Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases
Abstract
1. Introduction
2. Structure and Properties of Cyclodextrins
3. Chemical Modification of Cyclodextrins
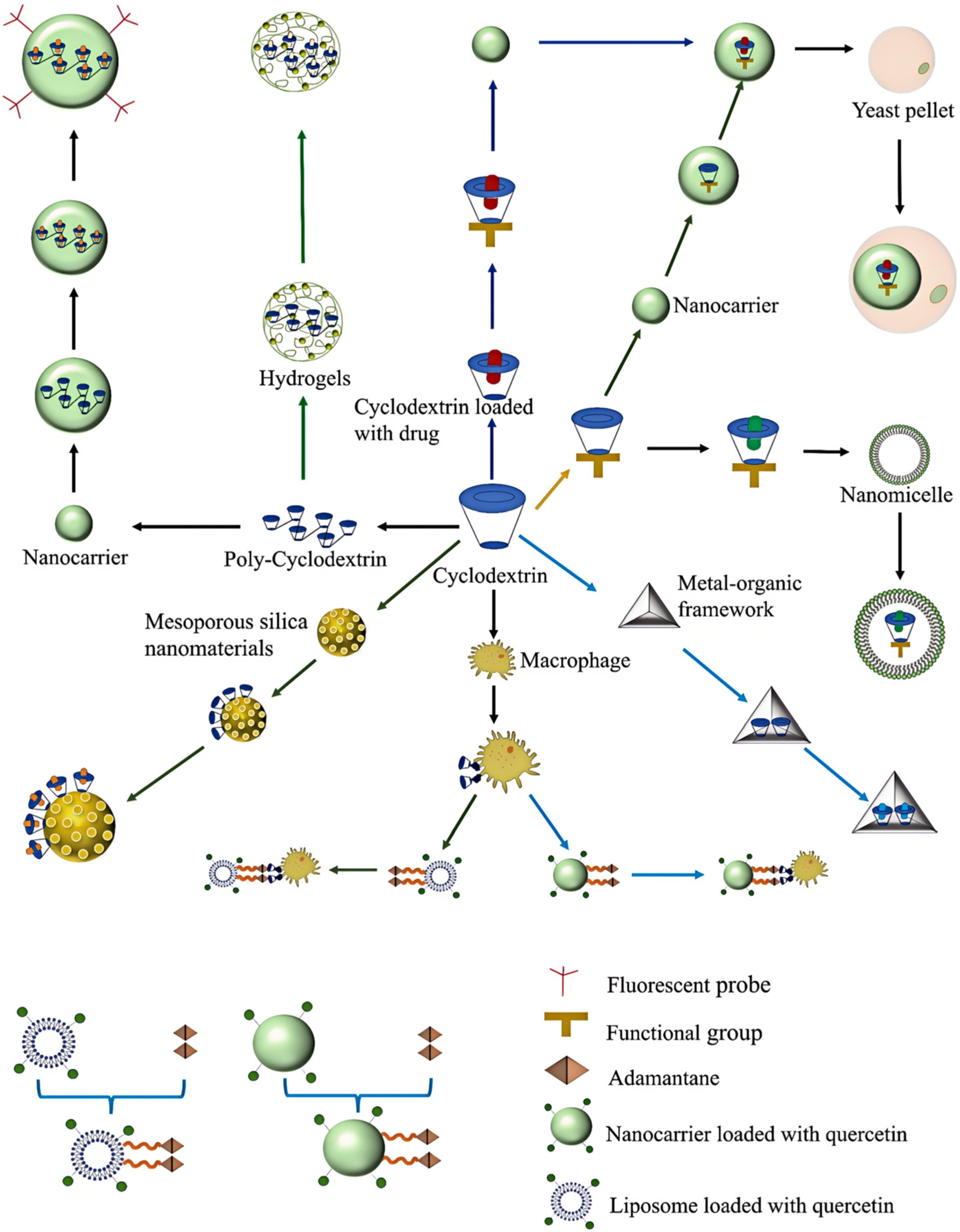
4. Nano-Delivery Systems Based on Cyclodextrins
4.1. Polymeric Nanosystems Based on Cyclodextrins
4.2. Graphene Derivative Delivery Systems Modified with Cyclodextrins
4.3. Cyclodextrin-Based Inorganic Nanoparticle Systems
4.4. Cyclodextrin–Liposome Systems
4.5. Other Nano-Delivery Systems Based on Cyclodextrins
5. Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases
5.1. Applications of Cyclodextrin-Based Delivery Systems in Respiratory Diseases
5.2. Applications of Cyclodextrin-Based Delivery Systems in Inflammatory Joint Diseases
5.3. Applications of Cyclodextrin-Based Delivery Systems in Inflammatory Bowel Diseases
5.4. Applications of Cyclodextrin-Based Delivery Systems in Inflammatory Eye Diseases
5.5. Applications of Cyclodextrin-Based Delivery Systems in Vascular Diseases
5.6. Applications of Cyclodextrin-Based Delivery Systems in Other Inflammatory Diseases
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dinarello, C.A. Anti-inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.K.; Behrens, T.W. Genetics of autoimmune diseases—Disorders of immune homeostasis. Nat. Rev. Genet. 2006, 7, 917–928. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Li, C.; Wang, Y.; Hu, Y.; Pu, W.; Zhang, Q.; Cai, J.; Lin, Y.; Li, G.; Wang, C.; et al. Pathologically triggered in situ aggregation of nanoparticles for inflammation-targeting amplification and therapeutic potentiation. Acta. Pharm. Sin. B 2023, 13, 390–409. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate immune receptors: Key regulators of metabolic disease progression. Cell Metab. 2013, 17, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [PubMed]
- Haley, R.M.; von Recum, H.A. Localized and targeted delivery of NSAIDs for treatment of inflammation: A review. Exp. Biol. Med. 2019, 244, 433–444. [Google Scholar] [CrossRef]
- Ye, J.; Gong, M.; Song, J.; Chen, S.; Meng, Q.; Shi, R.; Zhang, L.; Xue, J. Integrating Inflammation-Responsive Prodrug with Electrospun Nanofibers for Anti-Inflammation Application. Pharmaceutics 2022, 14, 1273. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Deng, Z.; Liu, G.; Hu, J.; Liu, S. Anti-inflammatory polymersomes of redox-responsive polyprodrug amphiphiles with inflammation-triggered indomethacin release characteristics. Biomaterials 2018, 178, 608–619. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Hammoud, Z.; Gharib, R.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Drug-in-hydroxypropyl-β-cyclodextrin-in-lipoid S100/cholesterol liposomes: Effect of the characteristics of essential oil components on their encapsulation and release. Int. J. Pharm. 2020, 579, 119151. [Google Scholar] [CrossRef]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 2007, 123, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Devika, V.; Sreelekshmi, P.J.; Gopalakrishnapai, R.; Archana, T.S.; Kavya, K.S.; Nair, P.B.; Velagaleti, C.S.L.; Sadanandan, S. Stimuli-responsive cyclodextrin-based materials for biomedical applications. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Allawadhi, P.; Singh, V.; Govindaraj, K.; Khurana, I.; Sarode, L.P.; Navik, U.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K.; Khurana, A. Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: Focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydr. Polym. 2022, 281, 118923. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Cui, R.; Luo, X.; Dong, J.; Wang, H.; Song, C.; Zhou, Y.; Cui, S. Dual-Sensitive Carbohydrate-Based Nanosystem for Targeted Drug Delivery to Potentiate the Therapeutic Efficacy of Ulcerative Colitis. Int. J. Nanomed. 2024, 19, 8555–8572. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Gao, X.; Fu, J.; Hu, L. Application of cyclodextrin in food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 2627–2640. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Alshati, F.; Alahmed, T.A.A.; Sami, F.; Ali, M.S.; Majeed, S.; Murtuja, S.; Hasnain, M.S.; Ansari, M.T. Guest-host Relationship of Cyclodextrin and its Pharmacological Benefits. Curr. Pharm. Des. 2023, 29, 2853–2866. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Jansook, P.; Loftsson, T. Self-assembled γ-cyclodextrin as nanocarriers for enhanced ocular drug bioavailability. Int. J. Pharm. 2022, 618, 121654. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł. A Review of Applications of Solid-State Nuclear Magnetic Resonance (ssNMR) for the Analysis of Cyclodextrin-Including Systems. Int. J. Mol. Sci. 2023, 24, 3648. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Meng, B.; Chen, Q. Cyclodextrin-Containing Drug Delivery Systems and Their Applications in Neurodegenerative Disorders. Int. J. Mol. Sci. 2024, 25, 10834. [Google Scholar] [CrossRef] [PubMed]
- Gavel, P.K.; Kumar, N.; Parmar, H.S.; Das, A.K. Evaluation of a Peptide-Based Coassembled Nanofibrous and Thixotropic Hydrogel for Dermal Wound Healing. ACS Appl. Bio Mater. 2020, 3, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, S.; Ren, J.; Zhai, Y.; Dong, S.; Wang, E. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host−Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Kovacs, T.; Nagy, P.; Panyi, G.; Szente, L.; Varga, Z.; Zakany, F. Cyclodextrins: Only Pharmaceutical Excipients or Full-Fledged Drug Candidates? Pharmaceutics 2022, 14, 2559. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, X.; Tian, B.R. Selective modifications at the different positions of cyclodextrins: A review of strategies. Turk. J. Chem. 2020, 44, 261–278. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Wang, L.; Li, S.; Chu, S.; Wang, J.; Li, Y.; Hou, J.; Luo, Q.; Liu, J. Design of Cyclodextrin-Based Functional Systems for Biomedical Applications. Front. Chem. 2021, 9, 635507. [Google Scholar] [CrossRef]
- Vecsernyés, M.; Fenyvesi, F.; Bácskay, I.; Deli, M.A.; Szente, L.; Fenyvesi, É. Cyclodextrins, blood-brain barrier, and treatment of neurological diseases. Arch. Med. Res. 2014, 45, 711–729. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J.; Kis, G.L. Spontaneous opalescence of aqueous gamma-cyclodextrin solutions: Complex formation or self-aggregation? J. Pharm. Sci. 1998, 87, 778–781. [Google Scholar]
- Parrot-Lopez, H.; Perret, F.; Bertino-Ghera, B. Amphiphilic cyclodextrins and their applications. Preparation of nanoparticles based on amphiphilic cyclodextrins for biomedical applications. Ann. Pharm. Fr. 2010, 68, 12–26. [Google Scholar] [PubMed]
- Tian, B.; Jiayue, L. The classification and application of cyclodextrin polymers: A review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2020, 15, 311–325. [Google Scholar] [CrossRef]
