Investigation of Cell Damage Induced by Silver Nanoparticles in a Model Cell System
Abstract
1. Introduction
2. Materials and Methods
2.1. Object of Study
2.2. Method for Determining Plasma Membrane Integrity
2.3. Cell Incubation Conditions and Their Modification
2.4. Method of Synthesizing AgNP
2.5. UV-Vis Absorption Spectroscopy Method
2.6. Scanning Electron Microscopy (SEM)
2.7. Transmission Electron Microscopy (TEM)
2.8. Dynamic Light Scattering Method (DLS)
2.9. Mathematical Model of AgNP Interaction with Macrophages
- -
- First Reaction: The diffusion transfer of nanoparticles to the cell surface and the formation of a “particle–membrane” complex, described within the framework of the Smoluchowski theory for diffusion-controlled processes [87]. To determine the rate constant, an analytical solution of the diffusion equation in spherical coordinates was used, taking into account boundary conditions corresponding to an irreversible reaction on the cell surface.
- -
- Second Reaction: This is based on the Arrhenius law, which accounts for the temperature dependence of the reaction [88]. This approach considers the generation of ROS upon contact of nanoparticles with the membrane and the subsequent overall work of the cell’s AOD system using a probabilistic approach. The actions of the AOD system protect potentially vulnerable cellular structures; however, cell death occurs if the AOD system is