Abstract
Background: Paullinia cupana Kunth, popularly known as guarana, a native Amazonian shrub cultivated by the Sateré-Mawé ethnic group, has been used in traditional medicine for various purposes, including stimulant and therapeutic actions, due to its chemical composition, which is rich in bioactive compounds. This study explored the reductive potential of guarana with nanobiotechnology and aimed to synthesize silver nanoparticles (AgNPs) using the aqueous extract of leaves collected during the dry and rainy seasons, assessing their biological and catalytic activities. Methods: The AgNPs were synthesized in a water bath at 70 °C for three hours and then characterized using techniques such as UV-Vis spectroscopy, DLS, zeta potential, MET, NTA, and EDX and had their effects on various biological systems assessed in vitro, as well as in catalytic tests aimed at indicating the probable influence of the time when the plant material was collected on the properties of the nanostructures. Results: The AgNPs had an average diameter between 39.33 and 126.2 nm, spherical morphology, absorption bands between 410 and 450 nm, and high colloidal stability over two years. The biological results showed antibacterial activity against all the species tested, as well as remarkable antioxidant action against DPPH and ABTS free radicals, in the same way as the aqueous leaf extracts of P. cupana, in addition to cytotoxic properties against cancerous (A431 and A549) and non-cancerous (HaCaT and HNTMC) cells. The AgNPs were active against promastigote forms of Leishmania (Leishmania) amazonensis while not affecting the viability of macrophages, and from the LC50 and LC90 values, the AgNPs were more effective than the metal salt solution in controlling Aedes aegypti larvae and pupae. We also reported that the catalytic degradation of the organic dyes methylene blue (MB) and methyl orange (MO) by AgNPs was over 90% after 40 or 14 min, respectively. Conclusions: Thus, our results support the potential of seasonal extracts of guarana leaves to produce AgNPs with diverse application possibilities for the health, industrial, and environmental sectors.
1. Introduction
The Amazon rainforest is home to a great deal of plant biodiversity, and guarana (Paullinia cupana Kunth), which belongs to the Sapindaceae family, stands out as one of the most promising native compounds in this region of Brazil. The plant’s use by traditional Sateré-Mawé people, mainly in the form of a drink, due to its stimulating and therapeutic effects, has been reported for a long time [1,2]. Because of this, guarana has been listed in the Brazilian Pharmacopoeia since 1977, and in 2005 it was recognized as a safe food by the National Health Surveillance Agency (ANVISA) and the Food and Drug Administration (FDA) [3,4].
The phytochemical composition of P. cupana extracts has been reported in some studies, showing differences in the molecular profile depending on the conditions of analysis. When evaluating the different parts of the plant, it is possible to report the occurrence of alkaloids, procyanidins, tannins, and flavonoids in the flower extract, while theobromine and caffeine have been detected in the plant’s nectar [5]. Other studies have described the presence of caffeine and tannins in guarana seeds, as well as methylxanthines in the leaves and pericarp, which stimulates new approaches to genetic improvement and pharmaceutical applications with a focus on different plant organs [6,7]. In addition, the phytochemical composition of guarana seeds from different geographical regions, such as the states of Amazonas, Bahia, and Mato Grosso, showed that dimers and trimers of procyanidins A and B, as well as catechin and epicatechin, were the main chemical markers [8,9]. In addition, higher levels of carbohydrates, caffeine, and tannins have been reported in plant samples from Bahia, while guarana from Amazonas has a high level of fatty acids and cyanolipids [10].
Nanotechnology applies scientific knowledge from various fields, manipulating materials made up of individual atoms, molecules, and molecular clusters to create structures with different or new properties. It is an emerging field, especially in the production and manipulation of nanoscale structures, where generally one of their dimensions must be in the range 1–100 nm [11,12]. Silver is a noble metal with a long history of use in different forms and for various purposes and has long been known for its beneficial properties acting in wound healing and infections, among others [13]. Silver nanoparticles (AgNPs) stand out due to their intrinsic properties such as high stability, strong absorption in the visible ultraviolet region, and broad potential for applications, which are made possible by the significant surface/volume ratio that gives nanoscale particles different attributes to those on a larger scale. In turn, the shape, size, distribution, and surface-related aspects of AgNPs are determined by the concentrations of reducing agents, metal precursors, and stabilizers used during synthesis [14,15].
The search for biocompatible and less toxic methods using natural sources and their derivatives to synthesize AgNPs with improved properties is desirable to maintain better conditions for both the environment and human health [16]. In this scenario, various parts of the same plant can be used to synthesize AgNPs, such as the leaf, bark, flower, seed, fruit, rhizome, and stem, with leaves being the most used materials due to the high volume of extraction, as well as the greater accumulation of biomolecules in their composition [17,18]. These secondary metabolites have the ability to protect plants in the control of various diseases [19], as well as having the ability to chelate with silver ions and subsequently act in the bioreduction and stabilization of AgNPs (Ag+ → Ag0), binding to their surface and preventing aggregation [20]. While the detailed mechanisms of this process are not yet fully understood, they are driving research into natural products and green nanobiotechnology.
In general, plant metabolism and, consequently, the presence/concentration of plant biomolecules can vary depending on biochemical, physiological, ecological, and evolutionary processes, including changes related to cultivation methods, extraction conditions, planting regions, seasonality, and environmental and genetic factors, among other characteristics such as the age of the plant, altitude, ultraviolet radiation, luminosity, and atmospheric pollution [21]. Thus, the aim of this study was to investigate the potential of P. cupana leaves used in the green synthesis of AgNPs by collecting the plant material in seasonal periods of drought and rain in the Amazon region, as well as to assess the possible differences in the characteristics of the nanostructures obtained and their effects on different biological systems in vitro and in the catalytic degradation of organic dyes in order to add value to the sustainable use of natural resources from Brazilian biodiversity.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemical reagents were used without further purification. Silver nitrate (AgNO3), DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), fetal bovine serum, penicillin/streptomycin antibiotic solution, methylene blue and methyl orange dyes, and phosphate-buffered saline (PBS) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol (CH3OH) and dimethyl sulphoxide (DMSO) were purchased from Dinamica (Indaiatuba, São Paulo, Brazil). Potassium persulphate (K2S2O8) was purchased from LabSynth (Diadema, São Paulo, Brazil). Sodium borohydride (NaBH4) was purchased from Alphatec (São Paulo, Brazil).
The bacteria Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus aureus, and Staphylococcus epidermidis were obtained from the American Type Culture Collection (ATCC), while the bacterium Bacillus cereus was isolated from the environment and kept in the laboratory. The Muller–Hinton agar culture medium was obtained from Difco (Difco, Franklin Lakes, NJ, USA).
The cell lines were acquired from the Rio de Janeiro Cell Bank (BCRJ) (Rio de Janeiro, Brazil). DMEM (Dulbecco’s Modified Eagle Medium) culture medium and MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) salt were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Only ultrapure water was used to prepare the plant extracts, the metal salt solution, and for other purposes during the study.
2.2. Preparation of Aqueous Extract of Paullinia cupana Leaves and Synthesis of AgNPs
The collection of plant material and the methodology used to prepare the aqueous extract of P. cupana leaves were carried out as described in our previous studies [22,23], with the aqueous extracts being called Ext-LD (aqueous extract of leaves collected during the dry season) and Ext-LR (aqueous extract of leaves collected during the rainy season). The biogenic synthesis of AgNPs was carried out using an aqueous solution of AgNO3 at 2 mM (340 µg/mL) and leaf extract at a concentration of 2 mg/mL in a final volume of 50 mL. In addition, experimental controls were also produced consisting of the following: (i) AgNO3 control—2 mM metal salt and ultrapure water and (ii) extract control—2 mg/mL aqueous extract and ultrapure water, both in a final volume of 50 mL. The flasks containing each solution were protected from light with aluminum foil and incubated in a water bath (555, Fisatom, São Paulo, Brazil) at 70 °C for 180 min. The obtained suspensions were named AgNPs-LD (nanoparticles synthesized using extract from leaves collected during the dry season) and AgNPs-LR (nanoparticles synthesized using extract from leaves collected during the rainy season).
2.3. Characterization of AgNPs
2.3.1. Ultraviolet–Visible Spectroscopy (UV-Vis)
The kinetics of AgNPs formation were monitored in a UV-Vis spectrophotometer (UV1800PC, Phenix, Blomberg, Germany) every 30 min during incubation in a water bath. Then, 2 mL aliquots of each sample, without prior dilution, were placed in a quartz cuvette and inserted into the equipment, and their absorbance was measured at a wavelength of 450 nm. At the end of the reactions, with the same volume of colloidal suspension, spectrophotometric analyses were carried out in the range between 350 and 550 nm to obtain the maximum absorption bands of each sample.
2.3.2. Dynamic Light Scattering (DLS) and Surface Zeta Potential
The colloidal characteristics of average hydrodynamic diameter (HD), polydispersity index (PdI), and surface zeta potential (ZP) of the AgNPs were measured using a ZetaSizer Nano ZS device (Malvern Instruments, Malvern, UK). For these analyses, the samples were initially diluted in a 1:10 (v/v) ratio in ultrapure water to a final volume of 1 mL, and then this solution was placed in a polystyrene cuvette to be inserted into the equipment, which was configured to take the readings at an angle of 90°, a temperature of 25 °C, with 120 s of stabilization, and readings in triplicate with 10 runs each.
To analyze the stability of the AgNPs, aliquots were separated into two storage conditions: at room temperature (25 °C) and under refrigeration (4 °C). Analyses were carried out over 730 days (2 years) from the initial synthesis of the nanostructures, and the data were represented as the mean ± standard deviation, processed using the ZetaSizer 7.13 software developed by the same equipment manufacturer.
2.3.3. Nanoparticle Tracking Analysis (NTA)
To determine the concentration, size and Span index of the AgNPs, a methodology adapted from a previous study was used [24]. Nano-Sight NS300 equipment (Malvern Instruments, Worcestershire, UK) equipped with a 532 nm diode laser (green) was used where the samples, diluted in a ratio of 1:100 (v/v) in ultrapure water to obtain a concentration of between 1 × 102 and 108 particles/mL, were injected with sterile syringes, and the captured videos were analyzed using NTA 3.2 software (Malvern Instruments, Malvern, UK).
The average size values obtained by the analysis software were based on the values calculated with the sizes of all the particles captured by the equipment and used to determine D10, D50, and D90 as average size distributions. The Span index, which denotes the dispersity of the populations of particles in suspension, was calculated using Equation (1). In turn, the results were presented as the mean ± standard deviation and the mode ± standard deviation from analyses carried out in triplicate for each type of AgNPs.
2.3.4. Transmission Electron Microscopy (TEM)
The morphological characterization of the AgNPs was carried out using a JEM-1011 transmission electron microscope (JEOL, Tokyo, Japan) operating at 80 kV. Briefly, 5 µL of each sample, without dilution, were placed on a 400-mesh copper screen covered with Formvar® film, and after drying for 24 h at room temperature, the nanostructures were visualized in the equipment.
Photomicrographs were taken at random and from the particle count of each group of AgNPs (between 340 and 450 particles) using ImageJ software version 1.8.0 (National Institute of Health, Bethesda, MD, USA). Size distribution histograms were constructed showing the mean ± standard deviation of the mean dry particle diameter.
2.3.5. Energy Dispersive X-Ray Spectroscopy (EDX)
The elemental composition of the atoms present in the colloidal suspensions was determined by EDX analyses carried out by viewing the samples in a scanning electron microscope (FEI, INSPECT S50 with Everhart-Thornley detector, Hillsboro, OR, USA) operating at 10 kV. Each AgNPs sample was placed separately on aluminum stubs without metallization and left in the dark for three days in closed containers until completely dry for subsequent analysis in the equipment.
2.4. Biological Studies of AgNPs
2.4.1. Antibacterial Activity
The broth microdilution method according to the Clinical Laboratory Standard Institute [25] was used for this test. The following bacteria were used Gram-negative: Acinetobacter baumannii (ATCC 19606), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603), Pseudomonas aeruginosa (ATCC 9027), and Salmonella enterica (ATCC 13076). The following bacteria were used Gram-positive: Bacillus cereus (environmentally isolated), Staphylococcus aureus (ATCC 25923), and Staphylococcus epidermidis (ATCC 12228).
Initially, to assess the minimum inhibitory concentration (MIC), the bacteria were seeded on Muller–Hinton (MH) agar and incubated for 24 h at 37 °C. After this period, around five bacterial colonies were collected and placed in saline solution (0.85% NaCl) with the McFarland scale adjusted to 0.5 or 1.5 × 108 CFU/mL. Then, 50 µL of MH was added to the wells of a 96-well microplate, and 50 µL of each sample was added afterwards, totaling 100 µL. Next, 50 µL of this solution was collected and placed in the next well and so on, carrying out a serial dilution with seven dilution points for the AgNPs and AgNO3 (42.5 to 0.33 µg/mL) and for the aqueous extracts (25,000 to 50 µg/mL). At the end, to complete the final volume of 100 µL per well, the saline solution with the bacteria was diluted to 1.5 × 106 CFU/mL, and 50 µL were added to each well, to which the sample to be tested had previously been added.
