High-Pressure Dielectric Spectroscopic Studies of Amorphous CBD: Investigating Molecular Dynamics and Physical Stability Under Manufacturing Conditions of the Pharmaceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Broadband Dielectric Spectroscopy (BDS)
2.2.3. High-Pressure Dielectric Studies (HP-BDS)
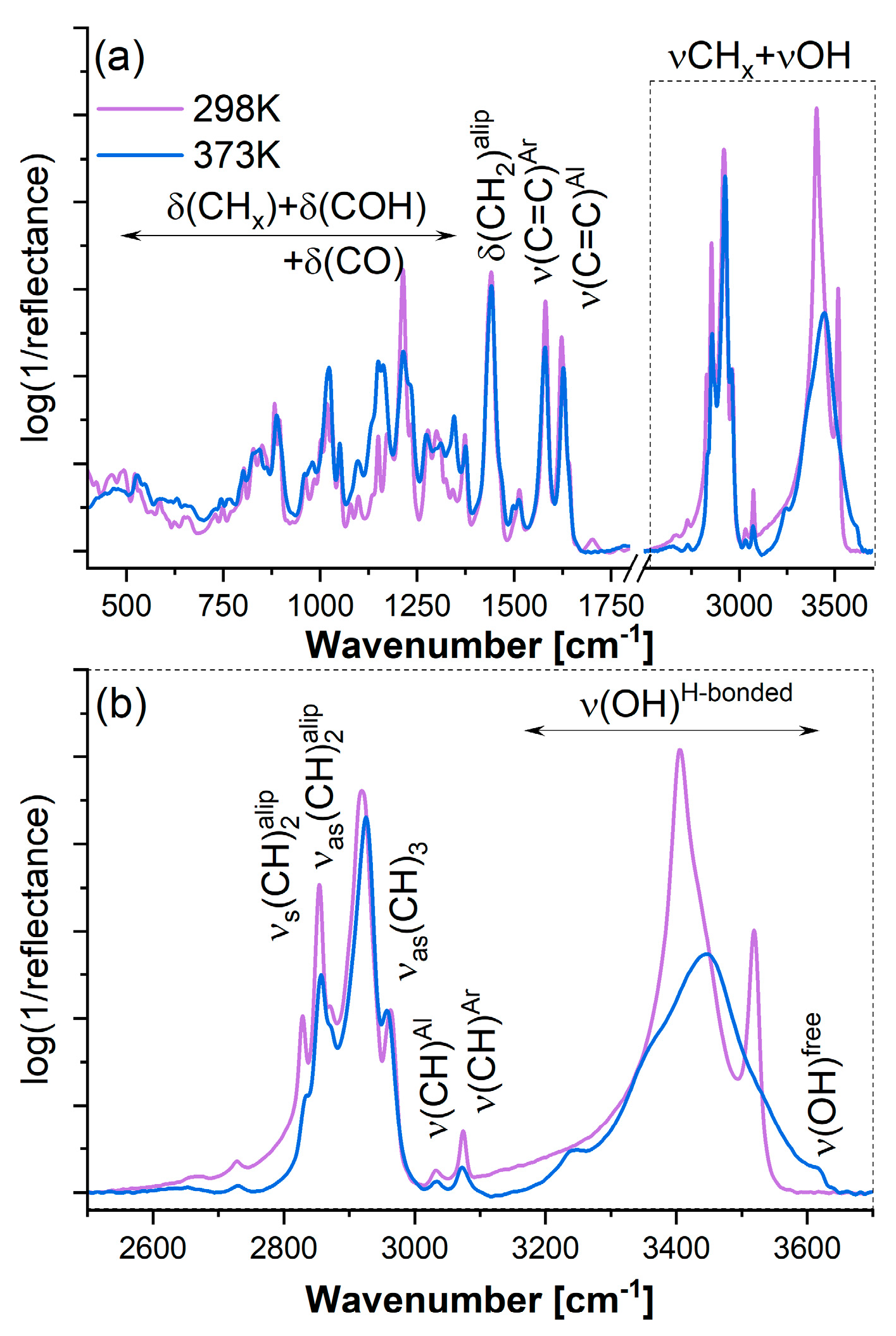
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Thermal Properties of Crystalline and Amorphous CBD
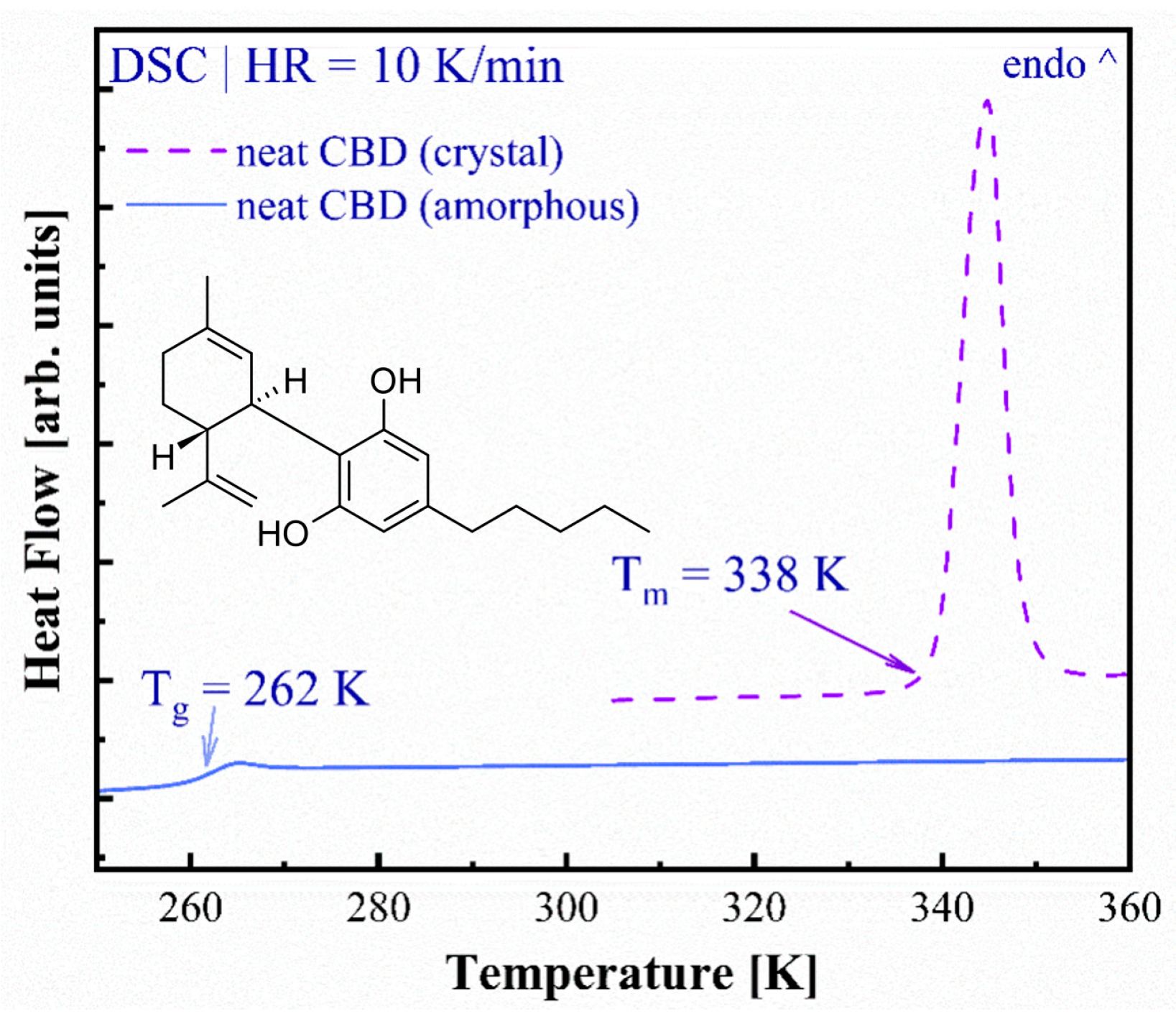
Molecular Features of Crystalline and Molten CDB
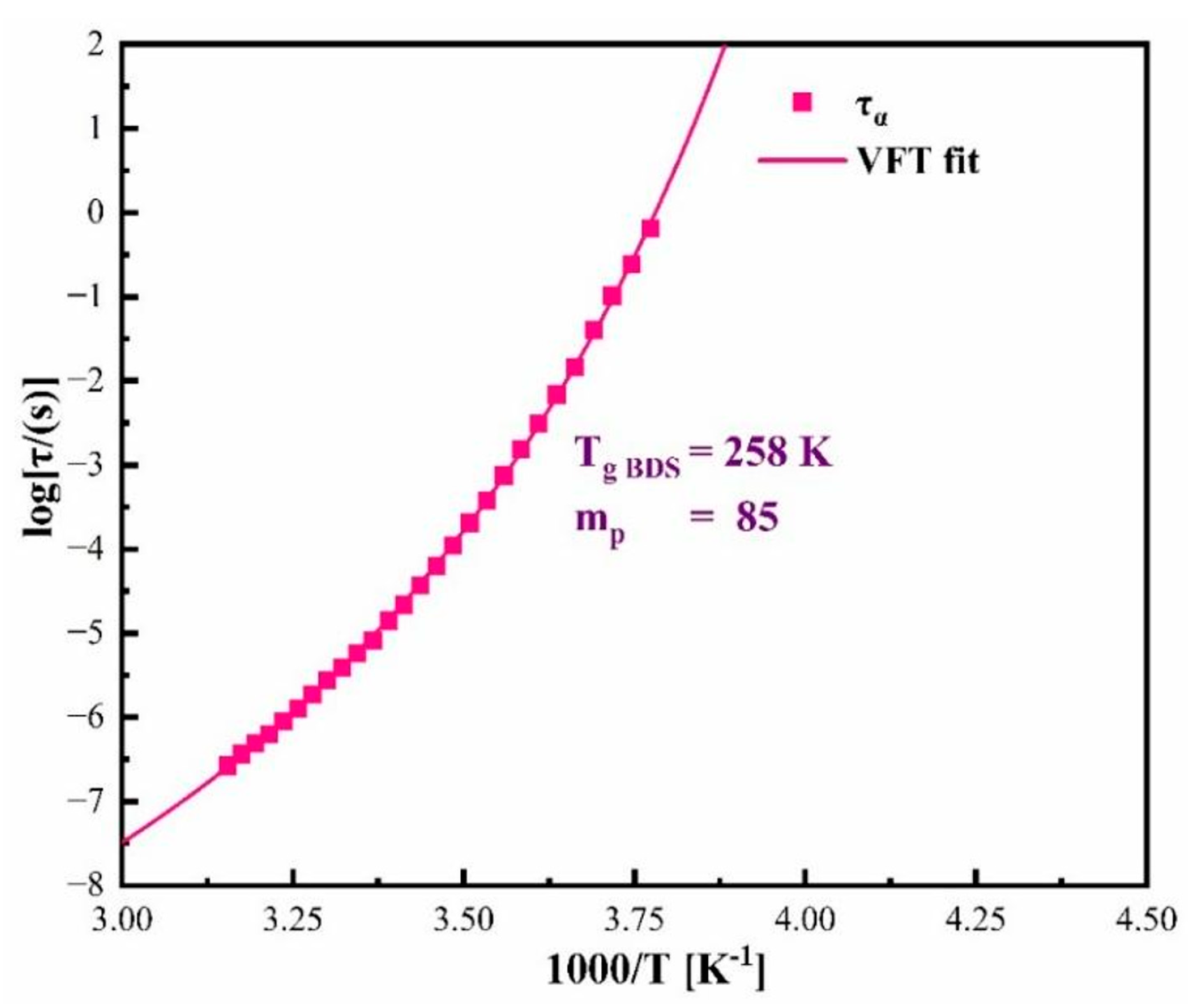
3.2. Molecular Dynamic Studies of Pure Amorphous CBD to Examine the Physical Stability
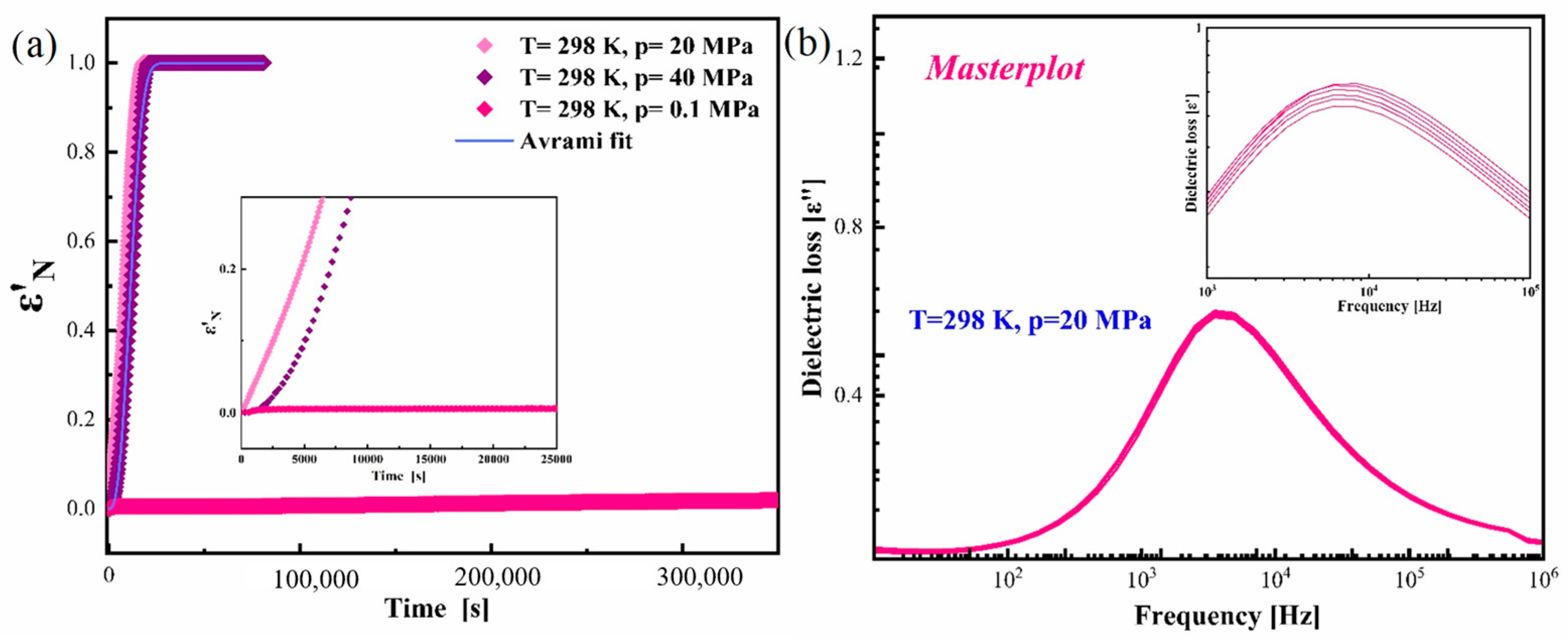
3.3. Effect of Elevated Pressure on the Re-Crystallization Tendency of Pure Amorphous CBD
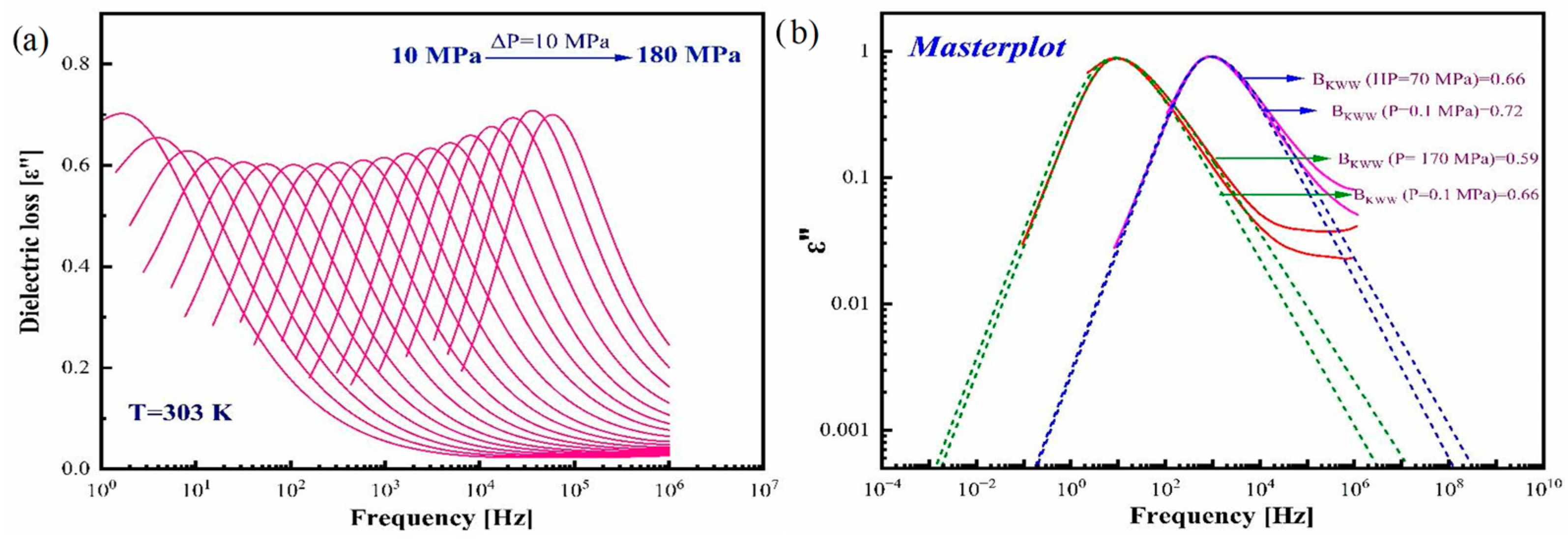
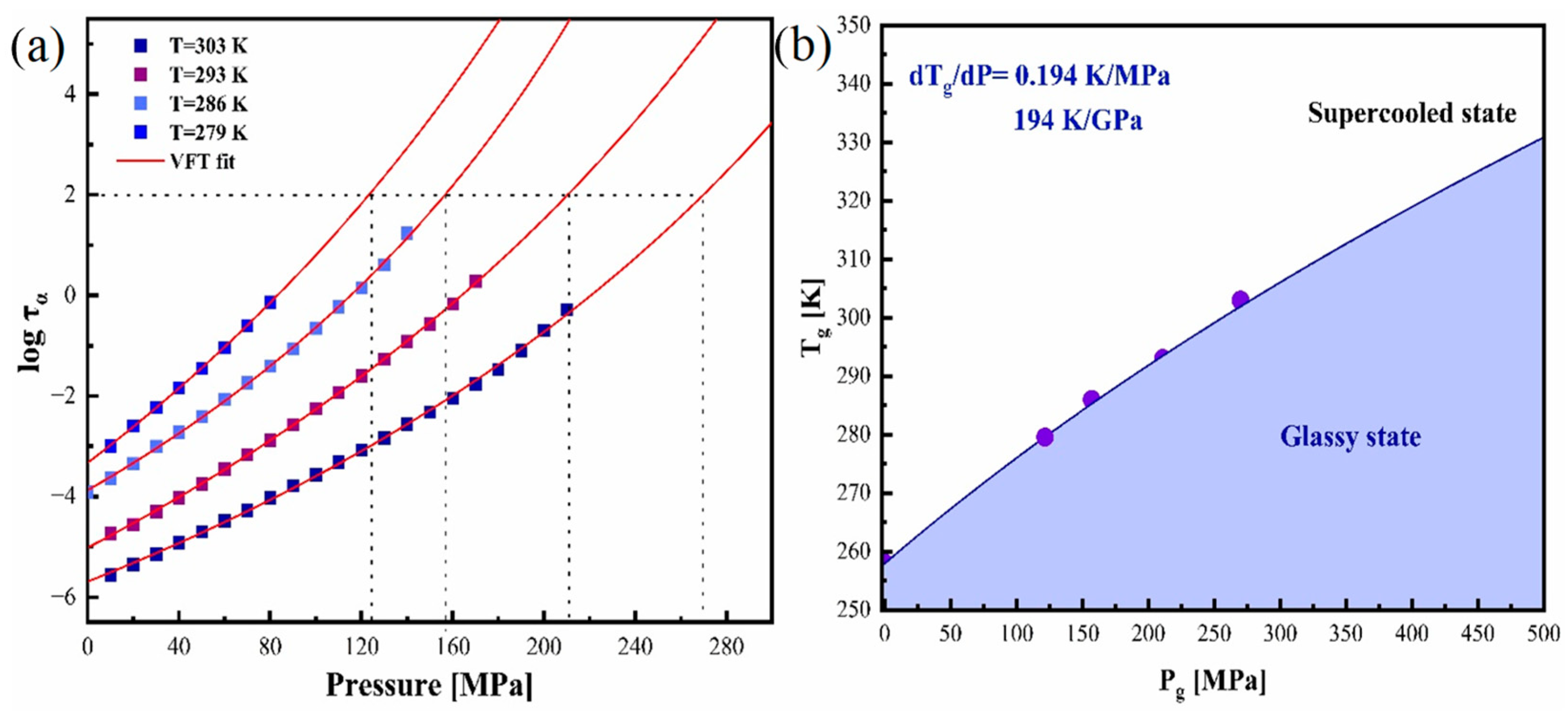
3.4. Effect of Elevated Pressure on the Structural Relaxation of Pure Amorphous CBD at Various Isothermal Conditions
3.5. Determination of Activation Volume of Amorphous CBD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villiers, M.M. De Oral Conventional Solid Dosage Forms: Powders and Granules, Tablets, Lozenges, and Capsules. In Theory and Practice of Contemporary Pharmaceutics; CRC Press: Boca Raton, FL, USA, 2021; pp. 279–331. [Google Scholar] [CrossRef]
- Lieberman, H.A.; Lachman, L.; Schwartz, J.B. Pharmaceutical Dosage Forms. Volumes 2: Tablets; Marcel Dekker: New York, NY, USA, 1990; ISBN 0824780442. [Google Scholar]
- Fabbiani, F.P.A.; Pulham, C.R. High-Pressure Studies of Pharmaceutical Compounds and Energetic Materials. Chem. Soc. Rev. 2006, 35, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Khodov, I.A.; Belov, K.V.; Dyshin, A.A.; Krestyaninov, M.A.; Kiselev, M.G. Pressure Effect on Lidocaine Conformational Equilibria in ScCO2: A Study by 2D NOESY. J. Mol. Liq. 2022, 367, 120525. [Google Scholar] [CrossRef]
