Silver Dimolybdate Nanorods: In Vitro Anticancer Activity Against Breast and Prostate Tumors and In Vivo Pharmacological Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis and Characterizations
2.3. Cell Culture
2.4. Cytotoxicity Assessment and Inhibitory Concentration (IC50) Determination
2.5. In Vivo Biodistribution: Tissue Deposition
2.5.1. Labeling Process with 99mTc
2.5.2. Quality Control of the Labeling Process with Tc-99m
2.5.3. Animals
2.5.4. Animal Preparation
2.5.5. Design Protocol
2.6. Radiopharmacokinetics
2.7. Statistical Analyses
3. Results and Discussion
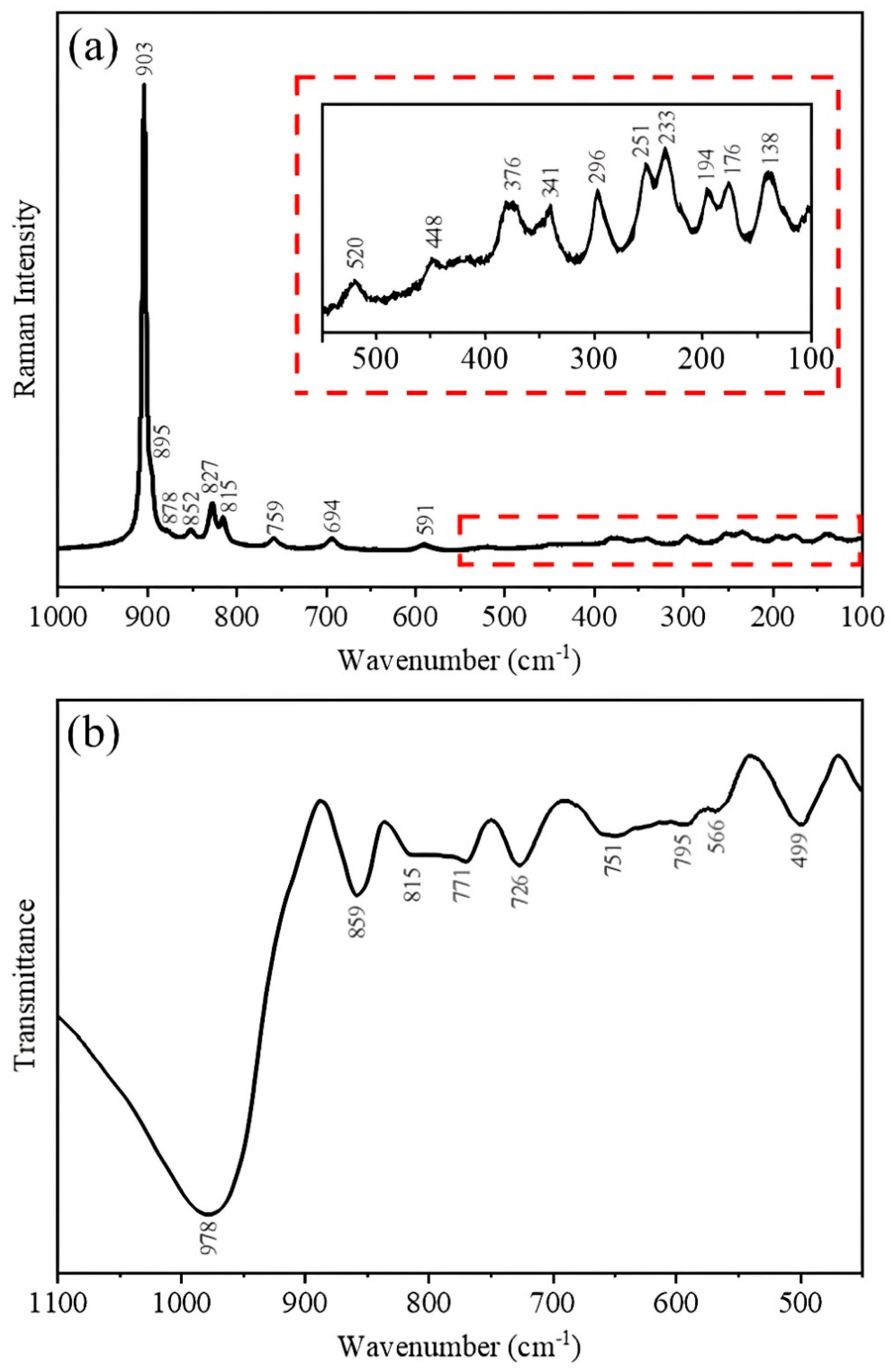
3.1. Characterization of Ag2Mo2O7 Nanorods
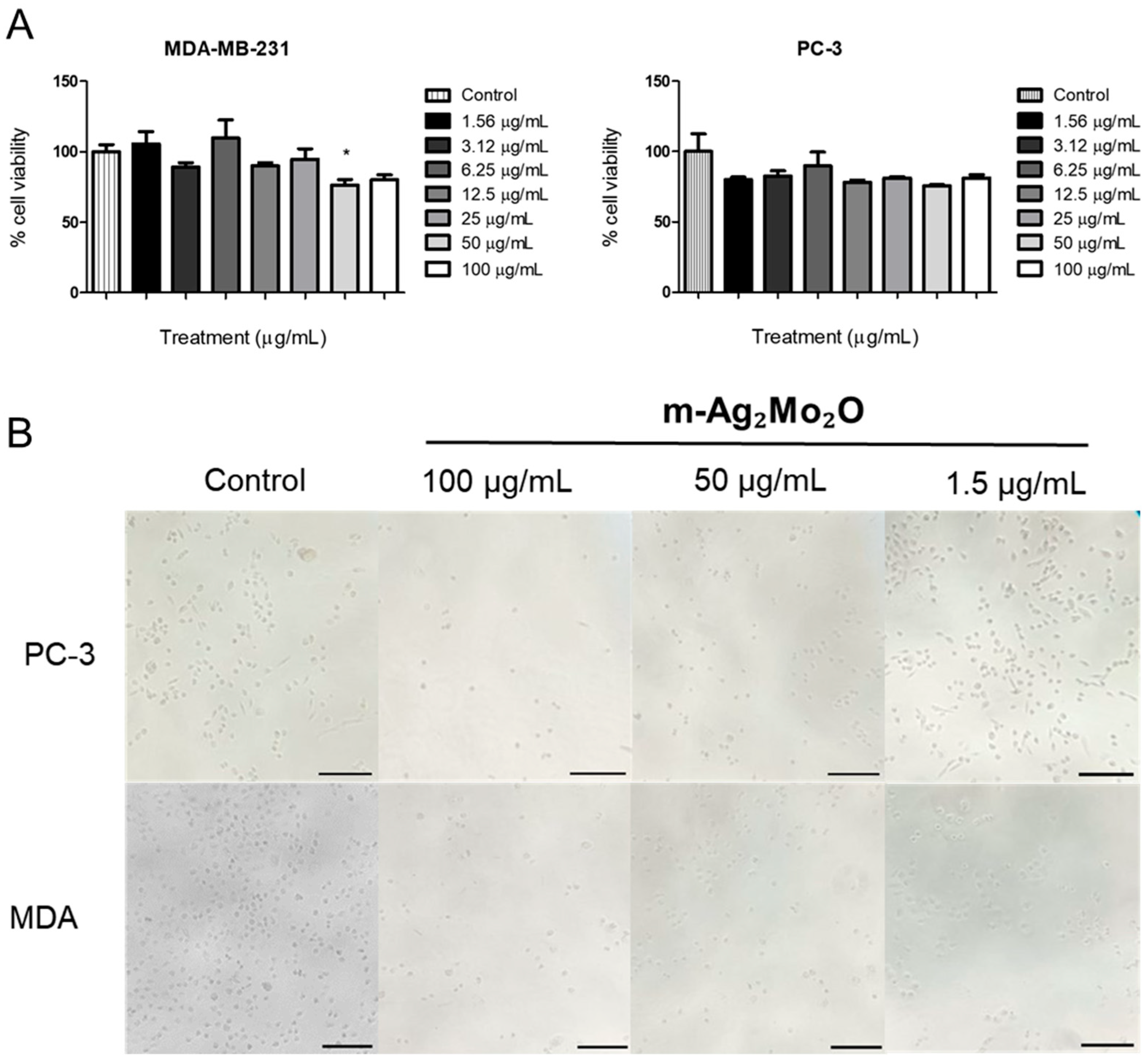
3.2. Cytotoxicity Effect on MDA-MB-231 and PC-3 Cells
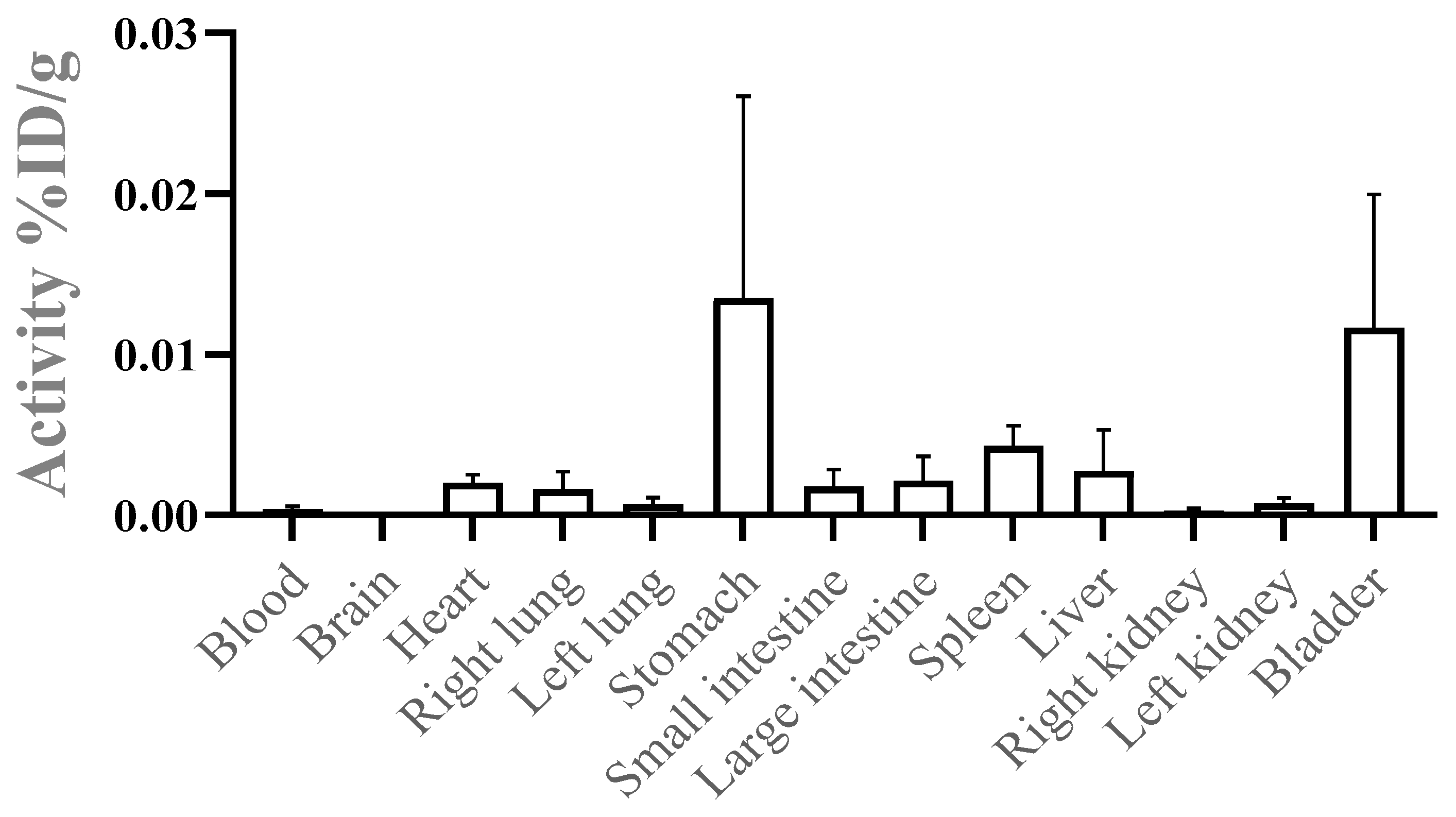
3.3. In Vivo Biodistribution: Tissue Deposition
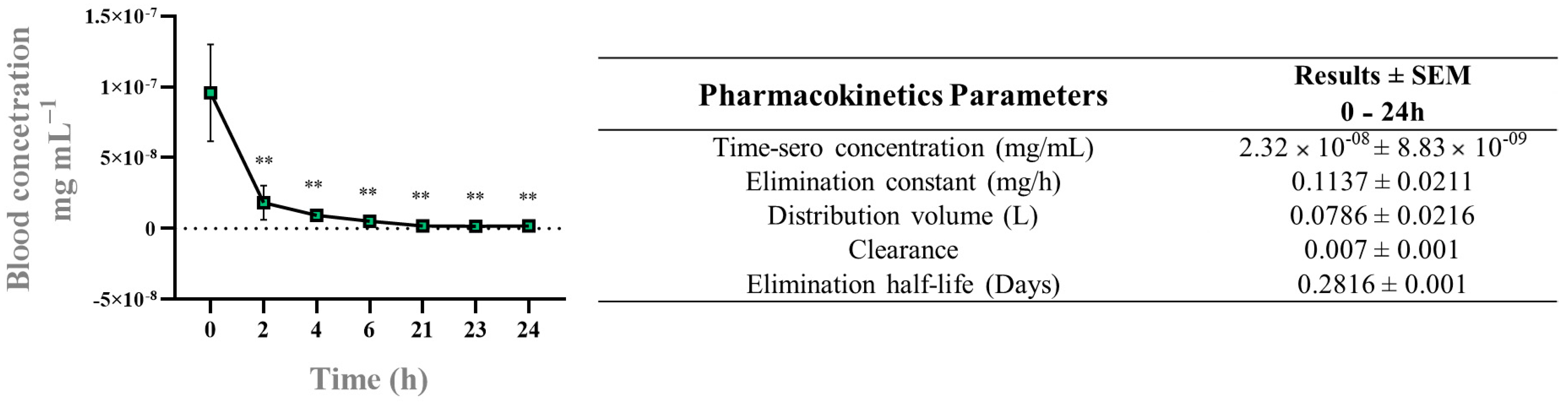
3.4. Radiopharmacokinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, R.; Demarchi, D.; Roch, M.R.; Aiassa, S.; Pagana, G. Nanomaterials to Fight Cancer: An Overview on Their Multifunctional Exploitability. J. Nanosci. Nanotechnol. 2021, 21, 2760–2777. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Huang, R.; Ding, Y.; Ruan, C.; Shen, X.-C. Mechanisms of Reactive Oxygen Species Generated by Inorganic Nanomaterials for Cancer Therapeutics. Front. Chem. 2021, 9, 630969. [Google Scholar] [CrossRef]
