Pharmacokinetics, Pharmacodynamics, and Bioequivalence of Test Insulin Glargine Versus Reference Preparation (Lantus®) in Healthy Male Volunteers—By Euglycemic Clamp Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Drugs
2.2. Study Subjects
2.3. Study Methods
2.3.1. Total Design
2.3.2. Clamping Test Process
2.3.3. Detection of Insulin Glargine and C-Peptide
2.3.4. Bioanalytical Methods
2.3.5. Safety Assessment
2.3.6. Statistical Analysis
- Log-Transform PK Parameter: log-transforming AUC and Cmax
- Check Normality Assumptions: Validate the normality of log-transformed parameters using the Shapiro–Wilk test.
- Fit a Mixed Model: Use a linear mixed effects model to account for crossover effects (sequence, period, treatment, and subject variability).
- Calculate the Geometric Mean Ratio (GMR): Extract the mean difference (estimate) for test vs. reference, computing the 90% CI on the log scale and exponentiate the values to convert to the original ratio scale after running the model.
3. Results
3.1. Basic Information of Subjects
3.2. Quality Evaluation of Clamp Test
3.2.1. Blood Glucose Level
3.2.2. C-Peptide Inhibition
3.3. Pharmacodynamic Evaluation
3.4. Pharmacokinetic Assessment
3.5. Bioequivalence Evaluation
3.6. Safety Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 6 September 2021).
- Home, P.D.; Mehta, R. Insulin therapy development beyond 100 years. Lancet Diabetes Endocrinol. 2021, 9, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Hilgenfeld, R.; Seipke, G.; Berchtold, H.; Owens, D.R. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs 2014, 74, 911–927. [Google Scholar] [CrossRef]
- Blair, H.A.; Keating, G.M. Insulin Glargine 300 U/mL: A Review in Diabetes Mellitus. Drugs 2016, 76, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.N.; Threatt, T.; Ward, E.; Shealy, K.M. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Glargine 300 U/mL. Clin. Pharmacokinet. 2017, 56, 449–458. [Google Scholar] [CrossRef]
- Owens, D.R. Pharmacokinetics and pharmacodynamics of insulin glargine 300 U/mL in the treatment of diabetes and their clinical relevance. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 977–987. [Google Scholar] [CrossRef]
- Sharma, A.K.; Taneja, G.; Kumar, A.; Sahu, M.; Sharma, G.; Kumar, A.; Sardana, S.; Deep, A. Insulin analogs: Glimpse on contemporary facts and future prospective. Life Sci. 2019, 219, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Porcellati, F.; Bolli, G.B.; Fanelli, C.G. Pharmacokinetics and pharmacodynamics of basal insulins. Diabetes Technol. Ther. 2011, 13 (Suppl. 1), S15–S24. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Liu, J.; Li, J.; Tan, H.; Yu, Y. Inhibiting effect of establishment of peripheral hyperinsulinemia on endogenous insulin secretion when using euglycemic clamp technique(in Chinese). Zhonghua Yi Xue Za Zhi 2018, 27, 167–172. [Google Scholar]
- Ponchner, M.; Heine, R.J.; Pernet, A.; Manning, I.; Francis, A.J.; Cook, D.; Ørskov, H.; Alberti, K.G.M.M. A comparison of the artificial pancreas (glucose controlled insulin infusion system) and a manual technique for assessing insulin sensitivity during euglycaemic clamping. Diabetologia 1984, 26, 420–425. [Google Scholar] [CrossRef]
- Becker, R.H.A.; Frick, A.D.; Teichert, L.; Nosek, L.; Heinemann, L.; Heise, T.; Rave, K. Fluctuation and reproducibility of exposure and effect of insulin glargine in healthy subjects. Diabetes Obes. Metab. 2008, 10, 1105–1113. [Google Scholar] [CrossRef]
- Crutchlow, M.F.; Palcza, J.S.; Mostoller, K.M.; Mahon, C.D.; Barbour, A.M.; Marcos, M.C.; Xu, Y.; Watkins, E.; Morrow, L.; Hompesch, M. Single-dose euglycaemic clamp studies demonstrating pharmacokinetic and pharmacodynamic similarity between MK-1293 insulin glargine and originator insulin glargine (Lantus) in subjects with type 1 diabetes and healthy subjects. Diabetes Obes. Metab. 2018, 20, 400–408. [Google Scholar] [CrossRef]
- Scholtz, H.E.; Pretorius, S.G.; Wessels, D.H.; Becker, R.H. Pharmacokinetic and glucodynamic variability: Assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia 2005, 48, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- China Center for Drug Evaluation. Guidance on the Clinical Study Design of Once-Daily Basal Insulin Biosimilars (Draft). Available online: https://www.cde.org.cn/main/news/viewInfoCommon/6d1b60aa84975dbdd7c37fc4fbaad781 (accessed on 18 January 2023).
