Prostate Cancer-Targeting Liposome Loaded with Zinc Ion-Coordinated Photosensitizer for Enhanced Chemo-Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Compound 2
2.2. Synthesis of Compound 3
2.3. Synthesis of Compound 4
2.4. Synthesis of TPEDPD
2.5. Preparation of Drug Loaded Liposome
2.6. Measurement of ROS Generation
2.7. Cell Culture
2.8. Cellular Uptake
2.9. In Vitro Cytotoxicity
2.10. PCI Enhanced Cytotoxicity
2.11. Biodistribution In Vivo
2.12. In Vivo Antitumor Efficacy
3. Results
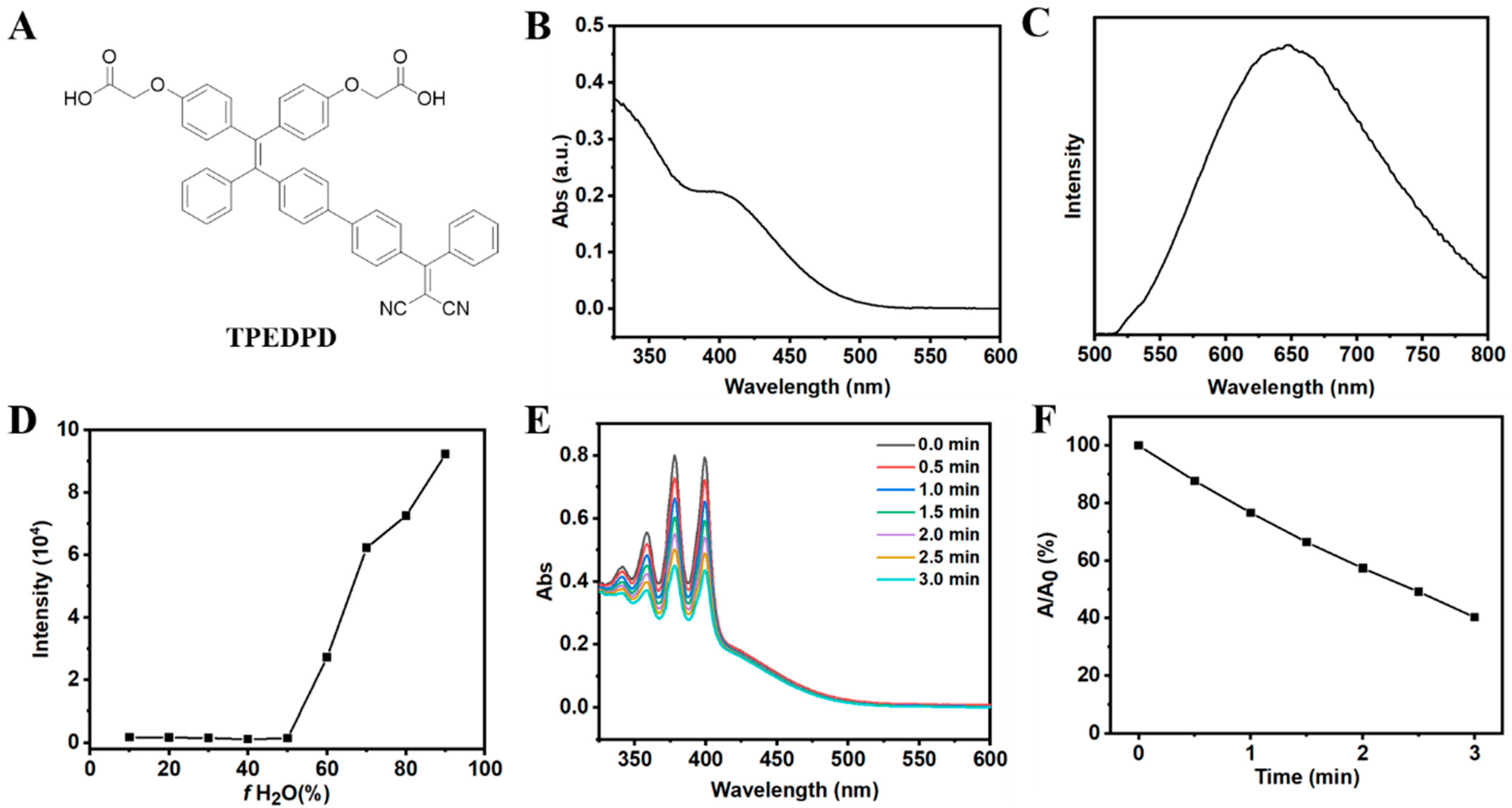
3.1. Synthesis and Characterization of AIE Photosensitizer (TPEDPD)
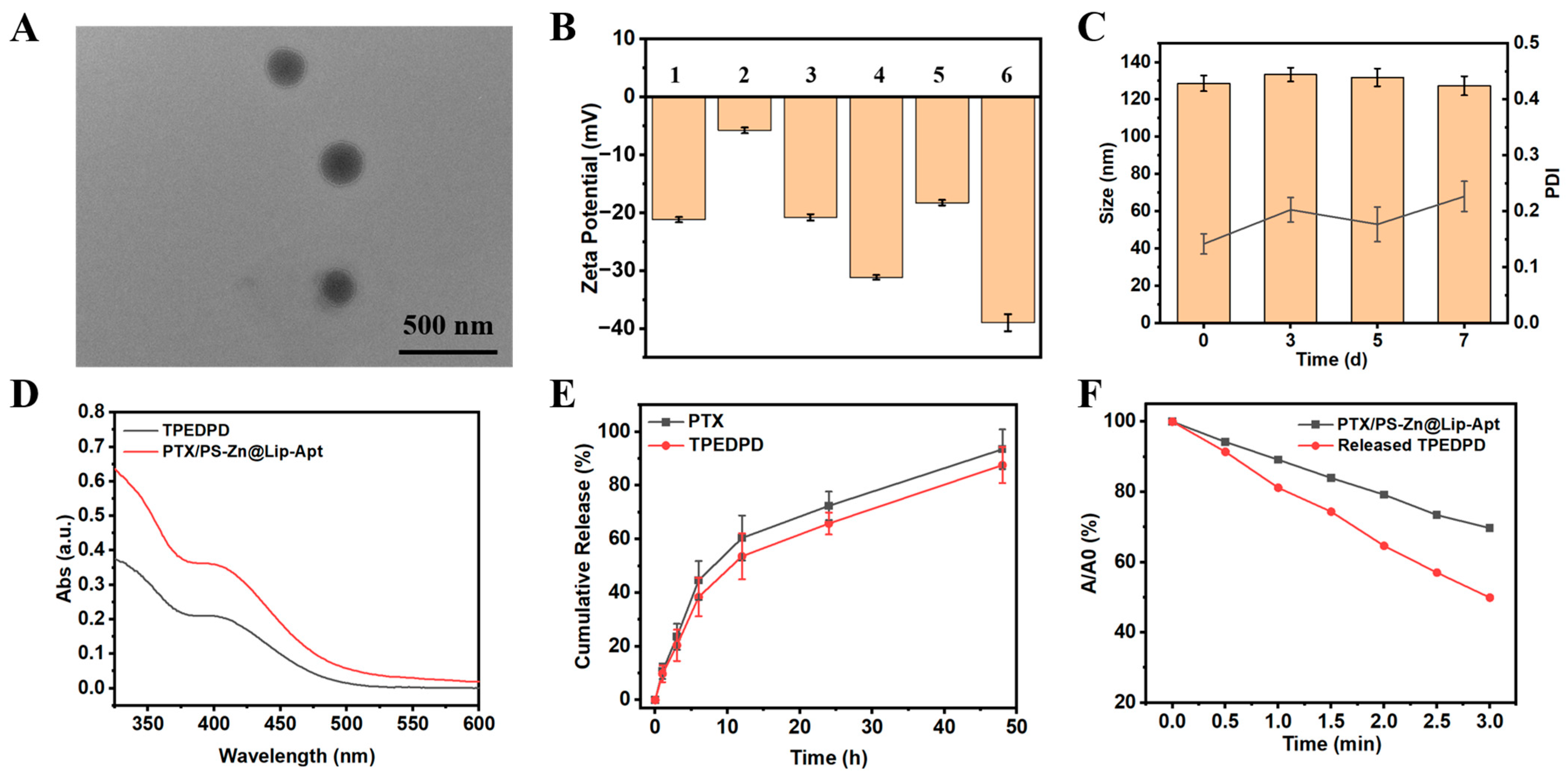
3.2. Preparation and Characterization of Liposome
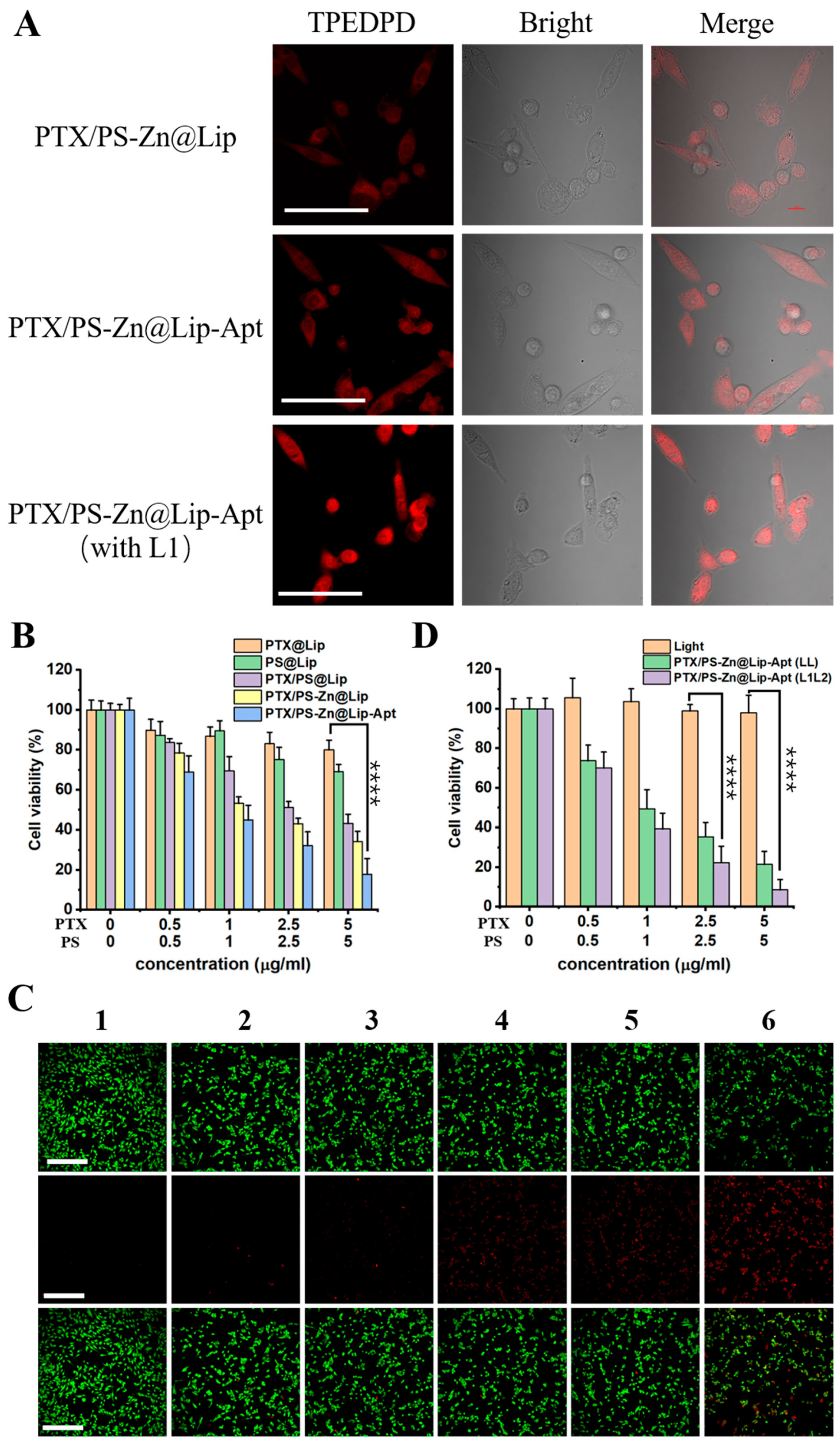
3.3. Cellular Uptake of PTX/PS-Zn@Lip-Apt NPs
3.4. In Vitro Therapeutic Effect
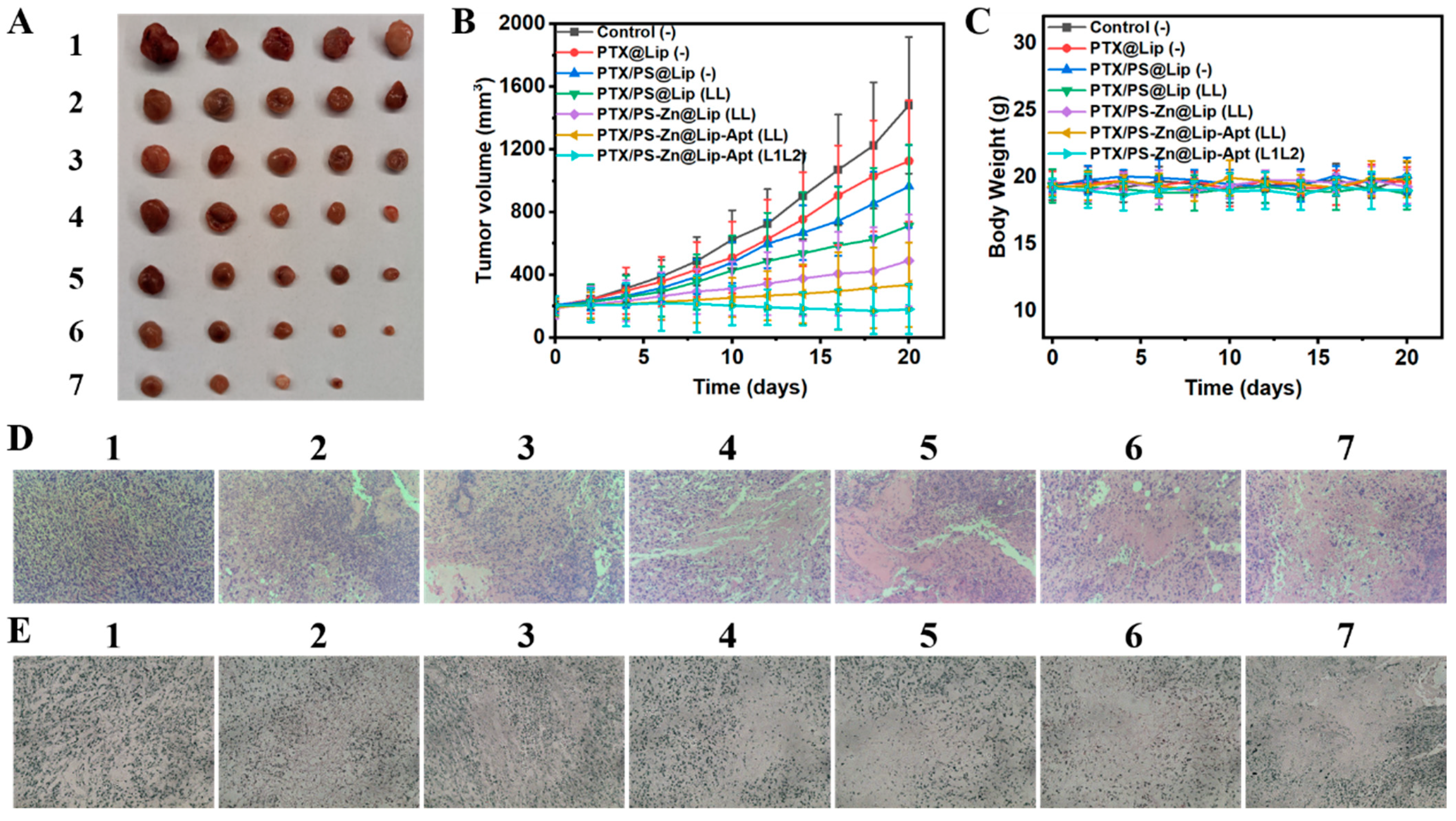
3.5. In Vivo Fluorescence Imaging
3.6. In Vivo Enhanced Anticancer Effect Based on the Combined Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCa | Prostate cancer |
| PTX | Paclitaxel |
| PCI | Photochemical internalization |
| PTT | Photothermal therapy |
| CDT | Chemodynamic therapy |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| Lip | Liposome |
| Zn | Zinc |
| ACQ | Aggregation-caused quenching |
| AIE | Aggregation-induced emission |
| AIEgens | AIE luminogens |
| RIM | Restriction of intramolecular motion |
| EPR | Enhanced permeability and retention |
| Apt | Aptamer |
| NCL | Nucleolin |
