Synergistic Effect of Conditioned Medium from Amniotic Membrane Mesenchymal Stromal Cells Combined with Paclitaxel on Ovarian Cancer Cell Viability and Migration in 2D and 3D In Vitro Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Human Amniotic Membrane (hAM) Fragment Preparation
2.3. Isolation and Culture of Human Amniotic Mesenchymal Stromal Cells (hAMSC)
2.4. Preparation of Conditioned Medium (CM)
2.5. Determination of Cellular Viability (MTT-Assay and CyQUANT-Assay)
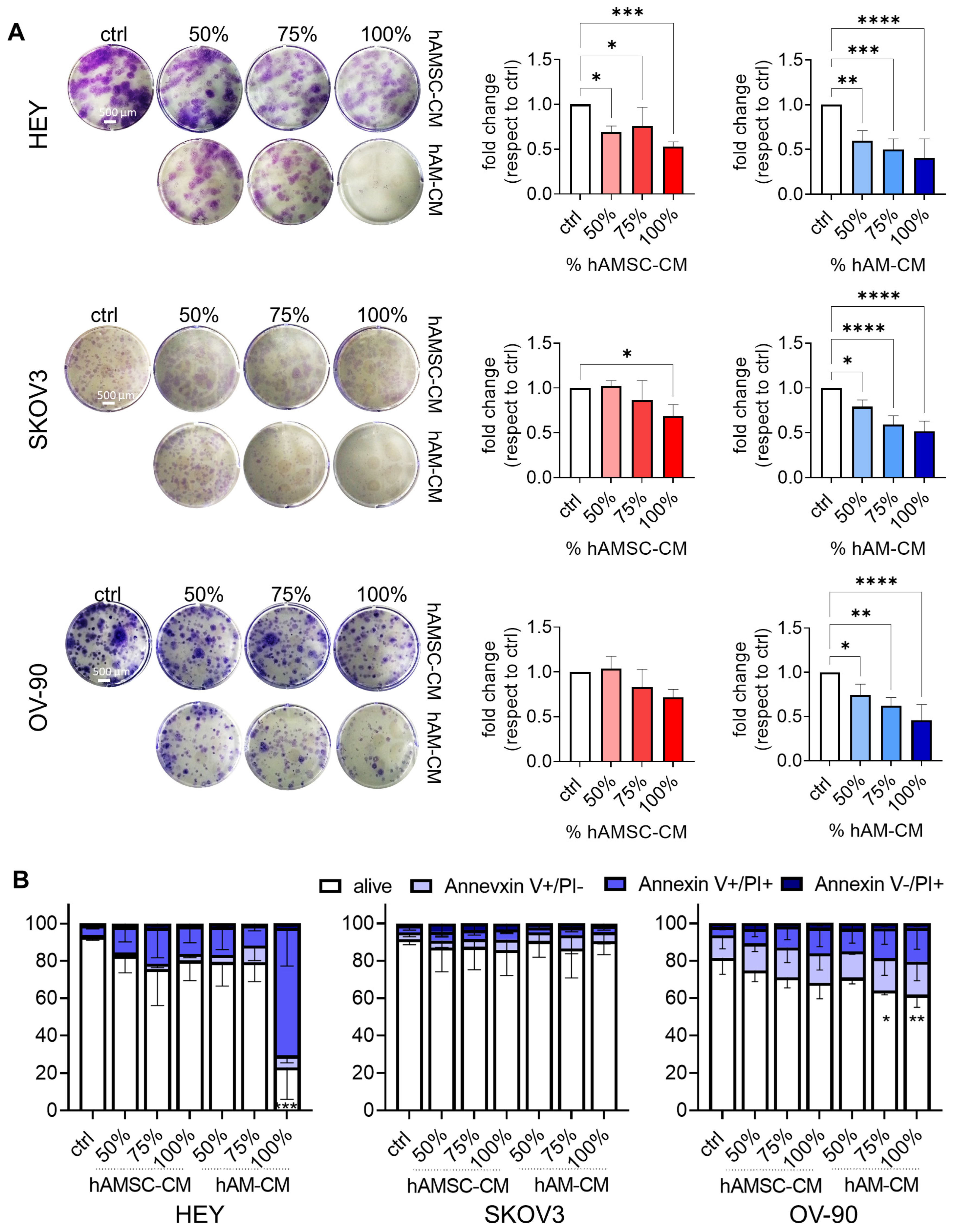
2.6. Colony Formation
2.7. Apoptosis Assay
2.8. Wound Healing Assay
2.9. Single-Cell Migration
2.10. Transwell Migration Assay
2.11. Western Blot
2.12. Spheroid Generation
