Nanotechnology-Based Therapies for Preventing Post-Surgical Adhesions
Abstract
1. Introduction
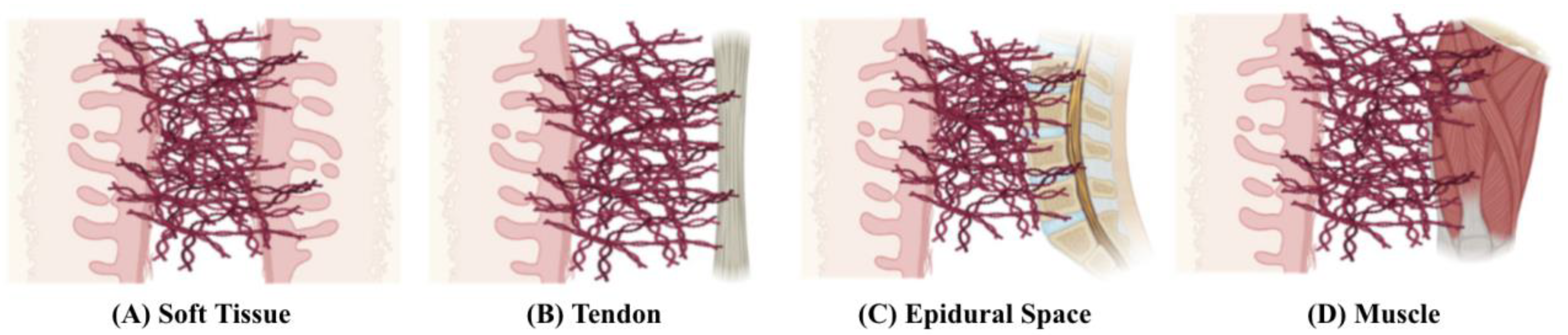
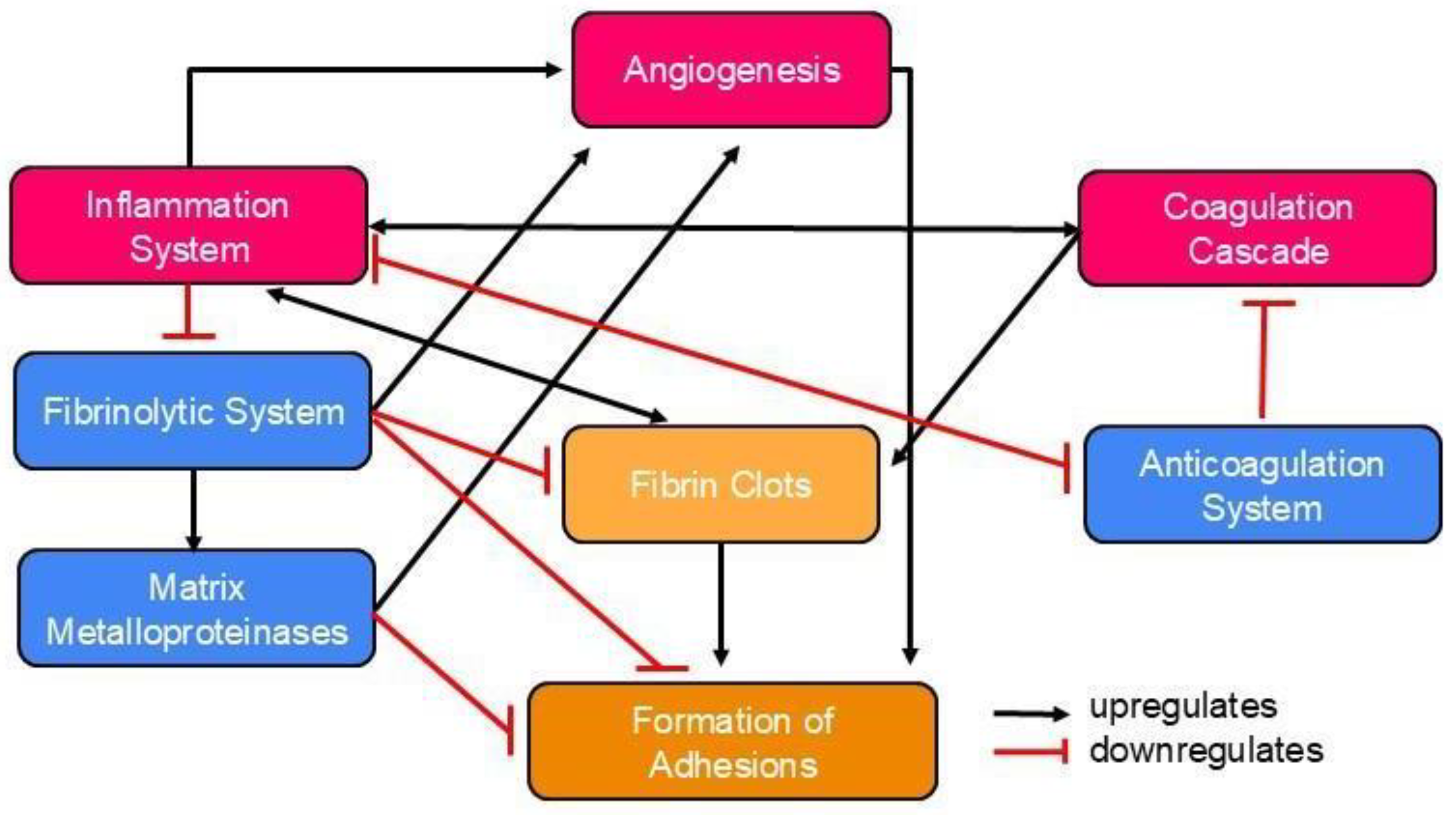
Pathophysiology of Postoperative Abdominal Adhesion Formation
2. Therapeutic Models to Prevent Postoperative Abdominal Adhesions
2.1. Surgical Techniques
2.2. Pharmaceutical Strategies
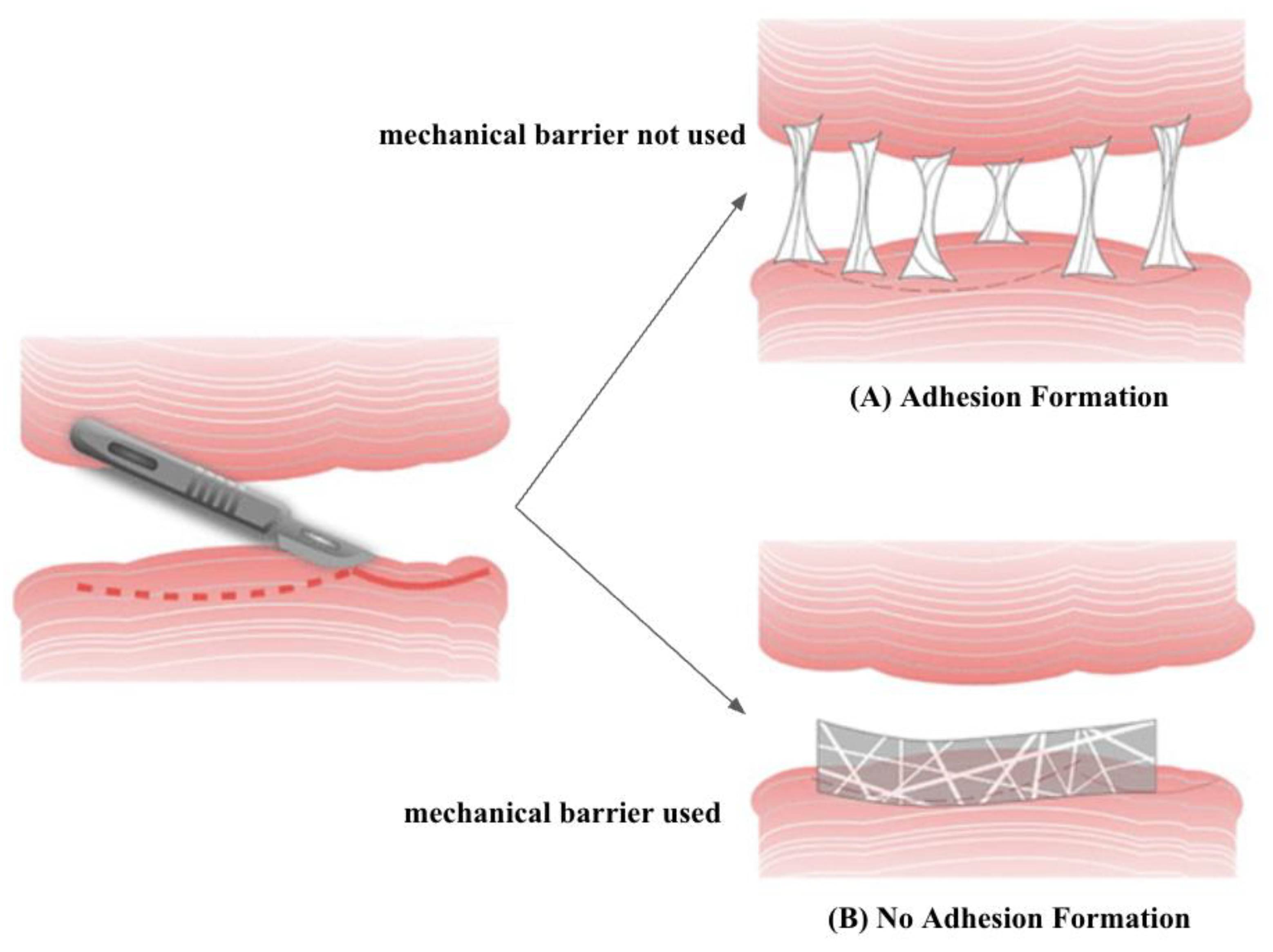
2.3. Mechanical Barriers
2.4. Gene Therapy
3. Nanotherapeutics for the Prevention of Abdominal Adhesions
3.1. Nanocomposites
3.2. Hydrogels
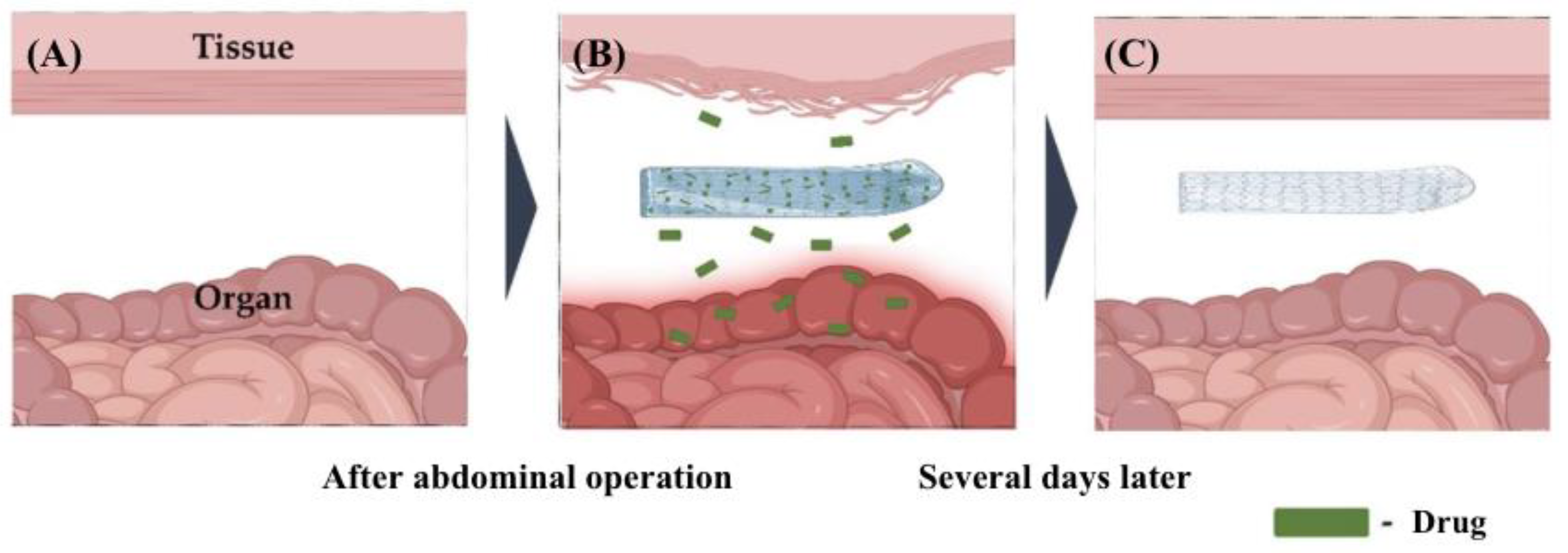
3.3. Nanofibers
4. Regulatory Pathways for Nanotherapeutics
5. Safety and Ethics of Nanotherapeutics
5.1. Safety Concerns
5.2. Ethical Concerns
6. Future Perspectives