insufficiently effective, particularly in cases with a significant accumulation of toxic peroxidation products over time.
2.10. UV-B Irradiation of Cells
2.11. Determination of ROS in Cell Suspensions
2.12. Statistical Data Processing
3. Results and Discussion
3.1. Study of the Size of Synthesized AgNP
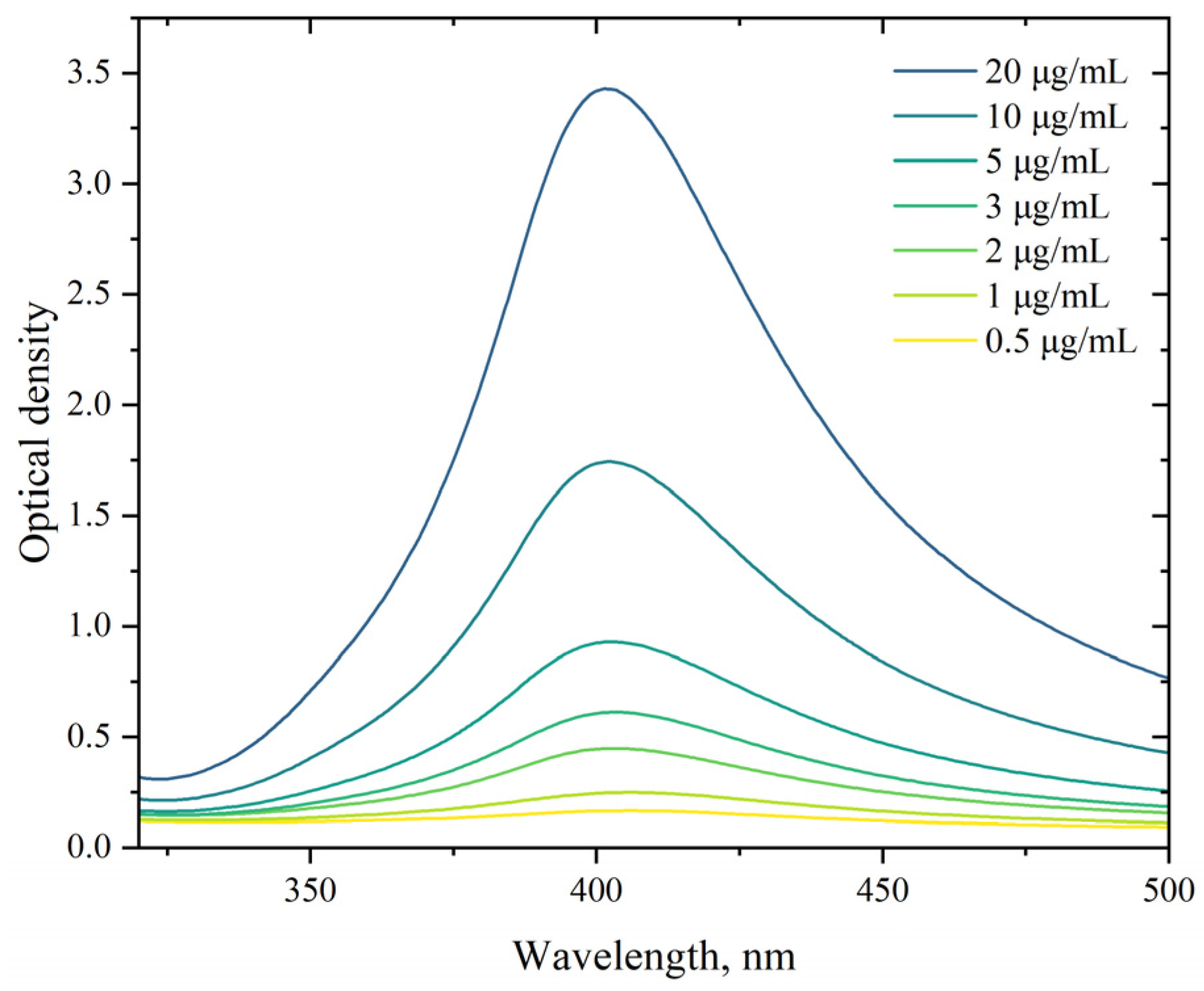
3.1.1. UV-Vis Absorption Spectroscopy
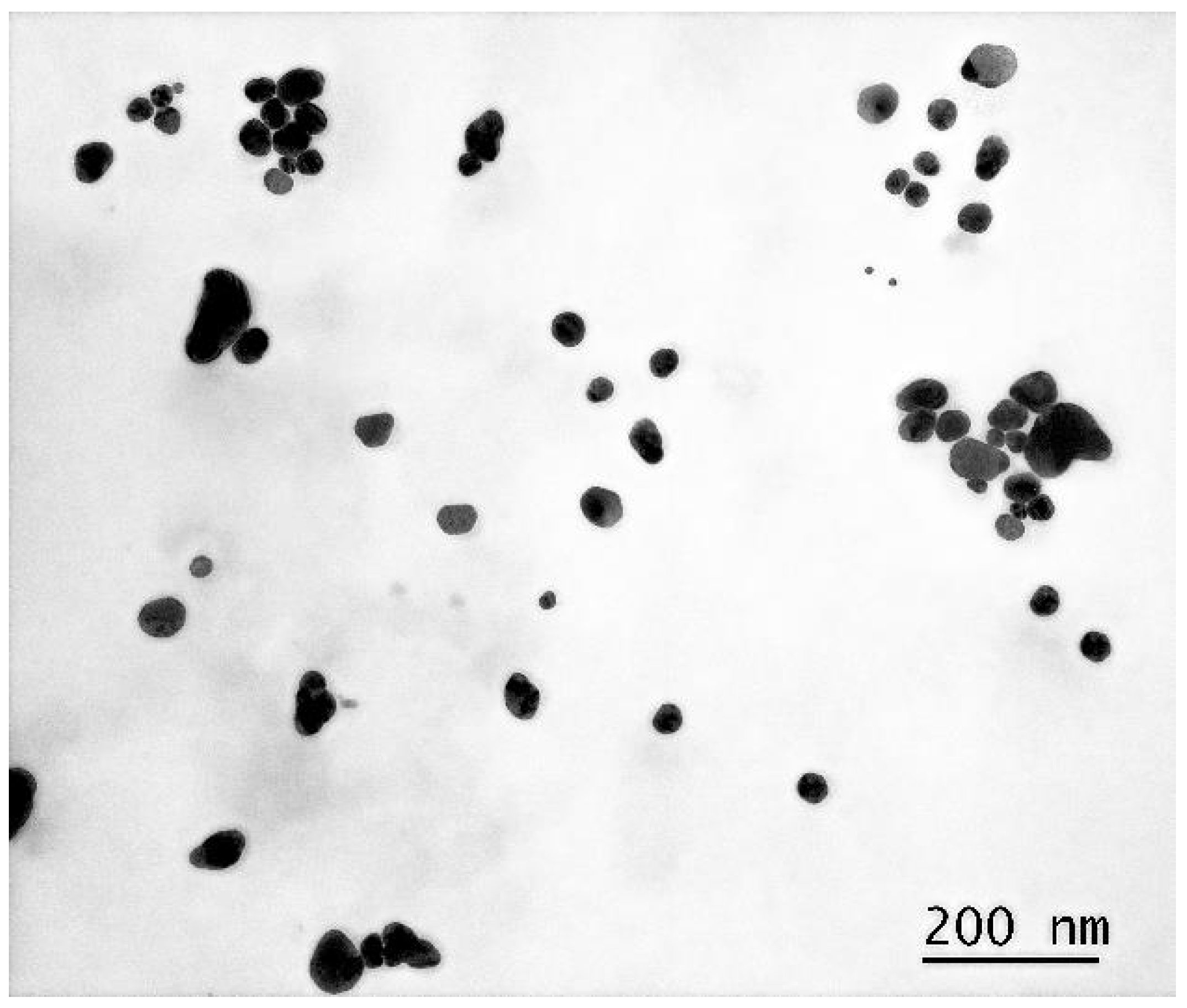
3.1.2. Transmission Electron Microscopy
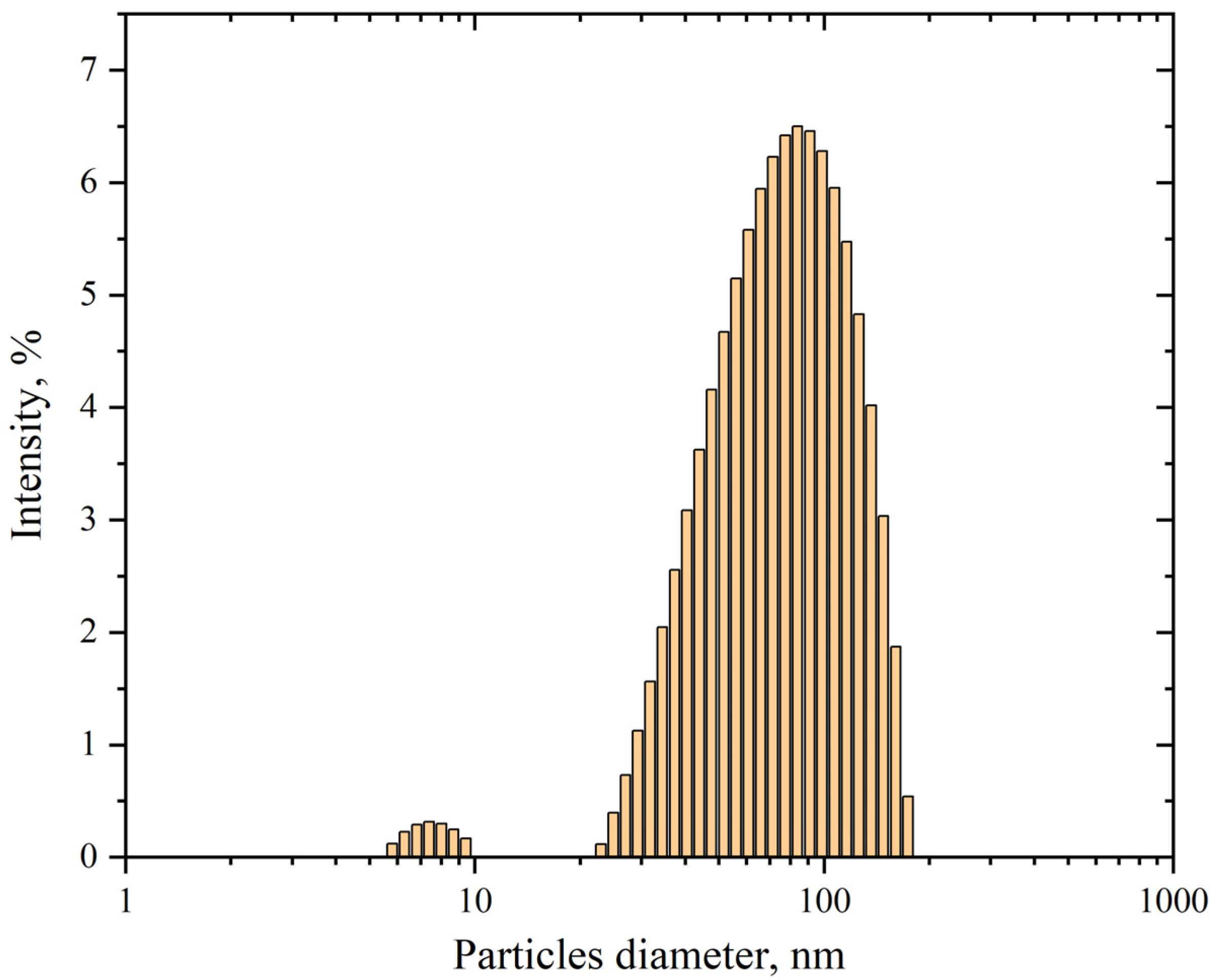
3.1.3. Determination of AgNP Size by Dynamic Light Scattering
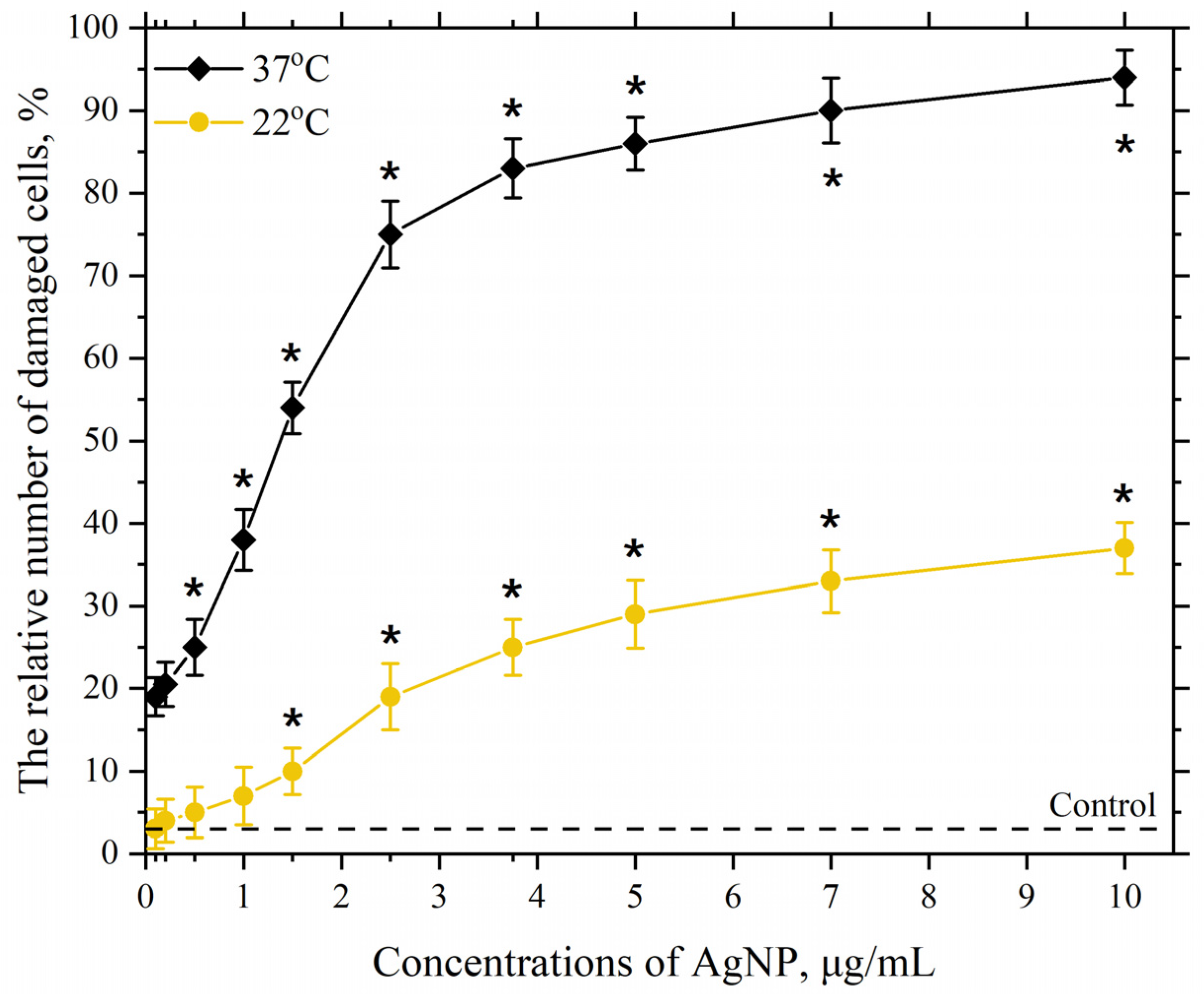
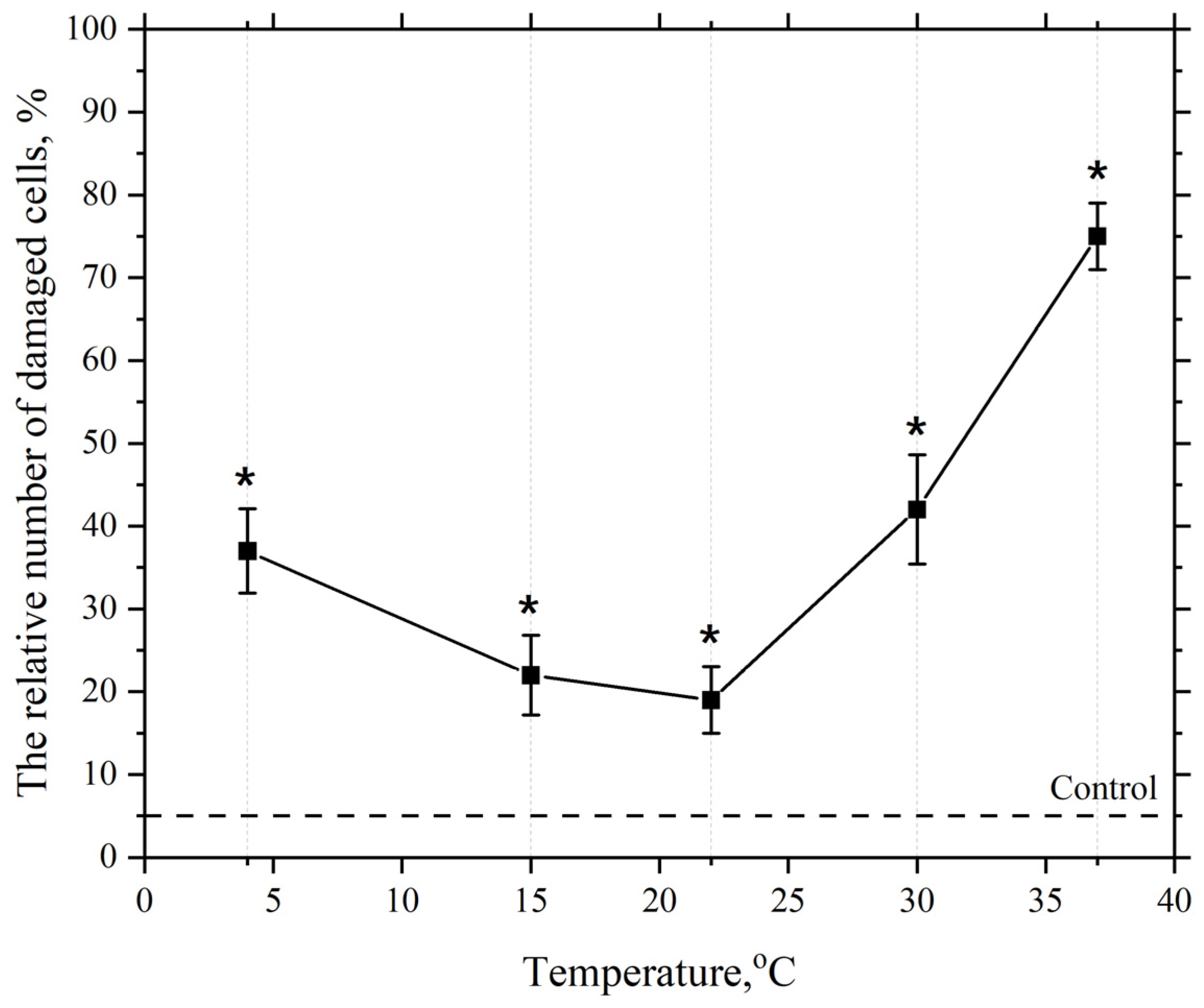
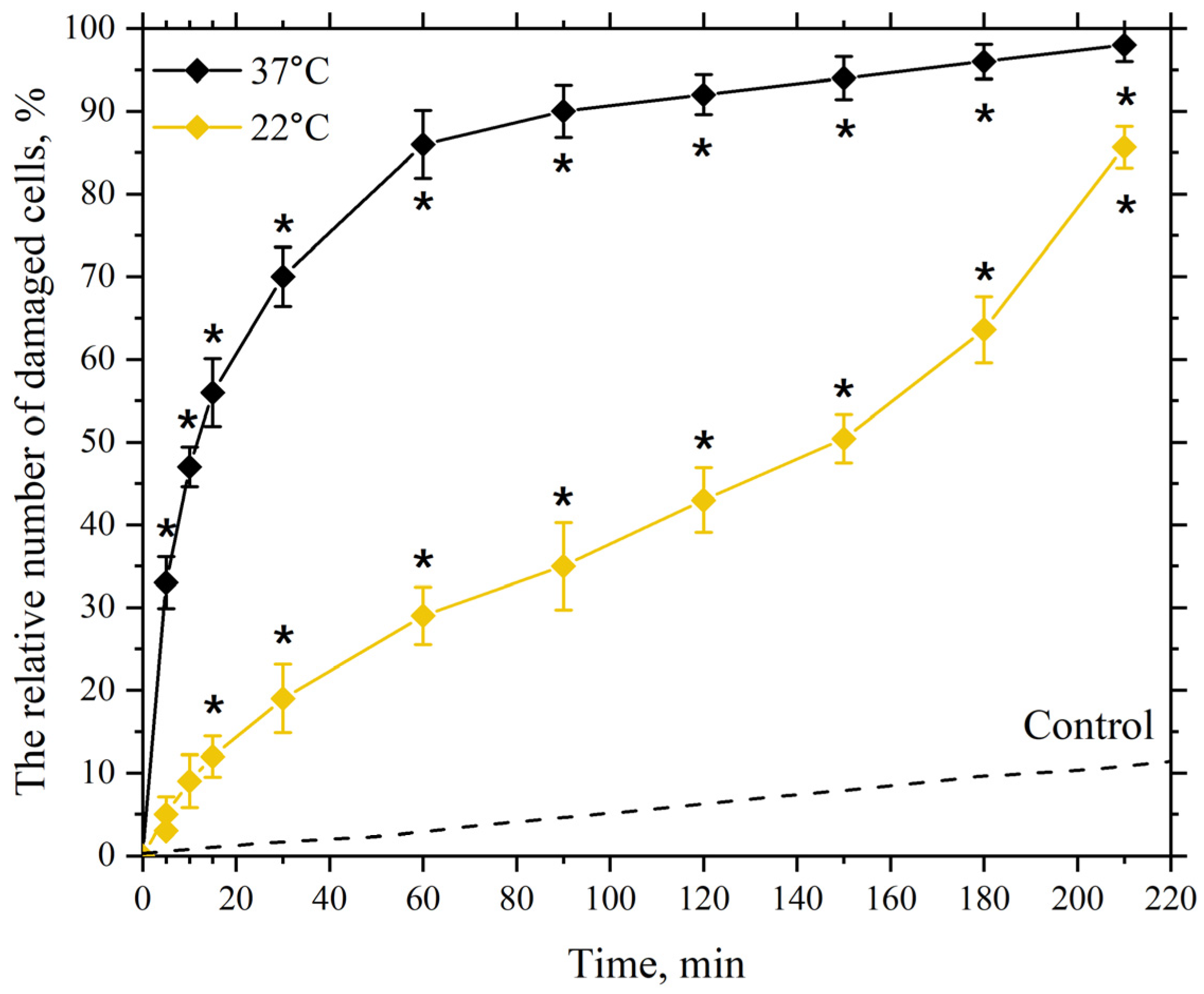
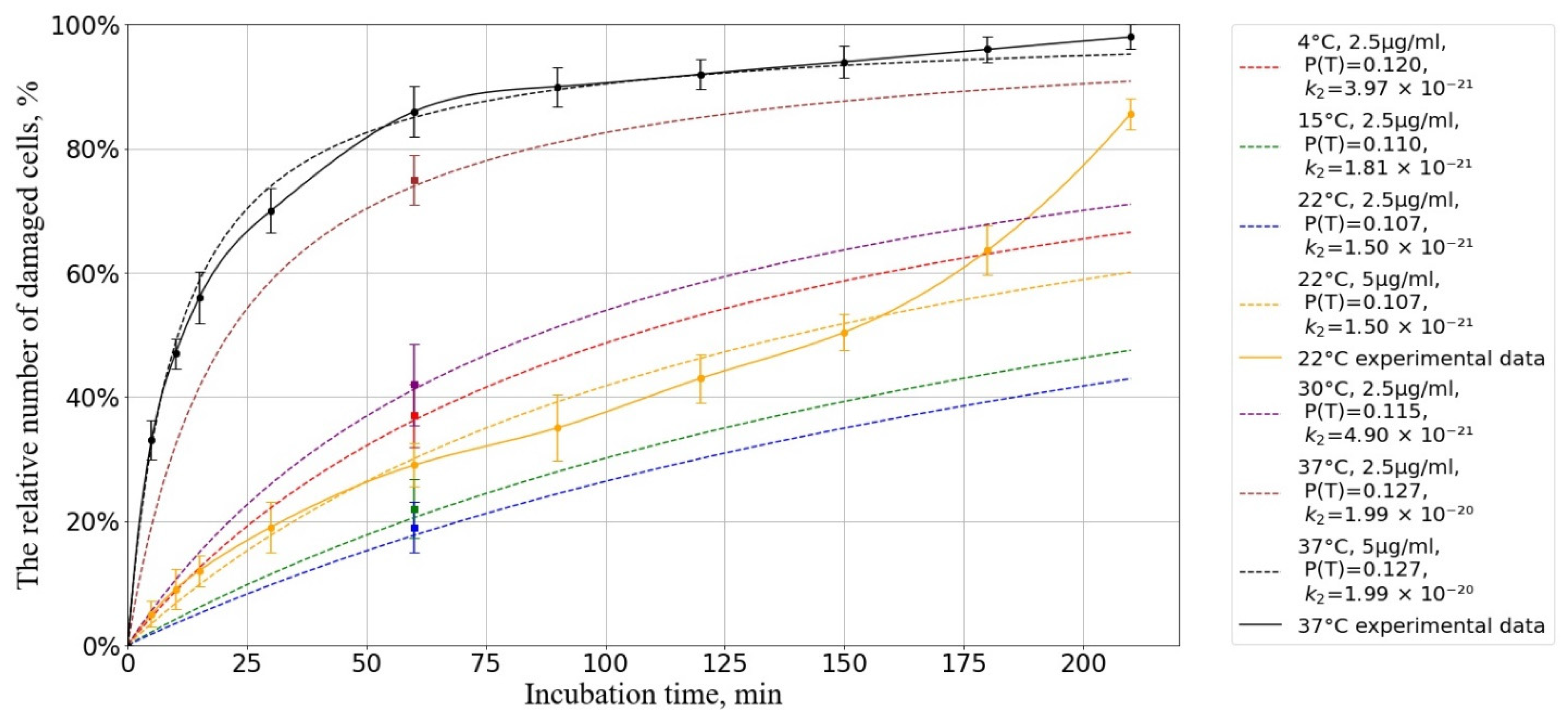
