Development and Evaluation of Fluconazole Co-Crystal for Improved Solubility and Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Co-Crystal
2.3. Evaluation of Co-Crystal
2.3.1. Solubility and Drug Content
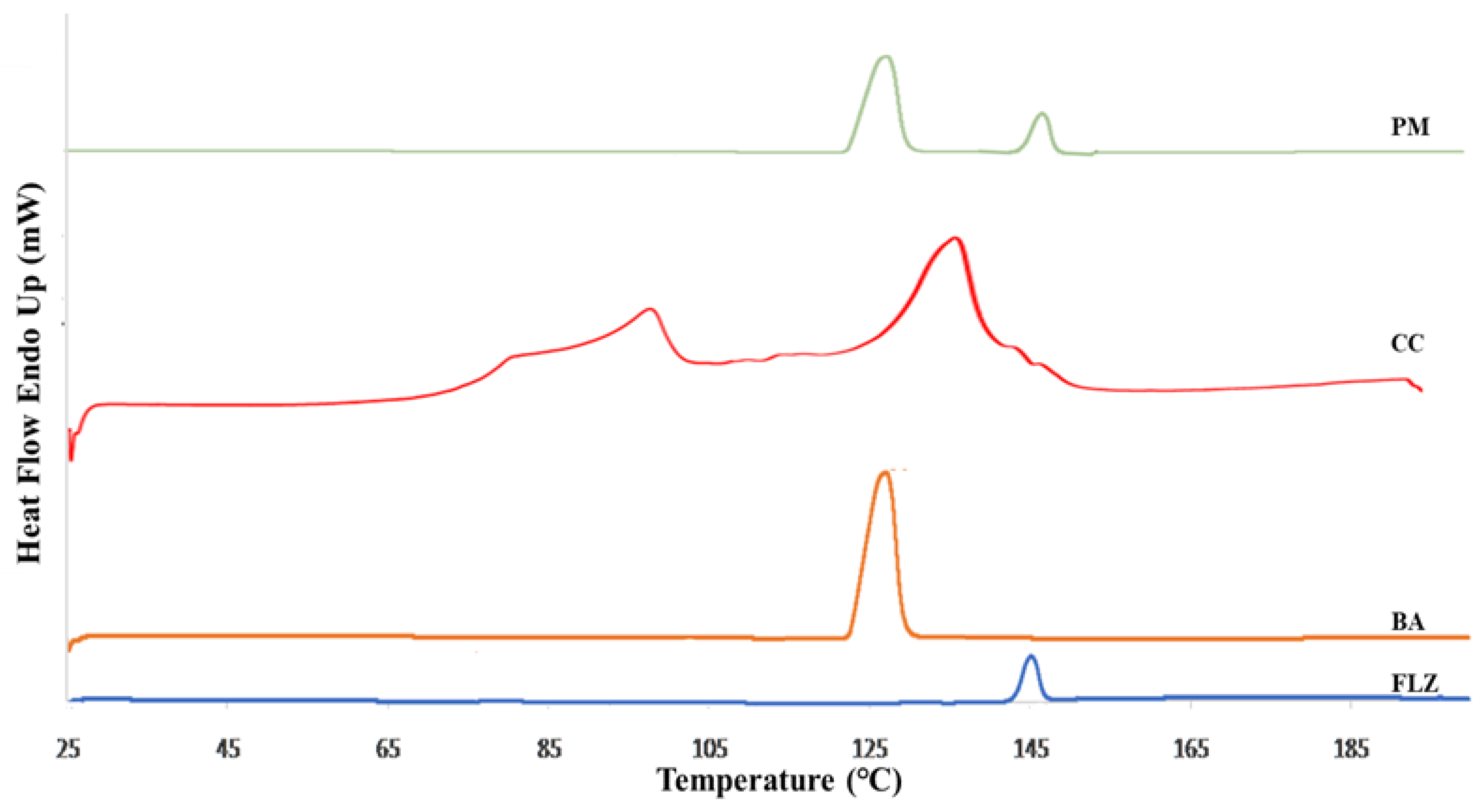
2.3.2. Differential Scanning Calorimetry
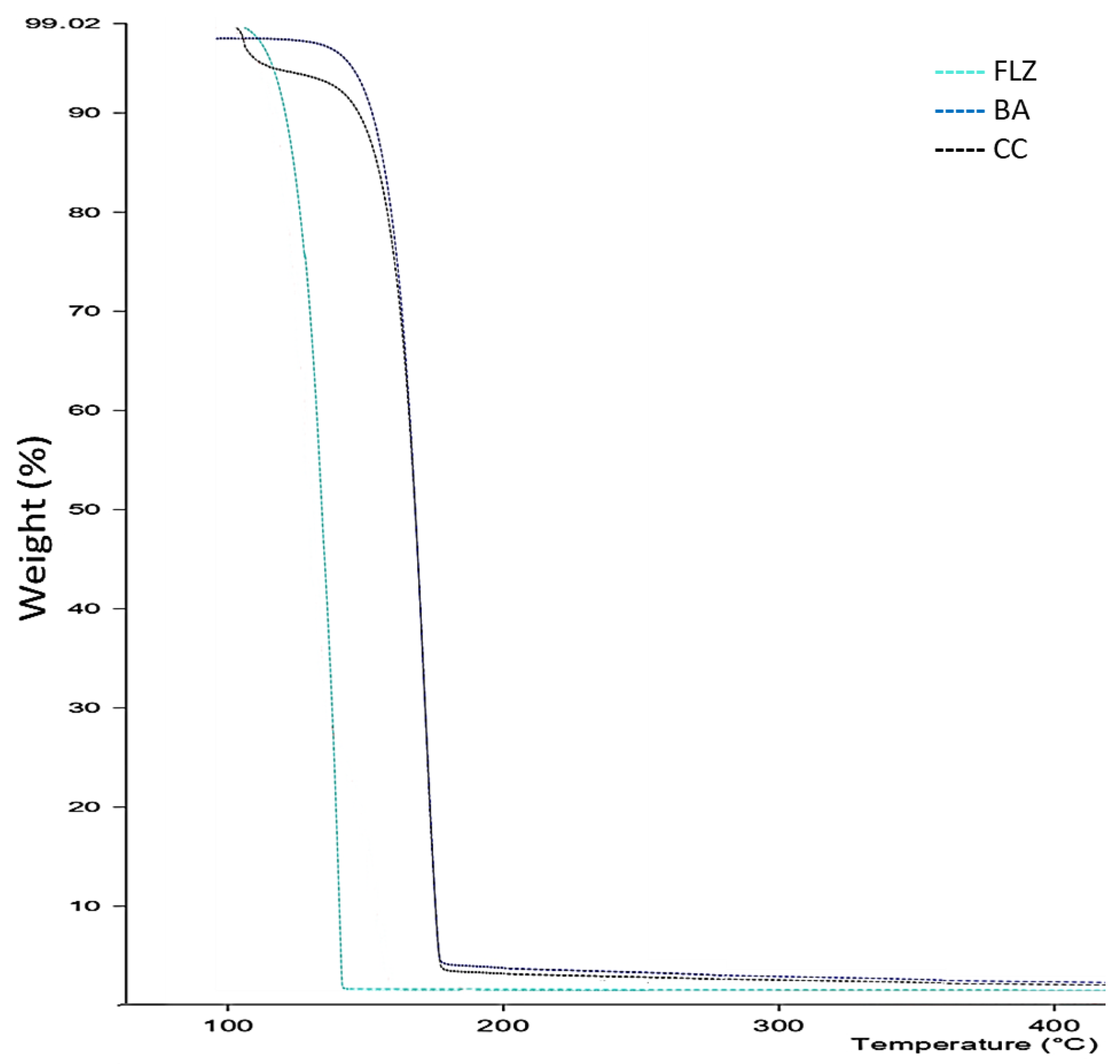
2.3.3. Thermogravimetric Analysis
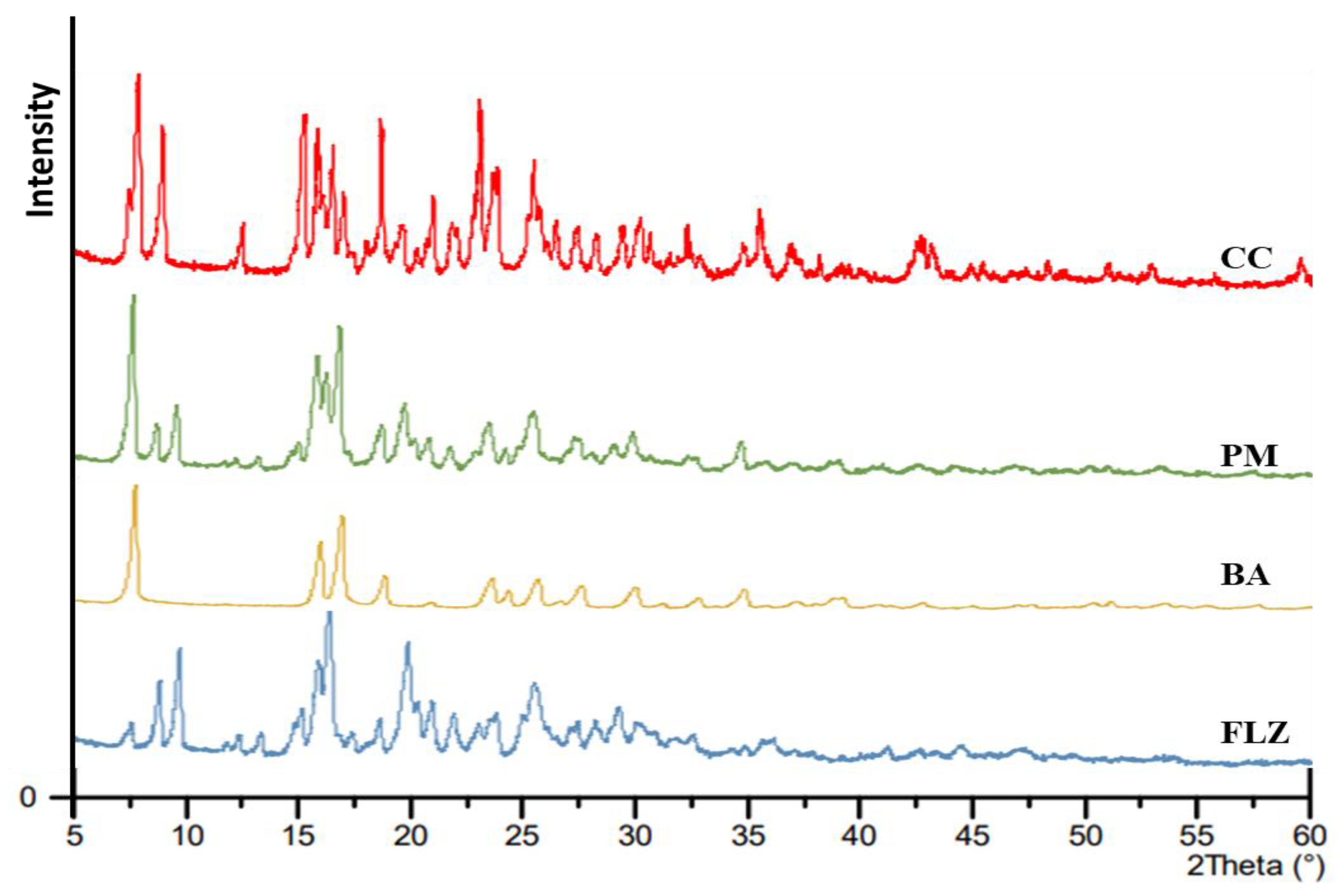
2.3.4. PXRD
2.3.5. SEM
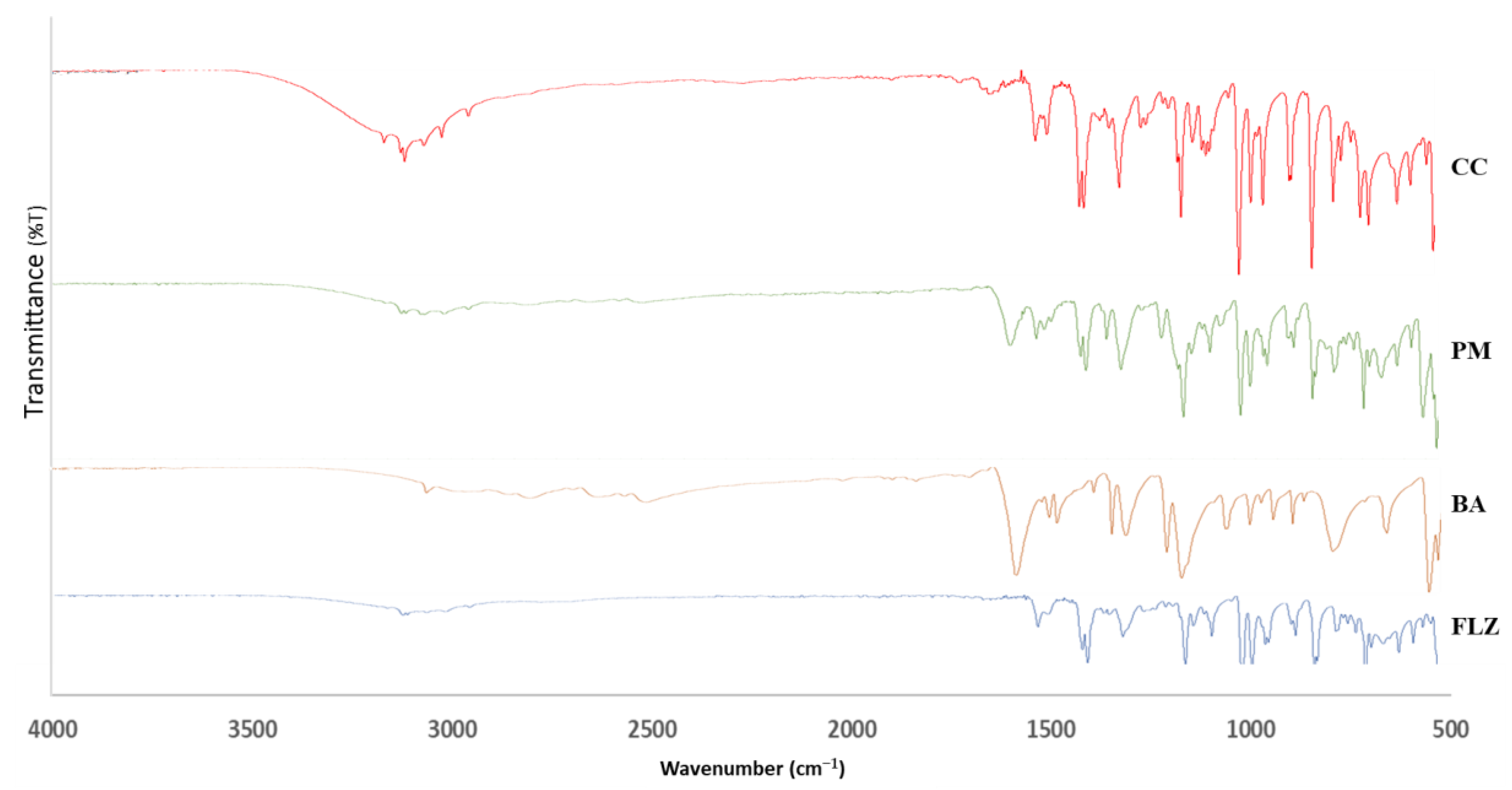
2.3.6. FTIR
2.3.7. In Vitro Anti-Fungal Activity
2.4. Powder Evaluation
2.4.1. Powder Flow Properties
2.4.2. Compaction Studies
2.5. In Vitro Drug Release
2.6. Stability
3. Results and Discussion
3.1. Solubility and Drug Content
3.2. Differential Scanning Calorimetry
3.3. TGA
3.4. PXRD
3.5. SEM
3.6. FTIR
3.7. In Vitro Anti-Fungal Activity
3.8. Powder Evaluation
3.8.1. Flow Characterization
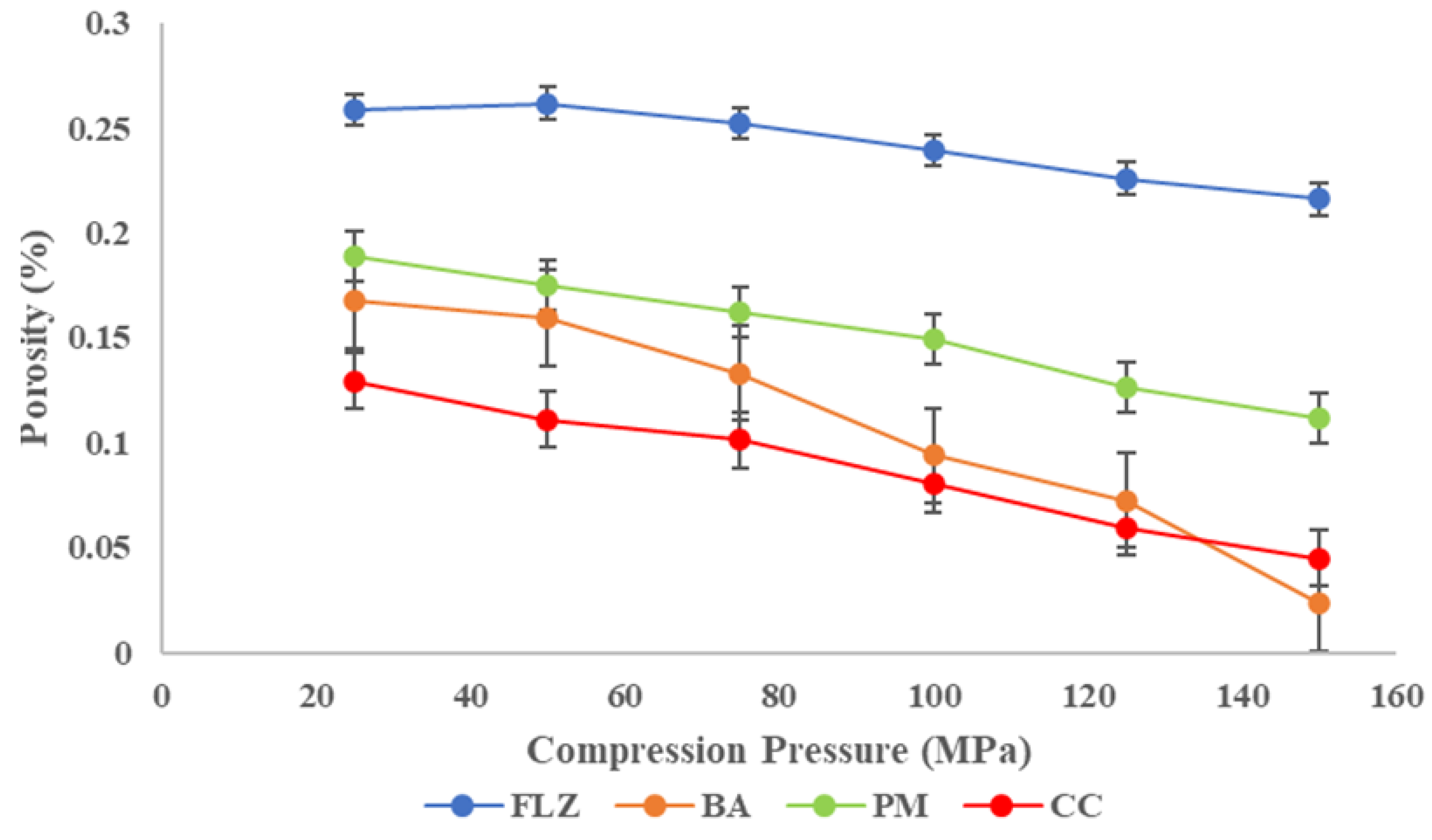
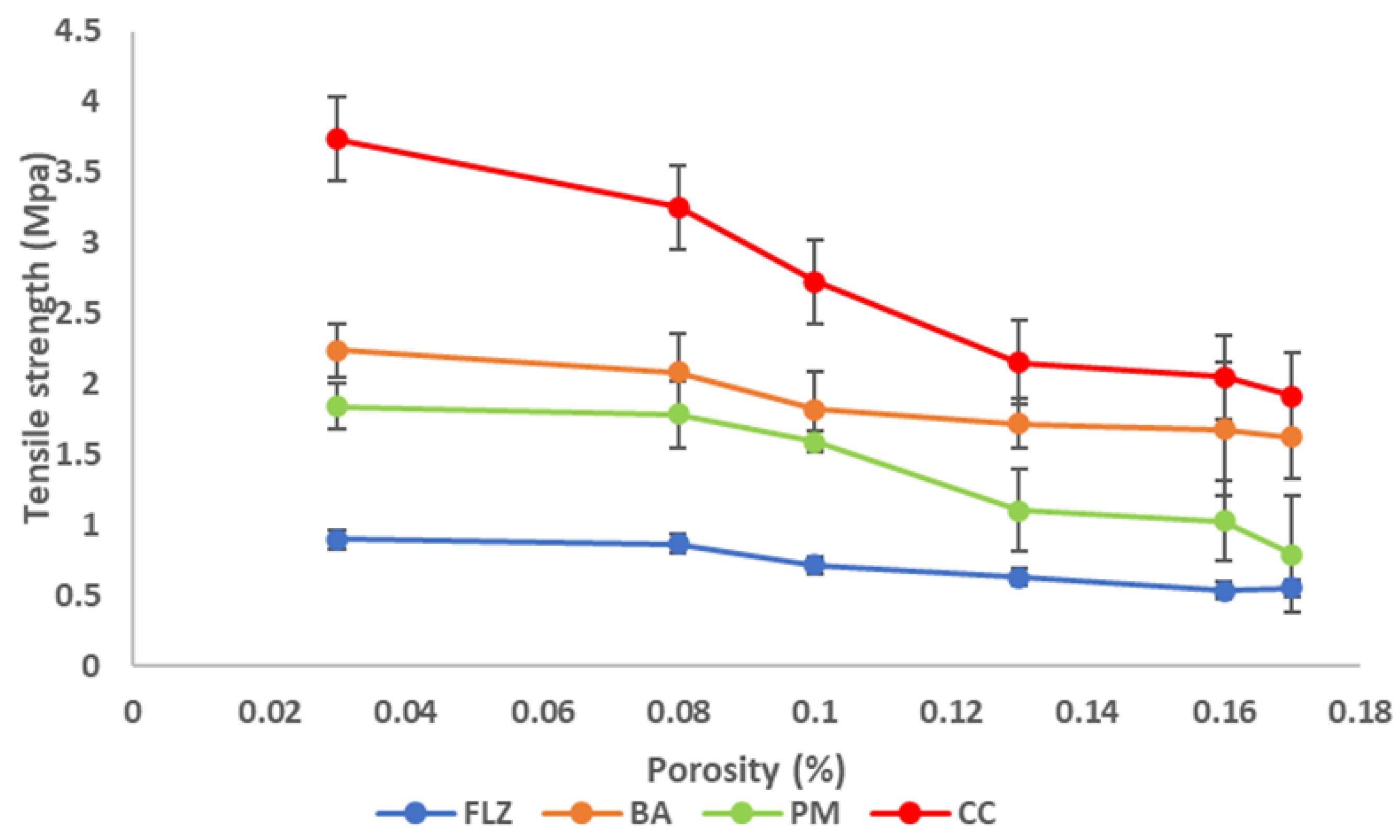
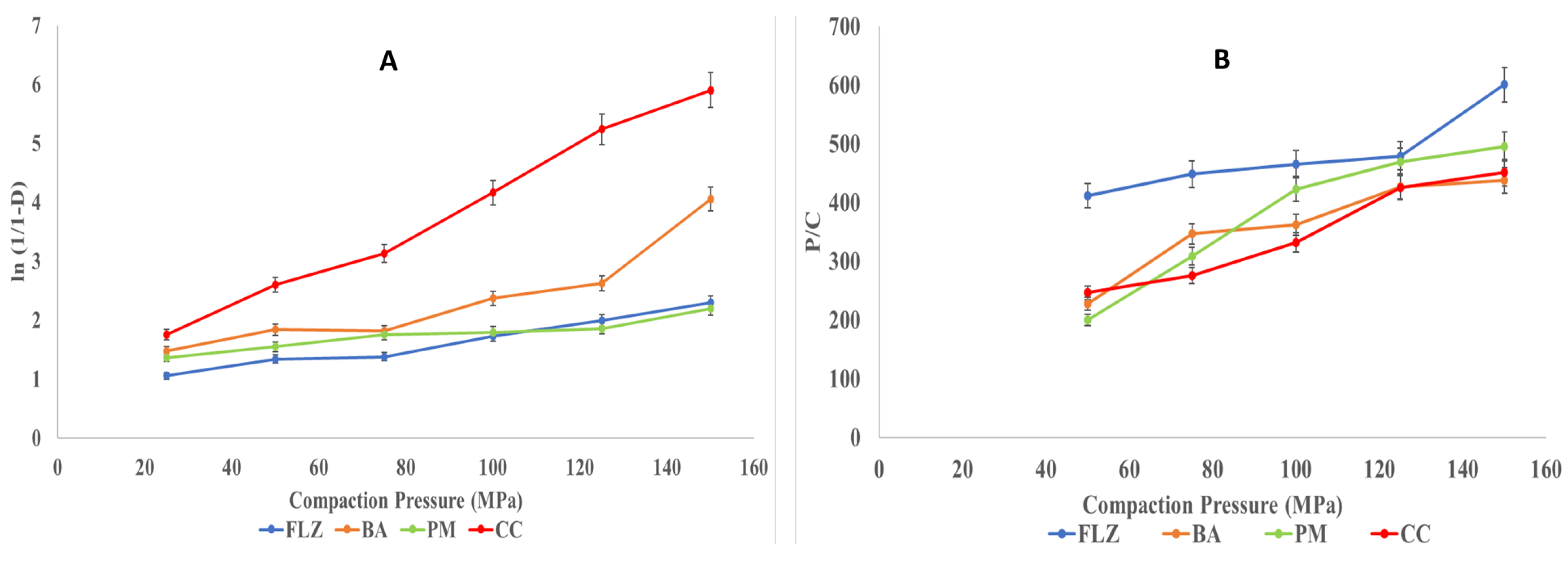
3.8.2. Compaction Studies
- Heckel analysis
- Kawakita Analysis
3.9. In Vitro Drug Release
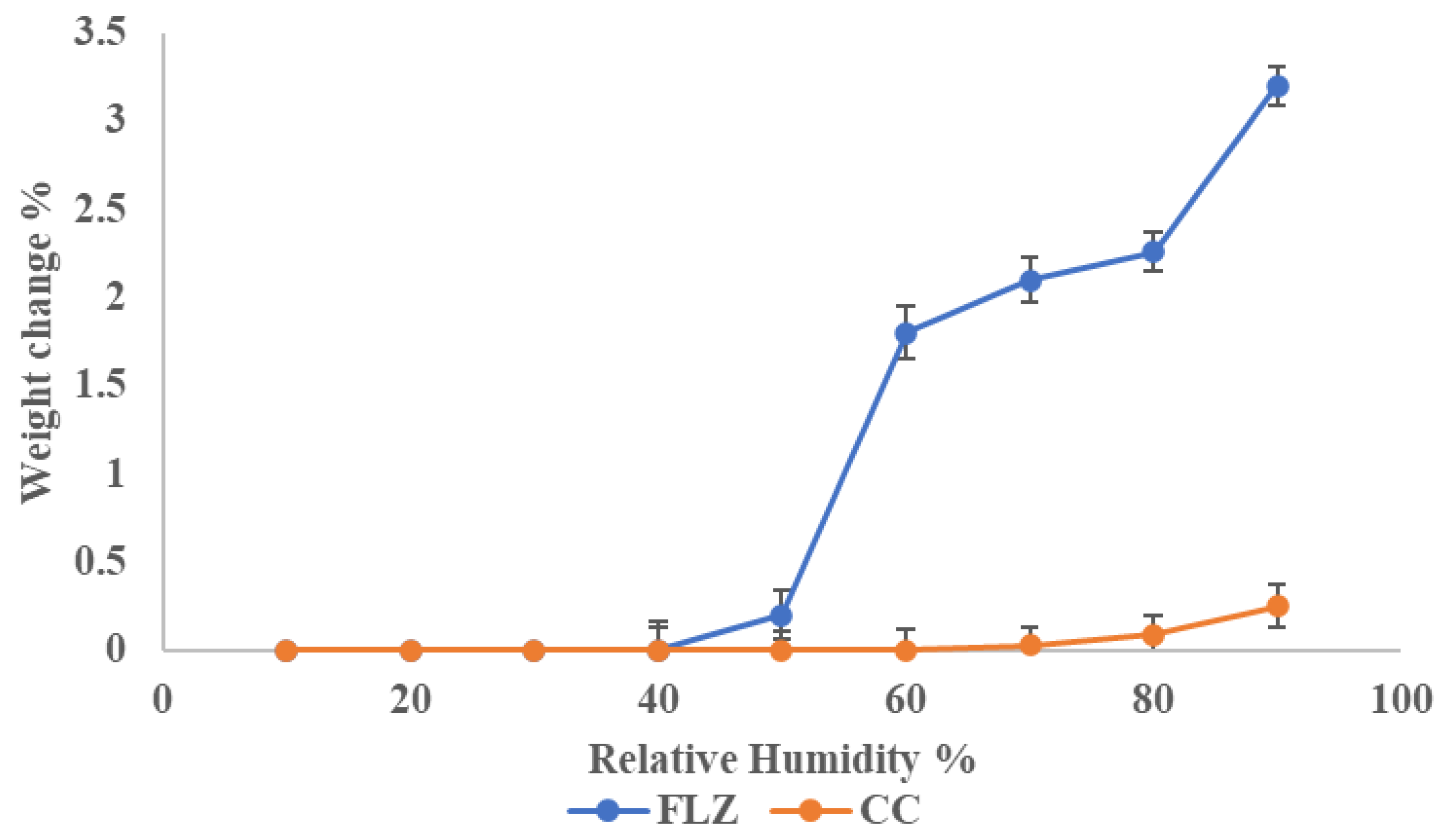
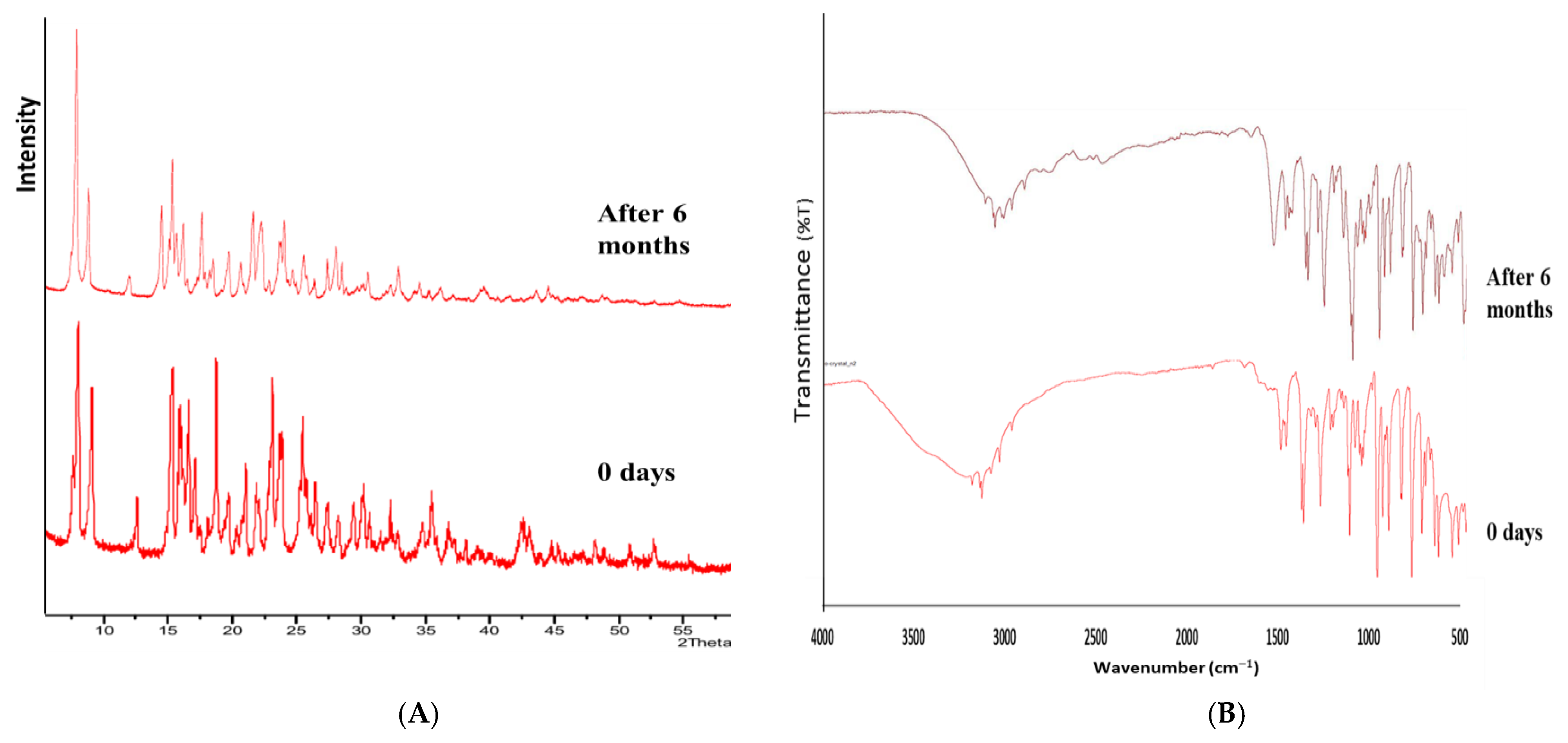
3.10. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyamba, I.; Sombié, C.B.; Yabré, M.; Zimé-Diawara, H.; Yaméogo, J.; Ouédraogo, S.; Lechanteur, A.; Semdé, R.; Evrard, B. Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Panzade, P.; Wagh, A.; Harale, P.; Bhilwade, S. Pharmaceutical cocrystals: A rising star in drug delivery applica-tions. J. Drug Target. 2024, 32, 115–127. [Google Scholar] [CrossRef]
- Rathi, R.; Kaur, S.; Singh, I. A review on co-crystals of Herbal Bioactives for solubility enhancement: Preparation methods and characterization techniques. Cryst. Growth Des. 2022, 22, 2023–2042. [Google Scholar] [CrossRef]