- Shen, S.; Zhu, C.; Huo, D.; Yang, M.; Xue, J.; Xia, Y. A Hybrid Nanomaterial for the Controlled Generation of Free Radicals and Oxidative Destruction of Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 8801–8804. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Q.; Wang, X.; Wen, T.; Yin, J.-J.; Wang, P.; Bai, R.; Zhang, X.-Q.; Zhang, L.-H.; Lu, A.-H.; et al. Using Hollow Carbon Nanospheres as a Light-Induced Free Radical Generator To Overcome Chemotherapy Resistance. J. Am. Chem. Soc. 2015, 137, 1947–1955. [Google Scholar] [CrossRef]
- Moura, J.; Ferreira, W.; da Silva-Filho, J.; Alabarse, F.; Freire, P.; Luz-Lima, C. Ag2Mo3O10·2H2O nanorods under high pressure: In situ Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 299, 122871. [Google Scholar] [CrossRef]
- Wang, L.; Peng, B.; Guo, X.; Ding, W.; Chen, Y. Ferric molybdate nanotubes synthesized based on the Kirkendall effect and their catalytic property for propene epoxidation by air. Chem. Commun. 2009, 12, 1565. [Google Scholar] [CrossRef]
- Geng, B.; He, Y.; Yan, F.; Zhu, C.; Zhang, X.; Zhang, X.; Chen, Y. Interface engineering of metallic nickel nanoparticles/semiconductive nickel molybdate nanowires for efficiently electrocatalytic water splitting. Mater. Today Nano 2022, 18, 100176. [Google Scholar] [CrossRef]
- Mokoba, T.; Liu, Y.; Wu, Y.; Zhang, T.C.; Yuan, S. Agave-Angustifolia-like Cu3Mo2O9 Nanoplate-Coated Copper Mesh for Effective Emulsion Separation and Photocatalytic Degradation of Soluble Dyes. Ind. Eng. Chem. Res. 2022, 61, 13635–13649. [Google Scholar] [CrossRef]
