Amikacin Dosing Adjustment in Critically Ill Oncologic Patients: A Study with Real-World Patients, PBPK Analysis, and Digital Twins
Abstract
1. Introduction
2. Methods
2.1. Clinical Protocol, Ethical Aspects, and Sample Collection
2.2. Physiologically Based Pharmacokinetic Model Development
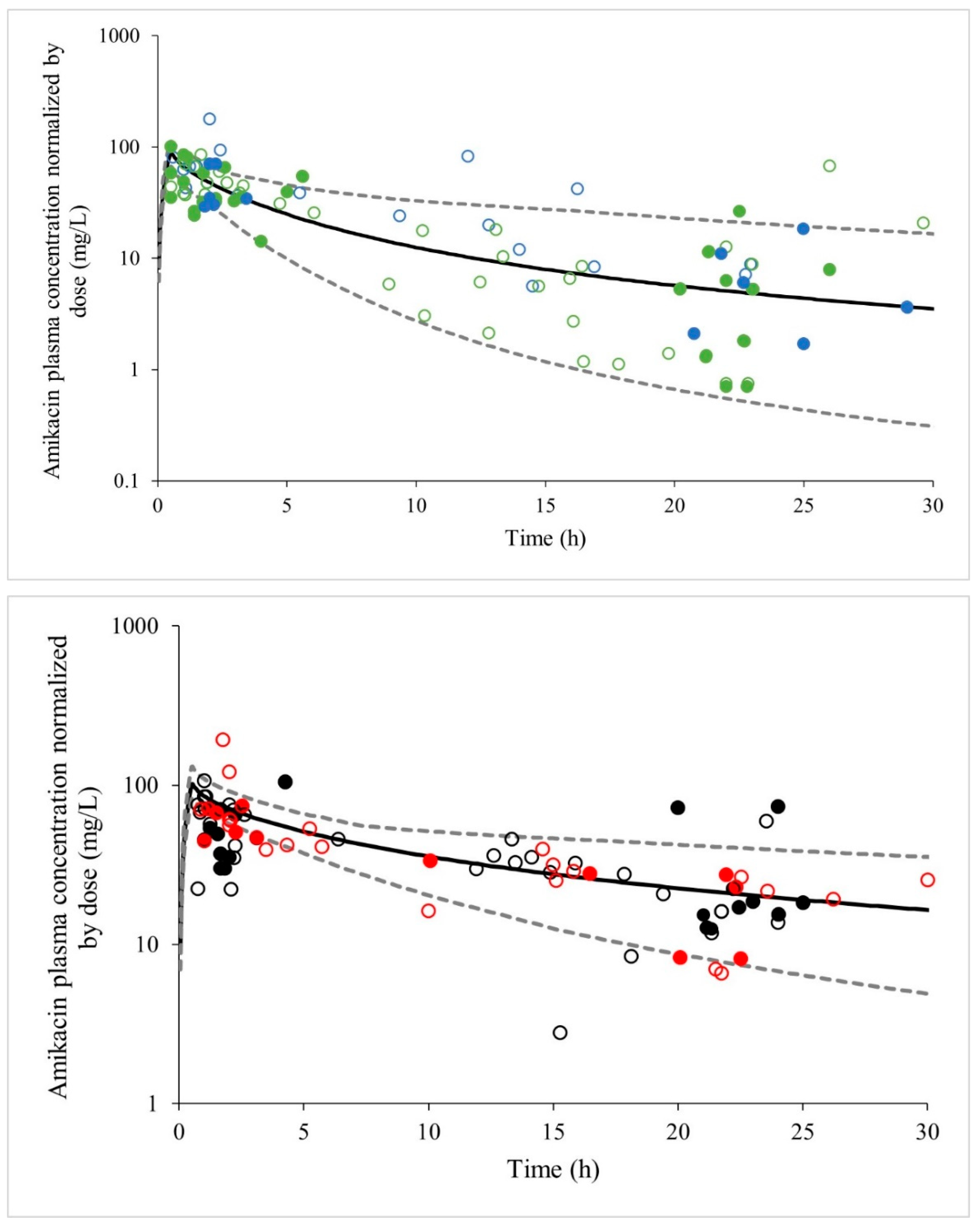
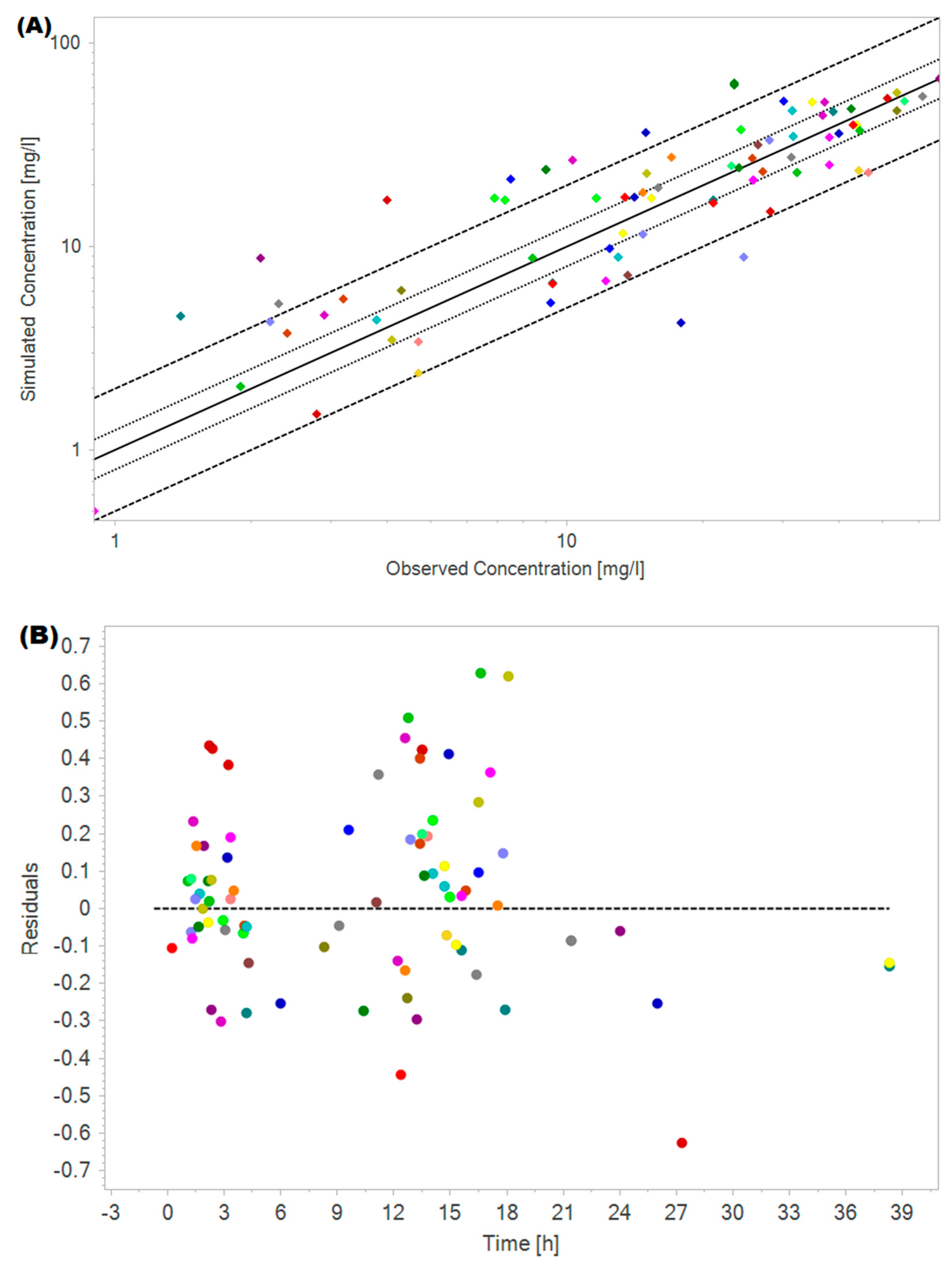
2.3. Amikacin PBPK Model Validation
2.4. Renal Impairment Population Model
2.5. Oncologic Population with Renal Impairment Model
Optimizing Amikacin Doses in Cancer Patients
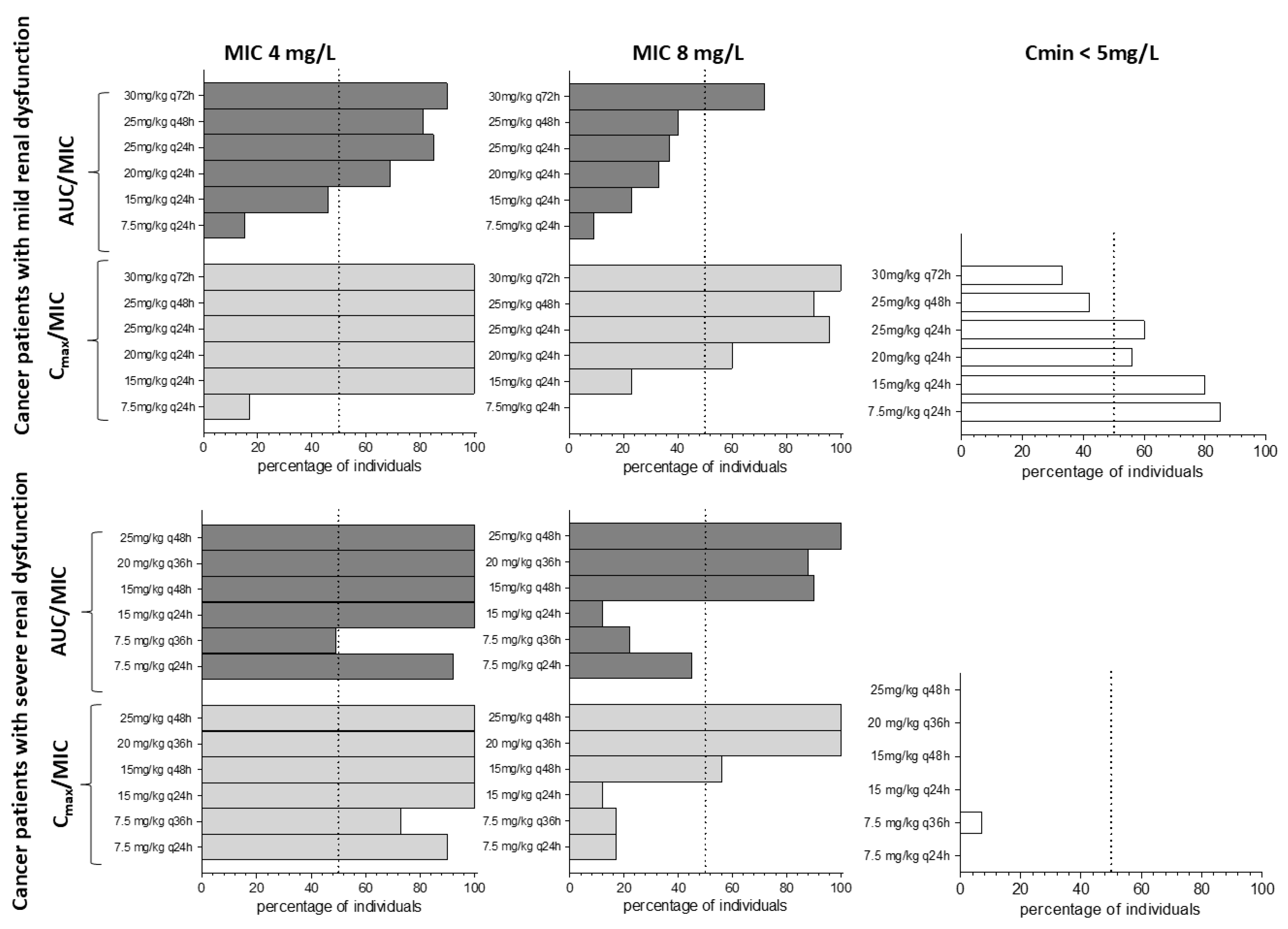
2.6. Application of the PBPK Model to Optimize Amikacin Doses Using a Digital Twin Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramirez, M.S.; Tolmasky, M.E. Amikacin: Uses, Resistance, and Prospects for Inhibition. Molecules 2017, 22, 2267. [Google Scholar] [CrossRef] [PubMed]
- Pyle-Eilola, A.L. Chapter 8—Guidelines for monitoring vancomycin, aminoglycosides, and other antibiotics. In Therapeutic Drug Monitoring, 2nd ed.; Dasgupta, A., Ed.; Academic Press: New York, NY, USA, 2024; pp. 197–215. [Google Scholar]