- European Medicines Agency. Guideline on Non-Clinical and Clinical Development of Similar Biological Medicinal Products Containing Recombinant Human Insulin and Insulin Analogues. Available online: https://www.ema.europa.eu/en/non-clinical-clinical-development-similar-biological-medicinal-products-containing-recombinant-human (accessed on 18 January 2023).
- Bhatia, A.; Tawade, S.; Mastim, M.; Kitabi, E.N.; Gopalakrishnan, M.; Shah, M.; Yeshamaina, S.; Gobburu, J.; Sahib, M.; Thakur, D.; et al. Comparative evaluation of pharmacokinetics and pharmacodynamics of insulin glargine (Glaritus®) and Lantus® in healthy subjects: A double-blind, randomized clamp study. Acta Diabetol. 2018, 55, 461–468. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Ding, J.; Li, X.; Guo, F.; Zhang, J.; Ding, L. Pharmacokinetic and pharmacodynamic similarity evaluation between an insulin glargine biosimilar product and Lantus® in healthy subjects: Pharmacokinetic parameters of both parent insulin glargine and M1 were used as endpoints. Front. Pharmacol. 2022, 13, 962201. [Google Scholar] [CrossRef]
- Benesch, C.; Heise, T.; Klein, O.; Heinemann, L.; Arnolds, S. How to Assess the Quality of Glucose Clamps? Evaluation of Clamps Performed With ClampArt, a Novel Automated Clamp Device. J. Diabetes Sci. Technol. 2015, 9, 792–800. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.L.; Han, L.N.; Zhang, S.Q.; Liu, J.L.; Li, J.Q.; Tan, H.W.; Yu, Y.R. Quality assessment of euglycemic glucose clamp test. J. Sichuan Univ. (Med. Sci. Ed.) 2019, 50, 588–593. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Sun, L.; Qiao, J.; Wan, S.; Li, S.; Li, J.; Tan, H.; Yu, Y. Similar pharmacokinetics and pharmacodynamics of a new biosimilar and reference insulin aspart in healthy Chinese males. Sci. Rep. 2021, 11, 9495. [Google Scholar] [CrossRef]
- Heinemann, L.; Linkeschova, R.; Rave, K.; Hompesch, B.; Sedlak, M.; Heise, T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000, 23, 644–649. [Google Scholar] [CrossRef]
- Zhang, X.; Lam, E.C.Q.; Seger, M.E.; Coutant, D.; Chua, L.; Tan, L.H.; Soon, D.; Linnebjerg, H. LY2963016 Insulin glargine and insulin glargine (Lantus) produce comparable pharmacokinetics and pharmacodynamics at two dose levels. Clin. Pharmacol. Drug Dev. 2017, 6, 556–563. [Google Scholar] [CrossRef]
- Lazzeroni, M.; Petrangolini, G.; Legarreta Iriberri, J.A.; Pascual Avellana, J.; Tost Robusté, D.; Cagnacci, S.; Macis, D.; Aristarco, V.; Bonanni, B.; Morazzoni, P.; et al. Development of an HPLC-MS/MS Method for the Determination of Silybin in Human Plasma, Urine and Breast Tissue. Molecules 2020, 25, 2918. [Google Scholar] [CrossRef]
- National Medical Products Administration. Circular on Therelease of Statistical Guidelines for Bioequivalence Studies and Technical Guidelines for Bioequivalence Studies of High-Variation Drugs. Available online: https://www.nmpa.gov.cn/yaopin/ypggtg/ypqtgg/20181029173101911.html (accessed on 18 January 2023).
