Clinical Applications of Targeted Nanomaterials
Abstract
1. Introduction
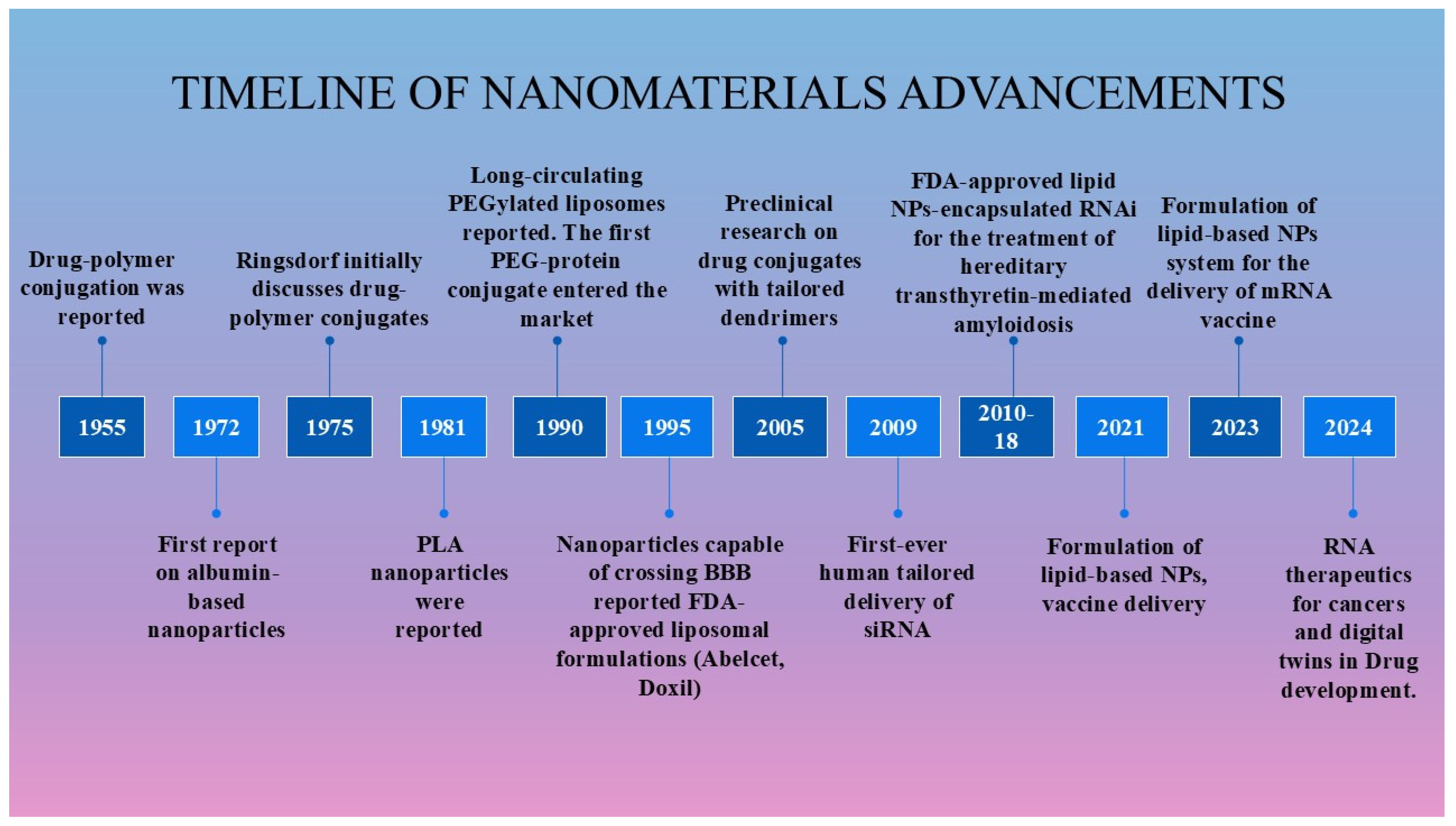
2. Historical Background of Programmable Nanomaterials
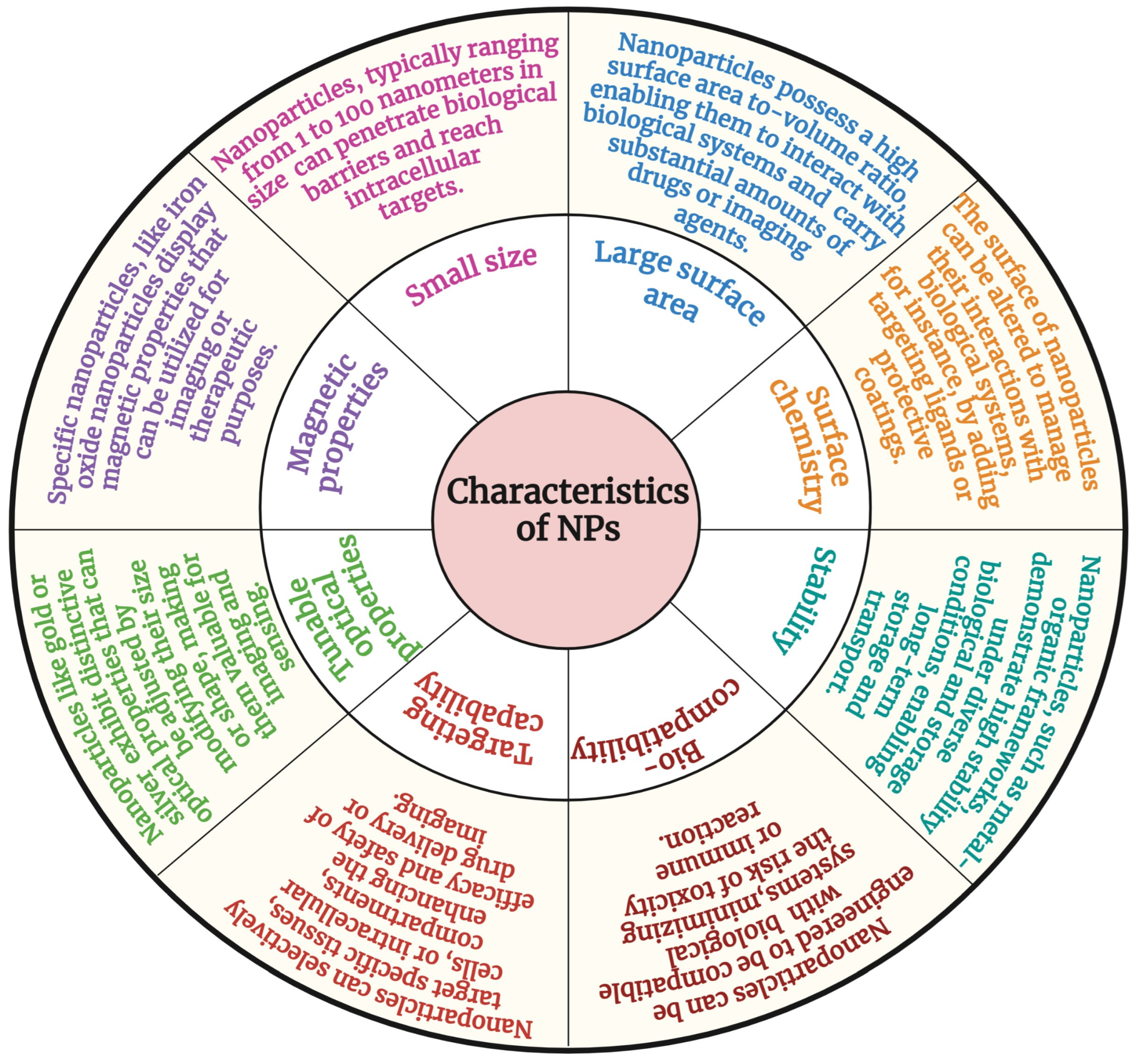
3. Characteristics of Nanomaterials
4. Surface Coating and Functionalization Strategies to Enhance Nanomaterial Properties
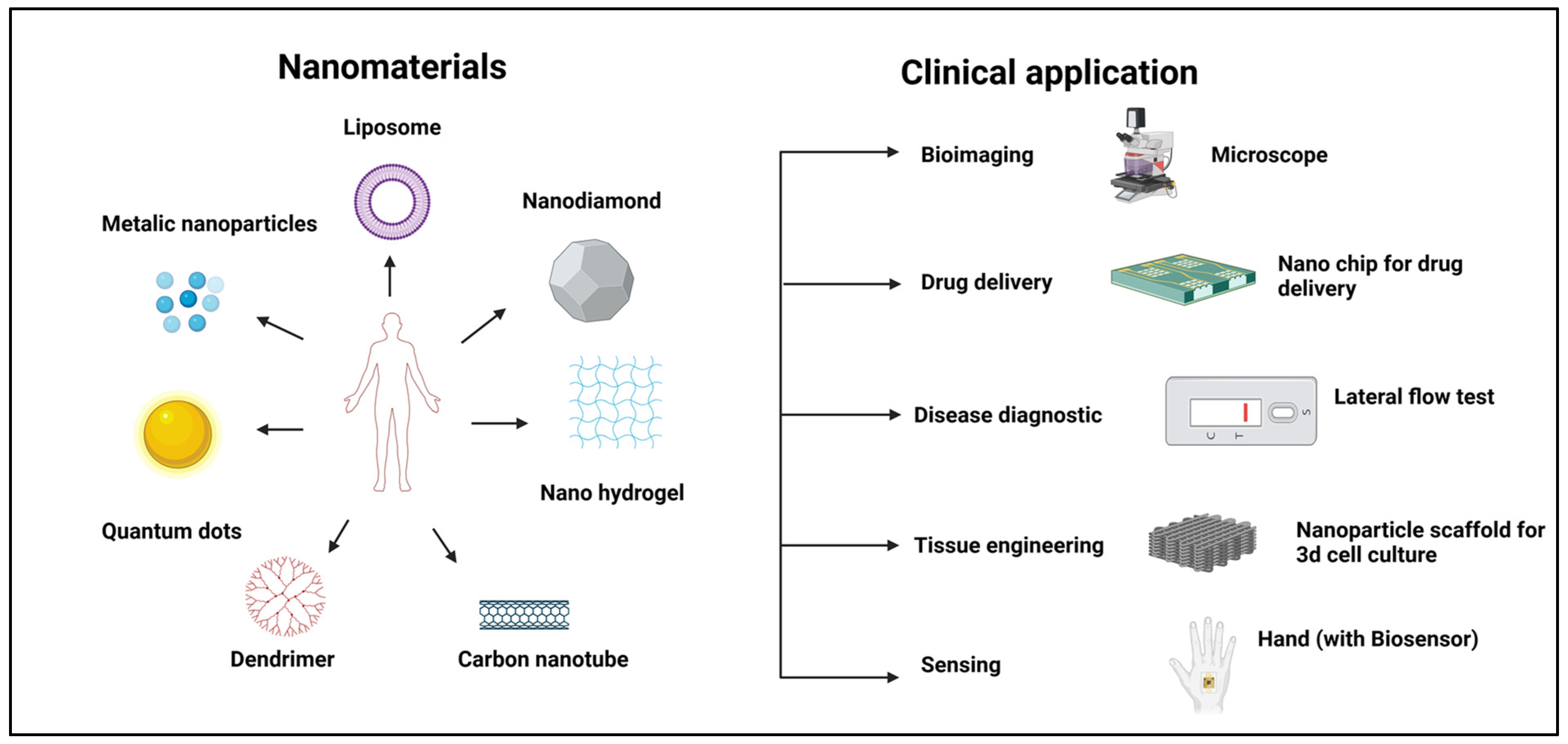
5. Different Types of Targeted Nanomaterials
5.1. Polymeric Nanomaterials
5.1.1. Clinical Examples
Genexol-PM and NK105
Abraxane (Nab-Paclitaxel)
5.2. Lipid-Based Nanomaterials
5.2.1. Clinical Examples
Doxil®
Vyxeos™ and Onivyde™
Marqibo®
5.3. Dendrimers
5.4. Nanogels
5.5. Carbon-Based Nanomaterials
5.5.1. Nanodiamonds (NDs)
5.5.2. Quantum Dots (QDs)
5.5.3. Carbon Nanotubes (CNTs)
5.6. Metallic Nanoparticles
5.6.1. Clinical Examples
NanoTherm
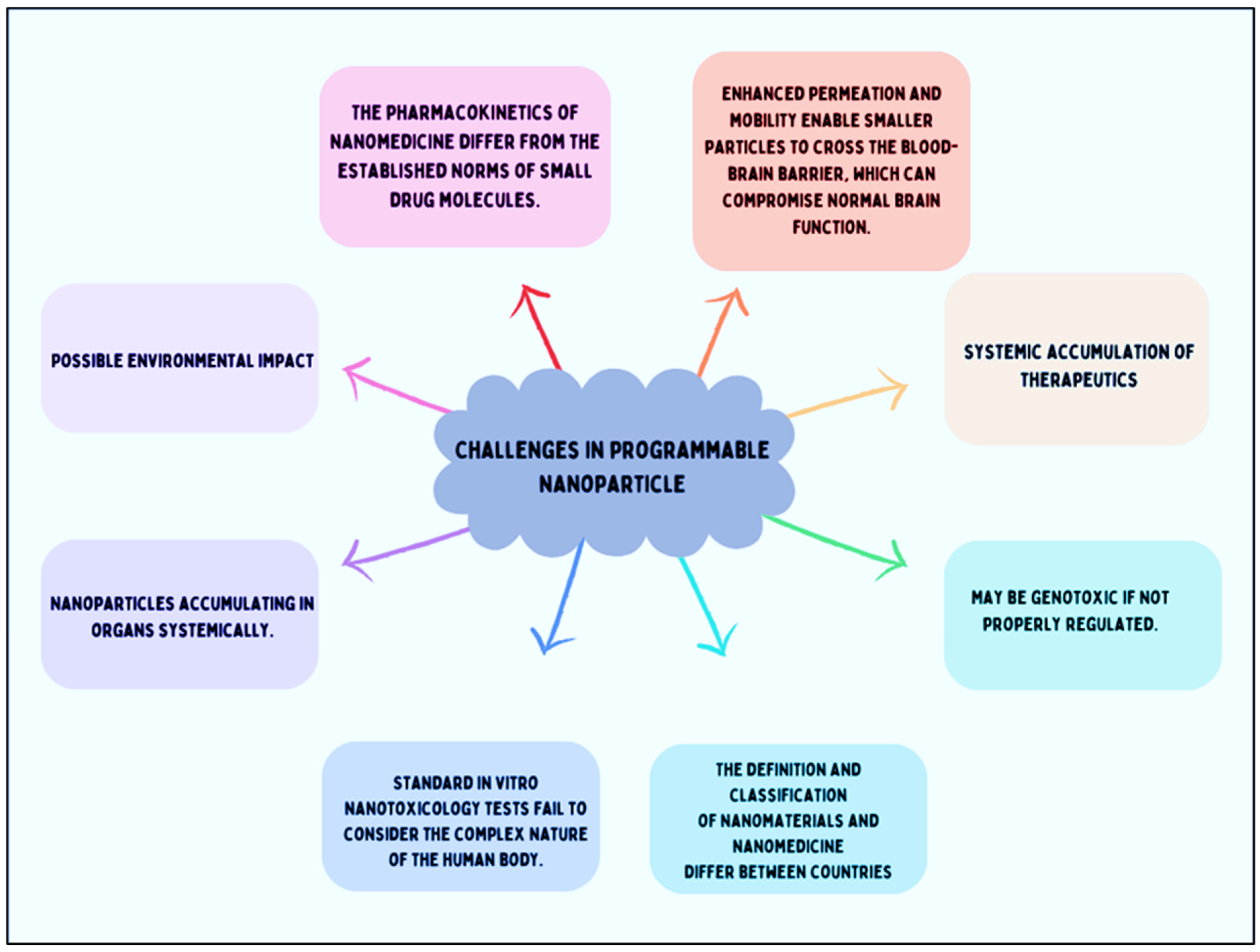
6. Limitations of Nanomaterials
7. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as Drug Delivery Systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in Medicine: Current Challenges Facing Inorganic Nanoparticle Toxicity Assessments and Standardizations. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; de Almeida-Cincotto, M.G.J.; Oshiro, J.A.; Taniguchi, C.Y.Y.; Chiavacci, L.A.; Isaac, V.L.B. Nanoparticles for Cosmetic Use and Its Application. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–146. [Google Scholar]
- Wang, J. Nanoparticle-Based Electrochemical DNA Detection. Anal. Chim. Acta 2003, 500, 247–257. [Google Scholar] [CrossRef]
- Peng, W.; Li, S.; Guan, C.; Shen, X.; Dai, Y.; Wang, Z. Improvement of Magnetorheological Finishing Surface Quality by Nanoparticle Jet Polishing. Opt. Eng. 2013, 52, 043401. [Google Scholar] [CrossRef]
- Ghosh, S.; Polaki, S.R.; Macrelli, A.; Casari, C.S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Nanoparticle-Enhanced Multifunctional Nanocarbons—Recent Advances on Electrochemical Energy Storage Applications. J. Phys. D Appl. Phys. 2022, 55, 413001. [Google Scholar] [CrossRef]
- Hanigan, D.; Truong, L.; Schoepf, J.; Nosaka, T.; Mulchandani, A.; Tanguay, R.L.; Westerhoff, P. Trade-Offs in Ecosystem Impacts from Nanomaterial versus Organic Chemical Ultraviolet Filters in Sunscreens. Water Res. 2018, 139, 281–290. [Google Scholar] [CrossRef]
- Willner, I.; Baron, R.; Willner, B. Integrated Nanoparticle–Biomolecule Systems for Biosensing and Bioelectronics. Biosens. Bioelectron. 2007, 22, 1841–1852. [Google Scholar] [CrossRef]
- Szelenyi, I. Nanomedicine: Evolutionary and Revolutionary Developments in the Treatment of Certain Inflammatory Diseases. Inflamm. Res. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current Status of Polymeric Gene Delivery Systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Parveen, S.; Sahoo, S.K. Polymeric Nanoparticles for Cancer Therapy. J. Drug Target 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional Nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. Ageing Int. 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, K.; Yokoe, J.; Ogawara, K.; Amano, S.; Takaguchi, M.; Higaki, K.; Kai, T.; Kimura, T. Effect of Coupling of Albumin onto Surface of PEG Liposome on Its in Vivo Disposition. Int. J. Pharm. 2007, 329, 110–116. [Google Scholar] [CrossRef]
- Jeong, J.H.; Mok, H.; Oh, Y.-K.; Park, T.G. SiRNA Conjugate Delivery Systems. Bioconjug. Chem. 2009, 20, 5–14. [Google Scholar] [CrossRef]
- Elegbede, A.I.; Banerjee, J.; Hanson, A.J.; Tobwala, S.; Ganguli, B.; Wang, R.; Lu, X.; Srivastava, D.K.; Mallik, S. Mechanistic Studies of the Triggered Release of Liposomal Contents by Matrix Metalloproteinase-9. J. Am. Chem. Soc. 2008, 130, 10633–10642. [Google Scholar] [CrossRef]
- Patil, M.L.; Zhang, M.; Minko, T. Multifunctional Triblock Nanocarrier (PAMAM-PEG-PLL) for the Efficient Intracellular SiRNA Delivery and Gene Silencing. ACS Nano 2011, 5, 1877–1887. [Google Scholar] [CrossRef]
- Stinchcombe, T.E. Nanoparticle Albumin-Bound Paclitaxel: A Novel Cremphor-EL®-Free Formulation of Paclitaxel. Nanomedicine 2007, 2, 415–423. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Gabizon-Peretz, S.; Modaresahmadi, S.; La-Beck, N.M. Thirty Years from FDA Approval of Pegylated Liposomal Doxorubicin (Doxil/Caelyx): An Updated Analysis and Future Perspective. BMJ Oncol. 2025, 4, e000573. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Koga, F.; Kawaguchi, Y.; Shimokawa, M.; Murayama, K.; Nakashita, S.; Oza, N.; Ureshino, N.; Takahashi, H.; Ueda, Y.; Nakazawa, J. Gemcitabine plus Nab-Paclitaxel in Older Patients with Metastatic Pancreatic Cancer: A Post-Hoc Analysis of the Real-World Data of a Multicenter Study (the NAPOLEON Study). J. Geriatr. Oncol. 2022, 13, 82–87. [Google Scholar] [CrossRef]
