PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
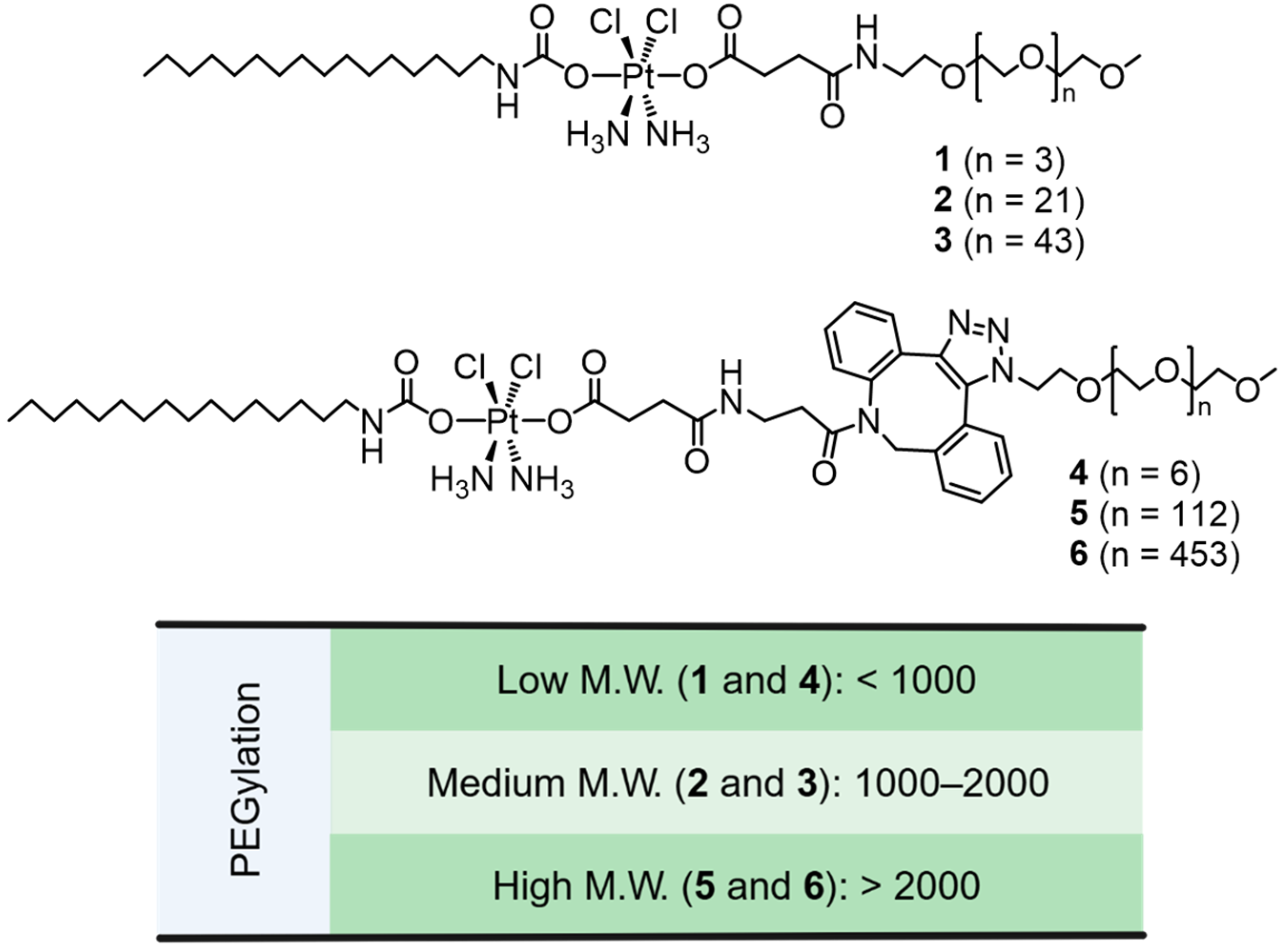
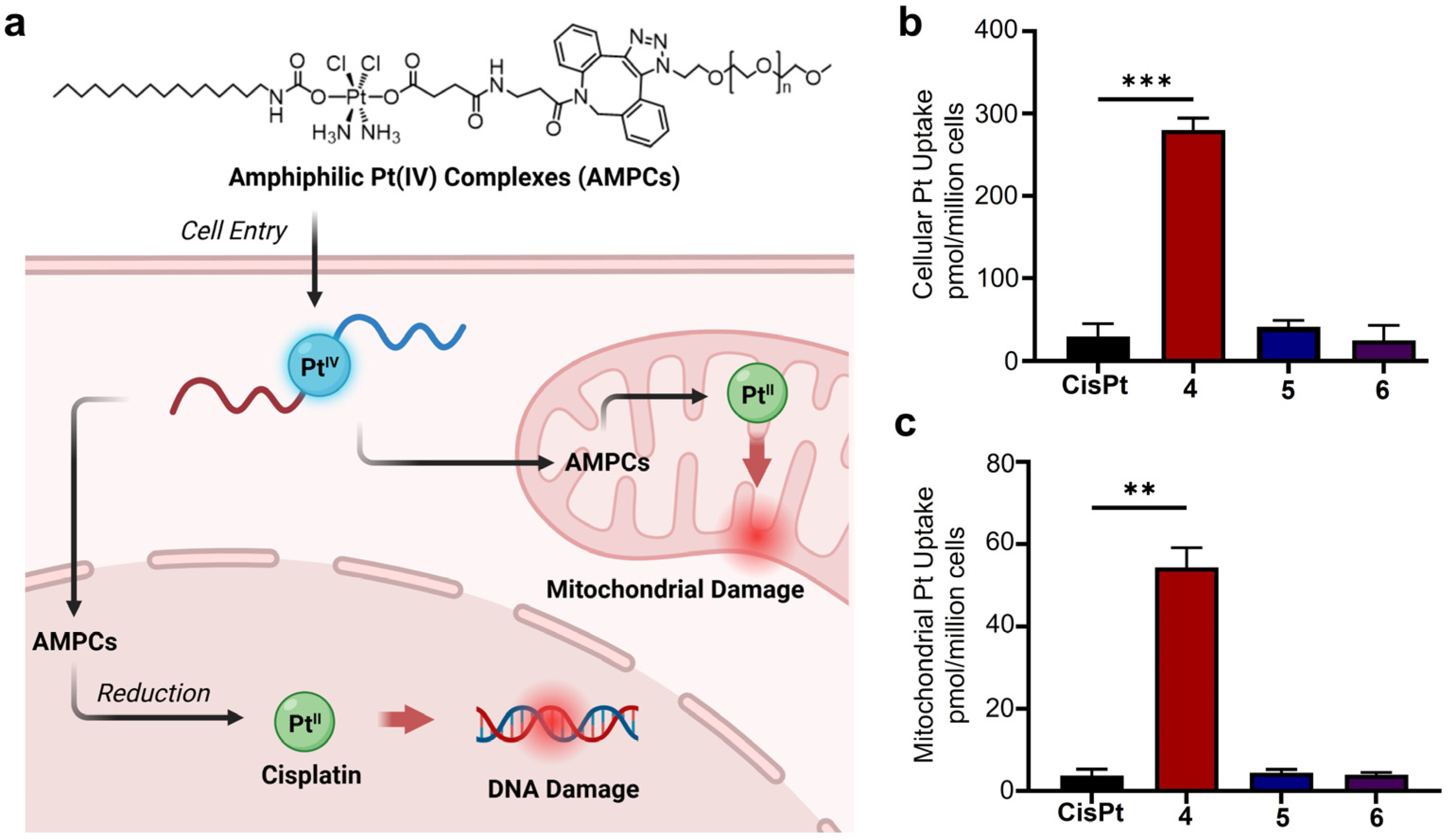
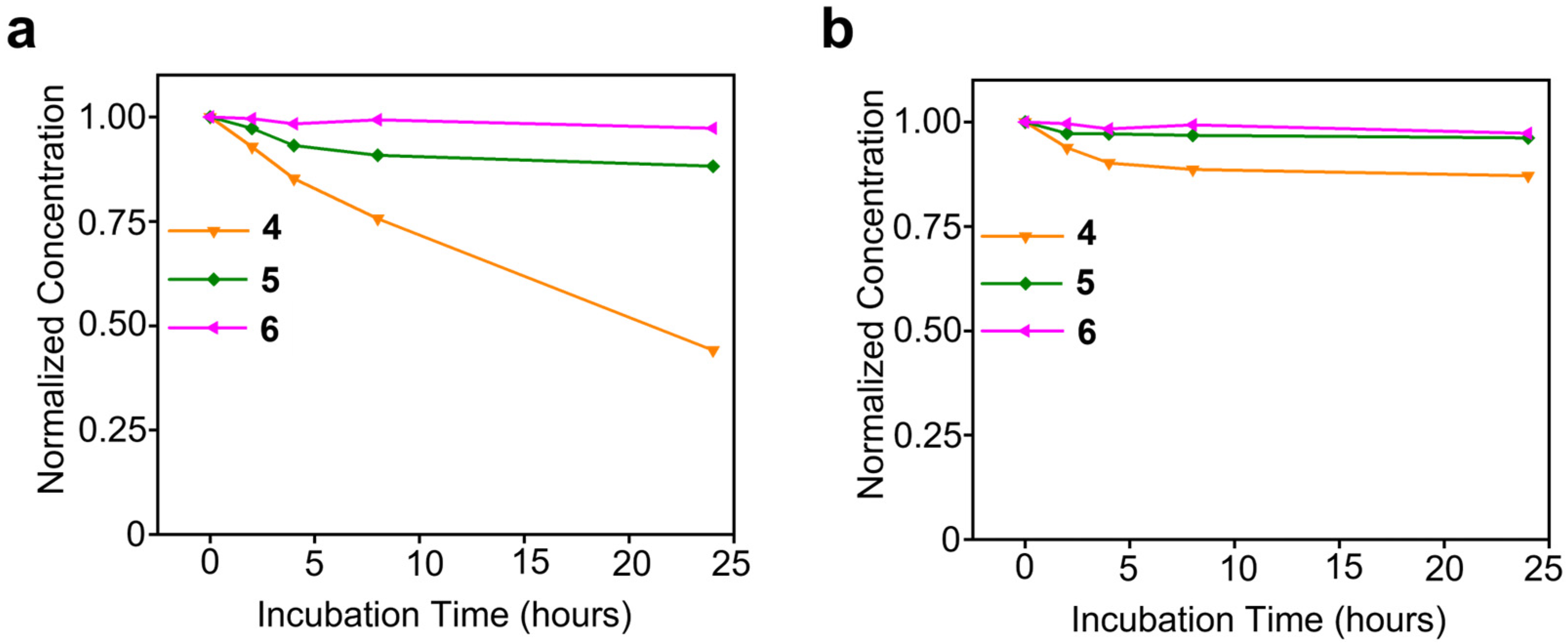
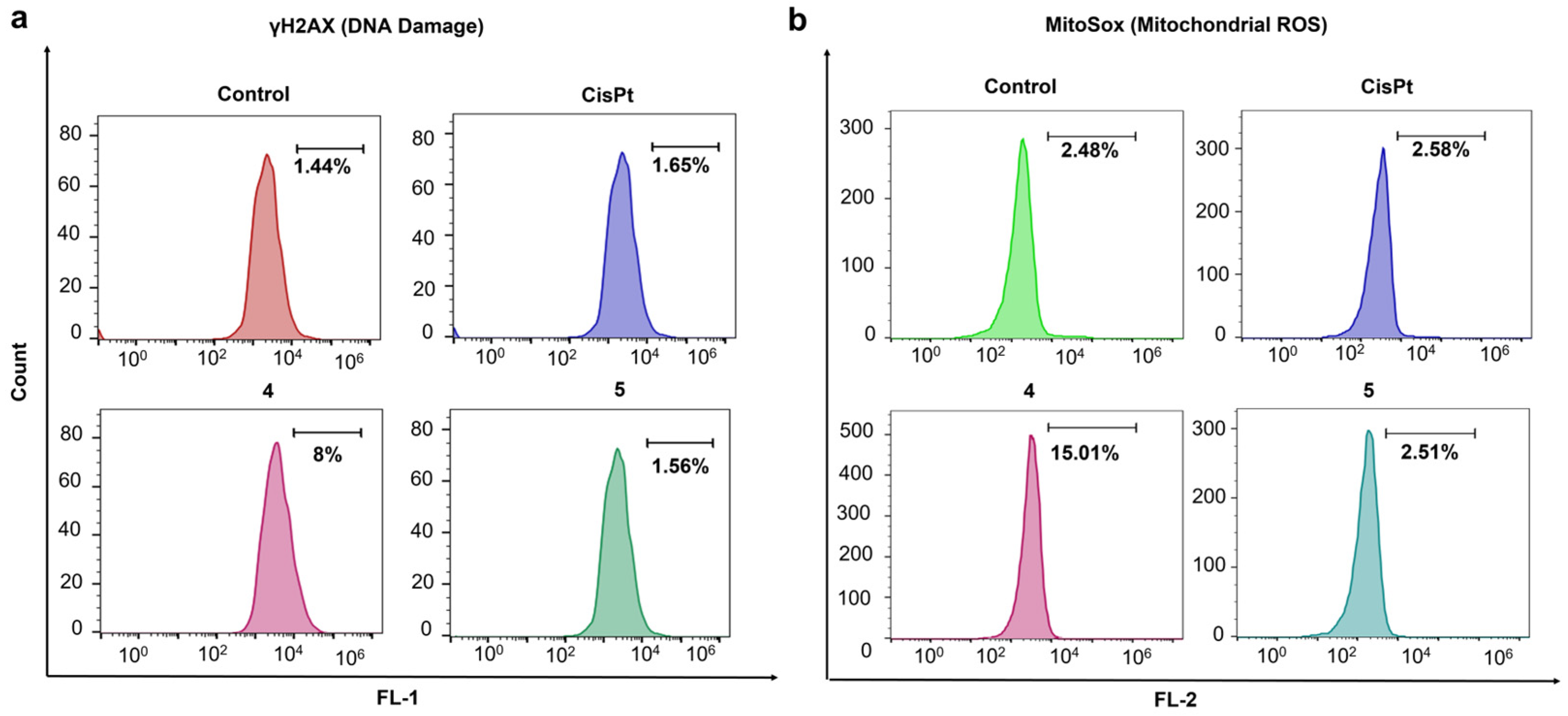
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, E.V.; Kopeina, G.S.; Imyanitov, E.N.; Zhivotovsky, B. Platinum drugs and taxanes: Can we overcome resistance? Cell Death Discov. 2021, 7, 155. [Google Scholar] [CrossRef]
- Alassadi, S.; Pisani, M.J.; Wheate, N.J. A chemical perspective on the clinical use of platinum-based anticancer drugs. Dalton Trans. 2022, 51, 10835–10846. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Chiorazzi, A.; Semperboni, S.; Marmiroli, P. Current View in Platinum Drug Mechanisms of Peripheral Neurotoxicity. Toxics 2015, 3, 304–321. [Google Scholar] [CrossRef]
- Li, H.; Cheng, S.; Zhai, J.; Lei, K.; Zhou, P.; Cai, K.; Li, J. Platinum based theranostics nanoplatforms for antitumor applications. J. Mater. Chem. B 2023, 11, 8387–8403. [Google Scholar] [CrossRef]
- Wang, T.; Wu, C.; Hu, Y.; Zhang, Y.; Ma, J. Stimuli-responsive nanocarrier delivery systems for Pt-based antitumor complexes: A review. RSC Adv. 2023, 13, 16488–16511. [Google Scholar] [CrossRef]
- Alfonso-Triguero, P.; Lorenzo, J.; Candiota, A.P.; Arús, C.; Ruiz-Molina, D.; Novio, F. Platinum-Based Nanoformulations for Glioblastoma Treatment: The Resurgence of Platinum Drugs? Nanomaterials 2023, 13, 1619. [Google Scholar] [CrossRef]
- Zhong, T.; Yu, J.; Pan, Y.; Zhang, N.; Qi, Y.; Huang, Y. Recent Advances of Platinum-Based Anticancer Complexes in Combinational Multimodal Therapy. Adv. Healthc. Mater. 2023, 12, e2300253. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, R.; Wang, D.; Wang, J.; Huang, Y.; Ding, Y.; Lu, B.; Sun, Y.; Stang, P.J.; Yao, Y. Platinum(II)-Metallaclip-Based Theranostics for Cell Imaging and Synergetic Chemotherapy-Photodynamic Therapy. Inorg. Chem. 2022, 62, 1786–1790. [Google Scholar] [CrossRef]
