Improved Photodynamic Therapy of Hepatocellular Carcinoma via Surface-Modified Protein Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Zein–PEG
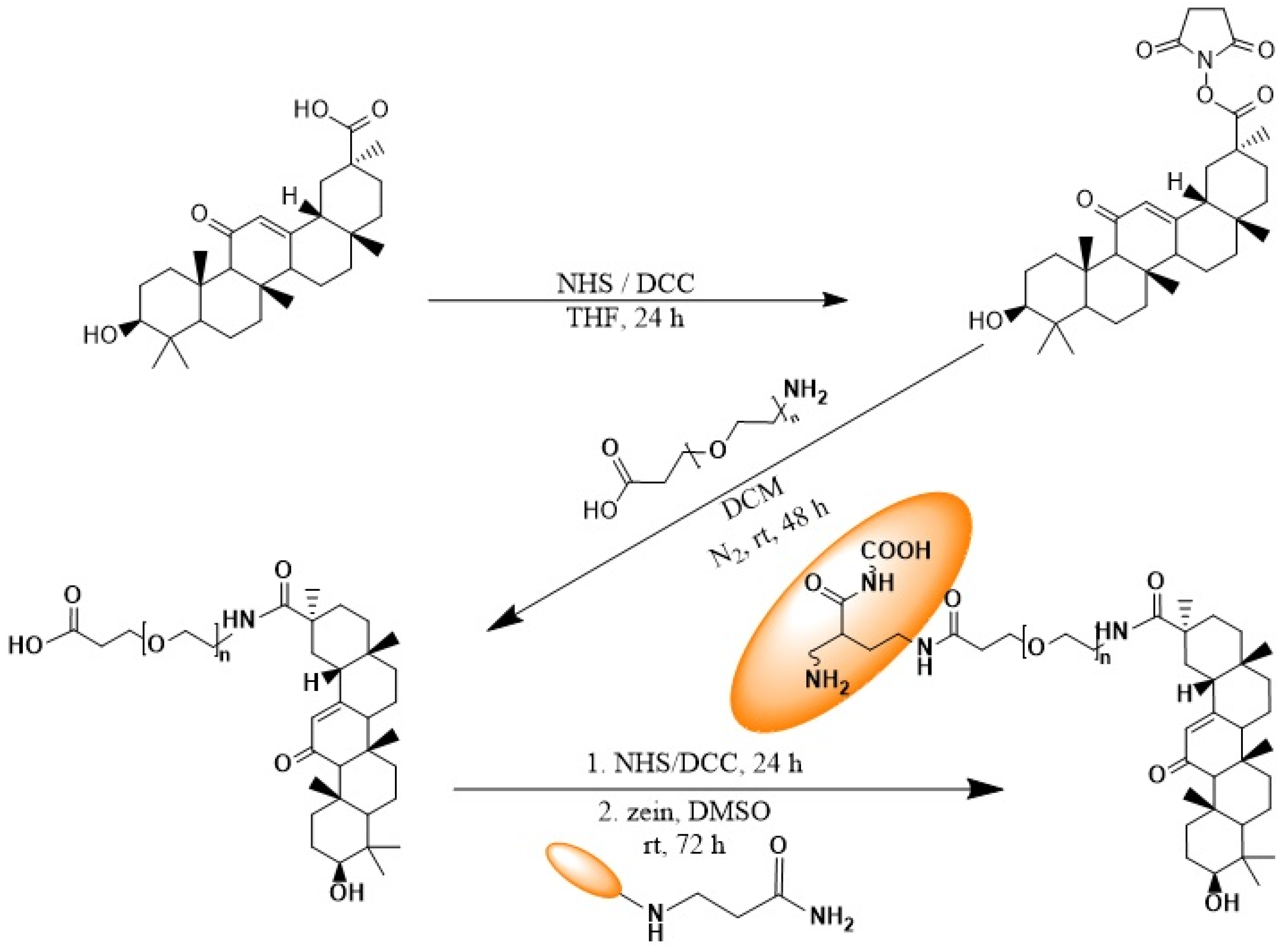
2.3. Synthesis of Glycyrrhetinic Acid–PEG–Zein Conjugate
2.3.1. Synthesis of Glycyrrhetinic Acid Succinimidyl Ester
2.3.2. Synthesis of Carboxy-PEG-GA Conjugate
2.3.3. Synthesis of the Z-PEG-GA Conjugate
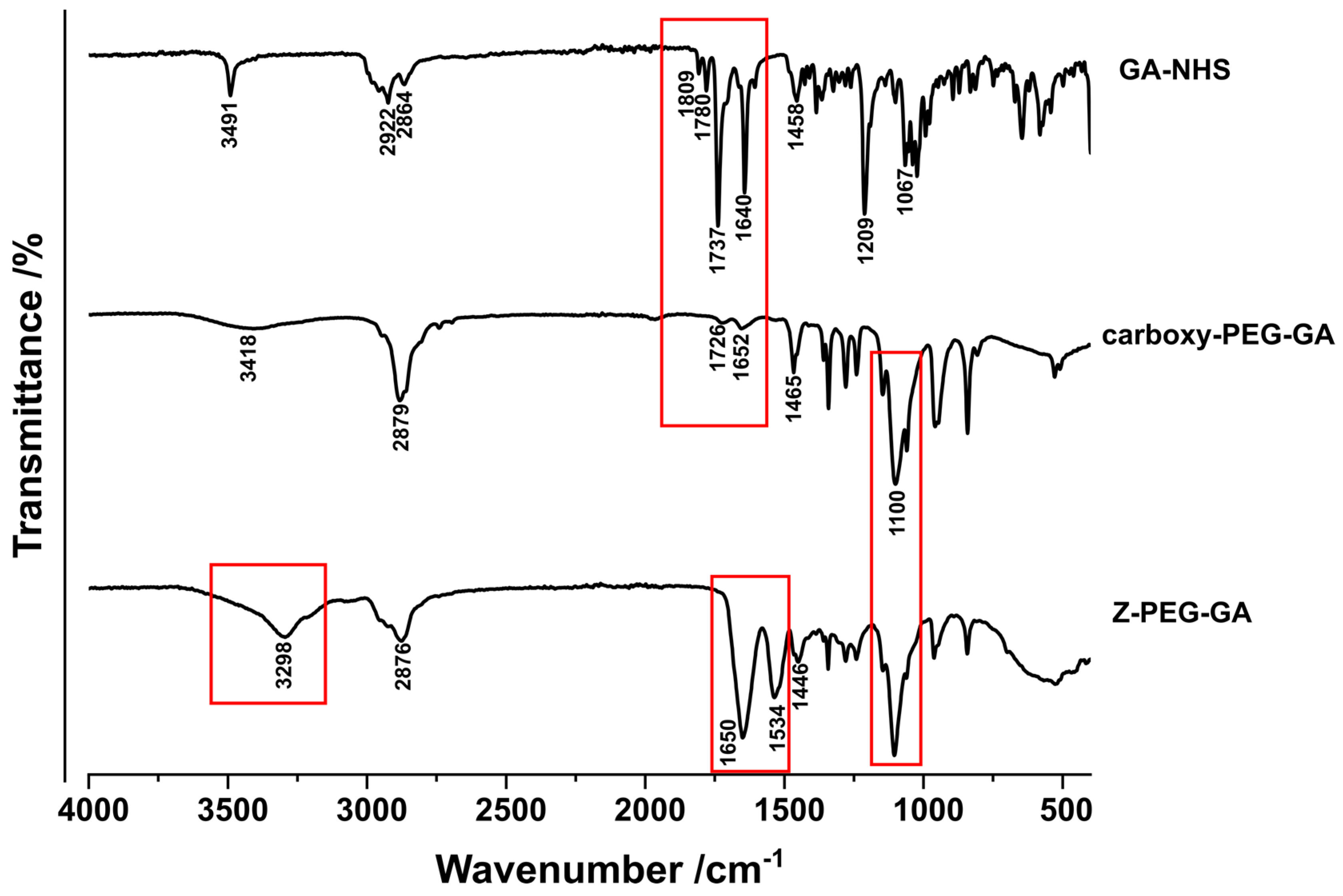
2.4. FT-IR and H1NMR Spectroscopy
2.5. Preparation and Characterization of Parietin-Loaded Z-PEG-GA Nanoparticles
2.5.1. Preparation of Z-PEG and Z-PEG-GA Nanoparticles Loaded with Parietin
2.5.2. Determination of Encapsulation and Loading Efficiency of Parietin
2.5.3. Dynamic Light Scattering (DLS)
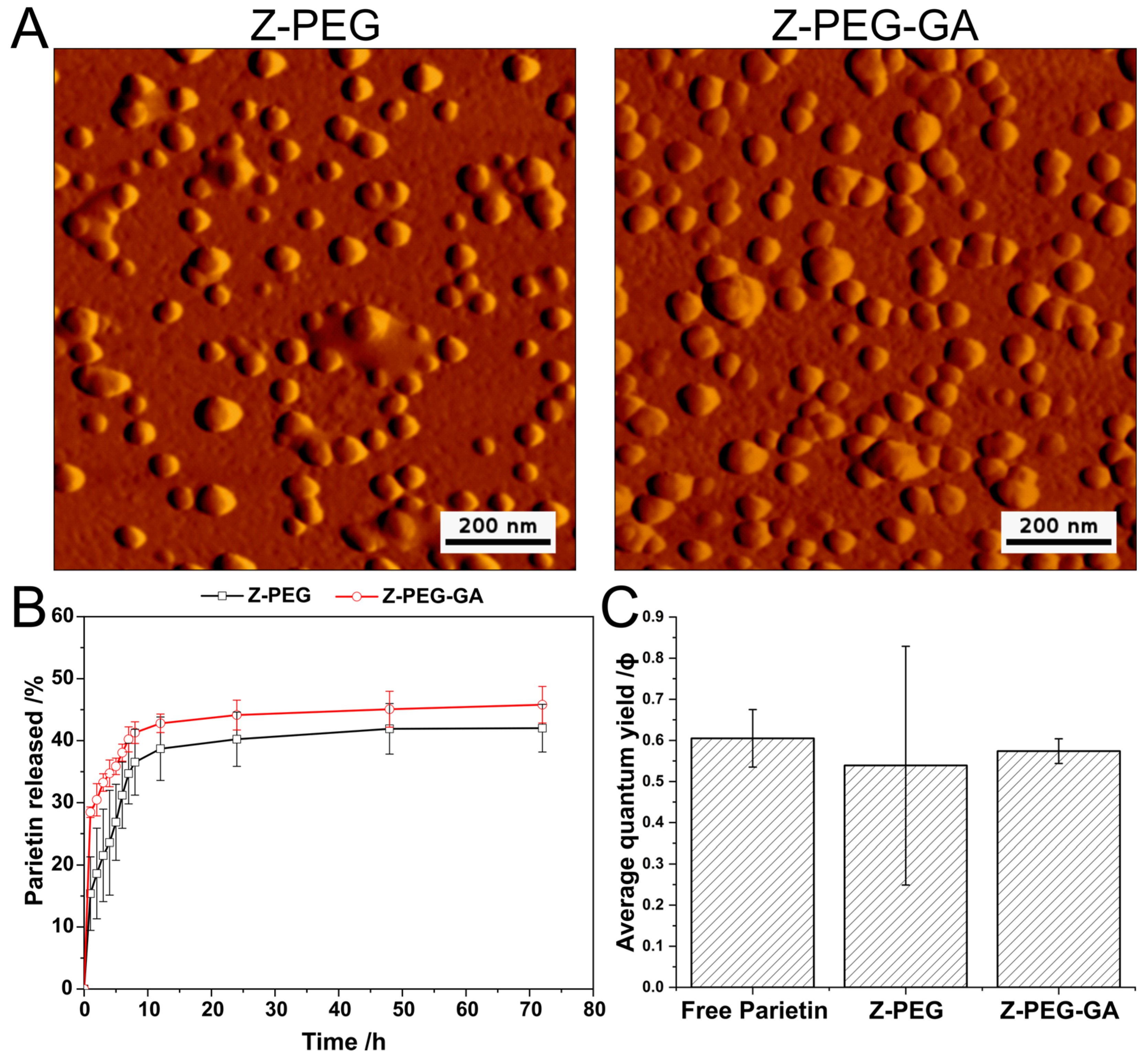
2.5.4. Atomic Force Microscopy (AFM)
2.5.5. Release Study of Parietin
2.5.6. Assay of Singlet Molecular Oxygen (1O2)
2.5.7. Stability of Zein Nanoparticles with Serum
2.6. In Vitro Biological Investigations
2.6.1. Cell Culture
2.6.2. LED Device
2.6.3. Cell Viability Assay
2.6.4. Uptake Study in HepG2 Monolayer
2.6.5. Intracellular ROS Assay
2.6.6. Mitochondrial Membrane Potential Assay (ΔΨm)
2.6.7. Immunocytochemistry Analysis of Cytochrome C Release
2.7. In Vivo Biodistribution Study
2.7.1. Animal Ethical Approval
2.7.2. Preparation of ICG-Loaded Z-PEG and Z-PEG-GA
2.7.3. In Vivo Optical Imaging
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Glycyrrhetinic Acid-Conjugated Zein
3.2. Preparation and Characterization of Parietin-Loaded Zein Nanoparticles
3.3. Generation of Singlet Molecular Oxygen 1O2
3.4. Stability of Zein Nanoparticles with Serum
3.5. In Vitro Biological Assessment of Glycyrrhetinic Acid-Tethered Zein Nanoparticles
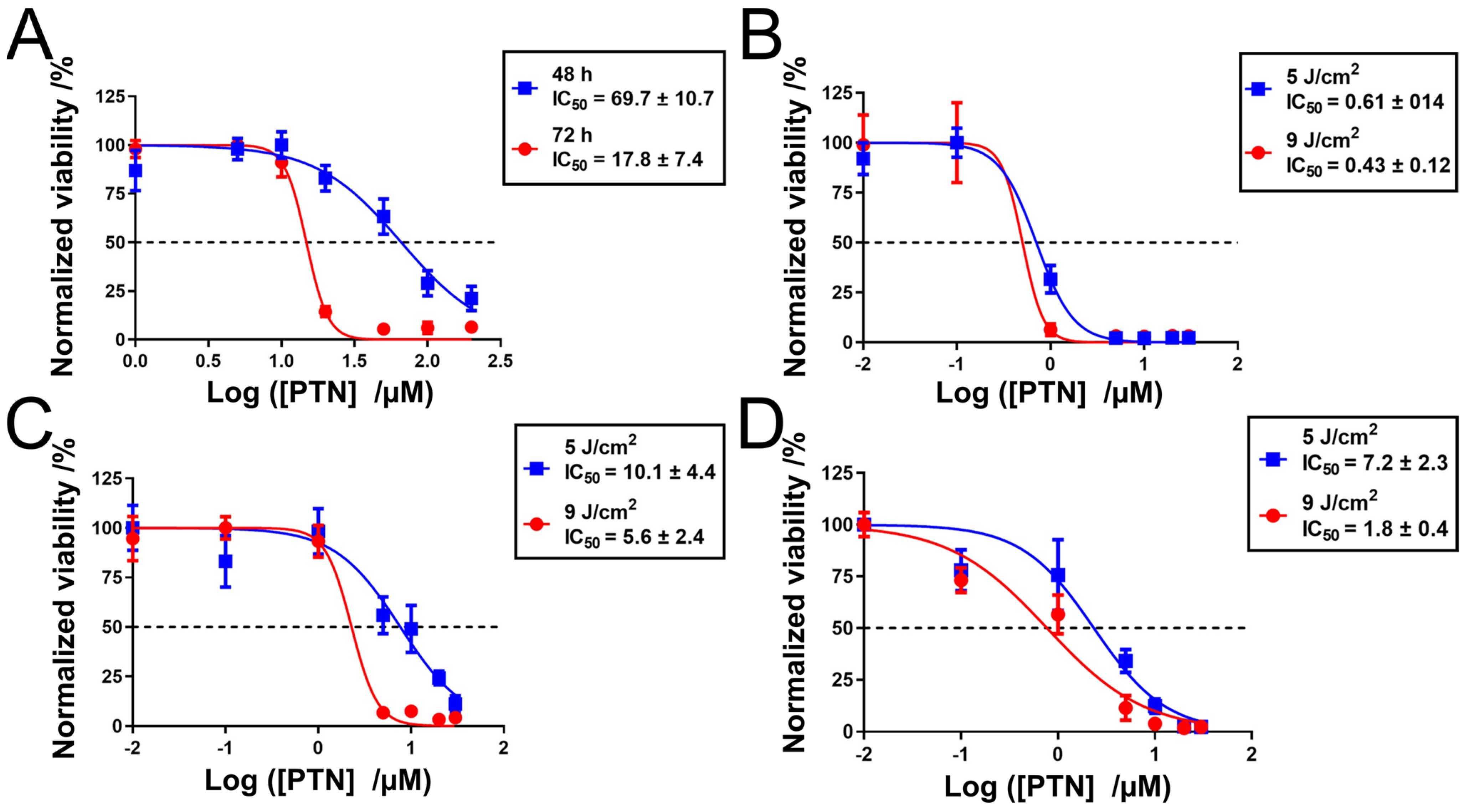
3.5.1. Cell Viability Study
3.5.2. Cellular Uptake Study
3.5.3. Generation of Intracellular Reactive Oxygen (ROS)
3.5.4. Mitochondrial Potential Disruption (ΔΨm) and Release of Cytochrome C
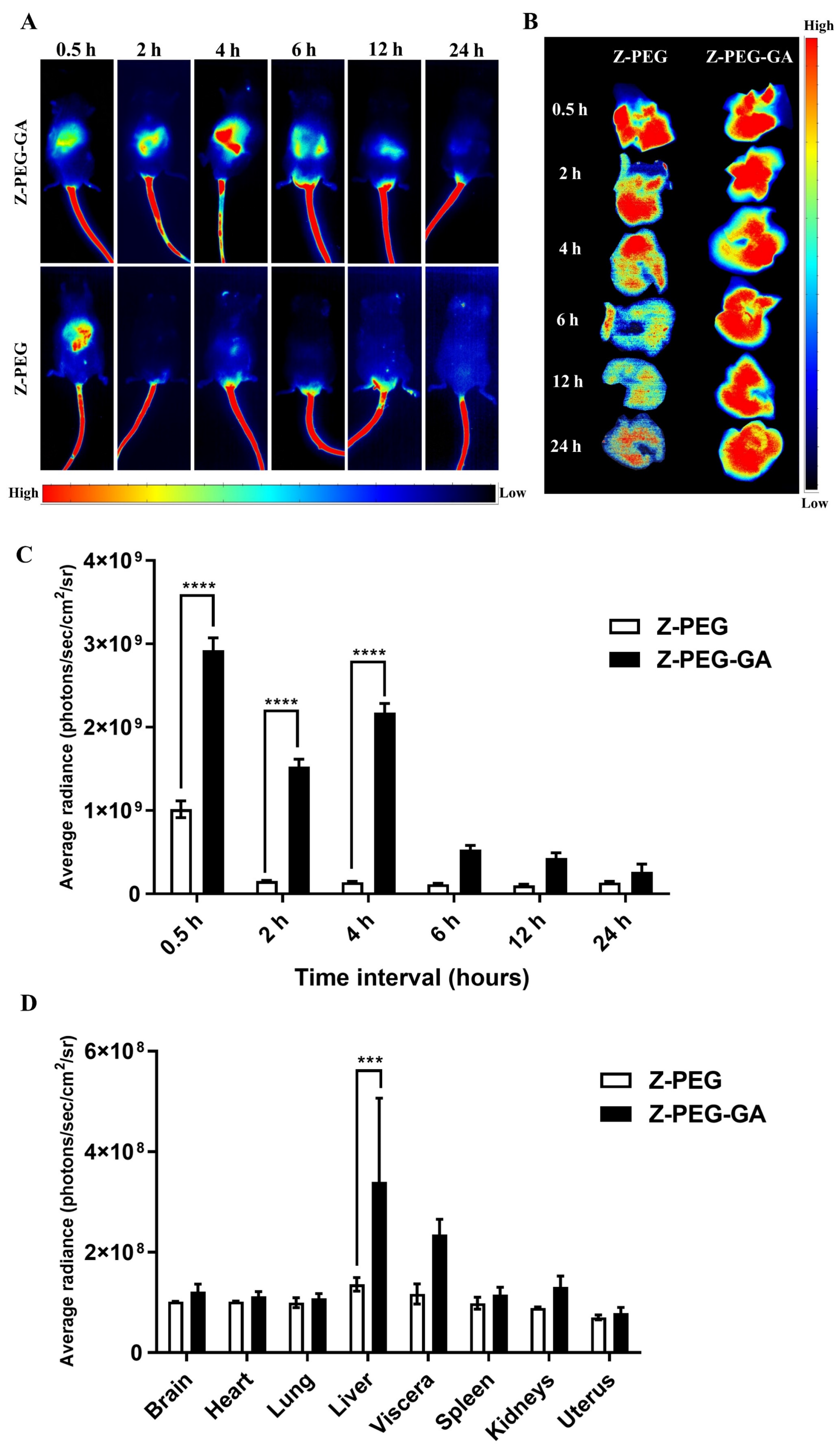
3.6. In Vivo Biodistribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef]