| DCM | Dichloromethane |
| TMS | Tetramethylsilane |
| DMSO | Dimethyl sulfoxide |
| DMF | Dimethylformamide |
| NMR | Nuclear magnetic resonance |
| PS | Photosensitizer |
| ABDA | 9,10-Anthracenediyl-bis(methylene)dimalonic acid |
| CLSM | Confocal laser scanning microscope |
| PBS | Phosphate buffer saline |
| PC3 | Human prostate cancer cells |
| CCK8 | Cell Counting Kit-8 |
| HR-MS | High-resolution mass spectroscopy |
| EEs | Encapsulation efficiencies |
| UV | Ultraviolet |
| vis | Visible |
| TEM | Transmission electron microscope |
| H&E | Hematoxylin and eosin |
| TUNEL | TdT-mediated dUTP Nick-End Labeling |
| TPEDPD | 2,2′-(((2-(4′-(2,2-dicyano-1-phenylvinyl)-[1,1′-biphenyl]-4-yl)-2-phenylethene-1,1-diyl)bis(4,1-phenylene))bis(oxy))diacetic acid |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of Androgen-Independent Prostate Cancer. eJIFCC Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2014, 25, 42–54. [Google Scholar]
- Xu, H.; Sheng, G.; Lu, L.; Wang, C.; Zhang, Y.; Feng, L.; Meng, L.; Min, P.; Zhang, L.; Wang, Y.; et al. GRPr-mediated photothermal and thermodynamic dual-therapy for prostate cancer with synergistic anti-apoptosis mechanism. Nanoscale 2021, 13, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zou, C.; Qin, S.; Meng, J.; Keller, E.T.; Zhang, J.; Lu, Y. Prostate cancer tends to metastasize in the bone-mimicking microenvironment via activating NF-kappa B signaling. J. Biomed. Res. 2018, 32, 343–353. [Google Scholar]