- Datta, R.S.; Ou, J.Z.; Mohiuddin, M.; Carey, B.J.; Zhang, B.Y.; Khan, H.; Syed, N.; Zavabeti, A.; Haque, F.; Daeneke, T.; et al. Two dimensional PbMoO4: A photocatalytic material derived from a naturally non-layered crystal. Nano Energy 2018, 49, 237–246. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A.; Mbese, J.Z. Impact of Molybdenum Compounds as Anticancer Agents. Bioinorg. Chem. Appl. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Belaidi, A.A. Molybdenum in Human Health and Disease. In Interrelations Between Essential Metal Ions and Human Diseases; Springer: Dordrecht, The Netherlands, 2013; pp. 415–450. [Google Scholar] [CrossRef]
- Fabbro, M.T.; Foggi, C.C.; Santos, L.P.S.; Gracia, L.; Perrin, A.; Perrin, C.; Vergani, C.E.; Machado, A.L.; Andrés, J.; Cordoncillo, E.; et al. Synthesis, antifungal evaluation and optical properties of silver molybdate microcrystals in different solvents: A combined experimental and theoretical study. Dalton Trans. 2016, 45, 10736–10743. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Xiong, Z.X. Preparation of Molybdates with Antibacterial Property. Key Eng. Mater. 2008, 368–372, 1516–1518. [Google Scholar] [CrossRef]
- Mao, G.; Xin, D.; Wang, Q.; Lai, D. Sodium molybdate inhibits the growth of ovarian cancer cells via inducing both ferroptosis and apoptosis. Free Radic. Biol. Med. 2022, 182, 79–92. [Google Scholar] [CrossRef]
- Kamaraju, J.R.M.; Kanchi, R.R.; Borra, R.K.; Reniguntla, P.S.; Rentala, S. Synthesis, characterization and anti-cancer applications of ytterbium doped gadolinium molybdate nanophosphor compound. Mater. Express 2019, 9, 882–894. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abu-Elghait, M.; El-Shahat, M. Engineering titanium-organic framework decorated silver molybdate and silver vanadate as antimicrobial, anticancer agents, and photo-induced hydroxylation reactions. J. Photochem. Photobiol. A Chem. 2021, 423, 113572. [Google Scholar] [CrossRef]
