Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Animals
2.3. In Vitro Accumulation of 99mTc-GSA in M. avium ATCC700898
2.4. Effect of the Asialoglycoprotein Receptor (ASGP-R) Inhibitor on 99mTc-GSA Accumulation
2.5. Biodistribution of 99mTc-GSA
2.6. SPECT Imaging of 99mTc-GSA in an M. avium ATCC700898 Mouse Thigh Infection Model
2.7. Counting of Viable Bacteria
2.8. Statistical Analysis
3. Results
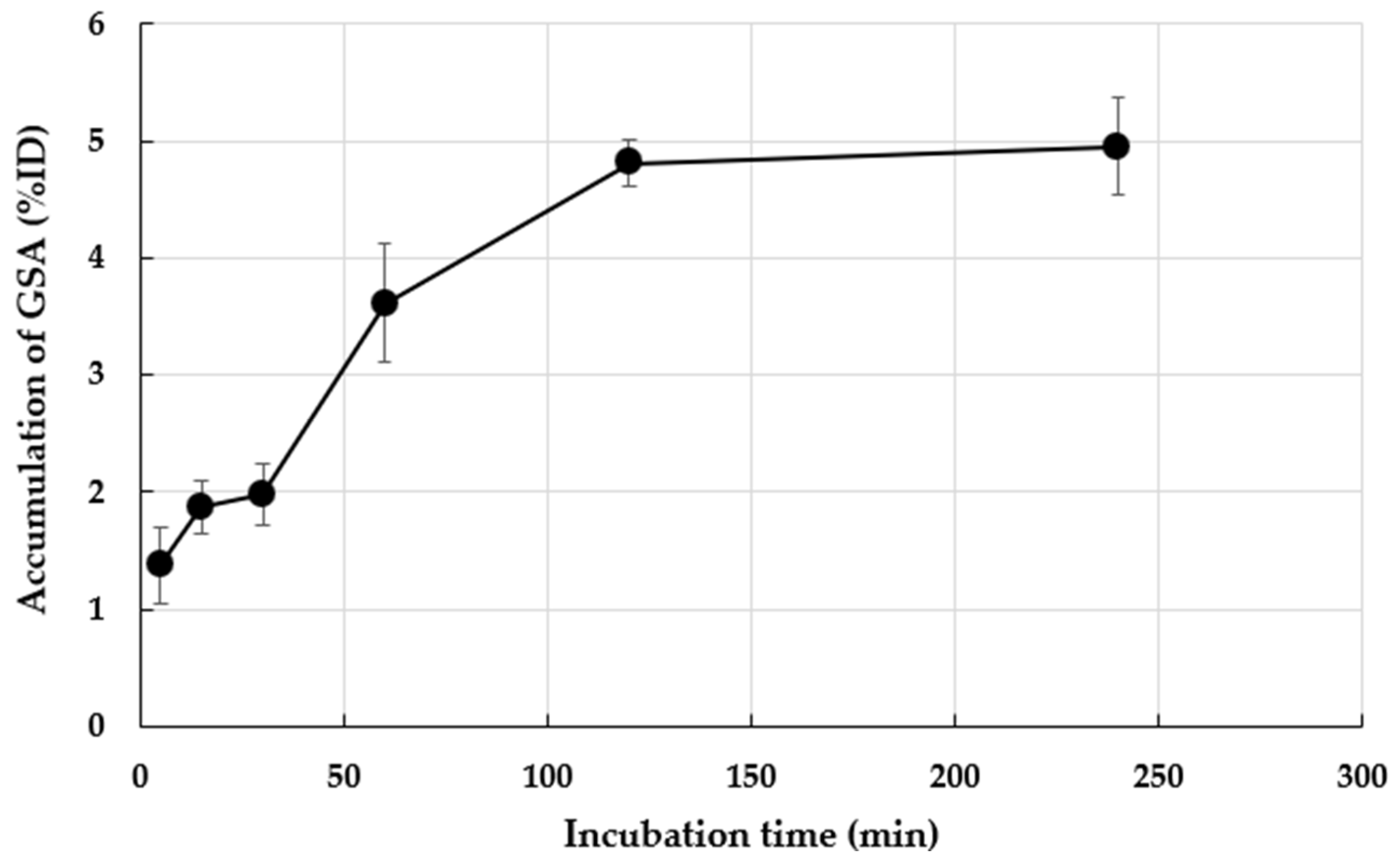
3.1. In Vitro Accumulation of 99mTc-GSA in M. avium ATCC70089
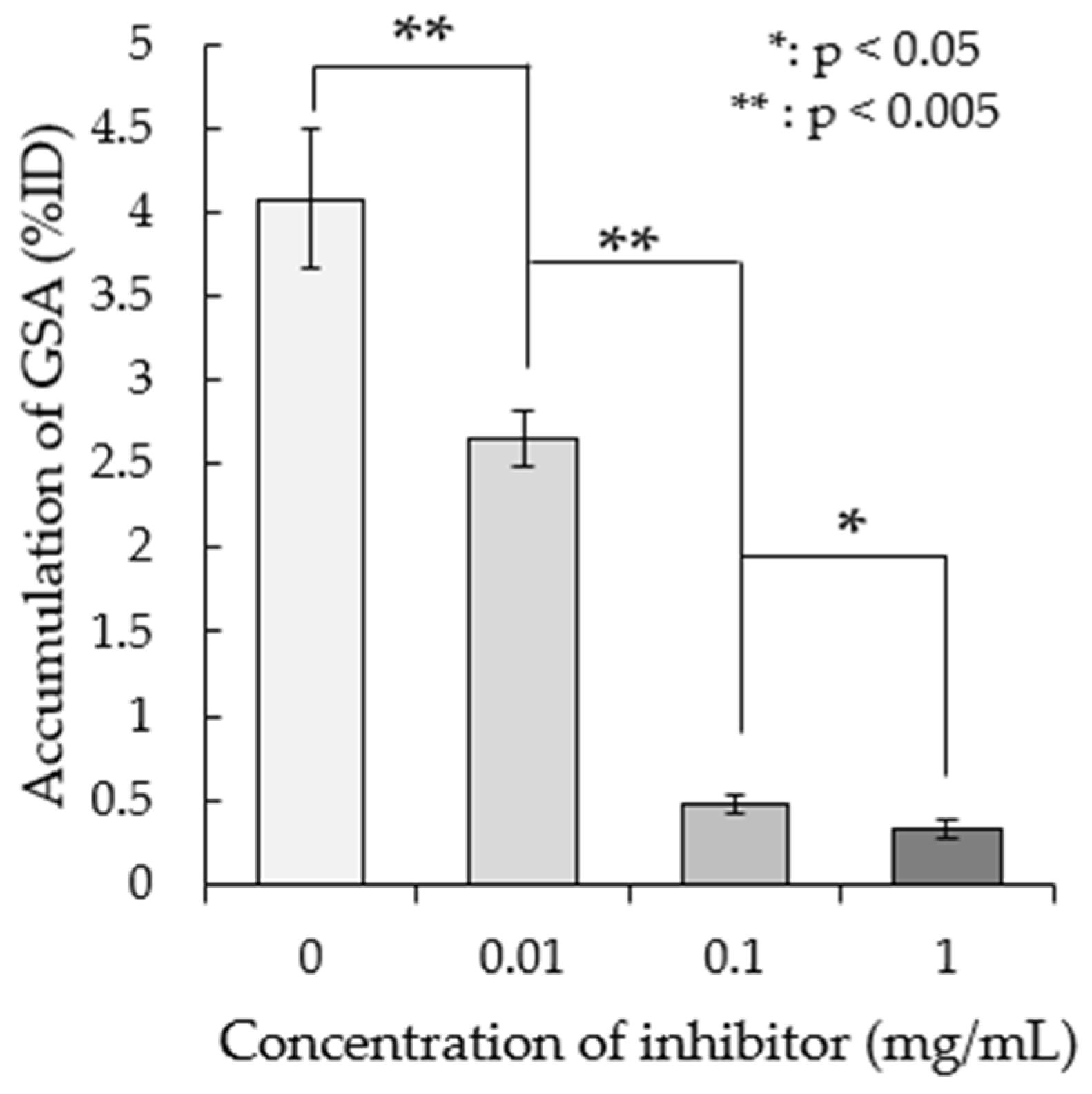
3.2. Effect of the Asialoglycoprotein Receptor (ASGP-R) Inhibitor on 99mTc-GSA Accumulation
3.3. Biodistribution of 99mTc-GSA
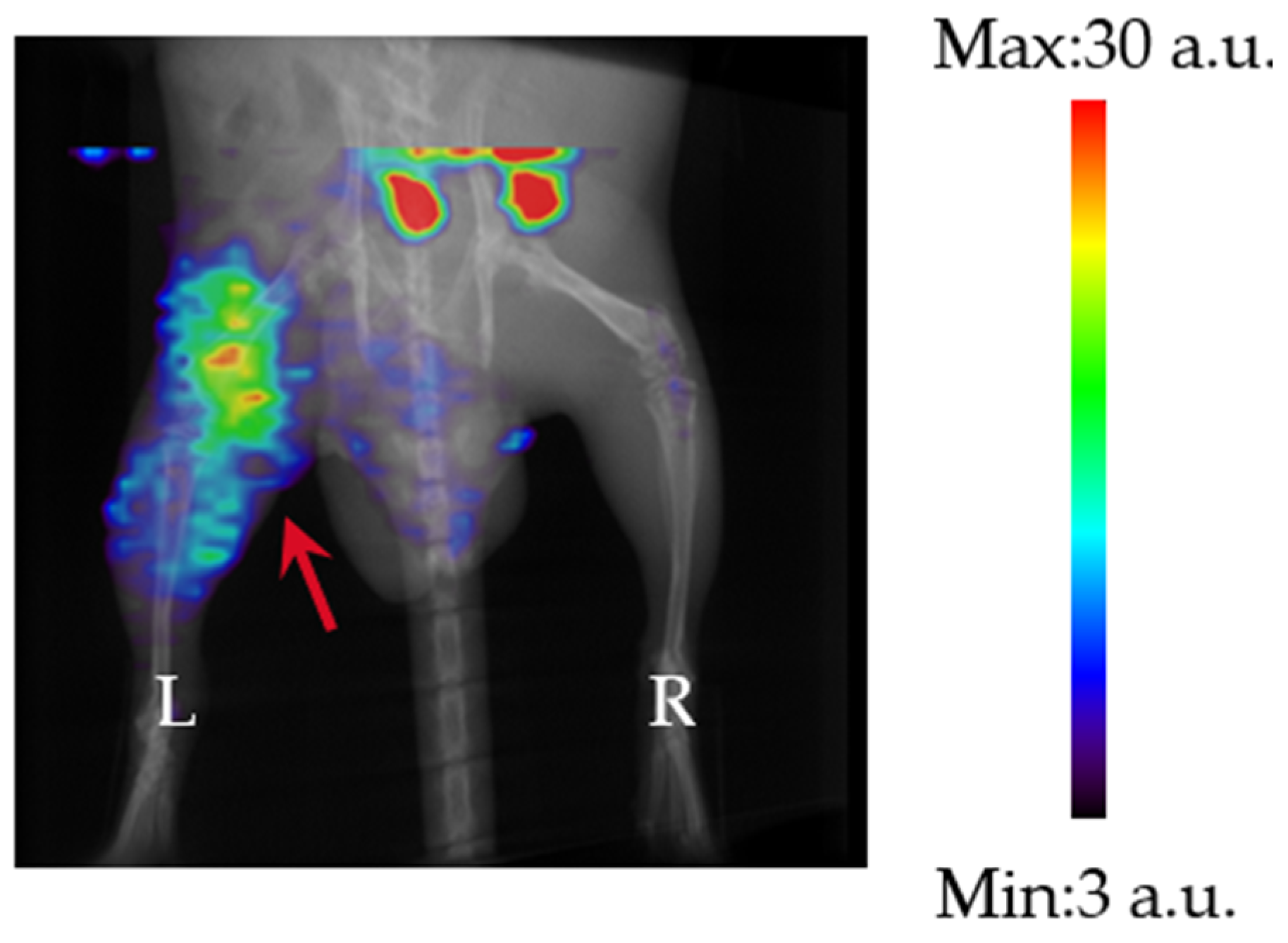
3.4. SPECT Imaging of 99mTc-GSA in an M. avium ATCC700898 Mouse Thigh Infection Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2023. Available online: https://www.who.int/publications/i/item/9789240083851 (accessed on 26 December 2024).
- Clinical Overview of Drug-Resistant Tuberculosis Disease. Available online: https://www.cdc.gov/tb/hcp/clinical-overview/drug-resistant-tuberculosis-disease.html (accessed on 26 December 2024).