The plates were incubated for between 18 and 24 h at 37 °C and then read visually, assessing the turbidity of the wells, where the absence of turbidity indicated inhibition of bacterial growth. All the tests were carried out in triplicate per concentration, with a positive control (only saline solution of bacteria) and a negative control (only MH medium). The minimum bactericidal concentration (MBC) was considered when bacterial death was ≥99.9% after 24 h of incubation.
2.4.2. Antioxidant Activity Against DPPH and ABTS Free Radicals
To determine antioxidant activity, 150 µL of a methanolic solution of the DPPH radical (0.1 mM) was added to a 96-well microplate, followed by 150 µL of each sample at different concentrations, with AgNPs (12.5, 25, 50, 75, 100, and 150 µg/mL), aqueous extracts (125, 250, 500, 750, 1000, and 1500 µg/mL), and ascorbic acid (positive control) at the same concentrations as the nanostructures, and then the microplates were covered with aluminum foil and incubated in the dark at room temperature for 30 min [26]. After this period, the absorbance of the wells was read at 517 nm using a spectrophotometer combined with a microplate reader (M3, Molecular Devices, San Jose, CA, USA). For this experiment, the negative control consisted of 150 µL of methanolic DPPH solution with 150 µL of methanol, and 300 µL of methanol was used as an experimental blank.
The antioxidant activity of the samples was also tested against the ABTS+ radical cation, formed from the reaction between aqueous solutions of ABTS (7 mM) and potassium persulphate (140 mM), according to previous studies with modifications [27,28]. In a 96-well microplate, 250 µL of the aqueous ABTS+ solution was deposited in the wells, followed by 50 µL of AgNPs (12.5, 25, 50, 75, 100, and 150 µg/mL), aqueous extracts (125, 250, 500, 750, 1000, and 1500 µg/mL), and ascorbic acid (positive control) at the same concentrations as the nanostructures. The microplates were then covered with aluminum foil and incubated for 7 min in the dark at room temperature, and then the absorbance of the wells was read at 734 nm on a spectrophotometer combined with a microplate reader (M3, Molecular Devices). The negative control was 250 µL of aqueous ABTS+ solution with 50 µL of potassium persulphate, and the experimental blank was 300 µL of potassium persulphate.
All experiments were carried out in triplicate per concentration for each sample and in three independent experiments. The results are presented as the mean ± standard deviation of the mean, with the antioxidant activities expressed as a percentage of free radical inhibition (%) based on the absorbance values corrected for their respective blanks. The antioxidant activities were calculated using Equation (2).
2.4.3. Anticancer Activity
The anticancer activity of AgNPs, AgNO3, and aqueous extracts was tested on A431 (non-melanoma skin carcinoma), HaCaT (human keratinocyte), A549 (lung adenocarcinoma), and HNTMC (human dental pulp fibroblast) cells using MTT colorimetric assay. The cell lines were grown in DMEM medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) antibiotic solution (100 U/mL penicillin and 100 µg/mL streptomycin) and kept in cell culture flasks in an incubator (Thermo Scientific, Waltham, MA, USA) at 37 °C with 80% humidity and 5% CO2.
A431, HaCaT, and A549 cells were plated at a density of 1 × 104 cells/well, while HNTMC cells were plated at 5 × 103 cells/well and incubated under the same conditions described above for 24 h at 200 µL per well in 96-well microplates. After this period, the supernatant was removed, and the cells were treated with 200 µL of new culture medium containing different concentrations of the samples, namely AgNPs and AgNO3 (2, 4, 6, 8, 10, and 20 µg/mL), as well as the aqueous extracts (200, 400, 600, 800, 1000, and 2000 µg/mL). The negative control was prepared only with supplemented culture medium without any treatment.
The MTT test was carried out after 24 h of incubating the cells with the experimental samples. Initially, the contents of the wells were discarded, and they were washed with PBS and then received 150 µL of 10% MTT solution (0.5 mg/mL) diluted in culture medium. The plates were incubated for 2 h under the same conditions as above, this time covered in aluminum foil. After this period, the supernatant from the wells was removed and replaced with 150 µL of DMSO to dissolve the formazan crystals that had formed. The contents of the wells were homogenized and, after five minutes, the absorbance was measured at 595 nm on a spectrophotometer combined with a microplate reader (Multiskan FC, Thermo Scientific, Waltham, MA, USA).
Three independent experiments were carried out for each cell line, with repetitions in quadruplicate per concentration for each sample. The results obtained were expressed as a percentage of cell viability (%) based on the absorbance values corrected for the control (wells with supplemented DMEM medium, without any treatment, with cell viability set at 100%) and presented as the mean ± standard deviation of the mean, according to Equation (3), as follows:
2.4.4. Antileishmanial Activity, Cytotoxicity, and Selectivity Index (SI)
Parasites of the species Leishmania (Leishmania) amazonensis (IFLA/BR/67/PH8) in promastigote form were used to determine antileishmanial activity. The cells were grown in supplemented Schneider’s medium (10% fetal bovine serum and 1% solution containing penicillin—100 U/mL—and streptomycin—100 ug/mL) at pH 7 and maintained at 26 °C in a biochemical oxygen demand (BOD) incubator (Eletrolab EL202, São Paulo, Brazil) with weekly relays [29].
The promastigote culture in logarithmic growth phase was grown in a 96-well microplate with 1 × 106 parasites/well in 100 µL of Schneider’s medium containing varying concentrations of the samples (AgNPs, AgNO3, or aqueous extracts) obtained by serial dilution from 100 to 1.56 µg/mL in four independent experiments in triplicate. The positive control was the drug miltefosine at the same concentrations as the AgNPs, and the negative control was the supplemented culture medium containing 0.2% DMSO (considered to be 100% viability of the leishmania). The plates were incubated in a BOD for 72 h at 26 °C. At the end of this time, 10 µL of MTT (5 mg/mL) was placed in each well, and the plates were then incubated for 4 h. After this period, the supernatant was removed from the wells, which received 50 µL of 10% sodium dodecyl sulphate (SDS) solution (w/v in distilled water) to solubilize the formazan crystals. The absorbance of the wells was measured in a microplate spectrophotometer at 540 nm (Biosystems ELx800, Curitiba, PR, Brazil).
For the cell viability tests to determine the selectivity index, the RAW 264.7 murine macrophage strain was used, which was maintained in cell culture flasks (Corning Glass Workers, New York, NY, USA) in supplemented DMEM medium (10% SFB, 1% antibiotic—100 U/mL penicillin and 100 μg/mL streptomycin), pH 7, at 37 °C, with 5% CO2 and 80% humidity. Recipes were carried out after the cells reached confluence, characterized by the formation of a cell monolayer around 48 to 72 h after the initial incubation [30].
Cytotoxicity was assessed in 96-well microplates in which 100 μL of DMEM medium supplemented with 1 × 105 macrophages were placed per well. After 4 h of incubation, the wells were washed three times with sterile PBS, and then 100 μL of DMEM medium supplemented with different serial concentrations of AgNPs, AgNO3, aqueous extracts (200 to 3.12 µg/mL), and the drug miltefosine (400 to 6.25 µg/mL) were added, followed by incubation for 72 h. At the end of the period, 10 μL of MTT (5 mg/mL) was applied to the wells, and the plates were incubated again for a further 4 h. The supernatant was then removed, and 100 μL of DMSO was added to each well. After 30 min of stirring, the absorbance was read at 540 nm on a plate reader (Biosystems ELx800, Curitiba, PR, Brazil). Only DMEM-supplemented medium containing 0.5% DMSO was used as a negative control (considered to possess 100% macrophage viability).
The selectivity index (SI) of each test sample was calculated by dividing the 50% cytotoxic concentration for macrophages (CC50) by the maximum concentration capable of inhibiting the growth of promastigote forms of leishmania by 50% (CI50).
2.4.5. Insecticidal Activity Against Aedes aegypti Larvae and Pupae
The colonies of Aedes aegypti (Rockefeller strain) used for the tests were kept in the laboratory, without exposure to any insecticide, under the following controlled conditions: temperature 28 °C (±2 °C), 70% (±10%) relative humidity, and 12/12 h photoperiod (light/dark). The eggs hatched in plastic trays containing tap water, and the larvae were fed fish food (0.5 g) every day. The newly formed pupae were separated into males and females (in a 1:3 ratio, respectively) and transferred to other trays. The adult insects were fed a 10% sugar solution, and the females were given equine blood (provided by the Veterinary Hospital of the University of Brasilia) three times a week for egg production and maturation.
Larvicide tests were carried out in accordance with the recommendations of the World Health Organization (WHO) [31] with modifications to determine the lethal concentration (LC50 and LC90). Briefly, 25 third instar larvae (L3) aged between 72 and 96 h were transferred to 200 mL transparent plastic cups containing different concentrations of AgNPs and AgNO3 (0.1, 0. 5, 2, 5, 10, 15, and 20 µg/mL), as well as the aqueous extract (200, 500, 1000, 1500, and 2000 µg/mL). The final volume was 120 mL/cup topped up with tap water, and the negative control was only tap water in the same volume as the cups with the test samples. Four independent experiments were carried out on different batches of larvae in quadruplicate for each sample tested, and larval mortality was assessed after 24, 48, and 72 h of exposure, with larvae that did not move and/or did not react to stimuli after gentle shaking of the beaker being considered non-living.
The pupicidal tests to determine the LC50 and LC90 values were carried out on 10 newly emerged pupae, no more than one day old, which were transferred to 50 mL plastic cups and then covered with a fine mesh. Different concentrations of AgNPs (0.156, 0.625, 2.5, 5, 10, and 20 µg/mL) and AgNO3 (0.468, 1.87, 3.75, 7.5, 15, and 30 µg/mL) were added to each cup, and the final volume was topped up to 20 mL/cup with tap water. Mortality was assessed after 24 and 48 h of exposure to the samples (the time limit for the pupae in the control not to turn into mosquitoes), and pupae that did not move and/or did not react to stimuli after lightly shaking the beaker were considered not to be alive. Only tap water in the same volume as the beakers containing the test samples was used as a negative control. Four independent experiments were carried out in quadruplicate per test sample, each using different batches of pupae.
2.5. Catalytic Activity
The catalytic activity of AgNPs was evaluated in the presence of sodium borohydride (NaBH4) as a substrate for the reduction of methylene blue (MB) and methyl orange (MO) dyes at room temperature. For the test with MB (λ = 664 nm), freshly prepared aqueous solutions of the dye were placed in a quartz cuvette (2.5 mL; 0.08 mM), plus NaBH4 (500 µL; 60 mM), and then each AgNPs suspension (500 µL; 100 µg/mL) was added individually in separate experiments, with the degradation reactions monitored using absorbance readings obtained by a UV-Vis spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan) in the 200–800 nm range at five-minute intervals [32]. For the MO tests (λ = 464 nm), a reaction mixture was prepared in a quartz cuvette with aqueous solutions of the organic dye (2.5 mL; 0.08 mM) and NaBH4 (500 µL; 60 mM), both freshly prepared, in addition to the individually arranged AgNPs suspensions (20 µL; 20 µg/mL), and immediately afterwards, the absorbance recordings were started on a UV-Vis spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan) in the 200–700 nm range with readings at two-minute intervals [33].
During the contact time between the nanocatalysts with the dye and substrate, there is a process of decolorization of the reaction mixtures, and the end of the reactions was defined by the transparent appearance of the solutions and the decrease in the intensity of the absorption bands characteristic of each dye. Control experiments were also carried out as mentioned above, but with the volume of nanocatalysts replaced by ultrapure water. Thus, with the absorbance data obtained at each reading time, it was possible to calculate the percentage degradation of each pollutant according to Equation (4), and the chemical kinetics of the reactions were obtained using three kinetic models: zero order, 1st order, and 2nd order, according to Equations (5)–(7), respectively [34].
where k is the rate constant of the reaction, t is the final reaction time, A0 is the initial absorbance of the pollutants at time 0, and At is the absorbance of the pollutants at time t. A0 and At are the absorbances measured at 664 and 464 nm for MB and MO, respectively, at time zero and at the aforementioned UV-Vis reading intervals during each reaction. Based on the curves constructed using linear regression, the k values were determined, as well as the correlation coefficients (R2), and the most appropriate kinetic model was chosen for each reaction involving the AgNPs, the substrate, and the pollutants tested.
2.6. Statistical Analysis
Statistical analyses of the DLS data (DH, PdI, and ZP) were carried out using a one-way ANOVA test followed by the Tukey test, with a significance level of 95% (p < 0.05). The CI50 values in the antioxidant, anticancer, and antileishmanial activity tests, as well as the CC50 data in the antileishmanial test and the LC50 and LC90 values in the insecticidal activity tests, were determined from a sigmoidal dose-dependent response by non-linear regression with four parameters, based on the normalized logarithmic analysis graphs (log (inhibitor) vs. variable slope) from the normalization of the values, transformation into log values, and then the calculation to define the 50% concentrations and R2 values. In these tests, the possible statistical differences between the experimental groups were based on one-way ANOVA (antileishmanial, larvicidal, and pupicidal) or two-way ANOVA (antioxidant and anticancer) tests, followed by Tukey or Bonferroni tests (specifically for the antileishmanial test) with a significance level of 95% (p < 0.05).
For all these analyses, the GraphPrism 8 statistical program (GraphPad Software, San Diego, CA, USA) was used for plotting and constructing the graphs/histograms, except for the construction of the histogram obtained by TEM and the graphs of the catalytic experiment in which the OriginPro 8.5 software (OriginLab Corporation, Northampton, MA, USA) was used.
