Development of Films for Wound Healing Based on Gelatin and Oil/Water Emulsions as Carriers of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatin Films with Emulsions (O/W)
2.3. Physicochemical Characterization
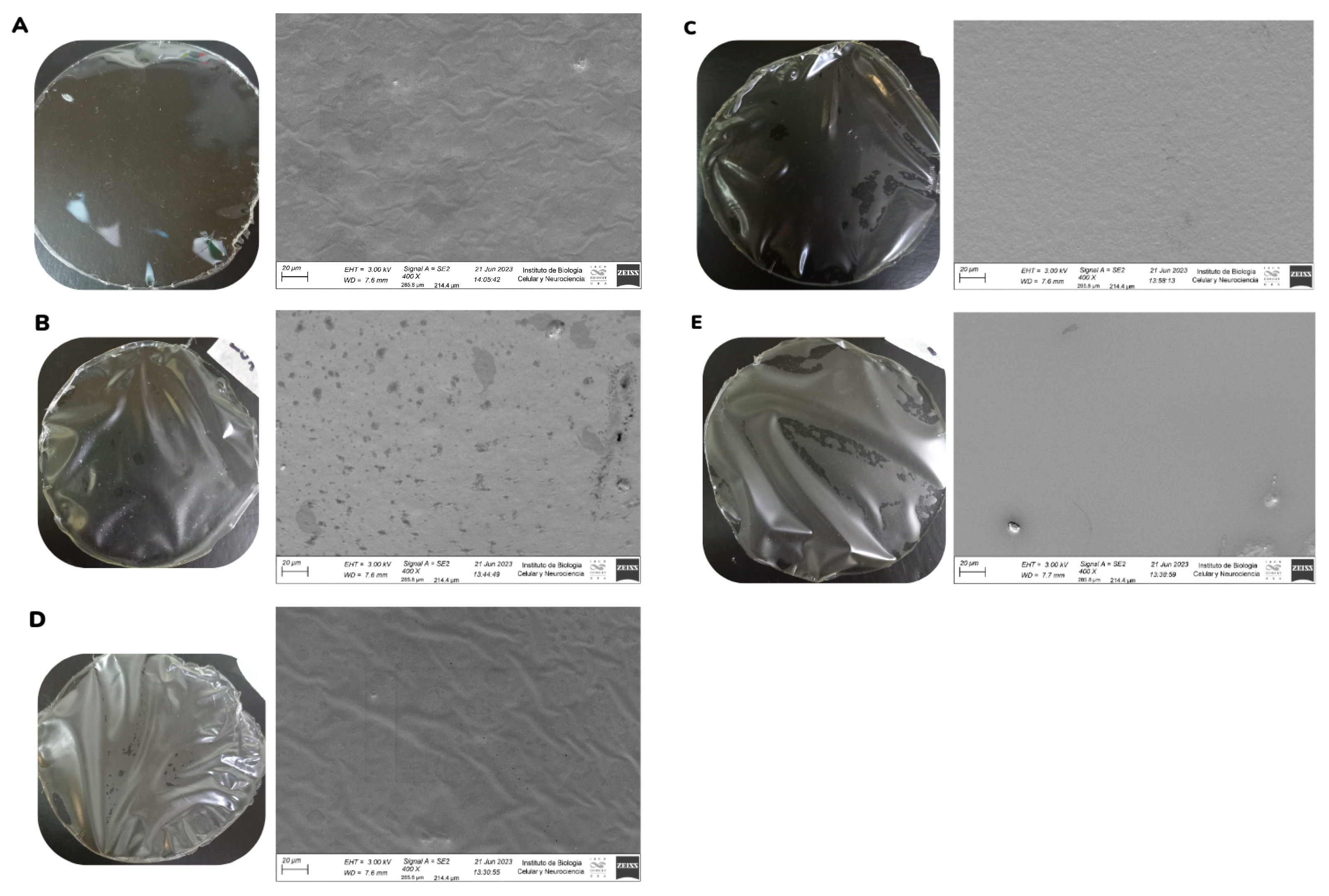
2.3.1. Field Emission Scanning Electron Microscopy (FESEM)
2.3.2. Mechanical Properties
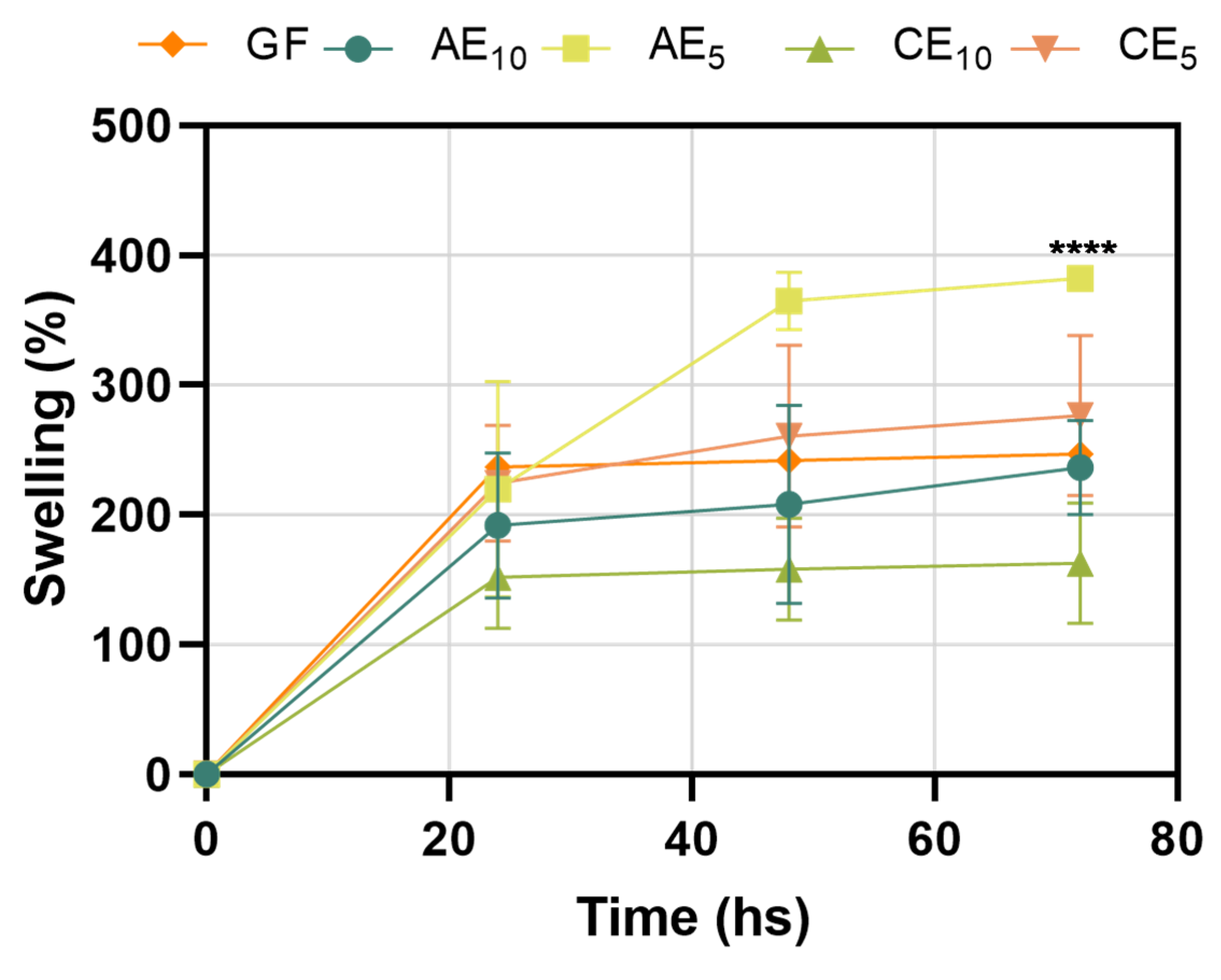
2.3.3. Swelling Capacity
2.3.4. Water Vapor Permeability (Pw)
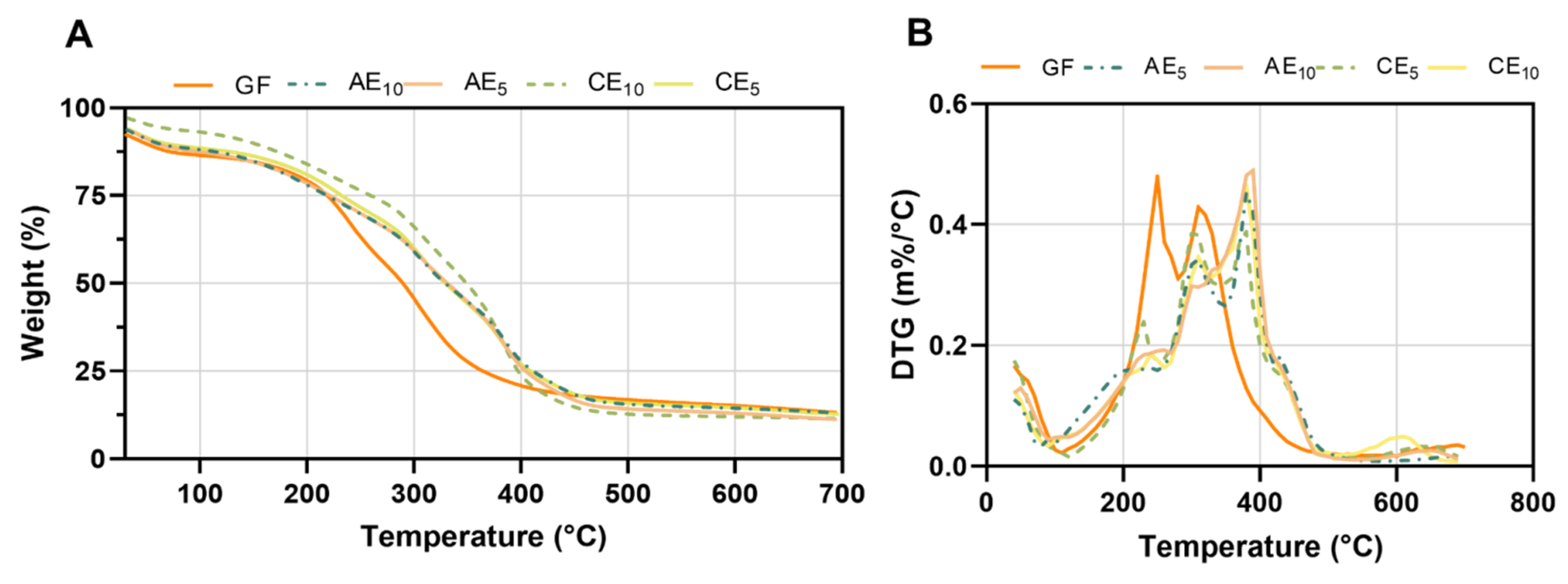
2.3.5. Thermal Gravimetric Analysis (TGA)
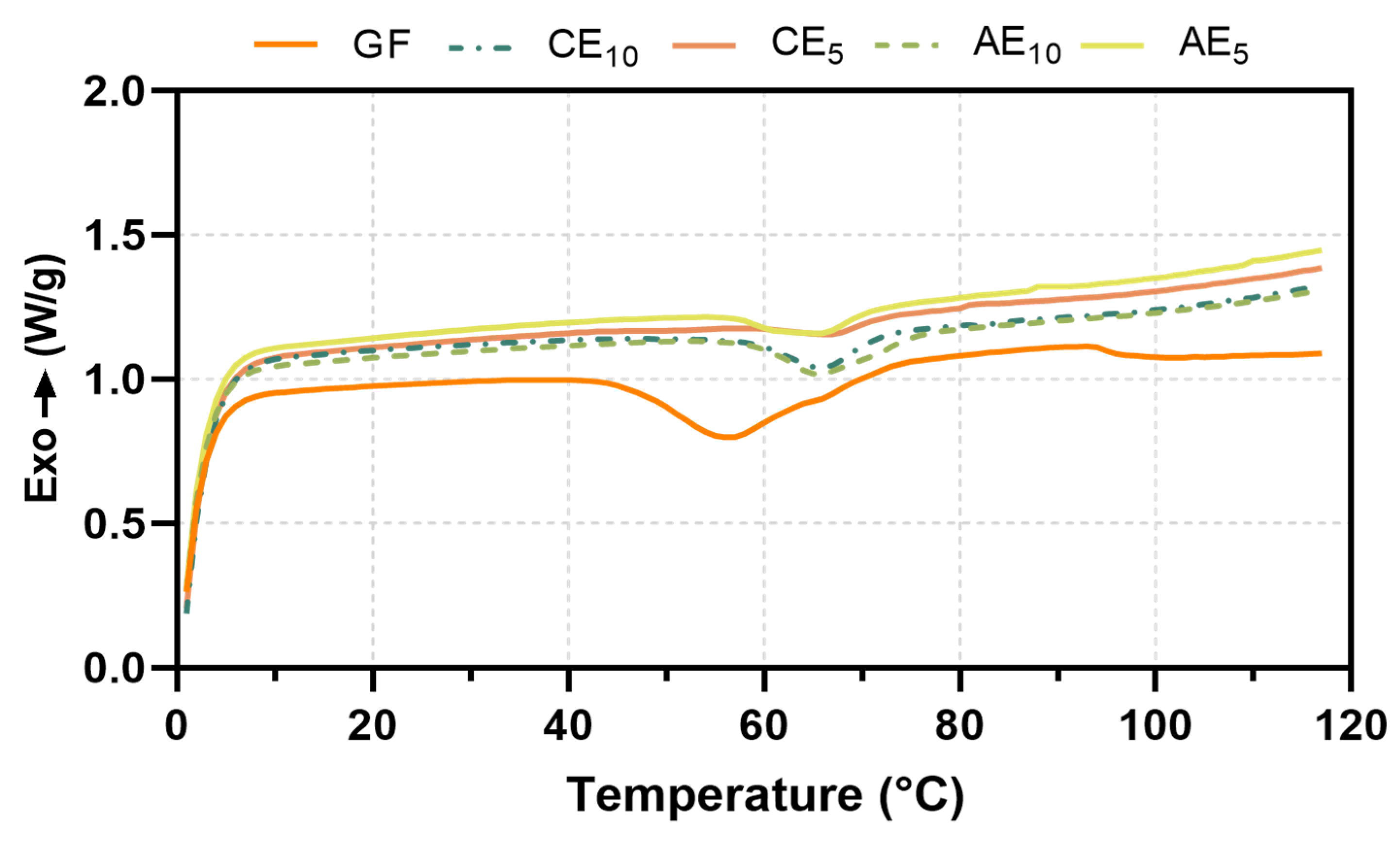
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.4. Cytotoxicity and Wound-Healing Assay
2.5. Toxicology Study in Zebrafish Model
2.6. Statistical Analysis
3. Results and Discussion
3.1. Field Emission Scanning Electron Microscopy (FESEM)
3.2. Mechanical Properties
3.3. Swelling Capacity
3.4. Water Vapor Permeability
3.5. Thermal Gravimetric Analysis (TGA)
3.6. Differential Scanning Calorimetry (DSC)
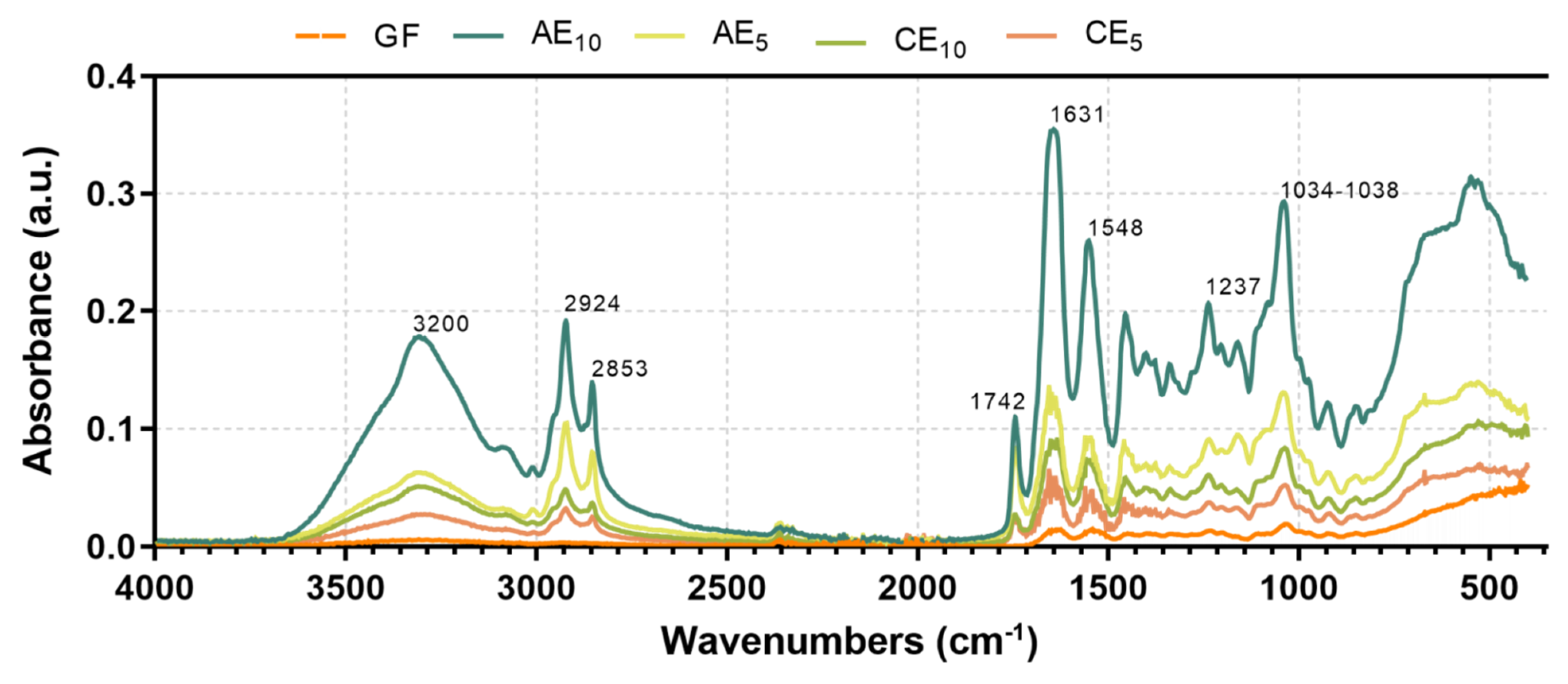
3.7. Fourier-Transform Infrared with Attenuated Total Reflectance (FTIR-ATR)
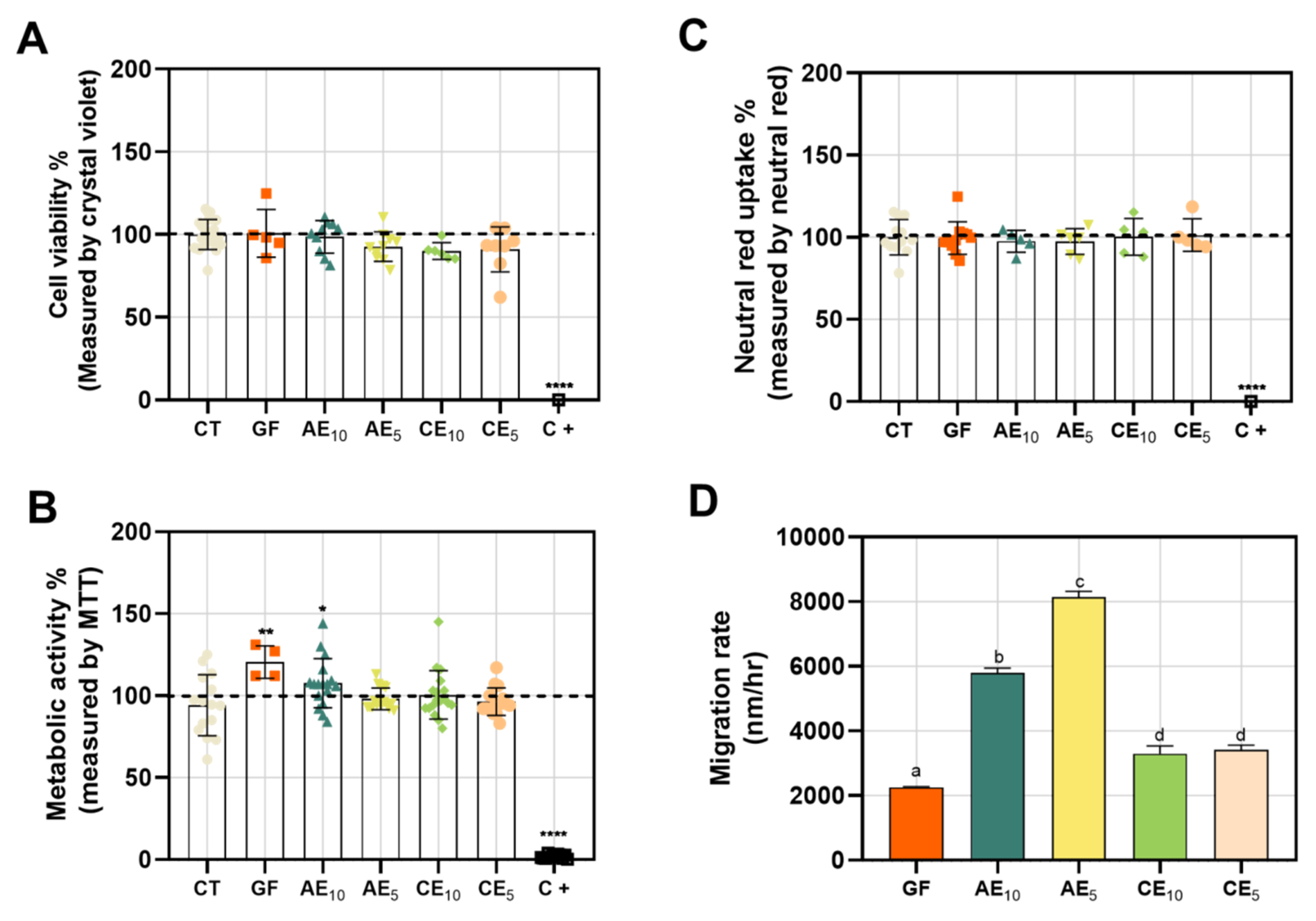
3.8. Cytotoxicity and Wound-Healing Assay
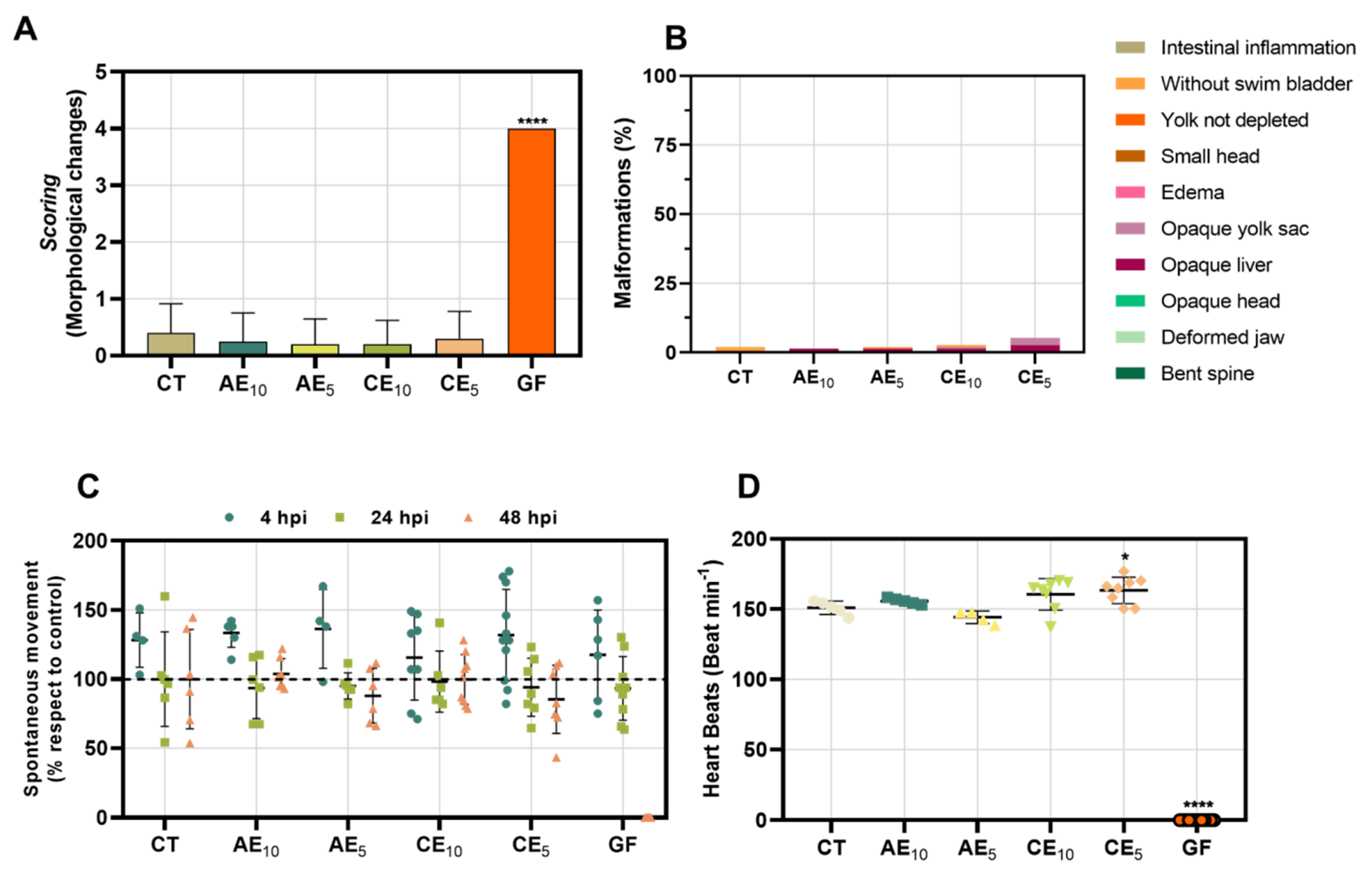
3.9. Toxicology Study in Zebrafish Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle, K.Z.M.; Saucedo Acuña, R.A.; Ríos Arana, J.V.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural Film Based on Pectin and Allantoin for Wound Healing: Obtaining, Characterization, and Rat Model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [PubMed]
- Kumar, S.P.; Asokan, Y.; Balamurugan, K.; Harsha, B. A Review of Wound Dressing Materials and Its Fabrication Methods: Emphasis on Three-Dimensional Printed Dressings. J. Med. Eng. Technol. 2022, 46, 318–334. [Google Scholar] [CrossRef]