- Yadav, A.; Shete, A.; Dabke, A.; Kulkarni, P.; Sakhare, S. Co-crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J. Pharm. Sci. 2009, 71, 359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, M. Co-Crystallization: A Novel Technique to Improvise the Pharmaceutical Characteristics of API’s. Curr. Drug Targets 2023, 24, 870–888. [Google Scholar] [CrossRef]
- Pandeya, A.; Puri, V.M. Feasibility of Relationships between Tablet Physical Quality Parameters and Mechanical Properties of Dry Powder Formulation. Kona Powder Part. J. 2013, 30, 211–220. [Google Scholar] [CrossRef]
- Abbas, N.; Latif, S.; Afzal, H.; Arshad, M.S.; Hussain, A.; Sadeeqa, S.; Bukhari, N.I. Simultaneously Improving Me-chanical, Formulation, and In Vivo Performance of Naproxen by Co-Crystallization. AAPS Pharmscitech 2018, 19, 3249–3257. [Google Scholar] [CrossRef]
- Connor, L.E.; Vassileiou, A.D.; Halbert, G.W.; Johnston, B.F.; Oswald, I.D. Structural investigation and compression of a co-crystal of indomethacin and saccharin. CrystEngComm 2019, 21, 4465–4472. [Google Scholar] [CrossRef]
- Rathi, R.; Singh, I. Multicomponent crystal compromising dasatinib and selected co-crystals formers: A patent evaluation of EP2861589B1. Pharm. Pat. Anal. 2022, 11, 15–21. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Yao, C.; Xie, G.; Song, S.; Li, H.; Qu, Y.; Tao, X. Novel formulations of the antiviral drug favipi-ravir: Improving permeability and tabletability. Cryst. Growth Des. 2021, 21, 3807–3817. [Google Scholar] [CrossRef]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Boycov, D.E.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical multicomponent crystals of antifungal drugs with improved dissolution performance. Cryst. Growth Des. 2021, 21, 7285–7297. [Google Scholar] [CrossRef]