- Lantus. Information of Lantus® (Insulin Glargine Injection) 100 Units/mL. Available online: https://www.lantus.com/ (accessed on 18 January 2023).
- Sinha, V.P.; Choi, S.L.; Soon, D.K.W.; Mace, K.F.; Yeo, K.P.; Lim, S.T.; Howey, D.C. Single-dose pharmacokinetics and glucodynamics of the novel, long-acting basal insulin LY2605541 in healthy subjects. J. Clin. Pharmacol. 2014, 54, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Linnebjerg, H.; Lam, E.C.Q.; Seger, M.E.; Coutant, D.; Chua, L.; Chong, C.L.; Ferreira, M.M.; Soon, D.; Zhang, X. Comparison of the Pharmacokinetics and Pharmacodynamics of LY2963016 Insulin Glargine and EU- and US-Approved Versions of Lantus Insulin Glargine in Healthy Subjects: Three Randomized Euglycemic Clamp Studies. Diabetes Care 2015, 38, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
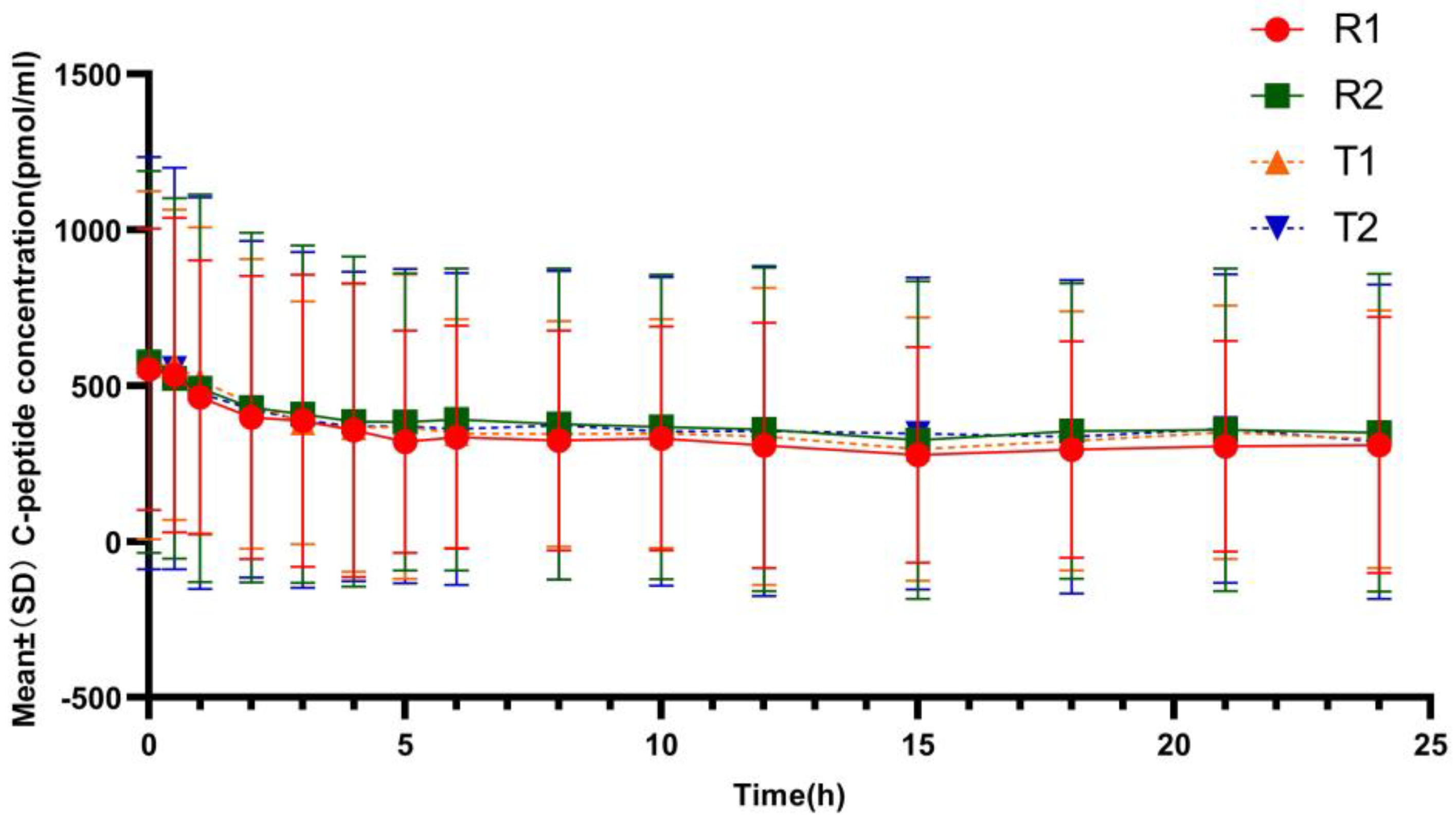
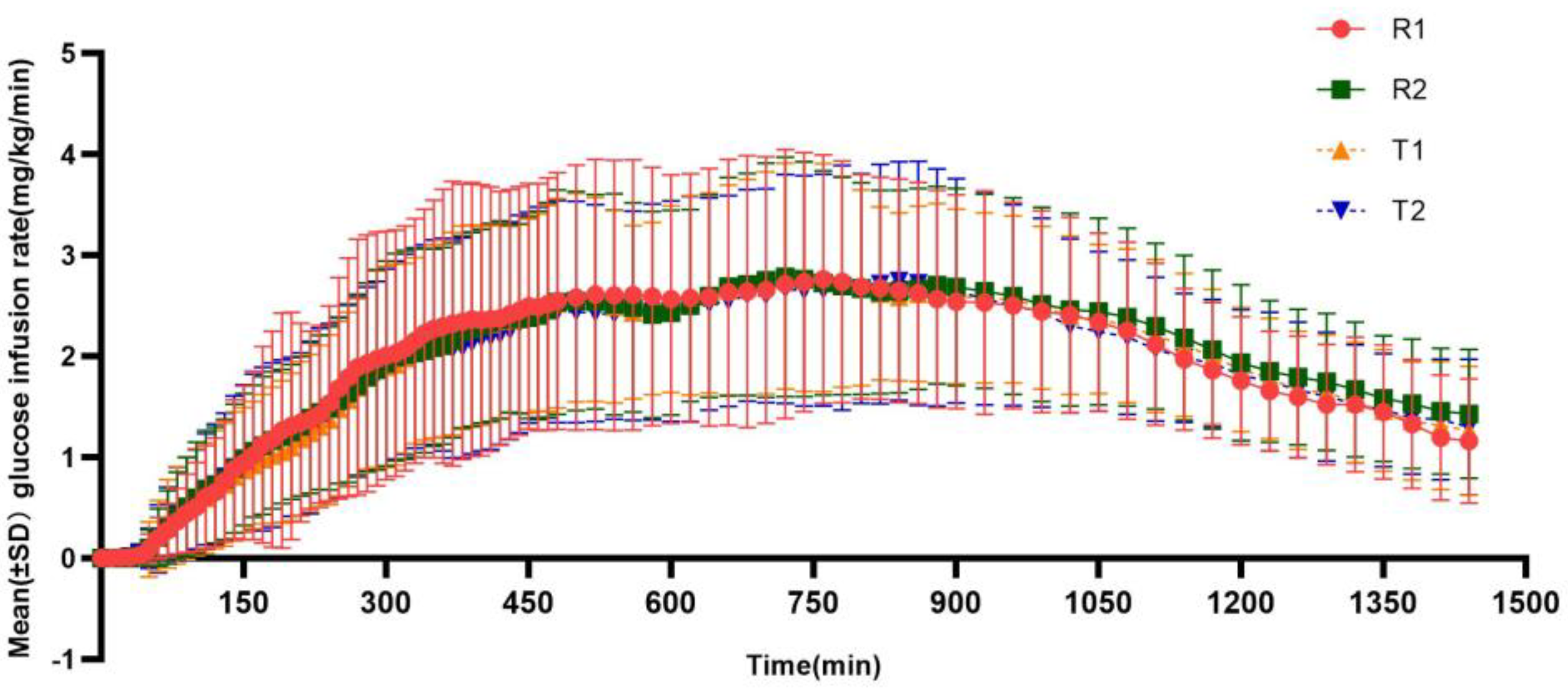

| Characteristics | TRTR (N = 19) | RTRT (N = 20) | Total (N = 39) |
|---|---|---|---|
| Age (year) | 26.5 (±4.48) | 28.7 (±5.74) | 27.6 (±5.21) |
| Hight (cm) | 169.21 (±6.04) | 169.88 (±5.41) | 169.55 (±5.66) |
| Weight(kg) | 62.95 (±5.30) | 64.30 (±5.73) | 63.64 (±5.49) |
| BMI (kg/m2) | 21.98 (±1.24) | 22.25 (±1.20) | 22.12 (±1.21) |