- Liao, R.; Lv, P.; Wang, Q.; Zheng, J.; Feng, B.; Yang, B. Cyclodextrin-based biological stimuli-responsive carriers for smart and precision medicine. Biomater. Sci. 2017, 5, 1736–1745. [Google Scholar] [CrossRef]
- Yu, H.S.; Lee, E.S. Honeycomb-like pH-responsive γ-cyclodextrin electrospun particles for highly efficient tumor therapy. Carbohydr. Polym. 2020, 230, 115563. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Yao, X.; Mu, J.; Zeng, L.; Lin, J.; Nie, Z.; Jiang, X.; Huang, P. Stimuli-responsive cyclodextrin-based nanoplatforms for cancer treatment and theranostics. Mater. Horiz. 2019, 6, 846–870. [Google Scholar] [CrossRef]
- Sun, H.L.; Zhang, Y.M.; Chen, Y.; Liu, Y. Polyanionic Cyclodextrin Induced Supramolecular Nanoparticle. Sci. Rep. 2016, 6, 27. [Google Scholar] [CrossRef]
- Li, B.; Feng, Z.; He, L.; Li, W.; Wang, Q.; Liu, J.; Huang, J.; Zheng, Y.; Ma, Y.; Yang, X.; et al. Self-Assembled Supramolecular Nanoparticles for Targeted Delivery and Combination Chemotherapy. ChemMedChem 2018, 13, 2037–2044. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, structural and in-vitro characterization of β-cyclodextrin grafted L-phenylalanine functionalized graphene oxide nanocomposite: A versatile nanocarrier for pH-sensitive doxorubicin delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef]
- Wang, P.; Huang, C.; Xing, Y.; Fang, W.; Ren, J.; Yu, H.; Wang, G. NIR-Light- and pH-Responsive Graphene Oxide Hybrid Cyclodextrin-Based Supramolecular Hydrogels. Langmuir 2019, 35, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Pan, L.; Ning, K.; Fang, Y.; Muhitdinov, B.; Liu, E.; Huang, Y. Asiatic acid cyclodextrin inclusion micro-cocrystal for insoluble drug delivery and acute lung injury therapy enhancement. J. Nanobiotechnol. 2024, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Liang, Y.; Meng, Z.; Chen, X.; Lu, M.; Han, X.; Deng, X.; Zhang, Q.; Zhu, H.; Fu, T. Inhalation of Tetrandrine-hydroxypropyl-β-cyclodextrin Inclusion Complexes for Pulmonary Fibrosis Treatment. Mol. Pharm. 2020, 17, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, T.; Zhang, K.; Liu, Y.; Wang, C.; Wu, L.; Zhang, J. Cyclodextrin MOFs modified dry powder inhalers quadruple bioavailability of luteolin to ameliorate fibrosing interstitial lung disease. Int. J. Pharm. 2023, 645, 123405. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, L.; Li, M.; Hu, Y.; Zhang, E.; Jiang, Q.; Han, G.; Jin, Y. Inhalable Andrographolide-β-cyclodextrin Inclusion Complexes for Treatment of Staphylococcus aureus Pneumonia by Regulating Immune Responses. Mol. Pharm. 2017, 14, 1718–1725. [Google Scholar] [CrossRef]
- Cordaro, A.; Zagami, R.; Malanga, M.; Venkatesan, J.K.; Alvarez-Lorenzo, C.; Cucchiarini, M.; Piperno, A.; Mazzaglia, A. Cyclodextrin Cationic Polymer-Based Nanoassemblies to Manage Inflammation by Intra-Articular Delivery Strategies. Nanomaterials 2020, 10, 1712. [Google Scholar] [CrossRef]
- Han, X.; Luo, R.; Qi, S.; Wang, Y.; Dai, L.; Nie, W.; Lin, M.; He, H.; Ye, N.; Fu, C.; et al. “Dual sensitive supramolecular curcumin nanoparticles” in “advanced yeast particles” mediate macrophage reprogramming, ROS scavenging and inflammation resolution for ulcerative colitis treatment. J. Nanobiotechnol. 2023, 21, 321. [Google Scholar] [CrossRef]