- Amarsy, I.; Papot, S.; Gasser, G. Stimuli-Responsive Metal Complexes for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205900. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.P.; Patra, M. Platinum glycoconjugates: “Sweet bullets” for targeted cancer therapy? Curr. Opin. Chem. Biol. 2023, 72, 102236. [Google Scholar] [CrossRef]
- Olelewe, C.; Awuah, S.G. Mitochondria as a target of third row transition metal-based anticancer complexes. Curr. Opin. Chem. Biol. 2023, 72, 102235. [Google Scholar] [CrossRef]
- Northcote-Smith, J.; Suntharalingam, K. Targeting chemotherapy-resistant tumour sub-populations using inorganic chemistry: Anti-cancer stem cell metal complexes. Curr. Opin. Chem. Biol. 2023, 72, 102237. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Chan, M.H.; Pan, M.; Li, Y.; Yam, V.W. Supramolecular Assembly of Organoplatinum(II) Complexes for Subcellular Distribution and Cell Viability Monitoring with Differentiated Imaging. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210703. [Google Scholar] [CrossRef] [PubMed]
- Momeni, B.Z.; Abd-El-Aziz, A.S. Recent advances in the design and applications of platinum-based supramolecular architectures and macromolecules. Coord. Chem. Rev. 2023, 486, 215113. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, G. Beyond mere DNA damage: Recent progress in platinum(IV) anticancer complexes containing multi-functional axial ligands. Curr. Opin. Chem. Biol. 2023, 74, 102303. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Deng, Z.; Zhu, G. Recent advances in the synthesis, stability, and activation of platinum(IV) anticancer prodrugs. Coord. Chem. Rev. 2021, 442, 213991. [Google Scholar] [CrossRef]