- Municoy, S.; Antezana, P.E.; Pérez, C.J.; Bellino, M.G.; Desimone, M.F. Tuning the Antimicrobial Activity of Collagen Biomaterials through a Liposomal Approach. J. Appl. Polym. Sci. 2021, 138, 50330. [Google Scholar] [CrossRef]
- El Kolli, H.; El Kolli, M. Preparation and Characterization of Gelatin-Based Films Cross-Linked by Two Essential Oils at Different Concentrations and Plasticized with Glycerol. Eng. Technol. Appl. Sci. Res. 2021, 11, 7489–7494. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, Y.; Xu, Z.; Chen, G.; Ge, L.; Mu, C.; Tian, Y.; Li, D. Emulsion Template Fabrication of Antibacterial Gelatin-Based Scaffolds with a Preferred Microstructure for Accelerated Wound Healing. ACS Appl. Polym. Mater. 2022, 4, 3885–3895. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Wairkar, S. Collagen Fabricated Delivery Systems for Wound Healing: A New Roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Ale, A.; Galdopórpora, J.M.; Desimone, M.F.; De La Torre, F.R.; Cazenave, J. Nanosilver and Silver Nitrate Toxicity in Ex Vivo-Exposed Gills of Fish and Mitigation by Humic Acids. Bull. Environ. Contam. Toxicol. 2021, 107, 421–426. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Ibar, A.; Tuttolomondo, M.V.; Desimone, M.F. Dual-Effect Core–Shell Polyphenol Coated Silver Nanoparticles for Tissue Engineering. Nano-Struct. Nano-Objects 2021, 26, 100716. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhao, J.; Xing, B. Environmental Processes and Toxicity of Metallic Nanoparticles in Aquatic Systems as Affected by Natural Organic Matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Sosa, A.M.; Igartúa, D.E.; Alonso, S.D.V.; Prieto, M.J.; Martinez, C.S. A Crossover Study of Antimicrobial Capacity and Biotoxicity of Silver Nanoparticles. Appl. Organomet. Chem. 2024, 38, e7377. [Google Scholar] [CrossRef]