- Davis, M.E. The First Targeted Delivery of SiRNA in Humans via a Self-Assembling, Cyclodextrin Polymer-Based Nanoparticle: From Concept to Clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Loretan, C.; Muller, A. Nano, Bits, and Feynman’s Dream: There’s Plenty of Room at the (Molecular) Bottom. J. Chem. Educ. 2023, 100, 1366–1370. [Google Scholar] [CrossRef]
- Pisano, R.; Durlo, A. Feynman’s Frameworks on Nanotechnology in Historiographical Debate. In Handbook for the Historiography of Science; Springer: Berlin/Heidelberg, Germany, 2023; pp. 441–478. [Google Scholar]
- Asadi, A.; Obidiro, O.; Elesho, R.; Agbaje, K.; Kochakzade, M.; Karla, P.K. Recent Advances and FDA Approvals in Nanoformulations for Drug Delivery. J. Nanopart. Res. 2025, 27, 12. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer–drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660-in10. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. Proc. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Duncan, R.; Ringsdorf, H.; Satchi-Fainaro, R. Polymer therapeutics: Polymers as drugs, drug and protein conjugates and gene delivery systems: Past, present and future opportunities. Polym. Ther. I 2006, 192, 1–8. [Google Scholar]
- Mahadik, N.; Barron, G.A.; Lin, P.K.T.; Thompson, C.J. Polymer–Drug Conjugates as Nano-Sized Multi-Targeting Systems for the Treatment of Alzheimer’s Disease. RSC Pharm. 2024, 1, 161–181. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric Drugs for Efficient Tumor-Targeted Drug Delivery Based on EPR-Effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Goldspiel, B.R.; Kohler, D.R. Goserelin Acetate Implant: A Depot Luteinizing Hormone-Releasing Hormone Analog for Advanced Prostate Cancer. DICP 1991, 25, 796–804. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-Platinate: A Novel Approach to Cancer Chemotherapy. Anticancer Drugs 1999, 10, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and Pharmacokinetic Study of Genexol-PM, a Cremophor-Free, Polymeric Micelle-Formulated Paclitaxel, in Patients with Advanced Malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I Dose Escalation and Pharmacokinetic Study of Pluronic Polymer-Bound Doxorubicin (SP1049C) in Patients with Advanced Cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer Nano-Delivery Systems Based on Bovine Serum Albumin Nanoparticles: A Critical Review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, S. Protein Nanocarriers for the Delivery of Phytoconstituents. In Nanotechnology Based Delivery of Phytoconstituents and Cosmeceuticals; Pooja, D., Kulhari, H., Eds.; Springer: Singapore, 2024; pp. 229–264. ISBN 978-981-99-5314-1. [Google Scholar]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The Landscape of Nanoparticle-Based SiRNA Delivery and Therapeutic Development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Gómez-Díaz, R.A.; Corral, P. New Therapies for Primary Hyperlipidemia. J. Clin. Endocrinol. Metab. 2022, 107, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.M.; Badkar, A.V.; Cirelli, D.; Combs, R.; Lerch, T.F. The Race to Develop the Pfizer-BioNTech COVID-19 Vaccine: From the Pharmaceutical Scientists’ Perspective. J. Pharm. Sci. 2023, 112, 640–647. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Cabral, H.; Li, J.; Miyata, K.; Kataoka, K. Controlling the Biodistribution and Clearance of Nanomedicines. Nat. Rev. Bioeng. 2024, 2, 214–232. [Google Scholar] [CrossRef]
- Mok, Z.H. The Effect of Particle Size on Drug Bioavailability in Various Parts of the Body. Pharm. Sci. Adv. 2024, 2, 100031. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical Approaches to Biomaterial Design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Göppert, T.M.; Müller, R.H. Polysorbate-Stabilized Solid Lipid Nanoparticles as Colloidal Carriers for Intravenous Targeting of Drugs to the Brain: Comparison of Plasma Protein Adsorption Patterns. J. Drug Target 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Patel, H.M. Serum-Mediated Recognition of Liposomes by Phagocytic Cells of the Reticuloendothelial System-The Concept of Tissue Specificity. Adv. Drug Deliv. Rev. 1998, 32, 45–60. [Google Scholar] [CrossRef]
- Wang, L.; Quine, S.; Frickenstein, A.N.; Lee, M.; Yang, W.; Sheth, V.M.; Bourlon, M.D.; He, Y.; Lyu, S.; Garcia-Contreras, L. Exploring and Analyzing the Systemic Delivery Barriers for Nanoparticles. Adv. Funct. Mater. 2024, 34, 2308446. [Google Scholar] [CrossRef] [PubMed]
- Awogbemi, O.; Von Kallon, D.V. Recent Advances in the Application of Nanomaterials for Improved Biodiesel, Biogas, Biohydrogen, and Bioethanol Production. Fuel 2024, 358, 130261. [Google Scholar] [CrossRef]
- Haider, A.; Ikram, M.; Rafiq, A. Properties of Nanomaterials. In Green Nanomaterials as Potential Antimicrobials; Springer: Berlin/Heidelberg, Germany, 2022; pp. 47–59. [Google Scholar]
- Hheidari, A.; Mohammadi, J.; Ghodousi, M.; Mahmoodi, M.; Ebrahimi, S.; Pishbin, E.; Rahdar, A. Metal-Based Nanoparticle in Cancer Treatment: Lessons Learned and Challenges. Front. Bioeng. Biotechnol. 2024, 12, 1436297. [Google Scholar] [CrossRef]