- Khodov, I.; Dyshin, A.; Efimov, S.; Ivlev, D.; Kiselev, M. High-Pressure NMR Spectroscopy in Studies of the Conformational Composition of Small Molecules in Supercritical Carbon Dioxide. J. Mol. Liq. 2020, 309, 113113. [Google Scholar] [CrossRef]
- Lura, A.; Tardy, G.; Kleinebudde, P.; Breitkreutz, J. Tableting of Mini-Tablets in Comparison with Conventionally Sized Tablets: A Comparison of Tableting Properties and Tablet Dimensions. Int. J. Pharm. X 2020, 2, 100061. [Google Scholar] [CrossRef]
- Huang, C.; Klinzing, G.; Procopio, A.; Yang, F.; Ren, J.; Burlage, R.; Zhu, L.; Su, Y. Understanding Compression-Induced Amorphization of Crystalline Posaconazole. Mol. Pharm. 2019, 16, 825–833. [Google Scholar] [CrossRef]
- Guerain, M. A Review on High Pressure Experiments for Study of Crystallographic Behavior and Polymorphism of Pharmaceutical Materials. J. Pharm. Sci. 2020, 109, 2640–2653. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of Nanoparticulate Strategies for Solubility Enhancement of Poorly Soluble Drugs. Life Sci. 2022, 291, 120301. [Google Scholar] [CrossRef]
- Kanaujia, P.; Poovizhi, P.; Ng, W.K.; Tan, R.B.H. Amorphous Formulations for Dissolution and Bioavailability Enhancement of Poorly Soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of Increased Bioavailability through Amorphous Solid Dispersions: A Review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility Advantage of Amorphous Pharmaceuticals: I. A Thermodynamic Analysis. J. Pharm. Sci. 2010, 99, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What Is the True Solubility Advantage for Amorphous Pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol Aqueous Solubility Enhancement: Comparison of Three Amorphous Formulations Strategies Using Different Type of Polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Modification of Physicochemical Characteristics of Active Pharmaceutical Ingredients and Application of Supersaturatable Dosage Forms for Improving Bioavailability of Poorly Absorbed Drugs. Adv. Drug Deliv. Rev. 2012, 64, 480–495. [Google Scholar] [CrossRef]
- Kawakami, K. Ultraslow Cooling for the Stabilization of Pharmaceutical Glasses. J. Phys. Chem. B 2019, 123, 4996–5003. [Google Scholar] [CrossRef]
- Chattoraj, S.; Sun, C.C. Crystal and Particle Engineering Strategies for Improving Powder Compression and Flow Properties to Enable Continuous Tablet Manufacturing by Direct Compression. J. Pharm. Sci. 2018, 107, 968–974. [Google Scholar] [CrossRef]
- Grzybowska, K.; Paluch, M.; Grzybowski, A.; Wojnarowska, Z.; Hawelek, L.; Kolodziejczyk, K.; Ngai, K.L. Molecular Dynamics and Physical Stability of Amorphous Anti-Inflammatory Drug: Celecoxib. J. Phys. Chem. B 2010, 114, 12792–12801. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Viciosa, M.T.; Danède, F.; Affouard, F.; Correia, N.T. Molecular Mobility of Amorphous S-Flurbiprofen: A Dielectric Relaxation Spectroscopy Approach. Mol. Pharm. 2014, 11, 112–130. [Google Scholar] [CrossRef]
- Chmiel, K.; Knapik-Kowalczuk, J.; Kamińska, E.; Tajber, L.; Paluch, M. High-Pressure Dielectric Studies—A Way to Experimentally Determine the Solubility of a Drug in the Polymer Matrix at Low Temperatures. Mol. Pharm. 2021, 18, 3050–3062. [Google Scholar] [CrossRef]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Hawelek, L.; Sawicki, W.; Wlodarski, K.; Markowski, J.; Paluch, M. Physical Stability of the Amorphous Anticholesterol Agent (Ezetimibe): The Role of Molecular Mobility. Mol. Pharm. 2014, 11, 4280–4290. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Wojnarowska, Z.; Rams-Baron, M.; Jurkiewicz, K.; Cielecka-Piontek, J.; Ngai, K.L.; Paluch, M. Atorvastatin as a Promising Crystallization Inhibitor of Amorphous Probucol: Dielectric Studies at Ambient and Elevated Pressure. Mol. Pharm. 