- Sun, Q.; Liu, F.; Wen, Z.; Xia, J.; Li, H.; Xu, Y.; Sun, S. Combined effect of heat shock protein inhibitor geldanamycin and free radicals on photodynamic therapy of prostate cancer. J. Mater. Chem. B 2022, 10, 1369–1377. [Google Scholar] [CrossRef]
- Jiang, R.; Dai, J.; Dong, X.; Wang, Q.; Meng, Z.; Guo, J.; Yu, Y.; Wang, S.; Xia, F.; Zhao, Z.; et al. Improving Image-Guided Surgical and Immunological Tumor Treatment Efficacy by Photothermal and Photodynamic Therapies Based on a Multifunctional NIR AIEgen. Adv. Mater. 2021, 33, 2101158. [Google Scholar] [CrossRef]
- He, X.; Situ, B.; Gao, M.; Guan, S.; He, B.; Ge, X.; Li, S.; Tao, M.; Zou, H.; Tang, B.Z.; et al. Stereotactic Photodynamic Therapy Using a Two-Photon AIE Photosensitizer. Small 2019, 15, 1905080. [Google Scholar] [CrossRef]
- Huang, J.; He, B.; Zhang, Z.; Li, Y.; Kang, M.; Wang, Y.; Li, K.; Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens Married to 2D Black Phosphorus Nanosheets for Highly Efficient Multimodal Theranostics. Adv. Mater. 2020, 32, 2003382. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Zhuang, J.; Yang, H.; Li, Y.; Wang, B.; Li, N.; Zhao, N. Efficient photosensitizers with aggregation-induced emission characteristics for lysosome- and Gram-positive bacteria-targeted photodynamic therapy. Chem. Commun. 2020, 56, 2630–2633. [Google Scholar]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [PubMed]