3. Results and Discussion
3.1. Visual Appearance and UV-Vis Spectrophotometry
Initially, the biogenic synthesis of the AgNPs was evaluated by means of the color change in the reaction medium, as illustrated in Figure 1, and among the effects observed, it is possible to notice the more intense shade of yellow in the tubes containing the AgNPs.
Figure 1.
Visual recording of the 2 mM silver nitrate solutions, plant extracts from Paullinia cupana leaves, and biogenic AgNPs.
This phenomenon, characterized by the change in color of colloidal suspensions, is known as Tyndall scattering and occurs from visual optical changes that are observed when light is deflected due to the reflection caused by the nanostructures suspended in an aqueous medium [35]. In addition, variations in color depend on the season in which the plant material was collected but also on the conditions adopted during synthesis, including the concentration of the metallic precursor agent, the concentration of the biological extract, time, and temperature [36].
Kinetic monitoring of the formation of AgNPs indicated that the aqueous extracts of P. cupana leaves have the potential to reduce metal ions and form nanostructures, regardless of when the plant material was collected. As shown in Figure 2, AgNPs-LR showed greater absorption intensity (1.572 a.u.) after 180 min of incubation compared to AgNPs-LD (0.648 a.u.), which tended to stabilize at the end of the synthesis time, while the experimental controls showed no change.
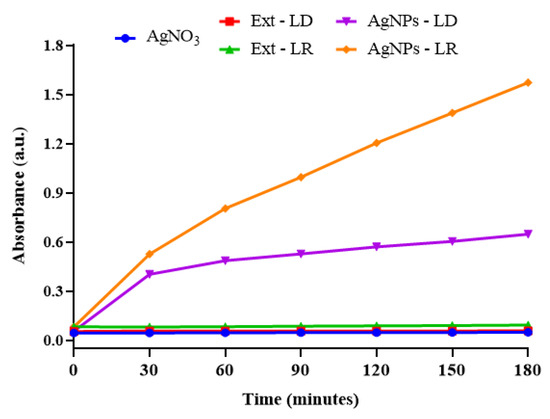
Figure 2.
Kinetic curves for the synthesis of AgNPs and their experimental controls obtained by spectrophotometric analysis at 450 nm for 180 min of reaction.
UV-Vis analysis made it possible to identify the spectral band characteristic of each type of nanostructure, and it is also an analysis that makes it possible to confirm the presence of AgNPs since, when they receive radiation, the free electrons oscillate and become excited, causing a phenomenon known as surface plasmon resonance (SPR) [37]. In Figure 3, the AgNPs-LD showed maximum absorption at a wavelength of 410 nm (0.787 a.u.), while a shift to a longer wavelength was observed for the AgNPs-LR with a band at 450 nm (0.700 a.u.), and in the spectra of the experimental controls, no considerable change in absorbance was obtained.
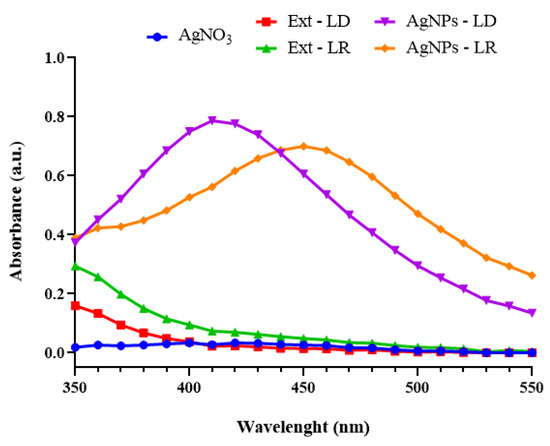
Figure 3.
Absorption curves of AgNPs and their experimental controls measured by spectrophotometry in the 350–550 nm range after 180 min of reaction.
In general, the absorbance spectra varied in the shape and symmetry of the curves, and the shifts in the wavelengths of maximum absorption, as well as the absence of well-defined spectral bands (broadening of the curves) represent, among other things, variations in the size, polydispersity, morphology, and, consequently, colloidal stability of the nanostructures [38]. In addition, the synthesis of AgNPs mediated by biological organisms can be affected by geographical and/or seasonal variations, resulting in different properties, as observed in studies in which AgNPs showed an absorbance band between 426 and 434 nm after using extracts of the plants Sisymbrium irio and Cleome viscosa collected in different seasons of the year [39,40].
3.2. Evaluation of Colloidal Stability by DLS and Surface Zeta Potential Analysis
The probable changes in the stability of AgNPs, such as their dimensional and electrical characteristics, are not desirable in biogenic synthesis, especially after long periods under different storage conditions. Table 1 and Table 2 show the HD, PdI, and ZP values of AgNPs-LD and AgNPs-LR, respectively, monitored for 730 days (2 years) under two storage conditions.

Table 1.
Monitoring the colloidal stability of AgNPs-LD by means of dynamic light scattering (DLS) and surface zeta potential analyses over two years with storage at room temperature (RT) or in a refrigerator (REF).

Table 2.
Monitoring the colloidal stability of AgNPs-LR by means of dynamic light scattering (DLS) and surface zeta potential analyses over two years with storage at room temperature (RT) or in a refrigerator (REF).
Regarding monitoring colloidal stability, the newly synthesized AgNPs-LD had an initial hydrodynamic diameter of 81.90 nm and suffered significant decreases (p < 0.05) only in the first two weeks after storage at room temperature. On the other hand, the AgNPs-LR initially measured 91.74 nm, and significant increases (p < 0.05) in the samples kept in the fridge were only seen after six months and two years. On the other hand, PdI did not show significant changes throughout the monitoring of the colloidal stability of AgNPs-LD and AgNPs-LR in relation to the measurement at the initial time, which was 0.448 and 0.295, respectively, indicating moderately polydisperse particle populations [41]. The ZP of the samples showed low and/or moderate stability, as recommended [42], showing significant momentary decreases (p < 0.05) in the surface charge of the AgNPs-LD, which was −38.1 mV, and for the AgNPs-LR in the first week and after one year in both storage conditions, in relation to the initial electrical charge of −34.9 mV.
Our results corroborate previous reports on AgNPs synthesized using extracts from Ceropegia debilis and Cymbopogon citratus leaves collected in summer. These studies reported an average nanoparticle diameter ranging from 88.2 to 100.6 nm, with a PdI between 0.193 and 0.3 [43,44]. On the other hand, the diameter of AgNPs produced from Olea europaea leaf extract and propolis collected in winter ranged from 12 to 68 nm, with a zeta potential between −15 and −52 mV [45,46].
It is important to note that the differences observed in the stability tests of the AgNPs synthesized with the extract of P. cupana leaves may be related to the following: (i) the time of collection/location of the plant material, (ii) the part of the plant used, (iii) the storage environment of the colloidal suspensions, and (iv) the reducing capacity of the metabolites in the plant extracts [8,47]. Our previous study [22] characterized the phytochemical profile of the aqueous extracts of P. cupana described here to assess their composition, and the results revealed a high content of phenolic compounds, as well as a high antioxidant potential against free radicals, in addition to the presence of a variety of secondary metabolites that were extracted using the decoction/boiling method, including alkaloids, flavonoids, terpenoids, carboxylic acids, and procyanidins. These bioactive compounds, as reported in previous research, belong to the well-known reducing agent classes of silver ions responsible for the successful synthesis of AgNPs [48,49,50,51,52].
3.3. Nanoparticle Tracking Analysis (NTA)
The size of the AgNPs was also analyzed by NTA, and values of 68.5 nm and 89.3 nm were observed for AgNPs-LD and AgNPs-LR, respectively, strongly corroborating the data obtained by DLS. The particle size found most frequently was 62.3 nm and 73.1 nm for the dry and rainy season nanostructures, respectively. In turn, the synthesis process resulted in a suspension of AgNPs-LD containing 5.33 × 107 particles/mL and 1.5 × 108 particles/mL for AgNPs-LR (Table 3).

Table 3.
Characteristics of the biosynthesized AgNPs obtained by nanoparticle tracking analysis.
Regarding the populations of particles formed, unimodal distribution is shown for the AgNPs-LD (Supplementary Figure S1A), while there is a multimodal distribution with populations of varying sizes in the AgNPs-LR sample (Supplementary Figure S1B). This behavior is in line with the results of the Span index, which were 0.443 and 0.523, respectively, since the more polydisperse the suspension, the higher this value.
NTA analysis is an important technique often used to estimate the size, distribution, and concentration of nanostructures in liquid media by tracking individual nanoparticles. In a previous study, a natural polysaccharide obtained from Anacardium occidentale, a plant found in the northeastern region of Brazil, was used to prepare AgNPs with a hydrodynamic diameter of 51.9 nm and 3.22 × 104 particles/mL [53], and more recently the leaf extract of Eucalyptus camaldulensis was used to synthesize AgNPs with an average size of 99 nm and 24.88 × 108 particles/mL [54].
3.4. Morphological Analysis by MET and Compositional Analysis by EDX
Figure 4A shows the TEM micrographs of the AgNPs-LD, revealing a particle size distribution between 22.5 and 92.5 nm, with a dry diameter of 39.33 nm, while in Figure 4B the size range of the AgNPs-LR was 17.5–97.5 nm, with a dry diameter of 42.43 nm. The morphology of the samples varied, with spherical, quasi-spherical, rod, triangular, prismatic, and hexagonal shapes, among others.
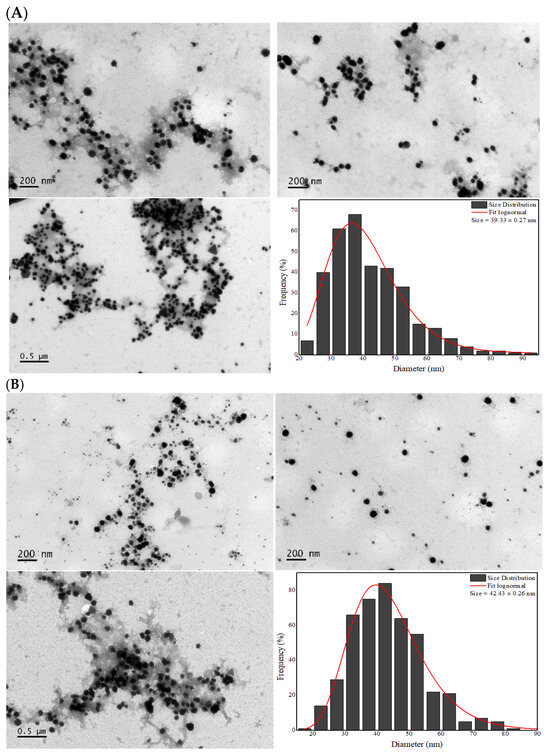
Figure 4.
TEM micrographs at 10,000× magnification and histograms of the dry diameter distribution of (A) AgNPs-LD and (B) AgNPs-LR.
Overall, the images showed morphologies to be clearly distinguished and confirm the small size of the AgNPs, but it is possible to observe a distribution in the size of the nanostructure populations that may be related to the broad bands shown in the UV-Vis spectra and the moderate PdI/Span index values that reflect the polydispersity of the samples [55]. In addition, in common with the images, the stabilization layer surrounding the surface of the AgNPs can be seen, which may refer to the biomolecules in the plant extract [56].
It is important to emphasize that even with the change in seasonal conditions and differences in phytochemical composition between the extracts, no significant changes were observed in the diameters of the AgNPs. Some research has shown that AgNPs synthesized with extracts from medicinal plants collected in summer had a diameter of between 11.36 and 75.7 nm [57,58], while AgNPs produced from citrus and aquatic plant species collected in winter ranged in size from 12 to 58.25 nm [59,60].
The information provided by EDX analysis reveals, based on the normalized weight percentage, the content of the atoms that represent the total composition of the samples by means of the intensity of the peaks obtained and allows the quantification of the chemical elements [61]. According to Figure 5A, the proportion of silver in the AgNPs-LD spectrum is 18.03%, and in Figure 5B, the silver content in AgNPs-LR is 26.5%, with both showing dispersion peaks at the start of the spectra and around 3 keV, typical of metallic silver [62].
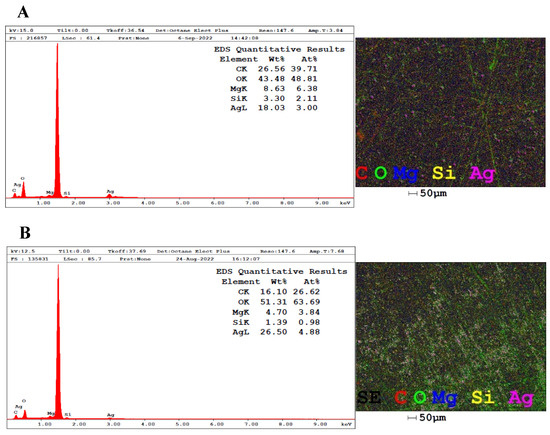
Figure 5.
EDX spectra and elemental map showing the distribution of the atoms present in the samples (A) AgNPs-LD and (B) AgNPs-LR.
The intense peak observed at around 1.5 keV indicates the presence of aluminum from the stub where the samples were previously deposited for reading on the equipment, and the other signals, referring to magnesium (Mg), silicon (Si), carbon (C), and oxygen (O) atoms, may come from the layer of biomolecules covering the surface of the nanostructures, as well as appearing as traces of the mineral content of the soil where the plant is grown and which may be absorbed by the plant tissues [63,64].