3.2. Dependence of the Membranotropic Action of AgNP on Concentration, Temperature, and Incubation Time
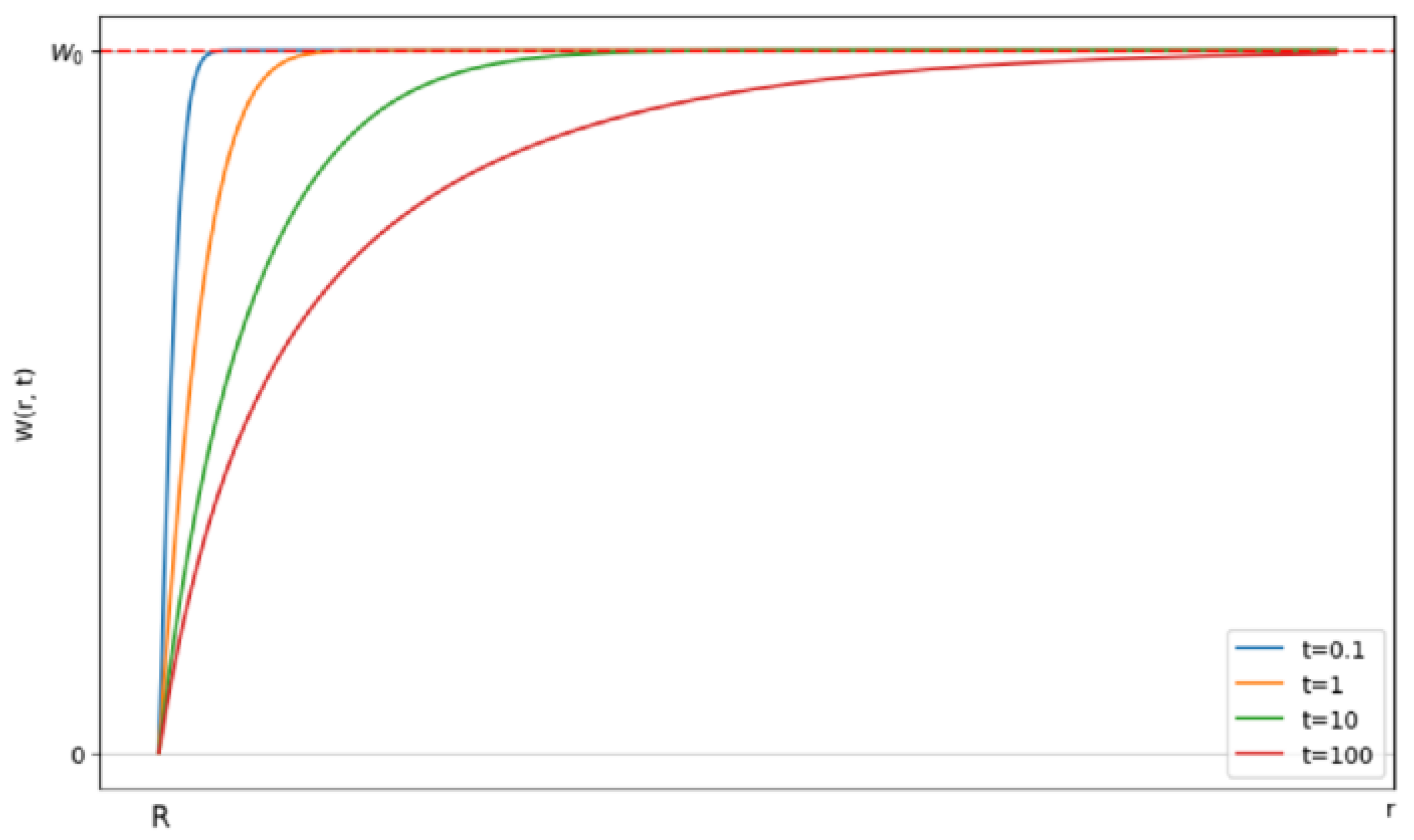
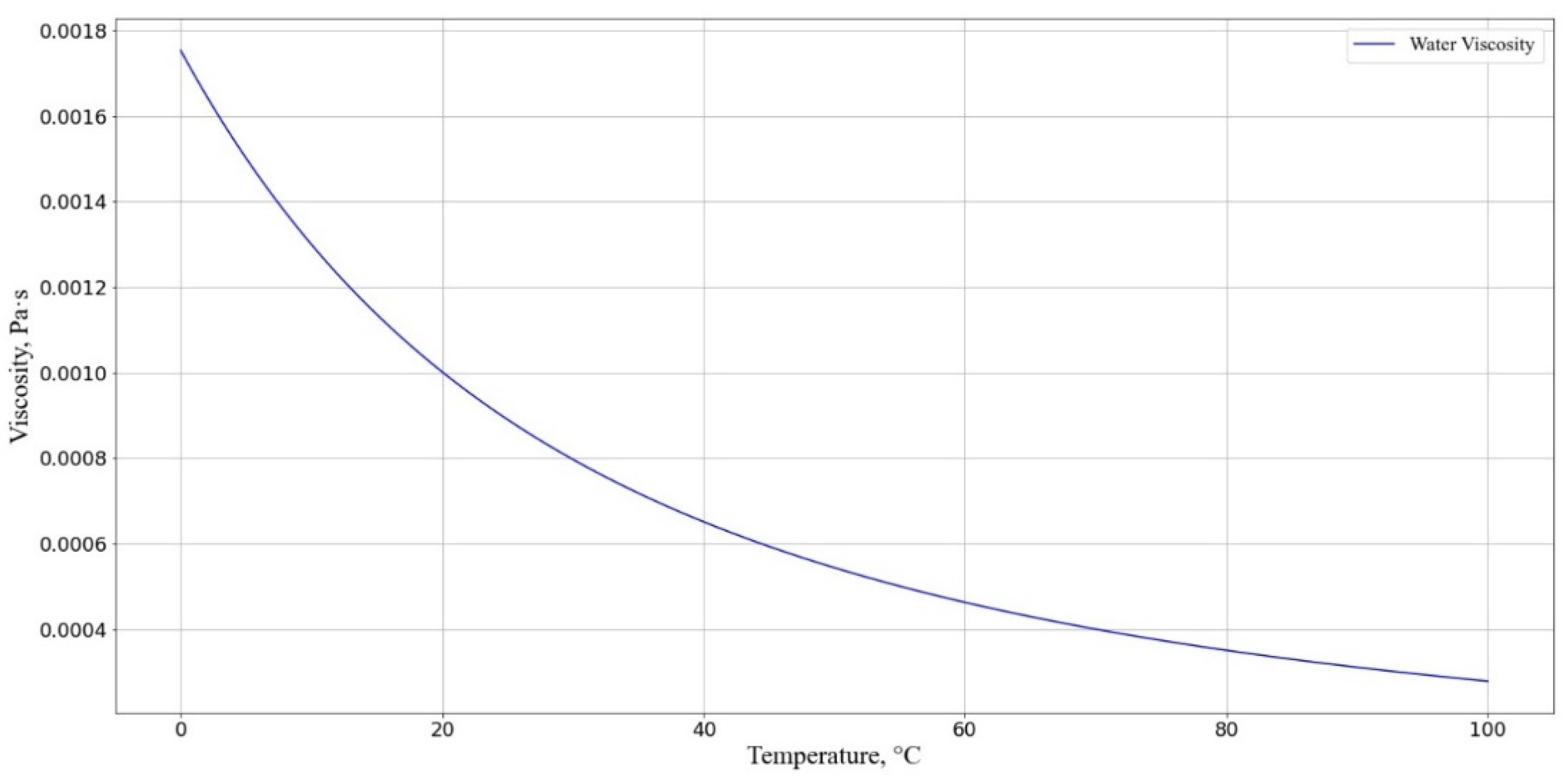
3.3. Mathematical Modeling of the Kinetics of a Diffusion-Controlled Reaction Between Mouse Macrophage Cells and AgNP in Solution
3.3.1. Rate Constant of the First Reaction
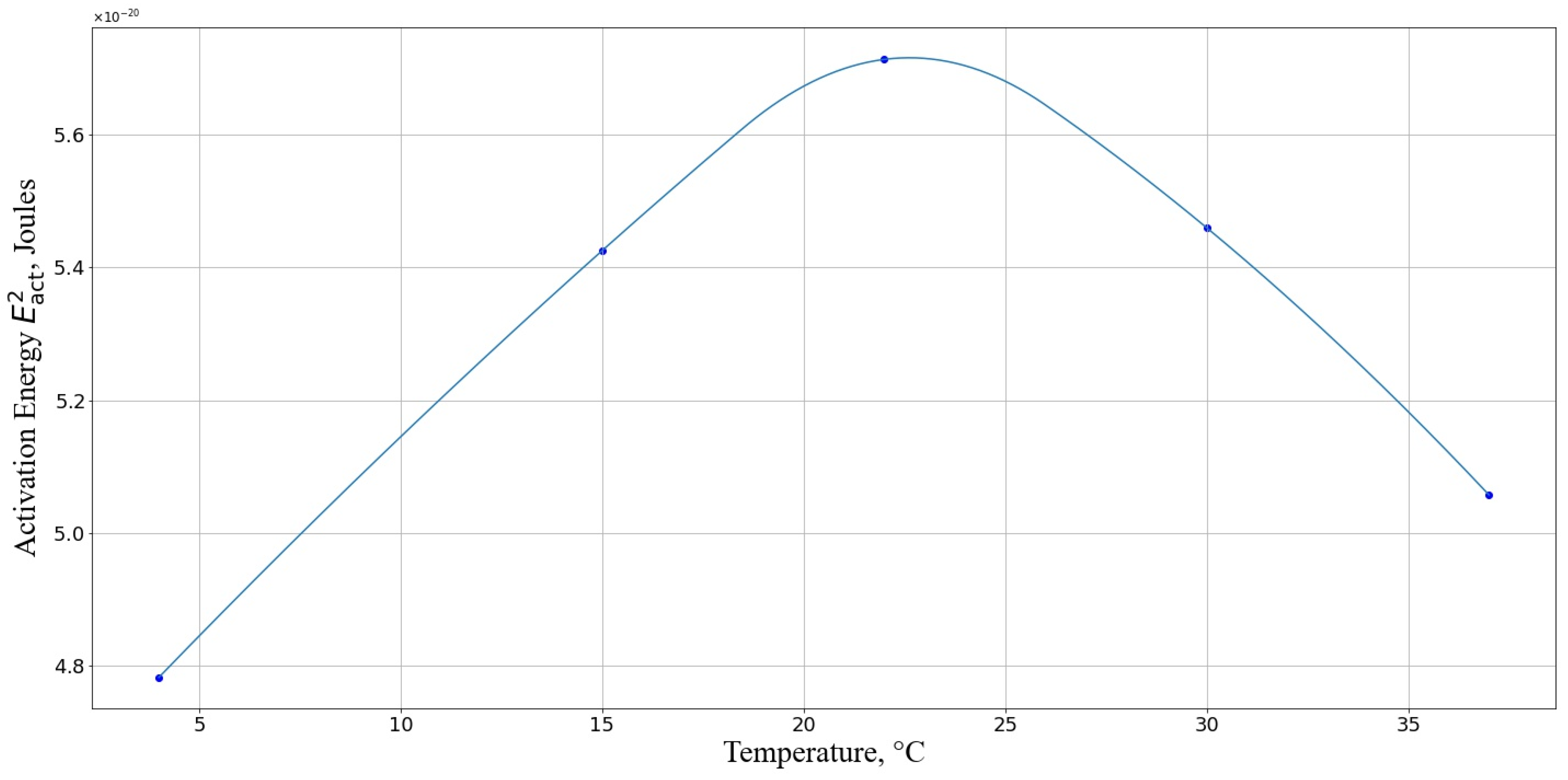
3.3.2. Rate Constant of the Second Reaction
3.3.3. Rate of AB Complex Formation
3.3.4. Numerical Results
- Nanoparticle concentration ( or μg/mL;
- Bulk density ( of silver nanoparticles kg/m3;
- Macrophage radius () m;
- Average radius of the silver particle () m;
- Macrophage count () units/mL.
