Topical Ocular Drug Delivery: The Impact of Permeation Enhancers
Abstract
1. Introduction
2. Ocular Anatomy and Physiology
2.1. Ocular Globe
2.2. Precorneal Tear Film (PTF)
2.3. Cornea
2.4. Conjunctiva
2.5. Sclera
2.6. Nasolacrimal Drainage System
3. Ocular Pharmacokinetics After Topical Administration
4. Assessment of Drug Permeability
4.1. In Vitro Models
4.1.1. Cell Culture Models
Apparent Permeability Coefficient (Papp)
Transepithelial Electrical Resistance (TEER)
4.2. Ex Vivo Models
4.3. In Vivo Models
5. Enhancement of Ocular Membrane Permeability: A Strategy to Improve Topical Ocular Drug Delivery
5.1. Cyclodextrins (CDs)
5.2. Chelating Agents
5.3. Crown Ethers (CEs)
5.4. Chitosan (CH)
5.5. Surface-Active Agents (SAAs)
5.5.1. Non-Ionic SAAs
Polyoxyethylene Alkyl Derivatives (PADs)
Polyoxyethylene Sorbitan Esters (Tween)
Sorbitan Fatty Acid Esters (Spans)
d-α-Tocopheryl Poly(Ethylene Glycol) 1000 Succinate (VE-TPGS 1000)
Labrasol®
N-Methyl-2-Pyrrolidone (NMP)
Lecithin
5.5.2. Cationic Surfactants
Benzalkonium Chloride (BAC)
Chlorobutanol (CB)
Cetylpyridinium Chloride (CPC)
Chlorhexidine (CX)
5.5.3. Bile Acids and Salts
5.5.4. Glycosides
Saponins (SPs)
Digitonin (DG)
Escin
5.6. Azone (1-Dodecylazacycloheptan-2-One)
5.7. Cell-Penetrating Peptides
5.7.1. Penetratin (PNT)
5.7.2. Trans-Activator of Transcription (TAT) Protein Transduction Domain
| Cargo and Molecular Weight | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| Human Acidic Fibroblast Growth Factor (aFGF19-154 or FGF-1) (16.0 kDa) | TAT aFGF-His showed a rapid ocular penetration, detected in retina within 30 min, mediating strong protection against retinal IR injury. | Not mentioned. | [263] |
| Endostatin (20 kDa) | Significantly enhanced penetration to retina and choroid. Micropinocytosis was the dominant uptake mechanism for TAT PTD. | Not mentioned. | [420] |
| Endostatin arginine–glycine–aspartic (20.3 kDa) | Improved permeability and higher inhibition of neovascularization. | Not mentioned. | [483] |
| Flurbiprofen (0.24 kDa) | TAT-functionalized, flurbiprofen-loaded liposomes reduced the drug loss rate from the eye surface and enhanced the intraocular delivery of flurbiprofen. | No toxicity observed. | [495] |
5.7.3. Polyarginines (PLAs)
5.7.4. Pep-1
5.8. Cytochalasins
5.9. Terpenes
5.9.1. Borneol
5.9.2. Menthol
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. World Report on Vision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [PubMed]
- Le Bourlais, C.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic Drug Delivery Systems—Recent Advances. Prog. Retin. Eye Res. 1997, 17, 33–58. [Google Scholar]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems—An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Anatomy of the Eye and the Role of Ocular Mucosa in Drug Delivery. Mucoadhesive Mater. Drug Deliv. Syst. 2014, 1, 40–59. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Lin, S.; Ge, C.; Wang, D.; Xie, Q.; Wu, B.; Wang, J.; Nan, K.; Zheng, Q.; Chen, W. Overcoming the Anatomical and Physiological Barriers in Topical Eye Surface Medication Using a Peptide-Decorated Polymeric Micelle. ACS Appl. Mater. Interfaces 2019, 11, 39603–39612. [Google Scholar]
- Hamalainen, K.M.; Kananen, K.; Auriola, S.; Kontturi, K.; Urtti, A. Characterization of Paracellular and Aqueous Penetration Routes in Cornea, Conjunctiva, and Sclera. Investig. Ophthalmol. Vis. Sci. 1997, 38, 627–634. [Google Scholar]
- Lee, V.H.L.; Robinson, J.R. Mechanistic and Quantitative Evaluation of Precorneal Pilocarpine Disposition in Albino Rabbits. J. Pharm. Sci. 1979, 68, 673–684. [Google Scholar]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [PubMed]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Zhang, C.; Zhang, B. Gelucire44/14 as a novel absorption enhancer for drugs with different hydrophilicities: In vitro and in vivo improvement on transcorneal permeation. J. Pharm. Sci. 2011, 100, 3186–3195. [Google Scholar] [PubMed]
- Kaur, I.P.; Smitha, R. Penetration Enhancers and Ocular Bioadhesives: Two New Avenues for Ophthalmic Drug Delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. [Google Scholar]
- Thareja, A.; Hughes, H.; Alvarez-Lorenzo, C.; Hakkarainen, J.J.; Ahmed, Z. Penetration enhancers for topical drug delivery to the ocular posterior segment—A systematic review. Pharmaceutics 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Nichols, J.J. Analysis of Meibum and Tear Lipids. Ocul. Surf. 2012, 10, 230–250. [Google Scholar] [PubMed]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional Aspects of the Tear Film Lipid Layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef]
- Cwiklik, L. Tear film lipid layer: A molecular level view. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2421–2430. [Google Scholar] [CrossRef]
- Foulks, G.N.; Bron, A.J. Meibomian gland dysfunction: A clinical scheme for description, diagnosis, classification, and grading. Ocul. Surf. 2003, 1, 107–126. [Google Scholar] [PubMed]
- Sun, X. Mechanism of Tear Electrolytes Concentration in Tear Formation on Ocular Surface. J. Eye Dis. Disord. 2023, 8, 197. [Google Scholar]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7012. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, G.; Zhang, D. Cloning, characterization, and production of a novel lysozyme by different expression hosts. J. Microbiol. Biotechnol. 2014, 24, 1405–1412. [Google Scholar]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar]
- Nautscher, N.; Bauer, A.; Steffl, M.; Amselgruber, W.M. Comparative morphological evaluation of domestic animal cornea. Vet. Ophthalmol. 2016, 19, 297–304. [Google Scholar]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the Cornea: Structure, Function, and Development. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; pp. 7–23. [Google Scholar]
- Yi, X.-J.; Wang, Y.; Yu, F.-S.X. Corneal Epithelial Tight Junctions and Their Response to Lipopolysaccharide Challenge. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4093–4100. [Google Scholar]
- Møller-Pedersen, T. Keratocyte reflectivity and corneal haze. Exp. Eye Res. 2004, 78, 553–560. [Google Scholar]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar]
- Fini, M.E.; Stramer, B.M. How the Cornea Heals: Cornea-Specific Repair Mechanisms Affecting Surgical Outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Tuft, S.J.; Coster, D.J. The Corneal Endothelium. Eye 1990, 4, 389–424. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and Clonal Analysis of Stem Cells and Their Differentiated Progeny in the Human Ocular Surface. J. Cell Biol. 1999, 145, 769–782. [Google Scholar] [CrossRef]
- Nichols, B.A. Conjunctiva. Microsc. Res. Tech. 1996, 33, 296–319. [Google Scholar] [CrossRef]
- Rusciano, G.; Zito, G.; Pesce, G.; Del Prete, S.; Cennamo, G.; Sasso, A. Assessment of conjunctival microvilli abnormality by micro-Raman analysis. J. Biophotonics 2016, 9, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hornof, M.; Toropainen, E.; Urtti, A. Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 2005, 60, 207–225. [Google Scholar] [CrossRef]
- Keeley, F.W.; Morin, J.D.; Vesely, S. Characterization of Collagen from Normal Human Sclera. Exp. Eye Res. 1984, 38, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M. The cornea and sclera. In Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008; pp. 359–396. [Google Scholar]
- Ross, R.; Bornstein, P. Elastic Fibers in the Body. Sci. Am. 1971, 224, 44–53. [Google Scholar]
- You, J.; Willcox, M.D.; Madigan, M.C.; Wasinger, V.; Schiller, B.; Walsh, B.J.; Graham, P.H.; Kearsley, J.H.; Li, Y. Tear Fluid Protein Biomarkers. In Advances in Clinical Chemistry; Academic Press: Cambridge, MA, USA, 2013; pp. 151–196. [Google Scholar]
- Watson, P.G.; Young, R.D. Scleral structure, organisation and disease. A review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef]
- Holly, F.J.; Lemp, M.A. Tear Physiology and Dry Eyes. Surv. Ophthalmol. 1977, 22, 69–87. [Google Scholar]
- Davis, K.; Carter, R.; Tully, T.; Negulescu, I.; Storey, E. Comparative evaluation of aqueous humor viscosity. Vet. Ophthalmol. 2015, 18, 50–58. [Google Scholar] [PubMed]
- Spreull, J.S.A. Symposium: The Corneal Ulcer*-I Anatomy and Physiology of the Cornea of the Dog. J. Small Anim. Pract. 1966, 7, 429–438. [Google Scholar]
- Labelle, P. The Eye. In Pathologic Basis of Veterinary Disease Expert Consult; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1265–1318.e1. [Google Scholar]
- Wilson, S.E. Bowman’s layer in the cornea- structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Gukasyan, H.J.; Kim, K.-J.; Lee, V.H. The Conjunctival Barrier in Ocular Drug Delivery. In Drug Absorption Studies: In Situ, In Vitro and In Silico Models; Springer: Boston, MA, USA, 2008; pp. 307–320. [Google Scholar]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.G.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. CLEAR—Anatomy and physiology of the anterior eye. Contact Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [PubMed]
- Boote, C.; Sigal, I.A.; Grytz, R.; Hua, Y.; Nguyen, T.D.; Girard, M.J.A. Scleral structure and biomechanics. Prog. Retin. Eye Res. 2020, 78, 100773. [Google Scholar] [CrossRef]
- Maliborski, A.; Różycki, R. Diagnostic imaging of the nasolacrimal drainage system. Part I. Radiological anatomy of lacrimal pathways. Physiology of tear secretion and tear outflow. Med. Sci. Monit. 2014, 20, 628–638. [Google Scholar]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar]
- Nagataki, S.; Mishima, S. Pharmacokinetics of instilled drugs in the human eye. Int. Ophthalmol. Clin. 1980, 20, 33–49. [Google Scholar]
- Mishima, S.; Gasset, A.; Klyce, S.D.; Baum, J.L. Determination of tear volume and tear flow. Investig. Ophthalmol. 1966, 5, 264–276. [Google Scholar]
- Takahashi, Y.; Kakizaki, H.; Nakano, T.; Asamoto, K.; Ichinose, A.; Iwaki, M. Anatomy of the vertical lacrimal canaliculus and lacrimal punctum: A macroscopic study. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 384–386. [Google Scholar] [PubMed]
- Tucker, N.A.; Tucker, S.M.; Linberg, J.V. The Anatomy of the Common Canaliculus. Arch. Ophthalmol. 1996, 114, 1231–1234. [Google Scholar] [PubMed]
- Mouly, S.; Mahé, I.; Haouchine, B.; Sanson-Le-Pors, M.J.; Blain, P.; Tillet, Y.; Dewailly, J.; Mongold, J.J.; Bergmann, J.F. Pharmacodynamics of a new ophthalmic mydriatic insert in healthy volunteers: Potential alternative as drug delivery system prior to cataract surgery. Basic Clin. Pharmacol. Toxicol. 2006, 98, 547–554. [Google Scholar] [PubMed]
- Ghate, D.; Edelhauser, H.F. Barriers to Glaucoma Drug Delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar]
- Maurice, D.M. Factors influencing the penetration of topically applied drugs. Int. Ophthalmol. Clin. 1980, 20, 21–32. [Google Scholar]
- Karki, R.; Meena, M.; Prakash, T.; Rajeswari, T.; Goli, D.; Kumar, S. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J. Adv. Pharm. Technol. Res. 2011, 2, 192–194. [Google Scholar]
- Shell, J.W. Pharmacokinetics of topically applied ophthalmic drugs. Surv. Ophthalmol. 1982, 26, 207–218. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Patton, T.F.; Robinson, J.R. Quantitative Precorneal Disposition of Topically Applied Pilocarpine Nitrate in Rabbit Eyes. J. Pharm. Sci. 1976, 65, 1295–1301. [Google Scholar] [CrossRef]
- Chrai, S.S.; Makoid, M.C.; Eriksen, S.P.; Robinson, J.R. Drop Size and Initial Dosing Frequency Problems of Topically Applied Ophthalmic Drugs. J. Pharm. Sci. 1974, 63, 333–338. [Google Scholar] [CrossRef]
- Van Santvliet, L.; Ludwig, A. Determinants of eye drop size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal Human Tear pH by Direct Measurement. Arch. Ophthalmol. 1981, 99, 302–303. [Google Scholar] [CrossRef]
- Beckwith-Cohen, B.; Elad, D.; Bdolah-Abram, T.; Ofri, R. Comparison of tear pH in dogs, horses, and cattle. Vet. Ophthalmol. 2014, 17, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Brennan, F.A. The effect of solution tonicity on the eye. Clin. Exp. Optom. 1993, 76, 115–120. [Google Scholar] [CrossRef]
- Maurice, D. The Tonicity of an Eye Drop and Its Dilution by Tears. Exp. Eye Res. 1971, 11, 30–33. [Google Scholar] [CrossRef]
- Green-Church, K.B.; Nichols, K.K.; Kleinholz, N.M.; Zhang, L.; Nichols, J.J. Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis. 2008, 14, 456–470. [Google Scholar]
- Kim, D.W.; Lee, S.H.; Ku, S.K.; Cho, S.H.; Cho, S.W.; Yoon, G.H.; Hwang, H.S.; Park, J.; Eum, W.S.; Kwon, O.S.; et al. Transduced PEP-1-FK506BP ameliorates corneal injury in Botulinum toxin A-induced dry eye mouse model. BMB Rep. 2013, 46, 124–129. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Vellonen, K.S.; Palmgrén, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hämäläinen, K.M.; Auriola, S.; Urtti, A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar]
- Ranta, V.P.; Toropainen, E.; Talvitie, A.; Auriola, S.; Urtti, A. Simultaneous determination of eight β-blockers by gradient high-performance liquid chromatography with combined ultraviolet and fluorescence detection in corneal permeability studies in vitro. J. Chromatogr. B 2002, 772, 81–87. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture Model of Human Corneal Epithelium for Prediction of Ocular Drug Absorption. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar]
- Khurana, V.; Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Functional characterization and molecular identification of Vitamin C transporter (SVCT2) in human corneal epithelial (HCEC) and retinal pigment epithelial (D407) cells. Curr. Eye Res. 2015, 40, 457–469. [Google Scholar]
- Zhou, X.; Li, X.; Xu, J.; Cheng, Y.; Cao, F. Latanoprost-loaded cyclodextrin microaggregate suspension eye drops for enhanced bioavailability and stability. Eur. J. Pharm. Sci. 2021, 159, 105758. [Google Scholar] [CrossRef]
- Kawazu, K.; Yamada, K.; Nakamura, M.; Ota, A. Characterization of Cyclosporin A Transport in Cultured Rabbit Corneal Epithelial Cells: P-Glycoprotein Transport Activity and Binding to Cyclophilin. Investig. Ophthalmol. Vis. Sci. 2000, 40, 1738–1744. [Google Scholar]
- Kawazu, K.; Shiono, H.; Tanioka, H.; Ota, A.; Ikuse, T.; Takashina, H.; Kawashima, Y. Beta adrenergic antagonist permeation across cultured rabbit corneal epithelial cells grown on permeable supports. Curr. Eye Res. 1998, 17, 125–131. [Google Scholar] [PubMed]
- Kawazu, K.; Midori, Y.; Ota, A. Cultured Rabbit Corneal Epithelium Elicits Levofloxacin Absorption and Secretion. J. Pharm. Pharmacol. 1999, 51, 791–796. [Google Scholar] [PubMed]
- Enríquez-de-Salamanca, A.; Calder, V.; Gao, J.; Galatowicz, G.; García-Vázquez, C.; Fernández, I.; Stern, M.E.; Diebold, Y.; Calonge, M. Cytokine responses by conjunctival epithelial cells: An in vitro model of ocular inflammation. Cytokine 2008, 44, 160–167. [Google Scholar]
- Civiale, C.; Paladino, G.; Marino, C.; Trombetta, F.; Pulvirenti, T.; Enea, V. Multilayer primary epithelial cell culture from bovine conjunctiva as a model for in vitro toxicity tests. Ophthalmic Res. 2003, 35, 126–136. [Google Scholar]
- Palumbo, P.; Picchini, U.; Beck, B.; Van Gelder, J.; Delbar, N.; DeGaetano, A. A general approach to the apparent permeability index. J. Pharmacokinet. Pharmacodyn. 2008, 35, 235–248. [Google Scholar]
- Resende, A.P.; Silva, B.; Braz, B.S.; Nunes, T.; Gonçalves, L.; Delgado, E. Ex vivo permeation of erythropoietin through porcine conjunctiva, cornea, and sclera. Drug Deliv. Transl. Res. 2017, 7, 625–631. [Google Scholar]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar]
- Powell, D.W. Barrier function of epithelia. Am. J. Physiol. 1981, 241, G275–G288. [Google Scholar] [PubMed]
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 582, 119020. [Google Scholar] [CrossRef]
- Liu, J.; Fu, S.; Wei, N.; Hou, Y.; Zhang, X.; Cui, H. The effects of combined menthol and borneol on fluconazole permeation through the cornea ex vivo. Eur. J. Pharmacol. 2012, 688, 1–5. [Google Scholar]
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703. [Google Scholar] [PubMed]
