Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of NLCs
Pre-Formulation Studies
2.3. Optimization of NLCs
2.4. Functionalization of NLCs
2.5. Characterization of NLCs
2.5.1. Particle Size Analysis
2.5.2. Zeta Potential Analysis
2.5.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.4. Morphological Analysis of NLCs
2.6. Quantification and Encapsulation Efficiency (EE%)
2.7. Stability Analysis
2.8. Protein Corona Evaluation
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
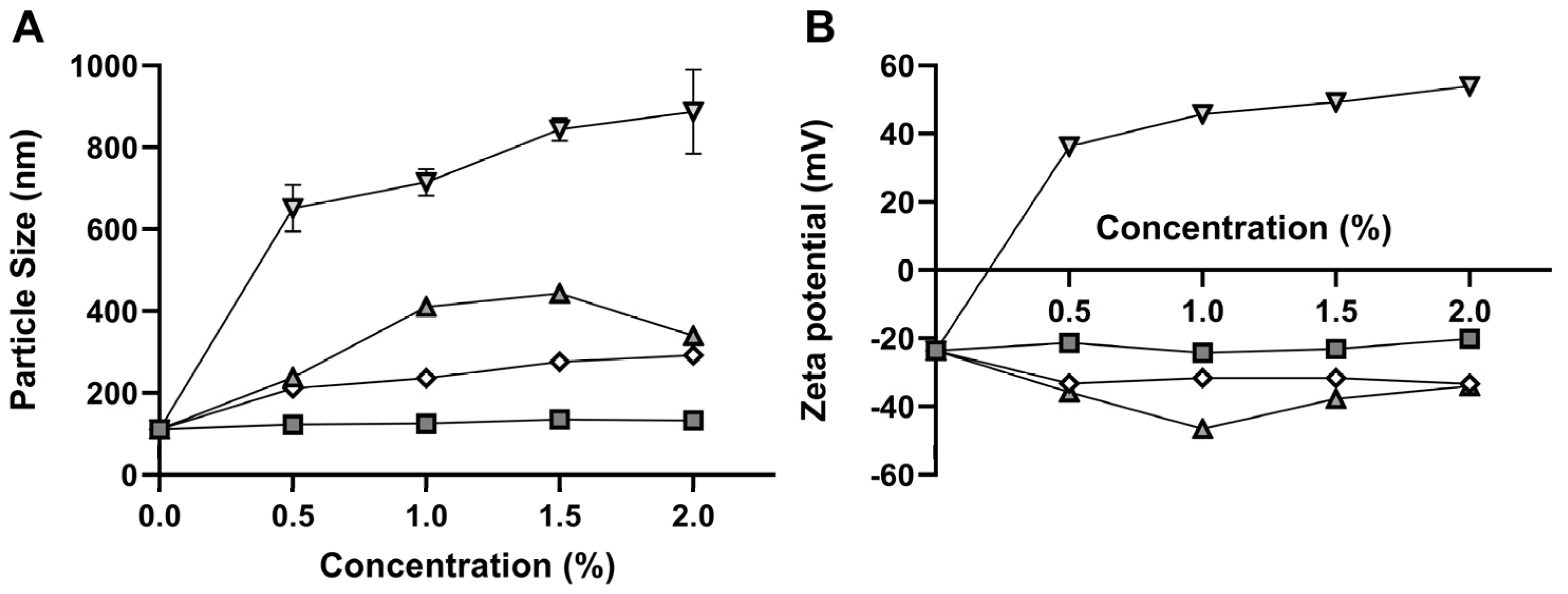
3.1. Pre-Formulation Studies
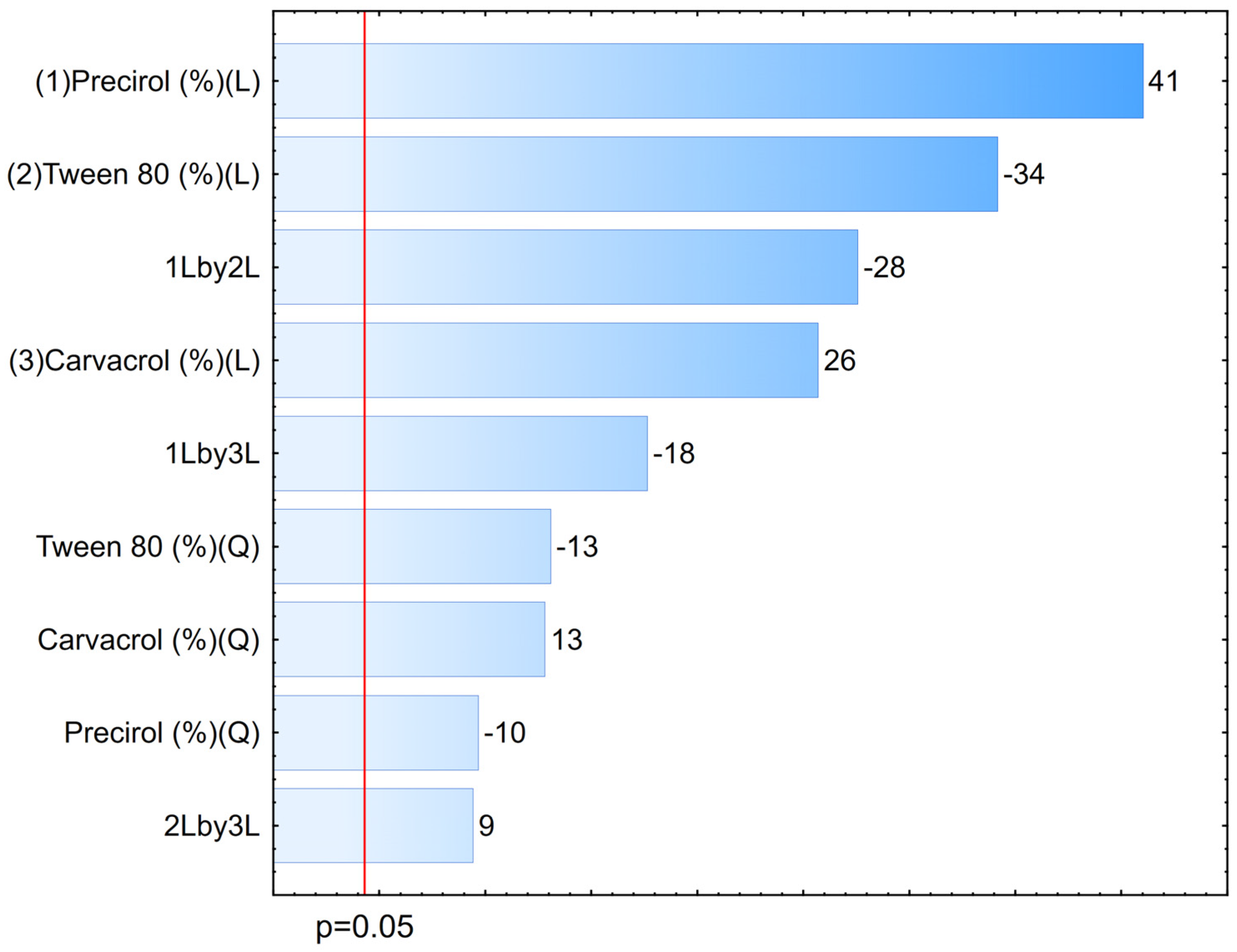
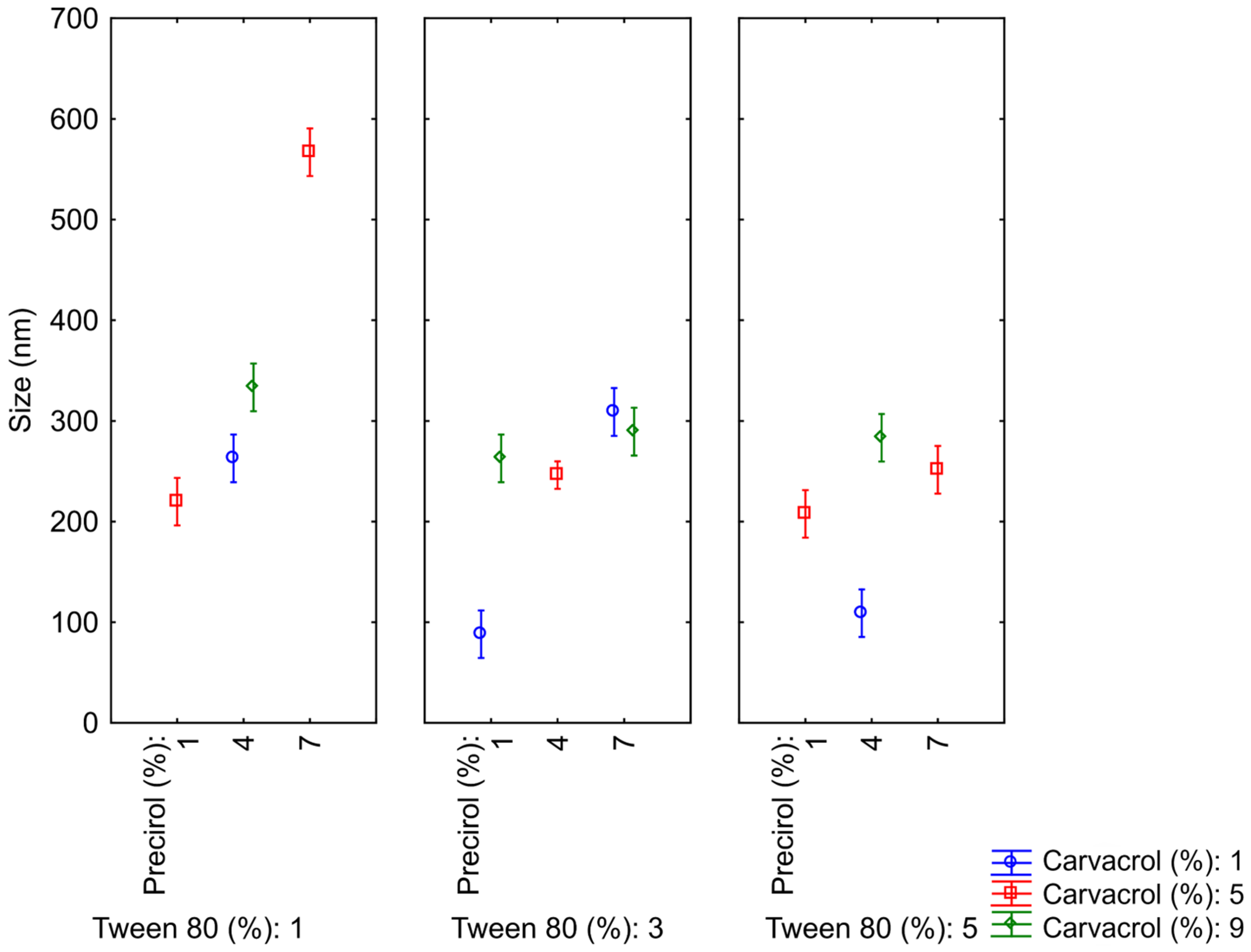
3.2. Optimization of NLCs
3.3. Functionalization of NLCs
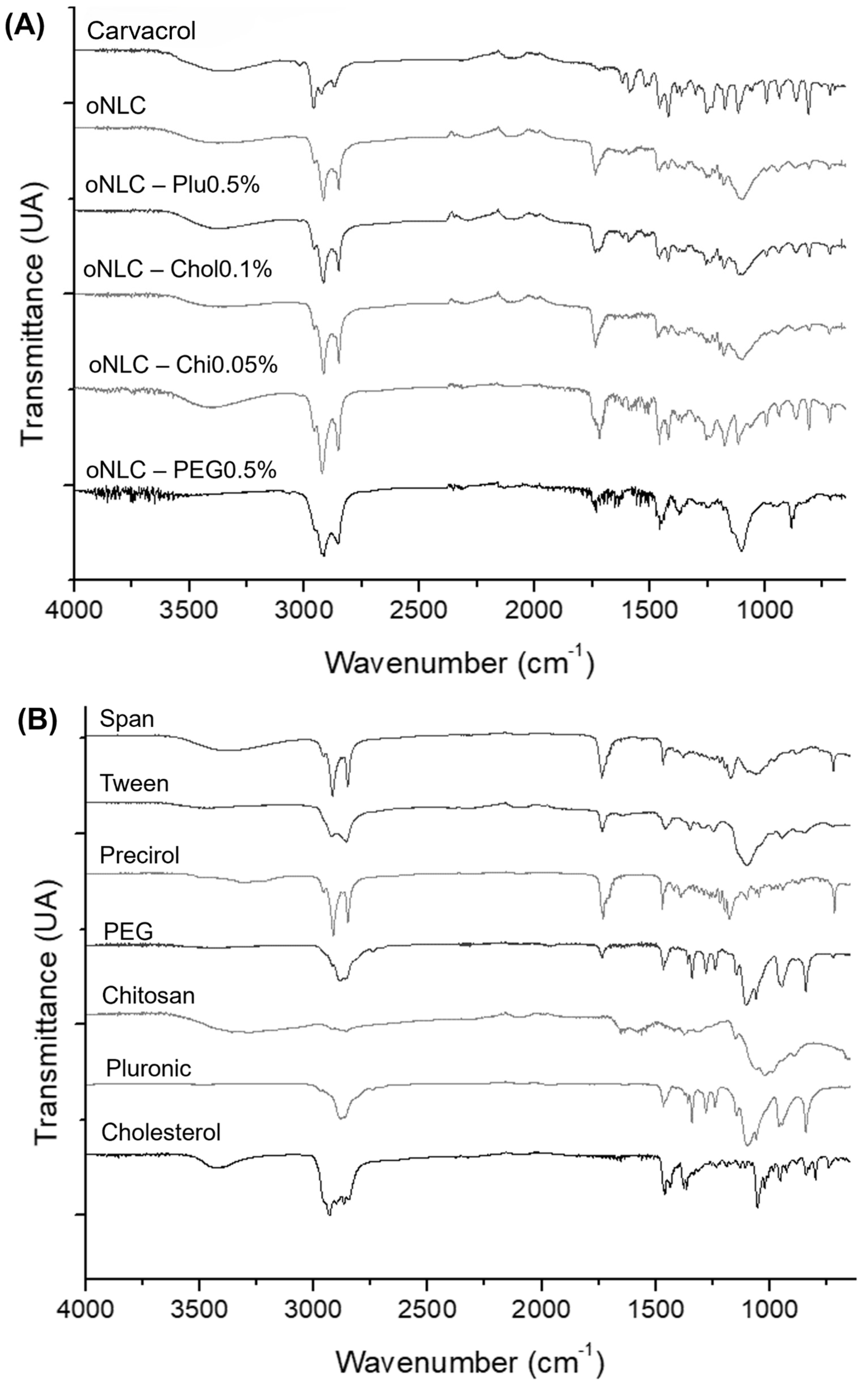
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
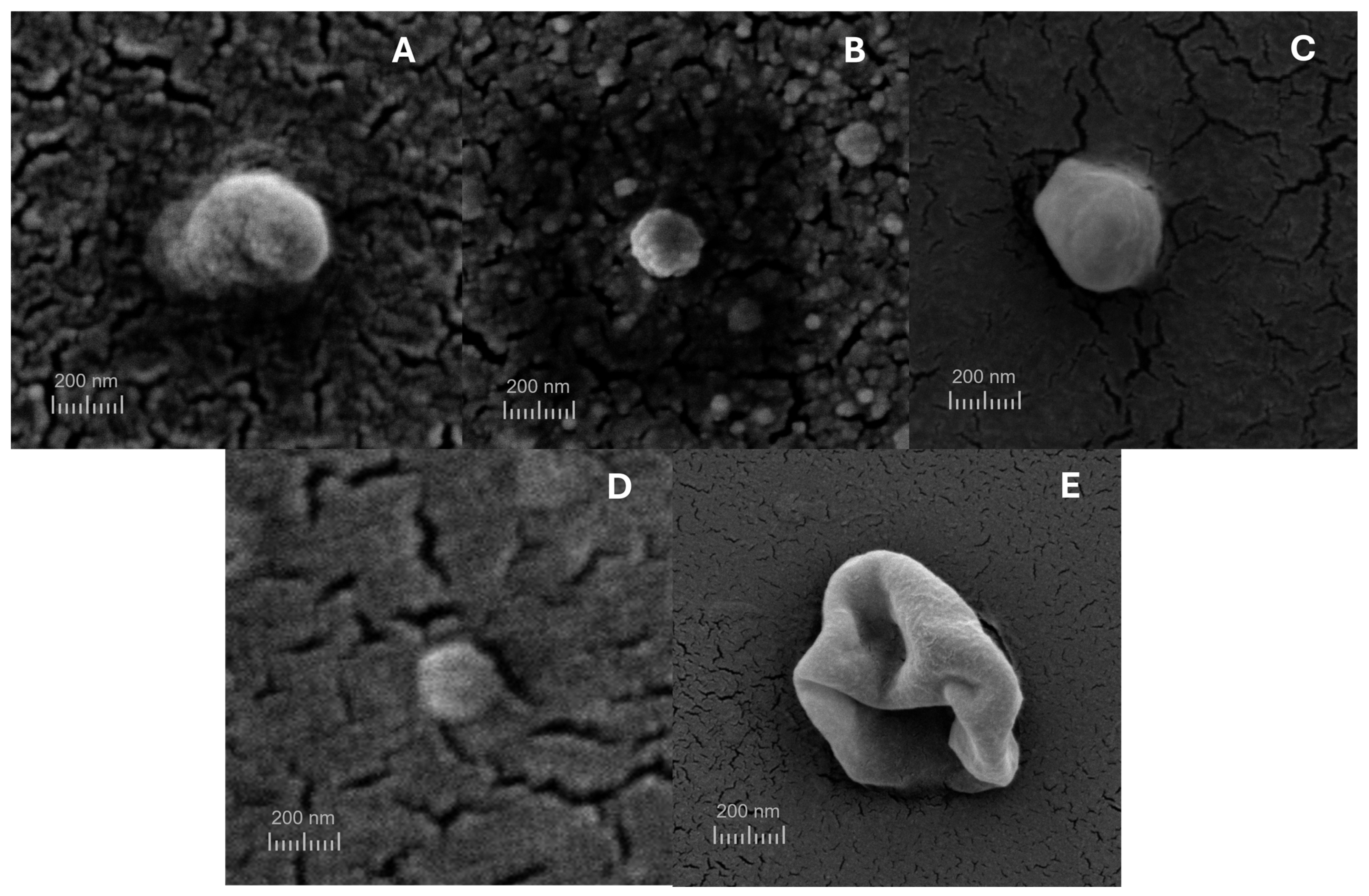
3.5. Scanning Electron Microscopy (SEM)
3.6. Quantification and Encapsulation Efficiency (EE%)
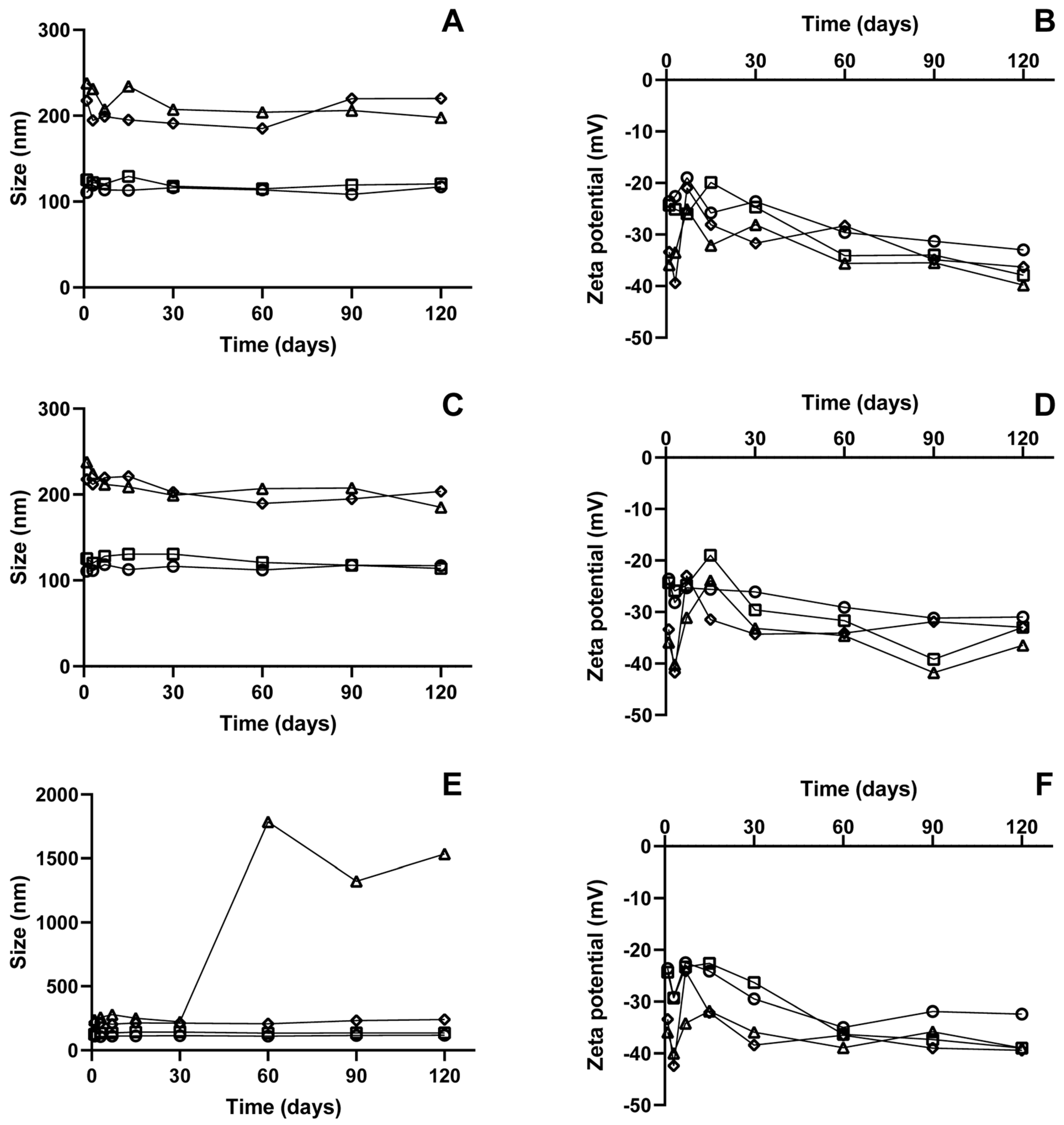
3.7. Stability Analysis
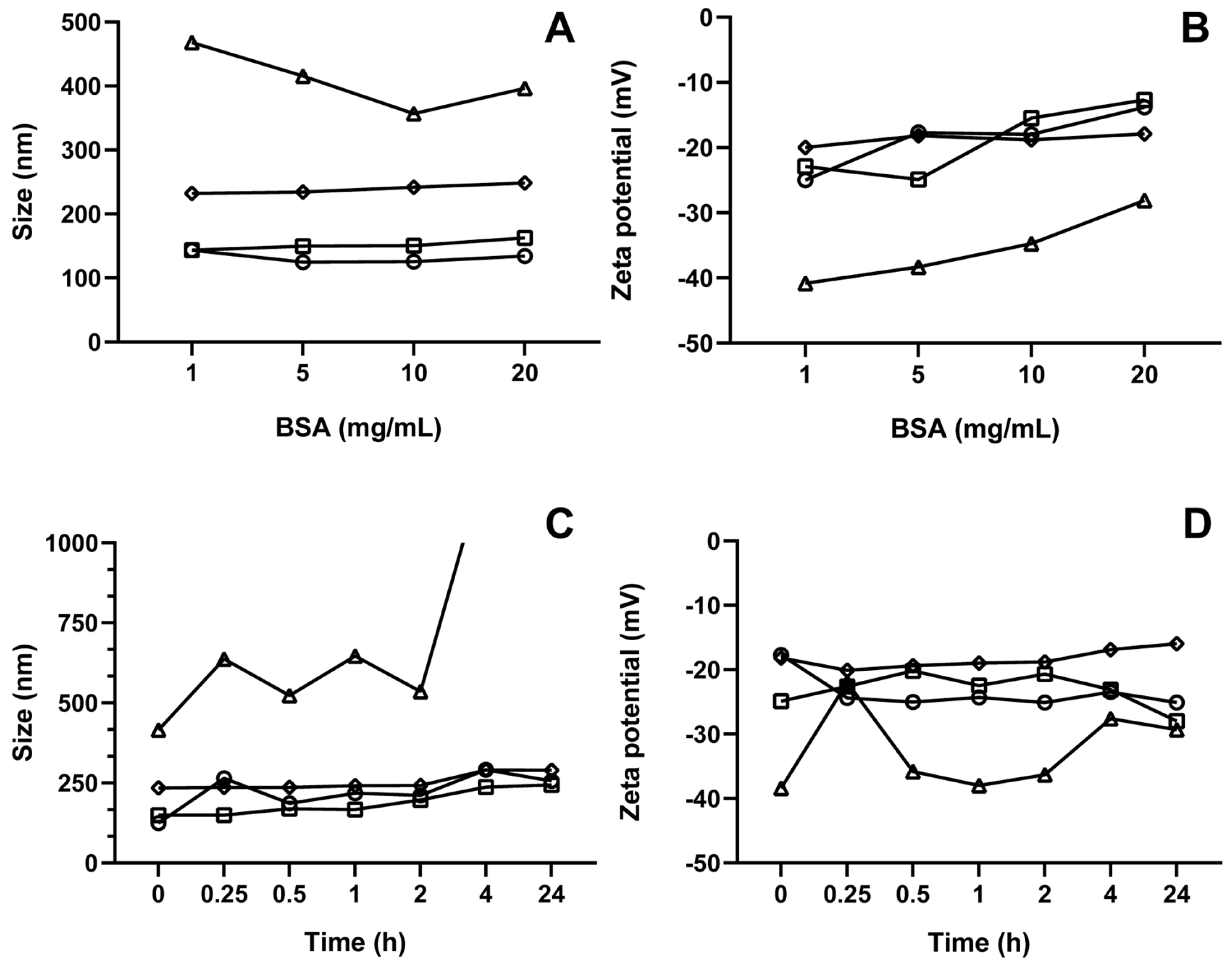
3.8. Corona Formation Evaluation
3.9. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NLC | Nanostructured lipid carrier |
| BSA | Bovine serum albumin |
| DLS | Dynamic light scattering |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| EE% | Encapsulation efficiency |
| FTIR | Fourier-transform infrared microscopy |
| HES | Hot emulsification followed by sonication |
| PBS | Phosphate-buffered saline |
| PdI | Polydispersity index |
| PEG | Polyethylene glycol |
| SDS | Sodium dodecyl sulfate |
| MCT | Medium-chain triglyceride |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the definition of cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Wani, S.U.D.; Gangadharappa, H.V.; Aldawsari, M.F.; Khafagy, E.S.; Lila, A.S.A. Enhanced cytotoxic activity of docetaxel-loaded silk fibroin nanoparticles against breast cancer cells. Polymers 2021, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Berko, Y.A.; Funmilola, A.F.; Akala, E.O. Fabrication of paclitaxel and 17aag-loaded poly-ε-caprolactone nanoparticles for breast cancer treatment. J. Pharm. Drug Deliv. Res. 2021, 10, 196. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.; Ullah, O.; Balant, S.; Rahman, H. Nanomedicine-based delivery strategies for breast cancer treatment and management. Int. J. Mol. Sci. 2022, 23, 2856. [Google Scholar] [CrossRef]