- Agnihotri, A.; Loren, D.E. Intraductal Therapies for Cholangiocarcinoma. Tech. Innov. Gastrointest. Endosc. 2022, 24, 200–210. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Shen, Y.; Tang, L.; Hu, Z.; Lin, G.; Law, W.-C.; Ma, M.; Dong, B.; Yong, K.-T.; et al. A biocompatible photosensitizer with a high intersystem crossing efficiency for precise two-photon photodynamic therapy. Mater. Horiz. 2022, 9, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Agel, M.R.; Baghdan, E.; Pinnapireddy, S.R.; Lehmann, J.; Schäfer, J.; Bakowsky, U. Curcumin loaded nanoparticles as efficient photoactive formulations against gram-positive and gram-negative bacteria. Colloids Surf. B Biointerfaces 2019, 178, 460–468. [Google Scholar] [CrossRef]
- Spring, B.Q.; Rizvi, I.; Xu, N.; Hasan, T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci. 2015, 14, 1476–1491. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations. Eur. Urol. Focus 2018, 4, 509–511. [Google Scholar] [CrossRef]
- Moore, C.M.; Pendse, D.; Emberton, M. Photodynamic therapy for prostate cancer—A review of current status and future promise. Nat. Rev. Urol. 2009, 6, 18–30. [Google Scholar] [CrossRef]
- Awad, M.; Thomas, N.; Barnes, T.J.; Prestidge, C.A. Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy. J. Control. Release 2022, 346, 300–316. [Google Scholar] [CrossRef]
- Edwards, H.G.; Newton, E.M.; Wynn-Williams, D.D.; Coombes, S.R. Molecular spectroscopic studies of lichen substances 1: Parietin and emodin. J. Mol. Struct. 2003, 648, 49–59. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Gutberlet, B.; Preis, E.; Abdelsalam, A.M.; Abu Dayyih, A.; Abdelkader, A.; Balash, A.; Schäfer, J.; Bakowsky, U. Parietin Cyclodextrin-Inclusion Complex as an Effective Formulation for Bacterial Photoinactivation. Pharmaceutics 2022, 14, 357. [Google Scholar] [CrossRef] [PubMed]
- Soomro, N.A.; Wu, Q.; Amur, S.A.; Liang, H.; Ur Rahman, A.; Yuan, Q.; Wei, Y. Natural drug physcion encapsulated zeolitic imidazolate framework, and their application as antimicrobial agent. Colloids Surf. B Biointerfaces 2019, 182, 110364. [Google Scholar] [CrossRef]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Zhang, C.; van Ta, Q.; Vo, T.-S.; Li, Y.-X.; Kim, S.-K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol. Res. 2014, 169, 255–261. [Google Scholar] [CrossRef]
- Mugas, M.L.; Calvo, G.; Marioni, J.; Céspedes, M.; Martinez, F.; Vanzulli, S.; Sáenz, D.; Di Venosa, G.; Nuñez Montoya, S.; Casas, A. Photosensitization of a subcutaneous tumour by the natural anthraquinone parietin and blue light. Sci. Rep. 2021, 11, 23820. [Google Scholar] [CrossRef]