- Boulet, M.H.C.; Bolland, H.R.; Hammond, E.M.; Sedgwick, A.C. Oxali(IV)Fluors: Fluorescence Responsive Oxaliplatin(IV) Complexes Identify a Hypoxia-Dependent Reduction in Cancer Cells. J. Am. Chem. Soc. 2023, 145, 12998–13002. [Google Scholar] [CrossRef]
- Su, S.; Chen, Y.; Zhang, P.; Ma, R.; Zhang, W.; Liu, J.; Li, T.; Niu, H.; Cao, Y.; Hu, B.; et al. The role of Platinum(IV)-based antitumor drugs and the anticancer immune response in medicinal inorganic chemistry. A systematic review from 2017 to 2022. Eur. J. Med. Chem. 2022, 243, 114680. [Google Scholar] [CrossRef]
- Deng, Z.; Li, H.; Chen, S.; Wang, N.; Liu, G.; Liu, D.; Ou, W.; Xu, F.; Wang, X.; Lei, D.; et al. Near-infrared-activated anticancer platinum(IV) complexes directly photooxidize biomolecules in an oxygen-independent manner. Nat. Chem. 2023, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, N.; Liu, Y.; Xu, Z.; Wang, Z.; Lau, T.C.; Zhu, G. A Photocaged, Water-Oxidizing, and Nucleolus-Targeted Pt(IV) Complex with a Distinct Anticancer Mechanism. J. Am. Chem. Soc. 2020, 142, 7803–7812. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Cheng, S.-C.; Xu, K.; Deng, Z.; Chen, S.; Xu, Z.; Xie, K.; Tse, M.-K.; Shi, P.; et al. Phorbiplatin, a Highly Potent Pt(IV) Antitumor Prodrug That Can Be Controllably Activated by Red Light. Chem 2019, 5, 3151–3165. [Google Scholar] [CrossRef]
- Gibson, D. Platinum(IV) anticancer agents; are we en route to the holy grail or to a dead end? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef]
- Babu, T.; Sarkar, A.; Karmakar, S.; Schmidt, C.; Gibson, D. Multiaction Pt(IV) Carbamate Complexes Can Codeliver Pt(II) Drugs and Amine Containing Bioactive Molecules. Inorg. Chem. 2020, 59, 5182–5193. [Google Scholar] [CrossRef] [PubMed]