2.13. Three-Dimensional Cell Viability Assay (ATPlite)
2.14. Three-Dimensional Co-Culture Angiogenesis Model
2.15. Statistical Analysis
3. Results
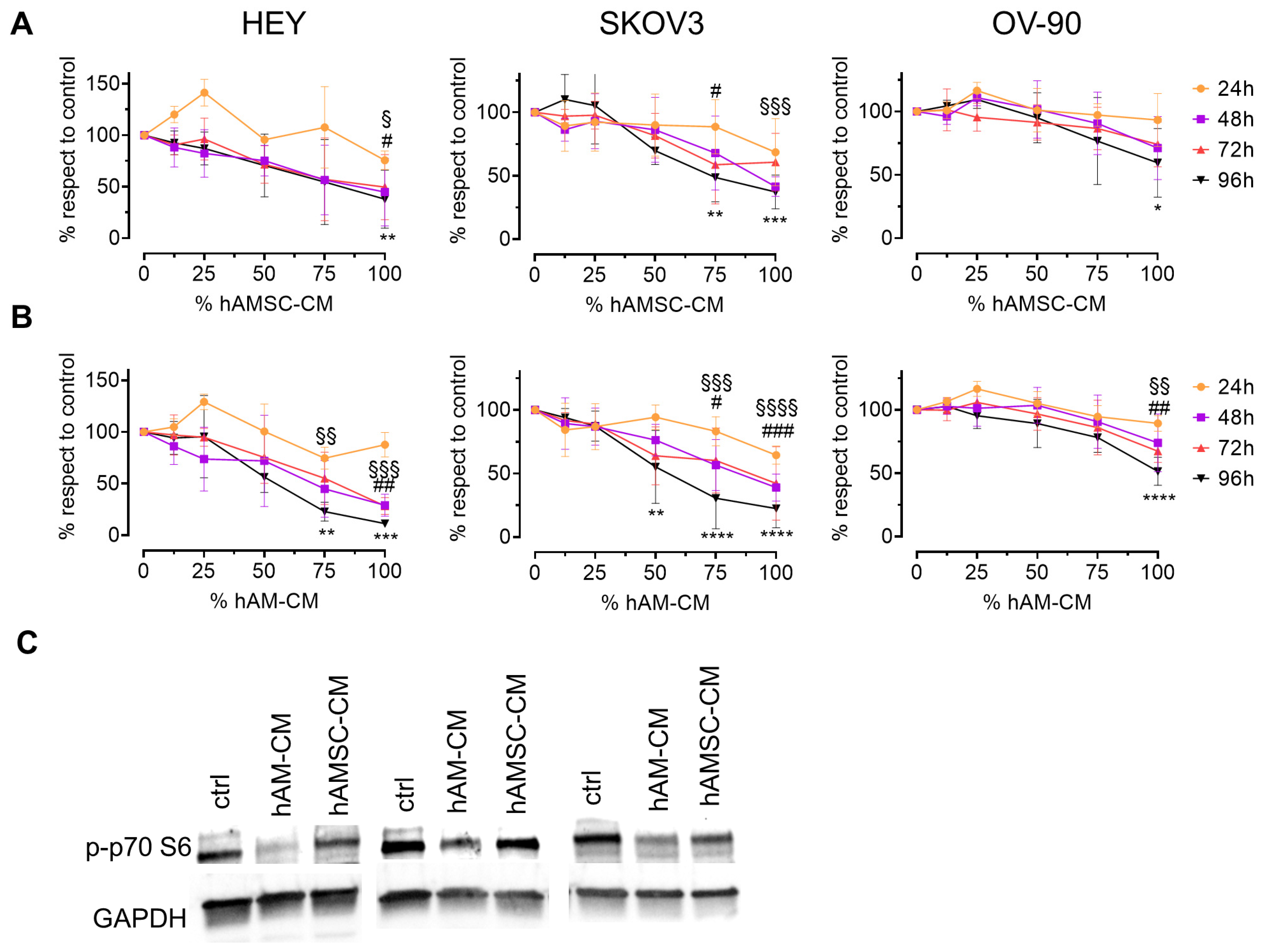
3.1. hAMSC-CM and hAM-CM Inhibit Ovarian Cancer Cell Proliferation
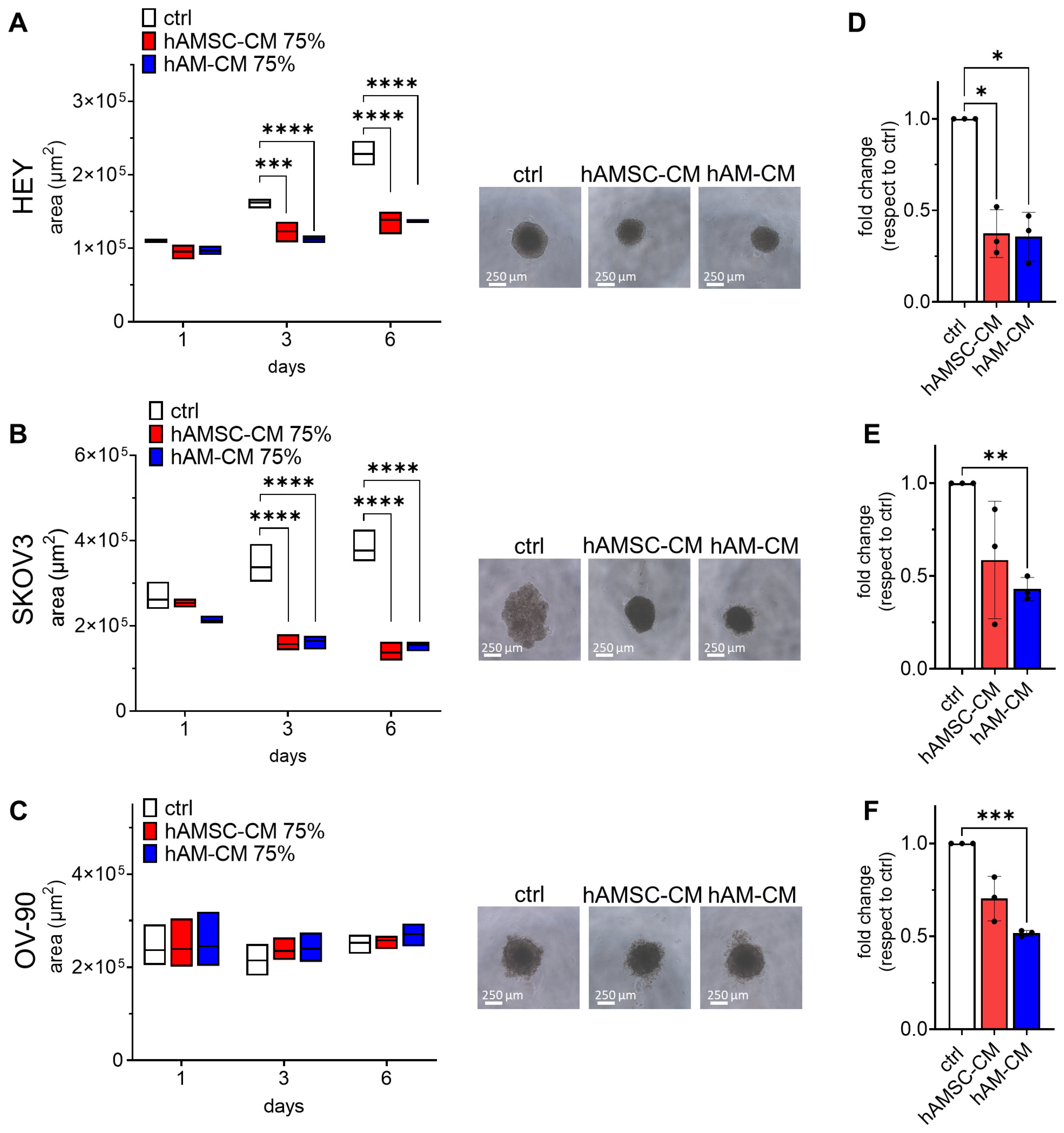
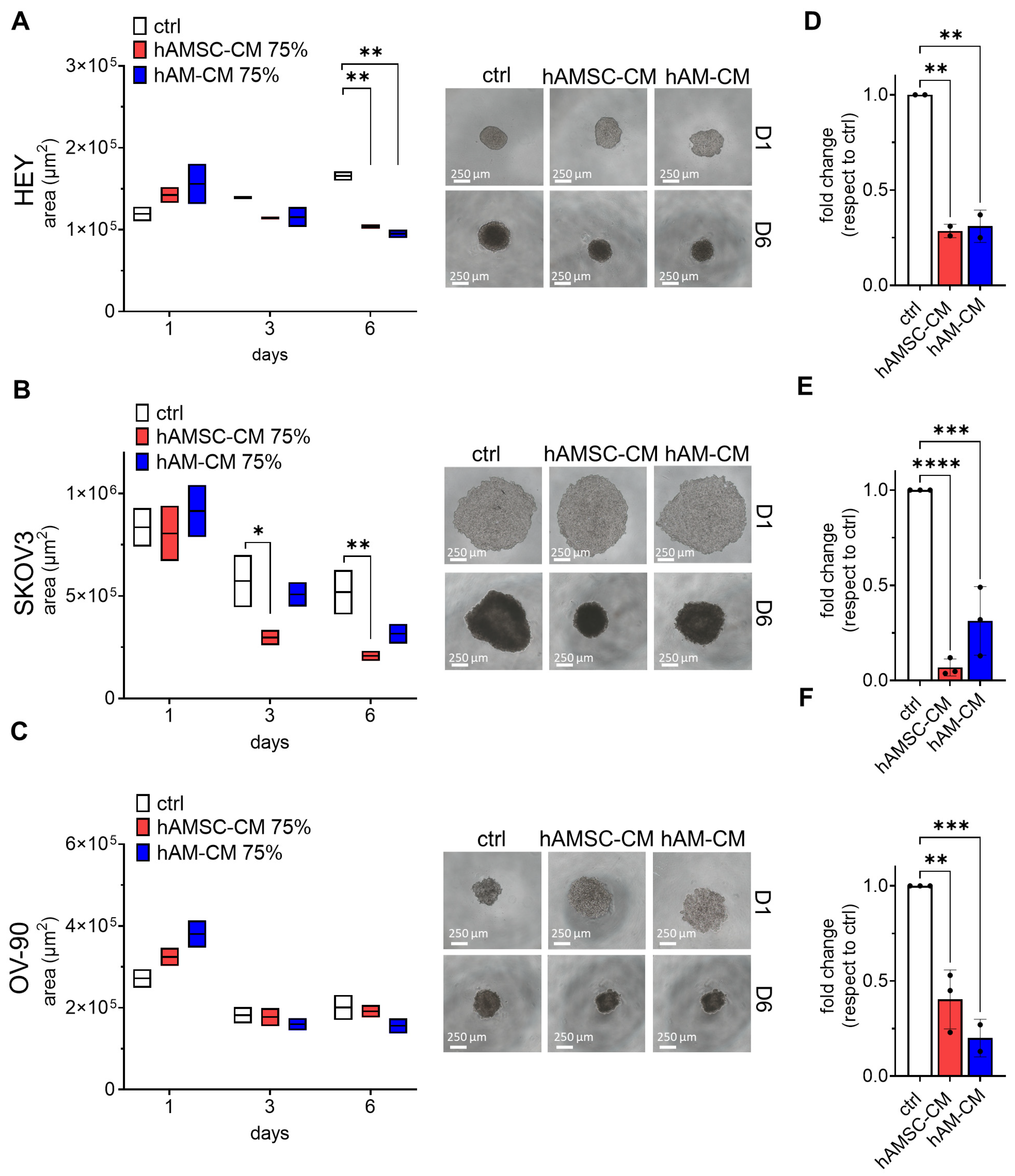
