conjugate active compounds and are capable of carrying multifunctional agents [67]. Known for their biocompatibility and biodegradability, they can conjugate, encapsulate, and carry various drugs or materials. Compared to conventional colloidal systems, they offer several advantages, including reduced toxicity, prolonged circulation times, enhanced cellular uptake via the EPR effect, improved bio-stability, uniform organ distribution, and controlled drug delivery [68].
3.1.1. Targeted Therapy Drugs
siRNAs are a crucial component of targeted therapy and have been extensively utilized in nanoparticle-based approaches for PDAC. CAFs are the predominant component of the PDAC stroma and contribute to PDAC progression through pro-tumor signaling and fibrosis production. Thus, the inhibition of CAFs is a promising therapeutic strategy [69]. SLC7A11 is a cystine transporter primarily expressed in CAFs that promotes their proliferation. Inhibiting its expression can reduce both CAF proliferation and PDAC proliferation and metastasis. Sharbeen et al. investigated Star3 nanoparticles complexed with SLC7A11 siRNA (Star3+SLC7A11-siRNA), which effectively downregulated SLC7A11 in PDAC mice, thereby reducing tumor growth, metastasis, and intratumoral fibrosis [47].
βIII-tubulin, which is linked to KRAS mutations, is overexpressed in PDAC tissues but is absent in normal acinar and ductal cells, facilitating cancer cell growth and metastasis [70]. Lo et al. designed a tandem peptide delivery system incorporating KRAS siRNA with an internalizing RGD peptide (iRGD)-targeting peptide. This construct successfully silenced the target gene in PDAC cell lines and immune-active genetically engineered mouse models, achieving significant therapeutic effects in 3D models [48]. Teo et al. developed star-poly (dimethylaminoethyl methacrylate) (POEGMA), which readily self-assembles with siRNA to form nanoparticles. Systemic administration of these nanoparticles in an orthotopic pancreatic tumor model resulted in high siRNA accumulation and silenced 80% of βIII-microtubule expression at both the gene and protein levels, thereby inhibiting tumor growth and metastasis [71].
MiR-21 targets and inhibits several tumor suppressor genes, including Programmed cell death protein 4(PDCD4), Phosphatase and tensin homolog deleted on chromosome ten (PTEN), and Tropomyosin 1 (TPM1) [72]. Li et al. provided a miR-21 antisense oligonucleotide (ASO-miR-21) combined with GEM and polyethylene glycol-polyethylenimine, which inhibited PDAC cell proliferation and metastasis [73]. Mutations in TGF-β are prevalent in patients with advanced PDAC and are correlated with poor survival. Ellermeier et al. The use of polyethylenimine (PEI)-coated delivery of TGF-β1 siRNA in an orthotopic PDAC model significantly reduced tumor TGF-β expression and prolonged mouse survival [49].
PRDM14 is overexpressed in various cancers but is undetectable in normal tissues and promotes PDAC metastasis. Taniguchi et al. developed a branched polyethylene glycol-conjugated poly-(l-ornithine) (PEG-PLO) nanoparticle for delivering PRDM14-targeted siRNA, achieving tumor-specific accumulation and effectively reducing tumor size and metastasis in vivo [73]. Similar outcomes were observed with calcium phosphate hybrid micelle-based delivery systems, which also improved the survival rates of mice [74].
In addition to the aforementioned siRNA formulations, proteins and signaling pathway inhibitors have been applied in nanoparticle-based therapies for PADC. Francisco et al. co-loaded the Poly (ADP-ribose) polymerase1 (PARP-1) inhibitor olaparib (OLA) and ascorbic acid (AA) into calcium phosphate-based nanoparticles (NP-ACP-OLA-AA). The resulting formulation exhibited prolonged in vitro activity, heightened genotoxicity, and enhanced apoptosis-mediated cytotoxicity [75].
Cyclopamine (CPA), a naturally occurring steroidal alkaloid, inhibits the hedgehog (Hh) pathway by targeting smoothened (SMO) receptors (Figure 3), thereby enhancing tumor perfusion and facilitating the accumulation and distribution of nanomedicines [76]. Zhang et al. utilized PEG-poly (lactic acid) (PEG-PLA) as a delivery vehicle for CPA. Although no significant reduction in tumor size was observed compared to the control group, this treatment effectively inhibited the Hh pathway and reduced the potential risk of PDAC invasiveness and metastasis [50].
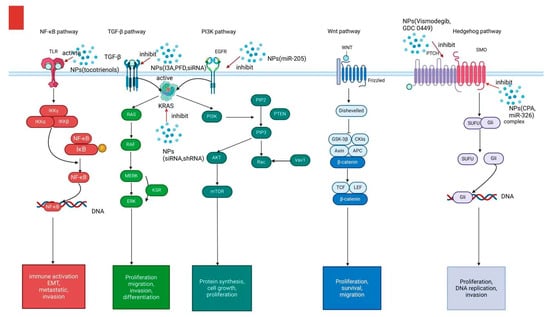
Figure 3.
Several signaling pathways (e.g., NF-κB, TGF-β,PI3K, Wnt, and Hedgehog signaling) contribute to the proliferation, invasion, migration, and Epithelial-mesenchymal transition (EMT) of PDAC. Different NPs have been developed to target these signaling pathways and inhibit tumorprogression.
Erlotinib, an EGFR inhibitor, blocks cell cycle progression by inducing apoptosis through the upregulation of p27 and caspase activation, while inhibiting EGFR phosphorylation. This mechanism suppresses DNA synthesis, cell growth, and angiogenesis in PDAC cells [77,78,79]. Noorani et al. loaded erlotinib onto albumin nanoparticles (ANPs) and demonstrated significantly increased cytotoxicity after 72 h in vitro [52].
3.1.2. Cytotoxic Chemotherapeutic Drugs
As a major component of the stroma of PDAC, CAFs represent a significant barrier to effective treatment of PDAC. To improve drug delivery through stromal depletion, a CAF-targeting nanopolymer, Cellax-docetaxel (DTX), was developed by conjugating DTX with acetylated carboxymethyl cellulose. Compared to the same dose of Nab-PTX, Cellax-DTX increased systemic drug exposure by 37 times and tumor uptake by 203-fold [80].
Chen et al. constructed an in vitro 3D PDAC model to evaluate the extracellular matrix (ECM) modulation capabilities of the GEM@nanogel (NGH) system. This system demonstrated robust ECM degradation, enhanced solid tumor penetration both in vitro and in vivo, and improved cytotoxicity by controlled GEM release and tumor cytoplasm disintegration [81]. The anticancer effects of doxorubicin (Dox) are often hindered by various drug resistance pathways, leading to poor treatment outcomes in PDAC. SiRNAs can reverse drug resistance by downregulating anti-apoptotic proteins when encapsulated into nanoparticles and co-delivered with siRNAs [82]. Thus, Hajar et al. used chitosan nanoparticles for dual delivery of insulin-like growth factor 1 receptor(IGFR1) siRNA and Dox, achieving favorable results in the A549 lung cancer cell line [83]. Given the high expression of IGFR1 in PDAC, we speculate that this therapeutic approach may also achieve favorable treatment outcomes in PDAC.