- Abu Ammar, A.; Raveendran, R.; Gibson, D.; Nassar, T.; Benita, S. A Lipophilic Pt(IV) Oxaliplatin Derivative Enhances Antitumor Activity. J. Med. Chem. 2016, 59, 9035–9046. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kong, X.; Li, X.; Zhang, B.; Deng, Y.; Wang, J.; Duan, C.; Zhang, D.; Liu, W. Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties. Molecules 2024, 29, 746. [Google Scholar] [CrossRef]
- Jogadi, W.; Kshetri, M.B.; Alqarni, S.; Sharma, A.; Cheline, M.; Amin, M.A.; Sheets, C.; Nsoure-Engohang, A.; Zheng, Y.R. Engineering Novel Amphiphilic Platinum(IV) Complexes to Co-Deliver Cisplatin and Doxorubicin. Molecules 2024, 29, 4095. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Yoo, H.; Lin, W.; Brooks, J.G.; Lippard, S.J. Pt(IV) Prodrugs Designed to Bind Non-Covalently to Human Serum Albumin for Drug Delivery. J. Am. Chem. Soc. 2014, 136, 8790–8798. [Google Scholar] [CrossRef]
- Fronik, P.; Poetsch, I.; Kastner, A.; Mendrina, T.; Hager, S.; Hohenwallner, K.; Schueffl, H.; Herndler-Brandstetter, D.; Koellensperger, G.; Rampler, E.; et al. Structure–Activity Relationships of Triple-Action Platinum(IV) Prodrugs with Albumin-Binding Properties and Immunomodulating Ligands. J. Med. Chem. 2021, 64, 12132–12151. [Google Scholar] [CrossRef] [PubMed]
- Navas, F.; Chocarro-Calvo, A.; Iglesias-Hernández, P.; Fernández-García, P.; Morales, V.; García-Martínez, J.M.; Sanz, R.; De la Vieja, A.; García-Jiménez, C.; García-Muñoz, R.A. Promising Anticancer Prodrugs Based on Pt(IV) Complexes with Bis-organosilane Ligands in Axial Positions. J. Med. Chem. 2024, 67, 6410–6424. [Google Scholar] [CrossRef]