3.2. hAMSC-CM and hAM-CM Differentially Affect 3D Spheroid Proliferation
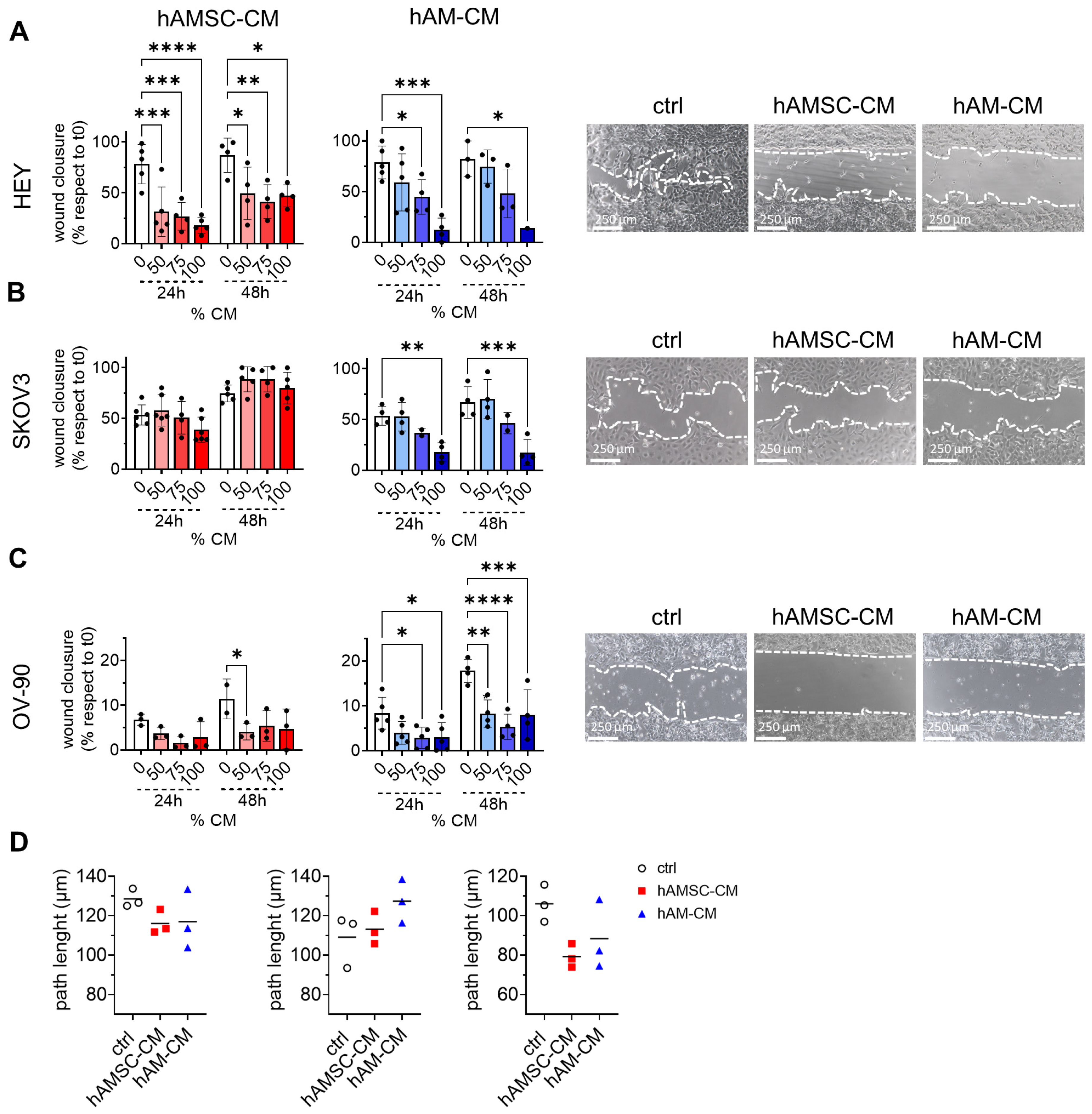
3.3. hAMSC-CM and hAM-CM Inhibit Ovarian Cancer Cell Migration
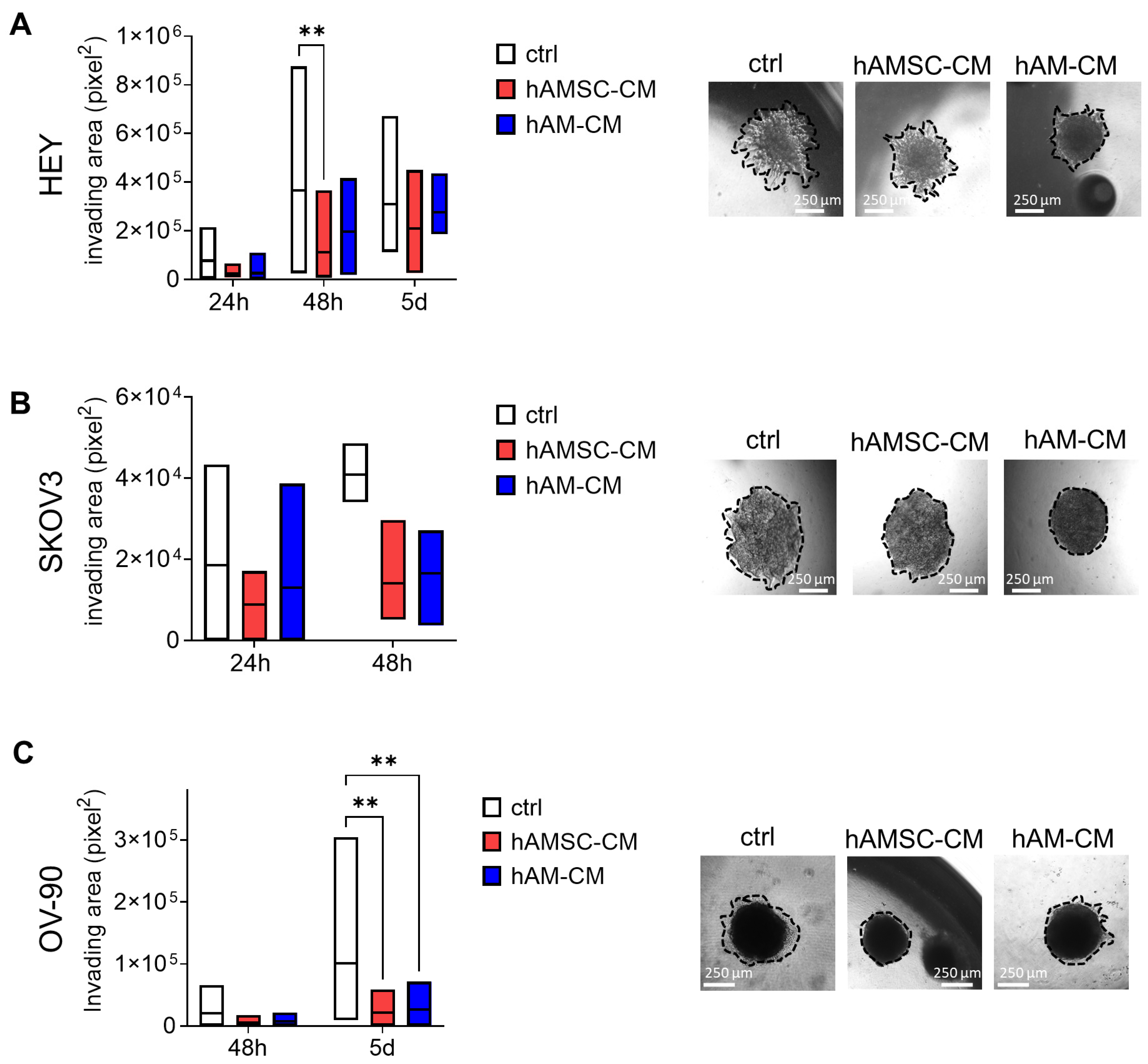
3.4. hAMSC-CM and hAM-CM Affect Spheroid Invasion
3.5. Effect of hAMSC-CM and hAM-CM in Combination with Paclitaxel on Ovarian Cancer Cell Lines in 2D and 3D