- Bhosale, V.A.; Srivastava, V.; Valamla, B.; Yadav, R.; Singh, S.B.; Mehra, N.K. Preparation and Evaluation of Modified Chitosan Nanoparticles using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics 2022, 14, 2802. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.K.; Majumdar, D.K. Effect of Formulation Factors on In Vitro Permeation of Moxifloxacin from Aqueous Drops Through Excised Goat, Sheep, and Buffalo Corneas. AAPS PharmSciTech 2006, 7, E1–E8. [Google Scholar]
- Barbalho, G.N.; Falcão, M.A.; Lopes, J.M.S.; Lopes, J.M.; Contarato, J.L.A.; Gelfuso, G.M.; Cunha-Filho, M.; Gratieri, T. Dynamic Ex Vivo Porcine Eye Model to Measure Ophthalmic Drug Penetration Under Simulated Lacrimal Flow. Pharmaceutics 2023, 15, 2325. [Google Scholar] [CrossRef]
- Zeiss, C.J. Translational models of ocular disease. Vet. Ophthalmol. 2013, 16, 15–33. [Google Scholar]
- del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar]
- Zernii, E.Y.; Baksheeva, V.E.; Iomdina, E.N.; Averina, O.A.; Permyakov, S.E.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I. Rabbit Models of Ocular Diseases: New Relevance for Classical Approaches. CNS Neurol. Disord. Drug Targets 2016, 15, 267–291. [Google Scholar]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Shimizu, S.; Ochiai, Y.; Kamijima, K.; Takai, N.; Watanabe, S.; Aihara, M. Development and characterization of a chronic high intraocular pressure model in New Zealand white rabbits for glaucoma research. Exp. Eye Res. 2024, 239, 109973. [Google Scholar] [CrossRef]
- Chen, W.L.; Lin, C.T.; Lin, N.T.; Tu, I.H.; Li, J.W.; Chow, L.P.; Liu, K.R.; Hu, F.R. Subconjunctival injection of bevacizumab (Avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1659–1665. [Google Scholar]
- Chen, J.; Ding, X.; Du, W.; Tang, X.; Yu, W.Z. Inhibition of corneal neovascularization by topical application of nintedanib in rabbit models. Int. J. Ophthalmol. 2021, 14, 1666–1673. [Google Scholar]
- Ashton, P. Huge Therapeutic Advances: Bigger Drug Delivery Opportunities. Ophthalmic Drug Deliv. 2015, 1, 4–6. [Google Scholar]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Rupenthal, I.D. Ocular Drug Delivery Technologies: Exciting Times Ahead. Ophthalmic Drug Deliv. 2015, 1, 7–11. [Google Scholar]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 323, 121500. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Effect of Cyclodextrins on Topical Drug Delivery to the Eye. Drug Dev. Ind. Pharm. 1997, 23, 473–481. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical Applications of Cyclodextrins. 2. In Vivo Drug Delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Centkowska, K. Use of cyclodextrins in topical formulations: Practical aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Owen, S.C. Handbook of Pharmaceutical Excipients, 5th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Vincze, A.; Facskó, R.; Budai-Szűcs, M.; Katona, G.; Gyarmati, B.; Csorba, A.; Zelkó, R.; Nagy, Z.Z.; Szente, L.; Balogh, G.T. Cyclodextrin-enabled nepafenac eye drops with improved absorption open a new therapeutic window. Carbohydr. Polym. 2023, 310, 120717. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Supramolecular cyclodextrin complex: Diversity, safety, and applications in ocular therapeutics. Exp. Eye Res. 2019, 189, 107829. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Maw, P.D.; Loftsson, T.; Jansook, P. A Current Overview of Cyclodextrin-Based Nanocarriers for Enhanced Antifungal Delivery. Pharmaceuticals 2022, 15, 1447. [Google Scholar] [CrossRef]
- Stefánsson, E.; Loftsson, T. Microspheres and nanotechnology for drug delivery. In Retinal Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2010; pp. 86–90. [Google Scholar]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef]
- Frijlink, H.W.; Eissens, A.C.; Schoonen, A.J.M.; Lerk, C.F. The effects of cyclodextrins on drug absorption II. In vivo observations. Int. J. Pharm. 1990, 64, 195–205. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Aqueous eye drops containing drug/cyclodextrin nanoparticles deliver therapeutic drug concentrations to both anterior and posterior segment. Acta Ophthalmol. 2022, 100, 7–25. [Google Scholar] [CrossRef]
- Fang, G.; Zhao, R.; Zhu, L.; Wang, Q.; Peng, S.; Kang, L.; Lu, H.; Zhang, G.; Tang, B. Nanoemulsion-based pseudopolyrotaxane hydrogel for enhanced corneal bioavailability and treatment of corneal inflammation. J. Control. Release 2025, 379, 14–29. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, B.; Deng, Q.; Wen, Y.; Feng, S.; Duan, C.; Zhao, B.; Li, H.; Gao, Y.; Ban, J. Polymer Nanoparticles with 2-HP-β-Cyclodextrin for Enhanced Retention of Uptake into HCE-T Cells. Molecules 2024, 29, 658. [Google Scholar] [CrossRef]
- Xiang, Y.; Qiu, Z.; Ding, Y.; Du, M.; Gao, N.; Cao, H.; Zuo, H.; Cheng, H.; Gao, X.; Zheng, S.; et al. Dexamethasone-loaded ROS stimuli-responsive nanogels for topical ocular therapy of corneal neovascularization. J. Control. Release 2024, 372, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, Y.; Luo, Q.; Liang, Z.; Lu, P.; Song, F.; Zhang, Z.; Zhou, T.; Zhang, J. Improving the solubility of vorinostat using cyclodextrin inclusion complexes: The physicochemical characteristics, corneal permeability and ocular pharmacokinetics of the drug after topical application. Eur. J. Pharm. Sci. 2022, 168, 106078. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Braz, D.; Zhu, L.; Gélizé, E.; Clarin, J.P.; Chatagnon, X.; Benzine, Y.; Rampignon, P.; Thouvenin, A.; Bourges, J.L.; Behar-Cohen, F.; et al. Spironolactone Eyedrop Favors Restoration of Corneal Integrity after Wound Healing in the Rat. Pharmaceuticals 2023, 16, 1446. [Google Scholar] [CrossRef]
- Mahfufah, U.; Sya’ban Mahfud, M.A.; Saputra, M.D.; Abd Azis, S.B.; Salsabila, A.; Asri, R.M.; Habibie, H.; Sari, Y.; Yulianty, R.; Alsayed, A.R.; et al. Incorporation of Inclusion Complexes in the Dissolvable Microneedle Ocular Patch System for the Efficiency of Fluconazole in the Therapy of Fungal Keratitis. ACS Appl. Mater. Interfaces 2024, 16, 25637–25651. [Google Scholar]
- Putri, R.A.; Enggi, C.K.; Sulistiawati, S.; Burhanuddin, H.; Iskandar, I.W.; Saputra, R.R.; Rahman, L.; Sartini, S.; Rifai, Y.; Aswad, M.; et al. Development of itraconazole ocular delivery system using β-cyclodextrin complexation incorporated into dissolving microneedles for potential improvement treatment of fungal keratitis. J. Biomater. Sci. Polym. Ed. 2024, 35, 2315–2342. [Google Scholar] [CrossRef]
- Xia, H.; Yang, J.; Song, F.; Pu, G.; Dong, F.; Liang, Z.; Zhang, J. Development of ion-triggered in situ gel containing ketoconazole/hydroxypropyl-β-cyclodextrin for ocular delivery: In vitro and in vivo evaluation. Drug Deliv. 2024, 31, 2424217. [Google Scholar]
- Gözcü, S.; Polat, H.K.; Gültekin, Y.; Ünal, S.; Karakuyu, N.F.; Şafak, E.K.; Doğan, O.; Pezik, E.; Haydar, M.K.; Aytekin, E.; et al. Formulation of hesperidin-loaded in situ gel for ocular drug delivery: A comprehensive study. J. Sci. Food Agric. 2024, 104, 5846–5859. [Google Scholar]
- Farkas, E.; Abboud, H.; Nagy, N.; Hofmeister, B.; Ostorházi, E.; Tóth, B.; Pinke, B.; Mészáros, L.; Zelkó, R.; Kazsoki, A. Formulation and Development of Nanofiber-Based Ophthalmic Insert for the Treatment of Bacterial Conjunctivitis. Int. J. Mol. Sci. 2024, 25, 9228. [Google Scholar] [CrossRef]
- Ye, X.; Li, F.; Li, M.; Zhang, G.; Wang, W.; Wang, Z.; Zhang, H.; Dong, L.; Lin, X.; Wu, L.; et al. Controlled release of vitamin A palmitate from crosslinked cyclodextrin organic framework for dry eye disease therapy. Int. J. Pharm. 2024, 659, 124279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tan, M.; Hu, Z.E.; Zhang, Y.T.; Qi, X.W.; Che, Y.T.; Li, J.; Zhang, S.; Li, B.J. A hyaluronic acid-modified cyclodextrin self-assembly system for the delivery of β-carotene in the treatment of dry eye disease. Int. J. Biol. Macromol. 2025, 287, 138428. [Google Scholar] [CrossRef]
- Chaudhari, P.; Birangal, S.; Mavlankar, N.; Pal, A.; Mallela, L.S.; Roy, S.; Kodoth, A.K.; Ghate, V.; Nampoothiri, M.; Lewis, S.A. Oil-free eye drops containing Cyclosporine A/cyclodextrin/PVA supramolecular complex as a treatment modality for dry eye disease. Carbohydr. Polym. 2022, 297, 120007. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Z.; Guan, B.; Liu, X.X.; Ke, L.N.; Wang, J.J.; Nan, K.H. A topical fluorometholone nanoformulation fabricated under aqueous condition for the treatment of dry eye. Colloids Surf. B Biointerfaces 2022, 212, 112351. [Google Scholar] [CrossRef]
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P.; Loftsson, T.; Stefánsson, E.; Ohira, A. Topical Dexamethasone-Cyclodextrin Microparticle Eye Drops for Diabetic Macular Edema Preparation of Dexamethasone-Cyclodextrin Microparticle Eye Drops. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7944–7948. [Google Scholar]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar]
- Shulman, S.; Jõhannesson, G.; Stefánsson, E.; Loewenstein, A.; Rosenblatt, A.; Habot-Wilner, Z. Topical dexamethasone-cyclodextrin nanoparticle eye drops for non-infectious Uveitic macular oedema and vitritis—A pilot study. Acta Ophthalmol. 2015, 93, 411–415. [Google Scholar]
- Loftsson, T.; Hreinsdóttir, D.; Stefánsson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2010, 59, 629–635. [Google Scholar]
- Yang, L.; Jonas, J.B.; Wei, W. Central serous chorioretinopathy and bright light: Authors reply. Acta Ophthalmol. 2014, 92, e689. [Google Scholar]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Kristinsdottir, I.M.; Fulop, Z.; Asgrimsdottir, G.M.; Thorsteindsottir, M.; Eiriksson, F.F.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: Dexamethasone concentrations in various eye tissues after topical administration for up to 15 days to rabbits. J. Drug Deliv. Sci. Technol. 2018, 45, 449–454. [Google Scholar]
- Lu, J.; Zhu, X.; Zhang, M.; Jiang, X.; Guo, W.; Jiang, F.; Cao, F. In vitro and in vivo assessment of structural integrity for HPCD complex@Liposome nanocomposites from ocular surface to the posterior segment of the eye. Carbohydr. Polym. 2023, 315, 120960. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Huang, J.; Yin, C.; Li, Y.; Guo, W.; Jiang, F.; Cao, F. FRET-based analysis on the fate of liposome and cyclodextrin@liposome nanocomposites from ocular surface to the posterior segment of the eye. J. Control. Release 2025, 377, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of Fenofibrate/Randomly Methylated β-Cyclodextrin-Loaded Eudragit® RL 100 Nanoparticles for Ocular Delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Lorenzo-Soler, L.; Praphanwittaya, P.; Olafsdottir, O.B.; Kristinsdottir, I.M.; Asgrimsdottir, G.M.; Loftsson, T.; Stefansson, E. Topical noninvasive retinal drug delivery of a tyrosine kinase inhibitor: 3% cediranib maleate cyclodextrin nanoparticle eye drops in the rabbit eye. Acta Ophthalmol. 2022, 100, 788–796. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Cantó, A.; Rodilla, V.; Miranda, M.; López-Castellano, A. Topical Ocular Administration of Progesterone Decreases Photoreceptor Cell Death in Retinal Degeneration Slow (rds) Mice. Pharmaceuticals 2022, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Goto, T.; Onodera, R.; Hirotsu, T.; Ikeda, H.O.; Motoyama, K. Sustained Release Formulation of Hydroxypropyl-β-cyclodextrin Eye Drops Using Xanthan Gum. Chem. Pharm. Bull. 2024, 72, 381–384. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef]
- Ghaffarieh, A.; Ciolino, J.B. Potential of Application of Iron Chelating Agents in Ophthalmic Diseases. Semin. Ophthalmol. 2021, 36, 157–161. [Google Scholar] [CrossRef]
- Meldolesi, J.; Castiglioni, G.; Parma, R.; Nassivera, N.; De, P. Ca++-Dependent Disassembly and Reassembly of Occluding Junctions in Guinea Pig Pancreatic Acinar Cells Effect of Drugs. J. Cell Biol. 1978, 79, 156–172. [Google Scholar] [CrossRef]
- Cereijido, M.; Robbins, S.; Dolan, W.J.; Rotunno, C.A.; Sabatini, D.D. Polarized Monolayers Formed by Epithelial Cells on a Permeable and Translucent Support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef]
- Martinez-Palomo, A.; Meza, I.; Beaty, G.; Cereijido, M. Experimental Modulation of Occluding Junctions in a Cultured Transporting Epithelium. J. Cell Biol. 1980, 87, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Chávez De Ramírez, B.; Cereijido, M. Tight Junction Formation in Cultured Epithelial Cells (MDCK). J. Membr. Biol. 1985, 86, 113–125. [Google Scholar] [PubMed]
- Ma, T.Y.; Tran, D.; Hoa, N.; Nguyen, D.; Merryfield, M.; Tarnawski, A. Mechanism of Extracellular Calcium Regulation of Intestinal Epithelial Tight Junction Permeability: Role of Cytoskeletal Involvement. Microcirculation 2000, 7, 45–56. [Google Scholar]
- Klingler, C.; Kniesel, U.; Bamforth, S.D.; Wolburg, H.; Engelhardt, B.; Risau, W. Disruption of epithelial tight junctions is prevented by cyclic nucleotide-dependent protein kinase inhibitors. Histochem. Cell Biol. 2000, 113, 349–361. [Google Scholar] [CrossRef]
- Overduin, M.; Harvey, T.S.; Bagby, S.; Tong, K.I.; Yau, P.; Takeichi, M.; Ikura, M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science 1995, 267, 253–256. [Google Scholar] [CrossRef]
- Contreras-Ruiz, L.; Schulze, U.; García-Posadas, L.; Arranz-Valsero, I.; López-García, A.; Paulsen, F.; Diebold, Y. Structural and functional alteration of corneal epithelial barrier under inflammatory conditions. Curr. Eye Res. 2012, 37, 971–981. [Google Scholar]
- Meng, W.; Takeichi, M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a002899. [Google Scholar] [CrossRef]
- Ramachandran, C.; Srinivas, S.P. Formation and disassembly of adherens and tight junctions in the corneal endothelium: Regulation by actomyosin contraction. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2139–2148. [Google Scholar]
- Morrison, P.W.J.; Khutoryanskiy, V.V. Enhancement in corneal permeability of riboflavin using calcium sequestering compounds. Int. J. Pharm. 2014, 472, 56–64. [Google Scholar]
- Rodriguez, I.; Antonio Vázquez, J.; Pastrana, L.; Khutoryanskiy, V.V. Enhancement and inhibition effects on the corneal permeability of timolol maleate: Polymers, cyclodextrins and chelating agents. Int. J. Pharm. 2017, 529, 168–177. [Google Scholar] [CrossRef]
- Ahuja, M.; Shridhar, A.; Kanti, D. Effect of Formulation Factors on In Vitro Permeation of Diclofenac from Experimental and Marketed Aqueous Eye Drops Through Excised Goat Cornea. Yakugaku Zasshi 2006, 126, 1369–1375. [Google Scholar]
- Malhotra, M.; Majumdar, D.K. Effect of preservative, antioxidant and viscolizing agents on in vitro transcorneal permeation of ketorolac tromethamine. J. Pharm. Pharmacol. 2002, 54, 1499–1505. [Google Scholar]
- Kikuchi, T.; Suzuki, M.; Kusai, A.; Iseki, K.; Sasaki, H. Synergistic effect of EDTA and boric acid on corneal penetration of CS-088. Int. J. Pharm. 2005, 290, 83–89. [Google Scholar]
- Malhotra, S.; Khare, A.; Grover, K.; Singh, I.; Pawar, P. Design and Evaluation of Voriconazole Eye Drops for the Treatment of Fungal Keratitis. J. Pharm. 2014, 2014, 490595. [Google Scholar]
- Scholz, M.; Chang Lin, J.-E.; Lee, V.H.L.; Keipert, S. Pilocarpine Permeability Across Ocular Tissues and Cell Cultures: Influence of Formulation Parameters. Pharm. Res. 2002, 19, 1195–1201. [Google Scholar]
- Pescina, S.; Carra, F.; Padula, C.; Santi, P.; Nicoli, S. Effect of pH and penetration enhancers on cysteamine stability and trans-corneal transport. Eur. J. Pharm. Biopharm. 2016, 107, 171–179. [Google Scholar]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Saettone, M.F. Ocular toxicity of some corneal penetration enhancers evaluated by electrophysiology measurements on isolated rabbit corneas. Toxicol. Vitr. 2003, 17, 497–504. [Google Scholar]
- Malhotra, M.; Majumdar, D.K. In Vivo Ocular Availability of Ketorolac Following Ocular Instillations of Aqueous, Oil, and Ointment Formulations to Normal Corneas of Rabbits: A Technical Note. AAPS PharmSciTech 2005, 6, E1–E6. [Google Scholar]