2017, 14, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowska, Z.; Adrjanowicz, K.; Wlodarczyk, P.; Kaminska, E.; Kaminski, K.; Grzybowska, K.; Wrzalik, R.; Paluch, M.; Ngai, K.L. Broadband Dielectric Relaxation Study at Ambient and Elevated Pressure of Molecular Dynamics of Pharmaceutical: Indomethacin. J. Phys. Chem. B 2009, 113, 12536–12545. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Hernando, K.; Bebin, E.M.; Gaston, T.E.; Grayson, L.E.; Ampah, S.B.; Moreadith, R. Higher Cannabidiol Plasma Levels Are Associated with Better Seizure Response Following Treatment with a Pharmaceutical Grade Cannabidiol. Epilepsy Behav. 2019, 95, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Verducci, C.; Thiele, E.A.; Laux, L.C.; Patel, A.D.; Filloux, F.; Szaflarski, J.P.; Wilfong, A.; Clark, G.D.; Park, Y.D.; et al. Open-Label Use of Highly Purified CBD (Epidiolex®) in Patients with CDKL5 Deficiency Disorder and Aicardi, Dup15q, and Doose Syndromes. Epilepsy Behav. 2018, 86, 131–137. [Google Scholar] [CrossRef]
- Sampson, P.B. Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the “Big Two”. J. Nat. Prod. 2021, 84, 142–160. [Google Scholar] [CrossRef]
- Sitovs, A.; Logviss, K.; Lauberte, L.; Mohylyuk, V. Oral Delivery of Cannabidiol: Revealing the Formulation and Absorption Challenges. J. Drug Deliv. Sci. Technol. 2024, 92, 105316. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising Nanocarriers to Enhance Solubility and Bioavailability of Cannabidiol for a Plethora of Therapeutic Opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef]
- Gabriela, L.; Bonilla, P.; Orozco, G.A.; Chaves, I.D.G.; Niñ, G.R. Experimental Solubility of Cannabidiol (CBD) in Different Organic Solvents. Chem. Res. 2023, 62, 12356–12364. [Google Scholar] [CrossRef]