- Li, Y.; Wu, Q.; Kang, M.; Song, N.; Wang, D.; Tang, B.Z. Boosting the photodynamic therapy efficiency by using stimuli-responsive and AIE-featured nanoparticles. Biomaterials 2020, 232, 119749. [Google Scholar]
- Yuan, G.; Lv, C.; Liang, J.; Zhong, X.; Li, Y.; He, J.; Zhao, A.; Li, L.; Shao, Y.; Zhang, X.; et al. Molecular Engineering of Efficient Singlet Oxygen Generators with Near-Infrared AIE Features for Mitochondrial Targeted Photodynamic Therapy. Adv. Funct. Mater. 2021, 31, 2104026. [Google Scholar]
- Wu, W.; Mao, D.; Xu, S.; Panahandeh-Fard, M.; Duan, Y.; Hu, F.; Kong, D.; Liu, B. Precise Molecular Engineering of Photosensitizers with Aggregation-Induced Emission over 800 nm for Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1901791. [Google Scholar]
- Li, Q.; Li, Y.; Min, T.; Gong, J.; Du, L.; Phillips, D.L.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; et al. Time-Dependent Photodynamic Therapy for Multiple Targets: A Highly Efficient AIE-Active Photosensitizer for Selective Bacterial Elimination and Cancer Cell Ablation. Angew. Chem. Int. Ed. 2020, 59, 9470–9477. [Google Scholar]
- Peng, Q.; Shuai, Z. Molecular mechanism of aggregation-induced emission. Aggregate 2021, 2, e91. [Google Scholar]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, 1801350. [Google Scholar]
- Hong, Y.; Zhang, P.; Wang, H.; Yu, M.; Gao, Y.; Chen, J. Photoswitchable AIE nanoprobe for lysosomal hydrogen sulfide detection and reversible dual-color imaging. Sens. Actuators B Chem. 2018, 272, 340–347. [Google Scholar]