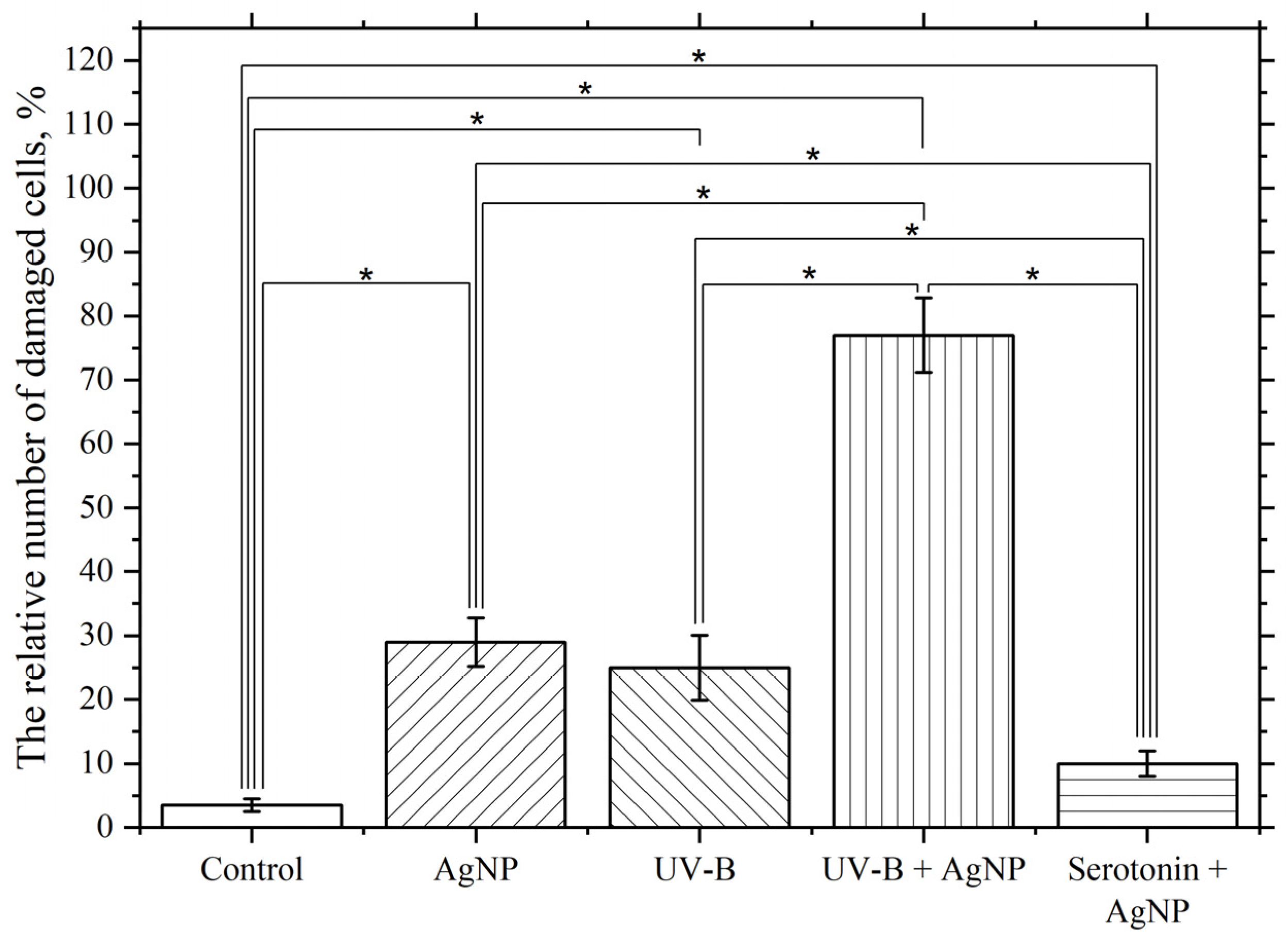
3.4. Influence of Pro- and Antioxidant Factors on the Toxic Effect of AgNP
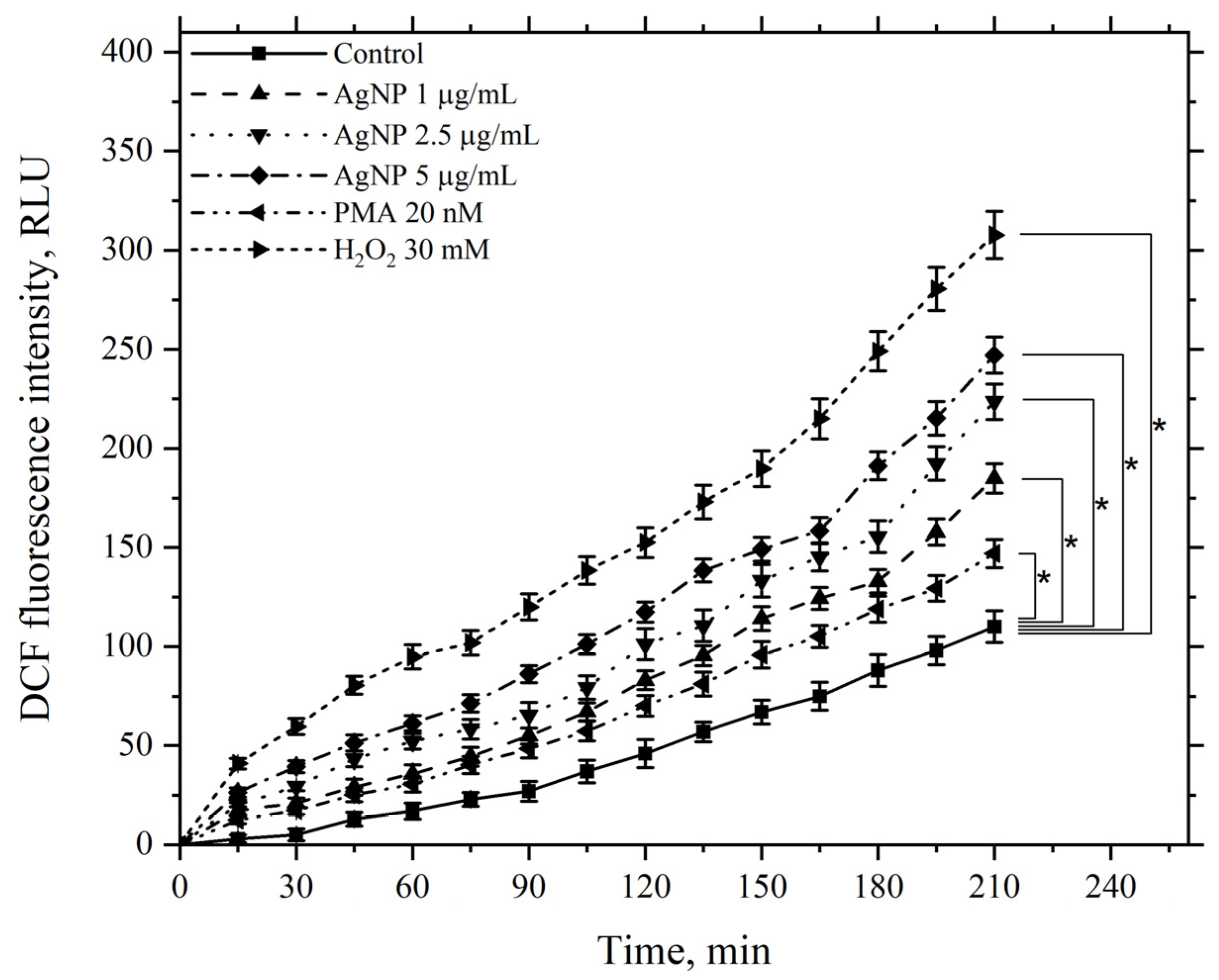
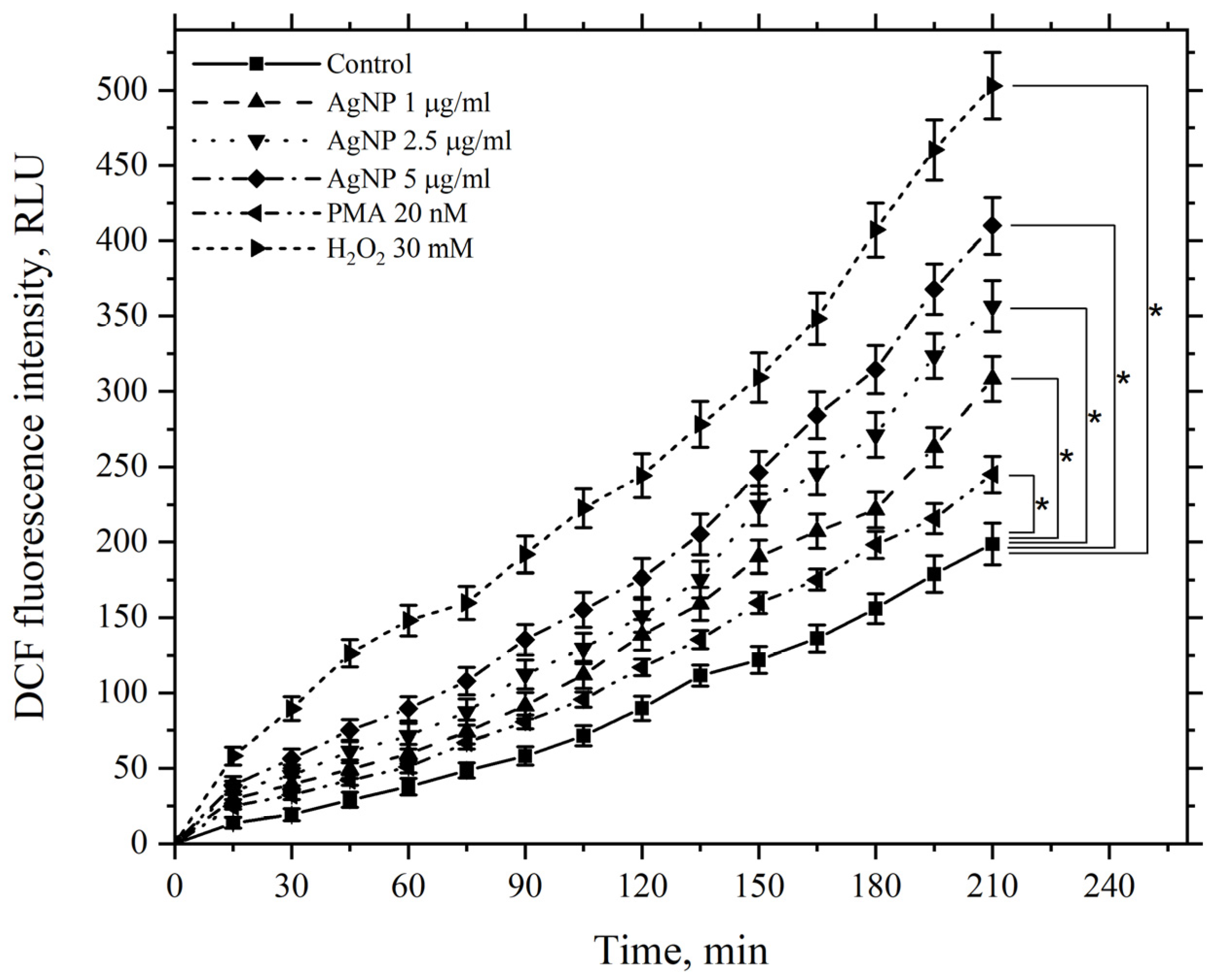
3.5. Study of the Effect of AgNP on ROS Levels in Cell Preparations and Cell Suspensions
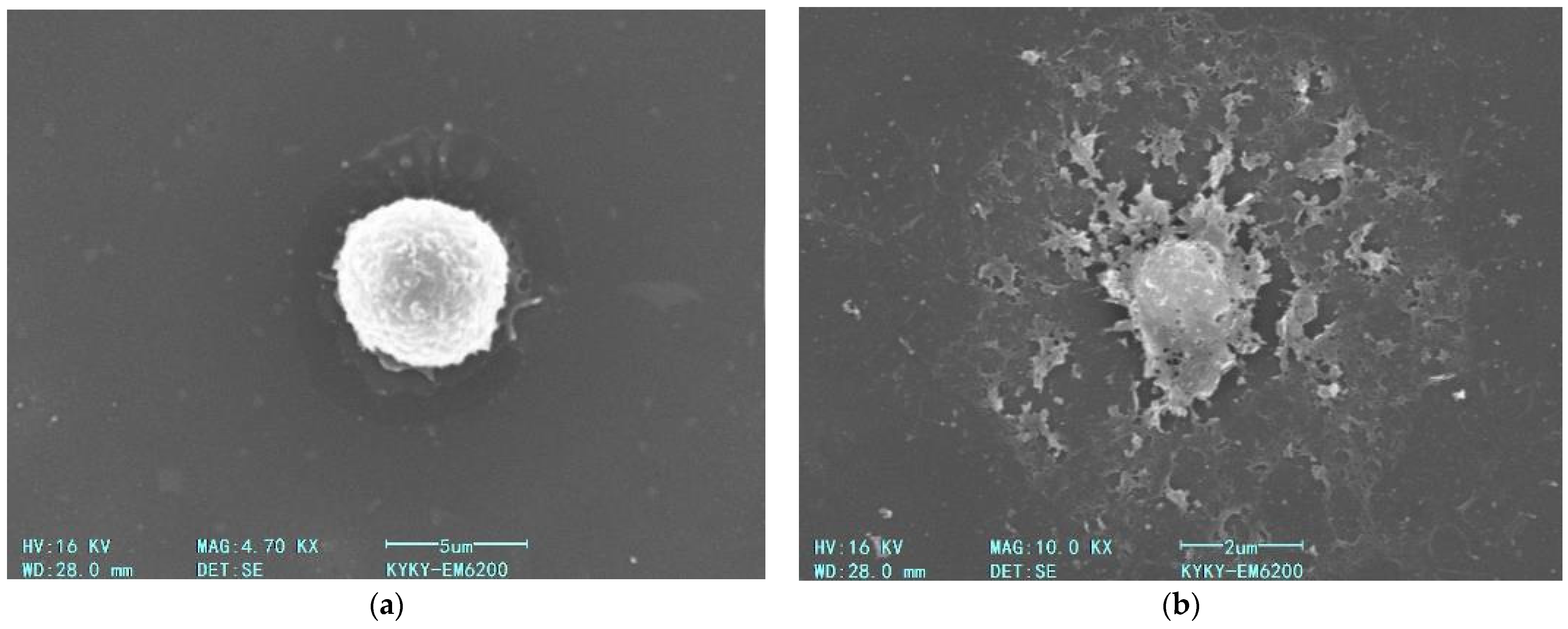
3.6. Investigation of AgNP Effects on Macrophage Membranes via Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Moore, M.N. Do Nanoparticles Present Ecotoxicological Risks for the Health of the Aquatic Environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Mahendra Kumar, S.; Rajni, Y.; Sandeep Prasad, T. Recent Advances in Nanotechnology. Int. J. Nanomater. Nanotechnol. Nanomed. 2023, 9, 015–023. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Shinde, M.U.; Patwekar, M.; Patwekar, F.; Bajaber, M.A.; Medikeri, A.; Mohammad, F.S.; Mukim, M.; Soni, S.; Mallick, J.; Jawaid, T. Nanomaterials: A Potential Hope for Life Sciences from Bench to Bedside. J. Nanomater. 2022, 2022, 5968131. [Google Scholar] [CrossRef]
- Behra, R.; Sigg, L.; Clift, M.J.D.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of Silver Nanoparticles and Ions: From a Chemical and Biochemical Perspective. J. R. Soc. Interface 2013, 10, 20130396. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Frattini, A.; Pellegri, N.; Nicastro, D.; Sanctis, O.D. Effect of Amine Groups in the Synthesis of Ag Nanoparticles Using Aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Capek, I. Preparation of Metal Nanoparticles in Water-in-Oil (w/o) Microemulsions. Adv. Colloid Interface Sci. 2004, 110, 49–74. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Joseph, T.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Spacciapoli, P.; Buxton, D.; Rothstein, D.; Friden, P. Antimicrobial Activity of Silver Nitrate against Periodontal Pathogens. J. Periodontal Res. 2001, 36, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the Anti-Bacterial Activity on the Nanosilver Shapes: Nanoparticles, Nanorods and Nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and Antifungal Properties of Silver Nanoparticles—Effect of a Surface-Stabilizing Agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver Nanoparticles—A Material of the Future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Kvitek, L.; Panacek, A.; Prucek, R.; Soukupova, J.; Vanickova, M.; Kolar, M.; Zboril, R. Antibacterial Activity and Toxicity of Silver—Nanosilver versus Ionic Silver. J. Phys. Conf. Ser. 2011, 304, 012029. [Google Scholar] [CrossRef]
- Alshareef, A.; Laird, K.; Cross, R.B.M. Shape-Dependent Antibacterial Activity of Silver Nanoparticles on Escherichia Coli and Enterococcus Faecium Bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively Charged Silver Nanoparticles with Potent Antibacterial Activity and Reduced Toxicity for Pharmaceutical Preparations. Int. J. Nanomed. 2007, 12, 2517–2530. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Xiu, Z.; Alvarez, P.J.J. Impacts of Silver Nanoparticles on Cellular and Transcriptional Activity of Nitrogen-cycling Bacteria. Environ. Toxicol. Chem. 2013, 32, 1488–1494. [Google Scholar] [CrossRef]
- Yin, N.; Liu, Q.; Liu, J.; He, B.; Cui, L.; Li, Z.; Yun, Z.; Qu, G.; Liu, S.; Zhou, Q.; et al. Silver Nanoparticle Exposure Attenuates the Viability of Rat Cerebellum Granule Cells through Apoptosis Coupled to Oxidative Stress. Small 2013, 9, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ferguson, S.A.; Watanabe, F.; Jones, Y.; Xu, Y.; Biris, A.S.; Hussain, S.; Ali, S.F. Silver Nanoparticles Decrease Body Weight and Locomotor Activity in Adult Male Rats. Small 2013, 9, 1715–1720. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory Effects of Silver Nanoparticles in Two Green Algae, Chlorella Vulgaris and Dunaliella Tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef]
- Jiang, H.; Qiu, X.; Li, G.; Li, W.; Yin, L. Silver Nanoparticles Induced Accumulation of Reactive Oxygen Species and Alteration of Antioxidant Systems in the Aquatic Plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-Dependent Antibacterial Activities of Silver Nanoparticles against Oral Anaerobic Pathogenic Bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Haase, A.; Rott, S.; Mantion, A.; Graf, P.; Plendl, J.; Thünemann, A.F.; Meier, W.P.; Taubert, A.; Luch, A.; Reiser, G. Effects of Silver Nanoparticles on Primary Mixed Neural Cell Cultures: Uptake, Oxidative Stress and Acute Calcium Responses. Toxicol. Sci. 2012, 126, 457–468. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-Ion-Mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-Coated Silver Nanoparticles and Silver Ions Induce Reactive Oxygen Species, Apoptosis and Necrosis in THP-1 Monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Onodera, A.; Nishiumi, F.; Kakiguchi, K.; Tanaka, A.; Tanabe, N.; Honma, A.; Yayama, K.; Yoshioka, Y.; Nakahira, K.; Yonemura, S.; et al. Short-Term Changes in Intracellular ROS Localisation after the Silver Nanoparticles Exposure Depending on Particle Size. Toxicol. Rep. 2015, 2, 574–579. [Google Scholar] [CrossRef]
- Hwang, I.; Lee, J.; Hwang, J.H.; Kim, K.; Lee, D.G. Silver Nanoparticles Induce Apoptotic Cell Death in Candida Albicans through the Increase of Hydroxyl Radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Reddy Mudiam, M.K.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver Nanoparticles Induced Alterations in Multiple Cellular Targets, Which Are Critical for Drug Susceptibilities and Pathogenicity in Fungal Pathogen (Candida Albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef]