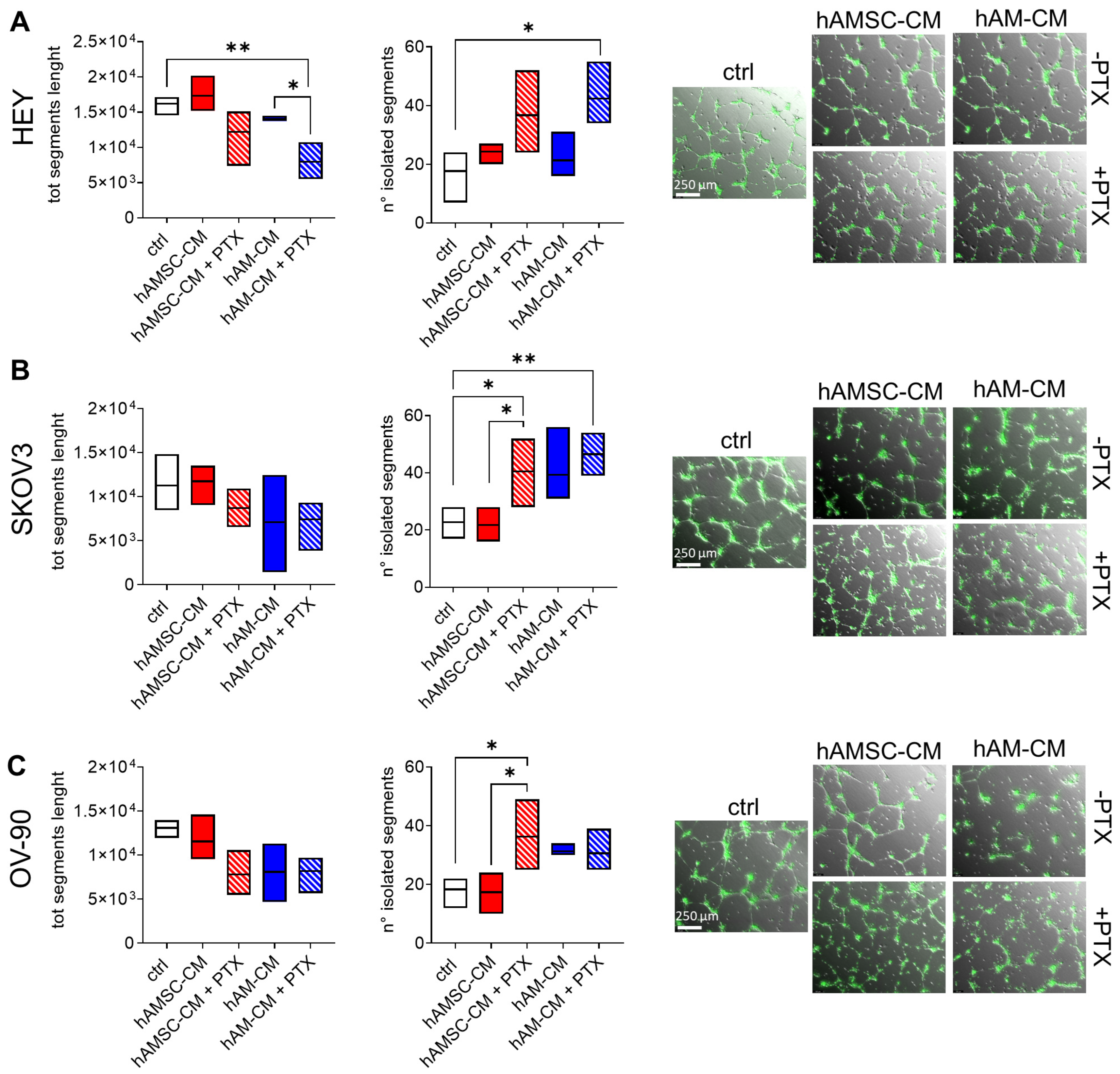
3.6. Effect of hAMSC-CM and hAM-CM in Combination with Paclitaxel in 3D Co-Culture Angiogenesis Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Ovarian Cancer. Available online: https://seer.cancer.gov/statfacts/html/ovary.html (accessed on 20 January 2025).