To addressing stromal barriers, a dual-drug delivery system combining the antifibrotic drug halofuginone (HF) and PTX was synthesized using methoxy polyethylene glycol-b-polycaprolactone. Pretreatment with HF nanoparticles restored stromal homeostasis by decreasing the major component of the ECM, substantially promoting PTX nanoparticle distribution, and penetrating cancer cells. This approach also improved cytotoxic T cell infiltration and resulted in significant tumor regression in two PDAC models [84].
Liu et al. explored the possibility of enhancing the chemotherapeutic efficacy of Mithramycin A (MIT) by encapsulating it into methoxy PEG-block-poly (d, l-lactic-co-glycolic acid) (mPEG-PLGA) NPs. By inhibiting the transcription factor Sp1, MITexhibited significantly better tumor suppression than free MIT (86% vs. 51%, p < 0.01) [51].
3.2. Nanoliposomal
Liposomes are double-layered lipid structures composed of phospholipids and cholesterol. Phospholipids form a bilayer structure that enhances the solubility and stability of anticancer drugs, facilitating effective drug encapsulation and delivery. Cholesterol, which is integrated within the bilayer, reduces the fluidity of the lipid membrane, thereby increasing the stability of nanoparticles in circulation. Additionally, it enhances the permeability of hydrophobic molecules across the plasma membrane, thereby improving drug transport and retention [68,85].
3.2.1. Nanoliposome Irinotecan
Irinotecan exerts its anticancer effects by stabilizing the complex between topoisomerase I (TOP1) and DNA, leading to DNA strand breaks and inhibition of replication. To enhance therapeutic efficacy and reduce toxicity, Ko et al. formulated irinotecan into liposomal nanoparticles (nal-IRI), which prolonged the exposure time of the active metabolite SN-38 in tumor tissues while minimizing systemic toxicity. Clinical outcomes showed that patients receiving nal-IRI plus 5-FU/LV had significantly improved OS and PFS compared to those treated with 5-FU/LV alone (0.67 and 0.56, respectively) [86].
To further augment therapeutic efficacy, Wang et al. developed a co-loaded nanoparticle drug combined with irinotecan and the Hedgehog pathway inhibitor, GDC-0449. This combination not only suppressed the expression of tumor-associated markers, including collagen, α-smooth muscle actin (α-SMA), and GLI family zinc finger 1 (GLI-1), but also promoted apoptosis in tumor cells [53].
3.2.2. Nab-PTX
Paclitaxel (PTX) enhances the efficacy of GEM by inhibiting tumor fibrosis and suppressing the expression of cytidine deaminase (CDA), an enzyme responsible for GEM inactivation [87]. However, systemic and repeated co-administration often leads to severe toxicity and limited therapeutic responses. Shabana et al. developed a thermosensitive, biodegradable hydrogel encapsulating PR_b-functionalized liposomes co-loaded with GEM and PTX for sustained local delivery to PDAC. The system exhibited prolonged drug release, enhanced cytotoxicity against PANC-1 cells, and greater tumor inhibition compared to non-targeted or free drug formulations [54].
Additionally, Yang et al. designed antibody fragment (AF)-conjugated liposomes encapsulating GEM and containing PTX (AF-GPL). Experimental results revealed an increased Bax/Bcl-2 ratio, demonstrating notable therapeutic effects in PDAC [55]. Similarly, Meng et al. used lipid-coated mesoporous silica nanoparticles (MSNP) for the co-delivery of PTX and GEM (LB-MSNP). Compared to free GEM, this system increased the active phosphorylated GEM metabolite by 13-fold, while reducing inactivated and deaminated metabolites by 4-fold, effectively inhibiting primary tumor growth and eliminating metastatic lesions [88].
In a clinical setting, Hingorani et al. conducted a randomized trial involving 279 patients with metastatic pancreatic cancer, evaluating polyethylene glycol-conjugated recombinant human hyaluronidase (PEGPH20) combined with nab-PTX/GEM. Combination therapy improved progression-free survival (PFS) with similar thromboembolic (TE) event rates compared to the control group [89]. Wei et al. designed a nanomedicine named TSL/HSA-PE, consisting of HSA complexes with PTX and ellagic acid (EA) co-encapsulated into thermosensitive liposomes (TSLs), which enhances drug retention and achieves better tumor accumulation and matrix penetration upon localized tumor heating [56].
3.2.3. Targeted Therapy Drugs
Lipid-based nanomedicines can enhance the cellular uptake of drugs like RNA, improving targeting and transfection efficiency and promoting drug release after uptake by macrophages into tumor tissues to improve activity [86]. Rao et al. incorporated bifunctional short hairpin RNA (bi-shRNA) into nanoliposomes to target and silence KRASG12mut, inhibiting tumor growth with low toxicity and offering a novel PDAC treatment [90].
Nuclear factor kappa-B (NF-κB) plays an important role in PDAC growth and chemotherapy resistance. Tocotrienols may enhance GEM antitumor activity via NF-κB activation. Maniam et al. used smoke nanoparticles to encapsulate GEM and tocotrienol. This nanomliposamal formulation showed a 2.78-fold increase in the anti-proliferative effect of GEM when combined with tocotrienol [57].
Stimulator of Interferon Genes (STING) is an endoplasmic reticulum receptor that induces inflammation and activates antitumor activity [91]. Shaji et al. encapsulated the STING agonist 2′3′-cyclic guanosine monophosphate (2′3′-cGAMP) in nanoliposomes, achieving efficient drug delivery and tumor proliferation inhibition through STING signaling activation [38,58].
3.2.4. Antifibrosis Drugs
Fibrotic stroma is a critical characteristic of the pancreatic tumor microenvironment. Therefore, Ji et al. synthesized a β-cyclodextrin (β-CD)-modified matrix metalloproteinase-2 (MMP-2) liposome, integrating antifibrosis agents and GEM for the treatment of PDAC. This approach demonstrated increased drug perfusion without severe adverse events [59].
3.3. Polymeric Micelles
Polymeric micelles are amphiphilic block copolymers with hydrophobic cores and hydrophilic shells for drug loading. These micelles have a high payload capacity, long circulation time, enhanced drug permeability, strong tumor penetration, and uniform distribution, making them a widely used nanocarrier system [92].
3.3.1. Cytotoxic Chemotherapeutic Drugs
Cytotoxic chemotherapeutic drugs are crucial for PDAC treatment. Chen et al. designed a micelle with polyethylene glycol-polyarginine-polylysine to deliver mono-phosphorylated GEM and PTX, releasing PTX in the tumor’s acidic microenvironment to inhibit metastasis [93]. Additionally, Li et al. designed a fluorouridine (FUDR) micelle in combination with the tumor-penetrating peptide iRGD to further enhance anti-tumor effects [94].
