Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NG FA Preparation
2.3. Characterization Techniques for NG FA
2.3.1. NG Size and Electrokinetic Potential
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.4. Thermal Properties of Dehydrated Samples: Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.4. Biopharmaceutical Evaluation
2.4.1. In Vitro FA Release Studies
2.4.2. NG Mucoadhesion Evaluation
2.4.3. Ex Vivo Trans-Corneal Permeation Studies
- (i)
- Steady-state flux (J): v/A (µg/min·cm2), where A is the effective available tissue surface area.
- (ii)
- Permeation rate (v): ∆Q/∆t (µg/min), where Q is the amount of FA diffuse through the cornea at time t.
- (iii)
- Apparent Permeability Coefficient (Papp): J/Ci, where Ci is the initial drug concentration of the donor medium.
2.5. Statistical Analysis
3. Results and Discussion
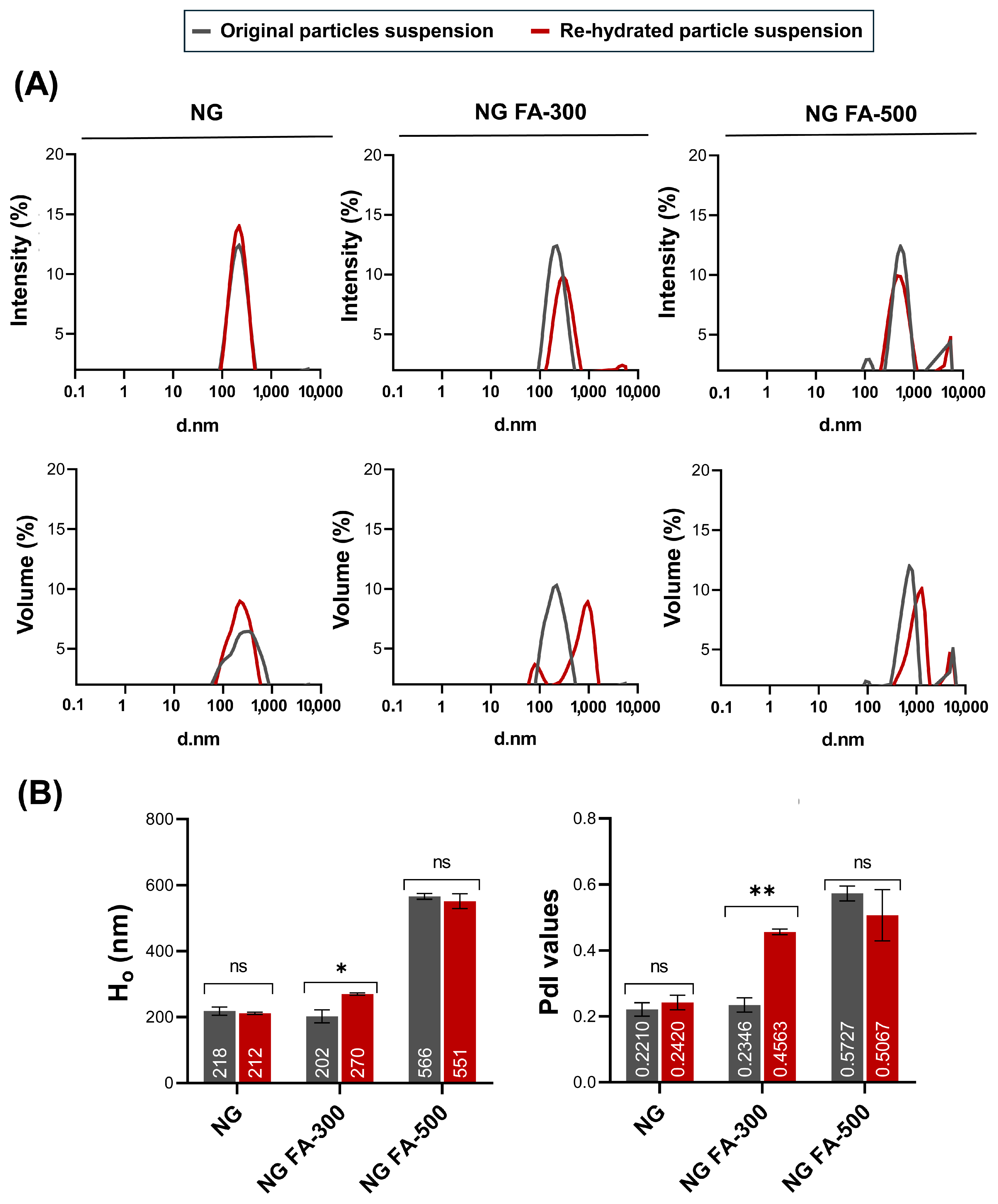
3.1. NG Size Distribution and PdI Determination
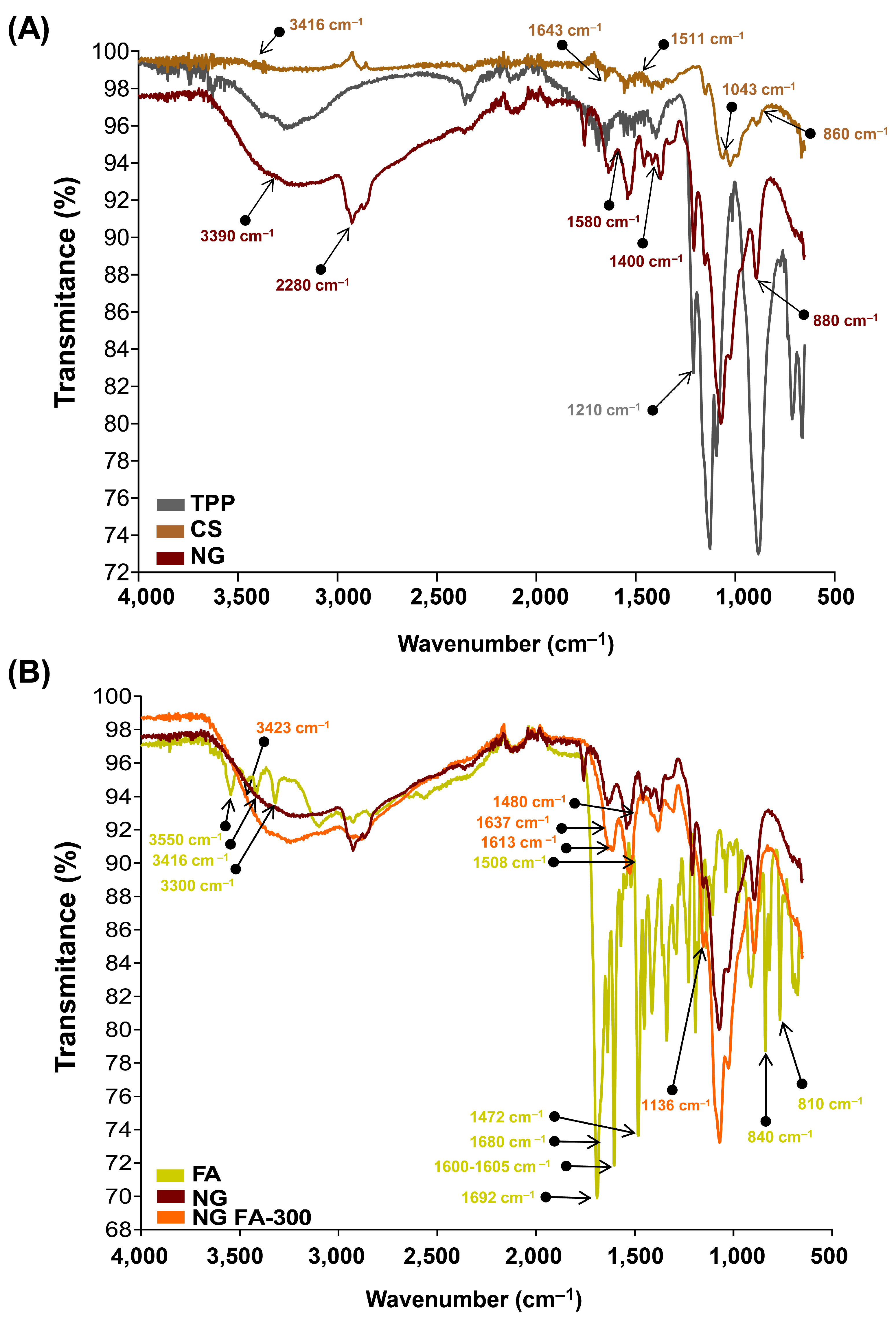
3.2. FTIR Analysis for Structural Characterization
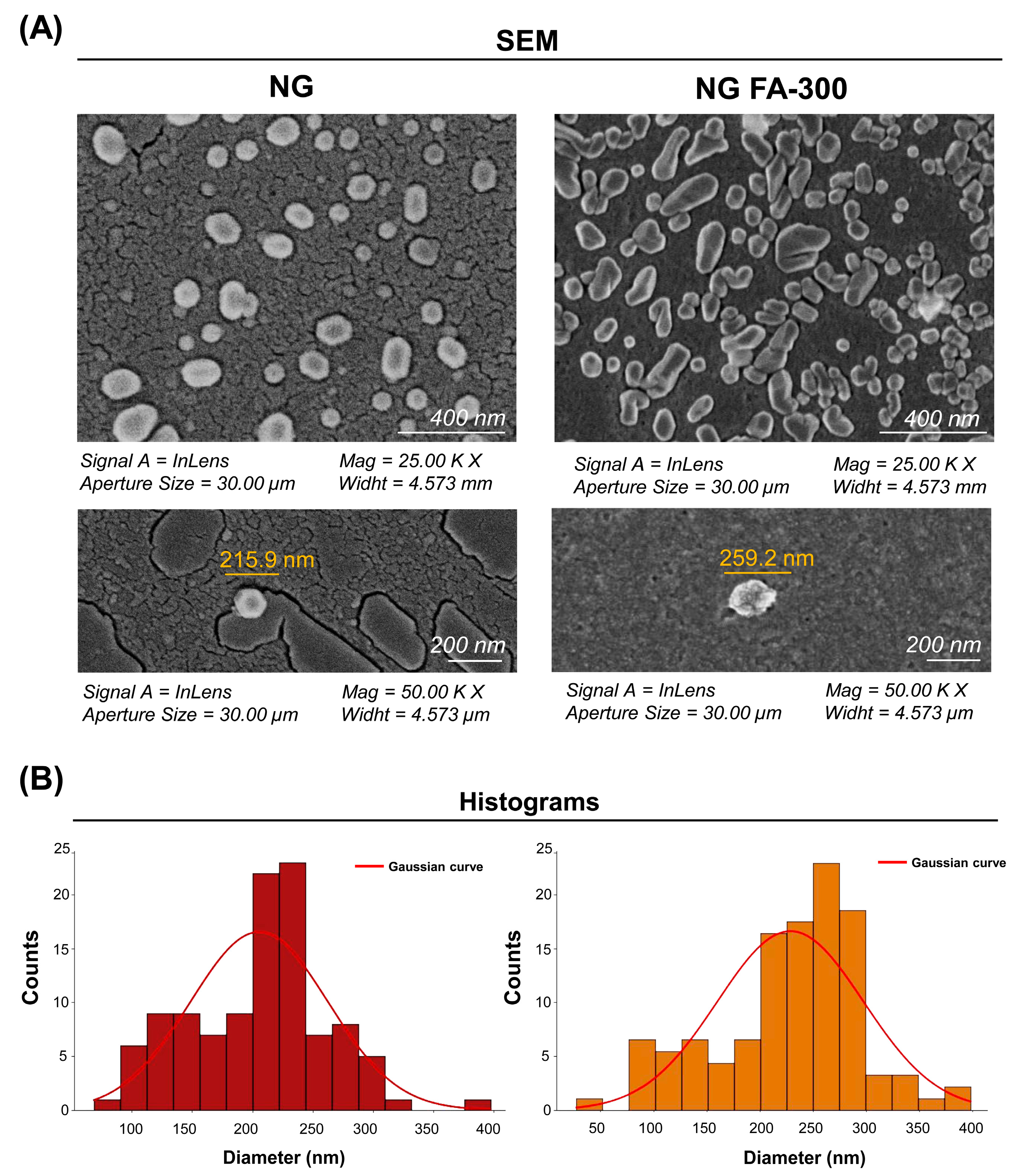
SEM Analysis of Ultrastructural Features
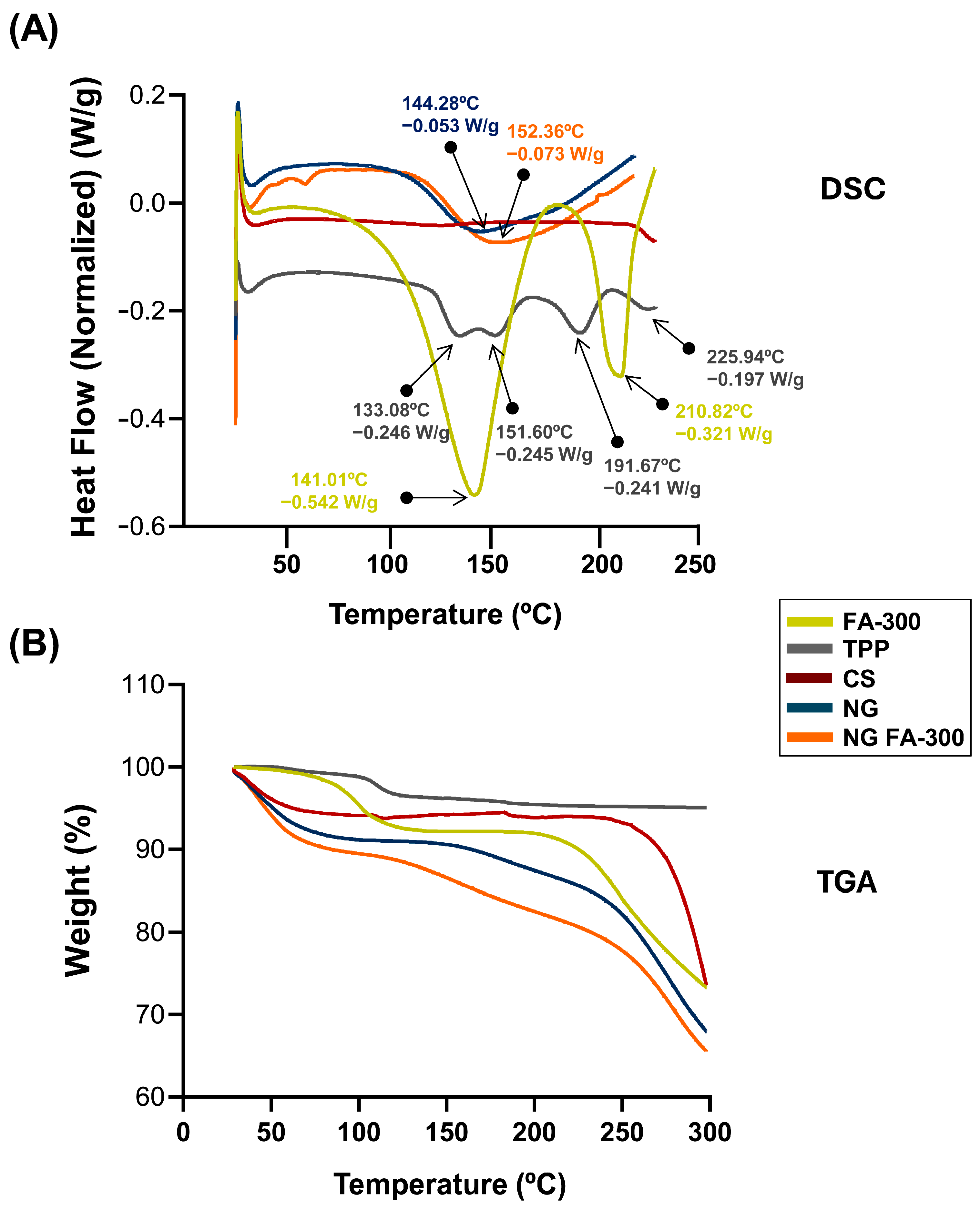
3.3. Thermal Behaviour Characterization
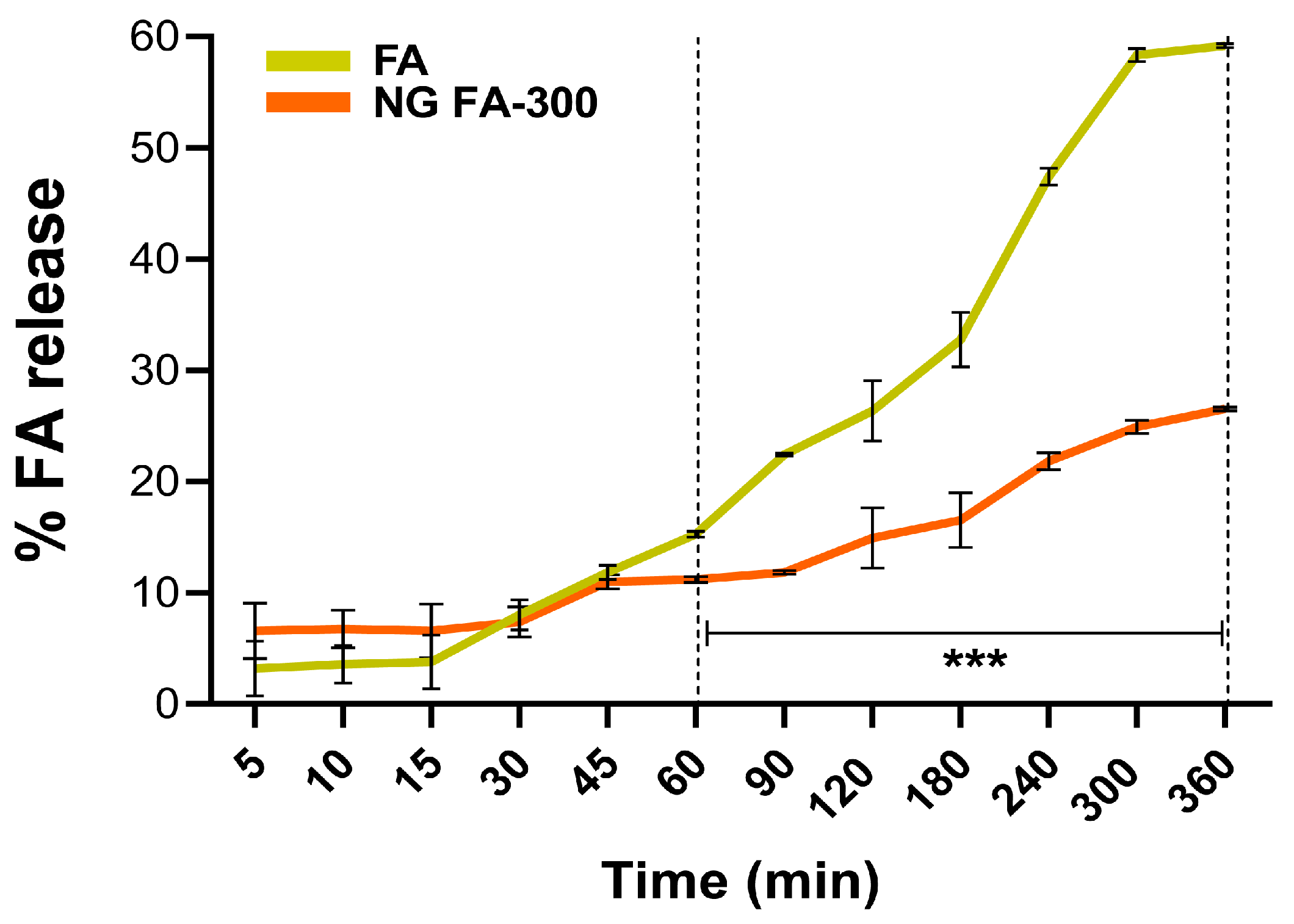
3.4. In Vitro FA Release from NG
3.5. Mucoadhesive Properties of NG FA
3.6. Ex Vivo Assays: Trans-Corneal Permeation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | chitosan |
| DD | degree of deacetylation |
| DLS | dynamic light scattering |
| DSC | differential scanning calorimetry |
| FA | folic acid |
| FT-IR | Fourier transform-infrared spectroscopy |
| HIUS | high-intensity ultrasound |