- Zinder, R.; Cooley, R.; Vlad, L.G.; Molnar, J.A. Vitamin A and Wound Healing. Nutr. Clin. Pract. 2019, 34, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Karnina, R.; Arif, S.K.; Hatta, M.; Bukhari, A. Molecular Mechanisms of Lidocaine. Ann. Med. Surg. 2021, 69, 102733. [Google Scholar] [CrossRef]
- Fuller, F.W. The Side Effects of Silver Sulfadiazine. J. Burn Care Res. 2009, 30, 464–470. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Kierul, K.; Bochynek, M.; Pannert, D.; Nowaczyk, P.; Łukaszewicz, M. Targeted Hybrid Nanocarriers as a System Enhancing the Skin Structure. Molecules 2021, 26, 1063. [Google Scholar] [CrossRef]
- Lillo, C.R.; Calienni, M.N.; Rivas Aiello, B.; Prieto, M.J.; Rodriguez Sartori, D.; Tuninetti, J.; Toledo, P.; Alonso, S.D.V.; Moya, S.; Gonzalez, M.C.; et al. BSA-Capped Gold Nanoclusters as Potential Theragnostic for Skin Diseases: Photoactivation, Skin Penetration, in Vitro, and in Vivo Toxicity. Mater. Sci. Eng. C 2020, 112, 110891. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F. Potential Applications of Multiple Emulsions in the Development of Healthy and Functional Foods. Food Res. Int. 2013, 52, 64–74. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Calienni, M.N.; Feas, D.A.; Chiaramoni, N.S.; Del Valle Alonso, S.; Prieto, M.J. Development of Nutraceutical Emulsions as Risperidone Delivery Systems: Characterization and Toxicological Studies. J. Pharm. Sci. 2015, 104, 4142–4152. [Google Scholar] [CrossRef] [PubMed]
- Okuma, C.H.; Andrade, T.A.M.; Caetano, G.F.; Finci, L.I.; Maciel, N.R.; Topan, J.F.; Cefali, L.C.; Polizello, A.C.M.; Carlo, T.; Rogerio, A.P.; et al. Development of Lamellar Gel Phase Emulsion Containing Marigold Oil (Calendula officinalis) as a Potential Modern Wound Dressing. Eur. J. Pharm. Sci. 2015, 71, 62–72. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid Addition to Improve Barrier Properties of Edible Starch-based Films and Coatings. J. Food Sci. 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; Wagner, J.R. Use of Edible Films and Coatings for Functional Foods Developments: A Review. In Functional Foods Sources, Health Effects and Future Perspectives; Nelson, D.L., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 1–26. [Google Scholar]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Sosa, A.M.; Igartúa, D.E.; Martínez, L.M.; Alonso, S.d.V.; Prieto, M.J.; Martinez, C.S. Multifunctional Emulsion for Potential Wound-Healing Applications: A Development for Co-Administration of Essential Vitamins, Silver Nanoparticles, and Potent Active Ingredients. J. Drug Deliv. Sci. Technol. 2024, 101, 106128. [Google Scholar] [CrossRef]
- Feas, D.A.; Igartúa, D.E.; Calienni, M.N.; Martinez, C.S.; Pifano, M.; Chiaramoni, N.S.; Del Valle Alonso, S.; Prieto, M.J. Nutraceutical Emulsion Containing Valproic Acid (NE-VPA): A Drug Delivery System for Reversion of Seizures in Zebrafish Larvae Epilepsy Model. J. Pharm. Investig. 2017, 47, 429–437. [Google Scholar] [CrossRef]
- Martinez, C.S.; Igartúa, D.E.; Czarnowski, I.; Feas, D.A.; Alonso, S.d.V.; Prieto, M.J. Biological Response and Developmental Toxicity of Zebrafish Embryo and Larvae Exposed to Multi-Walled Carbon Nanotubes with Different Dimension. Heliyon 2019, 5, e02308. [Google Scholar] [CrossRef]
- Dai, C.; Han, S.; Ma, C.; McClements, D.J.; Xu, D.; Chen, S.; Liu, X.; Liu, F. High Internal Phase Emulsions Stabilized by Pea Protein Isolate-EGCG-Fe3+ Complexes: Encapsulation of β-Carotene. Food Hydrocoll. 2024, 150, 109607. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Wen, X.; Li, X.; Fang, F.; Richel, A.; Xiao, N.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Incorporation of Cinnamon Essential Oil-Loaded Pickering Emulsion for Improving Antimicrobial Properties and Control Release of Chitosan/Gelatin Films. Food Hydrocoll. 2023, 138, 108438. [Google Scholar] [CrossRef]
