Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances
Abstract
1. Introduction
2. Challenges to the Oral Delivery of Peptide and Protein Therapeutics
2.1. Physicochemical Properties
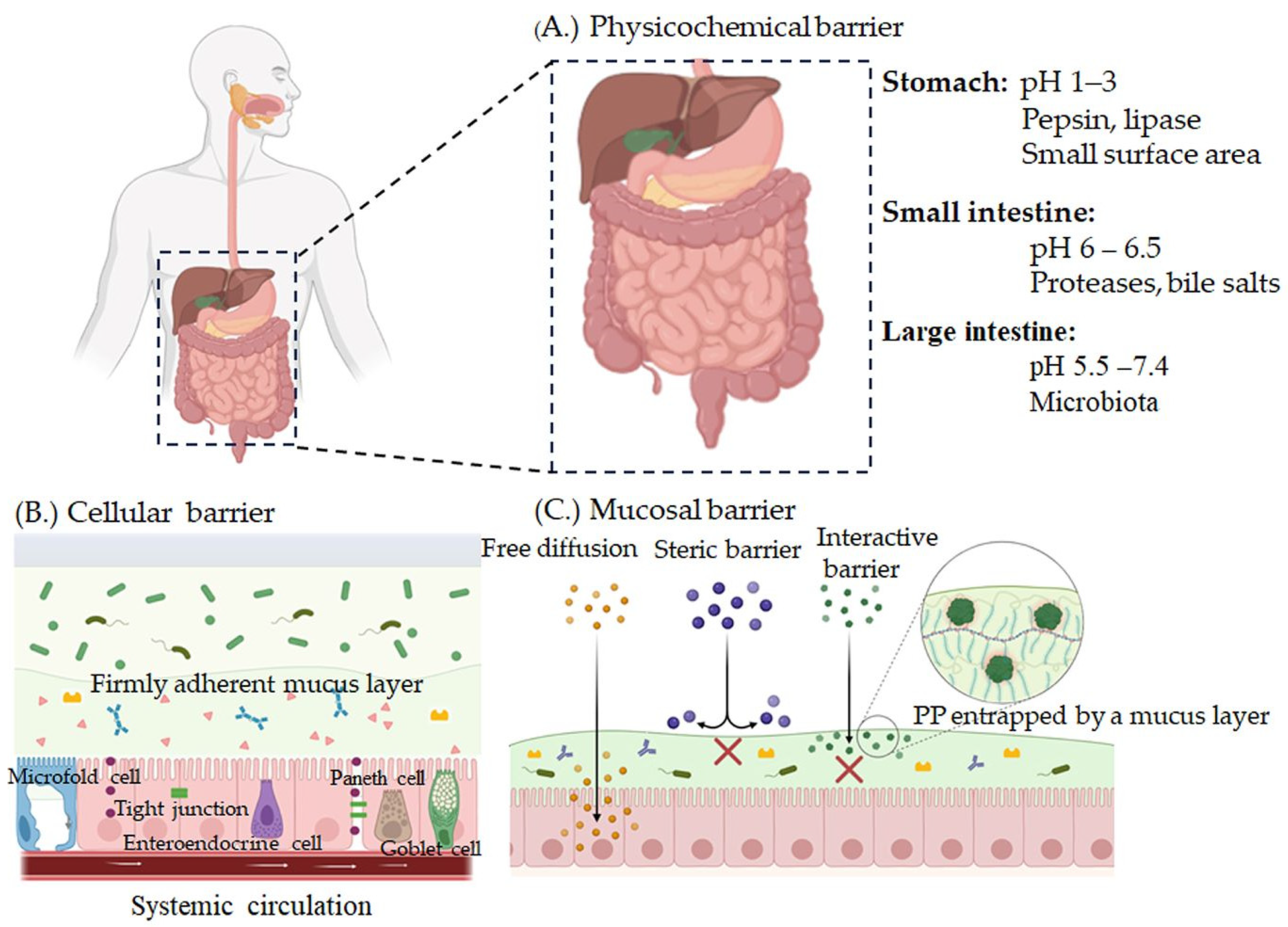
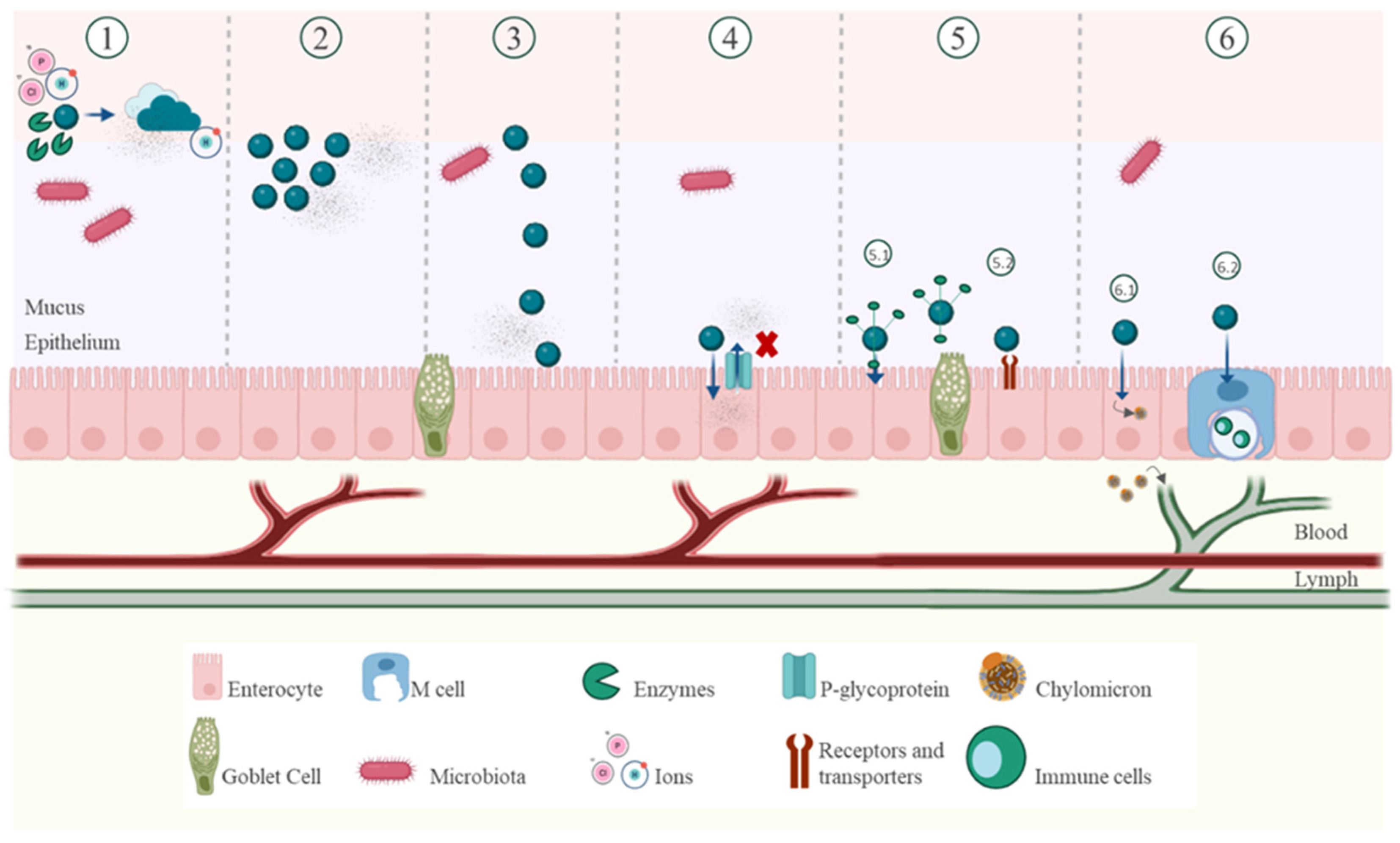
2.2. Biological Barriers
2.2.1. Luminal Barrier (pH)
2.2.2. Enzymatic Barrier
2.2.3. Epithelial Barrier
2.2.4. Mucus Barrier
2.2.5. Sulfhydryl Barrier
3. Strategies for Improving Oral PP Absorption
3.1. Stabilization
3.1.1. pH Modulation
3.1.2. Enzyme Inhibitors
3.1.3. Peptide Cyclization and Polyethylene Glycosylation
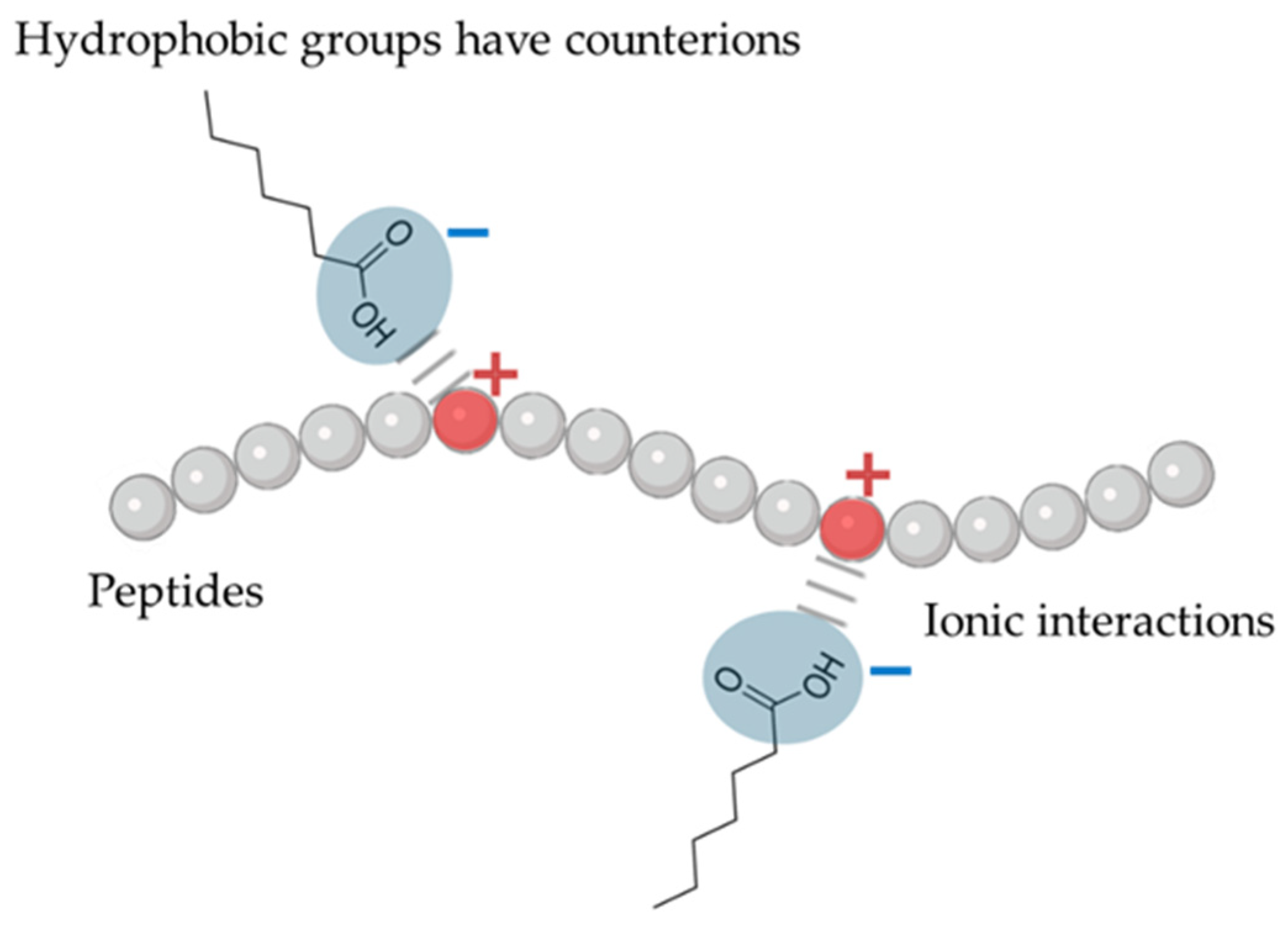
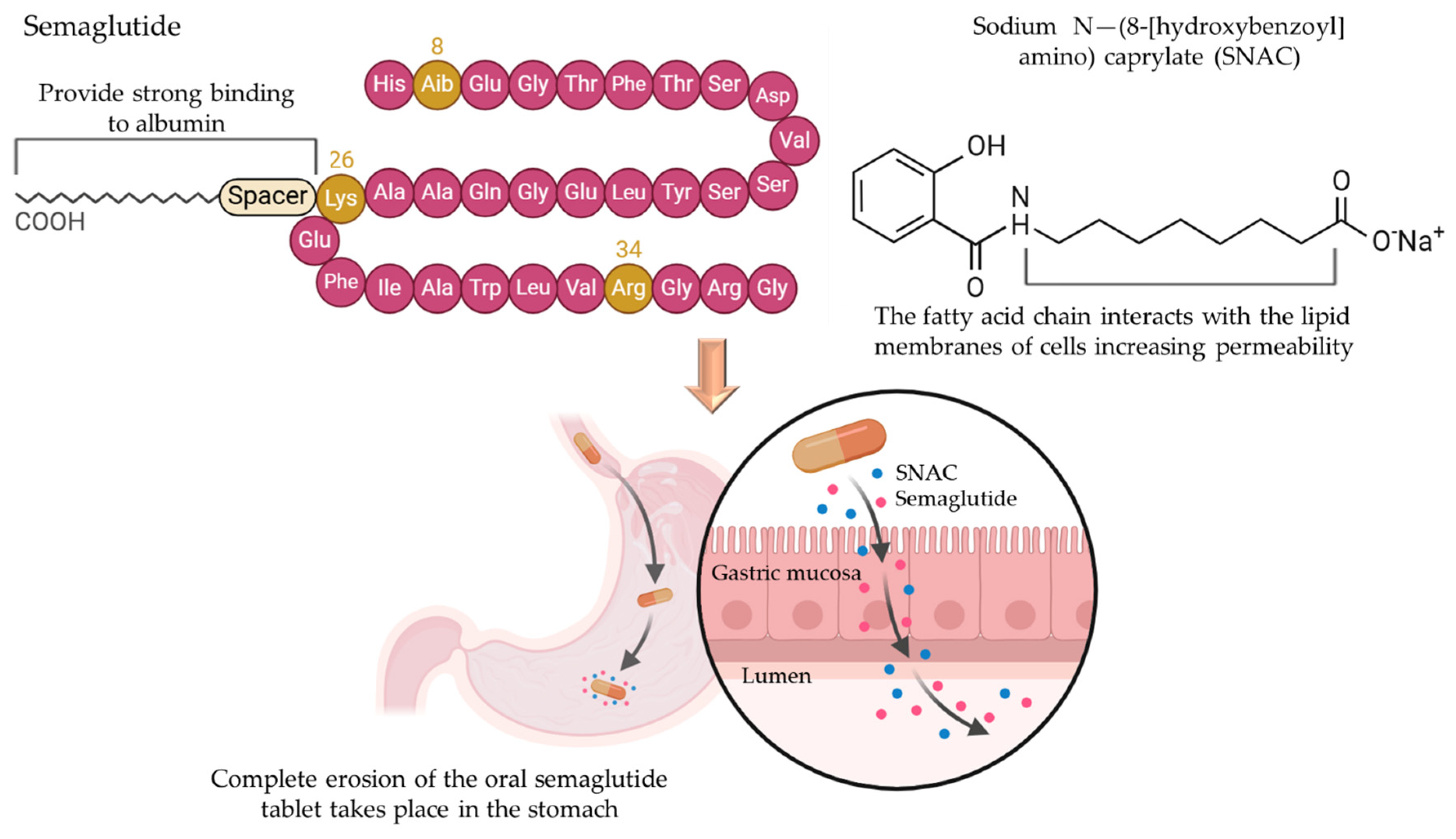
3.1.4. Lipidation
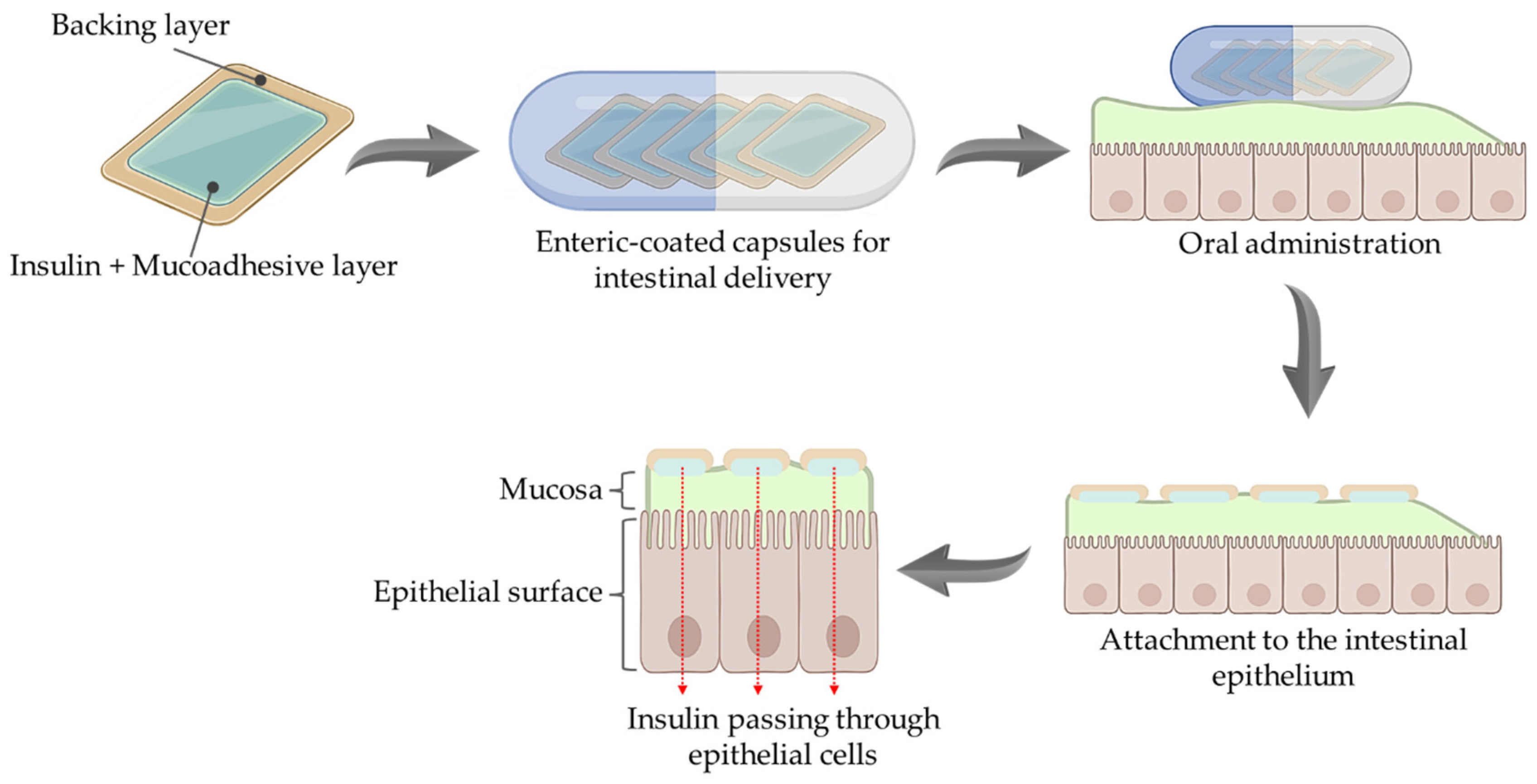
3.2. Mucus-Penetrating and Mucoadhesive Systems
3.2.1. Mucus-Penetrating Systems
3.2.2. Mucoadhesive Systems
3.3. Absorption Enhancement
3.3.1. Prodrugs
3.3.2. Absorption Enhancers
3.3.3. Site-Specific Delivery
3.3.4. Active Targeting
3.4. Lymphatic Transport
| Strategy | Examples | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| pH Coating | Eudragit®systems, and hypromellose phthalate | Protects PP against enzymatic degradation in the stomach; pH-triggered systems provide controlled and targeted release in the intestines | Requires precise coating technology; potential delays in drug release, requires protease inhibitor and permeation enhancer in conjunction | [65] |
| pH Modulation | Citric acid | Protects against degradation in the stomach; targeted release in the intestines | Not effective across all pH ranges | [35,66] |
| Enzymatic inhibitors | Cholic acids, bestatin, aprotinin inhibiting trypsin, soybean trypsin inhibitor, camostat mesylate | Inactivates target enzymes by binding to their specific sites either reversibly or irreversibly | Toxicity at high concentrations and unpredictable reactions with PPs may affect the normal digestion of nutritive proteins | [67] |
| PEGylation | PEGylated insulin, | Prolongs circulation time; reduces immunogenicity; enhances stability | PEGylation can minimize cellular uptake; activity may trigger immune responses to PEG; conjunction technique could be complex | [18,71,128] |
| Cyclization | Cyclized somatostatin | Improves stability against enzymatic degradation; enhanced membrane permeability, selectivity for their targets, bioavailability | Complex synthesis process; potential reduction in bioactivity | [129,130] |
| Lipidation | Lipidated GLP-1 | Enhances membrane permeability; improved oral bioavailability | Can affect peptide function; potential for increased lipid-induced toxicity | [131,132] |
| Mucopenetration | N-acetylcysteine, bromelain, papain | Enhances penetration through the mucus barrier | Risk of systemic absorption leading to side effects; complex formulation | [133] |
| Mucoadhesive | Chitosan, carbopol, polycarbophil, thiolated polymers, cellulose derivatives, pectin, xanthan gum | Increased PP concentration gradient at the epithelial barrier, prolongs retention time at the absorption site; enhanced membrane permeation | May cause local irritation; potential for unpredictable absorption due to mucus turnover at the absorption site | [134] |
| Prodrug | Dipeptide prodrugs, fatty acid prodrugs | Improved stability of PPs and bioavailability; targeted release | Difficult in prodrug design due to structural complexity; potential for toxic metabolites, and stability issues | [29] |
| Absorption Enhancer | Bile salts, fatty acids, surfactants, esters, cyclodextrin, dextran sulfate, crown ethers, EDTA, sodium caprate, and phosphatidyl choline | Improves intestinal permeability; enhanced bioavailability | Cause altered cell morphology, cause irritation or damage to the intestinal mucosa; transient effects, lack of specificity | [101,135] |
| Lymphatic Transport | Long-chain triglycerides based LDDSs | Avoid first-pass or presystemic metabolism | Limited to highly lipophilic drugs; complex formulation | [121,136] |
| Site-Specific Delivery | Folate-targeted nanoparticles, RGD vitamin B12, transferrin, invasins, and lectin | Lower systemic side effects; enhanced bioavailability | Requires specific targeting ligands; potential for off-target effects | [137,138] |
| Active Targeting | Peptide based-bioconjugates, Transferrin-Modified Carriers | Increases specificity to target tissues; improved therapeutic efficacy | Requires specific ligand-receptor interactions; complex manufacturing | [11,116,139] |
| Cell-penetrating peptides (CPPs) | TAT Peptide, Penetratin | Enhanced intramucosal delivery, and membrane permeability | Lack of cell specificity, endosomal entrapment, and potential immunogenicity | [140,141] |