- Desai, N.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic Nanoparticles as Drug Delivery System for the Treatment of Cancer. Expert Opin. Drug Deliv. 2021, 18, 1261–1290. [Google Scholar] [CrossRef]
- Chauhan, S.; Solanki, R.; Jangid, A.K.; Jain, P.; Pranjali, P.; Patel, S.; Guleria, A.; Pooja, D.; Kulhari, H. Manganese Nanocarrier for Matrix Metalloproteinase 9 Responsive Delivery of Irinotecan for Colon Cancer Treatment. J. Ind. Eng. Chem. 2023, 128, 258–267. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Trubetskoy, V.S. Which Polymers Can Make Nanoparticulate Drug Carriers Long-Circulating? Adv. Drug Deliv. Rev. 1995, 16, 141–155. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic Block Copolymers for Drug Delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Allouni, Z.E.; Cimpan, M.R.; Høl, P.J.; Skodvin, T.; Gjerdet, N.R. Agglomeration and Sedimentation of TiO2 Nanoparticles in Cell Culture Medium. Colloids Surf. B Biointerfaces 2009, 68, 83–87. [Google Scholar] [CrossRef]
- Taylor, R.A.; Jalali, M.; Marti, J.; Booth, T.J.; Baker, M.A.B. Nanotechnology: Creating, Manipulating, and Observing Nanostructured Systems in Biology and Medicine. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 727–747. [Google Scholar]
- Zain, M.; Yasmeen, H.; Yadav, S.S.; Amir, S.; Bilal, M.; Shahid, A.; Khurshid, M. Applications of Nanotechnology in Biological Systems and Medicine. In Nanotechnology for Hematology, Blood Transfusion, and Artificial Blood; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–235. [Google Scholar]
- Ma, X.; Zhao, Y.; Liang, X.-J. Theranostic Nanoparticles Engineered for Clinic and Pharmaceutics. Acc. Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Erkoc, P.; Ulucan-Karnak, F. Nanotechnology-Based Antimicrobial and Antiviral Surface Coating Strategies. Prosthesis 2021, 3, 25–52. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport, and Fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Solanki, R.; Bhatia, D. Stimulus-Responsive Hydrogels for Targeted Cancer Therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational Design of Multi-Stimuli-Responsive Nanoparticles for Precise Cancer Therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- Castillo, B.; Bromberg, L.; López, X.; Badillo, V.; González Feliciano, J.A.; González, C.I.; Hatton, T.A.; Barletta, G. Intracellular Delivery of SiRNA by Polycationic Superparamagnetic Nanoparticles. J. Drug Deliv. 2012, 2012, 218940. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Redox-Responsive Magnetic Nanoparticle for Targeted Convection-Enhanced Delivery of O6-Benzylguanine to Brain Tumors. ACS Nano 2014, 8, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Park, C.; Lee, H.; Park, H.J.; Kim, C. Glutathione-Induced Intracellular Release of Guests from Mesoporous Silica Nanocontainers with Cyclodextrin Gatekeepers. Adv. Mater. 2010, 22, 4280–4283. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in Redox-Responsive Drug Delivery Systems of Tumor Microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Choi, Y. Graphene Oxide–Photosensitizer Conjugate as a Redox-Responsive Theranostic Agent. Chem. Commun. 2012, 48, 9912. [Google Scholar] [CrossRef]
- Qian, C.; Yu, J.; Chen, Y.; Hu, Q.; Xiao, X.; Sun, W.; Wang, C.; Feng, P.; Shen, Q.; Gu, Z. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef]
- Zheng, X.; Tang, H.; Xie, C.; Zhang, J.; Wu, W.; Jiang, X. Tracking Cancer Metastasis In Vivo by Using an Iridium-Based Hypoxia-Activated Optical Oxygen Nanosensor. Angew. Chem. Int. Ed. 2015, 54, 8094–8099. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Bleich, H.L.; Moore, M.J.; Rodbard, D. The Role of Regional Body Temperature in the Pathogenesis of Disease. N. Engl. J. Med. 1981, 305, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hao, J.; Lee, L.A.; Biewer, M.C.; Wang, Q.; Stefan, M.C. Thermally Controlled Release of Anticancer Drug from Self-Assembled γ-Substituted Amphiphilic Poly(ε-Caprolactone) Micellar Nanoparticles. Biomacromolecules 2012, 13, 2163–2173. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Xu, L.; Gu, Y. The Targeted Behavior of Thermally Responsive Nanohydrogel Evaluated by NIR System in Mouse Model. J. Control. Release 2008, 131, 34–40. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, T.; Yang, W.; Zou, Y.; Wu, H.; Liu, X.; Zhu, F.; Qian, R.; Ling, D.; McDonald, K.; et al. The SiRNAsome: A Cation-Free and Versatile Nanostructure for SiRNA and Drug Co-delivery. Angew. Chem. Int. Ed. 2019, 58, 4938–4942. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Liu, G.; Hu, J.; Liu, S. Photo- and Thermo-Responsive Multicompartment Hydrogels for Synergistic Delivery of Gemcitabine and Doxorubicin. J. Control. Release 2017, 259, 149–159. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and Pseudo-Stealth Nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Abuchowski, A.; Van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of Immunological Properties of Bovine Serum Albumin by Covalent Attachment of Polyethylene Glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Illum, L.; Davis, S.S.; Müller, R.H.; Mak, E.; West, P. The Organ Distribution and Circulation Time of Intravenously Injected Colloidal Carriers Sterically Stabilized with a Blockcopolymer-Poloxamine 908. Life Sci. 1987, 40, 367–374. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S. Enzymatically Transformable Polymersome-Based Nanotherapeutics to Eliminate Minimal Relapsable Cancer. Adv. Mater. 2021, 33, 2105254. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Hazarika, P.; Dhanya, S.J.; Pooja, D.; Kulhari, H. Exploration of Sialic Acid Receptors as a Potential Target for Cancer Treatment: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 257, 128415. [Google Scholar] [CrossRef]
- Solanki, R.; Parmar, B.; Jadav, M.; Pooja, D.; Kulhari, H.; Patel, S. Berberine Encapsulated Phenylboronic Acid-Conjugated Pullulan Nanoparticles: Synthesis, Characterization and Anticancer Activity Validated in A431 Skin Cancer Cells and 3D Spheroids. Int. J. Biol. Macromol. 2024, 273, 132737. [Google Scholar] [CrossRef]
- Jangid, A.K.; Solanki, R.; Ghosh, M.; Jadav, M.; Patel, S.; Pooja, D.; Kulhari, H. Phenylboronic Acid Conjugated PAMAM G4 Dendrimers Augmented Usnic Acid Delivery to Gastric Cancer Cells. Eur. Polym. J. 2023, 192, 112073. [Google Scholar] [CrossRef]
- Jangid, A.K.; Solanki, R.; Jadav, M.; Bora, S.; Patel, S.; Pooja, D.; Kulhari, H. Phenyl Boronic Acid-PEG-Stearic Acid Biomaterial-Based and Sialic Acid Targeted Nanomicelles for Colon Cancer Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130445. [Google Scholar] [CrossRef]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent Advances in Folic Acid Engineered Nanocarriers for Treatment of Breast Cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Solanki, R.; Jangid, A.K.; Jadav, M.; Kulhari, H.; Patel, S. Folate Functionalized and Evodiamine-Loaded Pluronic Nanomicelles for Augmented Cervical Cancer Cell Killing. Macromol. Biosci. 2023, 23, 2300077. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Srivastav, A.K.; Patel, S.; Singh, S.K.; Jodha, B.; Kumar, U.; Patel, S. Folate Conjugated Albumin as a Targeted Nanocarrier for the Delivery of Fisetin: In Silico and in Vitro Biological Studies. RSC Adv. 2024, 14, 7338–7349. [Google Scholar] [CrossRef]
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Stability of Therapeutic Nano-Drugs during Storage and Transportation as Well as after Ingestion in the Human Body. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–102. [Google Scholar]
- Hu, X.; Liu, S.; Huang, Y.; Chen, X.; Jing, X. Biodegradable Block Copolymer-Doxorubicin Conjugates via Different Linkages: Preparation, Characterization, and in Vitro Evaluation. Biomacromolecules 2010, 11, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable Nanoparticles for Drug Delivery and Targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, R.; Li, C.; Qiao, R. Self-Assembled Polypeptide-Block-Poly (Vinylpyrrolidone) as Prospective Drug-Delivery Systems. Colloids Surf. B Biointerfaces 2009, 74, 284–292. [Google Scholar] [CrossRef]
- Gilding, D.K.; Reed, A.M. Biodegradable Polymers for Use in Surgery—Polyglycolic/Poly(Actic Acid) Homo- and Copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Duncan, R. Polymer Conjugates as Anticancer Nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional Micellar Nanomedicine for Cancer Therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef]
- Mao, C.Q.; Du, J.Z.; Sun, T.M.; Yao, Y.D.; Zhang, P.Z.; Song, E.W.; Wang, J. A Biodegradable Amphiphilic and Cationic Triblock Copolymer for the Delivery of SiRNA Targeting the Acid Ceramidase Gene for Cancer Therapy. Biomaterials 2011, 32, 3124–3133. [Google Scholar] [CrossRef]
- Park, M.R.; Han, K.O.; Han, I.K.; Cho, M.H.; Nah, J.W.; Choi, Y.J.; Cho, C.S. Degradable Polyethylenimine-Alt-Poly(Ethylene Glycol) Copolymers as Novel Gene Carriers. J. Control. Release 2005, 105, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Tam, Y.T.; Gao, J.; Kwon, G.S. Oligo(Lactic Acid)n-Paclitaxel Prodrugs for Poly(Ethylene Glycol)-Block-Poly(Lactic Acid) Micelles: Loading, Release, and Backbiting Conversion for Anticancer Activity. J. Am. Chem. Soc. 2016, 138, 8674–8677. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA Micelles and PLGA-b-PEG-b-PLGA Sol–Gels for Drug Delivery. J. Control. Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What Has Been Done and the Challenges Remain Ahead. Int. J. Pharm. 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Negishi, T.; Koizumi, F.; Uchino, H.; Kuroda, J.; Kawaguchi, T.; Naito, S.; Matsumura, Y. NK105, a Paclitaxel-Incorporating Micellar Nanoparticle, Is a More Potent Radiosensitising Agent Compared to Free Paclitaxel. Br. J. Cancer 2006, 95, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a Paclitaxel-Incorporating Micellar Nanoparticle Formulation, Can Extend in Vivo Antitumour Activity and Reduce the Neurotoxicity of Paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef]