- Castillo-Tobías, I.; Berlanga, L.; Poblano, J.; Rodríguez-Salazar, M.D.C.; Aguayo-Morales, H.; Cobos-Puc, L.E. Fundamental considerations of targeted drug therapies for breast cancer. Future Pharmacol. 2023, 3, 686–707. [Google Scholar] [CrossRef]
- Prayoga, D.K.; Aulifa, D.L.; Budiman, A.; Levita, J. Plants with anti-ulcer activity and mechanism: A review of preclinical and clinical studies. Drug Des. Devel Ther. 2024, 18, 193–213. [Google Scholar] [CrossRef]
- Capatina, L.; Napoli, E.M.; Ruberto, G.; Hritcu, L. Origanum vulgare ssp. Hirtum (Lamiaceae) essential oil prevents behavioral and oxidative stress changes in the scopolamine zebrafish model. Molecules 2021, 26, 7085. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.S.; Guimarães, A.G. Antitumor effects of carvacrol and thymol: A systematic review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Parseghyan, L.; Sevoyan, G.; Darbinyan, A.; Sahakyan, N.; Gaboyan, M.; Karabekian, Z.; Voskanyan, A. Antinociceptive, anti-inflammatory, and cytotoxic properties of Origanum vulgare essential oil, rich with β-caryophyllene and β-caryophyllene oxide. Korean J. Pain. 2022, 35, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, B.B.; Malekzadeh, R.; Azar, F.P.; Salehnia, F.; Naseri, A.R.; Ghorbani, M.; Hamishehkar, H.; Farajollahi, A.R. Main approaches to enhance radiosensitization in cancer cells by nanoparticles: A systematic review. Adv. Pharm. Bull. 2020, 13, 1. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In vivo efficacy and toxicity of curcumin nanoparticles in breast cancer treatment: A systematic review. Front. Oncol. 2021, 11, 612903. [Google Scholar] [CrossRef]
- Hamimed, S.; Jabberi, M.; Chatti, A. Nanotechnology in drug and gene delivery. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 769–787. [Google Scholar] [CrossRef]