- Kim, J.; Jo, Y.; Na, K. Photodynamic therapy with smart nanomedicine. Arch. Pharm. Res. 2020, 43, 22–31. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Amin, M.U.; Ambreen, G.; Dayyih, A.A.; Abdelsalam, A.M.; Somaida, A.; Engelhardt, K.; Wojcik, M.; Schäfer, J.; Bakowsky, U. Photodynamic and antiangiogenic activities of parietin liposomes in triple negative breast cancer. Biomater. Adv. 2022, 134, 112543. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Tariq, I.; Sohail, M.F.; Ali, M.Y.; Preis, E.; Ambreen, G.; Pinnapireddy, S.R.; Jedelská, J.; Schäfer, J.; et al. Lipoparticles for Synergistic Chemo-Photodynamic Therapy to Ovarian Carcinoma Cells: In vitro and in vivo Assessments. Int. J. Nanomed. 2021, 16, 951–976. [Google Scholar] [CrossRef]
- Raschpichler, M.; Preis, E.; Pinnapireddy, S.R.; Baghdan, E.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, A.M.; Somaida, A.; Ayoub, A.M.; Alsharif, F.M.; Preis, E.; Wojcik, M.; Bakowsky, U. Surface-Tailored Zein Nanoparticles: Strategies and Applications. Pharmaceutics 2021, 13, 1354. [Google Scholar] [CrossRef]
- Elzoghby, A.; Freag, M.; Mamdouh, H.; Elkhodairy, K. Zein-based Nanocarriers as Potential Natural Alternatives for Drug and Gene Delivery: Focus on Cancer Therapy. Curr. Pharm. Des. 2018, 23, 5261–5271. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, X.; Chang, Y.; Li, D.; Sun, X.; Liu, X. Zein-derived peptides as nanocarriers to increase the water solubility and stability of lutein. Food Funct. 2018, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, A.M.; Somaida, A.; Ambreen, G.; Ayoub, A.M.; Tariq, I.; Engelhardt, K.; Garidel, P.; Fawaz, I.; Amin, M.U.; Wojcik, M.; et al. Surface tailored zein as a novel delivery system for hypericin: Application in photodynamic therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112420. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Lee, M.-K.; Lim, S.-J. Enhanced Stability of Indocyanine Green by Encapsulation in Zein-Phosphatidylcholine Hybrid Nanoparticles for Use in the Phototherapy of Cancer. Pharmaceutics 2021, 13, 305. [Google Scholar] [CrossRef]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16, 248. [Google Scholar] [CrossRef]
- Yahanda, A.T.; Dunn, G.P.; Chicoine, M.R. Photosensitivity Reaction From Operating Room Lights After Oral Administration of 5-Aminolevulinic Acid for Fluorescence-Guided Resection of a Malignant Glioma. Cureus 2021, 13, e13442. [Google Scholar] [CrossRef]