- Yardley, D.A. Nab-Paclitaxel Mechanisms of Action and Delivery. J. Control. Release 2013, 170, 365–372. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Wee Ma, W.; Saleh, N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 18, 1691–1703. [Google Scholar] [CrossRef]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.-F.; Zhou, X. Lipid-Based Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Basu, B.; Prajapati, B.; Dutta, A.; Paliwal, H. Medical Application of Liposomes. J. Explor. Res. Pharmacol. 2024, 9, 30–39. [Google Scholar] [CrossRef]
- Wagner, A.; Platzgummer, M.; Kreismayr, G.; Quendler, H.; Stiegler, G.; Ferko, B.; Vecera, G.; Vorauer-Uhl, K.; Prof, H.K. GMP Production of Liposomes--a New Industrial Approach. J. Liposome Res. 2006, 16, 311–319. [Google Scholar] [CrossRef]
- Weiner, N.; Martin, F.; Riaz, M. Liposomes as a Drug Delivery System. Drug Dev. Ind. Pharm. 1989, 15, 1523–1554. [Google Scholar] [CrossRef]
- Anderson, M.; Omri, A. The Effect of Different Lipid Components on the in Vitro Stability and Release Kinetics of Liposome Formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Passero, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The Safety and Efficacy of Onivyde (Irinotecan Liposome Injection) for the Treatment of Metastatic Pancreatic Cancer Following Gemcitabine-Based Therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Sharma, S. Dendrimers in Nanomedicine: History, Concept and Properties of Dendrimers. In Dendrimers in Nanomedicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 41–51. [Google Scholar]
- Rastogi, V.; Yadav, P.; Porwal, M.; Sur, S.; Verma, A. Dendrimer as Nanocarrier for Drug Delivery and Drug Targeting Therapeutics: A Fundamental to Advanced Systematic Review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 310–332. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging Concepts in Dendrimer-Based Nanomedicine: From Design Principles to Clinical Applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Pavan, G.M.; Posocco, P.; Tagliabue, A.; Maly, M.; Malek, A.; Danani, A.; Ragg, E.; Catapano, C.V.; Pricl, S. PAMAM Dendrimers for SiRNA Delivery: Computational and Experimental Insights. Chem.—A Eur. J. 2010, 16, 7781–7795. [Google Scholar] [CrossRef]
- Jia, L.; Xu, J.-P.; Wang, H.; Ji, J. Polyamidoamine Dendrimers Surface-Engineered with Biomimetic Phosphorylcholine as Potential Drug Delivery Carriers. Colloids Surf. B Biointerfaces 2011, 84, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shao, J.; Jin, Q.; Wei, Q.; Tang, J.; Ji, J. Zwitterionic Phosphorylcholine as a Better Ligand for Gold Nanorods Cell Uptake and Selective Photothermal Ablation of Cancer Cells. Chem. Commun. 2010, 46, 1479–1481. [Google Scholar] [CrossRef]
- Mccarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as Drugs: Discovery and Preclinical and Clinical Development of Dendrimer-Based Microbicides for HIV and STI Prevention. Mol. Pharm. 2005, 2, 312–318. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Millwood, I.Y.; McDonald, H.M.; Price, C.F.; Kaldor, J.M.; Paull, J.R.A. Safety, Tolerability, and Pharmacokinetics of SPL7013 Gel (VivaGel®): A Dose Ranging, Phase I Study. Sex Transm. Dis. 2010, 37, 100–104. [Google Scholar] [CrossRef]
- Wrońska, N.; Majoral, J.P.; Appelhans, D.; Bryszewska, M.; Lisowska, K. Synergistic Effects of Anionic/Cationic Dendrimers and Levofloxacin on Antibacterial Activities. Molecules 2019, 24, 2894. [Google Scholar] [CrossRef]
- Zeng, Z.; Qi, D.; Yang, L.; Liu, J.; Tang, Y.; Chen, H.; Feng, X. Stimuli-Responsive Self-Assembled Dendrimers for Oral Protein Delivery. J. Control. Release 2019, 315, 206–213. [Google Scholar] [CrossRef]
- Bae, Y.; Thuy, L.T.; Lee, Y.H.; Ko, K.S.; Han, J.; Choi, J.S. Polyplexes of functional PAMAM dendrimer/apoptin gene induce apoptosis of human primary glioma cells in vitro. Polymers 2019, 11, 296. [Google Scholar] [CrossRef]
- Seixas, N.; Ravanello, B.B.; Morgan, I.; Kaluđerović, G.N.; Wessjohann, L.A. Chlorambucil conjugated Ugi dendrimers with PAMAM-NH2 core and evaluation of their anticancer activity. Pharmaceutics 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Thuy, L.T.; Choi, M.; Lee, M.; Choi, J.S. Preparation and Characterization of Polyamidoamine Dendrimers Conjugated with Cholesteryl-Dipeptide as Gene Carriers in HeLa Cells. J. Biomater. Sci. Polym. Ed. 2022, 33, 976–994. [Google Scholar] [CrossRef]