- He, S.; Li, C.; Zhang, Q.; Ding, J.; Liang, X.J.; Chen, X.; Xiao, H.; Zhou, D.; Huang, Y. Tailoring Platinum(IV) Amphiphiles for Self-Targeting All-in-One Assemblies as Precise Multimodal Theranostic Nanomedicine. ACS Nano 2018, 12, 7272–7281. [Google Scholar] [CrossRef]
- Wang, M.; Cai, Y.; He, T.; Zhang, Y.; Yi, L.; Li, W.; Zhou, P. Antitumor Effect of Platinum-Modified STING Agonist MSA-2. ACS Omega 2024, 9, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, D.; Yu, W.; Li, J.; Wang, X.; Li, Y.; Zhao, Z.; Xue, Q.; Zhao, J.; Li, J.P.; et al. Combining cisplatin and a STING agonist into one molecule for metalloimmunotherapy of cancer. Natl. Sci. Rev. 2024, 11, nwae020. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, J.; Li, R.; Tang, D.; Cao, Z.; Xu, C.; Xiao, H. Activating CD8. Adv. Mater. 2024, 36, e2311640. [Google Scholar] [CrossRef] [PubMed]
- Jogadi, W.; Zheng, Y.R. Supramolecular platinum complexes for cancer therapy. Curr. Opin. Chem. Biol. 2023, 73, 102276. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Yao, H.; Deng, Z.; Chen, S.; Wang, Y.; Peng, W.; Sun, G.; Tse, M.-K.; Chen, X.; et al. An ultrasound-activatable platinum prodrug for sono-sensitized chemotherapy. Sci. Adv. 2023, 9, eadg5964. [Google Scholar] [CrossRef] [PubMed]
- Kshetri, M.; Jogadi, W.; Alqarni, S.; Datta, P.; Cheline, M.; Sharma, A.; Betters, T.; Broyles, D.; Zheng, Y.R. Exploring the Impact of Head Group Modifications on the Anticancer Activities of Fatty-Acid-like Platinum(IV) Prodrugs: A Structure-Activity Relationship Study. Int. J. Mol. Sci. 2023, 24, 13301. [Google Scholar] [CrossRef]
- Jayawardhana, A.M.D.S.; Zheng, Y.R. Interactions between mitochondria-damaging platinum(IV) prodrugs and cytochrome c. Dalton Trans. 2022, 51, 2012–2018. [Google Scholar] [CrossRef]