| IG | ionic gelation |
| Ho | hydrodynamic diameter |
| MC | mucin |
| MW | molecular weight |
| NG | CS-based nanogels |
| NG FA-300 | folic acid (300 µM)-loaded chitosan-based nanogels |
| NG FA-500 | folic acid (500 µM)-loaded chitosan-based nanogels |
| NP | nanoparticles |
| PdI | polydispersity index |
| RT | room temperature |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| TPP | sodium tripolyphosphate |
| ζ-Pot | ζ-potential |
References
- Nettey, H.; Darko, Y.; Bamiro, O.A.; Addo, R.T. Ocular barriers. In Ocular Drug Delivery: Advances, Challenges and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 27–36. [Google Scholar] [CrossRef]
- Alaimo, A.; Pérez, O. Advanced Nanoformulations: Theranostic Nanosystems; Academic Press: Cambridge, MA, USA, 2022; Volume 3, Chapter 2. [Google Scholar]
- Alaimo, A.; Tettamanti, C.; Pérez, O.; Allemandi, D.; Quinteros, D. New Generation Nanocarriers for Ocular Delivery of Resveratrol. In Novel Phytopharmaceutical for Management of Disorders; Chauhan, D.N., Sharma, V., Chauhan, N., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–353. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Jakka, D.; Sandoval, M.A.; Kulkarni, V.R.; Bao, Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics 2024, 16, 1325. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef]
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: Implications in ocular disease therapy. J. Nanobiotechnol. 2021, 19, 10. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Paolicelli, P.; De La Fuente, M.; Sánchez, A.; Seijo, B.; Alonso, M.J. Chitosan nanoparticles for drug delivery to the eye. Expert Opin. Drug Deliv. 2009, 6, 239–253. [Google Scholar] [CrossRef]