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.N.; Mølhave, M.; Fløe, A.; van Ingen, J.; Schön, T.; Lillebaek, T.; Andersen, A.B.; Wejse, C. Global trends of pulmonary infections with nontuberculous mycobacteria: A systematic review. Int. J. Infect. Dis. 2022, 125, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Grigg, C.; Jackson, K.A.; Barter, D.; Czaja, C.A.; Johnston, H.; Lynfield, R.; Vagnone, P.S.; Tourdot, L.; Spina, N.; Dumyati, G.; et al. Epidemiology of pulmonary and extrapulmonary nontuberculous mycobacteria infections at 4 US emerging infections program sites: A 6-month pilot. Clin. Infect. Dis. 2023, 77, 629–637. [Google Scholar] [CrossRef]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, e1–e43. [Google Scholar] [CrossRef]
- The Japanese Society for Tuberculosis. Opinions regarding chemotherapy for adult pulmonary nontuberculous mycobacterial disease. Kekkaku 2023, 98, 1–11. [Google Scholar]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; McNulty, S.; Philley, J.V.; Killingley, J.; Wilson, R.W.; York, D.S.; Shepherd, S.; Griffith, D.E. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest 2014, 146, 276–282. [Google Scholar] [CrossRef]
- Uwamino, Y.; Hasegawa, N.; Kamoshita, Y.; Inose, R.; Aoki, W.; Nagata, M.; Namkoong, H.; Nishimura, T.; Matsushita, H. Optimal incubation duration of liquid cultures for assessing culture negative conversion in patients with Mycobacterium avium complex and Mycobacterium abscessus pulmonary diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 44, 45–51. [Google Scholar] [CrossRef]
- Davis, K.M.; Ryan, J.L.; Aaron, V.D.; Sims, J.B. PET and SPECT imaging of the brain: History, technical considerations, applications, and radiotracers. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 41, 521–529. [Google Scholar] [CrossRef]
- Kuwert, T. Skeletal SPECT/CT: A review. Clin. Transl. Imaging 2014, 2, 505–517. [Google Scholar] [CrossRef]
- Ashwell, G.; Morell, A.G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv. Enzymol. Relat. Areas. Mol. Biol. 1974, 41, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Stowell, C.P.; Lee, Y.C. The binding of d-glucosyl-neoglycoproteins to the hepatic asialoglycoprotein receptor. J. Biol. Chem. 1978, 253, 6107–6110. [Google Scholar] [CrossRef] [PubMed]
- Hilgard, P.; Schreiter, T.; Stockert, R.J.; Gerken, G.; Treichel, U. Asialoglycoprotein receptor facilitates hemolysis in patients with alcoholic liver cirrhosis. Hepatology 2004, 39, 1398–1407. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Mizutani, A.; Kobayashi, M.; Muranaka, Y.; Sato, K.; Maki, H.; Kawai, K. SPECT Imaging of P. aeruginosa infection in mice using 123I-BMIPP. Pharmaceutics 2024, 16, 656. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Uehara, T.; Okazaki, K.; Maki, H.; Abe, K.; Arano, Y.; Momosaki, S. In vivo monitoring of viable bacteria by SPECT using 99mTc-HYNIC(GH)2-UBI 29-41 and 99mTc-HYNIC(Tricine)2-UBI 29-41 in infected mice. J. Appl. Microbiol. Res. 2022, 5, 01–07. [Google Scholar]
- Torizuka, K.; Kawa, S.; Ikekubo, K.; Suga, Y.; Tanaka, Y.; Hino, M.; Ito, H.; Yamamoto, K.; Yonekura, Y. Phase I clinical study on 99mTc-GSA, a new agent for functional imaging of the liver. Jpn. J. Nucl. Med. 1991, 28, 1321–1331. [Google Scholar]
- Kwak, N.; Park, J.; Kim, E.; Lee, C.H.; Han, S.K.; Yim, J.J. Treatment outcomes of Mycobacterium avium complex lung disease: A systematic review and meta-analysis. Clin. Infect. Dis. 2017, 65, 1077–1084. [Google Scholar] [CrossRef]