- Epstein, S.P.; Ahdoot, M.; Marcus, E.; Asbell, P.A. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 2009, 25, 113–119. [Google Scholar]
- Grass, G.M.; Robinson, J.R. Mechanisms of Corneal Drug Penetration II: Ultrastructural Analysis of Potential Pathways for Drug Movement. J. Pharm. Sci. 1988, 77, 15–23. [Google Scholar]
- Grass, G.M.; Wood, R.W.; Robinson, J.R. Effects of calcium chelating agents on corneal permeability. Investig. Ophthalmol. Vis. Sci. 1985, 26, 110–113. [Google Scholar]
- Kralj, M.; Tušek-Božić, L.; Frkanec, L. Biomedical potentials of crown ethers: Prospective antitumor agents. ChemMedChem 2008, 3, 1478–1492. [Google Scholar] [CrossRef]
- Morrison, P.W.J.; Porfiryeva, N.N.; Chahal, S.; Salakhov, I.A.; Lacourt, C.; Semina, I.I.; Moustafine, R.I.; Khutoryanskiy, V.V. Crown Ethers: Novel Permeability Enhancers for Ocular Drug Delivery? Mol. Pharm. 2017, 14, 3528–3538. [Google Scholar] [PubMed]
- Steed, J.W. First-and second-sphere coordination chemistry of alkali metal crown ether complexes. Coord. Chem. Rev. 2001, 215, 171–221. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S. Crown Ethers, Cryptands and Other Compounds. In Macrocycles; Wiley: Hoboken, NJ, USA, 2011; pp. 34–76. [Google Scholar]
- Marjanović, M.; Kralj, M.; Supek, F.; Frkanec, L.; Piantanida, I.; Šmuc, T.; Tušek-Božić, L. Antitumor potential of crown ethers: Structure-activity relationships, cell cycle disturbances, and cell death studies of a series of ionophores. J. Med. Chem. 2007, 50, 1007–1018. [Google Scholar] [CrossRef]
- Song, M.Z.; Zhu, L.Y.; Gao, X.K.; Dou, J.M.; Sun, D.Z. Microcalorimetric study on host-guest complexation of naphtho-15-crown-5 with four ions of alkaline earth metal. J. Zhejiang Univ. Sci. B 2005, 6, 69–73. [Google Scholar] [PubMed]
- Ullah, F.; Khan, T.A.; Iltaf, J.; Anwar, S.; Khan, M.F.A.; Khan, M.R.; Ullah, S.; Rehman, M.F.U.; Mustaqeem, M.; Kotwica-Mojzych, K.; et al. Heterocyclic Crown Ethers with Potential Biological and Pharmacological Properties: From Synthesis to Applications. Appl. Sci. 2022, 12, 1102. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. The role of crown ethers in drug delivery. Supramol. Chem. 2019, 31, 221–238. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Afifi, S.A.; El Sayed, N.S. Crown ether nanovesicles (crownsomes) repositioned phenytoin for healing of corneal ulcers. Mol. Pharm. 2020, 17, 3952–3965. [Google Scholar] [CrossRef]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: A review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar]
- Domard, A.; Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Ma, G.H.; Su, Z.G. Preparation of uniform sized chitosan microspheres by membrane emulsification technique and application as a carrier of protein drug. J. Control. Release 2005, 106, 62–75. [Google Scholar] [CrossRef]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Pharm. 2012, 9, 1297–1306. [Google Scholar]
- Ricci, F.; Racaniello, G.F.; Lopedota, A.; Laquintana, V.; Arduino, I.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Sigurdsson, H.H.; Denora, N. Chitosan/sulfobutylether-β-cyclodextrin based nanoparticles coated with thiolated hyaluronic acid for indomethacin ophthalmic delivery. Int. J. Pharm. 2022, 617, 121905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, X.; Yang, Y.; Zhang, L.; Liu, R.; Li, Z. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 156, 749–761. [Google Scholar]
- Shinde, U.A.; Joshi, P.N.; Jain, D.D.; Singh, K. Preparation and Evaluation of N-Trimethyl Chitosan Nanoparticles of Flurbiprofen for Ocular Delivery. Curr. Eye Res. 2019, 44, 575–582. [Google Scholar]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef]
- Alhowyan, A.A.; Kalam, M.A.; Iqbal, M.; Raish, M.; El-Toni, A.M.; Alkholief, M.; Almomen, A.A.; Alshamsan, A. Mesoporous Silica Nanoparticles Coated with Carboxymethyl Chitosan for 5-Fluorouracil Ocular Delivery: Characterization, In Vitro and In Vivo Studies. Molecules 2023, 28, 1260. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Gonçalves, L.M.; Braz, B.S.; Delgado, E. Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma. Pharmaceuticals 2023, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, Y.S.; Prasad, Y.D.; Chandasana, H.; Vishvkarma, A.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 2015, 72, 1451–1458. [Google Scholar] [CrossRef]
- Alkholief, M.; Kalam, M.A.; Raish, M.; Ansari, M.A.; Alsaleh, N.B.; Almomen, A.; Ali, R.; Alshamsan, A. Topical Sustained-Release Dexamethasone-Loaded Chitosan Nanoparticles: Assessment of Drug Delivery Efficiency in a Rabbit Model of Endotoxin-Induced Uveitis. Pharmaceutics 2023, 15, 2273. [Google Scholar] [CrossRef]
- Rubenicia, A.M.L.; Cubillan, L.D.P.; Sicam, V.A.D.P.; Macabeo, A.P.G.; Villaflores, O.B.; Castillo, A.L. Intraocular pressure reduction effect of 0.005% latanoprost eye drops in a hyaluronic acid-chitosan nanoparticle drug delivery system in albino rabbits. Transl. Vis. Sci. Technol. 2021, 10, 4. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Z.; Ma, H.; Liu, Y.; Nwafor, E.O.; Zhu, S.; Jia, L.; Pang, X.; Han, Z.; Tian, B.; et al. Optimization and Characterization of Low-Molecular-Weight Chitosan-Coated Baicalin mPEG-PLGA Nanoparticles for the Treatment of Cataract. Mol. Pharm. 2022, 19, 3831–3845. [Google Scholar]
- Abdullah, T.; Ibrahim, N.; Warsi, M. Chondroitin sulfate-chitosan nanoparticles for ocular delivery of bromfenac sodium: Improved permeation, retention, and penetration. Int. J. Pharm. Investig. 2016, 6, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Schipper, N.G.M.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Varum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs 2: Mechanism of Absorption Enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar]
- Pratap-Singh, A.; Guo, Y.; Baldelli, A.; Singh, A. Mercaptonicotinic acid activated thiolated chitosan (MNA-TG-chitosan) to enable peptide oral delivery by opening cell tight junctions and enhancing transepithelial transport. Sci. Rep. 2023, 13, 44178. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar]
- Wang, S.; Gao, Z.; Liu, L.; Li, M.; Zuo, A.; Guo, J. Preparation, in vitro and in vivo evaluation of chitosan-sodium alginate-ethyl cellulose polyelectrolyte film as a novel buccal mucosal delivery vehicle. Eur. J. Pharm. Sci. 2022, 168, 106085. [Google Scholar] [CrossRef]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [PubMed]
- Shi, L.; Li, Z.; Liang, Z.; Zhang, J.; Liu, R.; Chu, D.; Han, L.; Zhu, L.; Shen, J.; Li, J. A dual-functional chitosan derivative platform for fungal keratitis. Carbohydr. Polym. 2022, 277, 118762. [Google Scholar] [CrossRef]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2017, 27, 228–233. [Google Scholar] [PubMed]
- Liu, Y.; Cui, X.; Zhao, L.; Zhang, W.; Zhu, S.; Ma, J. Chitosan Nanoparticles to Enhance the Inhibitory Effect of Natamycin on Candida Albicans. J. Nanomater. 2021, 2021, 6644567. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Kedar, A.; Yadav, K.S.; Krishna, A.; Akhir, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. Chitosan/HPMC-based mucoadhesive film co-loaded with fluconazole and ofloxacin for management of polymicrobial keratitis. Int. J. Biol. Macromol. 2022, 222, 2785–2795. [Google Scholar]
- Gao, N.; Ju, X.; Jiao, X.; Qi, Y.; Tian, Y.; Jiang, S.; Niu, Z.; Zhao, S.; Yang, R. Breaking Down the Barriers of Drug Resistance and Corneal Permeability with Chitosan-Poly(ethylene glycol)-LK13 Peptide Conjugate to Combat Fungal Keratitis. ACS Infect. Dis. 2024, 10, 2950–2960. [Google Scholar]
- Sun, X.; Sheng, Y.; Li, K.; Sai, S.; Feng, J.; Li, Y.; Zhang, J.; Han, J.; Tian, B. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022, 138, 193–207. [Google Scholar]
- Cui, X.; Li, X.; Xu, Z.; Guan, X.; Ma, J.; Ding, D.; Zhang, W. Fabrication and Characterization of Chitosan/Poly(Lactic-Co-Glycolic Acid) Core-Shell Nanoparticles by Coaxial Electrospray Technology for Dual Delivery of Natamycin and Clotrimazole. Front. Bioeng. Biotechnol. 2021, 9, 635485. [Google Scholar] [CrossRef]
- Latifi, A.; Esmaeili, F.; Mohebali, M.; Yasami-Khiabani, S.; Rezaeian, M.; Soleimani, M.; Kazemirad, E.; Amani, A. Chitosan nanoparticles improve the effectivity of miltefosine against Acanthamoeba. PLoS Negl. Trop. Dis. 2024, 18, e0011976. [Google Scholar] [CrossRef]
- Padaga, S.G.; Ch, S.; Paul, M.; Wable, B.D.; Ghosh, B.; Biswas, S. Chitosan oligosaccharide/pluronic F127 micelles exhibiting anti-biofilm effect to treat bacterial keratitis. Carbohydr. Polym. 2024, 327, 121818. [Google Scholar] [CrossRef]
- Padaga, S.G.; Bhatt, H.; Ch, S.; Paul, M.; Itoo, A.M.; Ghosh, B.; Roy, S.; Biswas, S. Glycol Chitosan-Poly(lactic acid) Conjugate Nanoparticles Encapsulating Ciprofloxacin: A Mucoadhesive, Antiquorum-Sensing, and Biofilm-Disrupting Treatment Modality for Bacterial Keratitis. ACS Appl. Mater. Interfaces 2024, 16, 18360–18385. [Google Scholar]
- Ch, S.; Padaga, S.G.; Ghosh, B.; Roy, S.; Biswas, S. Chitosan-poly(lactide-co-glycolide)/poloxamer mixed micelles as a mucoadhesive thermo-responsive moxifloxacin eye drop to improve treatment efficacy in bacterial keratitis. Carbohydr. Polym. 2023, 311, 120822. [Google Scholar] [CrossRef]
- Meng, S.; Hu, H.; Qiao, Y.; Wang, F.; Zhang, B.N.; Sun, D.; Zhou, L.; Zhao, L.; Xie, L.; Zhang, H.; et al. A Versatile Hydrogel with Antibacterial and Sequential Drug-Releasing Capability for the Programmable Healing of Infectious Keratitis. ACS Nano 2023, 17, 24055–24069. [Google Scholar] [PubMed]
- Chang, Y.F.; Cheng, Y.H.; Ko, Y.C.; Chiou, S.H.; Liu, C.J. Development of topical chitosan/β-glycerophosphate-based hydrogel loaded with levofloxacin in the treatment of keratitis: An ex-vivo study. Heliyon 2022, 8, e08697. [Google Scholar] [CrossRef]
- Lu, Y.; Geng, W.; Li, L.; Xie, F.; Zhang, M.; Xie, H.; Cai, J. Enhanced antibacterial and antibiofilm activities of quaternized ultra-highly deacetylated chitosan against multidrug-resistant bacteria. Int. J. Biol. Macromol. 2025, 254, 140052. [Google Scholar] [CrossRef]
- Sikhondze, S.S.; Makoni, P.A.; Walker, R.B.; Khamanga, S.M.M. Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole. Pharmaceutics 2023, 15, 1855. [Google Scholar] [CrossRef]
- Javed, S.; Abbas, G.; Shah, S.; Rasul, A.; Irfan, M.; Saleem, A.; Hosny, K.M.; Bukhary, S.M.; Safhi, A.Y.; Sabei, F.Y.; et al. Tobramycin-loaded nanoparticles of thiolated chitosan for ocular drug delivery: Preparation, mucoadhesion and pharmacokinetic evaluation. Heliyon 2023, 9, e19877. [Google Scholar] [CrossRef]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of chitosan/cyclodextrin nanospheres for levofloxacin ocular delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef]
- Kalam, M.A.; Iqbal, M.; Alshememry, A.; Alkholief, M.; Alshamsan, A. Development and Evaluation of Chitosan Nanoparticles for Ocular Delivery of Tedizolid Phosphate. Molecules 2022, 27, 2326. [Google Scholar] [CrossRef]
- Silva, B.; Gonçalves, L.M.; São Braz, B.; Delgado, E. Topical ocular delivery of nanoparticles with epoetin beta in Wistar Hannover rats. Sci. Rep. 2023, 13, 28845. [Google Scholar] [CrossRef]
- Omran, S.; Elnaggar, Y.S.R.; Abdallah, O.Y. Controlled release, chitosan-tethered luteolin phytocubosomes; Formulation optimization to in-vivo antiglaucoma and anti-inflammatory ocular evaluation. Int. J. Biol. Macromol. 2024, 254, 127930. [Google Scholar] [CrossRef]
- Rahbar, N.; Darvish, S.; Farrahi, F.; Kouchak, M. Chitosan/carbomer nanoparticles-laden in situ gel for improved ocular delivery of timolol: In vitro, in vivo, and ex vivo study. Drug Deliv. Transl. Res. 2024, 15, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Shajari, G.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In situ forming hydrogels based on modified gellan gum/chitosan for ocular drug delivery of timolol maleate. Int. J. Biol. Macromol. 2024, 254, 135071. [Google Scholar] [CrossRef]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar]
- Kailasam, V.; Kumara, B.N.; Prasad, K.S.; Nirmal, J. Combination of self-assembling system and N,O-carboxymethyl chitosan improves ocular residence of anti-glaucoma drug. Eur. J. Pharm. Biopharm. 2024, 196, 114208. [Google Scholar] [CrossRef]
- Badran, M.M.; Alomrani, A.H.; Almomen, A.; Bin Jardan, Y.A.; Abou El Ela, A.E.S. Novel Metoprolol-Loaded Chitosan-Coated Deformable Liposomes in Thermosensitive In Situ Gels for the Management of Glaucoma: A Repurposing Approach. Gels 2022, 8, 635. [Google Scholar] [CrossRef]
- Xiong, X.; Jiang, H.; Liao, Y.; Du, Y.; Zhang, Y.; Wang, Z.; Zheng, M.; Du, Z. Liposome-trimethyl chitosan nanoparticles codeliver insulin and siVEGF to treat corneal alkali burns by inhibiting ferroptosis. Bioeng. Transl. Med. 2023, 8, e10499. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Kumar, B.; Chitranshi, N.; Gupta, V.K.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; Vishwas, S.; et al. Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: A new dimension to an anticancer drug. Int. J. Biol. Macromol. 2023, 224, 810–830. [Google Scholar]
- Mohamed, H.B.; Shafie, M.A.A.; Mekkawy, A.I. Chitosan Nanoparticles for Meloxicam Ocular Delivery: Development, In Vitro Characterization, and In Vivo Evaluation in a Rabbit Eye Model. Pharmaceutics 2022, 14, 893. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Girgis, G.N.S.; El-Dahan, M.S. Chitosan-coated PLGA nanoparticles for enhanced ocular anti-inflammatory efficacy of atorvastatin calcium. Int. J. Nanomed. 2020, 15, 1335–1347. [Google Scholar] [CrossRef]
- Alqurshi, A.; Hanafy, A.F.; Abdalla, A.M.; Guda, T.K.; Gabr, K.E.; Royall, P.G. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int. J. Nanomed. 2019, 14, 3679–3689. [Google Scholar]
- Fathalla, Z.; Al Fatease, A.; Abdelkader, H. Formulation and In-Vitro/Ex-Vivo Characterization of Pregelled Hybrid Alginate-Chitosan Microparticles for Ocular Delivery of Ketorolac Tromethamine. Polymers 2023, 15, 2773. [Google Scholar] [CrossRef]
- Adwan, S.; Al-Akayleh, F.; Qasmieh, M.; Obeidi, T. Enhanced Ocular Drug Delivery of Dexamethasone Using a Chitosan-Coated Soluplus-Based Mixed Micellar System. Pharmaceutics 2024, 16, 1390. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhu, L.; Zhang, K.; Li, T.; Huang, S. Nanodelivery of triamcinolone acetonide with PLGA-chitosan nanoparticles for the treatment of ocular inflammation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 308–316. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-coated PLGA nanoparticles encapsulating triamcinolone acetonide as a potential candidate for sustained ocular drug delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ueda, K.; Isowaki, A.; Ohtori, A.; Takeuchi, H.; Ohguro, N.; Tojo, K. Mucoadhesive Properties of Chitosan-Coated Ophthalmic Lipid Emulsion Containing Indomethacin in Tear Fluid. J. Pharm. Soc. Jpn. 2009, 32, 1266–1271. [Google Scholar] [CrossRef]
- Rahman, S.N.R.; Agarwal, N.; Goswami, A.; Sree, A.; Jala, A.; Venuganti, A.; Deka, A.; Borkar, R.M.; Singh, V.; Das, D.; et al. Studies on spray dried topical ophthalmic emulsions containing cyclosporin A (0.05% w/w): Systematic optimization, in vitro preclinical toxicity and in vivo assessments. Drug Deliv. Transl. Res. 2023, 13, 1654–1674. [Google Scholar] [CrossRef]
- Teba, H.E.; Khalil, I.A.; Gebreel, R.M.; Fahmy, L.I.; Sorogy, H.M.E. Development of antifungal fibrous ocular insert using freeze-drying technique. Drug Deliv. Transl. Res. 2024, 14, 2520–2538. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.A.; Cronemberger, S.; Nogueira, J.C.; et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 567, 118662. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Taghe, S.; Asare-Addo, K.; Nokhodchi, A. Polyvinyl Alcohol/Chitosan Single-Layered and Polyvinyl Alcohol/Chitosan/Eudragit RL100 Multi-Layered Electrospun Nanofibers as an Ocular Matrix for the Controlled Release of Ofloxacin: An In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 218. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-loaded ocular inserts as sustained release drug delivery systems for glaucoma treatment: In Vitro and In Vivo evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef]