- Hamblin, M.R. Drug efflux pumps in photodynamic therapy. In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–276. ISBN 9780128164341. [Google Scholar]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef]
- El-Lakany, S.A.; Elgindy, N.A.; Helmy, M.W.; Abu-Serie, M.M.; Elzoghby, A.O. Lactoferrin-decorated vs PEGylated zein nanospheres for combined aromatase inhibitor and herbal therapy of breast cancer. Expert Opin. Drug Deliv. 2018, 15, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Ou, W.; Gautam, M.; Poudel, K.; Kim, B.K.; Le Pham, M.; Phung, C.D.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; et al. Development of Folate-Functionalized PEGylated Zein Nanoparticles for Ligand-Directed Delivery of Paclitaxel. Pharmaceutics 2019, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Irie, A.; Nagata, N.; Ichikawa, A. Specific binding of glycyrrhetinic acid to the rat liver membrane. Biochim. Biophys. Acta Biomembr. 1991, 1066, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, X.; Jiang, B.; Yan, J.; Zhang, Z.; Qin, J.; Yu, W.; Gao, Z. Glycyrrhetinic Acid Functionalized Nanoparticles for Drug Delivery to Liver Cancer. J. Biomed. Nanotechnol. 2018, 14, 1837–1852. [Google Scholar] [CrossRef] [PubMed]
- Yan, L. Preparation and characterization of dual pH-sensitive polymer-doxorubicin conjugate micelles. J. Chin. Pharm. Sci. 2018, 27, 530–539. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zheng, Y.; Geng, Y.; Han, C.; Shi, Y.; Sun, H.; Zhang, C.; Chen, Y.; Zhang, L.; et al. Glycyrrhetinic Acid Functionalized Graphene Oxide for Mitochondria Targeting and Cancer Treatment In Vivo. Small 2018, 14, 1703306. [Google Scholar] [CrossRef]
- Yang, T.; Lan, Y.; Cao, M.; Ma, X.; Cao, A.; Sun, Y.; Yang, J.; Li, L.; Liu, Y. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surf. B Biointerfaces 2019, 175, 106–115. [Google Scholar] [CrossRef]
- Zhu, X.; Tsend-Ayush, A.; Yuan, Z.; Wen, J.; Cai, J.; Luo, S.; Yao, J.; Bian, J.; Yin, L.; Zhou, J.; et al. Glycyrrhetinic acid-modified TPGS polymeric micelles for hepatocellular carcinoma-targeted therapy. Int. J. Pharm. 2017, 529, 451–464. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Snizhko, D.; Du, F.; Ma, X.; Lou, B.; Li, J.; Xu, G. Development of luminol-fluorescamine-PVP chemiluminescence system and its application to sensitive tyrosinase determination. Talanta 2020, 218, 121177. [Google Scholar] [CrossRef]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of Novel Biodegradable Methoxy Poly(ethylene