- Verma, D.; Stapleton, M.; Gadwa, J.; Vongtongsalee, K.; Schenkel, A.R.; Chan, E.D.; Ordway, D. Mycobacterium avium infection in a C3HeB/FeJ mouse model. Front. Microbiol. 2019, 10, 693. [Google Scholar] [CrossRef]
- McNabe, M.; Tennant, R.; Danelishvili, L.; Young, L.; Bermudez, L.E. Mycobacterium avium ssp. hominissuis biofilm is composed of distinct phenotypes and influenced by the presence of antimicrobials. Clin. Microbiol. Infect. 2011, 17, 697–703. [Google Scholar] [CrossRef]
- Limia, A.; Sangari, F.J.; Wagner, D.; Bermudez, L.E. Characterization and expression of secA in Mycobacterium avium. FEMS Microbiol. Lett. 2001, 197, 151–157. [Google Scholar] [CrossRef][Green Version]
- Tozawa, R.; Ishibashi, S.; Osuga, J.; Yamamoto, K.; Yagyu, H.; Ohashi, K.; Tamura, Y.; Yahagi, N.; Iizuka, Y.; Okazaki, H.; et al. Asialoglycoprotein receptor deficiency in mice lacking the major receptor subunit. Its obligate requirement for the stable expression of oligomeric receptor. J. Biol. Chem. 2001, 276, 12624–12628. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Yu, J.Y.; Jhun, B.W. Spontaneous Cultural Conversion Rate of Mycobacterium avium Complex Pulmonary Disease Based on BACES Severity. J. Clin. Med. 2023, 12, 7125. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.H.; Jeon, K.; Park, H.Y.; Kim, S.Y.; Lee, K.S.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 96–103. [Google Scholar] [CrossRef]
- Yagi, K.; Ishii, M.; Namkoong, H.; Asami, T.; Iketani, O.; Asakura, T.; Suzuki, S.; Sugiura, H.; Yamada, Y.; Nishimura, T.; et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect. Dis. 2017, 17, 558. [Google Scholar] [CrossRef]


| 99mTc-GSA Accumulation (%ID/g) | |||
|---|---|---|---|
| Organ | 1 h | 2 h | 4 h |
| Heart | 0.35 ± 0.06 | 0.26 ± 0.06 | 0.19 ± 0.04 |
| Lung | 0.44 ± 0.05 | 0.31 ± 0.03 | 0.22 ± 0.04 |
| Liver | 11.50 ± 1.10 | 12.71 ± 2.03 | 11.14 ± 1.52 |
| Kidney | 0.96 ± 0.07 | 1.16 ± 0.20 | 1.11 ± 0.20 |
| Blood | 0.52 ± 0.19 | 0.34 ± 0.11 | 0.32 ± 0.17 |
| Time After Injection (h) | Accumulation (%ID) | Contrast | |
|---|---|---|---|
| 1 | Infected | 1.73 ± 0.20 | 5.40 |
| Uninfected | 0.33 ± 0.07 | ||
| 2 | Infected | 1.22 ± 0.21 | 3.96 |
| Uninfected | 0.31 ± 0.07 | ||
| 4 | Infected | 0.88 ± 0.31 | 3.07 |
| Uninfected | 0.29 ± 0.10 |
| CFU/Mouse | Accumulation (%ID) | Contrast | |
|---|---|---|---|
| 108 | Infected | 1.73 ± 0.20 * | 5.40 |
| Uninfected | 0.33 ± 0.07 | ||
| 105 | Infected | 1.13 ± 0.19 | 3.11 |
| Uninfected | 0.36 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, Y.; Mizutani, A.; Kobayashi, M.; Kitagawa, M.; Muranaka, Y.; Sato, K.; Maki, H.; Kawai, K. Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography. Pharmaceutics 2025, 17, 362. https://doi.org/10.3390/pharmaceutics17030362
Nishiyama Y, Mizutani A, Kobayashi M, Kitagawa M, Muranaka Y, Sato K, Maki H, Kawai K. Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography. Pharmaceutics. 2025; 17(3):362. https://doi.org/10.3390/pharmaceutics17030362
Chicago/Turabian StyleNishiyama, Yuri, Asuka Mizutani, Masato Kobayashi, Miyu Kitagawa, Yuka Muranaka, Kakeru Sato, Hideki Maki, and Keiichi Kawai. 2025. "Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography" Pharmaceutics 17, no. 3: 362. https://doi.org/10.3390/pharmaceutics17030362
APA StyleNishiyama, Y., Mizutani, A., Kobayashi, M., Kitagawa, M., Muranaka, Y., Sato, K., Maki, H., & Kawai, K. (2025). Non-Invasive Mycobacterium avium Detection Using 99mTc-GSA on Single-Photon Emission Computed Tomography. Pharmaceutics, 17(3), 362. https://doi.org/10.3390/pharmaceutics17030362