- Cesar, A.L.A.; Navarro, L.C.; Castilho, R.O.; Goulart, G.A.C.; Foureaux, G.; Ferreira, A.J.; Cronemberger, S.; Gomes Faraco, A.A. New antiglaucomatous agent for the treatment of open angle glaucoma: Polymeric inserts for drug release and in vitro and in vivo study. J. Biomed. Mater. Res. A 2021, 109, 336–345. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Salema-Oom, M.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Diclofenac sustained release from sterilised soft contact lens materials using an optimised layer-by-layer coating. Int. J. Pharm. 2020, 587, 119506. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended wear therapeutic contact lens fabricated from timolol imprinted carboxymethyl chitosan-g-hydroxy ethyl methacrylate-g-poly acrylamide as a onetime medication for glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar]
- Behl, G.; Iqbal, J.; O’Reilly, N.J.; McLoughlin, P.; Fitzhenry, L. Synthesis and Characterization of Poly(2-hydroxyethylmethacrylate) Contact Lenses Containing Chitosan Nanoparticles as an Ocular Delivery System for Dexamethasone Sodium Phosphate. Pharm. Res. 2016, 33, 1638–1648. [Google Scholar] [CrossRef]
- Jiao, Z.; Huo, Q.; Lin, X.; Chu, X.; Deng, Z.; Guo, H.; Peng, Y.; Lu, S.; Zhou, X.; Wang, X.; et al. Drug-free contact lens based on quaternized chitosan and tannic acid for bacterial keratitis therapy and corneal repair. Carbohydr. Polym. 2022, 291, 119314. [Google Scholar] [CrossRef]
- Mehta, P.; Al-Kinani, A.A.; Arshad, M.S.; Singh, N.; van der Merwe, S.M.; Chang, M.W.; Alany, R.G.; Ahmad, Z. Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. J. Pharm. Sci. 2019, 108, 1540–1551. [Google Scholar]
- Hoyo, J.; Ivanova, K.; Guaus, E.; Tzanov, T. Multifunctional ZnO NPs-chitosan-gallic acid hybrid nanocoating to overcome contact lenses associated conditions and discomfort. J. Colloid Interface Sci. 2019, 543, 114–121. [Google Scholar]
- Ibrahim, S.S. The Role of Surface Active Agents in Ophthalmic Drug Delivery: A Comprehensive Review. J. Pharm. Sci. 2019, 108, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Tomer, S.; Dorsey, J.G. Polyoxyethylene alkyl ether nonionic surfactants: Physicochemical properties and use for cholesterol determination in food. Talanta 2001, 55, 69–83. [Google Scholar] [PubMed]
- Tomasino, C. Effect of wet processing and chemical finishing on fabric hand. In Effect of Mechanical and Physical Properties on Fabric Hand; Elsevier: Amsterdam, The Netherlands, 2005; pp. 289–341. [Google Scholar]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [PubMed]
- Kapoor, Y.; Howell, B.A.; Chauhan, A. Liposome assay for evaluating ocular toxicity of surfactants. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2727–2735. [Google Scholar] [CrossRef]
- Matsuda, S.; Hisama, M.; Shibayama, H.; Norihiko, I.; Iwaki, M. In Vitro Eye Irritancy Test of Lauryl Derivatives and Polyoxyethylene Alkyl Derivatives with the Reconstructed Rabbit Corneal Epithelium Model. J. Oleo Sci. 2009, 58, 437–442. [Google Scholar] [CrossRef][Green Version]
- Chiou, G.C.Y.; Shen, Z.F.; Zheng, Y.Q.; Chen, Y.J. Enhancement of Systemic Delivery of Peptide Drugs via Ocular Route with Surfactants. Drug Dev. Res. 1992, 25, 47–57. [Google Scholar]
- Chiou, G.C.Y.; Li, B.H.P. Chronic Systemic Delivery of Insulin Through the Ocular Route. J. Ocul. Pharmacol. 1993, 9, 85–90. [Google Scholar]
- Rohde, B.H.; Chiou, G.C.Y. Effect of Permeation Enhancers on Beta-Endorphin Systemic Uptake After Topical Application to the Eye. Ophthalmic Res. 1991, 23, 265–271. [Google Scholar] [CrossRef]
- Lee, Y.C.; Simamora, P.; Yalkowsky, S.H. Effect of Brij-78 on Systemic Delivery of Insulin from an Ocular Device. Int. J. Pharm. 1997, 159, 85–91. [Google Scholar]
- Srinivasan, R.; Jain, S.K. Insulin delivery through the ocular route. Drug Deliv. 1998, 5, 53–55. [Google Scholar] [CrossRef]
- Morgan, R.V. Delivery of Systemic Regular Insulin Via the Ocular Route in Cats. J. Ocul. Pharmacol. Ther. 1995, 11, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Furrer, P.; Mayer, J.M.; Plazonnet, B.; Gurny, R. Ocular Tolerance of Absorption Enhancers in Ophthalmic Preparations. Int. J. Pharm. 2002, 240, 67–74. [Google Scholar] [CrossRef]
- Chiou, G.C.Y.; Chlng, A.; Chuang, Y. Improvement of Systemic Absorption of Insulin Through Eyes with Absorption Enhancers. J. Pharm. Sci. 1989, 78, 815–818. [Google Scholar] [CrossRef]
- Chiou, G.C.Y.; Shen, Z.F.; Zheng, Y.Q. Systemic Absorption of Oxytocin and Vasopressin Through Eyes in Rabbits. J. Ocul. Pharmacol. 1991, 7, 351–360. [Google Scholar] [CrossRef]
- Pillion, D.J.; Atchison, J.A.; Stott, J.; McCracken, D.; Gargiulo, C.; Meezan, E. Efficacy of Insulin Eyedrops. J. Ocul. Pharmacol. 1994, 10, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.V.; Huntzicker, M.A. Delivery of Systemic Regular Insulin via the Ocular Route in Dogs. J. Ocul. Pharmacol. 1996, 12, 379–385. [Google Scholar] [CrossRef]
- Pillion, D.J.; McCracken, D.L.; Yang, M.; Atchison, J.A. Glucagon Administration to the Rat via Eye Drops. J. Ocul. Pharmacol. 1992, 8, 349–358. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.M.; Ang, H.; Lin, J.; Lui, Y.S.; Phua, J.L.; Chan, J.N.; Venkatraman, S.; Foin, N.; Huang, Y. Materials technology in drug eluting balloons: Current and future perspectives. J. Control. Release 2016, 239, 92–106. [Google Scholar] [CrossRef]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease-recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Zimmer, A.K.; Maincent, P.; Thouvenot, P.; Kreuter, J. Hydrocortisone delivery to healthy and inflamed eyes using a micellar polysorbate 80 solution or albumin nanoparticles. Int. J. Pharm. 1994, 110, 211–222. [Google Scholar] [CrossRef]
- Taniguchi, K.; Itakura, K.; Morisaki, K.; Hayashi, S. Effects of Tween 80 and Liposomes on the Corneal Permeability of Anti-Inflammatory Steroids. J. Pharmacobiodyn. 1988, 11, 330–337. [Google Scholar] [CrossRef]
- Barbalho, G.N.; Brugger, S.; Raab, C.; Lechner, J.S.; Gratieri, T.; Keck, C.M.; Rupenthal, I.D.; Agarwal, P. Development of Transferosomes for Topical Ocular Drug Delivery of Curcumin. Eur. J. Pharm. Biopharm. 2024, 205, 114535. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [PubMed]
- Ibrahim, S.S.; Abd-allah, H. Spanlastic nanovesicles for enhanced ocular delivery of vanillic acid: Design, in vitro characterization, and in vivo anti-inflammatory evaluation. Int. J. Pharm. 2022, 625, 122068. [Google Scholar] [CrossRef] [PubMed]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [PubMed]
- Abdelbari, M.A.; El-Mancy, S.S.; Elshafeey, A.H.; Abdelbary, A.A. Implementing spanlastics for improving the ocular delivery of clotrimazole: In vitro characterization, ex vivo permeability, microbiological assessment and in vivo safety study. Int. J. Nanomed. 2021, 16, 6249–6261. [Google Scholar] [CrossRef]
- Üstündağ-Okur, N.; Gökçe, E.H.; Bozbiyik, D.I.; Eğrilmez, S.; Özer, Ö.; Ertan, G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar]
- Hippalgaonkar, K.; Adelli, G.R.; Hippalgaonkar, K.; Repka, M.A.; Majumdar, S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: Development, characterization, and in vitro evaluation. J. Ocul. Pharmacol. Ther. 2013, 29, 216–228. [Google Scholar]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2019, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Trabado, J.; López-García, A.; Martín-Pastor, M.; Diebold, Y.; Sanchez, A. Sorbitan ester nanoparticles (SENS) as a novel topical ocular drug delivery system: Design, optimization, and in vitro/ex vivo evaluation. Int. J. Pharm. 2018, 546, 20–30. [Google Scholar]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Tayel, S.A.; El-Nabarawi, M.A.; Tadros, M.I.; Abd-Elsalam, W.H. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: Design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int. J. Pharm. 2013, 443, 293–305. [Google Scholar] [PubMed]
- Moghimipour, E.; Farsimadan, N.; Salimi, A. Ocular Delivery of Quercetin Using Microemulsion System: Design, Characterization, and Ex-Vivo Transcorneal Permeation. Iran. J. Pharm. Res. 2022, 21, e127486. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Kesavan, K. Phase-Transition W/O Microemulsions for Ocular Delivery: Evaluation of Antibacterial Activity in the Treatment of Bacterial Keratitis. Ocul. Immunol. Inflamm. 2017, 25, 463–474. [Google Scholar]
- Soliman, O.A.E.A.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro-in vivo evaluation. Pharm. Dev. Technol. 2019, 24, 48–62. [Google Scholar] [CrossRef]
- Mohammadi, M.; Elahimehr, Z.; Mahboobian, M.M. Acyclovir-Loaded Nanoemulsions: Preparation, Characterization and Irritancy Studies for Ophthalmic Delivery. Curr. Eye Res. 2021, 46, 1646–1652. [Google Scholar] [CrossRef]
- Agha, O.A.; Girgis, G.N.S.; El-Sokkary, M.M.A.; Soliman, O.A.E.A. Spanlastic-laden in situ gel as a promising approach for ocular delivery of Levofloxacin: In-vitro characterization, microbiological assessment, corneal permeability and in-vivo study. Int. J. Pharm. X 2023, 5, 100201. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Safety of surfactant excipients in oral drug formulations. Adv. Drug Deliv. Rev. 2023, 199, 115086. [Google Scholar] [CrossRef]
- Yasser, M.; El Naggar, E.E.; Elfar, N.; Teaima, M.H.; El-Nabarawi, M.A.; Elhabal, S.F. Formulation, optimization and evaluation of ocular gel containing nebivolol HCl-loaded ultradeformable spanlastics nanovesicles: In vitro and in vivo studies. Int. J. Pharm. X 2024, 7, 100228. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, R.; Elhabal, S.F.; Abdelmalak, N.S.; El-Nabarawi, M.A.; Teaima, M.H. Formulation and characterization of acetazolamide/carvedilol niosomal gel for glaucoma treatment: In vitro, and in vivo study. Pharmaceutics 2021, 13, 221. [Google Scholar] [CrossRef]
- Jain, N.; Verma, A.; Jain, N. Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: Intraocular pressure measurement in white albino rabbits. Drug Deliv. 2020, 27, 888–899. [Google Scholar]
- Aldawsari, M.F.; Moglad, E.H.; Alotaibi, H.F.; Alkahtani, H.M.; Khafagy, E.S. Ophthalmic Bimatoprost-Loaded Niosomal In Situ Gel: Preparation, Optimization, and In Vivo Pharmacodynamics Study. Polymers 2023, 15, 4336. [Google Scholar] [CrossRef]
- Sayed, S.; Abdelmoteleb, M.; Amin, M.M.; Khowessah, O.M. Effect of Formulation Variables and Gamma Sterilization on Transcorneal Permeation and Stability of Proniosomal Gels as Ocular Platforms for Antiglaucomal Drug. AAPS PharmSciTech 2020, 21, 67. [Google Scholar] [CrossRef]
- Yousry, C.; Zikry, P.M.; Salem, H.M.; Basalious, E.B.; El-Gazayerly, O.N. Integrated nanovesicular/self-nanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: Statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 801–814. [Google Scholar]
- Shukr, M.H. Novel in situ gelling ocular inserts for voriconazole-loaded niosomes: Design, in vitro characterisation and in vivo evaluation of the ocular irritation and drug pharmacokinetics. J. Microencapsul. 2016, 33, 71–79. [Google Scholar]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of dorzolamide-HCL via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar]
- Jin, Q.; Li, H.; Jin, Z.; Huang, L.; Wang, F.; Zhou, Y.; Liu, Y.; Jiang, C.; Oswald, J.; Wu, J.; et al. TPGS modified nanoliposomes as an effective ocular delivery system to treat glaucoma. Int. J. Pharm. 2018, 553, 21–28. [Google Scholar]
- Ostacolo, C.; Caruso, C.; Tronino, D.; Troisi, S.; Laneri, S.; Pacente, L.; Del Prete, A.; Sacchi, A. Enhancement of corneal permeation of riboflavin-5′-phosphate through vitamin E TPGS: A promising approach in corneal trans-epithelial cross linking treatment. Int. J. Pharm. 2013, 440, 148–153. [Google Scholar]
- Kumbhar, P.S.; Nadaf, S.; Manjappa, A.S.; Jha, N.K.; Shinde, S.S.; Chopade, S.S.; Shete, A.S.; Disouza, J.I.; Sambamoorthy, U.; Kumar, S.A. D-ɑ-tocopheryl polyethylene glycol succinate: A review of multifarious applications in nanomedicines. OpenNano 2022, 7, 100036. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: Formulation development and ocular biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar]
- Caruso, C.; Porta, A.; Tosco, A.; Eletto, D.; Pacente, L.; Bartollino, S.; Costagliola, C. A novel vitamin E TPGS-based formulation enhances chlorhexidine bioavailability in corneal layers. Pharmaceutics 2020, 12, 642. [Google Scholar] [CrossRef] [PubMed]
- Signorini, S.; Pescina, S.; Ricci, C.; del Favero, E.; Vivero-Lopez, M.; Alvarez-Lorenzo, C.; Santi, P.; Padula, C.; Nicoli, S. Innovative formulations for the ocular delivery of coenzyme Q10. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef]
- Lam, C.H.I.; Zuo, B.; Chan, H.H.L.; Leung, T.W.; Abokyi, S.; Catral, K.P.C.; Tse, D.Y.Y. Coenzyme Q10 eyedrops conjugated with vitamin E TPGS alleviate neurodegeneration and mitochondrial dysfunction in the diabetic mouse retina. Front. Cell. Neurosci. 2024, 18, 1404987. [Google Scholar] [CrossRef]
- Guo, P.; Li, N.; Fan, L.; Lu, J.; Liu, B.; Zhang, B.; Wu, Y.; Liu, Z.; Li, J.; Pi, J.; et al. Study of penetration mechanism of labrasol on rabbit cornea by Ussing chamber, RT-PCR assay, Western blot and immunohistochemistry. Asian J. Pharm. Sci. 2019, 14, 329–339. [Google Scholar]
- Liu, Z.; Zhang, X.; Li, J.; Liu, R.; Shu, L.; Jin, J. Effects of Labrasol on the corneal drug delivery of baicalin. Drug Deliv. 2009, 16, 399–404. [Google Scholar]
- Huang, L.; Bai, J.; Yang, H.; Liu, J.; Cui, H. Combined use of borneol or menthol with labrasol promotes penetration of baicalin through rabbit cornea in vitro. Eur. J. Pharm. Sci. 2015, 77, 1–8. [Google Scholar]
- Ibrahim, M.M.; Maria, D.N.; Wang, X.D.; Simpson, R.N.; Hollingsworth, T.J.; Jablonski, M.M. Enhanced corneal penetration of a poorly permeable drug using bioadhesive multiple microemulsion technology. Pharmaceutics 2020, 12, 704. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Shu, L.; Zhang, C.; Zhang, B. Effect of three penetration enhancers on corneal permeability of mangiferin in vitro. Zhongguo Zhongyao Zazhi 2010, 35, 3131–3135. [Google Scholar]
- Montenegro, L.; Bucolo, C.; Puglisi, G.; Montenegro, L. Enhancer effects on in vitro corneal permeation of timolol and acyclovir. Int. J. Pharm. 2003, 263, 23–28. [Google Scholar]
- Li, X.; Pan, W.; Ju, C.; Liu, Z.; Pan, H.; Zhang, H.; Nie, S. Evaluation of Pharmasolve® corneal permeability enhancement and its irritation on rabbit eyes. Drug Deliv. 2009, 16, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Méndez, N.; Chavez-Garay, D.R.; Leal-Ramos, M.Y. Lecithins: A comprehensive review of their properties and their use in formulating microemulsions. J. Food Biochem. 2022, 46, e14157. [Google Scholar] [CrossRef]
- List, G.R. Soybean Lecithin: Food, Industrial Uses, and Other Applications. In Polar Lipids: Biology, Chemistry, and Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–33. [Google Scholar]
- Caparosa, M.H.; Hartel, R.W. Characterizing Lecithin Interactions in Chocolate Using Interfacial Properties and Rheology. J. Am. Oil Chem. Soc. 2020, 97, 1309–1317. [Google Scholar] [CrossRef]
- Kent, C. Regulatory enzymes of phosphatidylcholine biosynthesis: A personal perspective. Biochim. Biophys. Acta 2005, 1733, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Vance, D.E. Dimethylethanolamine does not prevent liver failure in phosphatidylethanolamine N-methyltransferase-deficient mice fed a choline-deficient diet. Biochim. Biophys. Acta 2004, 1636, 175–182. [Google Scholar] [CrossRef]
- Exton, J.H. Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1994, 1212, 26–42. [Google Scholar] [CrossRef]
- Spernath, A.; Aserin, A.; Ziserman, L.; Danino, D.; Garti, N. Phosphatidylcholine embedded microemulsions: Physical properties and improved Caco-2 cell permeability. J. Control. Release 2007, 119, 279–290. [Google Scholar] [CrossRef]