- Consiglieri, V.O.; Mourão, S.; Sampaio, M.; Granizo, P.; Garcia, P.; Martinello, V.; Spricigo, R.; Ferraz, H.G. Improvement of fluconazole flowability and its effect on dissolution from tablets and capsules. Braz. J. Pharm. Sci. 2010, 46, 115–120. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Liu, M.; Xing, W.; Hu, K.; Yang, S.; He, G.; Gong, N.; Du, G.; Lu, Y. Design, preparation, characterization and evaluation of five cocrystal hydrates of fluconazole with hydroxybenzoic acids. Pharmaceutics 2022, 14, 2486. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Vasilev, N.A.; Churakov, A.V.; Perlovich, G.L. Cocrystals of fluconazole with aromatic carboxylic acids: Competition between anhydrous and hydrated solid forms. Cryst. Growth Des. 2019, 20, 1218–1228. [Google Scholar] [CrossRef]
- Srijana, P.; Narayana, B.; Sarojini, B.; Ramzi, F.D.B.; Quah, C.K.; Rajimon, K.; Thomas, R. Supramolecular and computational analysis of Fluconazole− 2− chloro− 5− nitrobenzoic acid cocrystal. J. Mol. Struct. 2025, 1321, 140143. [Google Scholar] [CrossRef]
- Ji, X.; Wu, D.; Li, C.; Li, J.; Sun, Q.; Chang, D.; Yin, Q.; Zhou, L.; Xie, C.; Gong, J. Enhanced solubility, dissolution, and permeability of abacavir by salt and cocrystal formation. Cryst. Growth Des. 2021, 22, 428–440. [Google Scholar] [CrossRef]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical co-crystals: A green way to enhance drug stability and solubility for improved therapeutic efficacy. J. Pharm. Pharmacol. 2024, 76, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, D.; Wang, H.; Jiao, L.; Zhao, X.; Song, J.; Yang, D.; Yang, H.; Yang, S.; Du, G. Cocrystal of apixaban–quercetin: Improving solubility and bioavailability of drug combination of two poorly soluble drugs. Molecules 2021, 26, 2677. [Google Scholar] [CrossRef]