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Col-APG-Cys@HHD | collagen aldehydeylated poly(ethylene glycol) cysteine HSA-18His protein and docetaxel |
| CaCl2 | calcium chloride |
| CUR | curcumin |
| DXP | dexamethasone 21-palmitate |
| ECM | extracellular matrix |
| EGCG | epigallocatechin-3-O-gallate |
| FDA | Food and Drug Administration |
| HA | hyaluronic acid |
| IND | investigational new drug |
| LNPs | lipid nanoparticles |
| mSiNPs | silica nanoparticles |
| NDAs | new drug applications |
| PA | plasminogen activators |
| PAI | plasminogen activator inhibitors |
| PAI-1 | plasminogen activator inhibitor 1 |
| PCL | poly(caprolactone) |
| pCNP | photo-crosslinkable nanopatch |
| PDA-KGF | poly(dopamine) human keratinocyte growth factor |
| PEG | poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PES | poly(ethylsulfone) |
| PLGA | poly(lactic-co-glycolic acid) |
| PLLA | poly(l-lactide) |
| PU | poly(urethane) |
| SLNM | nanofibers with superlubricated nano-skin |
| TiO-NPs | titanium dioxide nanoparticles |
| TNF-α | tumour necrosis factor-alpha |
| t-PA | tissue plasminogen activator |
| VEGF | vascular endothelial growth factor |
| β-GP | beta-glycerolphosphate disodium salt pentahydrate |
References
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to prevent post-operative adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef] [PubMed]
- Nahirniak, P.; Tuma, F. Adhesiolysis. StatPearls [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/n/statpearls/article-29/ (accessed on 16 December 2024).
- ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ 2013, 347, f5588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, X.; Luo, J.; Wen, Q.; Wu, Z.; Ding, Q.; Zhao, L.; Yang, L.; Wang, B.; Fu, S. Naproxen nanoparticle-loaded thermosensitive chitosan hydrogel for prevention of postoperative adhesions. ACS Biomater. Sci. Eng. 2019, 5, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.S.; Marshall, C.D.; Gulati, G.S.; Chinta, M.S.; Nguyen, A.; Salhotra, A.; Jones, R.E.; Burcham, A.; Lerbs, T.; Cui, L.; et al. Elucidating the fundamental fibrotic processes driving abdominal adhesion formation. Nat. Commun. 2020, 11, 4061. [Google Scholar] [CrossRef]
- Ellis, H.; Moran, B.J.; Thompson, J.N.; Parker, M.C.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’Brien, F.; et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet 1999, 353, 1476–1480. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.; Kooistra, T. Pathogenesis of postoperative adhesion formation. Br. J. Surg. 2011, 98, 1503–1516. [Google Scholar] [CrossRef]
- Wong, R.S.Y.; Tan, T.; Pang, A.S.R.; Srinivasan, D.K. The role of cytokines in wound healing: From mechanistic insights to therapeutic applications. Explor. Immunol. 2025, 5, 1003183. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B. The molecular basis of blood coagulation. Cell 1988, 53, 505–518. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; Büller, H.R. Bidirectional relation between inflammation and coagulation. Circulation 2004, 109, 2698–2704. [Google Scholar] [CrossRef]
- Esmon, C. The protein C pathway. Crit. Care Med. 2000, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry 2002, 67, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kisucka, J.; Butterfield, C.E.; Duda, D.G.; Eichenberger, S.C.; Saffaripour, S.; Ware, J.; Ruggeri, Z.M.; Jain, R.K.; Folkman, J.; Wagner, D.D. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. USA 2006, 103, 855–860. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Saed, G.M.; Diamond, M.P. Molecular characterization of postoperative adhesions: The adhesion phenotype. J. Am. Assoc. Gynecol. Laparosc. 2004, 11, 307–314. [Google Scholar] [CrossRef]
- Imudia, A.; Kumar, S.; Saed, G.; Diamond, M. Pathogenesis of intra-abdominal and pelvic adhesion development. Semin. Reprod. Med. 2008, 26, 289–297. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Gomel, V.; Ussia, A.; Adamyan, L. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil. Steril. 2016, 106, 998–1010. [Google Scholar] [CrossRef]
- Stommel, M.W.; ten Broek, R.P.; Strik, C.; Slooter, G.D.; Verhoef, C.; Grünhagen, D.J.; van Duijvendijk, P.; Bemelmans, M.H.; den Dulk, M.; Sietses, C.; et al. Multicenter observational study of adhesion formation after open and laparoscopic surgery for colorectal cancer. Ann. Surg. 2018, 267, 743–748. [Google Scholar] [CrossRef]
- Krielen, P.; Stommel, M.W.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; ten Broek, R.P. Adhesion-related readmissions after Open and laparoscopic surgery: A retrospective cohort study (scar update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.; Trimbos-Kemper, T.C.; Trimbos, J.B.M.; Emeis, J.J.; Kooistra, T. Use of fibrinolytic agents in the Prevention of Postoperative Adhesion Formation. Fertil. Steril. 2000, 74, 203–212. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chen, X.; Wang, G.; Fan, L.; Wang, K.; Li, X. Effect of resveratrol on the prevention of intra-abdominal adhesion formation in a rat model. Cell. Physiol. Biochem. 2016, 39, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, Z.; Zeren, S.; Kocak, F.E.; Kocak, C.; Akcılar, R.; Kargı, E.; Tiryaki, C.; Yaylak, F.; Akcılar, A. Antiadhesive and anti-inflammatory effects of pirfenidone in postoperative intra-abdominal adhesion in an experimental rat model. J. Surg. Res. 2016, 201, 348–355. [Google Scholar] [CrossRef]
- Imai, A.; Takagi, H.; Matsunami, K.; Suzuki, N. Non-barrier agents for postoperative adhesion prevention: Clinical and preclinical aspects. Arch. Gynecol. Obstet. 2010, 282, 269–275. [Google Scholar] [CrossRef]
- Atta, H.M. Prevention of peritoneal adhesions: A promising role for gene therapy. World J. Gastroenterol. 2011, 17, 5049. [Google Scholar] [CrossRef]
- Reid, R.L.; Hahn, P.M.; Spence JE, H.; Tulandi, T.; Yuzpe, A.A.; Wiseman, D.M. A randomized clinical trial of oxidized regenerated cellulose adhesion barrier (Interceed, TC7) alone or in combination with heparin. Fertil. Steril. 1997, 67, 23–29. [Google Scholar] [CrossRef]