3.3.2. Targeted Therapy Drugs
Sonic hedgehog (SHH), the most studied member of the Hh signaling pathway, is commonly overexpressed in PDAC [95]. Kumar et al. designed polymer micelles to co-deliver miRNA targeting let-7b and vismodegib, a hedgehog pathway inhibitor. These micelles were demonstrated in serum for more than 24 h, with high uptake and low cytotoxicity, effectively inhibiting tumor growth [96]. Wang et al. used polymer micelles encapsulating irinotecan and vismodegib suppressing tumor growth and metastasis by inhibiting glioma-associated protein 1 and glucuronyl transferase expression [53]. Ray et al. created pH-responsive micelles with GEM and the Hh inhibitor (GDC-0449), selectively releasing contents in the acidic PDAC microenvironment and inhibiting tumor proliferation [62]. Daman et al. loaded salinomycin (SAL) into PEG-PLA micelles, inhibited invasion, and harnessed EMT in cancer stem cells (CSCs), achieving therapeutic efficacy in Balb/c AsPC-1 xenograft mice [97].
Additionally, nanomicells targeting various molecular targets in tumors have been developed. Pittella et al. constructed a nano-micelle (PEG-CCP/CaP) composed of polyethylene glycol and calcium phosphate, designed to deliver siRNA targeting VEGF. After intravenous administration, this drug demonstrated a 70% gene silencing efficiency in the BxPC3 pancreatic tumor model, significantly inhibiting tumor growth [98]. In PDAC, the oncogene microRNA-34a (miR-34a) is notably downregulated and targets various oncogenes involved in tumor proliferation, apoptosis, and invasiveness. High expression levels of PLK1 are strongly correlated with poor short-term survival in patients with pancreatic cancer. In response, Xin et al. engineered a reactive oxygen species (ROS)-responsive polymer micelle to co-deliver the PLK1 inhibitor volasertib and miR-34a. This drug delivery system significantly inhibited tumor growth while minimizing systemic toxicity [64].
3.3.3. Immunomodulators
Ingenol-3-mebutate (I3A) acts as a novel immunomodulator by upregulating CD80 and CD86 expression on dendritic cells (DCs), which in turn activates CD8+ T cells and induces mitochondrial swelling, leading to tumor cell death. Yu et al. developed an I3A-loaded polymer micelle (I3A-PM) that promotes Th1 polarization. By upregulating Th1 cytokines (IL-12, IL-2, IFN-γ, and TNF-α), I3A-PM accelerates the expansion of CD4+ and CD8+ T cells and inhibits TGF-β signaling which leads to regulatory T cell and Th2 cytokine IL-6 depletion, ultimately suppressing tumor proliferation and metastasis [99]. Furthermore, Shen et al. created a nano-micelle system containing I3A modified with 2-3-((S)-5-amino-1-carboxy-pentyl)-ureido)-pentanedioate (ACUPA-) and triphenylphosphine (TPP+). This formulation significantly increased the infiltration of T lymphocytes into the tumor, activated adaptive immunity, and induced immunogenic cell death, achieving promising therapeutic effects [63].
3.3.4. Combination Drugs
Zhao et al. loaded CPA and PTX onto micelles to regulate CAFs in PDAC stroma. This nanomedicine was found to disrupt the communication between tumor cells and CAFs, breaking the cycle of tumor cell proliferation, stromal support, and metastasis [100]. Li et al. developed a nano-polymer micelle (M-CPA/PTX) for co-deliver CPA and PTX, which enhanced the intratumoral vasculature density, promoted CD8+ T cell infiltration, without depletion of tumor-restraining stroma [60]. GEM, a first-line PDAC treatment, was developed by Mondal et al. using a cetuximab (C225)-decorated micelle targeting EGFR, effectively improving drug accumulation in pancreatic tumor-bearing mice and suppressing tumor growth [61].
Resistance to GEM in PDAC is often associated with the activation of the STING signaling pathway, which induces chemokines like CCL2 and CCL7 [101]. This process leads to immune resistance by recruiting tumor-associated macrophages (TAM) and myeloid-derived suppressor cells (MDSC). To address this, Wan et al. incorporated the CCR2 antagonist PF-6309 (PF), which shares receptors with CCL2 and CCL7, into a GEM-conjugated polymer (PGEM) micelle system. This approach not only reduces the pancreatic tumor burden but also induces effective anti-tumor immunity [91].
3.4. Inorganic Nanoparticles
Inorganic nanoparticles have emerged as promising drug carriers, offering improved therapeutic efficacy and reduced side effects. Their advantages include simplified modification of target molecules, control over the drug release rate through different stimuli, and efficient delivery of targeted drugs. Common materials used in inorganic nanoparticles include gold, silica, and iron oxide [102].
3.4.1. AuNPs
Han et al. designed AuNPs coated with PEI and PEG to co-deliver all-trans retinoic acid (ATRA, which promotes PSC quiescence) and siRNA targeting heat shock protein 47 (HSP47, a collagen-specific chaperone). This nanoparticle system promotes PSC quiescence and suppresses ECM hyperplasia, thereby facilitating drug delivery and accumulation in pancreatic tumors and significantly boosting the effectiveness of chemotherapy agents [65].
The expression of SMO in the Hh pathway is regulated by various microRNAs, including miR-193b, miR-326, and miR-338-3p. Based on this, Mo et al. designed gold nanoparticles to carry miR-326. Although the study was conducted in hepatocellular carcinoma, the Hh pathway is also expressed in PDAC, suggesting the potential therapeutic relevance of this approach in PDAC treatment [66].
3.4.2. Iron Oxide NPs
Deby et al. conjugated relaxin 2 (RLX) with superparamagnetic iron oxide nanoparticles to target CAF precursors. This strategy not only delayed tumor growth but also enhanced anti-tumor effects when combined with GEM [103].
IGFR1 is highly expressed in 40–90% of PDAC tissues, as well as in both tumor and stromal cells, making it a potential biomarker for targeting tumor tissues. Zhou et al. developed magnetic iron oxide nanoparticles conjugated with recombinant human IGF1 and Dox. This nanosystem inhibited the proliferation of tumor tissues and enabled treatment response monitoring using MRI [104].
3.4.3. Tungsten Oxide NPs
Activation of the PI3K pathway can promote tumor invasion, migration, EMT, and drug resistance [105]. Huo et al. designed a nanoaggregated system using tungsten oxide nanoparticles modified with the hypoxia-directed chemokine CCL-28 ligand and MMP-2 cleavable peptides. This approach enables drug penetration into the deeper regions of tumors by targeting the hypoxic tumor microenvironment and alleviating hypoxic-induced resistance in a PI3K-dependent manner [106].