4. Clinical Applications of Oral PP Delivery
| Product | PP | Company | NCAT Number | Status | Indication |
|---|---|---|---|---|---|
| ORMD-0801 | Insulin | Oramed Ltd. | NCT01889667 | Phase 3 | T2DM |
| XW004 | Ecnoglutide | Sciwind Biosciences | NCT05184322 | Phase 1 | T2DM, obesity |
| TransCon hGH | Somatropin | Ascendis Pharma | NCT01247675 | Phase 2 | Growth hormone deficiency |
| Somatropin | PEG-somatropin | Changchun GeneScience | NCT01342146 | Phase 4 | Growth hormone deficiency |
| RaniPill™ Capsule (RT-102) | Parathyroid hormone (1–34) | RANI Therapeutics | NCT05164614 | Phase 1 | Osteoporosis |
| RaniPill™ Capsule | Octreotide | RANI Therapeutics | NCT03798912 | Phase 1 | Growth hormone disorder |
| RaniPill™ Capsule (RT-111) | Ustekinumab | RANI Therapeutics | NCT05890118 | Phase 1 | Psoriasis |
| Ovarest™ | Leuprolide | Enteris BioPharma Inc. | NCT02807363 | Phase 2 | Endometriosis |
| Product | PP | Therapeutic Indications | Strategy |
|---|---|---|---|
| Sandimmune®/Neoral® | Cyclosporin A | Immunosuppression | SNEDDS, systemic delivery |
| Minirin® | Desmopressin acetate (DDVAP) | Cranial diabetes insipidus or nocturia associated with multiple sclerosis | Chemical modification, systemic delivery |
| Ceredist®/Ceredist OD® | Taltirelin hydrate | Spino cerebellar ataxia | Chemical modification to avoid enzymatic hydrolysis, systemic delivery |
| MYCAPSSA® | Octreotide | Growth hormone disorder | Enteric-coated capsules containing oil suspension and sodium caprylate as a permeation enhancer, systemic delivery |
| ColomycinC | Colistin | Intestinal infection (caused by sensitive Gram-negative organisms) | Acts locally to GI tract |
| Linzess® | Linaclotide | Irritable bowel syndrome, chronic idiopathic constipation | Acts locally on the luminal surface of the intestinal epithelium |
| Vancocin® | Vancomycin | Staphylococcus aureus and chlostridium difficile infection | Acts locally by inhibition of cell-wall biosynthesis. |
| Tyrozets® | Tyrothricin | Pharyngitis | Acts locally on the throat |
| Technology | Strategy | Key Outcomes | Example | Reference |
|---|---|---|---|---|
| Transient Permeation Enhancer® (TPE® (Chiasma Pharmaceuticals)) | Oily suspension with permeation enhancer sodium caprylate and polysorbate-80, open tight junctions and altering intestinal mucus thickness | Improved oral bioavailability | MYCAPSSA® (octreotide) | [99] |
| Gastrointestinal Permeation Enhancement Technology (GIPET®) (Merrion Pharmaceuticals) | Incorporates medium-chain fatty acid derivatives coupled with salts and permeation enhancer (C10) to enhance hydrophobicity and epithelial tight junction opening. | Increased intestinal absorption, membrane fluidity, transcellular transport, and inhibited p-gp efflux | MER-101 (oral bisphosphonate for oncology, Phase-2), ACY-7 (Acyline, GnRHAntagonist, Phase-2) | [96,97,98] |
| Peptelligence™ Technology (Enteris Biopharma) | Enteric coating system containing pH-lowering agent (preferably citric acid), and permeation enhancer | Enhanced open tight junctions, facilitates paracellular transport and reduce protease activity and acid degradation. | TBRIATM (oral calcitonin, NDA), Ovarest® (leuprolide oral tablet, Phase-2) | [145,146] |
| ThioMatrix™ Technology (ThioMatrix GmbH) | Incorporates thiolated mucoadhesive polymers (thiomers) to form covalent bonds with intestinal mucus glycoproteins for enhanced adhesion and retention. | Provides mucoadhesive, prolonged GI retention, permeation enhancement, and efflux pump inhibitory properties. | Hydrophilic macromolecules (Preclinical studies) | [147] |
| Oramed Technology (Oramed Pharmaceuticals) | Incorporates protease inhibitors and an absorption enhancer. | Protect PP from acid degradation and enhance intestinal permeation. | ORMD-0801 (Oral insulin, T2DM, Phase 3), ORMD-0801 (Oral insulin, NASH, Phase 2) | [148,149] |
| Protein oral delivery (PODTM) technology (Oramed Pharmaceuticals) | Incorporates protease inhibitors to protect therapeutic PP in the GI tract system. | Protect orally delivered PP from enzymatic activity, enhanced intestinal absorption. | ORMD-0901 (Oral GLP-1 capsule) | [150] |
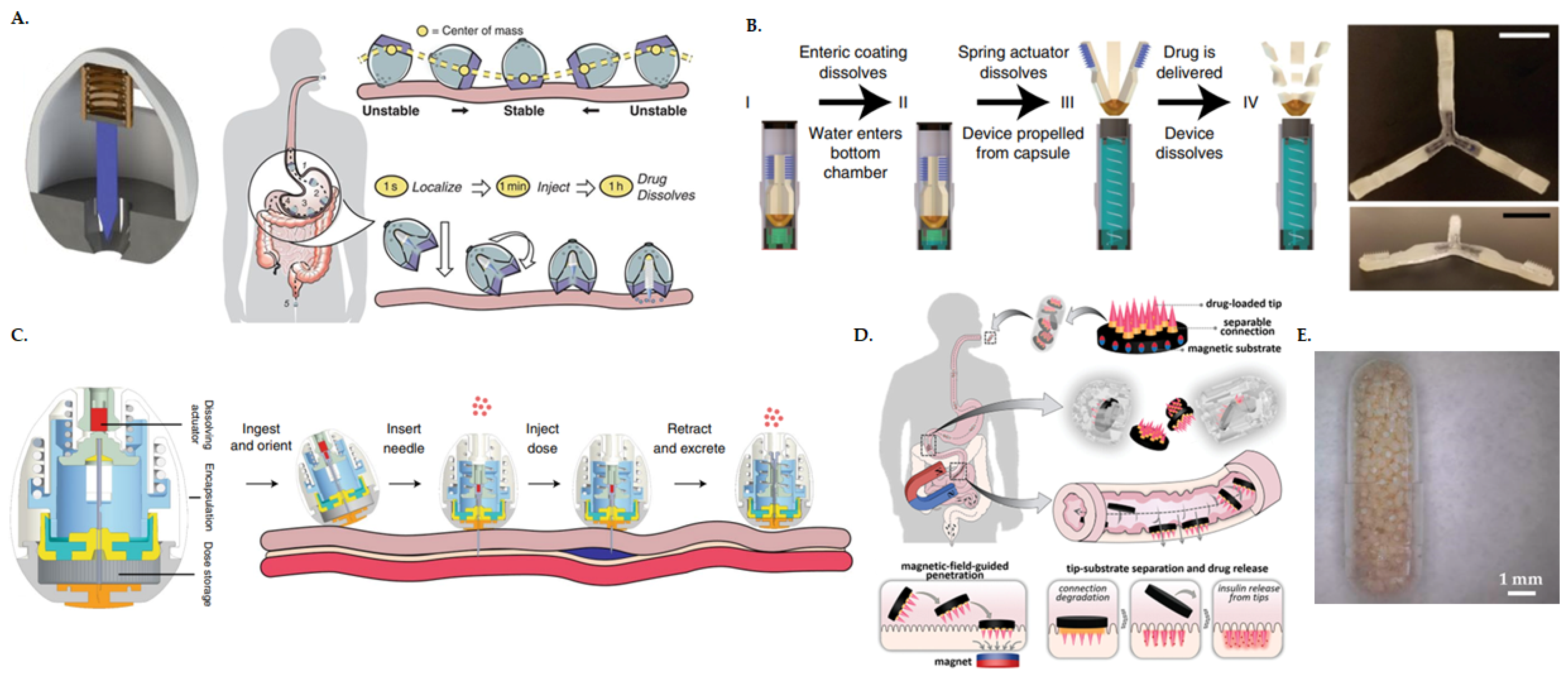
5. Advancement in Medical Device Technologies for Clinical Translation of Oral PP
6. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mullard, A. 2023 FDA approvals. Nat. Rev. Drug Discov. 2024, 23, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-R.; Seng, D.-J.; Xu, Y.; Zhang, Y.-D.; Zhou, W.-J.; Jia, Y.-Y.; Song, J.; He, Z.-X.; Liu, H.-M.; Yuan, S. A comprehensive review of small molecule drugs approved by the FDA in 2023: Advances and prospects. Eur. J. Med. Chem. 2024, 276, 116706. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Sig. Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, T.A.S.; Teijeiro-Osorio, D.; Rosa, M.; Coulter, I.S.; Alonso, M.-J.; Brayden, D.J. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trials. Adv. Drug Deliv. Rev. 2016, 106, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Alonso, M.-J. Oral delivery of peptides: Opportunities and issues for translation. Adv. Drug Deliv. Rev. 2016, 106, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, J.D.; Pamulapati, L.G.; Carter, N.; Malloy, K.; Dixon, D.L.; Sisson, E.M. Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist. Diabetes Technol. Ther. 2020, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Panchagnula, R. Peroral Route: An Opportunity for Protein and Peptide Drug Delivery. Chem. Rev. 2001, 101, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Chen, G.; Kang, W.; Li, W.; Chen, S.; Gao, Y. Oral delivery of protein and peptide drugs: From non-specific formulation approaches to intestinal cell targeting strategies. Theranostics 2022, 12, 1419–1439. [Google Scholar] [CrossRef] [PubMed]
- Asano, D.; Takakusa, H.; Nakai, D. Oral Absorption of Middle-to-Large Molecules and Its Improvement, with a Focus on New Modality Drugs. Pharmaceutics 2024, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.; Barnes, T.J.; Prestidge, C.A. Oral delivery of protein-based therapeutics: Gastroprotective strategies, physiological barriers and in vitro permeability prediction. Int. J. Pharm. 2020, 585, 119488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, T.; Gao, L.; Quan, D. Oral delivery of oil-based formulation for a novel synthetic cationic peptide of GnRH (gonadotropin-releasing hormone) antagonist for prostate cancer treatment. Int. J. Pharm. 2013, 450, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.; Beloqui, A.; Miolane, C.; Bourgeois, S.; Préat, V.; Fessi, H.; Jannin, V. Solid lipid nanocarriers diffuse effectively through mucus and enter intestinal cells—But where is my peptide? Int. J. Pharm. 2020, 586, 119581. [Google Scholar] [CrossRef] [PubMed]
- Santalices, I.; Vázquez-Vázquez, C.; Santander-Ortega, M.J.; Lozano, V.; Araújo, F.; Sarmento, B.; Shrestha, N.; Préat, V.; Chenlo, M.; Alvarez, C.V.; et al. A nanoemulsion/micelles mixed nanosystem for the oral administration of hydrophobically modified insulin. Drug Deliv. Transl. Res. 2021, 11, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Hill, T.A.; Fairlie, D.P.; Maher, S.; Mrsny, R.J. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Kammona, O.; Kiparissides, C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J. Control. Release 2012, 161, 781–794. [Google Scholar] [CrossRef]
- Kanugo, A.; Misra, A. New and Novel Approaches for Enhancing the Oral Absorption and Bioavailability of Protein and Peptides Therapeutics. Ther. Deliv. 2020, 11, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Reddy, I.K.; Khan, M.A. Oral delivery of proteins: Effect of chicken and duck ovomucoid on the stability of insulin in the presence of α-chymotrypsin and trypsin. Pharm. Pharmacol. Commun. 2000, 6, 223–227. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Alam, F.; Byun, Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv. Drug Deliv. Rev. 2013, 65, 845–864. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D.J. Recent advances in encapsulation, protection, and oral delivery of bioactive proteins and peptides using colloidal systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; McHugh, K.J. Strategies for overcoming protein and peptide instability in biodegradable drug delivery systems. Adv. Drug Deliv. Rev. 2023, 199, 114904. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular Uptake of Nanoparticles Versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Klepach, A.; Tran, H.; Mohammed, F.A.; ElSayed, M.E. Characterization and impact of peptide physicochemical properties on oral and subcutaneous delivery. Adv. Drug Deliv. Rev. 2022, 186, 114322. [Google Scholar] [CrossRef] [PubMed]
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Tibbitts, J.; Canter, D.; Graff, R.; Smith, A.; Khawli, L.A. Key factors influencing ADME properties of therapeutic proteins: A need for ADME characterization in drug discovery and development. mAbs 2016, 8, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef] [PubMed]