- Alaimo, A.; Pérez, O.E. Chitosan-based nanosuspensions for ocular diagnosis and therapy. In Advanced Nanoformulations; Academic Press: Cambridge, MA, USA, 2023; pp. 13–41. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A Review. Biomed. Pharmacother. 2021, 142, 11970. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Leithner, K.; Bernkop-Schnürch, A. Chitosan and Derivatives for Biopharmaceutical Use: Mucoadhesive Properties, Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; Wiley: Hoboken, NJ, USA, 2012; pp. 159–180. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; Liñares, G.G.; Acebedo, S.L.; Cataña, M.A.C.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol encapsulation in high molecular weight chitosan-based nanogels for applications in ocular treatments: Impact on human ARPE-19 culture cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The concept of folic acid in health and disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Martin, N.; Dodson, P.M. Homocysteine; folates and the eye. Eye 2008, 22, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Malm, E.; Ghosh, F. Chronic conjunctivitis in a patient with folic acid deficiency. Acta Ophthalmol. Scand. 2007, 85, 226. [Google Scholar] [CrossRef]
- Gu, J.; Lei, C.; Zhang, M. Folate and retinal vascular diseases. BMC Ophthalmol. 2023, 23, 413. [Google Scholar] [CrossRef]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef]
- Silva Nieto, R.; López, C.S.; Moretton, M.A.; Lizarraga, L.; Chiappetta, D.A.; Alaimo, A.; Pérez, O.E. Chitosan-Based Nanogels Designed for Betanin-Rich Beetroot Extract Transport: Physicochemical and Biological Aspects. Polymers 2023, 15, 3875. [Google Scholar] [CrossRef]
- Rivera López, E.; Samaniego López, C.; Spagnuolo, C.C.; Berardino, B.G.; Alaimo, A.; Pérez, O.E. Chitosan-Tricarbocyanine-Based Nanogels Were Able to Cross the Blood–Brain Barrier Showing Its Potential as a Targeted Site Delivery Agent. Pharmaceutics 2024, 16, 964. [Google Scholar] [CrossRef]
- De Matteo, R.; Rey, J.M.; Corfield, R.; Andrade, V.A.G.; Santagapita, P.R.; Di Salvo, F.; Pérez, O.E. Chitosan-inspired Matrices for Folic Acid. Insightful Structural Characterization and Ensured Bioaccessibility. Food Biophys. 2024, 19, 412–424. [Google Scholar] [CrossRef]
- Martinez, S.M.; Inda, A.; Ríos, M.N.; Bessone, C.d.V.; Bruera Bossio, A.; Guido, M.E.; Luna Pinto, J.D.; Allemandi, D.A.; Quinteros, D.A. Neuroprotective Effect of Melatonin Loaded in Human Serum Albumin Nanoparticles Applied Subconjunctivally in a Retinal Degeneration Animal Model. Pharmaceutics 2025, 17, 85. [Google Scholar] [CrossRef]
- Mora, M.J.; Tártara, L.I.; Onnainty, R.; Palma, S.D.; Longhi, M.R.; Granero, G.E. Characterization, dissolution and in vivo evaluation of solid acetazolamide complexes. Carbohydr. Polym. 2013, 98, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, D.A.; Manzo, R.H.; Allemandi, D.A. Interaction between Eudragit® E100 and anionic drugs: Addition of anionic polyelectrolytes and their influence on drug release performance. J. Pharm. Sci. 2011, 100, 4664–4673. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.V.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Exp. Eye Res. 2020, 200, 108222. [Google Scholar] [CrossRef]