- Gomaa, E.; Fathi, H.A.; Eissa, N.G.; Elsabahy, M. Methods for preparation of nanostructured lipid carriers. Methods 2022, 199, 3–8. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Sultan, M.H.; Albarraq, A.A.; Ahmed, R.A.; Alhazmi, H.A.; Alam, M.I. Preparation, Characterization, and Anti-Cancer Activity of Nanostructured Lipid Carriers Containing Imatinib. Pharmaceutics 2021, 13, 1086. [Google Scholar] [CrossRef]
- Uchôa, A.F.C.; Formiga, A.L.D.; Alves, Á.E.F.; Cardoso, A.L.M.R.; Pereira, G.M.d.A.; Carvalho, L.M.M.; da Silva, L.F.A.; Pereira, P.S.d.S.; de Souza, P.H.O.; Jales, S.T.L.; et al. Optimization and Functionalization of Copaiba Oil-Loaded Nanostructured Lipid Carriers to Improve Cytotoxicity against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2025, 105, 106575. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; D’Ambrosio, M.; Luceri, C.; Cirri, M. Evaluation and comparison of solid lipid nanoparticles (Slns) and nanostructured lipid carriers (Nlcs) as vectors to develop hydrochlorothiazide effective and safe pediatric oral liquid formulations. Pharmaceutics 2021, 13, 437. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Tazehjani, D.A.J.; Farahpour, M.R.; Hamishehkar, H. Effectiveness of topical caraway essential oil loaded into nanostructured lipid carrier as a promising platform for the treatment of infected wounds. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125748. [Google Scholar] [CrossRef]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanopart. Res. 2020, 22, 141. [Google Scholar] [CrossRef]
- Gavini, E.; Rassu, G.; Sanna, V.; Cossu, M.; Giunchedi, P. Mucoadhesive microspheres for nasal administration of an antiemetic drug, metoclopramide: In-vitro/ex-vivo studies. J. Pharm. Pharmacol. 2010, 57, 287–294. [Google Scholar] [CrossRef]
- Trivino, A.; Gumireddy, A.; Chauhan, H. Drug-lipid-surfactant miscibility for the development of solid lipid nanoparticles. AAPS PharmSciTech 2019, 20, 46. [Google Scholar] [CrossRef]