- Lee, B.; Lee, M.J.; Yun, S.J.; Kim, K.; Choi, I.-H.; Park, S. Silver Nanoparticles Induce Reactive Oxygen Species-Mediated Cell Cycle Delay and Synergistic Cytotoxicity with 3-Bromopyruvate in Candida Albicans, but Not in Saccharomyces Cerevisiae. Int. J. Nanomed. 2019, 14, 4801–4816. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kalishwaralal, K.; Barathmanikanth, S.; Gurunathani, S. Size-Based Cytotoxicity of Silver Nanoparticles in Bovine Retinal Endothelial Cells. Nanosci. Methods 2012, 1, 56–77. [Google Scholar] [CrossRef]
- Van Aerle, R.; Lange, A.; Moorhouse, A.; Paszkiewicz, K.; Ball, K.; Johnston, B.D.; de-Bastos, E.; Booth, T.; Tyler, C.R.; Santos, E.M. Molecular Mechanisms of Toxicity of Silver Nanoparticles in Zebrafish Embryos. Environ. Sci. Technol. 2013, 47, 8005–8014. [Google Scholar] [CrossRef]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of Engineered Nanoparticles to Human Lung Epithelial Cells: Influence of Chemical Composition and Catalytic Activity on Oxidative Stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef]
- Mao, B.-H.; Chen, Z.-Y.; Wang, Y.-J.; Yan, S.-J. Silver Nanoparticles Have Lethal and Sublethal Adverse Effects on Development and Longevity by Inducing ROS-Mediated Stress Responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
- Yu, N.-H.; Pei, H.; Huang, Y.-P.; Li, Y.-F. (-)-Epigallocatechin-3-Gallate Inhibits Arsenic-Induced Inflammation and Apoptosis through Suppression of Oxidative Stress in Mice. Cell. Physiol. Biochem. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Yumrutas, O.; Kara, M.; Atilgan, R.; Kavak, S.B.; Bozgeyik, I.; Sapmaz, E. Application of Talcum Powder, Trichloroacetic Acid and Silver Nitrate in Female Rats for Non-surgical Sterilization: Evaluation of the Apoptotic Pathway mRNA and Mi RNA Genes. Int. J. Exp. Pathol. 2015, 96, 111–115. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Redon, C.E.; Ferguson, N.F.; Kryston, T.B.; Parekh, P.; Dickey, J.S.; Nakamura, A.J.; Mitchell, J.B.; Bonner, W.M.; Martin, O.A. Systemic DNA Damage Accumulation under in Vivo Tumor Growth Can Be Inhibited by the Antioxidant Tempol. Cancer Lett. 2014, 353, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Gagné, F.; Auclair, J.; Turcotte, P.; Gagnon, C. Sublethal Effects of Silver Nanoparticles and Dissolved Silver in Freshwater Mussels. J. Toxicol. Environ. Health A 2013, 76, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sulik, K.K.; Chen, S. Nrf2-Mediated Transcriptional Induction of Antioxidant Response in Mouse Embryos Exposed to Ethanol in Vivo: Implications for the Prevention of Fetal Alcohol Spectrum Disorders. Antioxid. Redox Signal. 2008, 10, 2023–2033. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Himly, M.; Geppert, M.; Hofer, S.; Hofstätter, N.; Horejs-Höck, J.; Duschl, A. When Would Immunologists Consider a Nanomaterial to Be Safe? Recommendations for Planning Studies on Nanosafety. Small 2020, 16, 1907483. [Google Scholar] [CrossRef]
- Garcia-Manyes, S.; Oncins, G.; Sanz, F. Effect of Temperature on the Nanomechanics of Lipid Bilayers Studied by Force Spectroscopy. Biophys. J. 2005, 89, 4261–4274. [Google Scholar] [CrossRef]
- Schmidt, A.; Spinke, J.; Bayerl, T.; Sackmann, E.; Knoll, W. Streptavidin Binding to Biotinylated Lipid Layers on Solid Supports. A Neutron Reflection and Surface Plasmon Optical Study. Biophys. J. 1992, 63, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Roshchupkin, D.; Pelenitsyn, B.; Vladimirov, Y. Effect of Temperature and pH on the Lipid Photoperoxidation and Structural State of Erytrocyte Membranes. Stud. Biophys. 1978, 71, 23–37. [Google Scholar]
- To, K.T.; Truong, L.; Edwards, S.; Tanguay, R.L.; Reif, D.M. Multivariate Modeling of Engineered Nanomaterial Features Associated with Developmental Toxicity. NanoImpact 2019, 16, 100185. [Google Scholar] [CrossRef]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of Silver Nanoparticles on Human Cells: Effect of Particle Size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Kaba, S.; Egorova, E. In Vitro Studies of the Toxic Effects of Silver Nanoparticles on HeLa and U937 Cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef]
- Greulich, C.; Kittler, S.; Epple, M.; Muhr, G.; Köller, M. Studies on the Biocompatibility and the Interaction of Silver Nanoparticles with Human Mesenchymal Stem Cells (hMSCs). Langenbecks Arch. Surg. 2009, 394, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver Nanoparticles Induce Cytotoxicity by a Trojan-Horse Type Mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The Effect of Agglomeration State of Silver and Titanium Dioxide Nanoparticles on Cellular Response of HepG2, A549 and THP-1 Cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef]
- Le, A.-T.; Huy, P.T.; Tam, P.D.; Huy, T.Q.; Cam, P.D.; Kudrinskiy, A.A.; Krutyakov, Y.A. Green Synthesis of Finely-Dispersed Highly Bactericidal Silver Nanoparticles via Modified Tollens Technique. Curr. Appl. Phys. 2010, 10, 910–916. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of Silver Nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Interactions of Silver Nanoparticles with Primary Mouse Fibroblasts and Liver Cells. Toxicol. Appl. Pharmacol. 2009, 236, 310–318. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Podkopaev, D.O.; Shaburova, L.N.; Balandin, G.V.; Kraineva, O.V.; Labutina, N.V.; Suvorov, O.A.; Sidorenko, Y.I. Comparative Evaluation of Antimicrobial Activity of Silver Nanoparticles. Nanotechnol. Russ. 2014, 9, 93–97. [Google Scholar] [CrossRef]
- Suvorov, O.A.; Volozhaninova, S.Y.; Balandin, G.V.; Frolova, Y.V.; Kozlovskaya, A.E.; Fokina, E.N.; Labutina, N.V. Antibacterial Effect of Colloidal Solutions of Silver Nanoparticles on Microorganisms of Cereal Crops. Foods Raw Mater. 2017, 5, 100–107. [Google Scholar] [CrossRef]