- Nuvoli, L.; Conte, P.; Fadda, C.; Reglero Ruiz, J.A.; García, J.M.; Baldino, S.; Mannu, A. Structural, Thermal, and Mechanical Properties of Gelatin-Based Films Integrated with Tara Gum. Polymer 2021, 214, 123244. [Google Scholar] [CrossRef]
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; De La Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Lourenço, R.V.; Sobral, P.J.D.A. Active Gelatin Films Incorporated with Pickering Emulsions Encapsulating Hesperidin: Preparation and Physicochemical Characterization. J. Food Eng. 2019, 240, 9–20. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Jopling, D.W. The Swelling of Gelatin Films. The Effects of Drying Temperature and of Conditioning the Layers in Atmospheres of High Relative Humidity. J. Appl. Chem. 2007, 6, 79–84. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of Novel Alginate Based Hydrogel Films for Wound Healing Applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J. Physico-Mechanical, Morphological and Biomedical Properties of a Novel Natural Wound Dressing Material. J. Mech. Behav. Biomed. Mater. 2017, 65, 373–382. [Google Scholar] [CrossRef]
- Ganeson, K.; Razifah, M.R.; Mubarak, A.; Kam, A.; Vigneswari, S.; Ramakrishna, S. Improved Functionality of Cinnamon Oil Emulsion-Based Gelatin Films as Potential Edible Packaging Film for Wax Apple. Food Biosci. 2022, 47, 101638. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The Physiochemical and Preservation Properties of Anthocyanidin/Chitosan Nanocomposite-Based Edible Films Containing Cinnamon-Perilla Essential Oil Pickering Nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Mosleh, Y.; De Zeeuw, W.; Nijemeisland, M.; Bijleveld, J.C.; Van Duin, P.; Poulis, J.A. The Structure–Property Correlations in Dry Gelatin Adhesive Films. Adv. Eng. Mater. 2021, 23, 2000716. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Antibacterial Mechanism of Biogenic Copper Nanoparticles Synthesized Using Heliconia psittacorum Leaf Extract. Nanotechnol. Rev. 2016, 5, 529–536. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Zhang, H.; Dong, M.; Li, L.; Jia, P.; Bu, T.; Wang, X.; Wang, L. Development of Functional Gelatin-Based Composite Films Incorporating Oil-in-Water Lavender Essential Oil Nano-Emulsions: Effects on Physicochemical Properties and Cherry Tomatoes Preservation. LWT 2021, 142, 110987. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, Morphological and Thermal Behaviour Characterisations of Fish Gelatin Film Incorporated with Basil and Citronella Essential Oils as Affected by Surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Mo, X.; Peng, X.; Liang, X.; Fang, S.; Xie, H.; Chen, J.; Meng, Y. Development of Antifungal Gelatin-Based Nanocomposite Films Functionalized with Natamycin-Loaded Zein/Casein Nanoparticles. Food Hydrocoll. 2021, 113, 106506. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and Antifungal Properties of Bio-Nanocomposite Film Based on Gelatin-Chitin Nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The Importance of Both Fibroblasts and Keratinocytes in a Bilayered Living Cellular Construct Used in Wound Healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of Different Chitosan Derivatives on in Vitro Scratch Wound Assay: A Comparative Study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Barbosa, W.S.; Rangel, W.S.P.; Valle, I.M.M.; Matos, A.P.d.S.; Melgaço, F.G.; Dias, M.L.; Ricci Júnior, E.; Da Silva, L.C.P.; De Abreu, L.C.L.; et al. Polymeric Membrane Based on Polyactic Acid and Babassu Oil for Wound Healing. Mater. Today Commun. 2021, 26, 102173. [Google Scholar] [CrossRef]