- Nicze, M.; Borówka, M.; Dec, A.; Niemiec, A.; Bułdak, Ł.; Okopień, B. The Current and Promising Oral Delivery Methods for Protein-and Peptide-Based Drugs. Int. J. Mol. Sci. 2024, 25, 815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Zou, P.; Wu, M.; Zhang, Z.; Zhang, T. The Effects of Pharmaceutical Excipients on Gastrointestinal Tract Metabolic Enzymes and Transporters—An Update. AAPS J. 2016, 18, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Lee, C.C.; Ashaolu, J.O.; Tarhan, O.; Pourjafar, H.; Jafari, S.M. Pepsin: An excellent proteolytic enzyme for the production of bioactive peptides. Food Rev. Int. 2024, 40, 1875–1912. [Google Scholar] [CrossRef]
- Langguth, P.; Bohner, V.; Heizmann, J.; Merkle, H.P.; Wolffram, S.; Amidon, G.L.; Yamashita, S. The challenge of proteolytic enzymes in intestinal peptide delivery. J. Control. Release 1997, 46, 39–57. [Google Scholar] [CrossRef]
- Smart, A.L.; Gaisford, S.; Basit, A.W. Oral peptide and protein delivery: Intestinal obstacles and commercial prospects. Expert Opin. Drug Deliv. 2014, 11, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Woodley, J.F. Enzymatic barriers for GI peptide and protein delivery. Crit. Rev. Ther. Drug Carr. Syst. 1994, 11, 61–95. [Google Scholar]
- Mehrdadi, S. Lipid-Based Nanoparticles as Oral Drug Delivery Systems: Overcoming Poor Gastrointestinal Absorption and Enhancing Bioavailability of Peptide and Protein Therapeutics. Adv. Pharm. Bull. 2024, 14, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Leonaviciute, G.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems in oral (poly)peptide drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnurch, A.; Schmitz, T. Presystemic metabolism of orally administered peptide drugs and strategies to overcome it. Curr. Drug Metab. 2007, 8, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nielsen, H.M.; Müllertz, A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Csóka, I. Novel strategies in the oral delivery of antidiabetic peptide drugs–Insulin, GLP 1 and its analogs. Eur. J. Pharm. Biopharm. 2017, 115, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, Z.; Liu, W.; Zhang, Y.; Kang, Y.; Liu, J.; Hu, C.; Wang, R.; Zhang, M.; Chen, L.; et al. How nanoparticles open the paracellular route of biological barriers: Mechanisms, applications, and prospects. ACS Nano 2022, 16, 15627–15652. [Google Scholar] [CrossRef]
- Zhang, H.; Shahbazi, M.A.; Almeida, P.V.; Santos, H.A. Mucus as a Barrier for Biopharmaceuticals and Drug Delivery Systems. In Mucosal Delivery of Biopharmaceuticals: Biology, Challenges and Strategies; das Neves, J., Sarmento, B., Eds.; Springer: Boston, MA, USA, 2014; pp. 59–97. [Google Scholar]
- Johansson, M.E.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 2018, 124, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Zaiki, Y.; Lim, L.Y.; Wong, T.W. Critical material designs for mucus- and mucosa-penetrating oral insulin nanoparticle development. Int. Mater. Rev. 2023, 68, 121–139. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.M.; McKelvey, C.A.; Marsac, P.J.; Carrier, R.L. Size selectivity of intestinal mucus to diffusing particulates is dependent on surface chemistry and exposure to lipids. J. Drug Target. 2015, 23, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.; Lock, J.; Carrier, R. Engineering the mucus barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Fragner, R. Investigations into the diffusion behaviour of polypeptides in native intestinal mucus with regard to their peroral administration. Pharm. Pharmacol. Commun. 1996, 2, 361–363. [Google Scholar]
- Trivedi, M.V.; Laurence, J.S.; Siahaan, T.J. The role of thiols and disulfides on protein stability. Curr. Protein Pept. Sci. 2009, 10, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K.; et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018, 10, eaar7047. [Google Scholar] [CrossRef] [PubMed]
- Varamini, P.; Toth, I. Recent advances in oral delivery of peptide hormones. Expert Opin. Drug Deliv. 2016, 13, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Nimble Therapeutics. Available online: https://nimbletherapeutics.com/ (accessed on 11 March 2025).