glycol)–Zein Micelles for Effective Delivery of Curcumin. Mol. Pharmaceutics 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Zang, L.; Zhao, H.; Ji, X.; Cao, W.; Zhang, Z.; Meng, P. Photophysical properties, singlet oxygen generation efficiency and cytotoxic effects of aloe emodin as a blue light photosensitizer for photodynamic therapy in dermatological treatment. Photochem. Photobiol. Sci. 2017, 16, 1088–1094. [Google Scholar] [CrossRef]
- Rabello, B.R.; Gerola, A.P.; Pellosi, D.S.; Tessaro, A.L.; Aparício, J.L.; Caetano, W.; Hioka, N. Singlet oxygen dosimetry using uric acid as a chemical probe: Systematic evaluation. J. Photochem. Photobiol. A Chem. 2012, 238, 53–62. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 2020, 204, 111787. [Google Scholar] [CrossRef]
- Awad, M.; Barnes, T.J.; Joyce, P.; Thomas, N.; Prestidge, C.A. Liquid crystalline lipid nanoparticle promotes the photodynamic activity of gallium protoporphyrin against S. aureus biofilms. J. Photochem. Photobiol. B 2022, 232, 112474. [Google Scholar] [CrossRef]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Cha, R.; Du, T.; Li, J.; Yuan, Z. Synthesis and characterization of polypeptide containing liver-targeting group. Polym. Int. 2006, 55, 1057–1062. [Google Scholar] [CrossRef]
- Reboredo, C.; González-Navarro, C.J.; Martínez-Oharriz, C.; Martínez-López, A.L.; Irache, J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int. J. Pharm. 2021, 597, 120287. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Islam, M.S.; Podaralla, S.; Kaushik, R.S.; Reineke, J.; Woyengo, T.; Perumal, O. Food Protein Based Core–Shell Nanocarriers for Oral Drug Delivery: Effect of Shell Composition on in Vitro and in Vivo Functional Performance of Zein Nanocarriers. Mol. Pharm. 2017, 14, 757–769. [Google Scholar] [CrossRef]
- Lima, A.M.; Pizzol, C.D.; Monteiro, F.B.; Creczynski-Pasa, T.B.; Andrade, G.P.; Ribeiro, A.O.; Perussi, J.R. Hypericin encapsulated in solid lipid nanoparticles: Phototoxicity and photodynamic efficiency. J. Photochem. Photobiol. B 2013, 125, 146–154. [Google Scholar] [CrossRef]
- Dayyih, A.A.; Gutberlet, B.; Preis, E.; Engelhardt, K.H.; Amin, M.U.; Abdelsalam, A.M.; Bonsu, M.; Bakowsky, U. Thermoresponsive Liposomes for Photo-Triggered Release of Hypericin Cyclodextrin Inclusion Complex for Efficient Antimicrobial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 31525–31540. [Google Scholar] [CrossRef]