- Behvandi, S.; Sobhani-Nasab, A.; Karimi, M.A.; Sohouli, E.; Karimi, M.; Ganjali, M.R.; Ahmadi, F.; Rahimi-Nasrabadi, M. Synthesis and characterization of Sm2(MoO4)3, Sm2(MoO4)3/GO and Sm2(MoO4)3/C3N4 nanostructures for improved photocatalytic performance and their anti-cancer the MCF-7 cells. Polyhedron 2020, 180, 114424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ferreira AN, C.; Ferreira, W.C.; Duarte, A.V.; Santos, C.C.; Freire PT, C.; Luz-Lima, C.; Moura JV, B. In situ high-temperature Raman scattering study of monoclinic Ag2Mo2O7 nanorods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 295, 122632. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Magnetic Structure Determination from Powder Diffraction Using the Program Fullprof. In Applied Crystallography; World Scientific: Paris, France, 2001; pp. 30–36. [Google Scholar] [CrossRef]
- Helal-Neto, E.; de Barros, A.O.d.S.; Saldanha-Gama, R.; Brandão Costa, R.; Alencar, L.M.R.; dos Santos, C.C.; Martínez-Máñez, R.; Ricci-Junior, E.; Alexis, F.; Morandi, V.; et al. Molecular and Cellular Risk Assessment of Healthy Human Cells and Cancer Human Cells Exposed to Nanoparticles. Int. J. Mol. Sci. 2019, 21, 230. [Google Scholar] [CrossRef]
- Adhikari, S.; Mandal, S.; Kim, D.-H. Z-scheme 2D/1D MoS2 nanosheet-decorated Ag2Mo2O7 nanorods for efficient catalytic oxidation of levofloxacin. Chem. Eng. J. 2019, 373, 31–43. [Google Scholar] [CrossRef]