- De Matteo, R.P.O.; Corfield, R.; Schebor, C. Folic acid embedded in argentinian chitosan: Antioxidant capacity and cytotoxic effect in human Caco-2 cell line. In Symposium: Exploring the Frontiers of Chemistry; Peking University: Beijing, China, 2019; p. Abstract. [Google Scholar]
- Quinteros, D.A.; Tártara, L.I.; Palma, S.D.; Manzo, R.H.; Allemandi, D.A. Ocular delivery of flurbiprofen based on Eudragit® E-flurbiprofen complex dispersed in aqueous solution: Preparation, characterization, in vitro corneal penetration, and ocular irritation. J. Pharm. Sci. 2014, 103, 3859–3868. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodland Publishing: Salt Lake City, UT, USA, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Busolo, C.; Drago, F.; Salomone, S. Ocular Drug Delivery: A Clue From Nanotechnology. Front. Pharmacol. 2022, 3, 188. [Google Scholar] [CrossRef]
- da Silva, S.B.; Amorim, M.; Fonte, P.; Madureira, R.; Ferreira, D.; Pintado, M.; Sarmento, B. Natural extracts into chitosan nanocarriers for rosmarinic acid drug delivery. Pharm. Biol. 2015, 53, 642–652. [Google Scholar] [CrossRef]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Attama, A.A.; Nnamani, P.O.; Onugwu, S.O.; Onuigbo, E.B.; Khutoryanskiy, V.V. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J. Drug Deliv. Sci. Technol. 2022, 74, 103527. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Parize, A.L. In vitro cytotoxic and antioxidant evaluation of quercetin loaded in ionic cross-linked chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 74, 103561. [Google Scholar] [CrossRef]