- Comini, L.R.; Morán Vieyra, F.E.; Mignone, R.A.; Páez, P.L.; Laura Mugas, M.; Konigheim, B.S.; Cabrera, J.L.; Núñez Montoya, S.C.; Borsarelli, C.D. Parietin: An efficient photo-screening pigment in vivo with good photosensitizing and photodynamic antibacterial effects in vitro. Photochem. Photobiol. Sci. 2017, 16, 201–210. [Google Scholar] [CrossRef]
- Nuhn, L.; Gietzen, S.; Mohr, K.; Fischer, K.; Toh, K.; Miyata, K.; Matsumoto, Y.; Kataoka, K.; Schmidt, M.; Zentel, R. Aggregation Behavior of Cationic Nanohydrogel Particles in Human Blood Serum. Biomacromolecules 2014, 15, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.; Valizadeh, H.; Panahi, Y.; Fatahi, Y.; Chen, M.; Zarebkohan, A.; Gao, H. The impact of protein corona on the biological behavior of targeting nanomedicines. Int. J. Pharm. 2022, 614, 121458. [Google Scholar] [CrossRef] [PubMed]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of Nanoparticle Aggregation in Human Blood Serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef]
- Gräfe, C.; Weidner, A.; vd Lühe, M.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle–cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Meewan, J.; Somani, S.; Laskar, P.; Irving, C.; Mullin, M.; Woods, S.; Roberts, C.W.; Alzahrani, A.R.; Ferro, V.A.; McGill, S.; et al. Limited Impact of the Protein Corona on the Cellular Uptake of PEGylated Zein Micelles by Melanoma Cancer Cells. Pharmaceutics 2022, 14, 439. [Google Scholar] [CrossRef]
- Hasan, S.K.; Siddiqi, A.; Nafees, S.; Ali, N.; Rashid, S.; Ali, R.; Shahid, A.; Sultana, S. Chemopreventive effect of 18β-glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (HepG2). Mol. Cell. Biochem. 2016, 416, 169–177. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; He, Y.; Fang, X.; Wang, Y.; Chen, M. Glycyrrhetinic acid-decorated and reduction-sensitive micelles to enhance the bioavailability and anti-hepatocellular carcinoma efficacy of tanshinone IIA. Biomater. Sci. 2016, 4, 167–182. [Google Scholar] [CrossRef]
- Varol, M. Parietin as an efficient and promising anti-angiogenic and apoptotic small-molecule from Xanthoria parietina. Rev. Bras. Farmacogn. 2019, 29, 728–734. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural photosensitizers in photodynamic therapy: In vitro activity against monolayers and spheroids of human colorectal adenocarcinoma SW480 cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Du, J.; Jiang, F.; Xu, S.-S.; Huang, Z.-F.; Chen, L.-L.; Li, L. Tephrosin induces apoptosis of human pancreatic cancer cells through the generation of reactive oxygen species. J. Cancer 2021, 12, 270–280. [Google Scholar] [CrossRef]