- Huang, Z.; Staufenbiel, S.; Bodmeier, R. Combination of co-crystal and nanocrystal techniques to improve the solubility and dissolution rate of poorly soluble drugs. Pharm. Res. 2022, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Allu, S.; Nangia, A.K. Salts and cocrystal of etodolac: Advantage of solubility, dissolution, and permeability. Cryst. Growth Des. 2020, 20, 4512–4522. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.-R.; Mei, X. Enhancing the stability of active pharmaceutical ingredients by the cocrystal strategy. CrystEngComm 2022, 24, 2002–2022. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la O Contreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A green approach in the preparation of pharmaceutical cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Persson, A.-S.; Ahmed, H.; Velaga, S.; Alderborn, G. Powder compression properties of paracetamol, paracetamol hydrochloride, and paracetamol cocrystals and coformers. J. Pharm. Sci. 2018, 107, 1920–1927. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and mechanical properties of paracetamol cocrystal with 5-nitroisophthalic acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef]
- Latif, S.; Abbas, N.; Hussain, A.; Arshad, M.S.; Bukhari, N.I.; Afzal, H.; Riffat, S.; Ahmad, Z. Development of parace-tamol-caffeine co-crystals to improve compressional, formulation and in vivo performance. Drug Dev. Ind. Pharm. 2018, 44, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Sun, C.C. Material-sparing and expedited development of a tablet formulation of carbamazepine glutaric acid cocrystal–a QbD approach. Pharm. Res. 2020, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Liu, L.; Li, Y.; Chen, M.; Zhou, L.; Liu, Z.; Xu, L.; Shehzad, H. Cocrystals of carbamazepine: Structure, mechanical properties, fluorescence properties, solubility, and dissolution rate. Particuology 2024, 90, 20–30. [Google Scholar] [CrossRef]