- Huang, C.; Xu, J.; Li, J.; He, S.; Xu, H.; Ren, X.; Singh, V.; Wu, L.; Zhang, J. Hydrogen peroxide responsive covalent cyclodextrin framework for targeted therapy of inflammatory bowel disease. Carbohydr. Polym. 2022, 285, 119252. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- Glisoni, R.J.; García-Fernández, M.J.; Pino, M.; Gutkind, G.; Moglioni, A.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Sosnik, A. β-Cyclodextrin hydrogels for the ocular release of antibacterial thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Couceiro, R.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Poly(hydroxyethyl methacrylate-co-methacrylated-beta-cyclodextrin) hydrogels: Synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomater. 2008, 4, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Racaniello, G.F.; Lopedota, A.; Laquintana, V.; Arduino, I.; Lopalco, A.; Cutrignelli, A.; Franco, M.; Sigurdsson, H.H.; Denora, N. Chitosan/sulfobutylether-β-cyclodextrin based nanoparticles coated with thiolated hyaluronic acid for indomethacin ophthalmic delivery. Int. J. Pharm. 2022, 622, 121905. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and “Two-Pronged” Therapy. Small 2020, 16, e2003253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, K.; Wei, X.; Zhang, H.; Yang, H.; Jiao, K.; Liu, C.; Fan, Z.; Wu, J.; Zhou, T.; et al. Microneedle/CD-MOF-mediated transdural controlled release of methylprednisolone sodium succinate after spinal cord injury. J. Control. Release 2023, 360, 236–248. [Google Scholar] [CrossRef]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Seegobin, N.; Abdalla, Y.; Li, G.; Murdan, S.; Shorthouse, D.; Basit, A.W. Optimising the production of PLGA nanoparticles by combining design of experiment and machine learning. Int. J. Pharm. 2024, 667, 124905. [Google Scholar] [CrossRef]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of cyclodextrins and associated toxicities: A roadmap for high quality biomedical applications. Carbohydr. Polym. 2022, 295, 119880. [Google Scholar] [CrossRef]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Rohner, N.A.; Schomisch, S.J.; Marks, J.M.; von Recum, H.A. Cyclodextrin Polymer Preserves Sirolimus Activity and Local Persistence for Antifibrotic Delivery over the Time Course of Wound Healing. Mol. Pharm. 2019, 16, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Guo, T.; Wu, L.; Xu, J.; Liu, B.; Gref, R.; Zhang, J. Template-directed synthesis of a cubic cyclodextrin polymer with aligned channels and enhanced drug payload. RSC Adv. 2017, 7, 20789–20794. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar]
- Samuel, M.S.; Selvarajan, E.; Subramaniam, K.; Mathimani, T.; Seethappan, S.; Pugazhendhi, A. Synthesized β-cyclodextrin modified graphene oxide (β-CD-GO) composite for adsorption of cadmium and their toxicity profile in cervical cancer (HeLa) cell lines. Process Biochem. 2020, 93, 28–35. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloids Surf. B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455. [Google Scholar] [CrossRef]
- Yi, S.; Zheng, J.; Lv, P.; Zhang, D.; Zheng, X.; Zhang, Y.; Liao, R. Controlled Drug Release from Cyclodextrin-Gated Mesoporous Silica Nanoparticles Based on Switchable Host–Guest Interactions. Bioconjug. Chem. 2018, 29, 2884–2891. [Google Scholar] [CrossRef]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold nanoparticles interacting with β-cyclodextrin-phenylethylamine inclusion complex: A ternary system for photothermal drug release. ACS Appl. Mater. 2015, 7, 15177–15188. [Google Scholar] [CrossRef]