- Chen, T.-T.; Tian, X.; Liu, C.-L.; Ge, J.; Chu, X.; Li, Y. Fluorescence Activation Imaging of Cytochrome c Released from Mitochondria Using Aptameric Nanosensor. J. Am. Chem. Soc. 2015, 137, 982–989. [Google Scholar] [CrossRef]
- Jin, L.; Dai, L.; Ji, M.; Wang, H. Mitochondria-targeted triphenylphosphonium conjugated glycyrrhetinic acid derivatives as potent anticancer drugs. Bioorg. Chem. 2019, 85, 179–190. [Google Scholar] [CrossRef]
- Singh, H.; Kim, S.J.; Kang, D.H.; Kim, H.-R.; Sharma, A.; Kim, W.Y.; Kang, C.; Kim, J.S. Glycyrrhetinic acid as a hepatocyte targeting unit for an anticancer drug delivery system with enhanced cell type selectivity. Chem. Commun. 2018, 54, 12353–12356. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dai, C.; Zheng, Y.; Shi, S.; Hu, H.; Chen, D. Binding effect of fluorescence labeled glycyrrhetinic acid with GA receptors in hepatocellular carcinoma cells. Life Sci. 2017, 188, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xue, H.; Li, X.; Diao, W.; Jiang, B.; Wang, W.; Yu, W.; Bai, J.; Wang, Y.; Lian, B.; et al. Enhanced targeted delivery of adenine to hepatocellular carcinoma using glycyrrhetinic acid-functionalized nanoparticles in vivo and in vitro. Biomed. Pharmacother. 2020, 131, 110682. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Pan, R.; Zhang, B.; Qu, M.; Lian, B.; Jiang, H.; Gao, Z.; Wu, J. Liver-Targeted Combination Therapy Basing on Glycyrrhizic Acid-Modified DSPE-PEG-PEI Nanoparticles for Co-delivery of Doxorubicin and Bcl-2 siRNA. Front. Pharmacol. 2019, 10, 4. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Yan, S.-Z.; Tao, X.; Yang, Q.; Li, Q.; Wang, T.-S.; Yu, S.-Q.; Chen, S.-L. Evaluation of hypocrellin A-loaded lipase sensitive polymer micelles for intervening methicillin-resistant Staphylococcus Aureus antibiotic-resistant bacterial infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110230. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Otvagin, V.F.; Kuzmina, N.S.; Kudriashova, E.S.; Nyuchev, A.V.; Gavryushin, A.E.; Fedorov, A.Y. Conjugates of Porphyrinoid-Based Photosensitizers with Cytotoxic Drugs: Current Progress and Future Directions toward Selective Photodynamic Therapy. J. Med. Chem. 2022, 65, 1695–1734. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; Díaz-Quintana, A.; González-Arzola, K.; Velázquez-Campoy, A.; Orzáez, M.; López-Rivas, A.; Gil-Caballero, S.; de La Rosa, M.Á.; Díaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef] [PubMed]