3.5. Antibacterial Activity
In this study, the MIC results showed that AgNPs were effective against all bacterial species, with values between 10.60 and 42.50 µg/mL (Table 4). The MBC values were between 5.30 and 42.50, equal to or at least twice as high as the MIC concentrations obtained. In relation to the results for AgNO3, it was seen that, in general, the inhibitory effect on bacterial growth was greater than that observed after exposure to AgNPs, and in fact, due to their small size (around 0.26 nm), the bioavailability and internalization of Ag+ ions by bacterial cells can be increased [65].

Table 4.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of AgNPs and AgNO3 aqueous solution on various strains of bacteria.
Thus, it is possible to infer that there is no direct correlation between the two classes of AgNPs tested that could indicate which sample is more efficient against bacteria, but it is suggested that these nanostructures may be suitable for use as bacterial decontamination agents. Furthermore, it is worth pointing out that based on the MIC and MBC values, susceptibility was similar for both classes of bacteria tested (Gram-positive and Gram-negative).
The antimicrobial activity of silver has been known since antiquity and is interesting due to its high toxicity to microorganisms and low toxicity to human cells [66]. It is worth noting that the mechanism of action of Ag+ ions has been elucidated previously, but there is no consensus on the mechanisms related to the antibacterial effects of AgNPs, which, in general, are related to the small diameter combined with the high surface area of the nanostructures, which facilitates permeation into bacterial membranes [67,68].
Another important factor is the electrostatic attraction that can occur due to interactions between positively charged metal ions and the bacterial cell membrane, which is formed by molecules containing negatively charged functional groups (thiol, sulfhydryl, imidazole, amino, phosphate, teichoic acids, and lipopolysaccharides), which can lead to alterations in protein structure, changes in membrane permeability, the generation of reactive oxygen species (ROS), and the blocking of ATP synthesis, preventing cell division and causing the death of bacteria [44,69]. Furthermore, even if AgNPs have a negative charge, as in this study, bactericidal activity can occur since these nanostructures interact with sulfur-containing amino acids (cysteine, homocysteine, methionine, and taurine) causing damage to the bacterial membrane [70,71].
The aqueous extracts of P. cupana leaves showed no antibacterial action against any of the species tested similarly to what was reported in a recent study in which the extract of guarana seed hulls showed no activity against various bacteria [72]. However, other studies have used extracts of P. cupana seeds and obtained antibacterial efficacy resulting in MIC and MBC of 250 µg/mL against Gram-positive and Gram-negative bacteria [73], as well as MICs in the range of 16–128 µg/mL and an inhibitory effect of between 2.1 and 100% against various bacterial species [74,75].
3.6. Antioxidant Activity
As shown in Figure 6, the ability of the nanostructures to eliminate DPPH radicals increased in a dose-dependent manner, and at the highest concentration tested (150 µg/mL), the inhibition caused by AgNPs-LD and AgNPs-LR was 50.89% and 66.86%, respectively, with an IC50 of 43.16 and 36.93 µg/mL. In turn, the positive control ascorbic acid (AA) showed an elimination capacity of over 95%, with an IC50 of 22.43 µg/mL. After incubation with Ext-LD, the antioxidant activity ranged from 22.46 to 90.71% in the concentration range (125–1500 µg/mL), with an IC50 of 476.4 µg/mL, and for Ext-LR, the free radical scavenging was 93% from the concentration of 500 µg/mL, with an IC50 of 229.7 µg/mL.
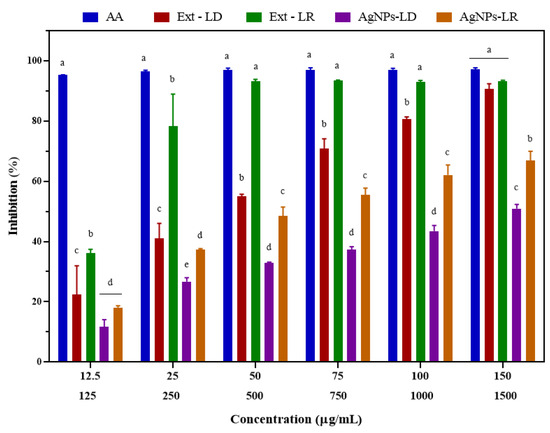
Figure 6.
Antioxidant activity of AgNPs, plant extracts of Paullinia cupana leaves collected in the dry and rainy seasons, and aqueous ascorbic acid (AA) solution against the DPPH free radical. The different letters indicate statistical significance (p < 0.05) between the treatments within each test concentration. On the X axis, the upper line indicates the test concentrations of AgNPs and AA. The lower line indicates the test concentrations of the plant extracts of Paullinia cupana leaves.
Figure 7 shows the ABTS radical inhibition results after exposure to the experimental groups, showing a dose-dependent behavior in which free radical elimination was similar when analyzed within the concentration range for AgNPs-LD (58.53–66.72%) and AgNPs-LR (55.48–73.62%), with an IC50 of 54.2 and 61.19 µg/mL, respectively. Inhibition of AA ranged from 69.95 to 100%, resulting in an IC50 of 24.98 µg/mL, and in relation to radical scavenging by Ext-LD, at a concentration of 1500 µg/mL, inhibition was 74.62%, with an IC50 of 414.5 µg/mL, while Ext-LR promoted elimination by 92.77% at the highest concentration, with an IC50 of 445.3 µg/mL.
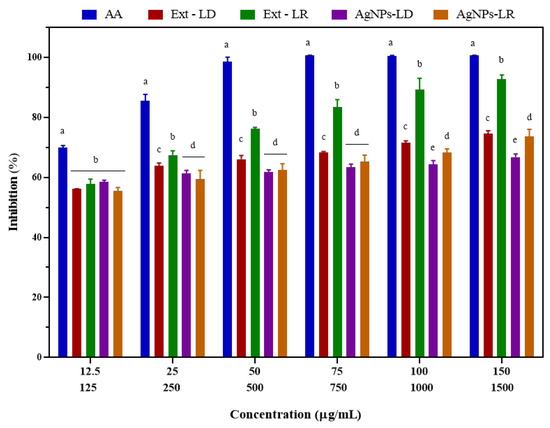
Figure 7.
Antioxidant activity of AgNPs, plant extracts of Paullinia cupana leaves collected in the dry and rainy seasons, and aqueous ascorbic acid (AA) solution against the ABTS free radical. The different letters indicate statistical significance (p < 0.05) between the treatments within each test concentration. On the X axis, the upper line indicates the test concentrations of AgNPs and AA. The lower line indicates the test concentrations of the plant extracts of Paullinia cupana leaves.
Our results show greater efficacy compared to previous research using Eupatorium adenophorum and Oxalis corniculata leaf extracts for the synthesis of AgNPs, in which 48.96 and 94.3 µg/mL were required to eliminate 50% of DPPH and ABTS radicals, respectively [76,77]. In turn, the methanolic extract of guarana seeds showed a strong DPPH radical scavenging capacity ranging from 83.4% to 90.9% [78], while another study estimated the antioxidant capacity of extracts from different parts of guarana and obtained 9534 µM eq. Trolox/g for the seed and 68 µM eq. Trolox/g for the bark against ABTS radicals [79].
Free radicals are chemical species with unpaired electrons, making them highly unstable and thus reactive in chemical reactions with other molecules. They can be derived from oxygen, nitrogen, and sulfur, for instance, creating distinctive reactive species which, in excess, can trigger various diseases since they mainly target proteins, DNA, and RNA [80,81]. In this sense, to nullify the harmful effects caused by oxidative stress, cells have developed an antioxidant defense machinery that involves enzymes (peroxidase, superoxide dismutase) and reducing agents, such as phenolic compounds [82]. The mechanism of action in which AgNPs are involved is mainly based on the transfer of free electrons and by donating hydrogen atoms to the free radical molecules, which act as acceptors and become reduced, with more stable structural characteristics and a change in the color of the final solution [83,84].
3.7. Cytotoxic Activity
The cytotoxic effects caused after exposures of the different cell lines to AgNPs and AgNO3 show a dose-dependent behavior within the range of concentrations tested, as well as non-specific activities, since the samples were effective on both cell types (cancerous and non-cancerous). In A431 cells, at the highest concentration (20 µg/mL), less than 20% of cells remained alive after incubation with the samples, and the IC50 values were 5.122 µg/mL (AgNO3), 5.510 µg/mL (AgNPs-LR), and 5.851 µg/mL (AgNPs-LD) (Figure 8A; Supplementary Table S1). On the other hand, against the HaCaT strain, there was a considerable reduction in viability from the intermediate concentration of 6 µg/mL, resulting in less than 8% of viable cells and an IC50 between 3.497 and 3.940 µg/mL (Figure 8B; Supplementary Table S1).
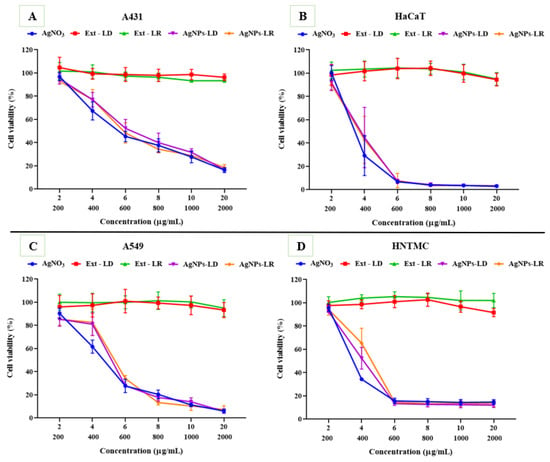
Figure 8.
Viability graphs of the cell lines (A) A431, (B) HaCaT, (C) A549, and (D) HNTMC after incubation with AgNPs, plant extracts of Paullinia cupana leaves collected in the dry and rainy seasons, and aqueous AgNO3 solution. On the X axis, the top line indicates the test concentrations of AgNPs and AgNO3. The lower line indicates the test concentrations of the Paullinia cupana leaf extracts.
The viability of A549 cells after incubation with the samples was reduced from 7% to 5% at the highest test concentration, with AgNPs-LD, AgNPs-LR, and AgNO3 having an IC50 of 5.436, 5.609, and 4.750 µg/mL, respectively (Figure 8C; Supplementary Table S1). For the HNTMC lineage, high susceptibility was observed from the concentration of 6 µg/mL onwards, as less than 15% of the cells remained alive, resulting in an IC50 of 3.490–4.231 µg/mL (Figure 8D; Supplementary Table S1).
Among the mechanisms proposed for the cytotoxic activity of AgNPs are oxidative stress, which may be responsible for the increase in pro-apoptotic genes [85,86], alterations in mitochondrial membrane potential, and the interruption of ATP synthesis through interaction with functional groups on the cell surface, generating free radicals [87,88]. In addition, after incubation with the nanostructures, the cell cycle may be interrupted in different phases. This leads to a reduction in angiogenesis and cell proliferation, preventing metastases [89,90]. Other intrinsic aspects of AgNPs allow for cytotoxic action, such as particle size [91], electrical charge [92], morphology [93], and surface coating [94].
As shown in Figure 8 and Supplementary Table S1, the aqueous extracts of P. cupana leaves did not significantly affect cell viability. After inoculation with the A431 and A549 cell lines, the extracts caused only about a 7% loss in cell viability even at the highest concentration tested (2000 µg/mL), resulting in an IC50 between 458.2 and 638.1 µg/mL for skin cancer cells and between 827.5 and 1017 µg/mL for lung cancer cells. Regarding non-cancerous cell lines, after incubation with the leaf extracts, both HaCaT and HNTMC remained with more than 90% of viable cells, with IC50 values set between 1052 and 1106 µg/mL in the keratinocyte assay and between 309.6 and 998.3 µg/mL for fibroblasts.
Our results support previous studies in which therapeutic safety was demonstrated after exposure of BV-2, MSCs, and NIH-3T3 cells to extracts of guarana and metabolite caffeine, resulting in a low reduction in viability or even inducing cell proliferation [95,96,97]. On the other hand, previous research has shown antiproliferative effects on HL-60, HT-29, and MCF-7 cancer cells after incubation with guarana seed extract [73,98], and in another study the alkaloid theobromine, described in the phytochemical composition of P. cupana, was responsible for inhibiting the growth of the U87MG strain, causing obvious morphological changes [99].
3.8. Leishmanicidal Activity
The antileishmanial activity was evaluated in promastigote cultures of L. amazonensis, and the results are shown in Table 5 and Supplementary Figure S2. The AgNPs-LD inhibited cell growth at concentrations of 100, 50, and 25 µg/mL, with a reduction of 76.03%, 49.40%, and 5.14%, respectively, resulting in a CI50 of 47.23 µg/mL. At these same concentrations, the AgNPs-LR showed cell death percentages of 84.06%, 52.91%, and 7.81%, with a CI50 of 43.79 µg/mL. In turn, the evaluation of the activity against L. amazonensis caused after incubation with AgNO3 resulted in a CI50 of 54.8 µg/mL, while the drug miltefosine was the most efficient in reducing the cell viability of the parasite, showing a CI50 of 17.25 µg/mL. Regarding the aqueous extracts, it was observed that after incubation with Ext-LD and Ext-LR at a concentration of 100 µg/mL, the inhibition was 7.82% and 22.76%, respectively, and from these values it was not possible to measure the IC50.