- Mat, D.J.; Cattenoz, T.; Souchon, I.; Michon, C.; Le Feunteun, S. Monitoring protein hydrolysis by pepsin using pH-stat: In vitro gastric digestions in static and dynamic pH conditions. Food Chem. 2018, 239, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, W.; Chen, Y.; Xu, Y.; Wang, B.; Zong, L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int. J. Biol. Macromol. 2018, 113, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, F.; Deng, T.; Zhu, S.; Liu, W.; Zhong, H.; Yu, H.; Luo, R.; Deng, Z. Eudragit S100-Coated Chitosan Nanoparticles Co-loading Tat for Enhanced Oral Colon Absorption of Insulin. AAPS PharmSciTech 2017, 18, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Welling, S.H.; Hubálek, F.; Jacobsen, J.; Brayden, D.J.; Rahbek, U.L.; Buckley, S.T. The role of citric acid in oral peptide and protein formulations: Relationship between calcium chelation and proteolysis inhibition. Eur. J. Pharm. Biopharm. 2014, 86, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kou, Y.; Zhang, X.; Cheng, H.; Chen, X.; Mao, S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin. Drug Deliv. 2018, 15, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; De Marco, R.; Cerisoli, L. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- White, C.J.; Yudin, A.K. Contemporary strategies for peptide macrocyclization. Nat. Chem. 2011, 3, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Abbaspourrad, A. Improved pH stability, heat stability, and functionality of phycocyanin after PEGylation. Int. J. Biol. Macromol. 2022, 222, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Zuma, L.K.; Gasa, N.L.; Makhoba, X.H.; Pooe, O.J. Protein PEGylation: Navigating recombinant protein stability, aggregation, and bioactivity. BioMed Res. Int. 2022, 2022, 8929715. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Guiotto, A.; Veronese, F.M. Protein, peptide and non-peptide drug PEGylation for therapeutic application. Expert Opin. Ther. Pat. 2004, 14, 859–894. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic Consequences of Pegylation. Drug Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Myšková, A.; Sýkora, D.; Kuneš, J.; Maletínská, L. Lipidization as a tool toward peptide therapeutics. Drug Deliv. 2023, 30, 2284685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bulaj, G. Converting peptides into drug leads by lipidation. Curr. Med. Chem. 2012, 19, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Shrawani, L.; Kim, J.H.; Lee, S. Recent progress in hydrophobic ion-pairing and lipid-based drug delivery systems for enhanced oral delivery of biopharmaceuticals. J. Pharm. Investig. 2022, 52, 75–93. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takada, K.; Kiso, Y.; Muranishi, S. Synthesis of palmitoyl derivatives of insulin and their biological activities. Pharm. Res. 1989, 6, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, O.; Bernkop-Schnürch, A. Lipophilic peptide character—What oral barriers fear the most. J. Control. Release 2017, 255, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed]
- Bandi, S.P.; Bhatnagar, S.; Venuganti, V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021, 119, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.; Alrabadi, N.; Haddad, R.; Malkawi, A.; Khaled, K.; Ovenseri, A.C. Development of Self-Emulsifying Drug Delivery Systems (SEDDSs) Displaying Enhanced Permeation of the Intestinal Mucus Following Sustained Release of Prototype Thiol-Based Mucolytic Agent Load. Molecules 2022, 27, 4611. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent advances in mucoadhesive interface materials, mucoadhesion characterization, and technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.R.; Yuan, F.; Qin, X.; Wang, M.; Huang, Y. In vitro and in vivo study of N-trimethyl chitosan nanoparticles for oral protein delivery. Int. J. Pharm. 2008, 349, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, J.; He, C.; Cui, L.; Tang, C.; Yin, C. Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 2009, 30, 5691–5700. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.-M. Lectin-mediated drug delivery: The second generation of bioadhesives. J. Control. Release 2000, 65, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Q.; Dong, Z.; Meng, T.; Hu, F.; Wang, J.; Yuan, H. Nanocarriers transport across the gastrointestinal barriers: The contribution to oral bioavailability via blood circulation and lymphatic pathway. Adv. Drug Deliv. Rev. 2023, 203, 115130. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.H.; Chung, L.Y.; Noordin, M.I.; Onuki, Y.; Morishita, M.; Takayama, K. Lectin-functionalized carboxymethylated kappa-carrageenan microparticles for oral insulin delivery. Carbohydr. Polym. 2011, 86, 555–565. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.M.; Gangwar, S.; Siahaan, T.J.; Aubé, J.; Borchardt, R.T. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv. Drug Deliv. Rev. 1997, 27, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, H.M.; Kennedy, A.R.; Shen, W.