- Pérez-Esteve, É.; Ruiz-Rico, M.; de la Torre, C.; Llorca, E.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Guillem, C.; Martínez-Máñez, R.; Barat, J.M. Stability of different mesoporous silica particles during an in vitro digestion. Microporous Mesoporous Mater. 2016, 230, 196–207. [Google Scholar] [CrossRef]
- Kuschnerus, I.; Giri, K.; Ruan, J.; Huang, Y.; Bedford, N.; Garcia-Bennett, A. On the growth of the soft and hard protein corona of mesoporous silica particles with varying morphology. J. Colloid Interface Sci. 2022, 612, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.-S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 4551. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef]
- Chen, X.; Zhouhua, W.; Jie, Z.; Xinlu, F.; Jinqiang, L.; Yuwen, Q.; Zhiying, H. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int. J. Nanomed. 2015, 10, 1–22. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin-drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. New “drug-in cyclodextrin-in deformable liposomes” formulations to improve the therapeutic efficacy of local anaesthetics. Int. J. Pharm. 2010, 395, 222–231. [Google Scholar] [CrossRef]
- Azzi, J.; Auezova, L.; Danjou, P.E.; Fourmentin, S.; Greige-Gerges, H. First evaluation of drug-in-cyclodextrin-in-liposomes as an encapsulating system for nerolidol. Food Chem. 2018, 255, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, Z.; Khreich, N.; Auezova, L.; Fourmentin, S.; Elaissari, A.; Greige-Gerges, H. Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int. J. Pharm. 2019, 564, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Fourmentin, S.; Charcosset, C.; Greige-Gerges, H. Effect of hydroxypropyl-β–cyclodextrin on lipid membrane fluidity, stability and freeze-drying of liposomes. J. Drug Deliv. Sci. Tec. 2018, 44, 101–107. [Google Scholar] [CrossRef]
- Hatzi, P.; Mourtas, S.; Klepetsanis, P.G.; Antimisiaris, S.G. Integrity of liposomes in presence of cyclodextrins: Effect of liposome type and lipid composition. Int. J. Pharm. 2007, 333, 167–176. [Google Scholar] [CrossRef]
- Mourtas, S.; Michanetzis, G.P.; Missirlis, Y.F.; Antimisiaris, S.G. Haemolytic activity of liposomes: Effect of vesicle size, lipid concentration and polyethylene glycol-lipid or arsonolipid incorporation. J. Biomed. Nanotechnol. 2009, 5, 409–415. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef]
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.M.; Terrér, J.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars-Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug Delivery. Chemistry 2019, 25, 8471–8478. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Sundaran, S.P.; Binoy, A.; Mishra, N.; Sujith, A. β-Cyclodextrin functionalized polyurethane nano fibrous membranes for drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102759. [Google Scholar]
- Xu, X.; Kwong, C.H.T.; Li, J.; Wei, J.; Wang, R. “Zombie” Macrophages for Targeted Drug Delivery to Treat Acute Pneumonia. ACS Appl. Mater. Interfaces 2023, 15, 29012–29022. [Google Scholar] [CrossRef]
- Suresh, M.V.; Francis, S.; Aktay, S.; Kralovich, G.; Raghavendran, K. Therapeutic potential of curcumin in ARDS and COVID-19. Clin. Exp. Pharmacol. Physiol. 2023, 50, 267–276. [Google Scholar] [CrossRef]