- Cañas-Arranz, R.; de León, P.; Forner, M.; Defaus, S.; Bustos, M.J.; Torres, E.; Andreu, D.; Blanco, E.; Sobrino, F. Immunogenicity of a Dendrimer B2T Peptide Harboring a T-Cell Epitope From FMDV Non-Structural Protein 3D. Front. Vet. Sci. 2020, 7, 498. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, Classification and Properties of Hydrogels: Their Applications in Drug Delivery and Agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Malpure, P.S.; Patil, S.S.; More, Y.M.; Nikam, P.P. A Review On-Hydrogel. Am. J. PharmTech Res. 2018, 8, 42–60. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The Science of Hyaluronic Acid Dermal Fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef]
- Culver, H.R.; Wechsler, M.E.; Peppas, N.A. Label-Free Detection of Tear Biomarkers Using Hydrogel Coated Gold Nanoshells in a Localized Surface Plasmon Resonance-Based Biosensor HHS Public Access. ACS Nano 2018, 12, 9342–9354. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Vela Ramirez, J.E.; Peppas, N.A. Uptake and Function of Membrane-destabilizing Cationic Nanogels for Intracellular Drug Delivery. Bioeng. Transl. Med. 2019, 4, 17–29. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Vela Ramirez, J.E.; Peppas, N.A. Cytoplasmic Delivery of Functional SiRNA Using PH-Responsive Nanoscale Hydrogels. Int. J. Pharm. 2019, 562, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging Trends in Nanomedicine: Carbon-Based Nanomaterials for Healthcare. Nanomaterials 2024, 14, 1085. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef]
- Hyder, A.; Ali, A.; Buledi, J.A.; Memon, A.A.; Iqbal, M.; Bangalni, T.H.; Solangi, A.R.; Thebo, K.H.; Akhtar, J. Nanodiamonds: A Cutting-Edge Approach to Enhancing Biomedical Therapies and Diagnostics in Biosensing. Chem. Rec. 2024, 24, e202400006. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The Properties and Applications of Nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.-W.; Lin, H.-H.; Chang, H.-C. Diamond Nanoparticles for Drug Delivery and Monitoring. In Carbon-Based Nanosensor Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 119–140. [Google Scholar]
- Rosenholm, J.M.; Vlasov, I.I.; Burikov, S.A.; Dolenko, T.A.; Shenderova, O.A. Nanodiamond-Based Composite Structures for Biomedical Imaging and Drug Delivery. J. Nanosci. Nanotechnol. 2014, 15, 959–971. [Google Scholar] [CrossRef]
- Su, L.-J.; Wu, M.-S.; Hui, Y.Y.; Chang, B.-M.; Pan, L.; Hsu, P.-C.; Chen, Y.-T.; Ho, H.-N.; Huang, Y.-H.; Ling, T.-Y.; et al. Fluorescent Nanodiamonds Enable Quantitative Tracking of Human Mesenchymal Stem Cells in Miniature Pigs. Sci. Rep. 2017, 7, 45607. [Google Scholar] [CrossRef]
- Boon Toh, T.; Jieh Lim, J.; Kai-Hua Chow, E. Epigenetics in Cancer Stem Cells. Mol. Cancer 2017, 16, 29. [Google Scholar] [CrossRef]
- Giammarco, J.; Mochalin, V.N.; Haeckel, J.; Gogotsi, Y. The Adsorption of Tetracycline and Vancomycin onto Nanodiamond with Controlled Release. J. Colloid Interface Sci. 2016, 468, 253–261. [Google Scholar] [CrossRef]
- Chen, M.; Pierstorff, E.D.; Lam, R.; Li, S.Y.; Huang, H.; Osawa, E.; Ho, D. Nanodiamond-Mediated Delivery of Water-Insoluble Therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef]
- Lim, D.G.; Jung, J.H.; Ko, H.W.; Kang, E.; Jeong, S.H. Paclitaxel-Nanodiamond Nanocomplexes Enhance Aqueous Dispersibility and Drug Retention in Cells. ACS Appl. Mater. Interfaces 2016, 8, 23558–23567. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3, 3001713. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mumper, R.J. Nanomedicinal Strategies to Treat Multidrug-Resistant Tumors: Current Progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef]
- Wang, H.; Lee, D.K.; Chen, K.Y.; Chen, J.Y.; Zhang, K.; Silva, A.; Ho, C.M.; Ho, D. Mechanism-Independent Optimization of Combinatorial Nanodiamond and Unmodified Drug Delivery Using a Phenotypically Driven Platform Technology. ACS Nano 2015, 9, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Coonrod, A.; Li, F.Q.; Horwitz, M. On the Mechanism of DNA Transfection: Efficient Gene Transfer without Viruses. Gene Ther. 1997, 4, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.Q.; Man, H.B.; Lam, R.; Chow, E.K.; Ho, D. Nanodiamond Vectors Functionalized with Polyethylenimine for SiRNA Delivery. J. Phys. Chem. Lett. 2010, 1, 3167–3171. [Google Scholar] [CrossRef]
- Alhaddad, A.; Adam, M.P.; Botsoa, J.; Dantelle, G.; Perruchas, S.; Gacoin, T.; Mansuy, C.; Lavielle, S.; Malvy, C.; Treussart, F.; et al. Nanodiamond as a Vector for SiRNA Delivery to Ewing Sarcoma Cells. Small 2011, 7, 3087–3095. [Google Scholar] [CrossRef]
- Alwani, S.; Kaur, R.; Michel, D.; Chitanda, J.M.; Verrall, R.E.; Karunakaran, C.; Badea, I. Lysine-Functionalized Nanodiamonds as Gene Carriers: Development of Stable Colloidal Dispersion for in Vitro Cellular Uptake Studies and SiRNA Delivery Application. Int. J. Nanomed. 2016, 11, 687. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, M.; Lam, R.; Xu, X.; Osawa, E.; Ho, D. Polymer-Functionalized Nanodiamond Platforms as Vehicles for Gene Delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef]