Table 5.
Antileishmanial activity (CI50), cytotoxicity against macrophages (CC50), and selectivity index (SI) values calculated after exposures for 72 h with AgNPs, plant extracts of Paullinia cupana leaves, aqueous AgNO3 solution, and the drug miltefosine.
To assess toxicity against macrophages, which are the main host cells for Leishmania sp. parasites, the MTT test was carried out for 72 h, and the results are described in Table 5 and Supplementary Figure S3. After exposure to AgNPs, the survival rate of RAW 264.7 macrophages remained close to 90% even at the highest concentrations. In addition, the aqueous extracts of P. cupana leaves showed viability of 96% at a concentration of 200 µg/mL. Based on these results, it was not possible to calculate the CC50 of the samples described above since higher concentrations would be needed to reduce cell viability, demonstrating the absence of cytotoxicity within the range of concentrations tested over the period evaluated.
On the other hand, exposure of macrophages to AgNO3 showed a reduction in cell viability from intermediate concentrations, with a CC50 of 61.35 µg/mL, suggesting greater toxicity than biogenic nanostructures. In addition, the action of miltefosine was evident, with a decrease in viability at the highest concentrations tested and a CC50 of 241.1 µg/mL. In short, as described and based on the SI values, the safety profile for the AgNPs tests was approximately twice as selective compared to incubation with the aqueous extracts and the metal salt, affecting the target cells to a lesser extent.
The information from this study corroborates other studies that have shown the leishmanicidal effect of biogenic AgNPs on the L. amazonensis species, even at varying concentrations and exposure times, without affecting macrophages [100,101]. Although extracts of P. cupana leaves did not have a significant effect on the loss of parasite viability, other studies have reported that plant extracts from Amazonian plants had an efficient antileishmanial action, with CI50 values between 5 and 20 µg/mL [102,103], as well as an absence of cytotoxicity for macrophages at concentrations of 10–400 µg/mL [104,105].
There are some suggested mechanisms of antileishmanial action caused by the size and surface charge of the nanostructures [106]; by the presence of phytochemicals used as coating agents, which can potentiate the activity through immunomodulatory effects [107]; and by the release of Ag+ ions when in contact with acidic organelles, such as the phagolysosome [108]. In addition, AgNPs can lead to a reduction in mitochondrial membrane potential, which maintains the vital energy of cells [109], causes an increase in the production of ROS and thus arrests cell cycle phases [110], increases the production of nitric oxide (NO) with the generation of microbicidal compounds [111], and inhibits the trypanothione/trypanothione reductase enzyme system [112].
3.9. Larvicidal and Pupicidal Activity Against Aedes aegypti
In this study, tests were carried out on third instar larvae of the Ae. aegypti mosquito after the organisms were exposed to AgNPs-LD and AgNO3. The results showed that the deleterious effects are time-dependent, with the LC50 and LC90—along with the lower and upper confidence limits—and R2 values shown in Table 6.

Table 6.
Insecticidal activity of AgNPs-LD and aqueous AgNO3 solution against third instar Aedes aegypti larvae at 24, 48, and 72 h of exposure.
The dose-response curves were similar in terms of mortality rates (%), reaching lethality above 95% from a concentration of 5 µg/mL, with higher mortality rates as concentration and time increased (Supplementary Figure S4A,B). Overall, the larvae showed greater susceptibility to the nanostructures compared to the aqueous solution of the metal salt. The LC50 values for AgNPs-LD were 9.936 µg/mL (24 h), 1.669 µg/mL (48 h), and 0.6097 µg/mL (72 h), while for AgNO3 they were 9.179 µg/mL (24 h), 2.682 µg/mL (48 h), and 0.9730 µg/mL (72 h) (Table 6). These results corroborate other studies that have reported time-dependent effectiveness in eliminating Ae. aegypti larvae when exposed to biogenic AgNPs synthesized from extracts of different plant species, in which the LC50 after 24, 48, and 72 h was 9.2, 1.51, and 0.61 µg/mL, respectively [113,114,115]. In addition, an LC50 of 21.23 µg/mL was reported for aqueous AgNO3 solution after exposure to Ae. aegypti larvae for 24 h [116].
To gain a deeper understanding of the larvicidal potential of the samples, the LC90 values were calculated after carrying out the experiments. The concentrations reflecting the efficacy of the AgNPs were 15.64, 3.867, and 1.065 µg/mL after 24, 48, and 72 h, respectively. In turn, the LC90 of the AgNO3 solution was 14.5 µg/mL (24 h), 6.955 µg/mL (48 h), and 2.457 µg/mL (72 h) (Table 6). Thus, our results agree with previous studies that showed LC90 values of 12.11 and 13.96 µg/mL after exposure for 24 h to AgNPs synthesized from leaf extracts of Derris trifoliata and Annona reticulata [117,118].
Regarding the possible mechanisms of action, larvicidal activity can be attributed to the interaction of AgNPs with the extracellular lipoprotein matrix, causing an increase in the permeability of the plasma membrane of larval cells [119]. In addition, the generalist food habits and acidic environment of the insect midgut, as well as the generally negative zeta potential and the small diameter of the nanostructures, facilitate the internalization of AgNPs, mainly through the insect cuticle (external part of the exoskeleton) [120,121]. In the intracellular space, AgNPs bind to sulfur-containing proteins or phosphorus-containing compounds, such as DNA, leading to the denaturation of organelles and enzymes, as well as reducing ATP synthesis and ion exchange, which results in loss of function, morphological changes, and cell death [122,123].
In addition, the effects on pupae after exposure to AgNPs-LD and AgNO3 are described, showing the LC50 and LC90, as well as the confidence intervals and R2 values adjusted for each exposure time (Table 7).

Table 7.
Insecticidal activity of AgNPs-LD and aqueous AgNO3 solution against Aedes aegypti pupae at 24 and 48 h of exposure.
In Supplementary Figure S5A,B, the dose-response curves show that the mortality rate at the end of the exposure period to the samples was 100% at the highest test concentrations and increased in a dose-dependent manner. As shown in Table 7, the greater the exposure time, the better the efficacy in eliminating pupae, with an LC50 of 6.885 µg/mL for AgNPs-LD after 24 h compared to AgNO3, which had 9.959 µg/mL. In turn, after 48 h, the LC50 was 2.840 and 4.194 µg/mL for the nanostructures and AgNO3, respectively. The LC90 values for the pupae were also defined based on the mortality rates after 24 and 48 h and reached, respectively, 11.98 µg/mL and 6.790 µg/mL for the AgNPs-LD and 14.82 µg/mL and 11.08 µg/mL for the metal salt.
Therefore, our results provide evidence that AgNPs synthesized with P. cupana leaf extract interfere in the Ae. aegypti life cycle and are in line with previous research in which biogenic AgNPs were tested against pupae of this mosquito, resulting in an LC50 of 13 µg/mL after 24 h of exposure [124,125]. In addition, delayed pupation and abnormal wing development of adult Ae. aegypti mosquitoes after incubation for 24 h with AgNPs synthesized from Artemisia nilagirica extract have already been reported [126].
In our study, we emphasized that the guarana leaf extract, when tested alone, did not show any activity against Ae. aegypti larvae, even at high concentrations (from 200 to 2000 µg/mL), and for this reason, this sample was not used in the pupicidal tests. It should be noted that the toxic mode of action of plant phytochemicals on insects can result in non-specific effects on a wide range of targets—including signaling molecules, ion channels, enzymes, proteins, nucleic acids, and biological membranes—that occur from the moment these secondary metabolites are ingested by herbivorous insects [127,128]. In addition, various polar biomolecules tend to dissolve in water and cause oxygen suppression, as well as penetrating the larvae via the respiratory tract, leading to lethal damage [129].
3.10. Catalytic Degradation of Organic Dyes
The catalytic degradation efficiency of AgNPs was investigated in the reduction of the cationic dye methylene blue (MB), and as shown in Figure 9A, the UV-Vis spectra of the dye alone over 90 min show no decrease in the absorption intensity of its characteristic bands around 290 and 664 nm, with a pronounced shoulder at 614 nm. On the other hand, the degradation process is accelerated with the addition of AgNPs, and after 40 min of reaction, it is possible to observe the disappearance of the blue color in the dye solutions regardless of the type of nanocatalyst used (Figure 9B), as well as a decay of the MB absorption bands in the UV-Vis spectra (Figure 9C,D). As the intense band around 664 nm decreases, a new intense band appears near 256 nm, which can be attributed to the formation of leucomethylene blue [32].
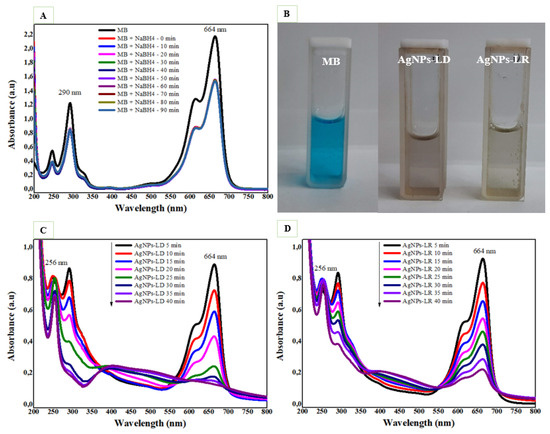
Figure 9.
Catalytic degradation of methylene blue dye (MB): (A) UV-Vis absorption spectra with the NaBH4 substrate only; (B) images of aqueous solutions of MB in the absence and presence of AgNPs (C) UV-Vis spectra with NaBH4 and the AgNPs-LD; and (D) AgNPs-LR nanocatalysts.
As shown in Supplementary Figure S6A, the percentage elimination of MB was 91.12% by AgNPs-LD and 93.52% by AgNPs-LR after 40 min of reaction. In addition, we found that the model best suited to the degradation of the pollutant followed second-order kinetics for AgNPs-LD, with an R2 of 0.9762 and a reaction rate constant (k) of 0.1218 min−1 (Supplementary Figure S6B), while for AgNPs-LR, first-order kinetics was more suitable, with R2 and rate constant values of 0.945 and 0.0579 min−1, respectively (Supplementary Figure S6C). Our findings are like recent reports in which biogenic nanocatalysts produced from Carissa opaca and Ficus sycomorus extracts promoted the complete reduction of MB after 50 min, with a rate constant of 0.031 min−1 and 12 min with a k value of 0.156 min−1, respectively [130,131].
The catalytic activity of AgNPs against the anionic dye methyl orange (MO) was also investigated, and as shown in Figure 10A, in the absence of the nanocatalysts, there is no decrease in absorbance intensity in the spectral bands characteristic of the organic molecule at 270 and 464 nm after 60 min. Successful degradation of the dye is achieved after 14 min of reaction in the presence of AgNPs, which considerably reduce the intensity of the color in the solutions (Figure 10B). In addition, the absorption band at 464 nm decays, and the band at 270 nm disappears, while a new band at 247 nm appears due to absorptions of the -NH2 group from hydrazine molecules, sulphanilic acid, and aromatic amines that are products of MO degradation (Figure 10C,D) [132].
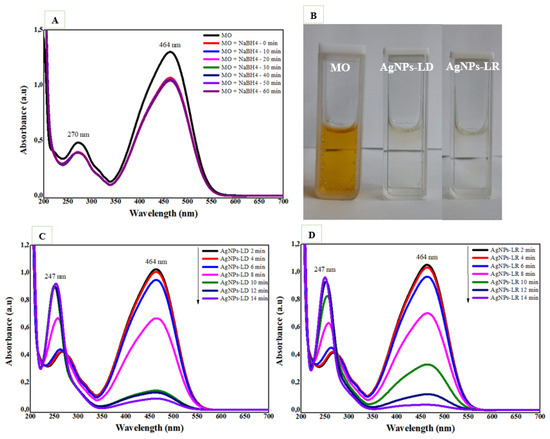
Figure 10.
Catalytic degradation of methyl orange dye (MO): (A) UV-Vis absorption spectra with the NaBH4 substrate only; (B) images of aqueous solutions of MO in the absence and presence of AgNPs (C) UV-Vis spectra with NaBH4 and the AgNPs-LD; and (D) AgNPs-LR nanocatalysts.
The percentage of MO degradation is time-dependent, and at the end of 14 min, it was 92.89% and 96.42% for AgNPs-LD and AgNPs-LR, respectively (Supplementary Figure S7A). When studying the models best suited to the chemical kinetics of each reaction, it was found that the zero-order model resulted in the best characteristics after incubation with both types of nanostructures, with R2 values of 0.9176 and 0.9498 for AgNPs-LD and AgNPs-LR, as well as reaction rate constant (k) values of 0.0945 min−1 and 0.0946 min−1, respectively (Supplementary Figure S7B,C). Recently, some studies have investigated the catalytic potential of AgNPs synthesized from extracts of Berberis vulgaris and Heterotheca subaxillaris, which resulted in complete degradation of the MO dye between 9 and 11 min, as well as reaction rate constants between 0.120 and 0.4109 min−1; the higher these values, the faster the degradation of the pollutants [133,134].