- Corrales, F.; Corrales, M.; Schirmer, C.C. Preventing intraperitoneal adhesions with vitamin E and sodium hyaluronate/carboxymethylcellulose: A comparative study in rats. Acta Cir. Bras. 2008, 23, 36–41. [Google Scholar] [CrossRef]
- Ward, B.C.; Panitch, A. Abdominal adhesions: Current and novel therapies. J. Surg. Res. 2011, 165, 91–111. [Google Scholar] [CrossRef]
- Pugliese, E.; Coentro, J.Q.; Zeugolis, D.I. Advancements and Challenges in Multidomain Multicargo Delivery Vehicles. Adv. Mater. 2018, 30, 1704324. [Google Scholar] [CrossRef]
- Coentro, J.Q.; Pugliese, E.; Hanley, G.; Raghunath, M.; Zeugolis, D.I. Current and upcoming therapies to modulate skin scarring and fibrosis. Adv. Drug Deliv. Rev. 2019, 146, 37–59. [Google Scholar] [CrossRef]
- Cheung, J.P.; Tsang, H.H.; Cheung, J.J.; Yu, H.H.; Leung, G.K.; Law, W.L. Adjuvant therapy for the reduction of postoperative intra-abdominal adhesion formation. Asian J. Surg. 2009, 32, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Klicova, M.; Rosendorf, J.; Erben, J.; Horakova, J. Antiadhesive nanofibrous materials for medicine: Preventing undesirable tissue adhesions. ACS Omega 2023, 8, 20152–20162. [Google Scholar] [CrossRef] [PubMed]
- ten Broek, R.P.; Bakkum, E.A.; Laarhoven, C.J.; van Goor, H. Epidemiology and prevention of postsurgical adhesions revisited. Ann. Surg. 2016, 263, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.-H.; Srinivasan, D.K. Nanoparticle-based Therapeutic Approach for diabetic wound healing. Nanomaterials 2020, 10, 1234. [Google Scholar] [CrossRef]
- Diamond, M.P.; The Seprafilm Adhesion Study Group. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (Hal-F): A blinded, prospective, randomized, Multicenter Clinical Study. Fertil. Steril. 1996, 66, 904–910. [Google Scholar] [CrossRef]
- Sekiba, K. Use of Interceed(TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. The Obstetrics and Gynecology Adhesion Prevention Committee. Obstet. Gynecol. 1992, 79, 518–522. [Google Scholar]
- Brown, C.B.; Luciano, A.A.; Martin, D.; Peers, E.; Scrimgeour, A.; diZerega, G.S.; Adept Adhesion Reduction Study Group. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: A double-blind, randomized, controlled study. Fertil. Steril. 2007, 88, 1413–1426. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Horbacka, K.; Karoń, J.; Malinger, S.; Antos, F.; Rudzki, S.; Kala, Z.; Stojcev, Z.; Kössi, J.; Krokowicz, P. Preliminary study with SprayShieldTM adhesion barrier system in the prevention of abdominal adhesions. Videosurg. Other Miniinvasive Tech. 2013, 8, 301–309. [Google Scholar] [CrossRef]
- Mais, V.; Bracco, G.L.; Litta, P.; Gargiulo, T.; Melis, G.B. Reduction of postoperative adhesions with an auto-crosslinked hyaluronan gel in gynaecological laparoscopic surgery: A blinded, controlled, randomized, multicentre study. Human Reprod. 2006, 21, 1248–1254. [Google Scholar] [CrossRef]
- Ruiz-Esparza, G.U.; Wang, X.; Zhang, X.; Jimenez-Vazquez, S.; Diaz-Gomez, L.; Lavoie, A.-M.; Afewerki, S.; Fuentes-Baldemar, A.A.; Parra-Saldivar, R.; Jiang, N.; et al. Nanoengineered shear-thinning hydrogel barrier for preventing postoperative abdominal adhesions. Nano-Micro Lett. 2021, 13, 212. [Google Scholar] [CrossRef]
- Liu, H.-J.; Wu, C.-T.; Duan, H.-F.; Wu, B.; Lu, Z.-Z.; Wang, L. Adenoviral-mediated gene expression of hepatocyte growth factor prevents postoperative peritoneal adhesion in a rat model. Surgery 2006, 140, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Gorgani, S.; Nazarnezhad, S.; Wang, A.Z. Biocompatible nanocomposites for postoperative adhesion: A state-of-the-art review. Nanomaterials 2023, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, Z.; Liu, R.; Zhou, C.; Li, E.; Shen, T.; Wang, X.; Wu, Y.; Li, X. A combination of hybrid polydopamine-human keratinocyte growth factor nanoparticles and sodium hyaluronate for the efficient prevention of postoperative abdominal adhesion formation. Acta Biomater. 2022, 138, 155–167. [Google Scholar] [CrossRef]
- Lopes, J.B.; Dallan, L.A.; Campana-Filho, S.P.; Lisboa, L.A.; Gutierrez, P.S.; Moreira, L.F.P.; Oliveira, S.A.; Stolf, N.A. Keratinocyte growth factor: A new mesothelial targeted therapy to reduce postoperative pericardial adhesions. Eur. J. Cardio-Thorac. Surg. 2009, 35, 313–318. [Google Scholar] [CrossRef]