- Ding, W.-Q.; Yu, H.-J.; Lind, S.E. Zinc-binding compounds induce cancer cell death via distinct modes of action. Cancer Lett. 2008, 271, 251–259. [Google Scholar] [PubMed]
- Fanzo, J.C.; Reaves, S.K.; Cui, L.B.; Zhu, L.; Wu, J.Y.J.; Wang, Y.R.; Lei, K.Y. Zinc status affects p53, gadd45, and c-fos expression and caspase-3 activity in human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2001, 281, C751–C757. [Google Scholar] [PubMed]
- Gao, L.; Teng, R.; Zhang, S.; Zhou, Y.; Luo, M.; Fang, Y.; Lei, L.; Ge, B. Zinc Ion-Stabilized Aptamer-Targeted Black Phosphorus Nanosheets for Enhanced Photothermal/Chemotherapy Against Prostate Cancer. Front. Bioeng. Biotechnol. 2020, 8, 769. [Google Scholar]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer. 2006, 5, 17. [Google Scholar]
- Zhao, Y.; Tan, Y.; Dai, J.; Wang, B.; Li, B.; Guo, L.; Cui, J.; Wang, G.; Li, W.; Cai, L. Zinc deficiency exacerbates diabetic down-regulation of Akt expression and function in the testis: Essential roles of PTEN, PTP1B and TRB3. J. Nutr. Biochem. 2012, 23, 1018–1026. [Google Scholar]
- Makhov, P.; Kutikov, A.; Golovine, K.; Uzzo, R.G.; Canter, D.J.; Kolenko, V.M. Docetaxel-Mediated Apoptosis in Myeloid Progenitor TF-I Cells Is Mitigated by Zinc: Potential Implication for Prostate Cancer Therapy. Prostate 2011, 71, 1413–1419. [Google Scholar]
- Franklin, R.B.; Ma, J.; Zou, J.; Guan, Z.; Kukoyi, B.I.; Feng, P.; Costello, L.C. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J. Inorg. Biochem. 2003, 96, 435–442. [Google Scholar]