- Sun, T.; Hu, D.; Guo, Z.; Gong, H.; Xin, Q.; Mu, Y.; Weng, J.; Li, J.; Chen, X. Hexapeptide decorated β-cyclodextrin delivery system for targeted therapy of bone infection. J. Control. Release 2023, 353, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, S.E.; Kim, H.J.; Song, G.G.; Jung, J.H. Intra-articular injection of stigmasterol-loaded nanoparticles reduce pain and inhibit the inflammation and joint destruction in osteoarthritis rat model: A pilot study. Drug Deliv. Transl. Res. 2024, 14, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, Y.; Kang, M.; Ding, H.; Gong, Y.; Yin, X.; Sun, R.; Qin, Y.; Wei, Y.; Huang, D. Gelatin-glucosamine hydrochloride/crosslinked-cyclodextrin metal-organic frameworks@IBU composite hydrogel long-term sustained drug delivery system for osteoarthritis treatment. Biomed. Mater. 2022, 17, 035003. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Kwong, C.H.T.; Gao, C.; Wei, J.; Yue, L.; Zhang, J.; Ye, R.D.; Wang, R. Amelioration of ulcerative colitis via inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics 2020, 10, 10106–10119. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J. Control. Release 2013, 168, 28–34. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Gözcü, S.; Polat, H.K.; Gültekin, Y.; Ünal, S.; Karakuyu, N.F.; Şafak, E.K.; Doğan, O.; Pezik, E.; Haydar, M.K.; Aytekin, E.; et al. Formulation of hesperidin-loaded in situ gel for ocular drug delivery: A comprehensive study. J. Sci. Food Agric. 2024, 104, 5846–5859. [Google Scholar] [CrossRef]
- Gao, C.; Liu, C.; Chen, Q.; Wang, Y.; Kwong, C.H.T.; Wang, Q.; Xie, B.; Lee, S.M.Y.; Wang, R. Cyclodextrin-mediated conjugation of macrophage and liposomes for treatment of atherosclerosis. J. Control. Release 2022, 349, 2–15. [Google Scholar] [CrossRef]
- Mou, N.; Duan, X.; Qu, K.; Chen, Q.; He, Z.; Cao, Y.; Zhang, K.; Qin, X.; Zhu, L.; Han, Z.; et al. Macrophage Membrane Spontaneously Encapsulated Cyclodextrin-Based Nanomedicines for Improving Lipid Metabolism and Inflammation in Atherosclerosis. ACS Appl. Mater. Interfaces 2024, 16, 49660–49672. [Google Scholar] [CrossRef]
- Feng, S.; Hu, Y.; Peng, S.; Han, S.; Tao, H.; Zhang, Q.; Xu, X.; Zhang, J.; Hu, H. Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis. Biomaterials 2016, 105, 167–184. [Google Scholar] [CrossRef]
- Wijetunge, S.S.; Wen, J.; Yeh, C.K.; Sun, Y. Wheat germ agglutinin liposomes with surface grafted cyclodextrins as bioadhesive dual-drug delivery nanocarriers to treat oral cells. Colloids Surf. B 2020, 185, 110572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly(lactic-co-glycolic acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Meng, Y.; Cui, J.; Li, R.; Ding, X.; Niu, B.; Chang, G.; Xu, N.; Li, G.; Wang, Y.; et al. Hepatic Stellate Cell- and Liver Microbiome-Specific Delivery System for Dihydrotanshinone I to Ameliorate Liver Fibrosis. ACS Nano 2023, 17, 23608–23625. [Google Scholar] [CrossRef]


| System | CD | APIs | Loading Capacity | Loading Efficiency | Drug Release Mechanism | In Vivo Studies | Ref |
|---|---|---|---|---|---|---|---|
| Polyanionic cyclodextrin supramolecular nanoparticles | Hepta-carboxyl-β-CD | HPTS | 66% | - | Diffusive release | - | [49] |
| Targeted self-assembled supramolecular nanoparticles | Poly-β-CD | DOX and DTX | - | 82.3% and 77.2%, respectively | pH change | - | [50] |
| GO-Phe-CD nanocarriers | β-CD | DOX | 85.2% | 78.7% | pH change | - | [51] |