- Okamoto-Schalch, N.O.; Pinho, S.G.B.; de Barros-Alexandrino, T.T.; Dacanal, G.C.; Assis, O.B.G.; Martelli-Tosi, M. Production and characterization of chitosan-TPP/cellulose nanocrystal system for encapsulation: A case study using folic acid as active compound. Cellulose 2020, 27, 5855–5869. [Google Scholar] [CrossRef]
- Fathima, E.; Nallamuthu, I.; Anand, T.; Naika, M.; Khanum, F. Enhanced cellular uptake, transport and oral bioavailability of optimized folic acid-loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2022, 208, 596–610. [Google Scholar] [CrossRef]
- Byrn, S.R.; Zografi, G.; Chen, X. Differential Scanning Calorimetry and Thermogravimetric Analysis. In Solid State Properties of Pharmaceutical Materials; Wiley: Hoboken, NJ, USA, 2017; pp. 124–141. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Lobry, M.; Colombeau, L.; Acherar, S.; Azaïs, H.; Mordon, S.; Arnoux, P.; Baros, F.; Vanderesse, R.; Frochot, C. Stability of folic acid under several parameters. Eur. J. Pharm. Sci. 2016, 93, 419–430. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Ullah, S.N.M.N.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Nimmansophon, P.; Wanasathop, A.; Li, S.K. Lateral Transport During Membrane Permeation in Diffusion Cell: In Silico Study on Edge Effect and Membrane Blocking. J. Pharm. Sci. 2023, 112, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.S.; D’Souza, A.; Aibani, N.; Nair, S.S.; Sandbhor, P.; Kumari, D.; Banerjee, R. Stable Liposome in Cosmetic Platforms for Transdermal Folic acid delivery for fortification and treatment of micronutrient deficiencies. Sci. Rep. 2018, 8, 16122. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef]