- Mi, Y.; Yang, F.; Bloomquist, C.; Xia, Y.; Sun, B.; Qi, Y.; Wagner, K.; Montgomery, S.A.; Zhang, T.; Wang, A.Z. Biologically targeted photo-crosslinkable nanopatch to prevent postsurgical peritoneal adhesion. Adv. Sci. 2019, 6, 1900809. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Wu, S.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(vinyl alcohol) hydrogels with broad-range tunable mechanical properties via the Hofmeister effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Chen, H.; Ling, Y.; Xi, Z.; Lv, M.; Chen, J. PH-responsive nanocomposite hydrogel for simultaneous prevention of postoperative adhesion and tumor recurrence. Acta Biomater. 2023, 158, 228–238. [Google Scholar] [CrossRef]
- Wang Yi Xu, Y.; Zhai, W.; Zhang, Z.; Liu, Y.; Cheng, S.; Zhang, H. In-situ growth of robust superlubricated nano-skin on electrospun nanofibers for post-operative adhesion prevention. Nat. Commun. 2022, 13, 5056. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H.; Hong, M.E.; Shin, H.Y.; Baek, C.W.; Jung, Y.H.; Lee, Y.; Kim, J.W.; Park, I.K.; Cho, W.J. Effects of a lidocaine-loaded poloxamer/alginate/CACL2 mixture on postoperative pain and adhesion in a rat model of incisional pain. Anesth. Analg. 2017, 125, 320–327. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Park, Y.; Kang, H.; Lee, D. Development of a lidocaine-loaded alginate/CMC/PEO electrospun nanofiber film and application as an anti-adhesion barrier. Polymers 2020, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Hashemi, S.M.; Seyedjafari, E.; Shabani, I.; Mohammadi-Sangcheshmeh, A.; Farhadian, S.; Soleimani, M. Function of poly (lactic-co-glycolic acid) nanofiber in reduction of adhesion bands. J. Surg. Res. 2012, 172, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Vargel Korkusuz, P.; Menceloğlu, Y.Z.; Pişkin, E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 81B, 530–543. [Google Scholar] [CrossRef]
- Babadi, D.; Rabbani, S.; Akhlaghi, S.; Haeri, A. Curcumin polymeric membranes for postoperative peritoneal adhesion: Comparison of nanofiber vs. film and phospholipid-enriched vs. non-enriched formulations. Int. J. Pharm. 2022, 614, 121434. [Google Scholar] [CrossRef]
- Shen, X.; Xu, Q.; Xu, S.; Li, J.; Zhang, N.; Zhang, L. Preparation and transdermal diffusion evaluation of the prazosin hydrochloride-loaded electrospun poly(vinyl alcohol) Fiber Mats. J. Nanosci. Nanotechnol. 2014, 14, 5258–5265. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Scarpa, M.; Abdelhakim, H.E.; Bukhary, H.A.; Craig, D.Q.; Barker, S.A.; Orlu, M. A potential alternative orodispersible formulation to prednisolone sodium phosphate orally disintegrating tablets. Pharmaceutics 2021, 13, 120. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, R.; Gong, M.; Zhang jingshuang Li, W.; Song, Q.; Wu, C.; Tian, W. Icariin-loaded electrospun PCL/gelatin sub-microfiber mat for preventing epidural adhesions after laminectomy. Int. J. Nanomed. 2018, 13, 4831–4844. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S.; et al. Phytosomes loaded with mitomycin C–soybean phosphatidylcholine complex developed for Drug Delivery. Mol. Pharm. 2012, 10, 90–101. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly(ε-caprolactone)/gum tragacanth nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Gül, M.; Yuksel, H.K.; Alabalik, U.; Ülger, B.V.; Uslukaya, O.; Avci, Y. Effect of intraperitoneal curcumin instillation on postoperative peritoneal adhesions. Med. Princ. Pract. 2014, 24, 153–158. [Google Scholar] [CrossRef]
- Reijnen, M.M.; Bleichrodt, R.P.; van Goor, H. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br. J. Surg. 2003, 90, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, W.; Yan, H.; Fan, C. Prevention of intra-abdominal adhesion by Bi-Layer Electrospun Membrane. Int. J. Mol. Sci. 2013, 14, 11861–11870. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Yang, W.J.; Lee, J.H.; Oh, J.-W.; Kim, T.W.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. PLGA nanofiber membranes loaded with epigallocatechin-3-o-gallate are beneficial to prevention of postsurgical adhesions. Int. J. Nanomed. 2014, 9, 4067–4078. [Google Scholar] [CrossRef]
- Li Jian Zhu, J.; He, T.; Li, W.; Zhao, Y.; Chen, Z.; Zhang, J.; Wan, H.; Li, R. Prevention of intra-abdominal adhesion using electrospun PEG/PLGA nanofibrous membranes. Mater. Sci. Eng. C 2017, 78, 988–997. [Google Scholar] [CrossRef]