- Chetoni, P.; Monti, D.; Tampucci, S.; Matteoli, B.; Ceccherini-Nelli, L.; Subissi, A.; Burgalassi, S. Liposomes as a potential ocular delivery system of distamycin A. Int. J. Pharm. 2015, 492, 120–126. [Google Scholar] [CrossRef]
- Tan, G.; Yu, S.; Pan, H.; Li, J.; Liu, D.; Yuan, K.; Yang, X.; Pan, W. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. Int. J. Biol. Macromol. 2017, 94, 355–363. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Sharma, S. Formulation, characterization, optimization and in-vivo evaluation of methazolamide liposomal in-situ gel for treating glaucoma. J. Drug Deliv. Sci. Technol. 2022, 67, 102951. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, T.; Wu, Y.; Wang, X.; Liu, R.; Jin, X. mPEG-CS-modified flexible liposomes-reinforced thermosensitive sol-gel reversible hydrogels for ocular delivery of multiple drugs with enhanced synergism. Colloids Surf. B 2023, 222, 113560. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular benzalkonium chloride exposure: Problems and solutions. Eye 2022, 36, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, F.; Schito, A.M.; Milanese, M.; Castellaro, S.; Alfei, S. Anti Gram-Positive Bacteria Activity of Synthetic Quaternary Ammonium Lipid and Its Precursor Phosphonium Salt. Int. J. Mol. Sci. 2024, 25, 2761. [Google Scholar] [CrossRef] [PubMed]
- Karamov, E.V.; Larichev, V.F.; Kornilaeva, G.V.; Fedyakina, I.T.; Turgiev, A.S.; Shibaev, A.V.; Molchanov, V.S.; Philippova, O.E.; Khokhlov, A.R. Cationic Surfactants as Disinfectants Against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 6645. [Google Scholar] [CrossRef]
- Barros, A.C.; Melo, L.F.; Pereira, A. A Multi-Purpose Approach to the Mechanisms of Action of Two Biocides (Benzalkonium Chloride and Dibromonitrilopropionamide): Discussion of Pseudomonas Fluorescens’ Viability and Death. Front. Microbiol. 2022, 13, 842414. [Google Scholar] [CrossRef]
- Schito, A.M.; Piatti, G.; Caviglia, D.; Zuccari, G.; Alfei, S. Broad-spectrum bactericidal activity of a synthetic random copolymer based on 2-methoxy-6-(4-vinylbenzyloxy)benzylammonium hydrochloride. Int. J. Mol. Sci. 2021, 22, 5021. [Google Scholar] [CrossRef]
- McCarlie, S.J.; du Preez, L.L.; Hernandez, J.C.; Boucher, C.E.; Bragg, R.R. Transcriptomic signature of bacteria exposed to benzalkonium chloride. Res. Microbiol. 2024, 175, 104151. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Green, K.; Chapman, J. Benzalkonium chloride kinetics in young and adult albino and pigmented rabbit eyes. Cutan. Ocul. Toxicol. 1986, 5, 133–142. [Google Scholar]
- Thacker, M.; Sahoo, A.; Reddy, A.A.; Bokara, K.K.; Singh, S.; Basu, S.; Singh, V. Benzalkonium chloride-induced dry eye disease animal models: Current understanding and potential for translational research. Indian J. Ophthalmol. 2023, 71, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Koev, K.; Kutsarova, E.; Ivanova, S.; Kyumurkov, A.; Jordanova, A.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of benzalkonium chloride with films of meibum, corneal cells lipids, and whole tears. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Tonjum, A. Influence of various agents on corneal permeability. Am. J. Ophthalmol. 1971, 72, 897–905. [Google Scholar] [PubMed]
- Majumdar, S.; Hippalgaonkar, K.; Repka, M.A. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int. J. Pharm. 2008, 348, 175–178. [Google Scholar]
- Roscoe, W.R.; Durstein, N.L. Preservative Alteration of Corneal Permeability in Humans and Rabbits. J. Toxicol. Cutan. Ocul. Toxicol. 1984, 5, 1453–1457. [Google Scholar]
- Saettone, M.F.; Chetoni, P.; Cerbai, R.; Mazzanti, G.; Braghiroli, L. Evaluation of ocular permeation enhancers: In vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int. J. Pharm. 1996, 142, 103–113. [Google Scholar]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Kristinsdottir, I.M.; Asgrimsdottir, G.M.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: The effect of benzalkonium chloride on topical dexamethasone penetration into the eye in vivo. J. Drug Deliv. Sci. Technol. 2018, 48, 125–127. [Google Scholar]
- Rouland, J.F.; Traverso, C.E.; Stalmans, I.; El Fekih, L.; Delval, L.; Renault, D.; Baudouin, C. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br. J. Ophthalmol. 2013, 97, 196–200. [Google Scholar]
- Aptel, F.; Choudhry, R.; Stalmans, I. Preservative-free versus preserved latanoprost eye drops in patients with open-angle glaucoma or ocular hypertension. Curr. Med. Res. Opin. 2016, 32, 1457–1463. [Google Scholar]
- Goldberg, I.; Gil Pina, R.; Lanzagorta-Aresti, A.; Schiffman, R.M.; Liu, C.; Bejanian, M. Bimatoprost 0.03%/timolol 0.5% preservative-free ophthalmic solution versus bimatoprost 0.03%/timolol 0.5% ophthalmic solution (Ganfort) for glaucoma or ocular hypertension: A 12-week randomised controlled trial. Br. J. Ophthalmol. 2014, 98, 926–931. [Google Scholar]
- Peace, J.H.; Ahlberg, P.; Wagner, M.; Lim, J.M.; Wirta, D.; Branch, J.D. Polyquaternium-1-Preserved Travoprost 0.003% or Benzalkonium Chloride-Preserved Travoprost 0.004% for Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2015, 160, 266–274.e1. [Google Scholar] [PubMed]
- Cordeiro, M.F.; Goldberg, I.; Schiffman, R.; Bernstein, P.; Bejanian, M. Efficacy of a preservative-free formulation of fixed-combination bimatoprost and timolol (Ganfort PF) in treatment-naïve patients vs previously treated patients. Clin. Ophthalmol. 2015, 9, 1605–1611. [Google Scholar]
- Tokuda, N.; Kitaoka, Y.; Matsuzawa, A.; Tsukamoto, A.; Sase, K.; Sakae, S.; Takagi, H. Changes in Ocular Surface Characteristics after Switching from Benzalkonium Chloride-Preserved Latanoprost to Preservative-Free Tafluprost or Benzalkonium Chloride-Preserved Tafluprost. J. Ophthalmol. 2017, 2017, 3540749. [Google Scholar] [CrossRef] [PubMed]
- Amiri, D.; Sessa, M.; Andersen, M.; Kolko, M. Persistence and adherence with Latanoprost: A Danish register-based cohort study in older patients with glaucoma. Acta Ophthalmol. 2024, 102, 172–178. [Google Scholar]
- Aptel, F.; Pfeiffer, N.; Schmickler, S.; Clarke, J.; Lavín-Dapena, C.; Moreno-Montañés, J.; Zarnowski, T.; Csutak, A.; Jugaste, T.; Volksone, L.; et al. Noninferiority of Preservative-free Versus BAK-preserved Latanoprost-timolol Fixed Combination Eye Drops in Patients with Open-angle Glaucoma or Ocular Hypertension. J. Glaucoma 2019, 28, 498–506. [Google Scholar]
- Kitazawa, Y.; Smith, P.; Sasaki, N.; Kotake, S.; Bae, K.; Iwamoto, Y. Travoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: A prospective, randomized, doubled-masked comparison of safety and efficacy. Eye 2011, 25, 1161–1169. [Google Scholar] [PubMed]
- Lewis, R.A.; Katz, G.J.; Weiss, M.J.; Landry, T.A.; Dickerson, J.E.; James, J.E.; Hua, S.Y.; Sullivan, E.K.; Montgomery, D.B.; Wells, D.T.; et al. Travoprost 0.004% With and Without Benzalkonium Chloride: A Comparison of Safety and Efficacy. J. Glaucoma 2007, 16, 98–103. [Google Scholar]
- Tonjum, A.M. Effects of Benzalkonium Chloride Upon The Corneal Epithelium Studied with Scanning Electron Microscopy. Acta Ophthalmol. 1975, 53, 358–366. [Google Scholar] [CrossRef]
- Debbasch, C.; Brignole, F.; Pisella, P.-J.; Warnet, J.-M.; Rat, P.; Baudouin, C. Quaternary Ammoniums and Other Preservatives’ Contribution in Oxidative Stress and Apoptosis on Chang Conjunctival Cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 642–652. [Google Scholar]
- Rogov, A.G.; Goleva, T.N.; Sukhanova, E.I.; Epremyan, K.K.; Trendeleva, T.A.; Ovchenkova, A.P.; Aliverdieva, D.A.; Zvyagilskaya, R.A. Mitochondrial Dysfunctions May Be One of the Major Causative Factors Underlying Detrimental Effects of Benzalkonium Chloride. Oxid. Med. Cell. Longev. 2020, 2020, 8956504. [Google Scholar] [CrossRef]
- Datta, S.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A.; Cortopassi, G.A. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2406–2412. [Google Scholar]
- Ammar, D.A.; Kahook, M.Y. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br. J. Ophthalmol. 2011, 95, 1466–1469. [Google Scholar]
- Ammar, D.A.; Noecker, R.J.; Kahook, M.Y. Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 2010, 27, 837–845. [Google Scholar]
- Ayaki, M.; Iwasawa, A. Cytotoxicity of prostaglandin analog eye drops preserved with benzalkonium chloride in multiple corneoconjunctival cell lines. Clin. Ophthalmol. 2010, 4, 919–924. [Google Scholar] [PubMed]
- Ayaki, M.; Iwasawa, A.; Inoue, Y. Toxicity of antiglaucoma drugs with and without benzalkonium chloride to cultured human corneal endothelial cells. Clin. Ophthalmol. 2010, 4, 1217–1222. [Google Scholar]
- Guzman-Aranguez, A.; Calvo, P.; Ropero, I.; Pintor, J. In vitro effects of preserved and unpreserved anti-allergic drugs on human corneal epithelial cells. J. Ocul. Pharmacol. Ther. 2014, 30, 790–798. [Google Scholar]
- Kim, J.H.; Kim, E.J.; Kim, Y.H.; Kim, Y.I.; Lee, S.H.; Jung, J.C.; Lee, K.W.; Park, Y.J. In Vivo Effects of Preservative-free and Preserved Prostaglandin Analogs: Mouse Ocular Surface Study. Korean J. Ophthalmol. 2015, 29, 270–279. [Google Scholar]
- Kim, Y.-H.; Jung, J.-C.; Jung, S.-Y.; Yu, S.; Lee, K.W.; Park, Y.J. Comparison of the Efficacy of Fluorometholone With and Without Benzalkonium Chloride in Ocular Surface Disease. J. Ocul. Pharmacol. Ther. 2015, 31, 634–641. [Google Scholar]
- Pauly, A.; Brasnu, E.; Riancho, L.; Brignole-Baudouin, F.; Baudouin, C. Multiple endpoint analysis of BAC-preserved and unpreserved antiallergic eye drops on a 3D-reconstituted corneal epithelial model. Mol. Vis. 2011, 17, 1575–1583. [Google Scholar]
- Izzotti, A.; La Maestra, S.; Micale, R.T.; Longobardi, M.G.; Saccà, S.C. Genomic and post-genomic effects of anti-glaucoma drugs preservatives in trabecular meshwork. Mutat. Res. 2015, 772, 1–9. [Google Scholar] [CrossRef]
- Kahook, M.Y.; Noecker, R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv. Ther. 2008, 25, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Brignole-Baudouin, F.; Riancho, L.; Baudouin, C. Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res. 2012, 48, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hedengran, A.; Freiberg, J.; May Hansen, P.; Boix-Lemonche, G.; Utheim, T.P.; Dartt, D.A.; Petrovski, G.; Heegaard, S.; Kolko, M. Comparing the effect of benzalkonium chloride-preserved, polyquad-preserved, and preservative-free prostaglandin analogue eye drops on cultured human conjunctival goblet cells. J. Optom. 2024, 17, 100481. [Google Scholar] [CrossRef]
- Nagai, N.; Murao, T.; Okamoto, N.; Ito, Y. Comparison of Corneal Wound Healing Rates after Instillation of Commercially Available Latanoprost and Travoprost in Rat Debrided Corneal Epithelium. J. Oleo Sci. 2010, 59, 135–141. [Google Scholar] [CrossRef]
- Jaenen, N.; Baudouin, C.; Pouliquen, P.; Manni, G.; Figueiredo, A.; Zeyen, T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 2007, 17, 341–349. [Google Scholar] [CrossRef]
- Uusitalo, H.; Egorov, E.; Kaarniranta, K.; Astakhov, Y.; Ropo, A. Benefits of switching from latanoprost to preservative-free tafluprost eye drops: A meta-analysis of two phase IIIb clinical trials. Clin. Ophthalmol. 2016, 10, 445–454. [Google Scholar] [CrossRef]
- Horsley, M.B.; Kahook, M.Y. Effects of prostaglandin analog therapy on the ocular surface of glaucoma patients. Clin. Ophthalmol. 2009, 3, 291–295. [Google Scholar] [PubMed]
- Hommer, A.; Kimmich, F. Switching patients from preserved prostaglandin-analog monotherapy to preservative-free tafluprost. Clin. Ophthalmol. 2011, 5, 623–631. [Google Scholar]
- Lopes, N.L.V.; Gracitelli, C.P.B.; Chalita, M.R.; Faria, N.V.L. Ocular Surface Evaluation After the Substitution of Benzalkonium Chloride Preserved Prostaglandin Eye Drops by a Preservative-free Prostaglandin Analogue. Discov. Innov. Ophthalmol. J. 2019, 8, 52. [Google Scholar]
- Rossi, G.C.M.; Scudeller, L.; Rolle, T.; Pasinetti, G.M.; Bianchi, P.E. From benzalkonium chloride-preserved Latanoprost to Polyquad-preserved Travoprost: A 6-month study on ocular surface safety and tolerability. Expert Opin. Drug Saf. 2015, 14, 619–623. [Google Scholar] [CrossRef]
- Aihara, M.; Oshima, H.; Araie, M. Effects of SofZia-preserved travoprost and benzalkonium chloride-preserved latanoprost on the ocular surface—A multicentre randomized single-masked study. Acta Ophthalmol. 2013, 91, e7–e14. [Google Scholar] [CrossRef]
- Tomić, M.; Kaštelan, S.; Metež Soldo, K.; Salopek-Rabatić, J. Influence of BAK-preserved prostaglandin analog treatment on the ocular surface health in patients with newly diagnosed primary open-angle glaucoma. Biomed Res. Int. 2013, 2013, 603782. [Google Scholar] [CrossRef]
- Economou, M.A.; Laukeland, H.K.; Grabska-Liberek, I.; Rouland, J.F. Better tolerance of preservative-free latanoprost compared to preserved glaucoma eye drops: The 12-month real-life FREE study. Clin. Ophthalmol. 2018, 12, 2399–2407. [Google Scholar] [PubMed]
- Lazreg, S.; Merad, Z.; Nouri, M.T.; Garout, R.; Derdour, A.; Ghroud, N.; Kherroubi, R.; Meziane, M.; Belkacem, S.; Ouhadj, O.; et al. Efficacy and safety of preservative-free timolol 0.1% gel in open-angle glaucoma and ocular hypertension in treatment-naïve patients and patients intolerant to other hypotensive medications. J. Fr. Ophtalmol. 2018, 41, 945–954. [Google Scholar] [CrossRef]
- Aihara, M.; Ikeda, Y.; Mizoue, S.; Arakaki, Y.; Kita, N.; Kobayashi, S. Effect of switching to travoprost preserved with SofZia in glaucoma patients with chronic superficial punctate keratitis while receiving BAK-preserved latanoprost. J. Glaucoma 2016, 25, e610–e614. [Google Scholar] [PubMed]
- Noecker, R. Effects of Common Ophthalmic Preservatives on Ocular Health. Adv. Ther. 2001, 18, 205–215. [Google Scholar]
- Chandran, S.; Roy, A.; Saha, R.N. Effect of pH and Formulation Variables on In Vitro Transcorneal Permeability of Flurbiprofen: A Technical Note. AAPS PharmSciTech 2008, 9, 1031–1034. [Google Scholar]
- Camber, O.; Edman, P. Influence of some preservatives on the corneal permeability of pilocarpine and dexamethasone, in vitro. Int. J. Pharm. 1987, 39, 229–234. [Google Scholar]
- Gasset, A.R.; Ishii, Y.; Kaufman, H.E.; Miller, T. Cytotoxicity of ophthalmic preservatives. Am. J. Ophthalmol. 1974, 78, 98–105. [Google Scholar] [CrossRef]
- Mao, X.; Aue, D.L.; Buchalla, W.; Hiller, K.A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Riveira-Muñoz, E.; Garcia-Vidal, E.; Bañó-Polo, M.; León, R.; Blanc, V.; Clotet, B.; Ballana, E. Cetylpyridinium Chloride-Containing Mouthwashes Show Virucidal Activity against Herpes Simplex Virus Type 1. Viruses 2023, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Moro, M.; Saracino, M.; Marmiere, M.; Cilona, M.B.; Lloyd-Jones, G.; Zangrillo, A. Efficacy of Cetylpyridinium Chloride mouthwash against SARS-CoV-2: A systematic review of randomized controlled trials. Mol. Oral Microbiol. 2023, 38, 171–180. [Google Scholar]
- Godbey, R.E.W.; Green, K.; Hull, D.S. Influence of Cetylpyridinium Chloride on Corneal Permeability to Penicillin. J. Pharm. Sci. 1973, 62, 177–179. [Google Scholar]
- Green, K.; Bowman, K.A.; Elijah, R.D.; Mermelstein, R.; Kilpper, R.W. Dose-effect response of the rabbit Eye to cetylpyridinium chloride. Cutan. Ocul. Toxicol. 1985, 4, 13–26. [Google Scholar]
- Li, X.; Muller, R.H.; Keck, C.M.; Bou-Chacra, N.A. Mucoadhesive dexamethasone acetate-polymyxin B sulfate cationic ocular nanoemulsion—Novel combinatorial formulation concept. Pharmazie 2016, 71, 327–333. [Google Scholar]
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of cationic nanocrystals for ocular delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222. [Google Scholar] [PubMed]
- Karpinski, T.M.; Szkaradkiewicz, A.K. Chlorhexidine—Pharmaco-biological activity and application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar]
- Ashton, P.; Diepold, R.; Platzer, A.; Lee, V.H.L. The Effect of Chlorhexidine Acetate on the Corneal Penetration of Sorbitol from an Arnolol Formulation in the Albino Rabbit. J. Ocul. Pharmacol. 1990, 6, 27–33. [Google Scholar]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar]