- Vlad, R.A.; Sovány, T.; Kristó, K.; Ibrahim, Y.H.E.Y.; Ciurba, A.; Aigner, Z.; Muntean, D.L.; Regdon, G. Structural and Thermal Analysis of Cannabidiol Orodispersible Formulations. Farmacia 2021, 69, 426–433. [Google Scholar] [CrossRef]
- Mayr, T.; Grassl, T.; Korber, N.; Christoffel, V.; Bodensteiner, M. Cannabidiol Revisited. IUCrdata 2017, 2, x170276. [Google Scholar] [CrossRef]
- Jones, P.G.; Falvello, L.; Kennard, O.; Sheldrick, G.M.; Mechoulam, R. Cannabidiol. Acta Crystallogr. B 1977, 33, 3211–3214. [Google Scholar] [CrossRef]
- Aguiar, A.S.N.; Veloso, C.M.; Tejota, Y.B.R.; Martins, J.L.R.; Fajemiroye, J.O.; Borges, L.L.; Camargo, A.J.; Dias, L.D.; Napolitano, H.B. A Comprehensive Molecular Analysis of Cannabidiol: From Solid State to Antioxidant Potential. Comput. Theor. Chem. 2024, 1241, 114890. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Szajna, E.; Matýsek, D.; Bogdał, D. Development of Stimuli-Responsive Chitosan/ZnO NPs Transdermal Systems for Controlled Cannabidiol Delivery. Polymers 2021, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Sangsanoh, P.; Chaiarwut, S.; Choipang, C.; Niyompanich, J.; Suwantong, O.; Lirdprapamongkol, K.; Svasti, J.; Chuysinuan, P.; Techasakul, S.; Supaphol, P. Cannabidiol/β-Cyclodextrin Inclusion Complex-Loaded Poly(Vinyl Alcohol) Semi-Solid Hydrogels for Potential Use in Wound Management. J. Polym. Environ. 2023, 31, 3982–3997. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Kaminski, K.; Paluch, M.; Wlodarczyk, P.; Grzybowska, K.; Wojnarowska, Z.; Hawelek, L.; Sawicki, W.; Lepek, P.; Lunio, R. Dielectric Relaxation Studies and Dissolution Behavior of Amorphous Verapamil Hydrochloride. J. Pharm. Sci. 2010, 99, 828–839. [Google Scholar] [CrossRef]