4. Summary and Future Directions
PDAC remains one of the most lethal cancers due to its late diagnosis, aggressive tumor biology, and profound resistance to conventional therapies. Nanomedicines have played a revolutionary role in addressing these challenges by enhancing diagnostic sensitivity, improving drug delivery efficiency, and overcoming TME-mediated therapeutic resistance.
From a diagnostic perspective, various nanoprobes—such as AuNPs, SPIONs, and BNTs—have been developed to target specific biomarkers, including CA19-9, claudin-4, and SMO, thereby improving early detection through enhanced imaging modalities like MRI, PET/CT, and NIR fluorescence imaging. These nanoparticles not only increase imaging sensitivity but also allow real-time monitoring of treatment responses. However, most diagnostic applications remain in the preclinical stages, with limited translation to clinical practice due to concerns about long-term toxicity, biodistribution, and regulatory approval processes.
The primary challenge in treating PDAC lies in its dense fibrotic stroma, which hinders perfusion and limits the penetration of chemotherapeutic agents. To overcome this obstacle, nanomedicine-based technologies have been developed to improve drug delivery and accumulation within tumor tissues via the EPR effect, while enabling controlled drug release through stimuli-responsive systems. Co-delivery platforms, such as micelles co-loaded with GEM and PTX or polymeric nanoparticles encapsulating both siRNA and chemotherapeutic agents, have demonstrated the ability to overcome drug resistance, remodel the fibrotic stroma, and enhance immune cell infiltration into the tumor microenvironment. Additionally, strategies targeting the TME plays an important role in chemotherapy resistance. ECMs driven by CAFs and aberrant oncogenic signaling pathways, such as Hh, enhances drug penetration and reduces stromal barriers. Approaches such as using relaxin-loaded nanoparticles to modulate CAFs or dual-drug systems to inhibit Hh signaling have shown promise in disrupting the ECM and enhancing drug accumulation within tumors. Research shows that inhibiting the Hh signaling pathway can reduce stromal barriers and improve perfusion, ultimately facilitating more effective drug delivery. Despite these advances, the heterogeneity of the TME, variability in the EPR effect among patients, and potential immunogenicity of certain nanocarriers remain hurdles.
One critical limitation of the clinical translation of nanomedicines is their complex manufacturing processes, scalability, and reproducibility. Furthermore, off-target accumulation and potential toxicity, especially for inorganic nanoparticles such as Au NPs and tungsten oxide nanoparticles, raise safety concerns that require thorough investigation. Moreover, single nanomedicine treatments are not effective in clinical settings and are typically used in combination with other chemotherapy drugs, which inevitably leads to further toxic adverse effects. The pharmacokinetics and long-term fates of these nanomaterials require comprehensive evaluations to ensure patient safety. Other technologies like interventional techniques, together with NPs, have been successfully applied clinically for the effective treatment of locally advanced PDAC, but their role in the metastasis stage is limited. Future research should focus on developing personalized nanomedicine approaches that account for interpatient variability in TME characteristics and nanoparticle uptake. The combination of nanomedicine with immunotherapy, targeted therapy, and radiotherapy could further potentiate therapeutic efficacy. In addition, the design of multi-functional nanoplatforms capable of simultaneous diagnosis, drug delivery, and real-time monitoring holds great promise. In recent years, TME-responsive nanoplatforms have demonstrated promising results in multimodal imaging-guided therapies, including photodynamic, photothermal, and chemodynamic treatments for various tumors. These nanoplatforms selectively release drugs or activate therapeutic functions in response to TME characteristics, resulting in enhanced selectivity, minimal side effects, and strong control. Furthermore, magnetic micro-robots that target folate receptors offer an innovative therapeutic approach that harness the properties of magnetic fields combined with folate receptor specificity. When integrated with imaging modalities, these microrobots enable real-time monitoring of drug delivery and treatment progression, significantly improving the precision of drug administration. To facilitate clinical translation, collaborative efforts between researchers, clinicians, and regulatory agencies are essential to establish standardized protocols for safety assessment and efficacy evaluation.
5. Conclusions
In conclusion, while nanotherapy has shown remarkable potential for improving the diagnosis and treatment of PDAC, addressing the current limitations and ensuring safe, effective, and patient-tailored solutions are critical for its successful clinical application.
Author Contributions
Conceptualization: H.X. and K.G.; investigation: K.G. and S.L.; writing—original draft preparation: K.G. and S.L.; writing—review and editing: X.W.; supervision: X.W. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Beijing Xisike Clinical Oncology Research Foundation, grant number Y-HR2020MS-1039.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PDAC | Pancreatic ductal adenocarcinoma |
| NPs | nanomedicines |
| EPR | Enhanced permeability and retention |
| GEM | gemcitabine |
| 5-FU | 5-fluorouracil |
| LV | leucovorin |
| CA19-9 | carbohydrate antigen 19-9 |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| US | Ultrasound |
| AuNPs | gold nanoparticles |
| CEA | carcinoembryonic antigen |
| ssDNA | single-stranded DNA |
| HRP | Horseradish peroxidase |
| SERS | Surface-enhanced Raman scattering |
| miRs | microRNAs |
| LSPR | localized surface plasmon resonance |
| αHL | α-hemolysin |
| EV | Extracellular vesicle |
| QD | Quantum dots |
| EphA2 | Erythropoietin-producing hepatocellular A 2 |
| EpCAM | Epithelial cell adhesion molecule |