- Steinsapir, K.D.; Woodward, J.A. Chlorhexidine Keratitis: Safety of Chlorhexidine as a Facial Antiseptic. Dermatol. Surg. 2017, 43, 1–6. [Google Scholar]
- Palka, L.; Nowakowska-Toporowska, A.; Dalewski, B. Is Chlorhexidine in Dentistry an Ally or a Foe? A Narrative Review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Review: Perspective on ocular toxicity of presurgical skin preparations utilizing Chlorhexidine Gluconate/Hibiclens/Chloraprep. Surg. Neurol. Int. 2021, 12, 566. [Google Scholar] [CrossRef]
- Bever, G.J.; Brodie, F.L.; Hwang, D.G. Corneal Injury from Presurgical Chlorhexidine Skin Preparation. World Neurosurg. 2016, 96, 610.e1–610.e4. [Google Scholar] [CrossRef]
- Romano, V.; Ferrara, M.; Gatti, F.; Airaldi, M.; Borroni, D.; Aragona, E.; Rocha-de-Lossada, C.; Gabrielli, F.; Papa, F.T.; Romano, M.R.; et al. Topical Antiseptics in Minimizing Ocular Surface Bacterial Load Before Ophthalmic Surgery: A Randomized Controlled Trial. Am. J. Ophthalmol. 2024, 261, 165–175. [Google Scholar] [CrossRef]
- de Buy Wenniger, L.M.; Pusl, T.; Beuers, U. Bile Salts. In Encyclopedia of Biological Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 167–171. [Google Scholar]
- Tazuma, S.; Takikawa, H. Bile Acids in Gastroenterology: Basic and Clinical; Springer: Tokyo, Japan, 2017. [Google Scholar] [CrossRef]
- Boatright, J.H.; Nickerson, J.M.; Moring, A.G.; Pardue, M.T. Bile acids in treatment of ocular disease. J. Ocul. Biol. Dis. Inform. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Valderrama, J.; Wilde, P.; MacIerzanka, A.; MacKie, A. The role of bile salts in digestion. Adv. Colloid Interface Sci. 2011, 165, 36–46. [Google Scholar] [CrossRef]
- Monte, M.J.; Marin, J.J.G.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.C.; Filipe, H.A.L.; Reis, R.L.; Ramalho, J.P.P.; Coreta-Gomes, F.; Moreno, M.J.; Loura, L.M.S. Interaction of bile salts with lipid bilayers: An atomistic molecular dynamics study. Front. Physiol. 2019, 10, 393. [Google Scholar] [CrossRef]
- Malik, N.A. Solubilization and Interaction Studies of Bile Salts with Surfactants and Drugs: A Review. Appl. Biochem. Biotechnol. 2016, 179, 179–201. [Google Scholar] [CrossRef]
- Macierzanka, A.; Torcello-Gómez, A.; Jungnickel, C.; Maldonado-Valderrama, J. Bile salts in digestion and transport of lipids. Adv. Colloid Interface Sci. 2019, 274, 102045. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.; Goločorbin-Kon, S.; Danić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Size and Structure of Bile Salt Micelles. In Bile Salt Chemistry; Plenum Press: New York, NY, USA, 1968; pp. 31–52. [Google Scholar]
- Faustino, C.; Serafim, C.; Rijo, P.; Reis, C.P. Bile acids and bile acid derivatives: Use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Deliv. 2016, 13, 1133–1148. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Müllertz, A.; Mu, H. Bile salts and their importance for drug absorption. Int. J. Pharm. 2013, 453, 44–55. [Google Scholar] [CrossRef]
- Dai, Y.; Zhou, R.; Liu, L.; Lu, Y.; Qi, J.; Wu, W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): In vitro characterization and improved corneal permeation. Int. J. Nanomed. 2013, 8, 1921–1933. [Google Scholar]
- Hayakawa, E.; Chien, D.S.; Inagaki, K.; Yamamoto, A.; Wang, W.; Lee, V.H.L. Conjunctival Penetration of Insulin and Peptide Drugs in the Albino Rabbit. Pharm. Res. 1992, 9, 769–775. [Google Scholar]
- Rojanasakul, Y.; Liaw, J.; Robinson, J.R. Mechanisms of action of some penetration enhancers in the cornea: Laser scanning confocal microscopic and electrophysiology studies. Int. J. Pharm. 1990, 66, 131–142. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [PubMed]
- Behl, T.; Kumar, K.; Brisc, C.; Rus, M.; Nistor-Cseppento, D.C.; Bustea, C.; Aron, R.A.C.; Pantis, C.; Zengin, G.; Sehgal, A.; et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed. Pharmacother. 2021, 133, 110959. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Farcas, A.C.; Socaci, S.A.; Tofana, M.; Diaconeasa, Z.M.; Pop, O.L.; Salanta, L.C. An Overview of Saponins—A Bioactive Group. Bull. UASVM Food Sci. Technol. 2020, 77, 25–36. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P. Flavonoids and saponins: What have we got or missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [CrossRef] [PubMed]
- Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Zhang, X.; Wang, M.; Liu, Y.; Shi, Y. Potentials of saponins-based adjuvants for nasal vaccines. Front. Immunol. 2023, 14, 1153042. [Google Scholar] [CrossRef]
- Grizzle, W.E. Systemic absorption of insulin delivered topically to the rat eye. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3021–3027. [Google Scholar]
- Pillion, D.J.; Recchia, J.; Wang, P.; Marcianit, D.J.; Kensils, C.R. DS-1, a Modified Quillaja Saponin, Enhances Ocular and Nasal Absorption of Insulin. J. Pharm. Sci. 1995, 84, 1276–1279. [Google Scholar] [CrossRef]
- Recchia, J.; Lurantos, M.H.A.; Amsden, J.A.; Storey, J.; Kensil, C.R. A Semisynthetic Quillaja Saponin as a Drug Delivery Agent for Aminoglycoside Antibiotics. Pharm. Res. 1995, 12, 1917–1923. [Google Scholar]
- Sasaki, H.; Igarashi, Y.; Nagano, T.; Nishida, K.; Nakamura, J. Different Effects of Absorption Promoters on Corneal and Conjunctival Penetration of Ophthalmic Beta-Blockers. Pharm. Res. 1995, 12, 1146–1150. [Google Scholar] [CrossRef]
- Lu, P.; Wang, R.; Xing, Y.; Gao, Y.; Zhang, Q.; Xing, B.; Zhang, Y.; Yu, C.; Cai, X.; Shang, Q.; et al. Development and evaluation of Panax notoginseng saponins contained in an in situ pH-Triggered gelling system for sustained ocular posterior segment drug delivery. Acupunct. Herb. Med. 2021, 1, 107–121. [Google Scholar]
- Sasaki, H.; Yamamura, K.; Tei, C.; Nishida, K.; Nakamura, J. Ocular Permeability of FITC-Dextran with Absorption Promoter for Ocular Delivery of Peptide Drug. J. Drug Target. 1995, 3, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yamamura, K.; Mukai, T.; Nishida, K.; Nakamura, J.; Nakashima, M.; Ichikawa, M. Modification of Ocular Permeability of Peptide Drugs by Absorption Promoters. Biol. Pharm. Bull. 2000, 23, 1524–1527. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Lan, J.; Li, X.; Xin, M.; Wang, H.; Zhang, F.; Lu, X.; Zhuang, Z.; Wu, X. Novel ultra-small micelles based on ginsenoside Rb1: A potential nanoplatform for ocular drug delivery. Drug Deliv. 2019, 26, 481–489. [Google Scholar] [CrossRef]
- Sudji, I.R.; Subburaj, Y.; Frenkel, N.; García-Sáez, A.J.; Wink, M. Membrane disintegration caused by the steroid saponin digitonin is related to the presence of cholesterol. Molecules 2015, 20, 20146–20160. [Google Scholar] [CrossRef] [PubMed]
- Wolosin, J.M. Membrane Biology Regeneration of Resistance and Ion Transport in Rabbit Corneal Epithelium after Induced Surface Cell Exfoliation. J. Membr. Biol. 1988, 104, 45–56. [Google Scholar] [CrossRef]
- Zuurendonk, P.F.; Tager, J.M. Rapid separation of particulate components and soluble cytoplasm of isolated rat-liver cells. Biochim. Biophys. Acta 1974, 333, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, W.P.; Cockrell, R.S. Ca2+ transport across plasma and mitochondrial membranes of isolated hepatocytes. FEBS Lett. 1975, 59, 39–43. [Google Scholar] [CrossRef]
- Siess, E.A.; Wieland, O.H. Phosphorylation State of Cytosolic and Mitochondrial Adenine Nucleotides and of Pyruvate Dehydrogenase in Isolated Rat Liver Cells. Biochem. J. 1976, 156, 91–102. [Google Scholar] [CrossRef]
- Murphy, E.; Coll, K.; Rich, T.L.; Williamson, J.R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J. Biol. Chem. 1980, 255, 6600–6608. [Google Scholar] [CrossRef]
- Akiyama, T.; Takagi, S.; Sankawa, U.; Inari, S.; Saito, H. Saponin-Cholesterol Interaction in the Multibilayers of Egg Yolk Lecithin As Studied by Deuterium Nuclear Magnetic Resonance: Digitonin and Its Analogues. Biochemistry 1980, 19, 1904–1911. [Google Scholar] [CrossRef]
- Frenkel, N.; Makky, A.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic investigation of interactions between steroidal saponin digitonin and cell membrane models. J. Phys. Chem. B 2014, 118, 14632–14639. [Google Scholar]
- Liaw, J.; Robinson, J.R. The effect of polyethylene glycol molecular weight on corneal transport and the related influence of penetration enhancers. Int. J. Pharm. 1992, 88, 125–140. [Google Scholar] [CrossRef]
- Thiel, M.A.; Coster, D.J.; Standfield, S.D.; Brereton, H.M.; Mavrangelos, C.; Zola, H.; Taylor, S. Penetration of engineered antibody fragments into the eye. Clin. Exp. Immunol. 2002, 128, 67–74. [Google Scholar] [PubMed]
- Gallelli, L.; Cione, E.; Wang, T.; Zhang, L. Glucocorticoid-like activity of escin: A new mechanism for an old drug. Drug Des. Devel. Ther. 2021, 15, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Jiang, N.; Wang, Z.; Chong, Y.; Fu, F. Anti-inflammatory effects of escin are correlated with the glucocorticoid receptor/NF-κB signaling pathway, but not the COX/PGF2α signaling pathway. Exp. Ther. Med. 2013, 6, 419–422. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Xu, S.Q.; Cheng, J.; Cao, X.L.; Zhang, Y.; Zhou, W.P.; Huang, Y.J.; Wang, J.; Hu, X.M. Anti-inflammatory effect of external use of escin on cutaneous inflammation: Possible involvement of glucocorticoids receptor. Chin. J. Nat. Med. 2018, 16, 105–112. [Google Scholar]
- Gallelli, L. Escin: A review of its anti-edematous, antiinflammatory, and venotonic properties. Drug Des. Devel. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Y.; Wang, W.; Ma, J.; Chen, M. Escin activates AKT-Nrf2 signaling to protect retinal pigment epithelium cells from oxidative stress. Biochem. Biophys. Res. Commun. 2015, 468, 541–547. [Google Scholar] [CrossRef]
- Zhang, F.; Man, X.; Yu, H.; Liu, L.; Li, Y. Synergistic protective effects of escin and low dose glucocorticoids against vascular endothelial growth factor induced blood retinal barrier breakdown in retinal pigment epithelial and umbilical vein endothelial cells. Mol. Med. Rep. 2015, 11, 1372–1377. [Google Scholar]
- Zhang, F.; Li, Y.; Zhang, L.; Mu, G. Synergistic protective effects of escin and low-dose glucocorticoids on blood-retinal barrier breakdown in a rat model of retinal ischemia. Mol. Med. Rep. 2013, 7, 1511–1515. [Google Scholar]
- Burgalassi, S.; Monti, D.; Brignoccoli, A.; Fabiani, O.; Lenzi, C.; Pirone, A.; Chetoni, P. Development of Cultured Rabbit Corneal Epithelium for Drug Permeation Studies: A Comparison with Excised Rabbit Cornea. J. Ocul. Pharmacol. Ther. 2004, 20, 514–524. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [PubMed]
- Narasimha Murthy, S.; Shivakumar, H.N. Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–36. [Google Scholar]
- Beastall, J.; Washington, C. Mechanism of action of Azone as a percutaneous penetration enhancer: Lipid bilayer fluidity and transition temperature effects. Int. J. Pharm. 1988, 43, 207–213. [Google Scholar]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. A review. Ski. Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Tang-Liu, D.D.-S.; Richman, J.B.; Weinkam, R.J.; Takruri, H. Effects of Four Penetration Enhancers on Corneal Permeability of Drugs in Vitro. J. Pharm. Sci. 1994, 83, 85–90. [Google Scholar] [CrossRef]
- Newton, C.; Gebhardt, B.M.; Kaufman, H.E. Topically Applied Cyclosporine in Azone Prolongs Corneal Allograft Survival. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1373–1376. [Google Scholar]
- Ismail, M.I.; Chen, C.-C.; Richman, J.B.; Andersen, J.S.; Tang-Liu, D.D.-S. Comparison of Azone and Hexamethylene Lauramide in Toxicologic Effects and Penetration Enhancement of Cimetidine in Rabbit Eyes. Pharm. Res. 1992, 9, 817–821. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, S.; Hen, H.; Du, L.; Li, G.; Li, B.; Zhang, H. Corneal permeability assay of topical eye drop solutions in rabbits by MRI. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 804–808. [Google Scholar] [CrossRef]
- Tang-Liu, D.D.-S.; Burke, P.J. The effect of azone on ocular levobunolol absorption: Calculating the area under the curve and its standard error using tissue sampling compartments. Pharm. Res. 1988, 5, 238–241. [Google Scholar] [PubMed]
- Afouna, M.I.; Hussein, A.K.; Ahmed, O.A. Influence of the Interplay between AzoneTM as Permeation Enhancer and Carbopol-974® as a Mucoadhesive upon the in vitro Transcorneal Release and the in vivo Antiglaucoma Effect of S-Timolol Maleate Ophthalmic Gel Formulations. Int. J. PharmTech Res. 2014, 6, 298–315. [Google Scholar]
- Afouna, M.I.; Roshdy, H.R.; Ibrahim, H.M.; Naim, A.B.; El-Marzoqi, A. Maximization of the in vitro transcorneal release and the in vivo IOP-lowering effects of Latanoprost ophthalmic gel formulations using Azone as a penetration enhancer and Carbopol-974® as a mucoadhesive. Drug Dev. Ind. Pharm. 2016, 42, 1963–1973. [Google Scholar]
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [PubMed]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar]
- Jiang, K.; Gao, X.; Shen, Q.; Zhan, C.; Zhang, Y.; Xie, C.; Wei, G.; Lu, W. Discerning the composition of penetratin for safe penetration from cornea to retina. Acta Biomater. 2017, 63, 123–134. [Google Scholar]
- Brasseur, R.; Divita, G. Happy birthday cell penetrating peptides: Already 20years. Biochim. Biophys. Acta Biomembr. 2010, 1798, 2177–2181. [Google Scholar]
- Jallouk, A.P.; Palekar, R.U.; Pan, H.; Schlesinger, P.H.; Wickline, S.A. Modifications of Natural Peptides for Nanoparticle and Drug Design. Adv. Protein Chem. Struct. Biol. 2015, 98, 57–91. [Google Scholar]
- Liu, C.; Tai, L.; Zhang, W.; Wei, G.; Pan, W.; Lu, W. Penetratin, a potentially powerful absorption enhancer for noninvasive intraocular drug delivery. Mol. Pharm. 2014, 11, 1218–1227. [Google Scholar]
- Morofuji, R.; Enomoto, H.; Honda, T.; Oyama, Y.; Ishida, R.; Kudo, K.; Okabe, K. Exploring Cell-Penetrating Peptides as Penetration Enhancers in Eye Drop Formulations Using a Reconstructed Human Corneal Epithelial Model. Biol. Pharm. Bull. 2023, 46, 1720–1730. [Google Scholar]
- Morofuji, R.; Kudo, K.; Honda, T.; Kinugasa, S.; Matsuo, T.; Okabe, K. Enhancing Corneal Drug Penetration Using Penetratin for Ophthalmic Suspensions. Biol. Pharm. Bull. 2024, 47, 123–131. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, K.; Tai, L.; Liu, Y.; Wei, G.; Lu, W.; Pan, W. Facile Noninvasive Retinal Gene Delivery Enabled by Penetratin. ACS Appl. Mater. Interfaces 2016, 8, 19256–19267. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Li, L.; Han, M.; Tang, S.; Wang, T.; Han, J.; He, X.; He, X.; Wang, A.; et al. A novel dendrimer-based complex co-modified with cyclic RGD hexapeptide and penetratin for noninvasive targeting and penetration of the ocular posterior segment. Drug Deliv. 2019, 26, 989–1001. [Google Scholar]
- Jiang, K.; Chen, J.; Tai, L.; Liu, C.; Chen, X.; Wei, G.; Lu, W.; Pan, W. Inhibition of post-trabeculectomy fibrosis via topically instilled antisense oligonucleotide complexes co-loaded with fluorouracil. Acta Pharm. Sin. B 2020, 10, 1754–1768. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, A.; Sun, R.; Xu, J.; Yin, T.; He, H.; Gou, J.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fan, X.; Jiang, K.; Hu, Y.; Liu, Y.; Lu, W.; Wei, G. Intraocular siRNA Delivery Mediated by Penetratin Derivative to Silence Orthotopic Retinoblastoma Gene. Pharmaceutics 2023, 15, 745. [Google Scholar] [CrossRef]
- Thareja, A.; Leigh, T.; Hakkarainen, J.J.; Hughes, H.; Alvarez-Lorenzo, C.; Fernandez-Trillo, F.; Blanch, R.J.; Ahmed, Z. Improving corneal permeability of dexamethasone using penetration enhancing agents: First step towards achieving topical drug delivery to the retina. Int. J. Pharm. 2024, 650, 124305. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, S.; Xu, N.; Liu, K.; Wei, F.; Zhang, X.; Zhang, J.; Gao, S.; Yu, Y.; Ding, X. Topical Ophthalmic Liposomes Dual-Modified with Penetratin and Hyaluronic Acid for the Noninvasive Treatment of Neovascular Age-Related Macular Degeneration. Int. J. Nanomed. 2024, 19, 1887–1908. [Google Scholar]