- da Silva, G.H.R.; de Moura, L.D.; de Carvalho, F.V.; Geronimo, G.; Mendonça, T.C.; de Lima, F.F.; de Paula, E. Antineoplastics encapsulated in nanostructured lipid carriers. Molecules 2021, 26, 6929. [Google Scholar] [CrossRef]
- Vinsova, J.; Vavrikova, E. Recent advances in drugs and prodrugs design of chitosan. Curr. Pharm. Des. 2008, 14, 1311–1326. [Google Scholar] [CrossRef]
- Valencia, M.S.; Júnior, M.F.d.S.; Júnior, F.H.X.; Veras, B.d.O.; Borba, E.F.d.O.; da Silva, T.G.; Xavier, V.L.; de Souza, M.P.; Carneiro-Da-Cunha, M.d.G. Bioactivity and cytotoxicity of quercetin-loaded, lecithin-chitosan nanoparticles. Biocatal. Agric. Biotechnol. 2021, 31, 101879. [Google Scholar] [CrossRef]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Pyo, Y.C.; Tran, P.; Kim, D.H.; Park, J.S. Chitosan-coated nanostructured lipid carriers of fenofibrate with enhanced oral bioavailability and efficacy. Colloids Surf. B Biointerfaces 2020, 196, 111331. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Selianitis, D.; Perinelli, D.R.; Bonacucina, G.; Pippa, N.; Gazouli, M.; Pispas, S. Non-ionic surfactant effects on innate pluronic 188 behavior: Interactions, and physicochemical and biocompatibility studies. Int. J. Mol. Sci. 2022, 23, 13814. [Google Scholar] [CrossRef] [PubMed]
- Sek, L.; Boyd, B.J.; Charman, W.N.; Porter, C.J.H. Examination of the impact of a range of Pluronic surfactants on the in-vitro solubilisation behaviour and oral bioavailability of lipidic formulations of atovaquone. J. Pharm. Pharmacol. 2006, 58, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Q.; Chen, J.; Liu, L.; Ding, L.; Shen, M.; Li, J.; Han, B.; Duan, Y. Temperature-sensitive gold nanoparticle-coated pluronic-pll nanoparticles for drug delivery and chemo-photothermal therapy. Theranostics 2017, 7, 4424–4444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, X.; Garamus, V.M.; Handge, U.A.; Bérengère, L.; Zhao, L.; Salamon, G.; Willumeit, R.; Zou, A.; Fan, S. Mixture of nonionic/ionic surfactants for the formulation of nanostructured lipid carriers: Effects on physical properties. Langmuir 2014, 30, 6920–6928. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Li, X.; Gao, W.; Zhen, Z.; Dong, D.; Huang, B.; Ma, Z.; Zhang, A.; Song, X.; et al. Cholesterol inhibits TCR signaling by directly restricting TCR-CD3 core tunnel motility. Mol. Cell 2022, 82, 1278–1287.e5. [Google Scholar] [CrossRef]
- Kuo, Y.; Wang, C. Cationic solid lipid nanoparticles with cholesterol-mediated surface layer for transporting saquinavir to the brain. Biotechnol. Prog. 2014, 30, 198–206. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from n-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Abumanhal-Masarweh, H.; da Silva, D.; Poley, M.; Zinger, A.; Goldman, E.; Krinsky, N.; Kleiner, R.; Shenbach, G.; Schroeder, J.E.; Shklover, J.; et al. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release 2019, 307, 331–341. [Google Scholar] [CrossRef]
- Karn-Orachai, K.; Smith, S.M.; Phunpee, S.; Treethong, A.; Puttipipatkhachorn, S.; Pratontep, S.; Ruktanonchai, U.R. The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers. J. Microencapsul. 2014, 31, 609–618. [Google Scholar] [CrossRef]
- Gardouh, A.R.; Faheim, S.H.; Noah, A.T.; Ghorab, M.M. Influence of formulation factors on the size of nanostructured lipid carriers and nanoemulsions prepared by high shear homogenization. Int. J. Pharm. Pharm. Sci. 2018, 10, 61. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(Ethylene glycol) alternatives for overcoming peg immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Hu, C.; Lei, T.; Wang, Y.; Cao, J.; Yang, X.; Qin, L.; Liu, R.; Zhou, Y.; Tong, F.; Umeshappa, C.S.; et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 2020, 255, 120159. [Google Scholar] [CrossRef]
- Kurczewska, J. Chitosan-based nanoparticles with optimized parameters for targeted delivery of a specific anticancer drug—A comprehensive review. Pharmaceutics 2023, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of active compounds of essential oils in antimicrobial food packaging using a chemometric method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the antimicrobial activity of oregano oil by encapsulation in chitosan—Alginate nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(Ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C 2021, 127, 112194. [Google Scholar] [CrossRef]