| Formulations | Diameter ± SD (nm) | PDI ± SD | ζ-Potential ± SD (mV) | EE ± SD (%) | DL (%) | |
|---|---|---|---|---|---|---|
| Z-PEG | blank | 82.8 ± 3.7 | 0.19 ± 0.02 | −10.7 ± 3.9 | ||
| parietin | 109.7 ± 16.9 | 0.24 ± 0.04 | −8.3 ± 4.2 | 70.3 ± 11.0 | 1.11 ± 0.29 | |
| Z-PEG-GA | blank | 94.7 ± 6.6 | 0.25 ± 0.04 | 7.1 ± 1.4 | ||
| parietin | 111.5 ± 6.3 | 0.19 ± 0.05 | −8.1 ± 0.5 | 86.1 ± 7.1 | 1.29 ± 0.24 | |
| Formulation | Parameters | Zero-Order | First Order | Higuchi Diffusion |
|---|---|---|---|---|
| % Released vs. Time | Log % Released vs. Time | (% Released vs. Time ½) | ||
| Z-PEG | Slope | 0.58291 ± 0.17881 | 0.0071 ± 0.00237 | 4.31347 ± 0.97279 |
| (r2) | 0.63916 | 0.59987 | 0.76618 | |
| (k) | 0.63916 | 0.01634 | 4.31347 | |
| Z-PEG-GA | Slope | 0.4767 ± 0.13568 | 0.00528 ± 0.00159 | 3.50077 ± 0.72689 |
| (r2) | 0.67293 | 0.64583 | 0.79448 | |
| (k) | 0.4767 | 0.01215 | 3.50077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, A.M.; Balash, A.; Khedr, S.M.; Amin, M.U.; Engelhardt, K.H.; Preis, E.; Bakowsky, U. Improved Photodynamic Therapy of Hepatocellular Carcinoma via Surface-Modified Protein Nanoparticles. Pharmaceutics 2025, 17, 370. https://doi.org/10.3390/pharmaceutics17030370
Abdelsalam AM, Balash A, Khedr SM, Amin MU, Engelhardt KH, Preis E, Bakowsky U. Improved Photodynamic Therapy of Hepatocellular Carcinoma via Surface-Modified Protein Nanoparticles. Pharmaceutics. 2025; 17(3):370. https://doi.org/10.3390/pharmaceutics17030370
Chicago/Turabian StyleAbdelsalam, Ahmed M., Amir Balash, Shaimaa M. Khedr, Muhammad Umair Amin, Konrad H. Engelhardt, Eduard Preis, and Udo Bakowsky. 2025. "Improved Photodynamic Therapy of Hepatocellular Carcinoma via Surface-Modified Protein Nanoparticles" Pharmaceutics 17, no. 3: 370. https://doi.org/10.3390/pharmaceutics17030370
APA StyleAbdelsalam, A. M., Balash, A., Khedr, S. M., Amin, M. U., Engelhardt, K. H., Preis, E., & Bakowsky, U. (2025). Improved Photodynamic Therapy of Hepatocellular Carcinoma via Surface-Modified Protein Nanoparticles. Pharmaceutics, 17(3), 370. https://doi.org/10.3390/pharmaceutics17030370