- Gholami, A.; Abdoluosefi, H.E.; Riazimontazer, E.; Azarpira, N.; Behnam, M.; Emami, F.; Omidifar, N. Prevention of postsurgical abdominal adhesion using electrospun TPU nanofibers in rat model. BioMed Res. Int. 2021, 2021, 9977142. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Zarzycki, A.N.; Jeon, K.; Dundas, J.A.; Vasanthan, V.; Deniset, J.F.; Fedak, P.W. Prevention of post-operative adhesions: A comprehensive review of present and emerging strategies. Biomolecules 2021, 11, 1027. [Google Scholar] [CrossRef]
- Tyner, K.M.; Zou, P.; Yang, X.; Zhang, H.; Cruz, C.N.; Lee, S.L. Product quality for nanomaterials: Current U.S. experience and perspective. WIREs Nanomed. Nanobiotechnol. 2015, 7, 640–654. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical trials legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez de la Ossa, D.H.; Pita, R.; Vidal, J.-M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and Nanosimilars: EU Regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef] [PubMed]
- D’Avenio, G.; Daniele, C.; Grigioni, M. Nanostructured Medical Devices: Regulatory Perspective and current applications. Materials 2024, 17, 1787. [Google Scholar] [CrossRef]
- Rocco, P.; Musazzi, U.M.; Minghetti, P. Medicinal products meet medical devices: Classification and nomenclature issues arising from their combined use. Drug Discov. Today 2022, 27, 103324. [Google Scholar] [CrossRef]
- Asmatulu, R. Toxicity of nanomaterials and recent developments in lung disease. In Bronchitis; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Schulte, P.A.; Salamanca-Buentello, F. Ethical and scientific issues of nanotechnology in the workplace. Ciência Saúde Coletiva 2006, 12, 1319–1332. [Google Scholar] [CrossRef]
- Asmatulu, R.; Zhang, B.; Asmatulu, E. Chapter 3—Safety and Ethics of Nanotechnology. In Nanotechnology Safety, 1st ed.; Asmatulu, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 31–41. [Google Scholar] [CrossRef]
- Abaszadeh, F.; Ashoub, M.H.; Khajouie, G.; Amiri, M. Nanotechnology development in surgical applications: Recent trends and developments. Eur. J. Med. Res. 2023, 28, 537. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Carbone, S.; Bosi, S.; Gatti, A.; Dinelli, G. Engineered nanoparticles effects in soil-plant system: Basil (Ocimum Basilicum L.) study case. Appl. Soil Ecol. 2018, 123, 551–560. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Mucha, A.P.; Francisco, T.; Gomes, C.R.; Almeida, C.M. Silver nanoparticles uptake by salt marsh plants–implications for phytoremediation processes and effects in Microbial Community Dynamics. Mar. Pollut. Bull. 2017, 119, 176–183. [Google Scholar] [CrossRef]
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.-B.; Hochepied, J.-F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the physicochemical features of TiO2 nanoparticles on their in vitro toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yan, R.; Fu, Z.; Wu, T.; Ren, C. Impact of physicochemical properties on biological effects of lipid nanoparticles: Are they completely safe. Sci. Total Environ. 2024, 927, 172240. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Claudel, M.; Ronzani, C.; Arezki, Y.; Lebeau, L.; Pons, F. Physicochemical characteristics that affect carbon dot safety: Lessons from a comprehensive study on a Nanoparticle Library. Int. J. Pharm. 2019, 569, 118521. [Google Scholar] [CrossRef] [PubMed]
- Breznan, D.; Das, D.D.; MacKinnon-Roy, C.; Bernatchez, S.; Sayari, A.; Hill, M.; Vincent, R.; Kumarathasan, P. Physicochemical properties can be key determinants of mesoporous silica nanoparticle potency in vitro. ACS Nano 2018, 12, 12062–12079. [Google Scholar] [CrossRef]
- Wasti, S.; Lee, I.H.; Kim, S.; Lee, J.-H.; Kim, H. Ethical and legal challenges in Nanomedical Innovations: A scoping review. Front. Genet. 2023, 14, 1163392. [Google Scholar] [CrossRef]
- Bragazzi, N.L. Nanomedicine: Insights from a bibliometrics-based analysis of Emerging Publishing and Research trends. Medicina 2019, 55, 785. [Google Scholar] [CrossRef]
- Atalla, K.; Chaudhary, A.; Eshaghian-Wilner, M.M.; Gupta, A.; Mehta, R.; Nayak, A.; Prajogi, A.; Ravicz, K.; Shiroma, B.; Trivedi, P. Chapter 20—Ethical, privacy, and intellectual property issues in nanomedicine. In Wireless Computing in Medicine: From Nano to Cloud with Ethical and Legal Implications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 567–600. [Google Scholar] [CrossRef]
- Resnik, D.B.; Tinkle, S.S. Ethics in Nanomedicine. Nanomedicine 2007, 2, 345–350. [Google Scholar] [CrossRef]