- Chan, K.; Ledesma, K.R.; Wang, W.; Tam, V.H. Characterization of Amikacin Drug Exposure and Nephrotoxicity in an Animal Model. Antimicrob. Agents Chemother. 2020, 64, e00859-20. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Agence française de sécurité sanitaire des produits de santé. Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med. Mal. Infect. 2012, 42, 301–308. [Google Scholar]
- Garraffo, R.; Drugeon, H.B.; Dellamonica, P.; Bernard, E.; Lapalus, P. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob. Agents Chemother. 1990, 34, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Guilhaumou, R.; Riff, C.; Blin, O. Amikacin in critically Ill patients: A review of population pharmacokinetic studies. Clin. Pharmacokinet. 2017, 56, 127–138. [Google Scholar] [CrossRef]
- Telles, J.P.; Diegues, M.S.; Migotto, K.C.; de Souza Borges, O.; Reghini, R.; Gavazza, B.V.; Pinto, L.; Caruso, P.; e Silva, I.L.F.; Schmidt, S.; et al. Failure to predict amikacin elimination in critically ill patients with cancer based on the estimated glomerular filtration rate: Applying PBPK approach in a therapeutic drug monitoring study. Eur. J. Clin. Pharmacol. 2023, 79, 1003–1012. [Google Scholar] [CrossRef]
- Pérez-Blanco, J.S.; Sáez-Fernández, E.M.; Calvo, M.V.; Lanao, J.M.; Martín-Suárez, A. Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin. Pharmaceutics 2021, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints and Dosing of Antibiotics. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf (accessed on 5 September 2024).
- Gilbert, D.N.; Chambers, D.N.; Eliopoulos, G.M.; Saag, M.S.; Pavia, A.T.; Black, D.; Freedman, D.O.; Kim, K.; Schwartz, B.S. The Sanford Guide to Antimicrobial Therapy 2019: 50 Years: 1969–2019; Antimicrobial Therapy: Madrid, Spain, 2019; 290p. [Google Scholar]
- Medication Safety Queensland. Aminoglycoside Dosing in Adults. Department of Health. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0019/713323/aminoglycoside-guidelines.pdf (accessed on 5 September 2024).
- Mensa, J.; Soriano, A.; García-Sánchez, J.E.; Letang, E.; López-Suñé, E.; Marco, F.; Llinares, E.; Barberán, J. Therapeutic Guide Mensa 2020; Antares: Barcelona, Spain, 2020; ISBN 978-84-88825-29-2. [Google Scholar]
- Taylor, Z.L.; Poweleit, E.A.; Paice, K.; Somers, K.M.; Pavia, K.; Vinks, A.A.; Punt, N.; Mizuno, T.; Girdwood, S.T. Tutorial on model selection and validation of model input into precision dosing software for model-informed precision dosing. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 1827–1845. [Google Scholar] [CrossRef]
- Polasek, T.M.; Rostami-Hodjegan, A. Virtual Twins: Understanding the Data Required for Model-Informed Precision Dosing. Clin. Pharmacol. Ther. 2020, 107, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Polasek, T.M.; Bousman, C.; Rostami-Hodjegan, A.; Sheffield, L.J.; Everall, I.; Pantelis, C.; Kirkpatrick, C.M.J. Delineating gene-environment effects using virtual twins of patients treated with clozapine. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-renal-function-study-design-data-analysis-and-impact-dosing (accessed on 6 September 2024).