| Indicators | Arithmetic Mean (SD) | |||
|---|---|---|---|---|
| T1 (n = 38) | T2 (n = 36) | R1 (n = 39) | R2 (n = 38) | |
| Mean pre-dose BG/mmol·L−1 | 5.31 (0.34) | 5.32 (0.33) | 5.29 (0.34) | 5.36 (0.30) |
| Target BG/mmol·L−1 | 5.03 (0.34) | 5.04 (0.33) | 5.01 (0.34) | 5.08 (0.30) |
| Mean post-dose BG/mmol·L−1 | 5.04 (0.32) | 5.05 (0.31) | 5.03 (0.33) | 5.07 (0.28) |
| Difference between the mean postdose BG and the target BG/mmol·L−1 | 0.01(0.04) | 0.02 (0.06) | 0.01(0.06) | 0.00 (0.03) |
| CVBG (%) | 3.58 | 3.55 | 3.73 | 3.58 |
| T Geo Mean (Geo CV, %) | R Geo Mean (Geo CV, %) | |||
|---|---|---|---|---|
| Parameters (Unit) | T1 (n = 38) | T2 (n = 36) | R1 (n = 39) | R2 (n = 38) |
| PD parameters | ||||
| GIRmax (mg/kg/min) | 2.912 (38.22) | 2.890 (39.14) | 3.067 (42.69) | 3.116 (34.50) |
| tGIRmax (h) a | 11.17 (5.50, 24.00) | 12.00 (4.83, 22.00) | 10.67 (3.17, 19.00) | 12.33 (3.67, 18.50) |
| AUCGIR,0–24h (h·mg/kg/min) | 42.368 (40.85) | 42.577 (42.62) | 43.849 (46.21) | 46.635 (34.94) |
| AUCGIR,0–12h (h·mg/kg/min) | 18.086 (58.51) | 18.646 (50.96) | 20.491 (53.56) | 20.383 (44.37) |
| AUCGIR,12–24h (h·mg/kg/min) | 23.658 (36.39) | 23.631 (40.02) | 24.005 (36.06) | 25.746 (34.42) |