- Jayawardhana, A.M.D.S.; Stilgenbauer, M.; Datta, P.; Qiu, Z.; Mckenzie, S.; Wang, H.; Bowers, D.; Kurokawa, M.; Zheng, Y.R. Fatty acid-like Pt(IV) prodrugs overcome cisplatin resistance in ovarian cancer by harnessing CD36. Chem. Commun. 2020, 56, 10706–10709. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, A.A.; Guin, S.; Ashokan, A.; Basu, U.; Surnar, B.; Delma, K.S.; Lima, L.M.; Kryvenko, O.N.; Dhar, S. New Pathway for Cisplatin Prodrug to Utilize Metabolic Substrate Preference to Overcome Cancer Intrinsic Resistance. ACS Cent. Sci. 2023, 9, 1297–1312. [Google Scholar] [CrossRef]
- Wei, D.; Yu, Y.; Zhang, X.; Wang, Y.; Chen, H.; Zhao, Y.; Wang, F.; Rong, G.; Wang, W.; Kang, X.; et al. Breaking the Intracellular Redox Balance with Diselenium Nanoparticles for Maximizing Chemotherapy Efficacy on Patient-Derived Xenograft Models. ACS Nano 2020, 14, 16984–16996. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, M.; Jayawardhana, A.M.D.S.; Datta, P.; Yue, Z.; Gray, M.; Nielsen, F.; Bowers, D.J.; Xiao, H.; Zheng, Y.R. A spermine-conjugated lipophilic Pt(iv) prodrug designed to eliminate cancer stem cells in ovarian cancer. Chem. Commun. 2019, 55, 6106–6109. [Google Scholar] [CrossRef]
- Couvreur, P.; Lepetre-Mouelhi, S.; Garbayo, E.; Blanco-Prieto, M.J. Self-assembled lipid–prodrug nanoparticles. Nat. Rev. Bioeng. 2023, 1, 749–768. [Google Scholar] [CrossRef]
- Coppens, E.; Desmaële, D.; Mougin, J.; Tusseau-Nenez, S.; Couvreur, P.; Mura, S. Gemcitabine Lipid Prodrugs: The Key Role of the Lipid Moiety on the Self-Assembly into Nanoparticles. Bioconjugate Chem. 2021, 32, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Fadzen, C.M.; Wolfe, J.M.; Zhou, W.; Cho, C.-F.; von Spreckelsen, N.; Hutchinson, K.T.; Lee, Y.-C.; Chiocca, E.A.; Lawler, S.E.; Yilmaz, O.H.; et al. A Platinum(IV) Prodrug—Perfluoroaryl Macrocyclic Peptide Conjugate Enhances Platinum Uptake in the Brain. J. Med. Chem. 2020, 63, 6741–6747. [Google Scholar] [CrossRef]