- Ratih, H.; Pamudji, J.S.; Alatas, F.; Soewandhi, S.N. Improving telmisartan mechanical properties through the formation of telmisartan and oxalic acid co-crystal by slow evaporation and ultrasound assisted co-crystallization from solution methods. Songklanakarin J. Sci. Technol. 2020, 42, 188–195. Available online: https://www.thaiscience.info/Journals/Article/SONG/10993058.pdf (accessed on 30 January 2025).
- Bhatt, J.A.; Bahl, D.; Morris, K.; Stevens, L.L.; Haware, R.V. Structure-mechanics and improved tableting performance of the drug-drug cocrystal metformin: Salicylic acid. Eur. J. Pharm. Biopharm. 2020, 153, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.M.; Chatale, B.C.; Amin, P.D. Comparative evaluation of ibuprofen co-crystals prepared by solvent evaporation and hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2022, 67, 103003. [Google Scholar] [CrossRef]
- Mehta, J.; Borkhataria, C.; Patel, A.; Manek, R.; Patel, N.; Sakhiya, D.; Shanishchara, K.; Mistry, B. Para-hydroxy ben-zoic acid coformer enable enhanced solubility, dissolution, and antifungal activity of ketoconazole cocrystals. J. Pharm. Innov. 2023, 18, 1602–1615. [Google Scholar] [CrossRef]
- Naksuriya, O.; Nitthikan, N.; Supadej, K.; Kheawfu, K.; Khonkarn, R.; Ampasavate, C.; Intasai, N.; Monton, C.; Kiattisin, K. Approach for Development of Topical Ketoconazole-Loaded Microemulsions and Its Antifungal Activity. Trends Sci. 2023, 20, 7046. [Google Scholar] [CrossRef]