- Gmoshinski, I.; Bagryantseva, O.; Arnautov, O.; Khotimchenko, S. Nanoclays in Food Products: Benefits and Possible Risks. Health Risk Anal. 2020, 1, 142–164. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological Properties of Engineered Nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [Google Scholar] [CrossRef]
- Cronin, J.G.; Jones, N.; Thornton, C.A.; Jenkins, G.J.S.; Doak, S.H.; Clift, M.J.D. Nanomaterials and Innate Immunity: A Perspective of the Current Status in Nanosafety. Chem. Res. Toxicol. 2020, 33, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Swartzwelter, B.J.; Fux, A.C.; Johnson, L.; Swart, E.; Hofer, S.; Hofstätter, N.; Geppert, M.; Italiani, P.; Boraschi, D.; Duschl, A.; et al. The Impact of Nanoparticles on Innate Immune Activation by Live Bacteria. Int. J. Mol. Sci. 2020, 21, 9695. [Google Scholar] [CrossRef]
- Swindle, E.J.; Hunt, J.A.; Coleman, J.W. A Comparison of Reactive Oxygen Species Generation by Rat Peritoneal Macrophages and Mast Cells Using the Highly Sensitive Real-Time Chemiluminescent Probe Pholasin: Inhibition of Antigen-Induced Mast Cell Degranulation by Macrophage-Derived Hydrogen Peroxide. J. Immunol. 2002, 169, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Grimm, J.B.; Heckman, L.M.; Lavis, L.D. The Chemistry of Small-Molecule Fluorogenic Probes. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1–34. ISBN 978-0-12-386932-6. [Google Scholar]
- Sundararajan, R.; Natarajan, A.; Sankaranarayanan, K.; Reece, L.M.; Vernon, B. Low and High Voltage Electroporation of in Vitro Human Ovarian Adenocarcinoma Cells. In Electroporation-Based Therapies for Cancer; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–218. ISBN 978-1-907568-15-2. [Google Scholar]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Evdokimov, P.V.; Tikhonova, S.A.; Kiseleva, A.K.; Filippov, Y.Y.; Novoseletskaya, E.S.; Efimenko, A.Y.; Putlayev, V.I. Effect of the Pore Size on the Biological Activity of β-Ca3(PO4)2-Based Resorbable Macroporous Ceramic Materials Obtained by Photopolymerization. Russ. J. Inorg. Chem. 2021, 66, 1609–1615. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Paltsev, O.S.; Marina, V.I.; Lukianov, D.A.; Moiseenko, A.V.; Shchelkunov, N.M.; Fedyanin, A.A.; Sybachin, A.V. Ag2O-Containing Biocidal Interpolyelectrolyte Complexes on Glass Surfaces—Adhesive Properties of the Coatings. Polymers 2023, 15, 4690. [Google Scholar] [CrossRef]
- Karmakar, S. Particle Size Distribution and Zeta Potential Based on Dynamic Light Scattering: Techniques to Characterise Stability and Surface Distribution of Charged Colloids. In Recent Trends in Materials Physics and Chemistry; Studium Press(India)Pvt Ltd.: New Delhi, India, 2019; pp. 117–159. ISBN 978-93-85046-32-2. [Google Scholar]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 237. [Google Scholar] [CrossRef]
- Bamford, C. Chapter 2 Diffusion-Controlled Reactions in Solution. In Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1985; Volume 25, pp. 3–46. ISBN 978-0-444-42354-2. [Google Scholar]
- Rohde, M.M.; Snyder, C.M.; Sloop, J.; Solst, S.R.; Donati, G.L.; Spitz, D.R.; Furdui, C.M.; Singh, R. The Mechanism of Cell Death Induced by Silver Nanoparticles Is Distinct from Silver Cations. Part. Fibre Toxicol. 2021, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.; Timashev, S.; Belyi, A. Kinetics of Diffusion Controlled Chemical Processes; Nova Science Publishers: Commack, NY, USA, 1989; ISBN 0-941743-52-7. [Google Scholar]
- Stiller, W. Arhenius Equation and Nonequilibrium Kinetics. Russ. J. Appl. Chem. 2001, 74, 1427. [Google Scholar] [CrossRef]
- Landreman, A.P.; Shafer, M.M.; Hemming, J.C.; Hannigan, M.P.; Schauer, J.J. A Macrophage-Based Method for the Assessment of the Reactive Oxygen Species (ROS) Activity of Atmospheric Particulate Matter (PM) and Application to Routine (Daily-24 h) Aerosol Monitoring Studies. Aerosol Sci. Technol. 2008, 42, 946–957. [Google Scholar] [CrossRef]
- Robinson, J.M.; Badwey, J.A. The NADPH Oxidase Complex of Phagocytic Leukocytes: A Biochemical and Cytochemical View. Histochem. Cell Biol. 1995, 103, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Z.-Y.; Zhong, Z.; Gates, B.; Xia, Y.; Venkateswaran, S. Synthesis and Characterization of Stable Aqueous Dispersions of Silver Nanoparticles through the Tollens process. J. Mater. Chem. 2002, 12, 522–527. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Choi, I. Green Synthesis of Silver Nanoparticles and Characterization of Their Inhibitory Effects on AGEs Formation Using Biophysical Techniques. Sci. Rep. 2016, 6, 20414. [Google Scholar] [CrossRef]
- Maarebia, R.Z.; Wahid Wahab, A.; Taba, P. Synthesis and Characterization Of Silver Nanoparticles Using Water Extract of Sarang Semut (Myrmecodia Pendans) For Blood Glucose Sensors. J. Akta Kim. Indones. Indones. Chim. Acta 2019, 12, 29–46. [Google Scholar] [CrossRef]
- Parvathalu, K.; Kumar, D.N.; Rajitha, K.; Kishan, M.G.; Kumar, B.N.; Bhemarajam, J.; Naidu, S.R.; Merlinsheeba, G.L.; Mandal, P.; Banne, S.; et al. Facile synthesis of silver nanoparticles using green tea leaf extract and evolution of antibacterial activity. Plasmonics 2023, 18, 1837–1845. [Google Scholar] [CrossRef]
- Qi, L.-X.; Wang, X.-T.; Huang, J.-P.; Yue, T.-Y.; Lu, Y.-S.; San, D.-M.; Xu, Y.-X.; Han, Y.-T.; Guo, X.-Y.; Xie, W.-D.; et al. Silver Nanoparticles Encapped by Dihydromyricetin: Optimization of Green Synthesis, Characterization, Toxicity, and Anti-MRSA Infection Activities for Zebrafish (Danio Rerio). Int. J. Mol. Sci. 2024, 25, 5255. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Cheke, R.A.; Tang, S. A Universal Delayed Difference Model Fitting Dose-Response Curves. Dose-Response 2021, 19, 155932582110627. [Google Scholar] [CrossRef]