- Wasserman, L. All of Statistics; Springer: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesive Polymers and Formulations for Transmucosal Drug Delivery; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Mendes, A.C.; Moreno, J.S.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, Mechanical and Mucoadhesive Properties of Electrospun Chitosan/Phospholipid Hybrid Nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Urimi, D.; Harde, H.; Kushwah, V.; Jain, S. Folate appended chitosan nanoparticles augment the stability, bioavailability and efficacy of insulin in diabetic rats following oral administration. RSC Adv. 2015, 5, 105179–105193. [Google Scholar] [CrossRef]
- de Britto, D.; de Moura, M.R.; Aouada, F.A.; Mattoso, L.H.C.; Assis, O.B.G. N,N,N-trimethyl chitosan nanoparticles as a vitamin carrier system. Food Hydrocoll. 2012, 27, 487–493. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.K.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Samaniego López, C.; Di Santo, M.; Pérez, O. Gellan gum-based dermatological formulations. In Application of Gellan Gum as a Biomedical Polymer, 1st ed.; Nayak, A.H.S., Ed.; Academic Press: Cambridge, MA, USA, 2024; p. 457. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S.; Costa-Pinto, R.; Oliveira, A.L. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]


| System | Mathematical Models of Drug Release | ||||||
|---|---|---|---|---|---|---|---|
| Zero-Order Plot | Higuchi Model | Korsmeyer–Peppas Model | |||||