- Hakouk, K.; Lajaunie, L.; El Bekkachi, H.; Serier-Brault, H.; Humbert, B.; Arenal, R.; Dessapt, R. Plasmonic properties of an Ag@Ag2Mo2O7 hybrid nanostructure easily designed by solid-state photodeposition from very thin Ag2Mo2O7 nanowires. J. Mater. Chem. C 2018, 6, 11086–11095. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yan, W.; Zhu, D.; Wang, Z.; Xia, Y.; Gui, D.-Y.; Luo, F.; Wang, C.-H. Ag2Mo2O7: An oxide solid-state Ag+ electrolyte. RSC Adv. 2022, 12, 3494–3499. [Google Scholar] [CrossRef]
- Fazio, E.; Speciale, A.; Spadaro, S.; Bonsignore, M.; Cimino, F.; Cristani, M.; Trombetta, D.; Saija, A.; Neri, F. Evaluation of biological response induced by molybdenum oxide nanocolloids on in vitro cultured NIH/3T3 fibroblast cells by micro-Raman spectroscopy. Colloids Surf. B Biointerfaces 2018, 170, 233–241. [Google Scholar] [CrossRef]
- Anh Tran, T.; Krishnamoorthy, K.; Song, Y.W.; Cho, S.K.; Kim, S.J. Toxicity of Nano Molybdenum Trioxide toward Invasive Breast Cancer Cells. ACS Appl. Mater. Interfaces 2014, 6, 2980–2986. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Z.; Wu, B.; Yu, Y.; Hou, H.; Zhang, X.-X.; Ren, H. Cytotoxicity and Efflux Pump Inhibition Induced by Molybdenum Disulfide and Boron Nitride Nanomaterials with Sheetlike Structure. Environ. Sci. Technol. 2017, 51, 10834–10842. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Saquib, Q.; Ahamed, M.; Farshori, N.N.; Ahmad, J.; Wahab, R.; Khan, S.T.; Alhadlaq, H.A.; Musarrat, J.; Al-Khedhairy, A.A.; et al. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloids Surf. B Biointerfaces 2014, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Fan, D.; Sun, H.; Yang, J.; Yu, Z.; Zhang, D.; Pu, X.; Li, H.; Cai, P. One-dimensional rod-shaped Ag2Mo2O7/BiOI n-n junctions for efficient photodegradation of tetracycline and rhodamine B under visible light. J. Alloys Compd. 2022, 912, 165184. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Dou, M.; Wang, J.; Cheng, Y.; Jönsson, P.G.; Zhao, Z. Visible light-driven g-C3N4/m-Ag2Mo2O7composite photocatalysts: Synthesis, enhanced activity and photocatalytic mechanism. RSC Adv. 2014, 4, 51008–51015. [Google Scholar] [CrossRef]
- Mardare, C.C.; Tanasic, D.; Rathner, A.; Müller, N.; Hassel, A.W. Growth inhibition of Escherichia coli by zinc molybdate with different crystalline structures. Phys. Status Solidi (A) 2016, 213, 1471–1478. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Kodama, R.; Kato, M.; Furuta, S.; Ueno, S.; Zhang, Y.; Matsuno, K.; Yabe-Nishimura, C.; Tanaka, E.; Kamata, T. ROS-generating oxidases Nox1 and Nox4 contribute to oncogenic Ras-induced premature senescence. Genes Cells 2012, 18, 32–41. [Google Scholar] [CrossRef]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of mesoporous silica nanoparticles; Towards clinical translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnol. 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.; Park, S.; Kocz, R. Drug Elimination. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://europepmc.org/books/nbk547662 (accessed on 15 January 2024).