- Garcia-Peiro, J.I.; Ortega-Liebana, M.C.; Adam, C.; Lorente-Macías, Á.; Travnickova, J.; Patton, E.E.; Guerrero-López, P.; Garcia-Aznar, J.M.; Hueso, J.L.; Santamaria, J.; et al. Dendritic Platinum Nanoparticles Shielded by Pt-S PEGylation as Intracellular Reactors for Bioorthogonal Uncaging Chemistry. Angew. Chem. Int. Ed. 2025, n/a, e202424037. [Google Scholar] [CrossRef]
- Li, W.; Zhan, P.; De Clercq, E.; Lou, H.; Liu, X. Current drug research on PEGylation with small molecular agents. Prog. Polym. Sci. 2013, 38, 421–444. [Google Scholar] [CrossRef]
- Wu, T.; Chen, K.; He, S.; Liu, X.; Zheng, X.; Jiang, Z.-X. Drug Development through Modification of Small Molecular Drugs with Monodisperse Poly(ethylene glycol)s. Org. Process Res. Dev. 2020, 24, 1364–1372. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Vinciguerra, B.; Cortinas, A.B.; Thapa, L.S.; Jhunjhunwala, S.; Isaacs, L.; Langer, R.; Anderson, D.G. Supramolecular PEGylation of biopharmaceuticals. Proc. Natl. Acad. Sci. USA 2016, 113, 14189–14194. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Zhu, L.; Zhu, X.; Sun, M.; Yan, D. Drug-Polymer Hybrid Macromolecular Engineering: Degradable PEG Integrated by Platinum(IV) for Cancer Therapy. Matter 2019, 1, 1618–1630. [Google Scholar] [CrossRef]
- Arduino, I.; Depalo, N.; Re, F.; Dal Magro, R.; Panniello, A.; Margiotta, N.; Fanizza, E.; Lopalco, A.; Laquintana, V.; Cutrignelli, A.; et al. PEGylated solid lipid nanoparticles for brain delivery of lipophilic kiteplatin Pt(IV) prodrugs: An in vitro study. Int. J. Pharm. 2020, 583, 119351. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, C.; Cao, Z.; Shen, S.; Li, L.; Li, D.; Wang, J.; Yang, X. On-demand PEGylation and dePEGylation of PLA-based nanocarriers. Theranostics 2019, 9, 8312–8320. [Google Scholar] [CrossRef]