| PK parameters | ||||
| Cmax (ng/mL) | 0.587 (28.79) | 0.570 (31.57) | 0.618 (31.59) | 0.609 (28.85) |
| AUC0–24h (h·ng/mL) | 9.919 (27.06) | 9.587 (25.41) | 10.494 (26.37) | 10.364 (27.20) |
| AUC0–12h (h·ng/mL) | 4.899 (27.51) | 4.766 (29.28) | 5.229 (32.30) | 5.042 (31.30) |
| AUC12–24h (h·ng/mL) | 4.980 (29.81) | 4.791 (23.75) | 5.209 (23.84) | 5.275 (26.37) |
| Tmax (h) a | 12.00 (4.00, 18.01) | 12.00 (4.01, 15.03) | 12.00 (4.00, 18.00) | 12.00 (3.00, 18.01) |
| t1/2 (h) b | 5.81 (±2.78) | 7.60 (±4.76) | 6.62(±4.29) | 7.05 (±4.26) |
| Parameters | GM | Intra-Subject Variability (%) | ||||
|---|---|---|---|---|---|---|
| T (n = 36) | R (n = 38) | GMR (%) | 90%CI of GMR | T | R | |
| PD parameters | ||||||
| AUCGIR, 0–24 h (h·mg/kg/min) | 42.748 | 45.279 | 94.41 | (88.33, 100.91) | 25.35 | 25.32 |
| GIRmax (mg/kg/min) | 2.924 | 3.096 | 94.45 | (88.78, 100.48) | 23.42 | 23.18 |
| PK parameters | ||||||
| AUC0–24 h (h·ng/mL) | 9.782 | 10.436 | 93.74 | (88.84, 98.91) | 18.90 | 22.16 |
| cmax (ng/mL) | 0.580 | 0.614 | 94.56 | (88.86, 100.63) | 21.39 | 25.