- Liang, D.; Feng, B.; Li, N.; Su, L.; Wang, Z.; Kong, F.; Bi, Y. Preparation, Characterization, and Biological Activity of Cinnamomum cassia Essential Oil Nano-Emulsion. Ultrason. Sonochem. 2022, 86, 106009. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.T.; Hooyberghs, J.; Blust, R.; Witters, H.E. Assessment of the Developmental Neurotoxicity of Compounds by Measuring Locomotor Activity in Zebrafish Embryos and Larvae. Neurotoxicol. Teratol. 2013, 37, 44–56. [Google Scholar] [CrossRef]

| Sample Names | Description |
|---|---|
| GF | Films based on gelatin without emulsion |
| CE5 | Films based on gelatin with CE without active compounds (5:1 mass ratio, film dispersion/emulsion) |
| CE10 | Films based on gelatin with CE without active compounds (10:1 mass ratio, film dispersion/emulsion) |
| AE5 | Films based on gelatin with AE with active compounds (5:1 mass ratio, film dispersion/emulsion) |
| AE10 | Films based on gelatin with AE with active compounds (10:1 mass ratio, film dispersion/emulsion) |
| Sample | TS (MPa) | YM (MPa) | ε (%) | Thickness (mm) |
|---|---|---|---|---|
| GF | 2.2 ± 0.4 a | 0.04 ± 0.01 a | 46 ± 13 a | 0.042 ± 0.003 a |
| AE10 | 3.6 ± 0.6 ab | 1.7 ± 0.4 b | 257 ± 20 b | 0.053 ± 0.003 b |
| CE10 | 3.9 ± 0.6 b | 1.7 ± 0.5 b | 338 ± 21 c | 0.057 ± 0.002 b |
| AE5 | 6.2 ± 0.5 c | 7.6 ± 0.8 c | 302 ± 32 c | 0.075 ± 0.002 c |
| CE5 | 2.6 ± 0.8 a | 3.9 ± 0.7 d | 306 ± 20 c | 0.075 ± 0.005 c |
| Sample | Pw 10−10(g s−1 m−1 Pa−1) | Thickness (mm) |
|---|---|---|
| GF | 1.43 ± 0.02 a | 0.032 ± 0.03 a |
| AE10 | 2.50 ± 0.01 b | 0.053 ± 0.003 b |