- Mansouri, L.; Xie, Y.; Rappolee, D. Adaptive and Pathogenic Responses to Stress by Stem Cells during Development. Cells 2012, 1, 1197–1224. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage Heterogeneity in Tissues: Phenotypic Diversity and Functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Millard, S.M.; Pettit, A.R. Macrophage Heterogeneity in the Single-Cell Era: Facts and Artifacts. Blood 2023, 142, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular Responses Induced by Silver Nanoparticles: In Vitro Studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef]
- Bidian, C.; Filip, G.A.; David, L.; Moldovan, B.; Olteanu, D.; Clichici, S.; Olănescu-Vaida-Voevod, M.-C.; Leostean, C.; Macavei, S.; Muntean, D.M.; et al. Green Synthesized Gold and Silver Nanoparticles Increased Oxidative Stress and Induced Cell Death in Colorectal Adenocarcinoma Cells. Nanomaterials 2023, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, P.; Żuberek, M.; Grzelak, A. Products of Lipid Peroxidation as a Factor in the Toxic Effect of Silver Nanoparticles. Materials 2020, 13, 2460. [Google Scholar] [CrossRef]
- Gurau, M.C.; Castellana, E.T.; Albertorio, F.; Kataoka, S.; Lim, S.-M.; Yang, R.D.; Cremer, P.S. Thermodynamics of Phase Transitions in Langmuir Monolayers Observed by Vibrational Sum Frequency Spectroscopy. J. Am. Chem. Soc. 2003, 125, 11166–11167. [Google Scholar] [CrossRef]
- Anderson, N.A.; Richter, L.J.; Stephenson, J.C.; Briggman, K.A. Determination of Lipid Phase Transition Temperatures in Hybrid Bilayer Membranes. Langmuir 2006, 22, 8333–8336. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Liu, Y.; Legleiter, J.; Nagle, J.F. Structure of Gel Phase DMPC Determined by X-Ray Diffraction. Biophys. J. 2002, 83, 3324–3335. [Google Scholar] [CrossRef]
- Khakbaz, P.; Klauda, J.B. Investigation of Phase Transitions of Saturated Phosphocholine Lipid Bilayers via Molecular Dynamics Simulations. Biochim. Biophys. Acta BBA—Biomembr. 2018, 1860, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Phase Transition Temperatures for Glycerophospholipids. Available online: https://avantiresearch.com/wp-content/uploads/2015/11/Phase_Transition_Temps_for_Glycerophospholipids_Table.pdf (accessed on 10 January 2025).
- Mabrey, S.; Sturtevant, J.M. Investigation of Phase Transitions of Lipids and Lipid Mixtures by Sensitivity Differential Scanning Calorimetry. Proc. Natl. Acad. Sci. USA 1976, 73, 3862–3866. [Google Scholar] [CrossRef]
- Silvius, J.R.; Mak, N.; McElhaney, R.N. Lipid and Protein Composition and Thermotropic Lipid Phase Transitions in Fatty Acid-Homogeneous Membranes of Acholeplasma Laidlawii B. Biochim. Biophys. Acta BBA—Biomembr. 1980, 597, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Vuleta, A.; Tucic, B. Thermal Dependence of the Antioxidant Enzymes Superoxide Dismutase, Catalase, and Peroxidase in Foliage of Iris pumila L. Arch. Biol. Sci. 2009, 61, 441–446. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of Reactive Species and Induction of Antioxidant Defence Systems in Polar and Temperate Marine Invertebrates and Fish. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef]
- Hale, J.P.; Winlove, C.P.; Petrov, P.G. Effect of Hydroperoxides on Red Blood Cell Membrane Mechanical Properties. Biophys. J. 2011, 101, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Pirutin, S.K.; Turovetskii, V.B.; Kudryashov, Y.B. Influence of pH on the UV Sensitivity of Peritoneal Macrophage Plasma Membrane. Biophysics 2010, 55, 148–150. [Google Scholar] [CrossRef]
- Punnonen, K.; Puntala, A.; Jansén, C.T.; Ahotupa, M. UVB Irradiation Induces Lipid Peroxidation and Reduces Antioxidant Enzyme Activities in Human Keratinocytes in Vitro. Acta Derm. Venereol. 1991, 71, 239–242. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis Oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and Membrane Binding Properties of Serotonin Protect Lipids from Oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef]
- Vašíček, O.; Lojek, A.; Číž, M. Serotonin and Its Metabolites Reduce Oxidative Stress in Murine RAW264.7 Macrophages and Prevent Inflammation. J. Physiol. Biochem. 2020, 76, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirutin, S.; Chaikovskii, D.; Shank, M.; Chivarzin, M.; Jia, S.; Yusipovich, A.; Suvorov, O.; Zhao, Y.; Bezryadnov, D.; Rubin, A. Investigation of Cell Damage Induced by Silver Nanoparticles in a Model Cell System. Pharmaceutics 2025, 17, 398. https://doi.org/10.3390/pharmaceutics17040398
Pirutin S, Chaikovskii D, Shank M, Chivarzin M, Jia S, Yusipovich A, Suvorov O, Zhao Y, Bezryadnov D, Rubin A. Investigation of Cell Damage Induced by Silver Nanoparticles in a Model Cell System. Pharmaceutics. 2025; 17(4):398. https://doi.org/10.3390/pharmaceutics17040398
Chicago/Turabian StylePirutin, Sergey, Dmitrii Chaikovskii, Mikhail Shank, Mikhail Chivarzin, Shunchao Jia, Alexander Yusipovich, Oleg Suvorov, Yuehong Zhao, Dmitry Bezryadnov, and Andrey Rubin. 2025. "Investigation of Cell Damage Induced by Silver Nanoparticles in a Model Cell System" Pharmaceutics 17, no. 4: 398. https://doi.org/10.3390/pharmaceutics17040398
APA StylePirutin, S., Chaikovskii, D., Shank, M., Chivarzin, M., Jia, S., Yusipovich, A., Suvorov, O., Zhao, Y., Bezryadnov, D., & Rubin, A. (2025). Investigation of Cell Damage Induced by Silver Nanoparticles in a Model Cell System. Pharmaceutics, 17(4), 398. https://doi.org/10.3390/pharmaceutics17040398