| GO hybrid CD-based supramolecular hydrogels | α-CD | 5-FU | - | - | NIR light-, temperature-, and pH-responsive release | - | [52] |
| Cyclodextrin inclusion micro-cocrystal | γ-CD | Asiatic acid | 91.2% | 11.4% | Diffusive release | Inflammatory factors and gene expression in lung tissue | [53] |
| Hydroxypropyl-β-cyclodextrin inclusion compound | HP-β-CD | Tetrandrine | - | 93.28 ± 0.58% | Diffusive release | Pharmacokinetics, biodistribution, and efficacy | [54] |
| γ-cyclodextrin metal–organic frameworks | γ-CD | Luteolin | 65% | - | Diffusive release | Pharmacokinetics, biodistribution, and efficacy | [55] |
| β-cyclodextrin inclusion complexes | β-CD | Andrographolide | 9.61 ± 1.99% | 63.92 ± 3.98% | Diffusive release | Anti-pneumonia efficacy and immune response | [56] |
| Cyclodextrin cationic polymer-based nanoassemblies | PolyCD | DCF | 92% | 100% | Diffusive release | - | [57] |
| Supramolecular nano-delivery systems | HP-β-CD | Curcumin | 90.24% ± 1.49% | 8.54 ± 0.08% | pH/ROS-responsive release | Biodistribution, anti-inflammatory antioxidant activities, and efficacy | [58] |
| H2O2-responsive covalent cyclodextrin frameworks | γ-CD | p-Hydroxybenzyl alcohol | - | - | H2O2-responsive release | Biodistribution, pharmacokinetics, and efficacy | [59] |
| A multiple-carbohydrate-based nanosystem | OX-CD | Dexamethasone | 86.3 ± 1.2% | 8.7 ± 0.1% | pH/ROS-responsive release | Biodistribution and therapeutic efficacy | [24] |
| pHEMA/β-CD contact lenses | β-CD | Puerarin | 4~5% | 18~30% | Diffusive release | Bioavailability and pharmacokinetics | [60] |
| pHEMA-co-β-CD | β-CD | Thiosemicarbazone | - | 5% | Diffusive release | - | [61] |
| pHEMA-co-beta-CD hydrogels | β-CD | Hydrocortisone and acetazolamide | - | Less than 10% | Diffusive release | - | [62] |
| Chitosan/sulfobutylether-β-cyclodextrin-based nanoparticles | β-CD | Indomethacin | 94.3% | 3.32% | Diffusive release | Mucoadhesive, release, and permeation studies | [63] |
| Two-photon fluorophore–cyclodextrin/prednisolone complexes | β-CD | Prednisolone | 93% | - | ROS-responsive release | Toxicity, pharmacokinetic study, and antiatherosclerosis activity | [64] |
| pH/ROS dual-responsive NPs | β-CD | Rapamycin | 8.7 ± 0.4% | - | pH/ROS-responsive release | Targeting capability and acute toxicity | [65] |
| Microneedles and β-cyclodextrin metal–organic frameworks | β-CD | Methylprednisolone sodium succinate | 60.49–72.66% | - | pH change | Biodistribution and therapeutic efficacy | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Yang, H.; Yin, P.; Liu, X.; Zhang, L.; Dou, Y.; Sun, S. Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases. Pharmaceutics 2025, 17, 378. https://doi.org/10.3390/pharmaceutics17030378
Dai Z, Yang H, Yin P, Liu X, Zhang L, Dou Y, Sun S. Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases. Pharmaceutics. 2025; 17(3):378. https://doi.org/10.3390/pharmaceutics17030378
Chicago/Turabian StyleDai, Zelan, Huijuan Yang, Peng Yin, Xingkang Liu, Ling Zhang, Youwei Dou, and Shibo Sun. 2025. "Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases" Pharmaceutics 17, no. 3: 378. https://doi.org/10.3390/pharmaceutics17030378
APA StyleDai, Z., Yang, H., Yin, P., Liu, X., Zhang, L., Dou, Y., & Sun, S. (2025). Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases. Pharmaceutics, 17(3), 378. https://doi.org/10.3390/pharmaceutics17030378