- Witika, B.A.; Walker, R.B. Preformulation characterization and identification of excipients for nevirapine loaded niosomes. Pharmazie 2021, 76, 77–83. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, H.; Li, F.; Sun, H.; Tong, L. Effect of milling intensity on the properties of chitin, chitosan and chitosan films obtained from grasshopper. Int. J. Biol. Macromol. 2023, 239, 124249. [Google Scholar] [CrossRef]
- Jia, X.; Tan, R.; Peng, B. Preparation and application of polyethylene glycol triazine derivatives as a chrome-free tanning agent for wet-white leather manufacturing. Environ. Sci. Pollut. Res. 2022, 29, 7732–7742. [Google Scholar] [CrossRef]
- Sobczyński, J.; Bielecka, G. Nanostructure lipid carriers. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–309. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lahtinen, M.H.; Agustin, M.B.; de Carvalho, D.M.; Hirvonen, S.; Penttilä, P.A.; Mikkonen, K.S. Green fabrication approaches of lignin nanoparticles from different technical lignins: A comparison study. ChemSusChem 2021, 14, 4718–4730. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid-PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Fayad, S.J. Obtenção e Caracterização de Micro e Nanopartículas a Base de Proteína Isolada de Soja [Obtaining and Characterizing Micro and Nanoparticles Based on Isolated Soy Protein]. Master’s Dissertation, Federal University of Santa Catarina, Florianópolis, Brazil, 2010. [Google Scholar]
- Conde, J.; Dias, J.T.; GrazÃo, V.; Moros, M.; Baptista, P.V.; De La Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 1–27. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [14C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotech 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “stealthy” nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Zhang, W.; Sui, X.; Yan, Z.; He, Z. Nanoparticle albumin-bound (Nab) technology is a promising method for anti-cancer drug delivery. Recent Patents Anti-Cancer Drug Discov. 2009, 4, 262–272. [Google Scholar] [CrossRef]