- Deka, P.; Gogoi, D.; Althubeiti, K.; Rao, D.R.; Thakuria, R. Mechanosynthesis, characterization, and physicochemical property investigation of a favipiravir cocrystal with theophylline and GRAS coformers. Cryst. Growth Des. 2021, 21, 4417–4425. [Google Scholar] [CrossRef]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.; Sun, C.C. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.P.; Ugale, B.; Nagaraja, C.; Dubey, G.; Bharatam, P.V.; Bansal, A.K. Molecular basis of water sorption behavior of rivaroxaban-malonic acid cocrystal. Mol. Pharm. 2019, 16, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Kuminek, G.; Cavanagh, K.L.; da Piedade, M.F.M.; Rodríguez-Hornedo, N. Posaconazole cocrystal with superior solubility and dissolution behavior. Cryst. Growth Des. 2019, 19, 6592–6602. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Bao, J.; Ding, Q.; Ren, G.; Mei, X. Pharmaceutical cocrystals of nicorandil with enhanced chemical stability and sustained release. Cryst. Growth Des. 2020, 20, 6995–7005. [Google Scholar] [CrossRef]
- Patel, R.D.; Raval, M.K. Formulation of diacerein cocrystal using β-resorcylic acid for improvement of physicome-chanical and biopharmaceutical properties. Org. Process Res. Dev. 2020, 25, 384–394. [Google Scholar] [CrossRef]
- Teleginski, L.K.; Maciel, A.B.; Mendes, C.; Silva, M.A.S.; Bernardi, L.S.; de Oliveira, P.R. Fluconazole–excipient compatibility studies as the first step in the development of a formulation candidate for biowaiver. J. Therm. Anal. Calorim. 2015, 120, 771–781. [Google Scholar] [CrossRef]
- Ainurofiq, A.; Mauludin, R.; Mudhakir, D.; Soewandhi, S.N. Synthesis, characterization, and stability study of desloratadine multicomponent crystal formation. Res. Pharm. Sci. 2018, 13, 93–102. [Google Scholar] [CrossRef]