| AC | Alternating current |
| MIF | macrophage migration inhibitory factor |
| GPC1 | Glypican1 |
| EGFR | Epidermal growth factor receptor |
| LAMP | liposome based on isothermal amplification |
| REG1A | Regenerating Family Member 1 Alpha |
| PCR | Polymerase chain reaction |
| SPIDE | screen-printed interdigitated electrode |
| CNO | carbon nano-onions |
| GO | graphene oxide |
| mAFTN | modified apoferritin |
| ZnS | CdSe |
| Ni-NTA | nickel-nitrilotriacetic acid |
| PEG | polyethylene glycol |
| FAP | fibroblast activation protein |
| NIR | near-infrared |
| PAI | photoacoustic imaging |
| FA | folic acid |
| CR | croconaine |
| CDH17 | Cadherin 17 |
| HSA | human serum albumin |
| CCK-B | cholecystokinin B |
| ICG | indocyanine green |
| CPSNP | calcium phosphate silica nanoparticles |
| Bi | bismuth |
| Pt | platinum |
| BNTs | bismuth subcarbonate nanotubes |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| NSF | nephrogenic systemic fibrosis |
| CXCR4 | Chemokine receptor 4 |
| ENO1 | enolase 1 |
| 18F | 18F-fluorodeoxyglucose |
| KRAS | Kirsten rats arcomaviral oncogene homolog |
| IGFR1 | insulin-like growth factor 1 receptor |
| TGF-β | transforming growth factor beta |
| OS | overall survival |
| CAFs | cancer-associated fibroblasts |
| PSCs | pancreatic stellate cells |
| TAMs | regulatory T cells, tumor-associated macrophages |
| TME | tumor microenvironment |
| EMT | Epithelial-mesenchymal transition |
| siRNA | small interfering RNA |
| iRGD | internalizing RGD peptide |
| PDCD4 | Programmed cell death protein 4 |
| PTEN | Phosphatase and tensin homolog deleted on chromosome ten |
| TPM1 | Tropomyosin 1 |
| PEI | polyethylenimine |
| Nab-PTX | Nab-paclitaxel |
| Hh | hedgehog |
| CPA | Cyclopamine |
| SMO | smoothened |
| DTX | docetaxel |
| NGH | nanogel |
| Dox | doxorubicin |
| ANPs | albumin nanoparticles |
| HF | halofuginone |
| OLA | olaparib |
| AA | ascorbic acid |
| nal-IRI | nanoparticles |
| α-SMA | α-smooth muscle actin |
| PTX | Paclitaxel |
| CDA | cytidine deaminase |
| AF | antibody fragment |
| PFS | progression-free survival |
| TE | thromboembolic |
| EA | ellagic acid |
| TSLs | thermosensitive liposomes |
| bi-shRNA | bifunctional short hairpin RNA |
| NF-κB | Nuclear factor kappa-B |
| MIT | Mithramycin |
| STING | Stimulator of Interferon Genes |
| β-CD | β-cyclodextrin |
| MMP-2 | matrix metalloproteinase-2 |
| MDSC | myeloid-derived suppressor cells |
| FUDR | fluorouridine |
| SHH | Sonic hedgehog |
| SAL | salinomycin |
| CSCs | cancer stem cells |
| I3A | Ingenol-3-mebutate |
| DC | dendritic cell |
| TPP+ | triphenylphosphine |
| ROS | reactive oxygen species |
| ATRA | all-trans retinoic acid |
| RLX | relaxin 2 |
References
- Quader, S.; Kataoka, K. Nanomaterial-Enabled Cancer Therapy. Mol. Ther. 2017, 25, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS Nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e712. [Google Scholar] [CrossRef]
- Luo, G.; Fan, Z.; Cheng, H.; Jin, K.; Guo, M.; Lu, Y.; Yang, C.; Fan, K.; Huang, Q.; Long, J.; et al. New observations on the utility of CA19-9 as a biomarker in Lewis negative patients with pancreatic cancer. Pancreatology 2018, 18, 971–976. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Chu, L.C.; Goggins, M.G.; Fishman, E.K. Diagnosis and Detection of Pancreatic Cancer. Cancer J. 2017, 23, 333–342. [Google Scholar] [CrossRef]
- Haqq, J.; Howells, L.M.; Garcea, G.; Metcalfe, M.S.; Steward, W.P.; Dennison, A.R. Pancreatic stellate cells and pancreas cancer: Current perspectives and future strategies. Eur. J. Cancer 2014, 50, 2570–2582. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Farhangnia, P.; Khorramdelazad, H.; Nickho, H.; Delbandi, A.A. Current and future immunotherapeutic approaches in pancreatic cancer treatment. J. Hematol. Oncol. 2024, 17, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, Z.; Zhao, C.; Han, W.; Lin, L.; Liu, A.; Weng, S.; Lin, X. Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19-9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int. J. Nanomed. 2017, 12, 3049–3058. [Google Scholar] [CrossRef]
- Liu, M.; Jia, C.; Huang, Y.; Lou, X.; Yao, S.; Jin, Q.; Zhao, J.; Xiang, J. Highly sensitive protein detection using enzyme-labeled gold nanoparticle probes. Analyst 2010, 135, 327–331. [Google Scholar] [CrossRef]
- Krasnoslobodtsev, A.V.; Torres, M.P.; Kaur, S.; Vlassiouk, I.V.; Lipert, R.J.; Jain, M.; Batra, S.K.; Lyubchenko, Y.L. Nano-immunoassay with improved performance for detection of cancer biomarkers. Nanomedicine 2015, 11, 167–173. [Google Scholar] [CrossRef]
- Joshi, G.K.; Deitz-McElyea, S.; Liyanage, T.; Lawrence, K.; Mali, S.; Sardar, R.; Korc, M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano 2015, 9, 11075–11089. [Google Scholar] [CrossRef]
- Xu, J.; Liao, K.; Fu, Z.; Xiong, Z. A new method for early detection of pancreatic cancer biomarkers: Detection of microRNAs by nanochannels. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2634–2640. [Google Scholar] [CrossRef]
- Rodrigues, M.; Richards, N.; Ning, B.; Lyon, C.J.; Hu, T.Y. Rapid Lipid-Based Approach for Normalization of Quantum-Dot-Detected Biomarker Expression on Extracellular Vesicles in Complex Biological Samples. Nano Lett. 2019, 19, 7623–7631. [Google Scholar] [CrossRef]
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320. [Google Scholar] [CrossRef]
- Li, T.D.; Zhang, R.; Chen, H.; Huang, Z.P.; Ye, X.; Wang, H.; Deng, A.M.; Kong, J.L. An ultrasensitive polydopamine bi-functionalized SERS immunoassay for exosome-based diagnosis and classification of pancreatic cancer. Chem. Sci. 2018, 9, 5372–5382. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Eck, W.; Craig, G.; Sigdel, A.; Ritter, G.; Old, L.J.; Tang, L.; Brennan, M.F.; Allen, P.J.; Mason, M.D. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano 2008, 2, 2263–2272. [Google Scholar] [CrossRef]