-C. Water-soluble fatty acid derivatives as acylating agents for reversible lipidization of polypeptides. FEBS Lett. 1995, 371, 283–286. [Google Scholar] [PubMed]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Li, Y.; Krishnamoorthy, R.; Chermak, T.; Mitra, A.K. Differential effects of anionic, cationic, nonionic, and physiologic surfactants on the dissociation, α-chymotryptic degradation, and enteral absorption of insulin hexamers. Pharm. Res. 1993, 10, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.G.; Adamczyk, B.E.; Chalasani, K.B.; Maher, S.; O ‘Toole, E.B.; Fox, J.S.; Leonard, T.W.; Brayden, D.J. Oral delivery of macromolecules: Rationale underpinning Gastrointestinal Permeation Enhancement Technology (GIPET). Ther. Deliv. 2011, 2, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K.; Leonard, T.W.; Page, S.T.; O’Toole, E.; McKenna, M.J.; Bremner, W.J. Oral administration of the GnRH antagonist acyline, in a GIPET®-enhanced tablet form, acutely suppresses serum testosterone in normal men: Single-dose pharmacokinetics and pharmacodynamics. Cancer Chemother. Pharmacol. 2009, 64, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Leonard, T.W.; Lynch, J.; McKenna, M.J.; Brayden, D.J. Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET™. Expert Opin. Drug Deliv. 2006, 3, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Maher, S. Transient Permeation Enhancer® (TPE®) technology for oral delivery of octreotide: A technological evaluation. Expert Opin. Drug Deliv. 2021, 18, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.; Jabbour, S.; Rosenstock, J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, A.T.; Pliatsika, D.; Gall, F.M.; Fischer, T.; Riedl, R. New perspectives in oral peptide delivery. Drug Discov. Today 2021, 26, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Noach, A.B.; Kurosaki, Y.; Blom-Roosemalen, M.C.; de Boer, A.G.; Breimer, D.D. Cell-polarity dependent effect of chelation on the paracellular permeability of confluent Caco-2 cell monolayers. Int. J. Pharm. 1993, 90, 229–237. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int. J. Toxicol. 2002, 21, 95–142. [Google Scholar] [PubMed]
- Doi, N.; Tomita, M.; Hayashi, M. Absorption enhancement effect of acylcarnitines through changes in tight junction protein in Caco-2 cell monolayers. Drug Metab. Pharmacokinet. 2011, 26, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Hwang, B.H.; Doshi, N.; Mitragotri, S. A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine. J. Control. Release 2013, 172, 541–549. [Google Scholar] [CrossRef] [PubMed]
- New, R.R.C. Dissolution Aids for Oral Peptide Delivery Comprising a Biguanide. U.S. Patent US20090041849A1, 12 February 2009. [Google Scholar]
- Axcess™ Oral Delivery System. Available online: https://www.diabetology.co.uk/ (accessed on 11 March 2025).
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-enhancing effects of bile salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, S.; Seo, J.E.; Choi, Y.W.; Lee, S. Bile acid transporter-mediated oral absorption of insulin via hydrophobic ion-pairing approach. J. Control. Release 2021, 338, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296–7301. [Google Scholar] [CrossRef] [PubMed]
- Uzzau, S.; Cappuccinelli, P.; Fasano, A. Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb. Pathog. 1999, 27, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kondoh, M.; Uchida, H.; Kakamu, Y.; Hamakubo, T.; Yagi, K. Mutated C-terminal fragments of Clostridium perfringens enterotoxin have increased affinity to claudin-4 and reversibly modulate tight junctions in vitro. Biochem. Biophys. Res. Commun. 2011, 410, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Wang, X.; Bzik, V.; McClean, S.; Brayden, D.J. Evaluation of intestinal absorption and mucosal toxicity using two promoters. II. Rat instillation and perfusion studies. Eur. J. Pharm. Sci. 2009, 38, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Singh, A.; Kumar, R.V.; Singh, S.; Kumria, R.; Bhinge, J.R. Oral colon-specific drug delivery of protein and peptide drugs. Crit. Rev. Ther. Drug Carr. Syst. 2007, 24, 63–92. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Del Curto, M.D.; Foppoli, A.; Gazzaniga, A. Oral colon delivery of insulin with the aid of functional adjuvants. Adv. Drug Deliv. Rev. 2012, 64, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, W. Ligand-mediated active targeting for enhanced oral absorption. Drug Discov. Today 2014, 19, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. Overcoming the intestinal barrier: A look into targeting approaches for improved oral drug delivery systems. J. Control. Release 2020, 322, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Gong, Y.C.; Xiong, X.Y.; Li, Z.L.; Luo, Y.Y.; Li, Y.P. Targeted folate-conjugated pluronic P85/poly (lactide-co-glycolide) polymersome for the oral delivery of insulin. Nanomedicine 2018, 13, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef] [PubMed]