- Dushna, O.; Dubenska, L.; Plotycya, S.; Kamiński, K.K.; Paluch, M.; Ziolo, J.; Ngai, K.L. Dielectric Studies of Molecular Motions in Glassy and Liquid Nicotine. J. Phys. Condens. Matter 2006, 18, 5607. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Hancock, B.C.; Dupuis, Y.; Pikal, M.J. Interpretation of Relaxation Time Constants for Amorphous Pharmaceutical Systems. J. Pharm. Sci. 2000, 89, 417–427. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Hawelek, L.; Paluch, M.; Sawicki, W.; Ngai, K.L. Molecular Dynamics at Ambient and Elevated Pressure of the Amorphous Pharmaceutical: Nonivamide (Pelargonic Acid Vanillylamide). J. Chem. Phys. 2011, 134, 44517. [Google Scholar] [CrossRef]
- Sailaja, U.; Shahin Thayyil, M.; Krishna Kumar, N.S.; Govindaraj, G. Molecular Dynamics in Liquid and Glassy States of Non-Steroidal Anti-Inflammatory Drug: Ketoprofen. Eur. J. Pharm. Sci. 2013, 49, 333–340. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Grzybowska, K.; Hawelek, L.; Dulski, M.; Wrzalik, R.; Gruszka, I.; Paluch, M.; Pienkowska, K.; Sawicki, W.; Bujak, P.; et al. Molecular Dynamics, Physical Stability and Solubility Advantage from Amorphous Indapamide Drug. Mol. Pharm. 2013, 10, 3612–3627. [Google Scholar] [CrossRef]
- Schönhals, A.; Kremer, F. Analysis of Dielectric Spectra. In Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003; pp. 59–98. [Google Scholar] [CrossRef]
- Yamamuro, O. Das Temperaturabhangigkeitsgesetz Der Viskositat von Flussigkeiten. Phys. Z. 1921, 22, 645–646. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tammann, G.; Hesse, W. Die Abhängigkeit Der Viscosität von Der Temperatur Bie Unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Kawakami, K.; Harada, T.; Yoshihashi, Y.; Yonemochi, E.; Terada, K.; Moriyama, H. Correlation between Glass-Forming Ability and Fragility of Pharmaceutical Compounds. J. Phys. Chem. B 2015, 119, 4873–4880. [Google Scholar] [CrossRef]