- Weissig, V.; Guzman-Villanueva, D. Nanopharmaceuticals (part 2): Products in the pipeline. Int. J. Nanomed. 2015, 10, 1245–1257. [Google Scholar] [CrossRef]
- Savin, G.; Sastourne-Array, O.; Caillol, S.; Bethry, A.; Assor, M.; David, G.; Nottelet, B. Evaluation of porous (poly(lactide-co-glycolide)-co-(ε-caprolactone)) polyurethane for use in orthopedic scaffolds. Molecules 2024, 29, 766. [Google Scholar] [CrossRef]
| Product | Type of Product | Trial Design Type | Advantages/Disadvantages | Ref. No. |
|---|---|---|---|---|
| Seprafilm® | Solid, hyaluronate carboxycellulose | Randomised clinical trial which involved 127 patients undergoing uterine myomectomy. | The incidence was significantly reduced in treated patients. | [36] |
| Interceed® | Solid, oxidised cellulose | Randomised multicentre clinical study which involved 63 patients undergoing bilateral pelvic sidewall adhesiolysis. | Reduced the formation of adhesions from 76% to 41%. | [37] |
| Adept® | Liquid, 4% icodextrin | Randomised double blind clinical study which involved 402 patients undergoing laparoscopic gynaecological surgery. | Safe to use and reduces adhesions. | [38] |
| SprayShield™ | Liquid, poly(ethylene glycol) | Randomised prospective multicentre single blind study which involved 11 patients diagnosed with ulcerative colitis or familial adenomatous polyposis. | Reduced incidence and severity of adhesion formation. | [39] |
| Hyalobarrier® | Gel, auto crosslinked hyaluronan gel | Randomised controlled multicentre blinded clinical study consisting of 52 patients undergoing laparoscopic gynaecological surgery. | Safe to use and showed anti-adhesive properties. | [40] |
| Nanotherapeutic | Nanoparticle Diameter (nm) | Nanofiber Diameter (nm) | Morphology | Pore Size (µm) | Water Contact Angle (°) | Drug Release | Ref. No. | |
|---|---|---|---|---|---|---|---|---|
| Nanocomposite | PDA-KGF NP with HA | 200 | - | Globular | - | - | Gradual KGF release. | [44] |
| pCNP NP-A | 166.1 ± 1.8 | - | - | - | - | - | [46] | |
| pCNP NP-B | 175.1 ± 28.7 | - | - | - | - | - | ||
| Hydrogel | Col-APG-Cys@HHD | 100 | - | Spherical | - | - | - | [49] |
| NAP-CS | 33 | - | - | - | - | 32% released in 24 h, reaching 68% over 6 days. | [4] | |
| SNP-PEO | 25 | - | Disc | - | - | - | [41] | |
| Nanofiber | SLNS | - | 360 | - | - | 0 | - | [50] |
| LID-loaded SA/CMC/ PEO film | - | - | - | - | - | Films with 1% CaCl2: 70% release in 1 h (higher than 3% and 5%).Films with 5% CaCl2: expected to ensure longer stable release. | [52] | |
| PCL | - | 549 ± 236 | - | 1.8 ± 0.8 | 132 ± 7 | - | [53] | |
| PES | - | 621 ± 118 | 1.5 ± 0.4 | 125 ± 6 | ||||
| PLGA | - | 608 ± 170 | 1.8 ± 0.7 | 138 ± 8 | ||||
| PLLA | - | 589 ± 200 | 2.0 ± 0.9 | 135 ± 6 | ||||
| PCL with ornidazole | - | 25,000 | - | - | - | 80% were released within 3 h, with complete release in 18 h. The rapid initial release likely caused a burst effect. | [54] | |
| CUR-PCL film | - | - | Rough surface | - | 78.0 ± 0.8 | 9% were released within 24 h, reaching 51% by day 30. | [55] | |
| CUR-PCL nanofiber | - | 830 ± 22 | No beads | - | 125.1 ± 1.6 | 13% were released within 24 h, reaching 45–50% by day 30. | ||
| Bi-layer nanofiber with inner PCL loaded with HA | - | - | Smooth surface with no beads | - | - | Enhanced controlled release. | [63] | |
| PLGA-EGCG | - | 300–500 | - | - | - | Minor burst effect of less than 10% on the first day, followed by sustained release after 7 days. | [64] | |
| PLGA-PEG | - | 810 | Smooth surface | - | 86 ± 1.5 | - | [65] | |
| PU | - | 360 | Smooth surface with beads | - | - | - | [66] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, Z.Y.; Senthilkumar, S.D.; Srinivasan, D.K. Nanotechnology-Based Therapies for Preventing Post-Surgical Adhesions. Pharmaceutics 2025, 17, 389. https://doi.org/10.3390/pharmaceutics17030389
Teo ZY, Senthilkumar SD, Srinivasan DK. Nanotechnology-Based Therapies for Preventing Post-Surgical Adhesions. Pharmaceutics. 2025; 17(3):389. https://doi.org/10.3390/pharmaceutics17030389
Chicago/Turabian StyleTeo, Zi Yi, Samyuktha Dhanalakshmi Senthilkumar, and Dinesh Kumar Srinivasan. 2025. "Nanotechnology-Based Therapies for Preventing Post-Surgical Adhesions" Pharmaceutics 17, no. 3: 389. https://doi.org/10.3390/pharmaceutics17030389
APA StyleTeo, Z. Y., Senthilkumar, S. D., & Srinivasan, D. K. (2025). Nanotechnology-Based Therapies for Preventing Post-Surgical Adhesions. Pharmaceutics, 17(3), 389. https://doi.org/10.3390/pharmaceutics17030389