The catalytic properties of metal nanoparticles depend on their redox potential, which must be higher than the reducing potential of the substrate and lower than that of the reactants [135]. According to our results, even though NaBH4 alone is a strong reducing agent, it is not able to reduce MB and MO, and this is due to the considerable difference in redox potential between them, which makes the reactions thermodynamically permissible but kinetically non-advantageous [136]. The accelerated rate of reduction of organic dyes in the presence of AgNPs can be attributed to the small diameter of these nanostructures and, consequently, their large surface area, which offers more active sites for the reactions to take place [137,138].
Catalytic activity can be studied based on the Langmuir–Hinshelwood (L-H) model, in which AgNPs act through the ‘electron relay’ effect [139,140]. Thus, the degradation of organic pollutants is based on an electron transfer process that occurs after the nanocatalysts are added to the reaction mixture, with subsequent adsorption of the dissociated NaBH4 molecules (nucleophiles) on their surface by means of electrostatic attraction. The AgNPs then act as mediators in the sharing of electrons for the dye molecules (electrophiles), which causes modifications to the chemical structures of the pollutants; the reduction products formed are stable and non-toxic, and when they are desorbed from the surface of the AgNPs, they melt into the solution, which becomes colorless [141,142,143].
4. Conclusions
The extracts of P. cupana leaves collected at different times of the year were efficient at reducing silver ions and stabilizing AgNPs, highlighting the importance of assessing this parameter to optimize the yield and final characteristics of the nanostructures formed using a plant native to Brazilian biodiversity with economic and social value.
The AgNPs showed similar characteristics, including high colloidal stability under the storage conditions evaluated, as well as varied morphologies and small diameters, which may have contributed to the biological activities investigated. Significant activities were observed in reducing bacterial growth of Gram-positive and Gram-negative species, as well as non-specific cytotoxic activity against cancerous and non-cancerous cells, in addition to significant elimination of free radicals in a dose-dependent manner, like that observed for plant extracts. The AgNPs showed insecticidal potential, affecting the development of disease-carrying mosquitoes, as well as marked activity against opportunistic parasites of human host cells and promising catalytic degradation rates of organic dyes at room temperature in short reaction times.
These findings broaden knowledge about the green synthesis of AgNPs, contributing to the continuity of studies related to the conditions in which plants are used to produce nanomaterials and reinforcing the need to promote the sustainable use of natural resources with a view to increasing biomedical and environmental applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics17030356/s1, Figure S1: Distribution histogram and 3D graphs of concentration/intensity versus hydrodynamic diameter of (A) AgNPs-LD and (B) AgNPs-LR; Table S1: Cytotoxic effects of AgNPs, Paullinia cupana plant extracts, and aqueous AgNO3 solution expressed as IC50 values; Figure S2: Antipromastigote activity of AgNPs, Paullinia cupana leaf extracts, aqueous AgNO3 solution, and miltefosine against Leishmania amazonensis after 72 h of incubation; Figure S3: Cytotoxicity of AgNPs, Paullinia cupana leaf extracts, aqueous AgNO3 solution, and miltefosine against RAW 264.7 macrophages after 72 h using the MTT colorimetric method; Figure S4: (A) Dose-response curve (mortality-%) of Aedes aegypti larvae after exposure for 24, 48, and 72 h to different concentrations of AgNPs-LD; (B) Dose-response curve (mortality-%) of Aedes aegypti larvae after exposure for 24, 48, and 72 h to different concentrations of AgNO3; Figure S5: (A) Dose-response curve (mortality-%) of Aedes aegypti pupae after exposure for 24 and 48 h to different concentrations of AgNPs-LD; (B) Dose-response curve (mortality-%) of Aedes aegypti pupae after exposure for 24 and 48 h to different concentrations of AgNO3; Figure S6: (A) Removal (%) of the methylene blue (MB) dye at different exposure times to the nanocatalysts; (B) Second-order kinetic graph of the linear regression of the logarithm of absorbance versus time (minutes) of MB degradation in the presence of AgNPs-LD; (C) First-order kinetic graph of the linear regression of the logarithm of absorbance versus time (minutes) of MB degradation in the presence of AgNPs-LR; Figure S7: (A) Removal (%) of methyl orange (MO) dye at different exposure times to the nanocatalysts; (B) Zero-order kinetic graph of the linear regression of the logarithm of absorbance versus time (minutes) of MO degradation in the presence of AgNPs-LD; (C) Zero-order kinetic graph of the linear regression of the logarithm of absorbance versus time (minutes) of MO degradation in the presence of AgNPs-LR.
Author Contributions
Conceptualization, A.K.O.L. and M.P.G.; methodology, A.K.O.L., Í.R.S.V., L.M.d.S.S., G.N., I.F., I.G.M.d.S., A.G.T.J., M.C., Y.A.A.M., K.A.d.F.R., L.S.E., L.C.A. and H.d.C.B., D.B.T.; formal analysis and investigation, A.K.O.L., Í.R.S.V., L.M.d.S.S., I.F., I.G.M.d.S., A.G.T.J., Y.A.A.M., L.C.d.S., P.S.T., L.C.A. and D.B.T.; resources, G.N., S.N.B., M.C., K.A.d.F.R., L.S.E., L.C.A., H.d.C.B., D.B.T. and M.P.G.; data curation, A.K.O.L., Í.R.S.V., L.M.d.S.S., I.F., I.G.M.d.S., A.G.T.J., Y.A.A.M., K.A.d.F.R., L.C.d.S., P.S.T., L.C.A. and D.B.T.; writing—original draft preparation, A.K.O.L. and M.P.G.; writing—review and editing, A.K.O.L., Í.R.S.V., L.M.d.S.S., G.N., I.F.S, S.N.B., A.G.T.J., M.C., K.A.d.F.R., L.C.d.S., P.S.T., L.C.A., L.A.M. and M.P.G.; funding acquisition, G.N., S.N.B., M.C., K.A.d.F.R., L.S.E., L.C.A., H.d.C.B., D.B.T., L.A.M. and M.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Brazilian agencies Financier of Studies and Projects—FINEP (01.08.0457.00), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Brazil—grant number [E-26/204.254/2021], and the Ministry of Health—Process TED 74/2016 & TED 42/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the University of Brasilia (UnB), which provided financial and infrastructural subsidies to carry out the research. The authors would also like to thank the Brazilian research institutions involved in the development of this study at all stages. The first author would especially like to thank Laís Bentes de Almeida and her family for providing the Paullinia cupana plant material and Thais Elias Almeida for the botanical identification of the plant.
Conflicts of Interest
The author Alan Kelbis Oliveira Lima was employed by the company Brazilian Agricultural Research Corporation (EMBRAPA). The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Salomão-Oliveira, A.; Lima, E.S.; Marinho, H.A.; Carvalho, R.P. Benefits and effectiveness of using Paullinia cupana: A review article. J. Food Nutr. Res. 2018, 6, 497–503. [Google Scholar] [CrossRef]
- Smith, N.; Atroch, A.L. Guaraná’s journey from regional tonic to aphrodisiac and global energy drink. Evid. Based Complement. Alternat. Med. 2010, 7, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Duchan, E.; Patel, N.D.; Feucht, C. Energy drinks: A review of use and safety for athletes. Phys. Sportsmed. 2010, 38, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.L.M.; Ferreira, E.D.F.; de Paula, M.N.; Klein, T.; de Mello, J.C.P. Paullinia cupana: A multipurpose plant—A review. Rev. Bras. Farmacogn. 2019, 29, 77–110. [Google Scholar] [CrossRef]
- de Oliveira, A.L.L.; Muniz, M.P.; da Silva, F.M.A.; do Nascimento, A.H.; dos Santos-Barnett, T.C.; Gomes, F.B.; Nunomura, S.M.; Krug, C. Chemical composition of guarana flowers and nectar and their ecological significance. Biochem. Syst. Ecol. 2024, 112, 104769. [Google Scholar] [CrossRef]
- dos Santos, G.A.N.; Schimpl, F.C.; Ribeiro, M.D.; Scherer Filho, C.; Valente, M.S.F.; da Silva, J.F. Methylxanthine and polyphenol distribution in guarana cultivars. Sci. Plena 2024, 20, 061503. [Google Scholar] [CrossRef]
- Rocha, C.A.M.; Santana, G.M.; Santos, H.M.; de Jesus, R.M.; Lôbo, I.P. Comprehensive evaluation of methylxanthines and phenolic compounds in Bahia’s guarana (Paullinia cupana), Brazil: Implications for variety selection and by-product utilization. Food Sci. Technol. 2024, 44, e00064. [Google Scholar] [CrossRef]
- de Oliveira Salles, R.C.; Muniz, M.P.; Nunomura, R.D.C.S.; Nunomura, S.M. Geographical origin of guarana seeds from untargeted UHPLC-MS and chemometrics analysis. Food Chem. 2022, 371, 131068. [Google Scholar] [CrossRef]
- da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef]
- da Silva, G.; Silva, L.M.A.; Alves Filho, E.G.; Canuto, K.M.; de Brito, E.S.; de Jesus, R. 1H quantitative nuclear magnetic resonance and principal component analysis as tool for discrimination of guarana seeds from different geographic regions of Brazil. In Proceedings of the XIII International Conference on the Applications of Magnetic Resonance in Food Science, Karlsruhe, Germany, 7–10 June 2016; Volume 6, pp. 21–25. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; da Silva, A.A.; da Silva, B.D.; Torres Neto, L.; Tessaro, L.; Lima, A.K.O.; Garcia, M.P.; Ribeiro, J.A.A.; Rodrigues, C.M.; de Sousa, A.M.F.; et al. Antioxidant, Antimicrobial, and Anticancer Potential of Green Synthesized ZnO Nanoparticles from Açaí (Euterpe oleracea Mart.) Berry Seed Residue Extract. Waste Biomass Valoriz. 2024, 15, 4717–4734. [Google Scholar] [CrossRef]
- Ettadili, F.E.; Aghris, S.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Recent advances in the nanoparticles synthesis using plant extract: Applications and future recommendations. J. Mol. Struct. 2022, 1248, 131538. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Khan, A.; Shahzad, A.; Alhodaib, A.; Qasim, M.; Naz, I.; Rehman, A. Phytogenic-mediated silver nanoparticles using Persicaria hydropiper extracts and its catalytic activity against multidrug resistant bacteria. Arab. J. Chem. 2022, 15, 104053. [Google Scholar] [CrossRef]
- Parmar, M.; Sanyal, M. Extensive study on plant mediated green synthesis of metal nanoparticles and their application for degradation of cationic and anionic dyes. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100624. [Google Scholar] [CrossRef]
- Githala, C.K.; Trivedi, R. Review on synthesis method, biomolecules involved, size affecting factors and potential applications of silver nanoparticles. Biocatal. Agric. Biotechnol. 2023, 54, 102912. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A. The recent progress on silver nanoparticles: Synthesis and electronic applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, J.; Yin, L.; Liu, J.; Chen, C. Silver nanoparticles: From in vitro green synthesis to in vivo biological effects in plants. Adv. Agrochem. 2023, 2, 313–323. [Google Scholar] [CrossRef]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Souza, L.M.D.S.; Reis, G.F.; Junior, A.G.T.; Araújo, V.H.S.; Santos, L.C.D.; Silva, V.R.P.; Chorilli, M.; Braga, H.C.; Tada, D.B.; et al. Synthesis of silver nanoparticles using extracts from different parts of the Paullinia cupana kunth plant: Characterization and in vitro antimicrobial activity. Pharmaceuticals 2024, 17, 869. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.K.O.; Silveira, A.P.; Silva, R.C.; Machado, Y.A.A.; de Araújo, A.R.; de Mendonça Araujo, S.S.; Vieira, I.R.S.; Araújo, J.L.; Santos, L.C.; Rodrigues, K.A.F.; et al. Phytosynthesis of silver nanoparticles using guarana (Paullinia cupana Kunth) leaf extract employing different routes: Characterization and investigation of in vitro bioactivities. Biomass Convers. Biorefin. 2024, 15, 4301–4317. [Google Scholar] [CrossRef]
- Ribeiro, T.D.C.; Sábio, R.M.; Luiz, M.T.; Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.A.; Planeta, C.S.; Chorilli, M. Curcumin-loaded mesoporous silica nanoparticles dispersed in thermo-responsive hydrogel as potential Alzheimer disease therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; p. 112.