| CE10 | 2.34 ±0.01 b | 0.057 ± 0.002 b |
| AE5 | 2.70 ± 0.01 b | 0.075 ± 0.002 c |
| CE5 | 2.60 ± 0.02 b | 0.075 ± 0.005 c |
| Formulation | Ti (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) |
|---|---|---|---|---|
| GF | 145 | 280 | 305 | - |
| AE10 | 140 | 325 | 385 | 435 |
| CE10 | 160 | 270 | 380 | 440 |
| AE5 | 155 | 310 | 390 | 431 |
| CE5 | 180 | 310 | 345 | 400 |
| Formulation | Tg (°C) | ΔH (J/g) |
|---|---|---|
| GF | 57 | 4.236 |
| AE10 | 62 | 5.043 |
| CE10 | 62 | 4.835 |
| AE5 | 67 | 6.758 |
| CE5 | 65 | 6.653 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, A.M.; Cottet, C.; Berin, B.E.; Martínez, L.M.; Peltzer, M.A.; Prieto, M.J.; Martinez, C.S. Development of Films for Wound Healing Based on Gelatin and Oil/Water Emulsions as Carriers of Bioactive Compounds. Pharmaceutics 2025, 17, 357. https://doi.org/10.3390/pharmaceutics17030357
Sosa AM, Cottet C, Berin BE, Martínez LM, Peltzer MA, Prieto MJ, Martinez CS. Development of Films for Wound Healing Based on Gelatin and Oil/Water Emulsions as Carriers of Bioactive Compounds. Pharmaceutics. 2025; 17(3):357. https://doi.org/10.3390/pharmaceutics17030357
Chicago/Turabian StyleSosa, Ayelen M., Celeste Cottet, Belén E. Berin, Luis M. Martínez, Mercedes A. Peltzer, María J. Prieto, and Carolina S. Martinez. 2025. "Development of Films for Wound Healing Based on Gelatin and Oil/Water Emulsions as Carriers of Bioactive Compounds" Pharmaceutics 17, no. 3: 357. https://doi.org/10.3390/pharmaceutics17030357
APA StyleSosa, A. M., Cottet, C., Berin, B. E., Martínez, L. M., Peltzer, M. A., Prieto, M. J., & Martinez, C. S. (2025). Development of Films for Wound Healing Based on Gelatin and Oil/Water Emulsions as Carriers of Bioactive Compounds. Pharmaceutics, 17(3), 357. https://doi.org/10.3390/pharmaceutics17030357