- Ma, M.Z.; Kong, X.; Weng, M.Z.; Cheng, K.; Gong, W.; Quan, Z.W.; Peng, C.H. Candidate microRNA biomarkers of pancreatic ductal adenocarcinoma: Meta-analysis, experimental validation and clinical significance. J. Exp. Clin. Cancer Res. 2013, 32, 71. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Hwang, M.P.; Lee, J.W.; Lee, K.E.; Lee, K.H. Think modular: A simple apoferritin-based platform for the multifaceted detection of pancreatic cancer. ACS Nano 2013, 7, 8167–8174. [Google Scholar] [CrossRef]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Yu, Z.; Wang, H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1527. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Dai, Y.; Xu, X.; Cao, X.; Zeng, Q.; He, H.; Pang, L.; Liang, J.; Chen, X.; et al. In vivo near infrared fluorescence imaging and dynamic quantification of pancreatic metastatic tumors using folic acid conjugated biodegradable mesoporous silica nanoparticles. Nanomedicine 2018, 14, 1867–1877. [Google Scholar] [CrossRef]
- Dong, Y.; Xia, P.; Xu, X.; Shen, J.; Ding, Y.; Jiang, Y.; Wang, H.; Xie, X.; Zhang, X.; Li, W.; et al. Targeted delivery of organic small-molecule photothermal materials with engineered extracellular vesicles for imaging-guided tumor photothermal therapy. J. Nanobiotechnol. 2023, 21, 442. [Google Scholar] [CrossRef]
- Clawson, G.A.; Abraham, T.; Pan, W.; Tang, X.; Linton, S.S.; McGovern, C.O.; Loc, W.S.; Smith, J.P.; Butler, P.J.; Kester, M.; et al. A Cholecystokinin B Receptor-Specific DNA Aptamer for Targeting Pancreatic Ductal Adenocarcinoma. Nucleic Acid. Ther. 2017, 27, 23–35. [Google Scholar] [CrossRef]
- Trono, J.D.; Mizuno, K.; Yusa, N.; Matsukawa, T.; Yokoyama, K.; Uesaka, M. Size, concentration and incubation time dependence of gold nanoparticle uptake into pancreas cancer cells and its future application to X-Ray Drug Delivery System. J. Radiat. Res. 2011, 52, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-Dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Song, W.; Lei, J.; Li, Z.; Cao, J.; Huang, S.; Meng, J.; Xu, H.; Jin, Z.; Xue, H. Anti-CXCR4 monoclonal antibody conjugated to ultrasmall superparamagnetic iron oxide nanoparticles in an application of MR molecular imaging of pancreatic cancer cell lines. Acta Radiol. 2012, 53, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, H.; Bi, R.; Gao, G.; Li, K.; Liu, H.L. ENO1-targeted superparamagnetic iron oxide nanoparticles for detecting pancreatic cancer by magnetic resonance imaging. J. Cell Mol. Med. 2020, 24, 5751–5757. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Han, H.; Wang, J.; Chen, T.; Yin, L.; Jin, Q.; Ji, J. Enzyme-sensitive gemcitabine conjugated albumin nanoparticles as a versatile theranostic nanoplatform for pancreatic cancer treatment. J. Colloid. Interface Sci. 2017, 507, 217–224. [Google Scholar] [CrossRef]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef]
- Abraham, T.; McGovern, C.O.; Linton, S.S.; Wilczynski, Z.; Adair, J.H.; Matters, G.L. Aptamer-Targeted Calcium Phosphosilicate Nanoparticles for Effective Imaging of Pancreatic and Prostate Cancer. Int. J. Nanomed. 2021, 16, 2297–2309. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Nanoparticulate X-ray computed tomography contrast agents: From design validation to in vivo applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef]
- Hu, X.; Sun, J.; Li, F.; Li, R.; Wu, J.; He, J.; Wang, N.; Liu, J.; Wang, S.; Zhou, F.; et al. Renal-Clearable Hollow Bismuth Subcarbonate Nanotubes for Tumor Targeted Computed Tomography Imaging and Chemoradiotherapy. Nano Lett. 2018, 18, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Yeh, R.; Dercle, L.; Garg, I.; Wang, Z.J.; Hough, D.M.; Goenka, A.H. The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2018, 43, 415–434. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Zhang, K.; Aruva, M.R.; Cardi, C.A.; Opitz, A.W.; Wagner, N.J.; Thakur, M.L.; Wickstrom, E. Radiohybridization PET imaging of KRAS G12D mRNA expression in human pancreas cancer xenografts with [(64)Cu]DO3A-peptide nucleic acid-peptide nanoparticles. Cancer Biol. Ther. 2007, 6, 948–956. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Abdel-Atti, D.; Tang, J.; Zhao, Y.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.M.; Reiner, T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun. 2016, 7, 11838. [Google Scholar] [CrossRef]
- Sharbeen, G.; McCarroll, J.A.; Akerman, A.; Kopecky, C.; Youkhana, J.; Kokkinos, J.; Holst, J.; Boyer, C.; Erkan, M.; Goldstein, D.; et al. Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma Determine Response to SLC7A11 Inhibition. Cancer Res. 2021, 81, 3461–3479. [Google Scholar] [CrossRef]
- Lo, J.H.; Hao, L.; Muzumdar, M.D.; Raghavan, S.; Kwon, E.J.; Pulver, E.M.; Hsu, F.; Aguirre, A.J.; Wolpin, B.M.; Fuchs, C.S.; et al. iRGD-guided Tumor-penetrating Nanocomplexes for Therapeutic siRNA Delivery to Pancreatic Cancer. Mol. Cancer Ther. 2018, 17, 2377–2388. [Google Scholar] [CrossRef]
- Ellermeier, J.; Wei, J.; Duewell, P.; Hoves, S.; Stieg, M.R.; Adunka, T.; Noerenberg, D.; Anders, H.J.; Mayr, D.; Poeck, H.; et al. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 2013, 73, 1709–1720. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, T.; Shen, S.; She, X.; Tuo, Y.; Hu, Y.; Pang, Z.; Jiang, X. Cyclopamine disrupts tumor extracellular matrix and improves the distribution and efficacy of nanotherapeutics in pancreatic cancer. Biomaterials 2016, 103, 12–21. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, L.; Liu, X.J.; Li, Y.; Zhao, C.Y.; Wang, R.Q.; Zhen, Y.S. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int. J. Nanomed. 2017, 12, 5255–5269. [Google Scholar] [CrossRef] [PubMed]
- Noorani, M.; Azarpira, N.; Karimian, K.; Heli, H. Erlotinib-loaded albumin nanoparticles: A novel injectable form of erlotinib and its in vivo efficacy against pancreatic adenocarcinoma ASPC-1 and PANC-1 cell lines. Int. J. Pharm. 2017, 531, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Zhou, Q.; Sui, M.; Lu, Z.; Zhou, Z.; Tang, J.; Miao, Y.; Zheng, M.; Wang, W.; et al. Terminating the criminal collaboration in pancreatic cancer: Nanoparticle-based synergistic therapy for overcoming fibroblast-induced drug resistance. Biomaterials 2017, 144, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Shabana, A.M.; Kambhampati, S.P.; Hsia, R.C.; Kannan, R.M.; Kokkoli, E. Thermosensitive and biodegradable hydrogel encapsulating targeted nanoparticles for the sustained co-delivery of gemcitabine and paclitaxel to pancreatic cancer cells. Int. J. Pharm. 2021, 593, 120139. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Q.; Xu, Y.; Liu, H.; Zhong, L. Antibody fragment-conjugated gemcitabine and paclitaxel-based liposome for effective therapeutic efficacy in pancreatic cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 328–335. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Xia, D.; Guo, S.; Wang, F.; Zhang, X.; Gan, Y. Thermosensitive Liposomal Codelivery of HSA-Paclitaxel and HSA-Ellagic Acid Complexes for Enhanced Drug Perfusion and Efficacy Against Pancreatic Cancer. ACS Appl. Mater. Interfaces 2017, 9, 25138–25151. [Google Scholar] [CrossRef]