- Ahangar, A.A.; Qadri, H.; Malik, A.A.; Mir, M.A.; Shah, A.H.; Dar, A.A. Physicochemical and Anti-fungal Studies of the Pharmaceutical Co-crystal/Salt of Fluconazole. Mol. Pharm. 2023, 20, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Alatas, F.; Suwartiningsih, N.; Ratih, H.; Sutarna, T.H. IAI Conference: Fluconazole-tartaric acid co-crystal formation and its mechanical properties. Pharm. Educ. 2021, 21, 116–122. [Google Scholar] [CrossRef]
- Upadhyay, P.; Khomane, K.S.; Kumar, L.; Bansal, A.K. Relationship between crystal structure and mechanical properties of ranitidine hydrochloride polymorphs. CrystEngComm 2013, 15, 3959–3964. [Google Scholar] [CrossRef]
- Sun, C.C. Decoding powder tabletability: Roles of particle adhesion and plasticity. J. Adhes. Sci. Technol. 2011, 25, 483–499. [Google Scholar] [CrossRef]
- Odeku, O.A. Assessment of Albizia zygia gum as binding agent in tablet formulations. Acta Pharm. 2005, 55, 263–276. [Google Scholar]




| S. No. | Compound | Drug Content (%) | Solubility (mg/mL) |
|---|---|---|---|
| 1 | FLZ | - | 0.21 ± 0.03 |
| 2 | PM | 70.34 ± 0.86 | 0.28 ± 0.07 |
| 3 | CC | 69.51 ± 0.53 | 2.82 ± 0.08 |
| S. No. | Sample Code | Antimicrobial Efficacy as Zone of Inhibition (in mm) at Different Concentrations of Samples Against Candida albicans | ||||
|---|---|---|---|---|---|---|
| 100 (μg/mL) | 50 (μg/mL) | 25 (μg/mL) | 12.5 (μg/mL) | 6.25 (μg/mL) | ||
| 1 | FLZ | 11 ± 0.2 | 9 ± 0.4 | 7 ± 0.2 | 0 | 0 |
| 2 | BA | 2 ± 0.6 | 0 | 0 | 0 | 0 |
| 3 | PM | 15 ± 0.4 | 8 ± 0.5 | 5 ± 0.8 | 4 ± 0.4 | 0 |
| 4 | CC | 20 ± 0.5 | 12 ± 0.2 | 10 ± 0.2 | 6 ± 0.6 | 0 |
| Parameters | FLZ | BA | PM | CC |
|---|---|---|---|---|
| Bulk density (g/cm3) | 0.41 ± 0.02 | 0.41 ± 0.05 | 0.62 ± 0.02 | 0.68 ± 0.04 |
| Tapped density (g/cm3) | 0.83 ± 0.02 | 0.83 ± 0.04 | 0.83 ± 0.06 | 0.83 ± 0.07 |