- Böhmer, R.; Ngai, K.L.; Angell, C.A.; Plazek, D.J. Nonexponential Relaxations in Strong and Fragile Glass Formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Knapik, J.; Wojnarowska, Z.; Grzybowska, K.; Jurkiewicz, K.; Tajber, L.; Paluch, M. Molecular Dynamics and Physical Stability of Coamorphous Ezetimib and Indapamide Mixtures. Mol. Pharm. 2015, 12, 3610–3619. [Google Scholar] [CrossRef]
- Kolodziejczyk, K.; Paluch, M.; Grzybowska, K.; Grzybowski, A.; Wojnarowska, Z.; Hawelek, L.; Ziolo, J.D. Relaxation Dynamics and Crystallization Study of Sildenafil in the Liquid and Glassy States. Mol. Pharm. 2013, 10, 2270–2282. [Google Scholar] [CrossRef]
- Grzybowska, K.; Capaccioli, S.; Paluch, M. Recent Developments in the Experimental Investigations of Relaxations in Pharmaceuticals by Dielectric Techniques at Ambient and Elevated Pressure. Adv. Drug Deliv. Rev. 2016, 100, 158–182. [Google Scholar] [CrossRef]
- Paluch, M.; Casalini, R.; Hensel-Bielowka, S.; Roland, C.M.; Casalini, R.; Hensel-Bielowka, S. Effect of Pressure on the α Relaxation in Glycerol and Xylitol. J. Chem. Phys 2002, 116, 9839–9844. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Kaminski, K.; Tarnacka, M.; Szutkowski, K.; Popenda, L.; Bartkowiak, G.; Paluch, M. The Effect of Hydrogen Bonding Propensity and Enantiomeric Composition on the Dynamics of Supercooled Ketoprofen—Dielectric, Rheological and NMR Studies. Phys. Chem. Chem. Phys. 2016, 18, 10585–10593. [Google Scholar] [CrossRef]
- Adrjanowicz, K.; Kaminski, K.; Paluch, M.; Niss, K. Crystallization Behavior and Relaxation Dynamics of Supercooled S-Ketoprofen and the Racemic Mixture along an Isochrone. Cryst Growth Des 2015, 15, 3257–3263. [Google Scholar] [CrossRef]