- Toffoletto, N.; Salema-Oom, M.; Nicoli, S.; Pescina, S.; González-Fernández, F.M.; Pinto, C.A.; Saraiva, J.A.; Alves de Matos, A.P.; Vivero-Lopez, M.; Huete-Toral, F.; et al. Dexamethasone phosphate and penetratin co-eluting contact lenses: A strategy to enhance ocular drug permeability. Int. J. Pharm. 2024, 650, 123685. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell 1988, 55, 1189–1193. [Google Scholar]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Cheng, Y.; Tan, H.; Li, Z.; Qu, Y.; Mu, G.; Wang, F. Tat PTD-endostatin: A novel anti-angiogenesis protein with ocular barrier permeability via eye-drops. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1140–1149. [Google Scholar] [CrossRef]
- Mann, D.A.; Frankel, A.D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991, 10, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, I.M.; Wadia, J.S.; Dowdy, S.F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release 2005, 102, 247–253. [Google Scholar] [CrossRef]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Lin, S.; Qu, J.; Xiao, J.; Huang, Y.; Xiao, Y.; Fu, X.; Yang, Y.; Li, X. Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia-reperfusion injury in rats. J. Cell. Mol. Med. 2010, 14, 1998–2005. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, Z.; Sheng, J.; Zhang, X.; Feng, D.; Zhang, X.; Yin, F.; Wang, A.; Wang, F. Tat PTD-Endostatin-RGD: A novel protein with anti-angiogenesis effect in retina via eye drops. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2137–2147. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, H.; Chen, Z.; Xia, X.; Deng, Q.; Shen, Y. A cell-permeable peptide inhibitor of p55PIK signaling alleviates ocular inflammation in mouse models of uveitis. Exp. Eye Res. 2020, 198, 108180. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lin, T.; Yin, H.; Pan, Y.; Zhu, M.; Zhang, M. A cell-permeable peptide inhibitor of p55PIK signaling alleviates suture-induced corneal neovascularization and inflammation. Heliyon 2023, 9, e14869. [Google Scholar] [CrossRef]
- Rohira, H.; Shankar, S.; Yadav, S.; Shah, S.G.; Chugh, A. Enhanced in vivo antifungal activity of novel cell penetrating peptide natamycin conjugate for efficient fungal keratitis management. Int. J. Pharm. 2021, 605, 120484. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Vega, E.; Pérez, Y.; Gómara, M.J.; García, M.L.; Haro, I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [PubMed]
- Chu, Y.; Chen, N.; Yu, H.; Mu, H.; He, B.; Hua, H.; Wang, A.; Sun, K. Topical ocular delivery to laser-induced choroidal neovascularization by dual internalizing rgd and tat peptide-modified nanoparticles. Int. J. Nanomed. 2017, 12, 1353–1368. [Google Scholar]
- Suda, K.; Murakami, T.; Gotoh, N.; Fukuda, R.; Hashida, Y.; Hashida, M.; Tsujikawa, A.; Yoshimura, N. High-density lipoprotein mutant eye drops for the treatment of posterior eye diseases. J. Control. Release 2017, 266, 301–309. [Google Scholar] [PubMed]
- Yang, H.; Wu, P.; Wang, T.; Yu, Y.; Li, J.; Liu, R.; Ruan, Q. Topical ophthalmic instillation of engineered hmscs-derived exosomes: A novel non-invasive therapeutic strategy for ocular posterior-segment disorder. Biochem. Biophys. Res. Commun. 2024, 723, 150212. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Pan, L.; Xiang, S.; Li, Y.; Jin, J.; Tan, J.; Huang, B.; Nan, K.; Lin, S. Suppression of NLRP3/Caspase-1/GSDMD Mediated Corneal Epithelium Pyroptosis Using Melatonin-Loaded Liposomes to Inhibit Benzalkonium Chloride-Induced Dry Eye Disease. Int. J. Nanomed. 2023, 18, 2447–2463. [Google Scholar]
- Li, Z.; Yu, H.; Liu, C.; Wang, C.; Zeng, X.; Yan, J.; Sun, Y. Efficiency co-delivery of ellagic acid and oxygen by a non-invasive liposome for ameliorating diabetic retinopathy. Int. J. Pharm. 2023, 641, 122987. [Google Scholar] [CrossRef]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The use of TAT peptide-functionalized graphene as a highly nuclear-targeting carrier system for suppression of choroidal melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef]
- Wu, B.; Li, M.; Li, K.; Hong, W.; Lv, Q.; Li, Y.; Xie, S.; Han, J.; Tian, B. Cell penetrating peptide TAT-functionalized liposomes for efficient ophthalmic delivery of flurbiprofen: Penetration and its underlying mechanism, retention, anti-inflammation and biocompatibility. Int. J. Pharm. 2021, 598, 120405. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Kreider, E.; VanDeusen, C.L.; Wright, L.; Wylie, B.L.; Wender, P.A. Arginine-rich molecular transporters for drug delivery: Role of backbone spacing in cellular uptake. J. Med. Chem. 2002, 45, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef] [PubMed]
- McEwan, G.T.; Jepson, M.A.; Hirst, B.H.; Simmons, N.L. Polycation-induced enhancement of epithelial paracellular permeability is independent of tight junctional characteristics. Biochim. Biophys. Acta 1993, 1148, 51–60. [Google Scholar] [CrossRef]
- Ohtake, K.; Maeno, T.; Ueda, H.; Natsume, H.; Morimoto, Y. Poly-L-Arginine Predominantly Increases the Paracellular Permeability of Hydrophilic Macromolecules across Rabbit Nasal Epithelium In Vitro. Pharm. Res. 2003, 20, 153–160. [Google Scholar] [CrossRef]
- Nemoto, E.; Takahashi, H.; Kobayashi, D.; Ueda, H.; Morimoto, Y. Effects of Poly-L-arginine on the Permeation of Hydrophilic Compounds through Surface Ocular Tissues. Biol. Pharm. Bull. 2006, 29, 155–160. [Google Scholar] [CrossRef]
- Liu, C.; Lan, Q.; He, W.; Nie, C.; Zhang, C.; Xu, T.; Jiang, T.; Wang, S. Octa-arginine modified lipid emulsions as a potential ocular delivery system for disulfiram: A study of the corneal permeation, transcorneal mechanism and anti-cataract effect. Colloids Surf. B Biointerfaces 2017, 160, 305–314. [Google Scholar] [CrossRef]
- Nemoto, E.; Ueda, H.; Masayuki, A.; Hideshi, N.; Morimoto, Y. Ability of Poly-L-arginine to Enhance Drug Absorption into Aqueous Humor and Vitreous Body after Instillation in Rabbits. Biol. Pharm. Bull. 2007, 30, 1768–1772. [Google Scholar] [CrossRef]
- Morris, M.C.; Depollier, J.; Mery, J.; Heitz, F.; Divita, G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001, 19, 1173–1176. [Google Scholar] [CrossRef]
- Pescina, S.; Sala, M.; Padula, C.; Scala, M.C.; Spensiero, A.; Belletti, S.; Gatti, R.; Novellino, E.; Campiglia, P.; Santi, P.; et al. Design and synthesis of new cell penetrating peptides: Diffusion and distribution inside the cornea. Mol. Pharm. 2016, 13, 3876–3883. [Google Scholar] [CrossRef]
- Henriques, S.T.; Quintas, A.; Bagatolli, L.A.; Homblé, F.; Castanho, M.A.R.B. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol. Membr. Biol. 2007, 24, 282–293. [Google Scholar] [PubMed]
- Henriques, S.T.; Castanho, M.A.R.B. Consequences of nonlytic membrane perturbation to the translocation of the cell penetrating peptide pep-1 in lipidic vesicles. Biochemistry 2004, 43, 9716–9724. [Google Scholar] [PubMed]
- Henriques, S.T.; Costa, J.; Castanho, M.A.R.B. Translocation of β-galactosidase mediated by the cell-penetrating peptide pep-1 into lipid vesicles and human HeLa cells is driven by membrane electrostatic potential. Biochemistry 2005, 44, 10189–10198. [Google Scholar]
- Weller, K.; Lauber, S.; Lerch, M.; Renaud, A.; Merkle, H.P.; Zerbe, O. Biophysical and biological studies of end-group-modified derivatives of Pep-1. Biochemistry 2005, 44, 15799–15811. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.H.; Ku, S.K.; Lee, J.E.; Cha, H.J.; Youn, J.K.; Kwon, H.Y.; Park, J.H.; Park, E.Y.; Cho, S.W.; et al. The effects of PEP-1-FK506BP on dry eye disease in a rat model. BMB Rep. 2015, 48, 153–158. [Google Scholar]
- Kim, D.W.; Lee, S.H.; Shin, M.J.; Kim, K.; Ku, S.K.; Youn, J.K.; Bin Cho, S.; Park, J.H.; Lee, C.H.; Son, O.; et al. PEP-1-FK506BP inhibits alkali burn-induced corneal inflammation on the rat model of corneal alkali injury. BMB Rep. 2015, 48, 618–623. [Google Scholar]
- Carter, S.B. Effects of Cytochalasins on Mammalian Cells. Nature 1967, 213, 261–264. [Google Scholar]
- Ito, S.; Sato, E.; Loewenstein, W.R. Studies on the Formation of a Permeable Cell Membrane Junction I. Coupling under Various Conditions of Membrane Contact. Effects of Colchicine, Cytochalasin B, Dinitrophenol. J. Membr. Biol. 1974, 19, 305–337. [Google Scholar]
- Stevenson, B.R.; Begg, D.A. Concentration-dependent effects of cytochalasin d on tight junctions and actin filaments in mdck epithelial cells. J. Cell Sci. 1994, 107, 367–375. [Google Scholar]
- Maclean-Fletcher, S.; Pollard, T.D. Mechanism of Action of Cytochalasin B on Actin. Cell 1980, 20, 329–341. [Google Scholar] [CrossRef]
- Lin, D.C.; Tobin, K.D.; Grumet, M.; Lin, S. Cytochalasins Inhibit Nuclei-Induced Actin Polymerization by Blocking Filament Elongation. J. Cell Biol. 1980, 84, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.S.; Spudich, J.A. Cytochalasin inhibits the rate of elongation of actin filament fragments. J. Cell Biol. 1979, 83, 657–662. [Google Scholar] [CrossRef]
- Cereijido, M.; Meza, I.; Martinez-Palomo, A. Occluding junctions in cultured epithelial monolayers. Am. J. Physiol. 1981, 241, C96–C102. [Google Scholar] [CrossRef]
- Meza, I.; Ibarra, G.; Sabanero, M.; Martinez-Palomo, A.; Cereijido, M. Occluding Junctions and Cytoskeletal Components in a Cultured Transporting Epithelium. J. Cell Biol. 1980, 87, 746–754. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar]
- Wickstead, B.; Gull, K. The evolution of the cytoskeleton. J. Cell Biol. 2011, 194, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, W.T. Roles of the actin cytoskeleton in aging and age-associated diseases. Ageing Res. Rev. 2020, 68, 101021. [Google Scholar] [CrossRef]
- Rojanasakul, Y.; Robinson, J.R. The cytoskeleton of the cornea and its role in tight junction permeability. Int. J. Pharm. 1991, 68, 135–146. [Google Scholar] [CrossRef]
- Lee, V.H.L.; Carson, L.W.; Takemoto, K.A. Macromolecular drug absorption in the albino rabbit eye. Int. J. Pharm. 1986, 29, 43–51. [Google Scholar]
- Liaw, J.; Chang, S.F.; Hsiao, F.C. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) polymeric micelles. Gene Ther. 2001, 8, 999–1004. [Google Scholar] [CrossRef][Green Version]
- Wang, C.J.; Huang, Q.W.; Qi, H.Y.; Guo, P.; Huang, S.X. Promoting effect of borneol on the permeability of puerarin eye drops and timolol maleate eye drops through the cornea in vitro. Pharmazie 2005, 61, 783–787. [Google Scholar]
- Qi, H.P.; Gao, X.C.; Zhang, L.Q.; Wei, S.Q.; Bi, S.; Yang, Z.C.; Cui, H. in vitro evaluation of enhancing effect of borneol on transcorneal permeation of compounds with different hydrophilicities and molecular sizes. Eur. J. Pharmacol. 2013, 705, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bi, H.; Xie, X.; Guo, J.; Wang, X.; Liu, D. Natural borneol enhances geniposide ophthalmic absorption in rabbits. Int. J. Pharm. 2013, 445, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xun, Y.; Li, Z.; Hang, T.; Zhang, X.; Cui, H. Influence of Borneol on In Vitro Corneal Permeability and on In Vivo and In Vitro Corneal Toxicity. J. Ocul. Pharmacol. Ther. 2009, 25, 429–435. [Google Scholar]
- Mao, Z.; Wang, X.; Liu, Y.; Huang, Y.; Liu, Y.; Di, X. Simultaneous determination of seven alkaloids from rhizoma corydalis decumbentis in rabbit aqueous humor by LC–MS/MS: Application to ocular pharmacokinetic studies. J. Chromatogr. B 2017, 1057, 46–53. [Google Scholar]
- Xu, X.; Yu, N.; Bai, Z.; Xun, Y.; Jin, D.; Li, Z.; Cui, H. Effect of menthol on ocular drug delivery. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1503–1510. [Google Scholar]
- Ahn, S.; Eom, Y.; Kang, B.; Park, J.; Lee, H.K.; Kim, H.M.; Song, J.S. Effects of Menthol-Containing Artificial Tears on Tear Stimulation and Ocular Surface Integrity in Normal and Dry Eye Rat Models. Curr. Eye Res. 2018, 43, 580–587. [Google Scholar]
- Bai, J.H.; Ding, X.M.; Mou, H.Y.; Wang, S.L.; Chen, S.H. Menthol in Combination with Iontophoresis Promotes Natamycin Penetration through the Cornea: In Vitro and In Vivo Studies. Bull. Exp. Biol. Med. 2022, 172, 318–323. [Google Scholar]
- Gelfuso, G.M.; Ferreira-Nunes, R.; Dalmolin, L.F.; Ré, A.C.S.; Santos, G.A.; Sá, F.A.P.; Cunha-Filho, M.; Alonso, A.; Neto, S.A.M.; Anjos, J.L.V.; et al. Iontophoresis enhances voriconazole antifungal potency and corneal penetration. Int. J. Pharm. 2020, 574, 118991. [Google Scholar] [CrossRef]
- Rojekar, S.; Parit, S.; Gholap, A.D.; Manchare, A.; Nangare, S.N.; Hatvate, N.; Sugandhi, V.V.; Paudel, K.R.; Ingle, R.G. Revolutionizing Eye Care: Exploring the Potential of Microneedle Drug Delivery. Pharmaceutics 2024, 16, 1398. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar]
- Alam, F.; Elsherif, M.; Alqattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [PubMed]
- Kashkooli, H.H.; Farokh, A.; Mohammadi, S.; Marcotulli, M.; Franco, S.; Angelini, R.; Ruocco, G.; Khalili, H.; Cidonio, G. Localised Therapies Using 3D-Printed Collagen-Based Micro-Implant for Ocular Indications. Macromol. Mater. Eng. 2025, 2400236. [Google Scholar] [CrossRef]
- Salahuddin, A.; Ashraf, A.; Ahmad, K.; Hou, H. Recent advances in chitosan-based smart hydrogel for drug delivery systems. Int. J. Biol. Macromol. 2024, 280, 135803. [Google Scholar]
- Kalyanwat, R.; Shrivastava, B.; Pathak, K. Preparation and Evaluation of Bioadhesive Ocular Inserts of Aceclofenac. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 207–213. [Google Scholar]
- Ioannou, N.; Luo, J.; Qin, M.; Di Luca, M.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D-printed long-acting 5-fluorouracil implant to prevent conjunctival fibrosis in glaucoma. J. Pharm. Pharmacol. 2023, 75, 276–286. [Google Scholar]
- Abrigo, N.A.; Dods, K.K.; Makovsky, C.A.; Lohan, S.; Mitra, K.; Newcomb, K.M.; Le, A.; Hartman, M.C.T. Development of a Cyclic, Cell Penetrating Peptide Compatible with In Vitro Selection Strategies. ACS Chem. Biol. 2023, 18, 746–755. [Google Scholar]
- Najjar, K.; Erazo-Oliveras, A.; Brock, D.J.; Wang, T.Y.; Pellois, J.P. An L- to D-amino acid conversion in an endosomolytic analog of the cell-penetrating peptide TAT influences proteolytic stability, endocytic uptake, and endosomal escape. J. Biol. Chem. 2017, 292, 847–861. [Google Scholar]
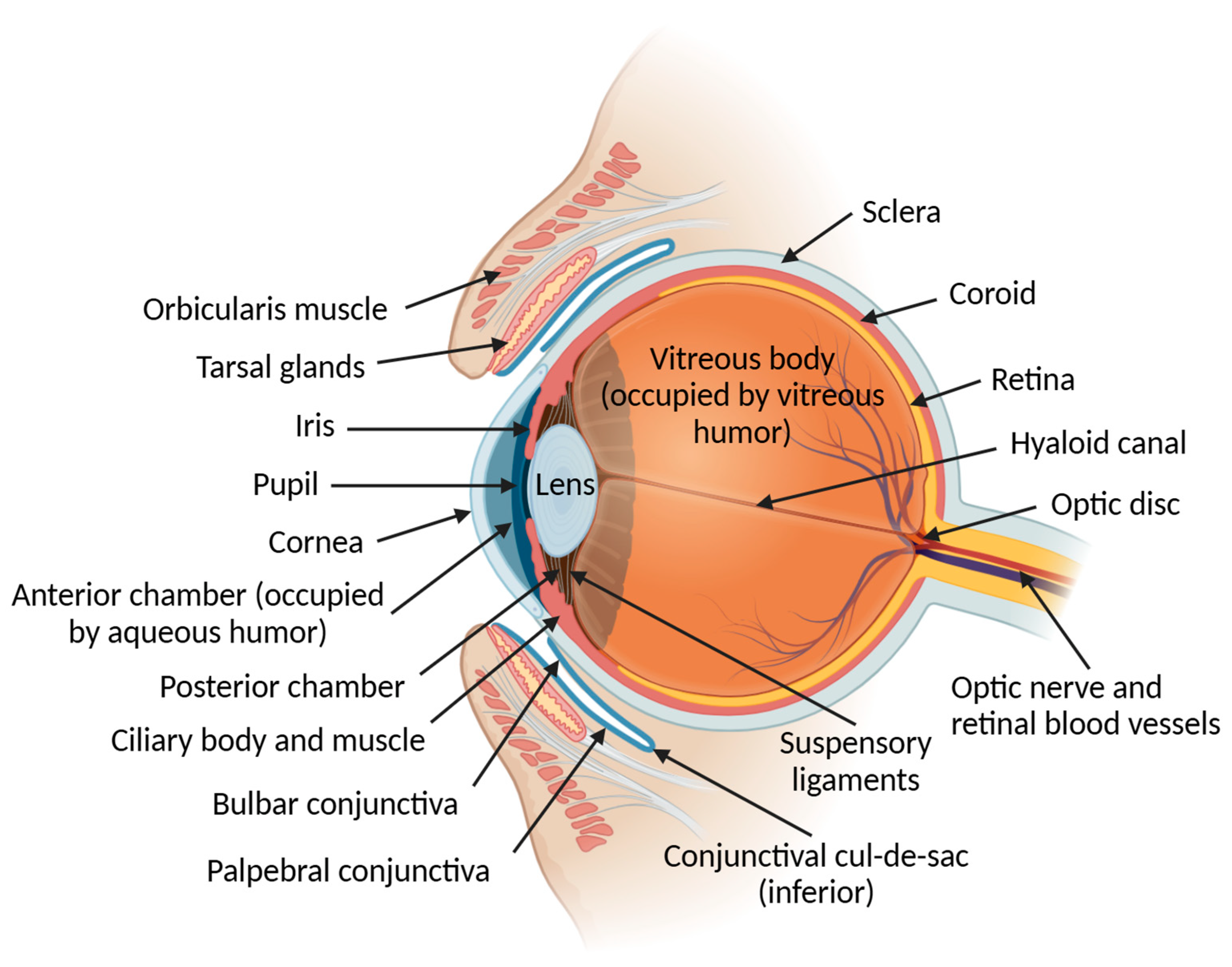
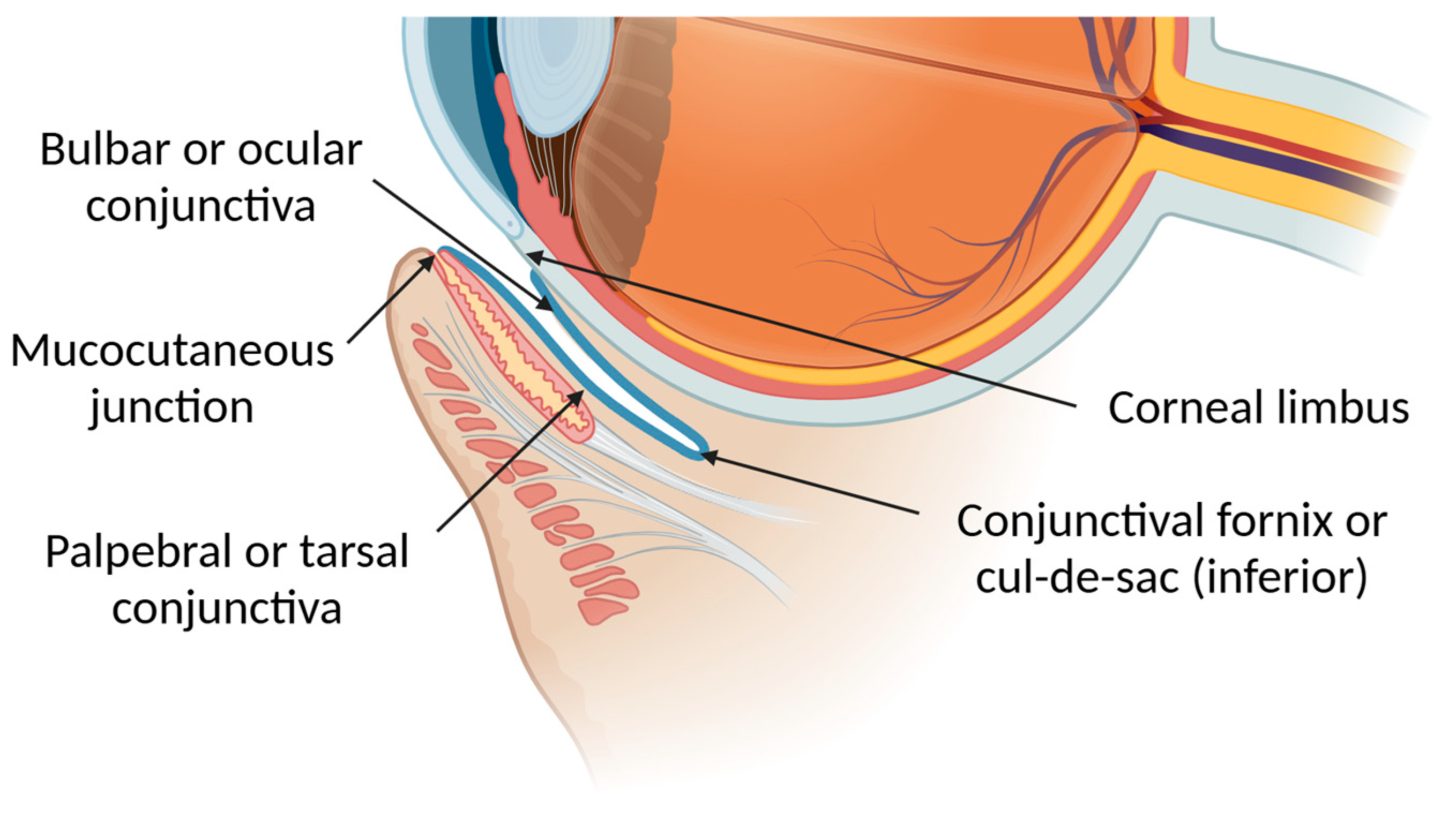
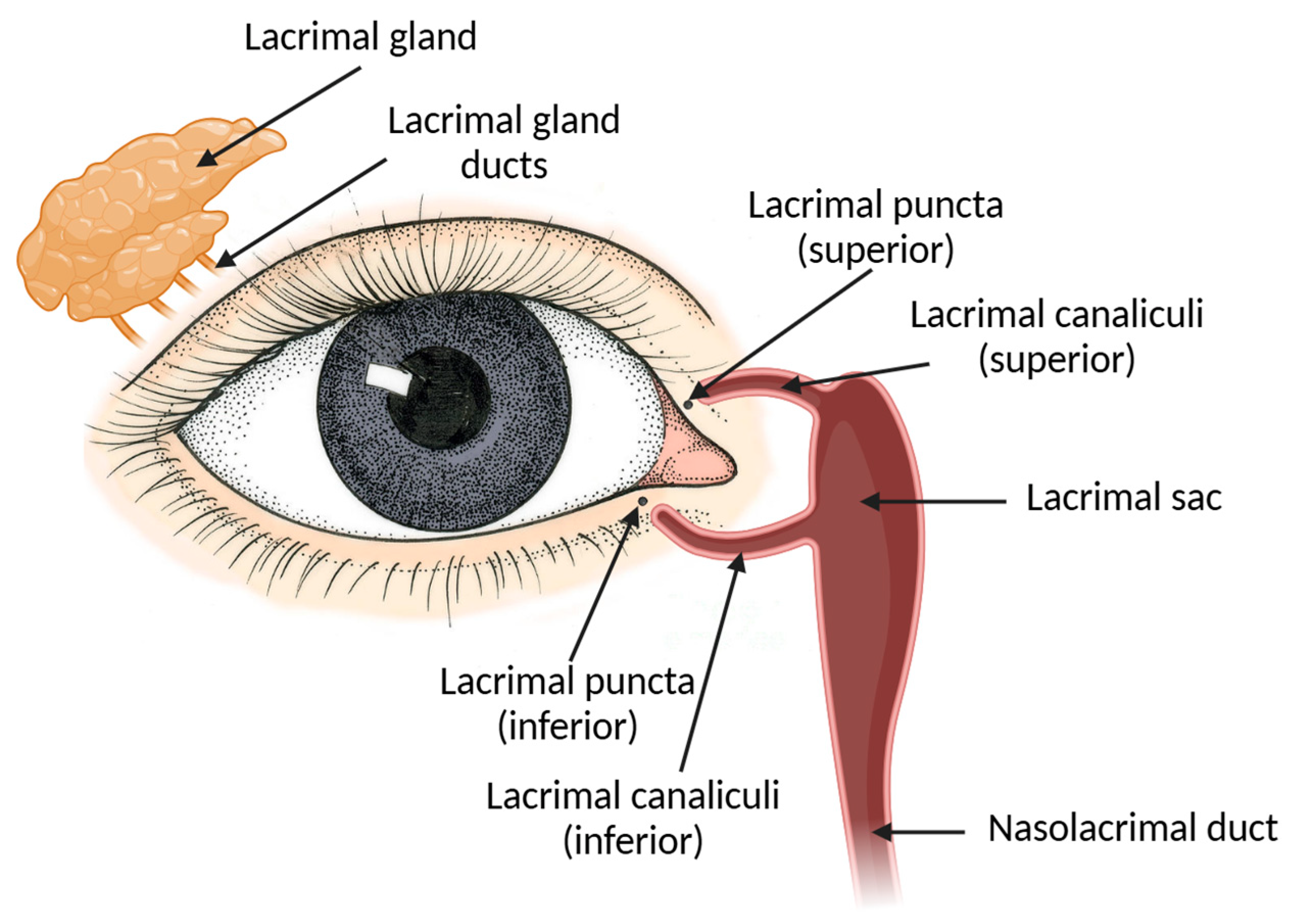
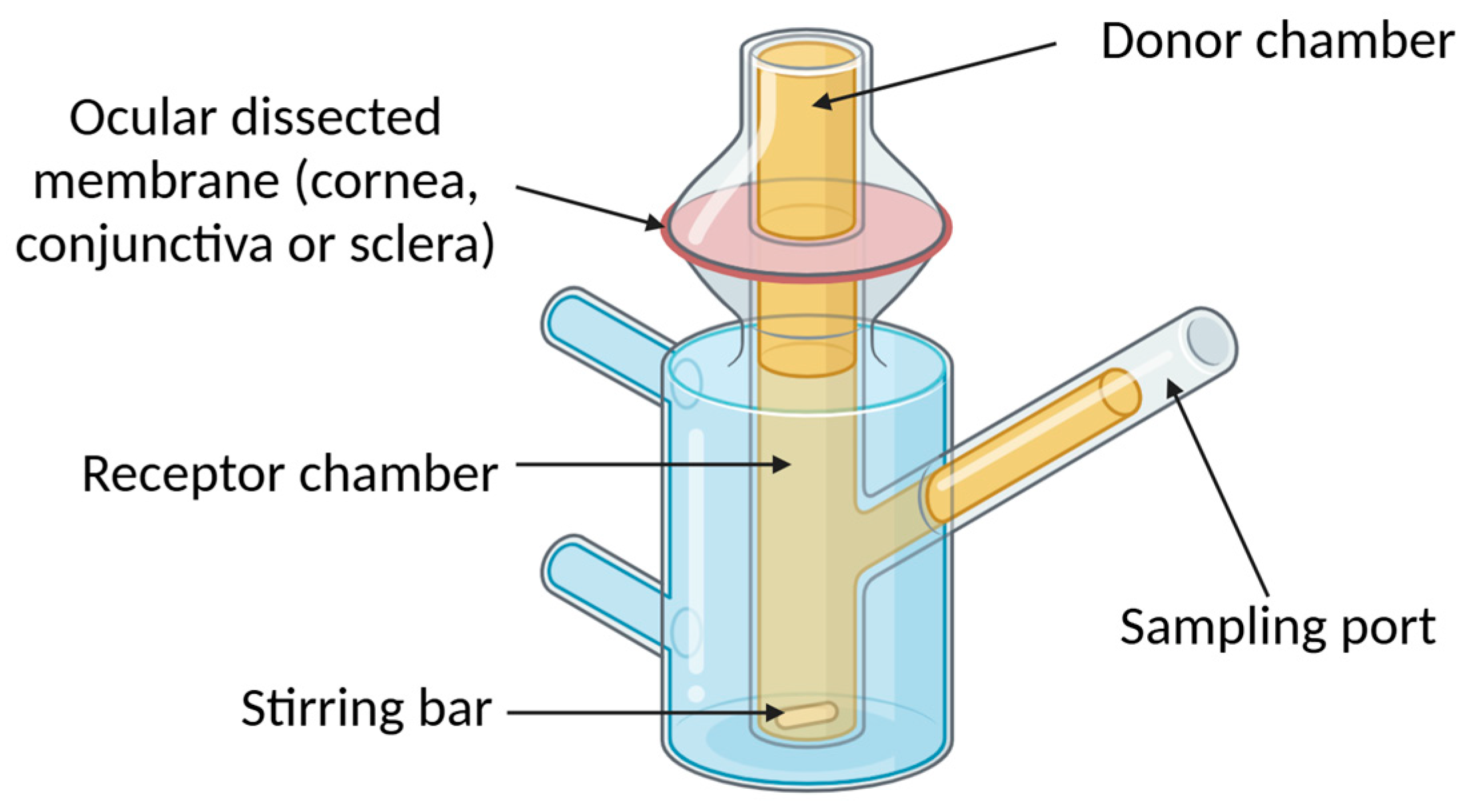
| Layer/Component | Description | Function | Key Components | References | |
|---|---|---|---|---|---|
| Precorneal Tear Film (PTF) | Tear Film Lipid Layer (TFLL) | Composed of meibomian gland secretions (polar and nonpolar lipids); forms the anterior layer of PTF, coating the aqueous layer as a continuous sheet spread by blinking. | The main function is to reduce PTF surface tension, but it also provides smooth optical surface, enhances stabilization, and aids the spreading of the PTF, delays aqueous layer evaporation, prevents tear spillover, and seals lid margins during sleep. | Glycerophospholipids, sphingophospholipids, u-hydroxy fatty acids, wax esters, cholesterol esters, triglycerides, free fatty acids, hydrocarbons. | [15,16,17,18] |
| Aqueous Middle Layer | Comprises 90% of PTF. Mainly produced by the lacrimal and by the third eyelid glands. | Lubricates ocular surface, washes away debris, nourishes the avascular cornea (oxygen, glucose, proteins, and salts), and provides antibacterial, antifungal, antiparasitic, and antiviral protection. | Water, electrolytes (sodium, potassium, calcium, magnesium, etc.), lysozyme, lactoferrin, lipocalin, secretory IgA, cytokines, vitamins, and peptide growth factors. | [19,20,21,22,23] | |
| Mucous Layer | Present in the apical epithelial ocular surface. Merges with the aqueous middle layer. | Protects against pathogens, offers an interface between tear film and ocular surface, helps the hydration of the ocular surface, and maintains tear film stability. | High-molecular-weight O-linked glycoproteins (Mucins). | [24,25] | |