- Managuli, R.S.; Raut, S.Y.; Reddy, M.S.; Mutalik, S. Targeting the intestinal lymphatic system: A versatile path for enhanced oral bioavailability of drugs. Expert Opin. Drug Deliv. 2018, 15, 787–804. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, Y.; Li, L.; Zhong, C.; Chen, C.; Gao, X. Lipid-based formulations: A promising approach for poorly soluble drug delivery via the intestinal lymphatic system. J. Drug Deliv. Sci. Technol. 2023, 87, 104770. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.A.; Wang, S.W.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Tang, H.; Zheng, Y.; Xiong, Y.; Cheng, H.; Li, J.; Zhang, Y.; Liu, G. Advances in nanomedicines for lymphatic imaging and therapy. J. Nanobiotechnol. 2023, 21, 292. [Google Scholar] [CrossRef]
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wu, J.; Fang, X.; Sha, X. A new function of Vitamin E-TPGS in the intestinal lymphatic transport of lipophilic drugs: Enhancing the secretion of chylomicrons. Int. J. Pharm. 2013, 445, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhuang, J.; Lv, Y.; Lu, Y.; Wu, W. Exploiting or overcoming the dome trap for enhanced oral immunization and drug delivery. J. Control. Release 2018, 275, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, T.; Tian, X.; An, W.; Wang, Z.; Han, B.; Tao, H.; Wang, J.; Wang, X. Research progress on the PEGylation of therapeutic proteins and peptides (TPPs). Front. Pharmacol. 2024, 15, 1353626. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, O.; Greenberg, S.; Laufer, B.; Gilon, C.; Hoffman, A.; Kessler, H. Improvement of drug-like properties of peptides: The somatostatin paradigm. Expert Opin. Drug Discov. 2010, 5, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Lalani, N.; Tivari, S.; Jain, V.; Jadeja, Y. Review on therapeutic potential of peptides: Advancements in synthesis methods, linear and cyclic peptides, and strategies for overcoming challenges. Pept. Sci. 2024, 116, e24343. [Google Scholar] [CrossRef]
- Menacho-Melgar, R.; Decker, J.S.; Hennigan, J.N.; Lynch, M.D. A review of lipidation in the development of advanced protein and peptide therapeutics. J. Control. Release 2019, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reddiar, S.B.; Abdallah, M.; Styles, I.K.; Müllertz, O.O.; Trevaskis, N.L. Lymphatic uptake of the lipidated and non-lipidated GLP-1 agonists liraglutide and exenatide is similar in rats. Eur. J. Pharm. Biopharm. 2024, 200, 114339. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yamada, K.; Miyake, M.; Onoue, S. Recent Advancements in the Development of Nanocarriers for Mucosal Drug Delivery Systems to Control Oral Absorption. Pharmaceutics 2023, 15, 2708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus mucopenetrating nanoparticles for oral delivery of insulin. Acta Biomater. 2021, 135, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Rubas, W.; Grass, G.M. Gastrointestinal lymphatic absorption of peptides and proteins. Adv. Drug Deliv. Rev. 1991, 7, 15–69. [Google Scholar] [CrossRef]
- Li, J.; Chen, B.; Yu, T.; Guo, M.; Zhao, S.; Zhang, Y.; Jin, C.; Peng, X.; Zeng, J.; Yang, J.; et al. An efficient controlled release strategy for hypertension therapy: Folate-mediated lipid nanoparticles for oral peptide delivery. Pharmacol. Res. 2020, 157, 104796. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Afshari, A.R.; Aminyavari, S.; Kesharwani, P.; Jamialahmadi, T.; Sahebkar, A. RGD-engineered nanoparticles as an innovative drug delivery system in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 84, 104562. [Google Scholar] [CrossRef]
- Yang, S.-B.; Banik, N.; Han, B.; Lee, D.N.; Park, J. Peptide-Based Bioconjugates and Therapeutics for Targeted Anticancer Therapy. Pharmaceutics 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Roesger, S.; Jones, N.; Hu, C.M.; Li, S.D. Cell-penetrating peptides for transmucosal delivery of proteins. J. Control. Release 2024, 366, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Guada, M.; Beloqui, A.; Kumar, M.R.; Préat, V.; del Carmen Dios-Viéitez, M.; Blanco-Prieto, M.J. Reformulating cyclosporine A (CsA): More than just a life cycle management strategy. J. Control. Release 2016, 225, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Granhall, C.; Donsmark, M.; Blicher, T.M.; Golor, G.; Søndergaard, F.L.; Thomsen, M.; Bækdal, T.A. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin. Pharmacokinet. 2019, 58, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Multiple Dose Trial Examining Dose Range, Escalation and Efficacy of Oral Semaglutide in Subjects with Type 2 Diabetes. Available online: https://www.clinicaltrials.gov (accessed on 3 February 2025).