- Huang, L.; Kirschke, C.P.; Zhang, Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: A possible role in prostate cancer progression. Cancer Cell Int. 2006, 6, 10. [Google Scholar]
- Sun, Y.; Qin, L.; Yang, Y.; Gao, J.; Zhang, Y.; Wang, H.; Wu, Q.; Xu, B.; Liu, H. Zinc-Based ROS Amplifiers Trigger Cancer Chemodynamic/Ion Interference Therapy Through Self-Cascade Catalysis. Small 2024, 20, 2402320. [Google Scholar]
- Xie, Y.; Wang, J.; Li, L.; Wang, M.; Sun, J.; Chang, J.; Lin, J.; Li, C. A Metal Chelation Therapy to Effectively Eliminate Breast Cancer and Intratumor Bacteria While Suppressing Tumor Metastasis by Copper Depletion and Zinc Ions Surge. Angew. Chem. Int. Ed. 2025, 64, e202417592. [Google Scholar]
- Zhang, P.; Li, Y.; Tang, X.; Guo, R.; Li, J.; Chen, Y.Y.; Guo, H.; Su, J.; Sun, L.; Liu, Y. Zinc enhances chemosensitivity to paclitaxel in PC-3 prostate cancer cells. Oncol. Rep. 2018, 40, 2269–2277. [Google Scholar]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar]
- Allen, T.M.; Mumbengegwi, D.R.; Charrois, G.J.R. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin. Cancer. Res. 2005, 11, 3567–3573. [Google Scholar]
- Moosavian, S.A.; Sahebkar, A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer Lett. 2019, 448, 144–154. [Google Scholar]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar]
- Hagtvet, E.; Evjen, T.J.; Olsen, D.R.; Fossheim, S.L.; Nilssen, E.A. Ultrasound enhanced antitumor activity of liposomal doxorubicin in mice. J. Drug Target. 2011, 19, 701–708. [Google Scholar]