- Mondal, J.; Lamba, R.; Yukta, Y.; Yadav, R.; Kumar, R.; Pani, B.; Singh, B. Advancements in Semiconductor Quantum Dots: Expanding Frontiers in Optoelectronics, Analytical Sensing, Biomedicine, and Catalysis. J. Mater. Chem. C Mater. 2024, 12, 10330–10389. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum Dots in Biomedical Applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Girija Aswathy, R.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Near-Infrared Quantum Dots for Deep Tissue Imaging. Anal. Bioanal. Chem. 2003, 397, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Palui, G.; Na, H.B. Luminescent Quantum Dots as Platforms for Probing in Vitro and in Vivo Biological Processes. Adv. Drug Deliv. Rev. 2012, 64, 138–166. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Wang, M.; Zhang, Y.; Chen, G.; Li, L.; Wu, D.; Wang, Q. In Vivo Real-Time Visualization of Tissue Blood Flow and Angiogenesis Using Ag2S Quantum Dots in the NIR-II Window. Biomaterials 2014, 35, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Ke, Y.; Yan, H.; Wang, Q.; Liu, Y.; Yan, H.; Ke, Y. Quantum Dot Bioconjugation during Core-Shell Synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 316. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Zheng, W.; Cong, L.; Guo, Z.; Xie, Y.; Wang, L.; Tang, R.; Feng, Q.; Hamada, Y.; et al. Thermo-Triggered Release of CRISPR-Cas9 System by Lipid-Encapsulated Gold Nanoparticles for Tumor Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 1491–1496. [Google Scholar] [CrossRef]
- Yao, J.; Fan, Y.; Li, Y.; Huang, L. Strategies on the Nuclear-Targeted Delivery of Genes. J. Drug Target 2013, 21, 926–939. [Google Scholar] [CrossRef]
- Medintz, I.L.; Mattoussi, H.; Clapp, A.R.; Clapp, A.; Medintz, I. Potential Clinical Applications of Quantum Dots. Int. J. Nanomed. 2008, 3, 151–167. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Islam, M.A.; Hasan, M.; Rahman, M.; Mobarak, M.H.; Mimona, M.A.; Hossain, N. Advances and Significances of Carbon Nanotube Applications: A Comprehensive Review. Eur. Polym. J. 2024, 220, 113443. [Google Scholar] [CrossRef]
- Gao, S.; Xu, B.; Sun, J.; Zhang, Z. Nanotechnological Advances in Cancer: Therapy a Comprehensive Review of Carbon Nanotube Applications. Front. Bioeng. Biotechnol. 2024, 12, 1351787. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Naik, S.S.; Lee, S.J.; Theerthagiri, J.; Yu, Y.; Choi, M.Y. Rapid and Highly Selective Electrochemical Sensor Based on ZnS/Au-Decorated f-Multi-Walled Carbon Nanotube Nanocomposites Produced via Pulsed Laser Technique for Detection of Toxic Nitro Compounds. J. Hazard Mater. 2021, 418, 126269. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Luo, X.J. Proteome-Wide Association Study Provides Insights into the Genetic Component of Protein Abundance in Psychiatric Disorders. Biol. Psychiatry 2021, 90, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kucukayan-Dogu, G.; Gozen, D.; Bitirim, V.; Akcali, K.C.; Bengu, E. A New Tool for Differentiating Hepatocellular Cancer Cells: Patterned Carbon Nanotube Arrays. Appl. Surf. Sci. 2015, 351, 27–32. [Google Scholar] [CrossRef]
- Kim, M.; Chen, C.; Wang, P.; Mulvey, J.J.; Yang, Y.; Wun, C.; Antman-Passig, M.; Luo, H.-B.; Cho, S.; Long-Roche, K. Detection of Ovarian Cancer via the Spectral Fingerprinting of Quantum-Defect-Modified Carbon Nanotubes in Serum by Machine Learning. Nat. Biomed. Eng. 2022, 6, 267–275. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Z.; Wang, P.; Mu, T.; Qin, H.; Zhu, Z.; Wang, J.; Sui, L. A Large-Inner-Diameter Multi-Walled Carbon Nanotube-Based Dual-Drug Delivery System with PH-Sensitive Release Properties. J. Mater. Sci. Mater. Med. 2017, 28, 110. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.Y.; Park, J.S.; Park, M.; Park, M.C. Antimicrobial and Antibiofilm Activities of Urinary Catheter Incorporated with ZnO-Carbon Nanotube. ACS Appl. Bio Mater. 2025, 8, 1397–1405. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Ru, Y.; Zhang, X.; Luo, F.; Chen, X.; Zhao, X.; Wang, C. Gold Nanoparticle-Modified Single-Walled Carbon Nanotube Terahertz Metasurface for Ultrasensitive Sensing of Trace Proteins. Talanta 2025, 286, 127549. [Google Scholar] [CrossRef]
- Patel, J.; Kumar, G.S.; Roy, H.; Maddiboyina, B.; Leporatti, S.; Bohara, R.A. From Nature to Nanomedicine: Bioengineered Metallic Nanoparticles Bridge the Gap for Medical Applications. Discover. Nano 2024, 19, 85. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, D.N.; Ion, D.; Niculescu, A.-G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Georgakilas, A.G. Editorial: Metal Nanoparticles in Cancer: Detection, Diagnosis, Therapy and Their Pharmacological Assessment. Front. Oncol. 2023, 13, 1344197. [Google Scholar] [CrossRef] [PubMed]