| k (min) | R2 | k (min−1/2) | R2 | k (min−n) | n | R2 | |
| Free FA-300 | 12.681 ± 0.520 | 0.978 | 20.072 ± 1.290 | 0.947 | 15.938 ± 0.228 | 0.781 ± 0.013 | 0.999 |
| NG FA-300 | 4.996 ± 0.523 | 0.483 | 8.263 ± 0.294 | 0.951 | 8.651 ± 0.472 | 0.441 ± 0.056 | 0.917 |
| System | Steady-state Flux (J) (μg/min) | Permeated (μg) (After 2h) | Apparent Permeability Coefficient, Papp (cm/min) (×10−4) |
|---|---|---|---|
| free FA-300 | 0.113 ± 0.004 | 33.848 ± 2.187 | 2.123 ± 0.032 |
| NG FA-300 | 0.0427 ± 0.003 | 14.611 ± 0.627 | 0.824 ± 0.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, S.G.; Martínez, S.M.; Costa Gobbato, C.; Quinteros, D.A.; Alaimo, A.; Pérez, O.E. Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment. Pharmaceutics 2025, 17, 424. https://doi.org/10.3390/pharmaceutics17040424
Bruno SG, Martínez SM, Costa Gobbato C, Quinteros DA, Alaimo A, Pérez OE. Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment. Pharmaceutics. 2025; 17(4):424. https://doi.org/10.3390/pharmaceutics17040424
Chicago/Turabian StyleBruno, Sebastián G., Sofía M. Martínez, Camila Costa Gobbato, Daniela A. Quinteros, Agustina Alaimo, and Oscar E. Pérez. 2025. "Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment" Pharmaceutics 17, no. 4: 424. https://doi.org/10.3390/pharmaceutics17040424
APA StyleBruno, S. G., Martínez, S. M., Costa Gobbato, C., Quinteros, D. A., Alaimo, A., & Pérez, O. E. (2025). Chitosan-TPP Nanogels for Ocular Delivery of Folic Acid: Release Profile, Corneal Permeation, and Mucoadhesion Assessment. Pharmaceutics, 17(4), 424. https://doi.org/10.3390/pharmaceutics17040424