- Kaminski, K.; Pawlus, S.; Adrjanowicz, K.; Wojnarowska, Z.; Wlodarczyk, P.; Paluch, M. The Importance of the Activation Volume for the Description of the Molecular Dynamics of Glass-Forming Liquids. J. Phys. Condens. Matter 2012, 24, 065105. [Google Scholar] [CrossRef] [PubMed]


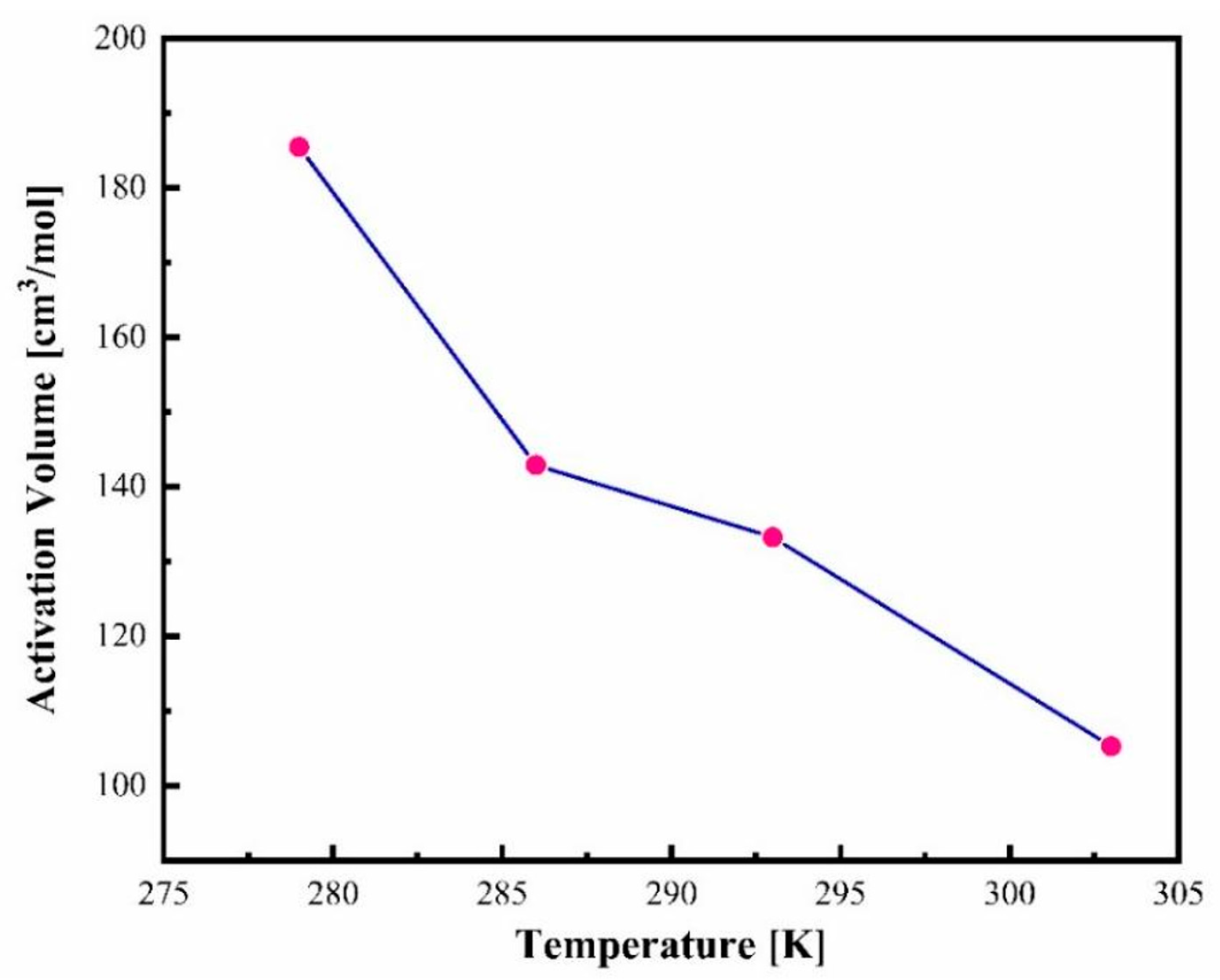
| Isotherms | τ0 | p0 | Dp | ΔV# (cm3/mol) | Pg (MPa) |
|---|---|---|---|---|---|
| 279 K | −3.33 ± 0.02 | 625 ± 80 | 49.9 ± 7.5 | 186 | 125 |
| 286 K | −387 ± 0.03 | 513 ± 38 | 30.8 ± 3.1 | 143 | 157 |
| 293 K | −5.01 ± 0.02 | 730 ± 33 | 39.9 ± 2.4 | 133 | 211 |
| 303 K | −5.69 ± 0.03 | 741 ± 49 | 31.0 ± 2.8 | 105 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, M.; Knapik-Kowalczuk, J.; Dulski, M.; Paluch, M. High-Pressure Dielectric Spectroscopic Studies of Amorphous CBD: Investigating Molecular Dynamics and Physical Stability Under Manufacturing Conditions of the Pharmaceuticals. Pharmaceutics 2025, 17, 358. https://doi.org/10.3390/pharmaceutics17030358
Mathew M, Knapik-Kowalczuk J, Dulski M, Paluch M. High-Pressure Dielectric Spectroscopic Studies of Amorphous CBD: Investigating Molecular Dynamics and Physical Stability Under Manufacturing Conditions of the Pharmaceuticals. Pharmaceutics. 2025; 17(3):358. https://doi.org/10.3390/pharmaceutics17030358
Chicago/Turabian StyleMathew, Mariya, Justyna Knapik-Kowalczuk, Mateusz Dulski, and Marian Paluch. 2025. "High-Pressure Dielectric Spectroscopic Studies of Amorphous CBD: Investigating Molecular Dynamics and Physical Stability Under Manufacturing Conditions of the Pharmaceuticals" Pharmaceutics 17, no. 3: 358. https://doi.org/10.3390/pharmaceutics17030358
APA StyleMathew, M., Knapik-Kowalczuk, J., Dulski, M., & Paluch, M. (2025). High-Pressure Dielectric Spectroscopic Studies of Amorphous CBD: Investigating Molecular Dynamics and Physical Stability Under Manufacturing Conditions of the Pharmaceuticals. Pharmaceutics, 17(3), 358. https://doi.org/10.3390/pharmaceutics17030358