- Maniam, G.; Mai, C.W.; Zulkefeli, M.; Fu, J.Y. Co-encapsulation of gemcitabine and tocotrienols in nanovesicles enhanced efficacy in pancreatic cancer. Nanomedicine 2021, 16, 373–389. [Google Scholar] [CrossRef]
- Shaji, S.G.; Patel, P.; Mamani, U.F.; Guo, Y.; Koirala, S.; Lin, C.Y.; Alahmari, M.; Omoscharka, E.; Cheng, K. Delivery of a STING Agonist Using Lipid Nanoparticles Inhibits Pancreatic Cancer Growth. Int. J. Nanomed. 2024, 19, 8769–8778. [Google Scholar] [CrossRef]
- Ji, T.; Li, S.; Zhang, Y.; Lang, J.; Ding, Y.; Zhao, X.; Zhao, R.; Li, Y.; Shi, J.; Hao, J.; et al. An MMP-2 Responsive Liposome Integrating Antifibrosis and Chemotherapeutic Drugs for Enhanced Drug Perfusion and Efficacy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2016, 8, 3438–3445. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, Z.; Li, T.; Chen, H.; Yuan, Y.; Wang, Y.A.; Hsiao, C.H.; Chow, D.S.; Overwijk, W.W.; Li, C. Stromal Modulation Reverses Primary Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. ACS Nano 2018, 12, 9881–9893. [Google Scholar] [CrossRef]
- Mondal, G.; Almawash, S.; Chaudhary, A.K.; Mahato, R.I. EGFR-Targeted Cationic Polymeric Mixed Micelles for Codelivery of Gemcitabine and miR-205 for Treating Advanced Pancreatic Cancer. Mol. Pharm. 2017, 14, 3121–3133. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Confeld, M.; Borowicz, P.; Wang, T.; Mallik, S.; Quadir, M. PEG-b-poly (carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surf. B Biointerfaces 2019, 174, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, C.; Wang, Z.; Chu, Z.; Liu, C.; Xu, X.; Xia, M.; Zhao, M.; Wang, C. Sequential receptor-mediated mixed-charge nanomedicine to target pancreatic cancer, inducing immunogenic cell death and reshaping the tumor microenvironment. Int. J. Pharm. 2021, 601, 120553. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Lin, F.; Wang, Q.; Yin, L.; Mahato, R.I. ROS-Responsive Polymeric Micelles for Triggered Simultaneous Delivery of PLK1 Inhibitor/miR-34a and Effective Synergistic Therapy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2019, 11, 14647–14659. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Xu, Y.; Zhao, X.; Zhang, Y.; Yang, X.; Wang, Y.; Zhao, R.; Anderson, G.J.; Zhao, Y.; et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018, 9, 3390. [Google Scholar] [CrossRef]
- Mo, Y.; He, L.; Lai, Z.; Wan, Z.; Chen, Q.; Pan, S.; Li, L.; Li, D.; Huang, J.; Xue, F.; et al. Gold nano-particles (AuNPs) carrying miR-326 targets PDK1/AKT/c-myc axis in hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2830–2837. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Afzal, M.; Ameeduzzafar; Alharbi, K.S.; Alruwaili, N.K.; Al-Abassi, F.A.; Al-Malki, A.A.L.; Kazmi, I.; Kumar, V.; Kamal, M.A.; Nadeem, M.S.; et al. Nanomedicine in treatment of breast cancer—A challenge to conventional therapy. Semin. Cancer Biol. 2021, 69, 279–292. [Google Scholar] [CrossRef]
- Barman, S.; Fatima, I.; Singh, A.B.; Dhawan, P. Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 4765. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Sharbeen, G.; Liu, J.; Youkhana, J.; Goldstein, D.; McCarthy, N.; Limbri, L.F.; Dischl, D.; Ceyhan, G.O.; Erkan, M.; et al. βIII-tubulin: A novel mediator of chemoresistance and metastases in pancreatic cancer. Oncotarget 2015, 6, 2235–2249. [Google Scholar] [CrossRef]
- Teo, J.; McCarroll, J.A.; Boyer, C.; Youkhana, J.; Sagnella, S.M.; Duong, H.T.; Liu, J.; Sharbeen, G.; Goldstein, D.; Davis, T.P.; et al. A Rationally Optimized Nanoparticle System for the Delivery of RNA Interference Therapeutics into Pancreatic Tumors In Vivo. Biomacromolecules 2016, 17, 2337–2351. [Google Scholar] [CrossRef] [PubMed]
- Frampton, A.E.; Castellano, L.; Colombo, T.; Giovannetti, E.; Krell, J.; Jacob, J.; Pellegrino, L.; Roca-Alonso, L.; Funel, N.; Gall, T.M.; et al. MicroRNAs cooperatively inhibit a network of tumor suppressor genes to promote pancreatic tumor growth and progression. Gastroenterology 2014, 146, 268–277.e218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L.; et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Natori, Y.; Miyagi, Y.; Hayashi, K.; Nagamura, F.; Kataoka, K.; Imai, K. Treatment of primary and metastatic breast and pancreatic tumors upon intravenous delivery of a PRDM14-specific chimeric siRNA/nanocarrier complex. Int. J. Cancer 2021, 149, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Quiñonero, F.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Garcés, V.; Delgado-López, J.M.; Jiménez-Luna, C.; Perazzoli, G.; Melguizo, C.; Prados, J.; Ortíz, R. Combining Olaparib and Ascorbic Acid on Nanoparticles to Enhance the Drug Toxic Effects in Pancreatic Cancer. Int. J. Nanomed. 2023, 18, 5075–5093. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Z.; Yang, X.; Yuan, Z.; Wang, M.; Chen, C.; Xie, L.; Gao, Y.; Li, W.; Bai, Y.; et al. Smad4 regulates TGF-β1-mediated hedgehog activation to promote epithelial-to-mesenchymal transition in pancreatic cancer cells by suppressing Gli1 activity. Comput. Struct. Biotechnol. J. 2024, 23, 1189–1200. [Google Scholar] [CrossRef]
- Torres, C.; Linares, A.; Alejandre, M.J.; Palomino-Morales, R.J.; Delgado, J.R.; Perales, S. Interplay Between Gemcitabine and Erlotinib Over Pancreatic Adenocarcinoma Cells. Pancreas 2016, 45, 269–280. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Ijichi, H.; Mohri, D.; Tada, M.; Yamamoto, K.; Asaoka, Y.; Ikenoue, T.; Tateishi, K.; Nakai, Y.; Isayama, H.; et al. Erlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signals. Cancer Res. 2013, 73, 2221–2234. [Google Scholar] [CrossRef]