- Selbo, P.K.; Weyergang, A.; Hogset, A.; Norum, O.-J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release 2010, 148, 2–12. [Google Scholar]
- Lu, H.-L.; Syu, W.-J.; Nishiyama, N.; Kataoka, K.; Lai, P.-S. Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo. J. Control. Release 2011, 155, 458–464. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Tang, Z.; Xiao, D.; Chen, X.; Zhu, Y. Prostate Cancer-Targeting Liposome Loaded with Zinc Ion-Coordinated Photosensitizer for Enhanced Chemo-Photodynamic Therapy. Pharmaceutics 2025, 17, 448. https://doi.org/10.3390/pharmaceutics17040448
Gao L, Tang Z, Xiao D, Chen X, Zhu Y. Prostate Cancer-Targeting Liposome Loaded with Zinc Ion-Coordinated Photosensitizer for Enhanced Chemo-Photodynamic Therapy. Pharmaceutics. 2025; 17(4):448. https://doi.org/10.3390/pharmaceutics17040448
Chicago/Turabian StyleGao, Li, Zhisheng Tang, Dongming Xiao, Xu Chen, and Yizhun Zhu. 2025. "Prostate Cancer-Targeting Liposome Loaded with Zinc Ion-Coordinated Photosensitizer for Enhanced Chemo-Photodynamic Therapy" Pharmaceutics 17, no. 4: 448. https://doi.org/10.3390/pharmaceutics17040448
APA StyleGao, L., Tang, Z., Xiao, D., Chen, X., & Zhu, Y. (2025). Prostate Cancer-Targeting Liposome Loaded with Zinc Ion-Coordinated Photosensitizer for Enhanced Chemo-Photodynamic Therapy. Pharmaceutics, 17(4), 448. https://doi.org/10.3390/pharmaceutics17040448