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, M.; Tao, Y.; Wan, L.; Chen, Y.; Zhu, M.; Zhao, H.; Tang, C. Pharmacokinetics, Pharmacodynamics, and Bioequivalence of Test Insulin Glargine Versus Reference Preparation (Lantus®) in Healthy Male Volunteers—By Euglycemic Clamp Technique. Pharmaceutics 2025, 17, 418. https://doi.org/10.3390/pharmaceutics17040418
Li Z, Liu M, Tao Y, Wan L, Chen Y, Zhu M, Zhao H, Tang C. Pharmacokinetics, Pharmacodynamics, and Bioequivalence of Test Insulin Glargine Versus Reference Preparation (Lantus®) in Healthy Male Volunteers—By Euglycemic Clamp Technique. Pharmaceutics. 2025; 17(4):418. https://doi.org/10.3390/pharmaceutics17040418
Chicago/Turabian StyleLi, Zhongping, Min Liu, Yi Tao, Lei Wan, Yuan Chen, Mingxue Zhu, Hongtao Zhao, and Chengyong Tang. 2025. "Pharmacokinetics, Pharmacodynamics, and Bioequivalence of Test Insulin Glargine Versus Reference Preparation (Lantus®) in Healthy Male Volunteers—By Euglycemic Clamp Technique" Pharmaceutics 17, no. 4: 418. https://doi.org/10.3390/pharmaceutics17040418
APA StyleLi, Z., Liu, M., Tao, Y., Wan, L., Chen, Y., Zhu, M., Zhao, H., & Tang, C. (2025). Pharmacokinetics, Pharmacodynamics, and Bioequivalence of Test Insulin Glargine Versus Reference Preparation (Lantus®) in Healthy Male Volunteers—By Euglycemic Clamp Technique. Pharmaceutics, 17(4), 418. https://doi.org/10.3390/pharmaceutics17040418