- Ding, H.M.; Ma, Y.Q. Computer simulation of the role of protein corona in cellular delivery of nanoparticles. Biomaterials 2014, 35, 8703–8710. [Google Scholar] [CrossRef]
- Shourni, S.; Javadi, A.; Hosseinpour, N.; Bahramian, A.; Raoufi, M. Characterization of protein corona formation on nanoparticles via the analysis of dynamic interfacial properties: Bovine serum albumin—Silica particle interaction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128273. [Google Scholar] [CrossRef]
- Yu, Y.; Luan, Y.; Dai, W. Dynamic process, mechanisms, influencing factors and study methods of protein corona formation. Int. J. Biol. Macromol. 2022, 205, 731–739. [Google Scholar] [CrossRef]



| NLC | Carvacrol (%) | Precirol® (%) | Lipoid® S-100 (%) | Kolliphor® ELP (%) | Span® 60 (%) | Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|---|
| PF01-L | 2 | 1 | 1 | - | - | 273 ± 2 | 0.41 ± 0.03 | −21 ± 1 |
| PF01-K | 2 | 1 | - | 1 | - | 173 ± 1 | 0.15 ± 0.01 | −18 ± 1 |
| PF01-S | 2 | 1 | - | - | 1 | 115 ± 1 | 0.25 ± 0.01 | −24 ± 1 |
| PF02-L | 5 | 4 | 1 | - | - | 289 ± 6 | 0.40 ± 0.08 | −36 ± 0.2 |
| PF02-K | 5 | 4 | - | 1 | - | 224 ± 3 | 0.24 ± 0.01 | −20 ± 0.1 |
| PF02-S | 5 | 4 | - | - | 1 | 208 ± 5 | 0.25 ± 0.01 | −22 ± 1 |
| PF03-L | 9 | 7 | 1 | - | - | 310 ± 4 | 0.35 ± 0.02 | −29 ± 1 |
| PF03-K | 9 | 7 | - | 1 | - | 300 ± 1 | 0.33 ± 0.01 | −24 ± 0.4 |
| PF03-S | 9 | 7 | - | - | 1 | 283 ± 2 | 0.24 ± 0.01 | −30 ± 0.5 |
| Variables | Size (nm) | PdI | Zeta Potential (mv) | |||
|---|---|---|---|---|---|---|
| NLC | Carvacrol (%) | Precirol® (%) | Tween® 80 (%) | |||
| 1 | 5 | 1 | 1 | 220 ± 9 | 0.24 ± 0.04 | −36 ± 1 |
| 2 | 5 | 4 | 3 | 248 ± 7 | 0.23 ± 0.02 | −28 ± 0.2 |
| 3 | 5 | 1 | 5 | 208 ± 6 | 0.25 ± 0.04 | −22 ± 1 |
| 4 | 9 | 4 | 5 | 283 ± 2 | 0.24 ± 0.01 | −30 ± 1 |
| 5 | 9 | 4 | 1 | 333 ± 12 | 0.10 ± 0.07 | −34 ± 1 |