- Andrade, C. The Practical Importance of Half-Life in Psychopharmacology. J. Clin. Psychiatry 2022, 83, 41940. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Obach, R.S.; Shalaeva, M.Y.; Gao, F. Prediction of Volume of Distribution Values in Humans for Neutral and Basic Drugs Using Physicochemical Measurements and Plasma Protein Binding Data. J. Med. Chem. 2002, 45, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; Mahabadi, N. Volume of Distribution. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545280/ (accessed on 15 January 2024).
- Celestin, M.N.; Musteata, F.M. Impact of Changes in Free Concentrations and Drug-Protein Binding on Drug Dosing Regimens in Special Populations and Disease States. J. Pharm. Sci. 2021, 110, 3331–3344. [Google Scholar] [CrossRef]
- Holt, K.; Nagar, S.; Korzekwa, K. Methods to Predict Volume of Distribution. Curr. Pharmacol. Rep. 2019, 5, 391–399. [Google Scholar] [CrossRef]

| Method | Concentration (µg/mL) | Cell Viability (%) | Cell Death (%) | ||
|---|---|---|---|---|---|
| PC-3 | MDA-MB-231 | PC-3 | MDA-MB-231 | ||
| MTT | 100 | 81.2 ± 2.4 | 80.1 ± 3.5 | 18.8 ± 2.4 | 19.9 ± 3.5 |
| 50 | 75.5 ± 1.1 | 76.5 ± 3.8 * | 24.5 ± 1.1 | 23.5 ± 3.8 * | |
| 25 | 81.1 ± 1.2 | 94.6 ± 7.4 | 18.9 ± 1.2 | 5.4 ± 7.4 | |
| 12.5 | 78.2 ± 1.5 | 89.9 ± 2.2 | 21.8 ± 1.5 | 10.1 ± 2.2 | |
| 6.25 | 89.8 ± 9.8 | 109.8 ± 12.8 | 10.2 ± 9.8 | ND | |
| 3.125 | 82.6 ± 3.7 | 89.1 ± 3.2 | 17.4 ± 3.7 | 10.9 ± 3.2 | |
| 1.56 | 80.2 ± 1.6 | 105.6 ± 8.7 | 19.8 ± 1.6 | ND | |
| Controls | SC | 100 ± 12.5 | 100 ± 5.0 | --- | --- |
| DMSO | ND | ND | --- | --- | |
| IC50 | >100 | >100 | --- | --- | |
| NOAEC | >100 | ≥25 | --- | --- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, J.V.B.; Gomes-da-Silva, N.C.; Rebêlo Alencar, L.M.; Ferreira, W.C.; da Luz Lima, C.; Santos-Oliveira, R. Silver Dimolybdate Nanorods: In Vitro Anticancer Activity Against Breast and Prostate Tumors and In Vivo Pharmacological Insights. Pharmaceutics 2025, 17, 298. https://doi.org/10.3390/pharmaceutics17030298
Moura JVB, Gomes-da-Silva NC, Rebêlo Alencar LM, Ferreira WC, da Luz Lima C, Santos-Oliveira R. Silver Dimolybdate Nanorods: In Vitro Anticancer Activity Against Breast and Prostate Tumors and In Vivo Pharmacological Insights. Pharmaceutics. 2025; 17(3):298. https://doi.org/10.3390/pharmaceutics17030298
Chicago/Turabian StyleMoura, João Victor Barbosa, Natália Cristina Gomes-da-Silva, Luciana Magalhães Rebêlo Alencar, Wellington Castro Ferreira, Cleânio da Luz Lima, and Ralph Santos-Oliveira. 2025. "Silver Dimolybdate Nanorods: In Vitro Anticancer Activity Against Breast and Prostate Tumors and In Vivo Pharmacological Insights" Pharmaceutics 17, no. 3: 298. https://doi.org/10.3390/pharmaceutics17030298
APA StyleMoura, J. V. B., Gomes-da-Silva, N. C., Rebêlo Alencar, L. M., Ferreira, W. C., da Luz Lima, C., & Santos-Oliveira, R. (2025). Silver Dimolybdate Nanorods: In Vitro Anticancer Activity Against Breast and Prostate Tumors and In Vivo Pharmacological Insights. Pharmaceutics, 17(3), 298. https://doi.org/10.3390/pharmaceutics17030298