- Roshani, M.; Rezaian-Isfahni, A.; Lotfalizadeh, M.H.; Khassafi, N.; Abadi, M.H.J.N.; Nejati, M. Metal Nanoparticles as a Potential Technique for the Diagnosis and Treatment of Gastrointestinal Cancer: A Comprehensive Review. Cancer Cell Int. 2023, 23, 1–23. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Singh, S.; Singh, A. Utilizing Nanomaterials for Cancer Treatment and Diagnosis: An Overview. Discov. Nano 2024, 19, 1–32. [Google Scholar] [CrossRef]
- Fernandes, D.A. Multifunctional Gold Nanoparticles for Cancer Theranostics. 3 Biotech 2024, 14, 1–31. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- El-Boubbou, K. Magnetic Iron Oxide Nanoparticles As Drug Carriers: Clinical Relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy Using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kataoka, K. Chemo-Physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The next Generation. J. Am. Chem. Soc. 2020, 143, 538–559. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of Nanomaterials and Biological Systems: Implications to Personalized Nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA Damaging Potential of Engineered Nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef] [PubMed]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 2019, 21, 347–355. [Google Scholar] [CrossRef]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Göbbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity Screening of 23 Engineered Nanomaterials Using a Test Matrix of Ten Cell Lines and Three Different Assays. Part. Fibre Toxicol. 2011, 8, 9. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Dickinson, A.M.; Godden, J.M.; Lanovyk, K.; Ahmed, S.S. Assessing the Safety of Nanomedicines: A Mini Review. Appl. In Vitro Toxicol. 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The Magic Bullets Reaching Their Target? Eur J. Pharm. Sci. 2019, 128, 73–80. [Google Scholar] [CrossRef]
- Siegrist, S.; Cörek, E.; Detampel, P.; Sandström, J.; Wick, P.; Huwyler, J. Preclinical Hazard Evaluation Strategy for Nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef]
- Rannard, S.; Owen, A. Nanomedicine: Not a Case of “One Size Fits All”. Nano Today 2009, 4, 382–384. [Google Scholar] [CrossRef]
- Ali, F. Regulatory Perspectives of Nanomaterials for Theranostic Application. In Nanotheranostics for Treatment and Diagnosis of Infectious Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 373–384. [Google Scholar] [CrossRef]
| Name | Drug | Nanocarrier | Application | Manufacturing Company |
|---|---|---|---|---|
| Myocet® | Doxorubicin | Liposome | Breast cancer | Teva |
| Marqibo® | Vincristine sulfate | Liposome | Acute lymphoblastic leukemia (ALL) | Talon Therapeutics |
| Ambisome | Amphotericin B | Liposome | Fungal infection | Gilead Sciences |
| Onivyde® | Irinotecan | Liposome | Pancreatic cancer | Merrimack Pharmaceuticals |
| Doxil® | Doxorubicin | Liposome | Various cancers, ALL, AML, breast cancer, ovarian cancer | Johnson & Johnson |
| Genexol PM® | Paclitaxel | mPEG–PLA | Metastatic breast cancer | Samyang Corporation |
| Eligard® | Leuprolide acetate | PLGA | Prostate cancer | Tolmar |
| Vyxeos® | Cytarabine and daunorubicin | Liposome | AML | Jazz Pharmaceuticals |
| Abraxane® | Paclitaxel | Albumin | Various cancers, breast cancer | Abraxis BioScience |
| Name | Drug | Nanocarrier | Application in | Manufacture Company |
|---|---|---|---|---|
| Genexol-PM® | Paclitaxel | Polymeric micelles | Breast, lung, and ovarian cancer | Samyang |
| NK-105® | Paclitaxel | Micelle: PEG– polyaspartate | Metastatic breast cancer | Nippon Kayaku Co. |
| NanoTherm | Aminosilane- coated SPIONs | Metallic nanoparticle | GBM and prostate cancer | MagForce Nanotechnologies |
| ThermoDox | Doxorubicin | Thermosensitive liposome | Hepatocellular carcinoma | Celsion |
| Lipoplatin® | Cisplatin | Liposome | Breast, pancreatic, urinary bladder, and gastrointestinal cancer | Regulon Inc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Shahvej, S.; Yadav, P.; Modi, U.; Yadav, A.K.; Solanki, R.; Bhatia, D. Clinical Applications of Targeted Nanomaterials. Pharmaceutics 2025, 17, 379. https://doi.org/10.3390/pharmaceutics17030379
Kumar A, Shahvej S, Yadav P, Modi U, Yadav AK, Solanki R, Bhatia D. Clinical Applications of Targeted Nanomaterials. Pharmaceutics. 2025; 17(3):379. https://doi.org/10.3390/pharmaceutics17030379
Chicago/Turabian StyleKumar, Ankesh, SK Shahvej, Pankaj Yadav, Unnati Modi, Amit K. Yadav, Raghu Solanki, and Dhiraj Bhatia. 2025. "Clinical Applications of Targeted Nanomaterials" Pharmaceutics 17, no. 3: 379. https://doi.org/10.3390/pharmaceutics17030379
APA StyleKumar, A., Shahvej, S., Yadav, P., Modi, U., Yadav, A. K., Solanki, R., & Bhatia, D. (2025). Clinical Applications of Targeted Nanomaterials. Pharmaceutics, 17(3), 379. https://doi.org/10.3390/pharmaceutics17030379