| 6 | 1 | 4 | 5 | 109 ± 2 | 0.24 ± 0.03 | −18 ± 0.4 |
| 7 | 5 | 4 | 3 | 240 ± 9 | 0.24 ± 0.03 | −28 ± 0.1 |
| 8 | 1 | 1 | 3 | 88 ± 2 | 0.30 ± 0.01 | −19 ± 1 |
| 9 | 1 | 7 | 3 | 309 ± 12 | 0.47 ± 0.06 | −24 ± 2 |
| 10 | 9 | 1 | 3 | 263 ± 2 | 0.24 ± 0.03 | −39 ± 1 |
| 11 | 5 | 7 | 5 | 251 ± 12 | 0.47 ± 0.05 | −20 ± 1 |
| 12 | 1 | 4 | 1 | 263 ± 12 | 0.49 ± 0.06 | −11 ± 1 |
| 13 | 5 | 7 | 1 | 567 ± 24 | 0.21 ± 0.03 | −24 ± 1 |
| 14 | 5 | 4 | 3 | 250 ± 8 | 0.22 ± 0.04 | −32 ± 0.3 |
| 15 | 9 | 7 | 3 | 289 ± 1 | 0.05 ± 0.07 | −28 ± 1 |
| NLCs | IC50 (μg/mL) |
|---|---|
| Carvacrol | 77 ± 1 |
| oNLC | 7 ± 1 |
| oNLC MCT | >25 |
| oNLC-Plu0.05% | 6 ± 0.4 |
| oNLC-Chol0.1% | 6 ± 0.4 |
| oNLC-PEG0.5% | 7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchôa, A.F.C.; Formiga, A.L.D.; Cardoso, A.L.M.R.; Pereira, G.M.A.; Carvalho, L.M.M.; Souza, P.H.O.; Silva, A.L.; Souza, R.R.M.; Sobral, M.V.; Silva, M.S.; et al. Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells. Pharmaceutics 2025, 17, 363. https://doi.org/10.3390/pharmaceutics17030363
Uchôa AFC, Formiga ALD, Cardoso ALMR, Pereira GMA, Carvalho LMM, Souza PHO, Silva AL, Souza RRM, Sobral MV, Silva MS, et al. Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells. Pharmaceutics. 2025; 17(3):363. https://doi.org/10.3390/pharmaceutics17030363
Chicago/Turabian StyleUchôa, Ana F. C., Allessya L. D. Formiga, Anny L. M. R. Cardoso, Graziela M. A. Pereira, Lucas M. M. Carvalho, Pedro H. O. Souza, Anauara L. Silva, Ramon R. M. Souza, Marianna V. Sobral, Marcelo S. Silva, and et al. 2025. "Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells" Pharmaceutics 17, no. 3: 363. https://doi.org/10.3390/pharmaceutics17030363
APA StyleUchôa, A. F. C., Formiga, A. L. D., Cardoso, A. L. M. R., Pereira, G. M. A., Carvalho, L. M. M., Souza, P. H. O., Silva, A. L., Souza, R. R. M., Sobral, M. V., Silva, M. S., Barbosa-Filho, J. M., & Xavier-Júnior, F. H. (2025). Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells. Pharmaceutics, 17(3), 363. https://doi.org/10.3390/pharmaceutics17030363