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Abdullah, H.S.T.S.H.; Asseri, S.N.A.R.M.; Mohamad, W.N.K.W.; Kan, S.Y.; Azmi, A.A.; Julius, F.S.Y.; Chia, P.W. Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ. Pollut. 2021, 271, 116295. [Google Scholar] [CrossRef]
- Torres, P.B.; Pires, J.S.; Santos, D.Y.A.C.; Chow, F. Ensaio do Potencial Antioxidante de Extratos de Algas Através do Sequestro do ABTS•+ em Microplaca; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; pp. 1–4. [Google Scholar] [CrossRef]
- de Lima Nunes, T.A.; Costa, L.H.; De Sousa, J.M.S.; De Souza, V.M.R.; Rodrigues, R.R.L.; Val, M.D.C.A.; Pereira, A.C.T.C.; Ferreira, G.P.; da Silva, M.V.; da Costa, J.M.A.R.; et al. Eugenia piauhiensis Vellaff. essential oil and γ-elemene its major constituent exhibit antileishmanial activity, promoting cell membrane damage and in vitro immunomodulation. Chem. Biol. Interact. 2021, 339, 109429. [Google Scholar] [CrossRef]
- da Franca Rodrigues, K.A.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; de Amorim Carvalho, F.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005; pp. 1–41. [Google Scholar]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, antimicrobial, antioxidant, and catalytic activities of green-synthesized silver and gold nanoparticles using Bauhinia purpurea leaf extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef]
- Paramesh, C.C.; Halligudra, G.; Gangaraju, V.; Sriramoju, J.B.; Shastri, M.; Rangappa, D.; Subbegowda, R.K.; Shivaramu, P.D. Silver nanoparticles synthesized using saponin extract of Simarouba glauca oil seed meal as effective, recoverable and reusable catalyst for reduction of organic dyes. Results Surf. Interfaces 2021, 3, 100005. [Google Scholar] [CrossRef]
- Alamier, W.M.; Hasan, N.; Ali, S.K.; Oteef, M.D. Biosynthesis of Ag nanoparticles using Caralluma acutangula extract and its catalytic functionality towards degradation of hazardous dye pollutants. Crystals 2022, 12, 1069. [Google Scholar] [CrossRef]
- Evensen, H.T. A Hands-on, Introductory Course for First-year Engineering Students in Microsystems and Nanomaterials. In Proceedings of the 2013 ASEE Annual Conference & Exposition, Atlanta, GA, USA, 23–26 June 2013; pp. 23–53. [Google Scholar]
- Pourmortazavi, S.M.; Taghdiri, M.; Makari, V.; Rahimi-Nasrabadi, M. Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, J.; Udhayakumar, G.; Suja, R.; Kannaki, K.; Sreenathkumar, C.; Gokulakumar, B. An investigation of silver nanoparticles made from Plectranthus amboinicus leaves and their antibacterial and photocatalytic activities. J. Indian Chem. Soc. 2024, 101, 101252. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Shailajha, S.; Shanavas, S.; Gurung, J. Biosynthesis of silver nanoparticles by Cyperus pangorei and its potential in structural, optical and catalytic dye degradation. Appl. Nanosci. 2021, 11, 477–491. [Google Scholar] [CrossRef]
- Mickymaray, S. One-step synthesis of silver nanoparticles using Saudi Arabian desert seasonal plant Sisymbrium irio and antibacterial activity against multidrug-resistant bacterial strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef]
- Yarrappagaari, S.; Gutha, R.; Narayanaswamy, L.; Thopireddy, L.; Benne, L.; Mohiyuddin, S.S.; Vijayakumar, V.; Saddala, R.R. Eco-friendly synthesis of silver nanoparticles from the whole plant of Cleome viscosa and evaluation of their characterization, antibacterial, antioxidant and antidiabetic properties. Saudi J. Biol. Sci. 2020, 27, 3601–3614. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Contr. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Oil and Grease and Petroleum Hydrocarbons in Water; American Society for Testing and Materials: West Conshohocken, PA, USA, 1985; pp. 3921–3985. [Google Scholar]
- Al-Otibi, F.; Albulayhid, L.S.; Alharbi, R.I.; Almohsen, A.A.; AlShowiman, G.M. Biological Activity of Biosynthesized Silver Nanoaggregates Prepared from the Aqueous Extract of Cymbopogon citratus against Candida spp. Nanomaterials 2023, 13, 2198. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Losetty, V. Synthesis of sustainable silver nanoparticles using plant extract and their antimicrobial, anticancer, and photocatalytic dye degradation efficiency analysis. Process Biochem. 2024, 144, 64–78. [Google Scholar] [CrossRef]
- De Matteis, V.; Rizzello, L.; Ingrosso, C.; Liatsi-Douvitsa, E.; De Giorgi, M.L.; De Matteis, G.; Rinaldi, R. Cultivar-dependent anticancer and antibacterial properties of silver nanoparticles synthesized using leaves of different Olea Europaea trees. Nanomaterials 2019, 9, 1544. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Gaona-Ochoa, J.; Juarez, J.; Gastelum-Cabrera, M.; Montaño-Leyva, B.; Arenas-Hernández, M.; Caporal-Hernandez, L.; Ortega-García, J.; Barrios-Villa, E.; et al. Biosynthesis of Silver Nanoparticles Using Seasonal Samples of Sonoran Desert Propolis: Evaluation of Its Antibacterial Activity Against Clinical Isolates of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 1853. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Ayma, F.C.; Osorio Anaya, A.M.; Peiter, G.C.; Jaerger, S.; Schneider, R. Green Synthesis and Characterization of Silver Nanoparticles from Minthostachys acris Schmidt Lebuhn (Muña) and Its Evaluation as a Bactericidal Agent Against Escherichia coli and Staphylococus aureus. Micro 2024, 4, 706–720. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M.G. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 2012, 4, 1351–1357. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Sethuraman, M.G.; Lee, Y.R. NaBH4 reduction of ortho and para-nitroaniline catalyzed by silver nanoparticles synthesized using Tamarindus indica seed coat extract. Res. Chem. Intermed. 2016, 42, 713–724. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Behpour, M.; Khayatkashani, M. Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary application in electrochemistry. Phys. E Low Dimens. Syst. Nanostruct. 2011, 44, 97–104. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanoparticle Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- de Jesus Oliveira, A.C.; de Araújo, A.R.; Quelemes, P.V.; Nadvorny, D.; Soares-Sobrinho, J.L.; de Almeida Leite, J.R.S.; Silva-Filho, E.C.; da Silva, D.A. Solvent-free production of phthalated cashew gum for green synthesis of antimicrobial silver nanoparticles. Carbohydr. Polym. 2019, 213, 176–183. [Google Scholar] [CrossRef]
- Zein, R.; Alghoraibi, I.; Soukkarieh, C.; Ismail, M.T.; Alahmad, A. Influence of polyvinylpyrrolidone concentration on properties and anti-bacterial activity of green synthesized silver nanoparticles. Micromachines 2022, 13, 777. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Trieu, Q.A.; Le, C.T.B.; Pham, C.M.; Bui, T.H. Photocatalytic degradation of methylene blue and antibacterial activity of silver nanoparticles synthesized from Camellia sinensis leaf extract. J. Exp. Nanosci. 2023, 18, 2225759. [Google Scholar] [CrossRef]
- Chaudhari, R.K.; Shah, P.A.; Shrivastav, P.S. Green synthesis of silver nanoparticles using Adhatoda vasica leaf extract and its application in photocatalytic degradation of dyes. Discov. Nano 2023, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Heikal, Y.M.; Şuţan, N.A.; Rizwan, M.; Elsayed, A. Green synthesized silver nanoparticles induced cytogenotoxic and genotoxic changes in Allium cepa L. varies with nanoparticles doses and duration of exposure. Chemosphere 2020, 243, 125430. [Google Scholar] [CrossRef] [PubMed]
- Mogole, L.; Omwoyo, W.; Viljoen, E.; Moloto, M. Green synthesis of silver nanoparticles using aqueous extract of Citrus sinensis peels and evaluation of their antibacterial efficacy. Green Process. Synth. 2021, 10, 851–859. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef]
- İpek, P.; Yıldız, R.; Baran, M.F.; Hatipoğlu, A.; Baran, A.; Sufianov, A.; Beylerli, O. Green synthesis of silver nanoparticles derived from Papaver rhoeas L. leaf extract: Cytotoxic and antimicrobial properties. Molecules 2023, 28, 6424. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Green biosynthesis of silver nanoparticles using Prunus cerasifera pissardii nigra leaf and their antimicrobial activities against some food pathogens. Czech J. Food Sci. 2022, 40, 383–391. [Google Scholar] [CrossRef]
- Pathak, M.; Pathak, P.; Khalilullah, H.; Grishina, M.; Potemkin, V.; Kumar, V.; Majee, R.; Ramteke, P.W.; Abdellattif, M.H.; Mohd Shahbaaz, M.; et al. Green synthesis of silver nanoformulation of Scindapsus officinalis as potent anticancer and predicted anticovid alternative: Exploration via experimental and computational methods. Biocatal. Agric. Biotechnol. 2021, 35, 102072. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Ma, J.; Alvarez, P.J. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ. Sci. Technol. 2011, 45, 9003–9008. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Balu, S.K.; Andra, S.; Damiri, F.; Sivaramalingam, A.; Sudandaradoss, M.V.; Kumarasamy, K.; Bhakthavachalam, K.; Ali, F.; Kundu, M.K.; Rahman, M.H.; et al. Size-dependent antibacterial, antidiabetic, and toxicity of silver nanoparticles synthesized using solvent extraction of Rosa indica L. Petals. Pharmaceuticals 2022, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively charged silver nanoparticles with potent antibacterial activity and reduced toxicity for pharmaceutical preparations. Int. J. Nanomed. 2017, 2017, 2517–2530. [Google Scholar] [CrossRef]
- Nogueira, R.B.; Manzato, L.; Gurgel, R.S.; Albuquerque, P.M.; Mendes, F.M.T.; Hotza, D. Optimized green synthesis of silver nanoparticles from guarana seed skin extract with antibacterial potential. Green Process. Synth. 2025, 14, 20230210. [Google Scholar] [CrossRef]
- Carvalho, L.V.D.N.; Cordeiro, M.F.; e Lins, T.U.L.; Sampaio, M.C.P.D.; de Mello, G.S.V.; da Costa, V.D.C.M.; Marques, L.L.M.; Klein, T.; de Mello, J.C.P.; Cavalcanti, I.M.F.; et al. Evaluation of antibacterial, antineoplastic, and immunomodulatory activity of Paullinia cupana seeds crude extract and ethyl-acetate fraction. Evid.-Based Complement. Alternat. Med. 2016, 2016, 1203274. [Google Scholar] [CrossRef]
- Basile, A.; Ferrara, L.; Del Pezzo, M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar] [CrossRef]
- Majhenič, L.; Škerget, M.; Knez, Ž. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem. 2007, 104, 1258–1268. [Google Scholar] [CrossRef]
- Dua, T.K.; Giri, S.; Nandi, G.; Sahu, R.; Shaw, T.K.; Paul, P. Green synthesis of silver nanoparticles using Eupatorium adenophorum leaf extract: Characterizations, antioxidant, antibacterial and photocatalytic activities. Chem. Pap. 2023, 77, 2947–2956. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Bakhshi, N.; Ramzanpoor, M. Biogenic silver nanoparticles using Oxalis corniculata characterization and their clinical implications. J. Drug Deliv. Sci. Technol. 2019, 54, 101263. [Google Scholar] [CrossRef]
- Dalonso, N.; de Oliveira Petkowicz, C.L. Guarana powder polysaccharides: Characterisation and evaluation of the antioxidant activity of a pectic fraction. Food Chem. 2012, 134, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Martelli-Tosi, M.; Massarioli, A.P.; Melo, P.S.; Alencar, S.M.; Favaro-Trindade, C.S. Co-encapsulation of guaraná extracts and probiotics increases probiotic survivability and simultaneously delivers bioactive compounds in simulated gastrointestinal fluids. LWT 2022, 161, 113351. [Google Scholar] [CrossRef]
- Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B. Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 2012, 11, 148–173. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Silver nanoparticles: An instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Chang, X.; Wang, X.; Li, J.; Shang, M.; Niu, S.; Zhang, W.; Li, Y.; Sun, Z.; Gan, J.; Li, W.; et al. Silver nanoparticles induced cytotoxicity in HT22 cells through autophagy and apoptosis via PI3K/AKT/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111696. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.D.; Shahriar, S.M.; Ahammad, A.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Jain, P.; Rajput, D.; Patil, U.K. Green synthesized plant-based silver nanoparticles: Therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst. Lett. 2021, 9, 5. [Google Scholar] [CrossRef]
- Mishra, V.; Nayak, P.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A.; Chellappan, D.K.; Dua, K. Pharmaceutical aspects of green synthesized silver nanoparticles: A boon to cancer treatment. Anti-Cancer Agents Med. Chem. 2021, 21, 1490–1509. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 1–17. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S. Silver Nanoparticles Biosynthesized Using Azadirachta indica Fruit and Leaf Extracts: Optimization, Characterization, and Anticancer Activity. J. Nanomater. 2023, 2023, 9916777. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Part. Fibre Toxicol. 2011, 8, 1–15. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Seligy, V.L.; Massarsky, A.; Moon, T.W.; Rippstein, P.; Tan, J.; Tayabali, A.F. Comparison of toxicity of uncoated and coated silver nanoparticles. J. Phys. Conf. Ser. 2013, 429, 012025. [Google Scholar] [CrossRef]