- Slama, Y.; Ah-Pine, F.; Khettab, M.; Arcambal, A.; Begue, M.; Dutheil, F.; Gasque, P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 3511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, X.; Song, H.; Bie, Q.; Zhang, B. Dual Role of MSC-Derived Exosomes in Tumor Development. Stem Cells Int. 2020, 2020, 8844730. [Google Scholar] [CrossRef]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y. Mesenchymal stem cells as a double-edged sword in tumor growth: Focusing on MSC-derived cytokines. Cell. Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef]
- Papait, A.; Stefani, F.R.; Cargnoni, A.; Magatti, M.; Parolini, O.; Silini, A.R. The Multifaceted Roles of MSCs in the Tumor Microenvironment: Interactions with Immune Cells and Exploitation for Therapy. Front. Cell Dev. Biol. 2020, 8, 447. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Liu, M.; Jia, Y.; He, J.; Xu, X.; Shi, H.; Zhang, J.; Liu, Y. Inhibition of STAT3 signaling as critical molecular event in HUC-MSCs suppressed Glioblastoma Cells. J. Cancer 2023, 14, 611–627. [Google Scholar] [CrossRef]
- Aslam, N.; Abusharieh, E.; Abuarqoub, D.; Alhattab, D.; Jafar, H.; Alshaer, W.; Masad, R.J.; Awidi, A.S. An In Vitro Comparison of Anti-Tumoral Potential of Wharton’s Jelly and Bone Marrow Mesenchymal Stem Cells Exhibited by Cell Cycle Arrest in Glioma Cells (U87MG). Pathol. Oncol. Res. 2021, 27, 584710. [Google Scholar] [CrossRef]
- Janev, A.; Ramuta, T.Z.; Jerman, U.D.; Obradovic, H.; Kamensek, U.; Cemazar, M.; Kreft, M.E. Human amniotic membrane inhibits migration and invasion of muscle-invasive bladder cancer urothelial cells by downregulating the FAK/PI3K/Akt/mTOR signalling pathway. Sci. Rep. 2023, 13, 19227. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Alidoust Saharkhiz Lahiji, M.; Safari, F. Potential therapeutic effects of hAMSCs secretome on Panc1 pancreatic cancer cells through downregulation of SgK269, E-cadherin, vimentin, and snail expression. Biologicals 2022, 76, 24–30. [Google Scholar] [CrossRef]
- Bu, S.; Zhang, Q.; Wang, Q.; Lai, D. Human amniotic epithelial cells inhibit growth of epithelial ovarian cancer cells via TGF-beta1-mediated cell cycle arrest. Int. J. Oncol. 2017, 51, 1405–1414. [Google Scholar] [CrossRef]
- Riedel, R.; Perez-Perez, A.; Carmona-Fernandez, A.; Jaime, M.; Casale, R.; Duenas, J.L.; Guadix, P.; Sanchez-Margalet, V.; Varone, C.L.; Maymo, J.L. Human amniotic membrane conditioned medium inhibits proliferation and modulates related microRNAs expression in hepatocarcinoma cells. Sci. Rep. 2019, 9, 14193. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; De Munari, S.; Vertua, E.; Parolini, O. Amniotic membrane-derived cells inhibit proliferation of cancer cell lines by inducing cell cycle arrest. J. Cell. Mol. Med. 2012, 16, 2208–2218. [Google Scholar] [CrossRef]
- Ramuta, T.Z.; Jerman, U.D.; Tratnjek, L.; Janev, A.; Magatti, M.; Vertua, E.; Bonassi Signoroni, P.; Silini, A.R.; Parolini, O.; Kreft, M.E. The Cells and Extracellular Matrix of Human Amniotic Membrane Hinder the Growth and Invasive Potential of Bladder Urothelial Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 554530. [Google Scholar] [CrossRef]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Perucca Orfei, C.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Papait, A.; Perucca Orfei, C.; Silini, A.R.; Colombini, A.; Vigano, M.; Libonati, F.; Parolini, O.; de Girolamo, L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: Anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl. Med. 2021, 10, 1044–1062. [Google Scholar] [CrossRef]
- Buick, R.N.; Pullano, R.; Trent, J.M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985, 45, 3668–3676. [Google Scholar]
- Magatti, M.; Pianta, S.; Silini, A.; Parolini, O. Isolation, Culture, and Phenotypic Characterization of Mesenchymal Stromal Cells from the Amniotic Membrane of the Human Term Placenta. Methods Mol. Biol. 2016, 1416, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Pianta, S.; Magatti, M.; Sedlmayr, P.; Parolini, O. Characterization of the conditioned medium from amniotic membrane cells: Prostaglandins as key effectors of its immunomodulatory activity. PLoS ONE 2012, 7, e46956. [Google Scholar] [CrossRef]
- Lim, G.J.; Kang, S.J.; Lee, J.Y. Novel invasion indices quantify the feed-forward facilitation of tumor invasion by macrophages. Sci. Rep. 2020, 10, 718. [Google Scholar] [CrossRef]
- Wan, X.; Bovornchutichai, P.; Cui, Z.; O’Neill, E.; Ye, H. Morphological analysis of human umbilical vein endothelial cells co-cultured with ovarian cancer cells in 3D: An oncogenic angiogenesis assay. PLoS ONE 2017, 12, e0180296. [Google Scholar] [CrossRef]