- Li, J.M.; Chen, W.; Wang, H.; Jin, C.; Yu, X.J.; Lu, W.Y.; Cui, L.; Fu, D.L.; Ni, Q.X.; Hou, H.M. Preparation of albumin nanospheres loaded with gemcitabine and their cytotoxicity against BXPC-3 cells in vitro. Acta Pharmacol. Sin. 2009, 30, 1337–1343. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Hoang, B.; Lohse, I.; Undzys, E.; Cao, P.; Do, T.; Gill, B.; Pintilie, M.; Hedley, D.; Li, S.D. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J. Control Release 2015, 206, 122–130. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, X.; Tao, W.; Kong, Y.; Huag, Y.; Zhang, Y.; Liu, R.; Jiang, L.; Tang, Y.; Yu, H.; et al. Regulation of pancreatic cancer microenvironment by an intelligent gemcitabine@nanogel system via in vitro 3D model for promoting therapeutic efficiency. J. Control Release 2020, 324, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, D.; Peng, Y.; Wang, S.; Hu, S.; Liu, M.; Ding, J.; Zhou, W. Metal organic framework coated MnO(2) nanosheets delivering doxorubicin and self-activated DNAzyme for chemo-gene combinatorial treatment of cancer. Int. J. Pharm. 2020, 585, 119513. [Google Scholar] [CrossRef] [PubMed]
- Shali, H.; Shabani, M.; Pourgholi, F.; Hajivalili, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Baradaran, B.; Movassaghpour Akbari, A.A.; Younesi, V.; Yousefi, M. Co-delivery of insulin-like growth factor 1 receptor specific siRNA and doxorubicin using chitosan-based nanoparticles enhanced anticancer efficacy in A549 lung cancer cell line. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhang, X.; Zhong, H.; Mu, J.; Li, X.; Liu, T.; Shi, X.; Liang, X.J.; Guo, S. Stromal Homeostasis-Restoring Nanomedicine Enhances Pancreatic Cancer Chemotherapy. Nano Lett. 2022, 22, 8744–8754. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal Nanoformulation as a Carrier for Curcumin and pEGCG-Study on Stability and Anticancer Potential. Nanomaterials 2022, 12, 1274. [Google Scholar] [CrossRef]
- Ko, A.H. Nanomedicine developments in the treatment of metastatic pancreatic cancer: Focus on nanoliposomal irinotecan. Int. J. Nanomed. 2016, 11, 1225–1235. [Google Scholar] [CrossRef]
- Frese, K.K.; Neesse, A.; Cook, N.; Bapiro, T.E.; Lolkema, M.P.; Jodrell, D.I.; Tuveson, D.A. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012, 2, 260–269. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Rao, D.D.; Luo, X.; Wang, Z.; Jay, C.M.; Brunicardi, F.C.; Maltese, W.; Manning, L.; Senzer, N.; Nemunaitis, J. KRAS mutant allele-specific expression knockdown in pancreatic cancer model with systemically delivered bi-shRNA KRAS lipoplex. PLoS ONE 2018, 13, e0193644. [Google Scholar] [CrossRef]
- Wan, Z.; Huang, H.; West, R.E., 3rd; Zhang, M.; Zhang, B.; Cai, X.; Zhang, Z.; Luo, Z.; Chen, Y.; Zhang, Y.; et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater. Today 2023, 62, 33–50. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, W.; Liang, C.; Shi, S.; Yu, X.; Chen, Q.; Sun, T.; Lu, Y.; Zhang, Y.; Guo, Q.; et al. Codelivery Nanosystem Targeting the Deep Microenvironment of Pancreatic Cancer. Nano Lett. 2019, 19, 3527–3534. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Zheng, S.; Mu, J.; Yu, N.; Guo, S. Tumor-penetrating iRGD facilitates penetration of poly(floxuridine-ketal)-based nanomedicine for enhanced pancreatic cancer therapy. J. Control Release 2024, 369, 444–457. [Google Scholar] [CrossRef]
- Vetvicka, D.; Sivak, L.; Jogdeo, C.M.; Kumar, R.; Khan, R.; Hang, Y.; Oupický, D. Gene silencing delivery systems for the treatment of pancreatic cancer: Where and what to target next? J. Control Release 2021, 331, 246–259. [Google Scholar] [CrossRef]
- Kumar, V.; Mondal, G.; Slavik, P.; Rachagani, S.; Batra, S.K.; Mahato, R.I. Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol. Pharm. 2015, 12, 1289–1298. [Google Scholar] [CrossRef]
- Daman, Z.; Montazeri, H.; Azizi, M.; Rezaie, F.; Ostad, S.N.; Amini, M.; Gilani, K. Polymeric Micelles of PEG-PLA Copolymer as a Carrier for Salinomycin Against Gemcitabine-Resistant Pancreatic Cancer. Pharm. Res. 2015, 32, 3756–3767. [Google Scholar] [CrossRef]
- Pittella, F.; Miyata, K.; Maeda, Y.; Suma, T.; Watanabe, S.; Chen, Q.; Christie, R.J.; Osada, K.; Nishiyama, N.; Kataoka, K. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. J. Control Release 2012, 161, 868–874. [Google Scholar] [CrossRef]
- Yu, M.; Zhao, M.; Yu, R.; Chu, S.; Xu, J.; Xia, M.; Wang, C. [Nanotechnology-mediated immunochemotherapy with Ingenol-3-Mebutate for Systematic Anti-tumor Effects]. J. Control Release 2019, 304, 242–258. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Hsiao, C.H.; Chow, D.S.; Koay, E.J.; Kang, Y.; Wen, X.; Huang, Q.; Ma, Y.; Bankson, J.A.; et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials 2018, 159, 215–228. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Xu, J.; Song, X.; Wan, Z.; Du, Y.; Ma, W.; Li, X.; Zhang, L.; Li, S. High Loading of Hydrophobic and Hydrophilic Agents via Small Immunostimulatory Carrier for Enhanced Tumor Penetration and Combinational Therapy. Theranostics 2020, 10, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.; Estévez, S.V.; Del Pilar Chantada, M. Inorganic nanoparticles in diagnosis and treatment of breast cancer. J. Biol. Inorg. Chem. 2018, 23, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Mardhian, D.F.; Storm, G.; Bansal, R.; Prakash, J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Control Release 2018, 290, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, D.; Cai, Y.; Zhu, L.; Xiao, Q.; Peng, W.; Chen, B. A multi-omic analysis reveals that Gamabufotalin exerts anti-hepatocellular carcinoma effects by regulating amino acid metabolism through targeting STAMBPL1. Phytomedicine 2024, 135, 156094. [Google Scholar] [CrossRef]
- Huo, D.; Liu, S.; Zhang, C.; He, J.; Zhou, Z.; Zhang, H.; Hu, Y. Hypoxia-Targeting, Tumor Microenvironment Responsive Nanocluster Bomb for Radical-Enhanced Radiotherapy. ACS Nano 2017, 11, 10159–10174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).