| True density (g/cm3) | 1.47 ± 0.07 | 1.32 ± 0.08 | 1.45 ± 0.03 | 1.56 ± 0.05 |
| Carr’s index (%) | 50.0 ± 1.2 | 7.69 ± 1.6 | 21.87 ± 1.3 | 17.24 ± 1.5 |
| Hausner’s ratio | 2.0 ± 0.09 | 1.08 ± 0.05 | 1.28 ± 0.04 | 1.14 ± 0.07 |
| Angle of repose (deg) | 76.1 ± 1.2 | 31.28 ± 1.8 | 41.5 ± 1.4 | 33.0 ± 1.6 |
| Type of Flow | very poor | good | passable | good |
| K | Py (MPa) | A | e−A | DA | DB | |
|---|---|---|---|---|---|---|
| FLZ | 0.0131 | 76.36 | 0.418 | 0.62 | 0.34 | 0.19 |
| BA | 0.021 | 46.96 | 0.82 | 0.65 | 0.37 | 0.15 |
| PM | 0.0112 | 89.29 | 0.66 | 0.51 | 0.35 | 0.16 |
| CC | 0.037 | 26.66 | 0.44 | 0.64 | 0.48 | 0.20 |
| Di | Pk | |
|---|---|---|
| FLZ | 0.51 | 0.040 |
| BA | 0.76 | 0.009 |
| PM | 0.68 | 0.0214 |
| CC | 0.98 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathi, R.; Singh, I.; Sangnim, T.; Huanbutta, K. Development and Evaluation of Fluconazole Co-Crystal for Improved Solubility and Mechanical Properties. Pharmaceutics 2025, 17, 371. https://doi.org/10.3390/pharmaceutics17030371
Rathi R, Singh I, Sangnim T, Huanbutta K. Development and Evaluation of Fluconazole Co-Crystal for Improved Solubility and Mechanical Properties. Pharmaceutics. 2025; 17(3):371. https://doi.org/10.3390/pharmaceutics17030371
Chicago/Turabian StyleRathi, Ritu, Inderbir Singh, Tanikan Sangnim, and Kampanart Huanbutta. 2025. "Development and Evaluation of Fluconazole Co-Crystal for Improved Solubility and Mechanical Properties" Pharmaceutics 17, no. 3: 371. https://doi.org/10.3390/pharmaceutics17030371
APA StyleRathi, R., Singh, I., Sangnim, T., & Huanbutta, K. (2025). Development and Evaluation of Fluconazole Co-Crystal for Improved Solubility and Mechanical Properties. Pharmaceutics, 17(3), 371. https://doi.org/10.3390/pharmaceutics17030371