- Carpentier, G.M.M.; Courty, J.; Cascone, I. Angiogenesis Analyzer for ImageJ. In Proceedings of the 4th ImageJ User and Developer Conference proceeding, Luxembourg, 24–26 October 2012; pp. 198–201. [Google Scholar]
- Ng, T.Y.; Ngan, H.Y.; Cheng, D.K.; Wong, L.C. Clinical applicability of the ATP cell viability assay as a predictor of chemoresponse in platinum-resistant epithelial ovarian cancer using nonsurgical tumor cell samples. Gynecol. Oncol. 2000, 76, 405–408. [Google Scholar] [CrossRef]
- Kalamegam, G.; Sait, K.H.W.; Ahmed, F.; Kadam, R.; Pushparaj, P.N.; Anfinan, N.; Rasool, M.; Jamal, M.S.; Abu-Elmagd, M.; Al-Qahtani, M. Human Wharton’s Jelly Stem Cell (hWJSC) Extracts Inhibit Ovarian Cancer Cell Lines OVCAR3 and SKOV3 in vitro by Inducing Cell Cycle Arrest and Apoptosis. Front. Oncol. 2018, 8, 592. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Wallis, B.; Bowman, K.R.; Lu, P.; Lim, C.S. The Challenges and Prospects of p53-Based Therapies in Ovarian Cancer. Biomolecules 2023, 13, 159. [Google Scholar] [CrossRef]
- Mullany, L.K.; Wong, K.K.; Marciano, D.C.; Katsonis, P.; King-Crane, E.R.; Ren, Y.A.; Lichtarge, O.; Richards, J.S. Specific TP53 Mutants Overrepresented in Ovarian Cancer Impact CNV, TP53 Activity, Responses to Nutlin-3a, and Cell Survival. Neoplasia 2015, 17, 789–803. [Google Scholar] [CrossRef]
- Shojaei, S.; Moradi-Chaleshtori, M.; Paryan, M.; Koochaki, A.; Sharifi, K.; Mohammadi-Yeganeh, S. Mesenchymal stem cell-derived exosomes enriched with miR-218 reduce the epithelial-mesenchymal transition and angiogenesis in triple-negative breast cancer cells. Eur. J. Med. Res. 2023, 28, 516. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef]
- Reza, A.; Choi, Y.J.; Yasuda, H.; Kim, J.H. Human adipose mesenchymal stem cell-derived exosomal-miRNAs are critical factors for inducing anti-proliferation signalling to A2780 and SKOV-3 ovarian cancer cells. Sci. Rep. 2016, 6, 38498. [Google Scholar] [CrossRef] [PubMed]
- Kalamegam, G.; Sait, K.H.W.; Anfinan, N.; Kadam, R.; Ahmed, F.; Rasool, M.; Naseer, M.I.; Pushparaj, P.N.; Al-Qahtani, M. Cytokines secreted by human Wharton’s jelly stem cells inhibit the proliferation of ovarian cancer (OVCAR3) cells in vitro. Oncol. Lett. 2019, 17, 4521–4531. [Google Scholar] [CrossRef]
- Rahimi Lifshagerd, M.; Safari, F. Therapeutic effects of hAMSCs secretome on proliferation of MDA-MB-231 breast cancer cells by the cell cycle arrest in G1/S phase. Clin. Transl. Oncol. 2023, 25, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.W.; Li, J.Y.; Zhang, X.C.; Liu, Y.; Liu, Q.Y.; Xiao, L.; Zhang, W.J.; Wu, H.Y.; Deng, K.Y.; Xin, H.B. Human amniotic mesenchymal stem cells inhibit hepatocellular carcinoma in tumour-bearing mice. J. Cell. Mol. Med. 2020, 24, 10525–10541. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Giuffrida, D.; Giuffrida, M.C.; Todros, T.; Calogero, A.E. New perspectives for prostate cancer treatment: In vitro inhibition of LNCaP and PC3 cell proliferation by amnion-derived mesenchymal stromal cells conditioned media. Aging Male 2014, 17, 94–101. [Google Scholar] [CrossRef]
- Pashaei-Asl, R.; Pashaiasl, M.; Ebrahimie, E.; Lale Ataei, M.; Paknejad, M. Apoptotic effects of human amniotic fluid mesenchymal stem cells conditioned medium on human MCF-7 breast cancer cell line. Bioimpacts 2023, 13, 191–206. [Google Scholar] [CrossRef]
- Bolouri, M.R.; Ghods, R.; Zarnani, K.; Vafaei, S.; Falak, R.; Zarnani, A.H. Human amniotic epithelial cells exert anti-cancer effects through secretion of immunomodulatory small extracellular vesicles (sEV). Cancer Cell Int. 2022, 22, 329. [Google Scholar] [CrossRef]
- Palmirotta, R.; Silvestris, E.; D’Oronzo, S.; Cardascia, A.; Silvestris, F. Ovarian cancer: Novel molecular aspects for clinical assessment. Crit. Rev. Oncol. Hematol. 2017, 117, 12–29. [Google Scholar] [CrossRef]
- Florkemeier, I.; Antons, L.K.; Weimer, J.P.; Hedemann, N.; Rogmans, C.; Kruger, S.; Scherliess, R.; Dempfle, A.; Arnold, N.; Maass, N.; et al. Multicellular ovarian cancer spheroids: Novel 3D model to mimic tumour complexity. Sci. Rep. 2024, 14, 23526. [Google Scholar] [CrossRef]
- Moss, N.M.; Barbolina, M.V.; Liu, Y.; Sun, L.; Munshi, H.G.; Stack, M.S. Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: A potential role in I.p. metastatic dissemination. Cancer Res. 2009, 69, 7121–7129. [Google Scholar] [CrossRef] [PubMed]
- Usui, A.; Ko, S.Y.; Barengo, N.; Naora, H. P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions. Mol. Cancer Res. 2014, 12, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Sterzynska, K.; Rucinski, M.; Andrzejewska, M.; Nowicki, M.; Januchowski, R. The response and resistance to drugs in ovarian cancer cell lines in 2D monolayers and 3D spheroids. Biomed. Pharmacother. 2023, 165, 115152. [Google Scholar] [CrossRef]