- Moretton, A.; Slyskova, J.; Simaan, M.E.; Arasa-Verge, E.A.; Meyenberg, M.; Cerrón-Infantes, D.A.; Unterlass, M.M.; Loizou, J.I. Clickable Cisplatin Derivatives as Versatile Tools to Probe the DNA Damage Response to Chemotherapy. Front. Oncol. 2022, 12, 874201. [Google Scholar]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Pathak, R.K.; McNitt, C.D.; Popik, V.V.; Dhar, S. Copper-Free Click-Chemistry Platform to Functionalize Cisplatin Prodrugs. Chem.-Eur. J. 2014, 20, 6861–6865. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Al Amin, M.; Kshetri, M.B.; Alqarni, S.; Jogadi, W.; Solmen, J.; Lin, Z.; Akter, S.; Zheng, Y.-R. PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity. Pharmaceutics 2025, 17, 440. https://doi.org/10.3390/pharmaceutics17040440
Sharma A, Al Amin M, Kshetri MB, Alqarni S, Jogadi W, Solmen J, Lin Z, Akter S, Zheng Y-R. PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity. Pharmaceutics. 2025; 17(4):440. https://doi.org/10.3390/pharmaceutics17040440
Chicago/Turabian StyleSharma, Arpit, Md Al Amin, Man B. Kshetri, Suha Alqarni, Wjdan Jogadi, Jordan Solmen, Zexin Lin, Shirin Akter, and Yao-Rong Zheng. 2025. "PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity" Pharmaceutics 17, no. 4: 440. https://doi.org/10.3390/pharmaceutics17040440
APA StyleSharma, A., Al Amin, M., Kshetri, M. B., Alqarni, S., Jogadi, W., Solmen, J., Lin, Z., Akter, S., & Zheng, Y.-R. (2025). PEGylation Effects on Amphiphilic Platinum(IV) Complexes: Influence on Uptake, Activation, and Cytotoxicity. Pharmaceutics, 17(4), 440. https://doi.org/10.3390/pharmaceutics17040440