- Ganesh, A.N.; Heusser, C.; Garad, S.; Sánchez-Félix, M.V. Patient-centric design for peptide delivery: Trends in routes of administration and advancement in drug delivery technologies. Med. Drug Discov. 2021, 9, 100079. [Google Scholar] [CrossRef]
- An Open-Label Dose-Finding Study to Evaluate the Pharmacodynamic (PD) Profiles and Efficacy of Different Dosing Regimens of Leuprolide Oral Tablets (Ovarest®) in Women with Endometriosis. Available online: https://clinicaltrials.gov/study/NCT05096065?term=nct05096065&rank=1 (accessed on 6 February 2025).
- Maher, S.; Ryan, B.; Duffy, A.; Brayden, D.J. Formulation Strategies to Improve Oral Peptide Delivery. Pharm. Pat. Anal. 2014, 3, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Eldor, R.; Francis, B.H.; Fleming, A.; Neutel, J.; Homer, K.; Kidron, M.; Rosenstock, J. Oral insulin (ORMD-0801) in type 2 diabetes mellitus: A dose-finding 12-week randomized placebo-controlled study. Diabetes Obes. Metab. 2023, 25, 943–952. [Google Scholar] [CrossRef] [PubMed]
- ORMD 0801—Oral Insulin Capsule for NASH. Available online: https://oramed.com/pipeline/ (accessed on 6 February 2025).
- ORMD 0901—Oral GLP-1 for T2DM. Available online: https://www.oramed.com/pipeline/ormd-0901/ (accessed on 6 February 2025).
- Hashim, M.; Korupolu, R.; Syed, B.; Horlen, K.; Beraki, S.; Karamchedu, P.; Dhalla, A.K.; Ruffy, R.; Imran, M. Jejunal wall delivery of insulin via an ingestible capsule in anesthetized swine—A pharmacokinetic and pharmacodynamic study. Pharmacol. Res. Perspect. 2019, 7, e00522. [Google Scholar] [CrossRef] [PubMed]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubálek, F.; Water, J.J.; et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Abramson, A.; Caffarel-Salvador, E.; Soares, V.; Minahan, D.; Tian, R.Y.; Lu, X.; Dellal, D.; Gao, Y.; Kim, S.; Wainer, J.; et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019, 25, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shang, L. Smart ingestible devices: Orally delivering macromolecules and beyond. Matter 2021, 4, 3379–3381. [Google Scholar] [CrossRef]
- Abramson, A.; Frederiksen, M.R.; Vegge, A.; Jensen, B.; Poulsen, M.; Mouridsen, B.; Jespersen, M.O.; Kirk, R.K.; Windum, J.; Hubálek, F.; et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat. Biotechnol. 2022, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Fu, X.; Wang, Y.; Zhao, Y. Magneto-Responsive microneedle robots for intestinal macromolecule delivery. Adv. Mater. 2021, 33, 2104932. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.R.; Yu, F.; Venkatasubramanian, R.; Nielsen, L.H.; Nielsen, H.M.; Boisen, A.; Rades, T.; Müllertz, A. In Vitro, Ex Vivo and In Vivo Evaluation of Microcontainers for Oral Delivery of Insulin. Pharmaceutics 2020, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Mitragotri, S. Intestinal patch systems for oral drug delivery. Curr. Opin. Pharmacol. 2017, 36, 58–65. [Google Scholar] [CrossRef]
- Banerjee, A.; Wong, J.; Gogoi, R.; Brown, T.; Mitragotri, S. Intestinal micropatches for oral insulin delivery. J. Drug Target. 2017, 25, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Hwang, B.H.; Doshi, N.; Banerjee, A.; Anselmo, A.C.; Mitragotri, S. Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann. Biomed. Eng. 2016, 44, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Chen, R.; Arafin, S.; Mitragotri, S. Intestinal iontophoresis from mucoadhesive patches: A strategy for oral delivery. J. Control. Release 2019, 297, 71–78. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baral, K.C.; Choi, K.Y. Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances. Pharmaceutics 2025, 17, 397. https://doi.org/10.3390/pharmaceutics17040397
Baral KC, Choi KY. Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances. Pharmaceutics. 2025; 17(4):397. https://doi.org/10.3390/pharmaceutics17040397
Chicago/Turabian StyleBaral, Kshitis Chandra, and Ki Young Choi. 2025. "Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances" Pharmaceutics 17, no. 4: 397. https://doi.org/10.3390/pharmaceutics17040397
APA StyleBaral, K. C., & Choi, K. Y. (2025). Barriers and Strategies for Oral Peptide and Protein Therapeutics Delivery: Update on Clinical Advances. Pharmaceutics, 17(4), 397. https://doi.org/10.3390/pharmaceutics17040397