- Lima, N.D.S.; Numata, E.D.P.; Mesquita, L.M.D.S.; Dias, P.H.; Vilegas, W.; Gambero, A.; Ribeiro, M.L. Modulatory effects of guarana (Paullinia cupana) on adipogenesis. Nutrients 2017, 9, 635. [Google Scholar] [CrossRef]
- Roggia, I.; Dalcin, A.J.F.; de Souza, D.; Machado, A.K.; de Souza, D.V.; da Cruz, I.B.M.; Ribeiro, E.E.; Ourique, A.F.; Gomes, P. Guarana: Stability-Indicating RP-HPLC method and safety profile using microglial cells. J. Food Compos. Anal. 2020, 94, 103629. [Google Scholar] [CrossRef]
- Sirena, D.H.; Araujo, A.B.; Silveira, A.D.; Serafini, M.A.; Silva, M.D.; Silveira, A.K.; Filippi-Chiela, E.; Moreira, J.C.F.; Paz, A.H. Guarana (Paullinia cupana) as a potential tool for mesenchymal stromal cells priming in regenerative medicine. Braz. J. Med. Biol. Res. 2024, 57, e13286. [Google Scholar] [CrossRef] [PubMed]
- Cadoná, F.C.; Rosa, J.L.; Schneider, T.; Cubillos-Rojas, M.; Sánchez-Tena, S.; Azzolin, V.F.; Assmann, C.E.; Machado, A.K.; Ribeiro, E.E.; da Cruz, I.B.M. Guaraná, a highly caffeinated food, presents in vitro antitumor activity in colorectal and breast cancer cell lines by inhibiting AKT/mTOR/S6K and MAPKs pathways. Nutr. Cancer 2017, 69, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Miwa, S.; Hitomi, Y.; Nakamura, H.; Tsuchiya, H.; Yachie, A. Theobromine, the primary methylxanthine found in Theobroma cacao, prevents malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinase, Akt/mammalian target of rapamycin kinase, and nuclear factor-kappa B. Nutr. Cancer 2014, 66, 419–423. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Sousa, P.S.; Rodrigues, R.R.L.; de Souza, V.M.R.; de Mendonça Araujo, S.S.; Franco, M.S.C.R.; Dos Santos, L.B.P.; Ribeiro, F.O.S.; Paiva Junior, J.R.; Araujo-Nobre, A.R.; Rodrigues, K.A.F.; et al. Antimicrobial activity of nanoparticles based on carboxymethylated cashew gum and epiisopiloturine: In vitro and in silico studies. Int. J. Biol. Macromol. 2024, 274, 133048. [Google Scholar] [CrossRef]
- Gélvez, A.P.C.; Farias, L.H.S.; Pereira, V.S.; da Silva, I.C.M.; Costa, A.C.; Dias, C.G.B.T.; Costa, R.M.R.; da Silva, S.H.M.; Rodrigues, A.P.D. Biosynthesis, characterization and leishmanicidal activity of a biocomposite containing AgNPs-PVP-glucantime. Nanomedicine 2018, 13, 373–390. [Google Scholar] [CrossRef]
- Costa, E.V.; Pinheiro, M.L.B.; Silva, J.R.D.A.; Maia, B.H.L.D.N.S.; Duarte, M.C.T.; Amaral, A.C.F.; Machado, G.M.C.; Leon, L.L. Antimicrobial and antileishmanial activity of essential oil from the leaves of Annona foetida (Annonaceae). Quim. Nova 2009, 32, 78–81. [Google Scholar] [CrossRef]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Junior, V.F.V.; Pinto, A.C.; Nakamura, C.V. Effect of Brazilian copaiba oils on Leishmania amazonensis. J. Ethnopharmacol. 2008, 120, 204–208. [Google Scholar] [CrossRef]
- Lima, G.S.; Castro-Pinto, D.B.; Machado, G.C.; Maciel, M.A.; Echevarria, A. Antileishmanial activity and trypanothione reductase effects of terpenes from the Amazonian species Croton cajucara Benth (Euphorbiaceae). Phytomedicine 2015, 22, 1133–1137. [Google Scholar] [CrossRef]
- Zyoud, S.E.H.; Al-Jabi, S.W.; Sweileh, W.M. Scientific publications from Arab world in leading journals of Integrative and Complementary Medicine: A bibliometric analysis. BMC Complement. Altern. Med. 2015, 15, 308. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Ustundag, C.B.; Kaya, C.; Kaya, F.; Rafailovich, M. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int. J. Nanomed. 2011, 6, 2705–2714. [Google Scholar] [CrossRef]
- Alti, D.; Veeramohan Rao, M.; Rao, D.N.; Maurya, R.; Kalangi, S.K. Gold–silver bimetallic nanoparticles reduced with herbal leaf extracts induce ROS-mediated death in both promastigote and amastigote stages of Leishmania donovani. ACS Omega 2020, 5, 16238–16245. [Google Scholar] [CrossRef] [PubMed]
- Fanti, J.R.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Cataneo, A.H.D.; de Jesus Andrade, C.G.T.; Panis, C.; Rodrigues, J.H.S.; Wowk, P.F.; Kuczera, D.; Costa, I.N.; et al. Biogenic silver nanoparticles inducing Leishmania amazonensis promastigote and amastigote death in vitro. Acta Trop. 2018, 178, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.B.; da Silva Bortoleti, B.T.; Tomiotto-Pellissier, F.; Ganaza, A.F.M.; Gonçalves, M.D.; Carloto, A.C.M.; Rodrigues, A.C.J.; Silva, T.F.; Nakazato, G.; Kobayashi, R.K.T.; et al. Synergistic antileishmanial effect of oregano essential oil and silver nanoparticles: Mechanisms of action on Leishmania amazonensis. Pathogens 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef]
- Horta, M.F.; Mendes, B.P.; Roma, E.H.; Noronha, F.S.M.; Macêdo, J.P.; Oliveira, L.S.; Duarte, M.M.; Vieira, L.Q. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J. Parasitol. Res. 2012, 2012, 203818. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Advances 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Amarasinghe, L.D.; Wickramarachchi, P.A.S.R.; Aberathna, A.A.A.U.; Sithara, W.S.; De Silva, C.R. Comparative study on larvicidal activity of green synthesized silver nanoparticles and Annona glabra (Annonaceae) aqueous extract to control Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Heliyon 2020, 6, e04322. [Google Scholar] [CrossRef]
- Azarudeen, R.M.S.T.; Govindarajan, M.; AlShebly, M.M.; AlQahtani, F.S.; Amsath, A.; Senthilmurugan, S.; Vijayan, P.; Benelli, G. Size-controlled biofabrication of silver nanoparticles using the Merremia emarginata leaf extract: Toxicity on Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) and non-target mosquito predators. J. Asia-Pac. Entomol. 2017, 20, 359–366. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, S.; Tripathi, P. A facile and rapid method for green synthesis of Achyranthes aspera stem extract-mediated silver nano-composites with cidal potential against Aedes aegypti L. Saudi J. Biol. Sci. 2019, 26, 698–708. [Google Scholar] [CrossRef]
- Elumalai, D.; Ashok, K.; Suresh, A.; Hemavathi, M. Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng. Agric. Environ. Food 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Kumar, V.A.; Ammani, K.; Jobina, R.; Subhaswaraj, P.; Siddhardha, B. Photo-induced and phytomediated synthesis of silver nanoparticles using Derris trifoliata leaf extract and its larvicidal activity against Aedes aegypti. J. Photochem. Photobiol. B 2017, 171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, E.; Manivannan, N.; Ramanibai, R.; Mathivanan, N. Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol. Rep. 2019, 21, e00297. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Hoti, S.L.; Rajeswary, M.; Benelli, G. One-step synthesis of polydispersed silver nanocrystals using Malva sylvestris: An eco-friendly mosquito larvicide with negligible impact on non-target aquatic organisms. Parasitol. Res. 2016, 115, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- de Assunção, M.A.S.; Dourado, D.; Dos Santos, D.R.; Faierstein, G.B.; Braga, M.E.M.; Junior, S.A.; Barbosa, R.M.R.; de Sousa, H.J.C.; Formiga, F.R. Green synthesis of silver nanoparticles derived from algae and their larvicidal properties to control Aedes aegypti. Beilstein J. Nanotechnol. 2024, 15, 1566–1575. [Google Scholar] [CrossRef]
- Azarudeen, R.M.S.T.; Govindarajan, M.; Amsath, A.; Muthukumaran, U.; Benelli, G. Single-step biofabrication of silver nanocrystals using Naregamia alata: A cost effective and eco-friendly control tool in the fight against malaria, Zika virus and St. Louis encephalitis mosquito vectors. J. Clust. Sci. 2017, 28, 179–203. [Google Scholar] [CrossRef]
- Sap-Iam, N.; Homklinchan, C.; Larpudomlert, R.; Warisnoicharoen, W.; Sereemaspun, A.; Dubas, S.T. UV irradiation-induced silver nanoparticles as mosquito larvicides. J. Appl. Sci. 2010, 10, 3132–3136. [Google Scholar] [CrossRef]
- Sareen, S.J.; Pillai, R.K.; Chandramohanakumar, N.; Balagopalan, M. Larvicidal potential of biologically synthesised silver nanoparticles against Aedes albopictus. Res. J. Recent Sci. 2012, 1, 52–56. [Google Scholar]
- Murugan, K.; Priyanka, V.; Dinesh, D.; Madhiyazhagan, P.; Panneerselvam, C.; Subramaniam, J.; Suresh, U.; Chandramohan, B.; Roni, M.; Nicoletti, M.; et al. Predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector, Aedes aegypti, in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol. Res. 2015, 114, 3601–3610. [Google Scholar] [CrossRef]
- Suresh, U.; Murugan, K.; Benelli, G.; Nicoletti, M.; Barnard, D.R.; Panneerselvam, C.; Kumar, P.M.; Subramaniam, J.; Dinesh, D.; Chandramohan, B. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 1551–1562. [Google Scholar] [CrossRef]
- Nalini, M.; Lena, M.; Sumathi, P.; Sundaravadivelan, C. Effect of phyto-synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 2017, 4, 212–218. [Google Scholar] [CrossRef]
- Koul, O. Phytochemicals and insect control: An antifeedant approach. Crit. Rev. Plant Sci. 2008, 27, 10. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Kumar, P.M.; Dinesh, D.; Chandramohan, B.; Suresh, U.; Nicoletti, M.; et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: Towards an integrative approach? Environ. Sci. Pollut. Res. 2015, 22, 20067–20083. [Google Scholar] [CrossRef]
- Ullah, I.; Tahir, K.; Khan, A.U.; Albalawi, K.; Li, B.; El-Zahhar, A.A.; Jevtovic, V.; Al-Shehri, H.S.; Asghar, B.H.; Alghamdi, M.M. Facile fabrication of Ag nanoparticles: An advanced material for antioxidant, infectious therapy and photocatalytic applications. Inorg. Chem. Commun. 2022, 141, 109539. [Google Scholar] [CrossRef]
- Zayed, M.F.; Shalby, O.M.; Eisa, W.H.; El-Kousy, S.M.; Eltorgoman, A.M. Controlled synthesis, spectral studies, and catalytic activity of silver and gold nanoparticles biosynthesized using Ficus sycomorus leaf extract. J. Appl. Spectrosc. 2022, 89, 381–390. [Google Scholar] [CrossRef]
- Maruthai, J.; Muthukumarasamy, A.; Baskaran, B. Fabrication and characterisation of silver nanoparticles using bract extract of Musa paradisiaca for its synergistic combating effect on phytopathogens, free radical scavenging activity, and catalytic efficiency. IET Nanobiotechnol. 2019, 13, 134–143. [Google Scholar] [CrossRef]
- Hashemi, Z.; Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Sustainable biosynthesis of metallic silver nanoparticles using barberry phenolic extract: Optimization and evaluation of photocatalytic, in vitro cytotoxicity, and antibacterial activities against multidrug-resistant bacteria. Inorg. Chem. Commun. 2022, 139, 109320. [Google Scholar] [CrossRef]
- Rajasekar, R.; Thanasamy, R.; Samuel, M.; Edison, T.N.J.I.; Raman, N. Ecofriendly synthesis of silver nanoparticles using Heterotheca subaxillaris flower and its catalytic performance on reduction of methyl orange. Biochem. Eng. J. 2022, 187, 108447. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, H.P.; Sharma, R.K. Metal nanoparticles with high catalytic activity in degradation of methyl orange: An electron relay effect. J. Mol. Catal. A Chem. 2011, 335, 248–252. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Junejo, Y.; Safdar, M. Highly effective heterogeneous doxycycline stabilized silver nanocatalyst for the degradation of ibuprofen and paracetamol drugs. Arab. J. Chem. 2019, 12, 2823–2832. [Google Scholar] [CrossRef]
- Naseem, K.; Zia Ur Rehman, M.; Ahmad, A.; Dubal, D.; AlGarni, T.S. Plant extract induced biogenic preparation of silver nanoparticles and their potential as catalyst for degradation of toxic dyes. Coatings 2020, 10, 1235. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Charti, I.; Azouzi, A.; Belghiti, A.; Sair, S.; Abboud, Y.; El Bouari, A. Ecofriendly synthesis of stabilized silver nanoparticles and the evaluation of their potential applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100102. [Google Scholar] [CrossRef]
- Kumar, P.; Dixit, J.; Singh, A.K.; Rajput, V.D.; Verma, P.; Tiwari, K.N.; Mishra, S.K.; Minkina, T.; Mandzhieva, S. Efficient catalytic degradation of selected toxic dyes by green biosynthesized silver nanoparticles using aqueous leaf extract of Cestrum nocturnum L. Nanomaterials 2022, 12, 3851. [Google Scholar] [CrossRef]
- Seku, K.; Hussaini, S.S.; Hussain, M.; Siddiqui, M.A.; Golla, N.; Ravinder, D.; Reddy, B. Synthesis of Frankincense gum stabilized AgNPs by microwave irradiation and their catalytic, antioxidant, and antibacterial properties. Phys. E Low Dimens. Syst. Nanostruct. 2022, 140, 115169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).