- Sliwa, A.; Szczerba, A.; Pieta, P.P.; Bialas, P.; Lorek, J.; Nowak-Markwitz, E.; Jankowska, A. A Recipe for Successful Metastasis: Transition and Migratory Modes of Ovarian Cancer Cells. Cancers 2024, 16, 783. [Google Scholar] [CrossRef]
- Moffitt, L.; Karimnia, N.; Stephens, A.; Bilandzic, M. Therapeutic Targeting of Collective Invasion in Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 1466. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Bhat, R. Ovarian cancer cells exhibit diverse migration strategies on stiff collagenous substrata. Biophys. J. 2024, 123, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Shakery, T.; Sayadamin, N. Evaluating the effect of secretome of human amniotic mesenchymal stromal cells on apoptosis induction and epithelial-mesenchymal transition inhibition in LNCaP prostate cancer cells based on 2D and 3D cell culture models. Cell Biochem. Funct. 2021, 39, 813–820. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef]
- Jantalika, T.; Manochantr, S.; Kheolamai, P.; Tantikanlayaporn, D.; Saijuntha, W.; Pinlaor, S.; Chairoungdua, A.; Paraoan, L.; Tantrawatpan, C. Human chorion-derived mesenchymal stem cells suppress JAK2/STAT3 signaling and induce apoptosis of cholangiocarcinoma cell lines. Sci. Rep. 2022, 12, 11341. [Google Scholar] [CrossRef]
- Salkin, H.; Satir-Basaran, G.; Korkmaz, S.; Burcin Gonen, Z.; Erdem Basaran, K. Mesenchymal stem cell-derived conditioned medium and Methysergide give rise to crosstalk inhibition of 5-HT2A and 5-HT7 receptors in neuroblastoma cells. Brain Res. 2023, 1808, 148354. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Park, S.H.; Lee, H.J.; Lee, D.W.; Lee, H.N. Inhibition of Phospho-S6 Kinase, a Protein Involved in the Compensatory Adaptive Response, Increases the Efficacy of Paclitaxel in Reducing the Viability of Matrix-Attached Ovarian Cancer Cells. PLoS ONE 2016, 11, e0155052. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Silini, A.; Vertua, E.; Signoroni, P.B.; Cocce, V.; Cavicchini, L.; Sisto, F.; Alessandri, G.; Pessina, A.; Parolini, O. Human amniotic mesenchymal stromal cells (hAMSCs) as potential vehicles for drug delivery in cancer therapy: An in vitro study. Stem Cell Res. Ther. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Muntiu, A.; Papait, A.; Vincenzoni, F.; Rossetti, D.V.; Romele, P.; Cargnoni, A.; Silini, A.; Parolini, O.; Desiderio, C. Proteomic analysis of the human amniotic mesenchymal stromal cell secretome by integrated approaches via filter-aided sample preparation. J. Proteom. 2025, 310, 105339. [Google Scholar] [CrossRef]
- Marassi, V.; La Rocca, G.; Placci, A.; Muntiu, A.; Vincenzoni, F.; Vitali, A.; Desiderio, C.; Maraldi, T.; Beretti, F.; Russo, E.; et al. Native characterization and QC profiling of human amniotic mesenchymal stromal cell vesicular fractions for secretome-based therapy. Talanta 2024, 276, 126216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiodelli, P.; Bonassi Signoroni, P.; Scalvini, E.; Farigu, S.; Giuzzi, E.; Paini, A.; Papait, A.; Stefani, F.R.; Silini, A.R.; Parolini, O. Synergistic Effect of Conditioned Medium from Amniotic Membrane Mesenchymal Stromal Cells Combined with Paclitaxel on Ovarian Cancer Cell Viability and Migration in 2D and 3D In Vitro Models. Pharmaceutics 2025, 17, 420. https://doi.org/10.3390/pharmaceutics17040420
Chiodelli P, Bonassi Signoroni P, Scalvini E, Farigu S, Giuzzi E, Paini A, Papait A, Stefani FR, Silini AR, Parolini O. Synergistic Effect of Conditioned Medium from Amniotic Membrane Mesenchymal Stromal Cells Combined with Paclitaxel on Ovarian Cancer Cell Viability and Migration in 2D and 3D In Vitro Models. Pharmaceutics. 2025; 17(4):420. https://doi.org/10.3390/pharmaceutics17040420
Chicago/Turabian StyleChiodelli, Paola, Patrizia Bonassi Signoroni, Elisa Scalvini, Serafina Farigu, Elisabetta Giuzzi, Alice Paini, Andrea Papait, Francesca Romana Stefani, Antonietta Rosa Silini, and Ornella Parolini. 2025. "Synergistic Effect of Conditioned Medium from Amniotic Membrane Mesenchymal Stromal Cells Combined with Paclitaxel on Ovarian Cancer Cell Viability and Migration in 2D and 3D In Vitro Models" Pharmaceutics 17, no. 4: 420. https://doi.org/10.3390/pharmaceutics17040420
APA StyleChiodelli, P., Bonassi Signoroni, P., Scalvini, E., Farigu, S., Giuzzi, E., Paini, A., Papait, A., Stefani, F. R., Silini, A. R., & Parolini, O. (2025). Synergistic Effect of Conditioned Medium from Amniotic Membrane Mesenchymal Stromal Cells Combined with Paclitaxel on Ovarian Cancer Cell Viability and Migration in 2D and 3D In Vitro Models. Pharmaceutics, 17(4), 420. https://doi.org/10.3390/pharmaceutics17040420