| Cornea | Epithelium | The most superficial layers are flat with apical microvilli covered by a glycocalyx, which maximizes the surface area contacting the mucinous layer of the tear film. | Forms the first anatomical line of defense, offering a highly resistant barrier against foreign substances and preventing the transcellular and paracellular transport of many drugs, mainly due to its lipophilic character and the existence of strong intercellular junctions. | It is characterized as a non-keratinizing stratified epithelium consisting of three layers: the stratum superficiale (superficial nonkeratinized stratified squamous cells), the stratum intermedius (wing cells), and the stratum basale (the basal cell layer) | [11,26,27,28] |
| Stroma | Constitutes 90% of the cornea’s volume. | Provides structural corneal integrity and transparency. | Highly ordered Type I and V collagen fibrils aligned in lamellae, proteoglycans (e.g., lumican, keratocan, and mimecan) that regulate water retention and collagen organization, keratocytes that maintain the extracellular matrix, monocytes, and dendritic cells. | [29,30,31,32] | |
| Descemet’s Membrane | Elastic and acellular structure that anchors the corneal endothelium to the corneal stroma. | Regulates the bidirectional flow of nutrients, growth factors, cytokines, and macromolecules between the aqueous humor and stroma. | Type IV collagen and laminin, produced by endothelial cells. | [32,33] | |
| Endothelium | Hexagonal-shaped lipophilic monolayer of cells that represents the innermost sheet of the cornea, forming a barrier between the stroma and aqueous humor. | Maintains corneal transparency by actively transporting ions and passively regulating water movement to keep the stroma in a relative dehydrated state. | Endothelial cells. | [6,34,35] | |
| Conjunctiva | Epithelium | Outer layer of conjunctiva | Provides a barrier function and assists in tear film adherence and spreading through microvilli. Goblet cells produce mucin. | Epithelial cells and goblet cells. | [36,37,38] |
| Submucosal Lamina Propria (Stroma) | Underlying connective tissue layer supporting the conjunctival epithelium. | Provides structural integrity and contains blood and lymphatic vessels. | Connective tissue, blood vessels, and lymphatic vessels. | [36,39] | |
| Sclera | Maintains intraocular pressure, protects intraocular contents, prevents deformations caused by muscle contractions, and reduces internal light scattering to ensure optimal retinal imaging. | Dense Types I (>90%), III, V, and VI collagen bundles irregularly interspersed with elastic fibers and microfibrils in a hydrated matrix of proteoglycans (the water content of sclera is around 68%), glycoproteins, and elastin, interspersed with fibroblasts. | [40,41,42,43,44] | ||
| Drug | Cyclodextrin | Trade Name | Company |
|---|---|---|---|
| Chloramphenicol | RM-β-CD | Clorocil® | Oftalder (Lisbon, Portugal) |
| Diclofenac | HPγ-CD | Voltaren®/ Voltarol® | Novartis (Basel, Schwitzerland) |
| Indomethacin | HP-β-CD | Indocid®/ Indocyllir® | Baush & Lomb (Bridgewater, NJ, USA) |
| Naphasoline hydrochloride | β-CD | Clear eyes® | Prestige Consumer Healthcare Inc. (Tarrytown, NY, USA) |
| Thimerosal | β-CD | Vitaseptol® | Novartis (Basel, Schwitzerland) |
| Cyclodextrin | Drug Model | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|---|
| SBE-β-CD | Cyclosporine A | Enhanced corneal permeation, improved tear volume, reduced inflammation, and better dry eye management. | Not mentioned. | [131] |
| HP-γ-CD | Spironolactone | Superior therapeutic effects in corneal wound healing compared to potassium canrenoate. | Well-tolerated. | [123] |
| HP-β-CD | Ketoconazole | Increased precorneal retention, corneal permeation, and ocular bioavailability using ion-sensitive gel. | Well-tolerated. | [126] |
| β-CD | Itraconazole | Improved ocular kinetics with dissolving microneedle system | Well-tolerated. | [125] |
| SBE-β-CD | Fluconazole | Faster drug delivery and higher bioavailability using ocular patch system. | Well-tolerated. | [124] |
| γ-CD | Vitamin A palmitate | Faster recovery of dry eye disease using crosslinked γ-CD framework. | Well-tolerated. | [129] |
| β-CD | Hesperidin | Sustained release and enhanced corneal permeation using in situ gel. | Well-tolerated. | [144] |
| HP-β-CD | Vorinostat | Increased corneal and conjunctival bioavailability compared to suspension. | Well-tolerated. | [122] |
| HP-β-CD | Nepafenac | Improved corneal permeability and bioavailability compared to suspension eye drop. | Not mentioned. | [111] |
| 2-HP-β-CD | Triamcinolone acetonide | Increased retention time, prolonged release, and enhanced corneal permeability using PLGA nanoparticles. | Not mentioned. | [120] |
| β-CD | Dexamethasone | Reduced vessel area in the cornea using ROS-responsive nanogel with β-CD (once-daily) compared to free drug solution (twice-daily) | Well-tolerated. | [121] |
| SBE-β-CD | Dexamethasone | Prolonged residence time and enhanced permeability using chitosan/SBE-β-CD nanoparticles. | Well-tolerated. | [119] |
| γ-CD | Dexamethasone | Improved bioavailability and anti-inflammatory effect using nanoemulsion-based pseudopolyrotaxane hydrogel. | Well-tolerated. | [118] |
| Crown Ether Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 12C4, 15C5, and 18C6 1 mg/mL and 30 mg/mL | Enhanced calcium sequestration and riboflavin permeability in bovine corneas; 12C4 showed highest efficiency. | 12C4, 15C5, and 18C6 induced similar epithelial changes. Similar epithelial changes. Less toxic than BAC > 0.1% or 1 mg/mL EDTA. 12C4 was the least toxic. | [171] |
| 18C6 1 mM | Enhanced corneal permeability of phenytoin sodium and promoted healing of alkali-induced corneal ulcers. | Minimal irritation and safe ophthalmic use. | [178] |
| VE-TPGS 1000 Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.01–1% (w/w) | Increased riboflavin and chlorhexidine permeability, peaking at 0.5%. There was no added benefit at 1%. | No irritation reported. | [300,303] |
| 1% | Brinzolamide-loaded nanoliposomes containing TPGS (T-LPs/Brz) showed 5-fold higher permeation than Brz alone and reduced IOP for 4–10 h. | T-LPs/Brz caused mild conjunctival inflammation. The corneal epithelium retained an intact structure. | [299] |
| 0.01–1% (w/w) | Enhanced the accumulation of chlorhexidine gluconate in the cornea in a concentration-dependent manner up to 0.5% (w/w). | No treatment-related alterations or irritation reported. | [303] |
| Labrasol® Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.5%, 2% and 8% (w/v) | Enhanced fluorescein sodium permeation in a concentration-dependent manner | Not mentioned. | [306] |
| 0.5–5.0% (v/v) | Increased corneal permeability of baicalin. The highest improvement was seen at 2%. | Non-irritant up to 3%. Slightly irritant at 5%. | [308,310] |
| 4.5% (w/w) | The formulated Labrasol containing ribavirin microemulsion improved corneal permeability 3-fold compared to aqueous solution. | No irritation or allergic reaction observed. | [309] |
| NMP Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.1–10% (w/w) | No significant enhancement for timolol maleate or acyclovir. | Non-irritant up to 10%. NMP 10% significantly increased percentage hydration level. | [311] |
| 2.5%, 5% and 10% (v/v) | Improved corneal permeability of ibuprofen, ribavirin, puerarin, and enoxacin in a concentration-dependent manner, with maximum at 10%. | Non-irritant at concentrations of 0–10% (v/v), slightly irritant at 15% (v/v), and moderately irritant at 20% (v/v). | [312] |
| BS Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| GCA and CA 1% (w/v) | Not mentioned. | Caused mild irritation in a concentration-dependent manner. | [262] |
| DCA, TCA, UDC, TDC 0.05% | TDC enhanced the in vitro corneal permeation of atenolol. DCA and UDC enhanced the in vitro corneal permeation of timolol maleate and betaxolol hydrochloride. | DCA showed higher toxicity. | [337] |
| GCA, DCA and TCA | Improved permeability of liposomes compared to cholesterol-containing liposomes. | DCA showed greater toxicity. | [410] |
| GCA and TCA 0.5% | Enhanced permeability of PCL-PF68 nanoparticles in cornea, iris, and ciliary body. | Not mentioned. | [413] |
| SP Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.5% and 1% | Not mentioned. | Significant corneal damage (>30%). | [262] |
| 0.01–1% | SP 0.05% increased permeability of atenolol and timolol maleate and, to a smaller extent, betaxolol hydrochloride. | SP 0.015% caused corneal hydration to increase. SP 0.25% caused slight irritation. | [337] |
| 0.5% | Enhanced corneal permeability of atenolol, carteolol, and tilisolol. | Slight ocular irritation after 12 h. | [423] |
| 0.5% | Enhanced corneal permeability of FITC-dextran-4 and FITC-dextran-10. | Not mentioned. | [425] |
| 0.5% | Enhanced corneal permeability of Thyrotropin-releasing Hormone and Luteinizing-Hormone-releasing Hormone in a molecular-weight-dependent manner. | Not mentioned. | [426] |
| 30 mg/mL | SP micelles improved diclofenac permeation with enhanced anti-inflammatory effects. | No significant ocular irritation. | [427] |
| DG Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.0025% (w/v) | Slightly increased timolol maleate and betaxolol permeability. | DG 0.0025% increased the corneal hydration value. Concentrations > 0.015% caused significant swelling and opacification of the cornea. | [337] |
| 1 mM | Increased corneal permeation of PEG 200–1000 in a molecular-weight-dependent manner. | Severe corneal epithelial damage. | [436] |
| 0.1 mM | Had no effect as a corneal permeation enhancer for monovalent single-chain variable region (scFv) antibody fragments and divalent miniantibodies. | Not mentioned. | [437] |
| Escin Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.05% (w/v) | Enhanced corneal permeability of atenolol, timolol, and levobunolol. | Increased corneal hydration. Showed a similar irritation profile as digitonin | [337] |
| 0.015% | Increased corneal permeability of timolol maleate. | Not mentioned. | [445] |
| Azone Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 1% | Enhanced ex vivo corneal permeation and in vivo anti-inflammatory efficiency compared to the azone-free formulations. | No signs of ocular irritancy. | [451] |
| 0.1% | Enhanced the corneal penetration of hydrophilic compounds (acetazolamide, sulfacetamide, guanethidine and cimetidine). | No in vivo irritating signs. | [452] |
| 5% | Enhanced cyclosporine corneal permeation, leading to faster steady state. | No toxicity to corneal epithelium. | [453] |
| 0.1%, 0.4% and 0.7% | Increased the in vivo ocular bioavailability of cimetidine 3.9- and 22-fold, respectively. Above 0.1%, did not produce higher ocular permeation | Exerted toxic and irritating effects on the cornea in a concentration-dependent manner. The highest dose caused moderate congestion. | [454] |
| 0.2% | Improved permeability of Gd-DTPA. | No corneal toxicity. | [455] |
| 0.025% | No significant effect on levobunolol corneal permeability. | Not mentioned. | [456] |
| 0.125–0.625% | Increased corneal permeability of S-timolol maleate (STM). | No signs of ophthalmic irritation. | [457] |
| 0.125–0.625% | increased the in vitro corneal permeation of Latanoprost in a concentration-dependent manner up 0.5%. | No signs of ophthalmic irritation. | [458] |
| Cytochalasin Concentration | Permeation Outcomes | Adverse Reactions | References |
|---|---|---|---|
| Cytochalasin B 0.1–1 mM | Reduced transcorneal electrical resistance in a concentration-dependent manner. The effect of cytochalasin B was predominantly on the junctional portion of the corneal epithelium. | From all permeation enhancers tested (EDTA, digitonin, and deoxycholic acid), cytochalasin B appeared to present the least harmful effects to the corneal tissue. | [412] |
| Cytochalasin B 1 mM | Improved corneal permeation of PEG 400 to PEG 700. Above the molecular weight of PEG 700, there was no significant change. | Not mentioned. | [436] |
| Cytochalasin B 10 μg/mL | Enhanced inulin corneal permeation but had no effect on glucose or epinephrine. | Not mentioned. | [524] |
| Cytochalasin B 3 nM | Increased in vivo gene delivery into ocular tissues by eye drops of plasmid/poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (Pluronic® P105) polymeric micelles. | Not mentioned. | [525] |
| Borneol Concentration | Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.05% and 0.1% | 0.05% and 0.1% had no effect on the fluconazole corneal permeability. Higher and significant corneal-permeation-enhancing effects when combined with menthol. | No significant differences in corneal hydration levels, in vivo irritation, and blinking frequency. | [89] |
| 0.05% and 0.1% | The optimal penetration-enhancing concentration of borneol was 0.1%. The penetration-enhancing effects of borneol were increased when used in combination with Labrasol. | The maximum safe concentration of borneol was 0.2%. No obvious corneal or iris irritation symptoms occurred when using 0.1% borneol. | [308] |
| 0.025%, 0.05% and 0.1% | Improved the penetration of puerarin eye drops. 0.1% led to an increase in timolol maleate eye drop corneal permeability when compared to the control group. | No impact on corneal hydration. | [526] |
| 0.05%, 0.1% and 0.2% | Improved the corneal permeability of rhodamine B, sodium fluorescein, and FITC-dextrans of 4 kDa in a borneol-concentration-dependent manner. The paracellular permeability was correlated to the molecular weight of the drugs tested. | No damage was observed to the epithelium or endothelium. Ocular irritation tests indicated good corneal biocompatibility. | [527] |
| 0.01%, 0.02% and 0.04% | Increased the corneal permeability of geniposide. This enhancing effect was temporary and closely dose-related. | All formulations were well tolerated, and no ocular damage or clinically abnormal signs were observed. | [528] |
| 0.1% | Both natural and synthetic borneol increased corneal permeability of dexamethasone, ribavirin, and tobramycin. | No ocular irritation observed. No damage to the corneal epithelium was found. Neither toxic nor inflammatory reactions were found. | [529] |
| 0.04% | Increased the concentrations of the seven alkaloids studied in the aqueous humor. | Not mentioned. | [530] |
| Menthol Concentration | Outcomes | Adverse Reactions | References |
|---|---|---|---|
| 0.05% and 0.1% | Enhanced fluconazole permeation. Higher effects when combined with borneol. | No irritation, no impact on corneal hydration. | [89] |
| 0.1% and 0.2% | The optimal penetration-enhancing concentration of menthol was found to be 0.2%. The penetration-enhancing effects of menthol were increased when used in combination with Labrasol. | The maximum safe concentration of menthol was 0.4%. There was no significant tissue irritation or damage. | [308] |
| 0.025%, 0.05% and 0.1% | Increased permeability of dexamethasone disodium phosphate. | Mild irritation, resolved in 48 h. | [531] |
| 0.1%, 0.2%, and 0.3%. | Improved natamycin penetration under iontophoresis. | The maximum safe concentration of menthol was 0.4%. There was no significant tissue damage. | [533] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, G.; Delgado, E.; Silva, B.; Braz, B.S.; Gonçalves, L. Topical Ocular Drug Delivery: The Impact of Permeation Enhancers. Pharmaceutics 2025, 17, 447. https://doi.org/10.3390/pharmaceutics17040447
Santos G, Delgado E, Silva B, Braz BS, Gonçalves L. Topical Ocular Drug Delivery: The Impact of Permeation Enhancers. Pharmaceutics. 2025; 17(4):447. https://doi.org/10.3390/pharmaceutics17040447
Chicago/Turabian StyleSantos, Gonçalo, Esmeralda Delgado, Beatriz Silva, Berta São Braz, and Lídia Gonçalves. 2025. "Topical Ocular Drug Delivery: The Impact of Permeation Enhancers" Pharmaceutics 17, no. 4: 447. https://doi.org/10.3390/pharmaceutics17040447
APA StyleSantos, G., Delgado, E., Silva, B., Braz, B. S., & Gonçalves, L. (2025). Topical Ocular Drug Delivery: The Impact of Permeation Enhancers. Pharmaceutics, 17(4), 447. https://doi.org/10.3390/pharmaceutics17040447