- Lode, H.; Grunert, K.; Koeppe, P.; Langmaack, H. Pharmacokinetic and clinical studies with amikacin, a new aminoglycoside antibiotic. J. Infect Dis. 1976, 134, S316–S322. [Google Scholar] [CrossRef] [PubMed]
- Delattre, I.K.; Musuamba, F.T.; Nyberg, J.; Taccone, F.S.; Laterre, P.F.; Verbeeck, R.K.; Jacobs, F.; Wallemacq, P.E. Population pharmacokinetic modeling and optimal sampling strategy for Bayesian estimation of amikacin exposure in critically ill septic patients. Ther. Drug Monit. 2010, 32, 749–756. [Google Scholar] [CrossRef]
- Mahmoudi, L.; Mohammadpour, A.H.; Ahmadi, A.; Niknam, R.; Mojtahedzadeh, M. Influence of sepsis on higher daily dose of amikacin pharmacokinetics in critically ill patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 285–291. [Google Scholar]
- De Winter, S.; van Hest, R.; Dreesen, E.; Annaert, P.; Wauters, J.; Meersseman, W.; Van den Eede, N.; Desmet, S.; Verelst, S.; Vanbrabant, P.; et al. Quantification and Explanation of the Variability of First-Dose Amikacin Concentrations in Critically Ill Patients Admitted to the Emergency Department: A Population Pharmacokinetic Analysis. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 653–663. [Google Scholar] [CrossRef]
- Wu, C.; Liu, H.; Yu, S.; Ren, C.; Zhang, J.; Wang, G.; Li, B.; Liu, Y. Prediction of pharmacokinetics and pharmacodynamics of trelagliptin and omarigliptin in healthy humans and in patients with renal impairment using physiologically based pharmacokinetic combined DPP-4 occupancy modeling. Biomed. Pharmacother. 2022, 153, 113509. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Chen, Y.; Chen, J.; Dixit, V.; Parrott, N.; Peters, S.A.; Poggesi, I.; Sharma, P.; Snoeys, J.; Shebley, M.; et al. Physiologically-Based Pharmacokinetic Modeling in Renal and Hepatic Impairment Populations: A Pharmaceutical Industry Perspective. Clin. Pharmacol. Ther. 2021, 110, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Goebel, U.; Hassenzahl, L.O.; Jung, C.; David, S.; Feldheiser, A.; Stopfkuchen-Evans, M.; Wollborn, J. Capillary leak and endothelial permeability in critically ill patients: A current overview. Intensive Care Med. Exp. 2023, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Martins, H.; Oliveira, J.C.; Lapa, R.; Vale, N. PBPK Modeling and simulation of antibiotics amikacin, gentamicin, tobramycin, and vancomycin used in hospital practice. Life 2021, 11, 1130. [Google Scholar] [CrossRef]
- Wu, M.; Feng, K.; Wu, X.; Liu, C.; Zhu, S.; Martins, F.S.; Yu, M.; Lv, Z.; Yan, M.; Sy, S.K.B. Prediction of tissue exposures of polymyxin-B, amikacin and sulbactam using physiologically-based pharmacokinetic modeling. Front. Microbiol. 2024, 15, 1435906. [Google Scholar] [CrossRef]
- Meraz-Munoz, A.; Langote, A.D.; Jhaveri, K.; Izzedine, H.; Gudsoorkar, P. Acute Kidney Injury in the Patient with Cancer. Diagnostics 2021, 11, 611. [Google Scholar] [CrossRef]
- Soares, M.; Salluh, J.I.; Carvalho, M.S.; Darmon, M.; Rocco, J.R.; Spector, N. Prognosis of critically ill patients with cancer and acute renal dysfunction. J. Clin. Oncol. 2006, 24, 4003–4010. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, W.; Yang, L.; Li, J.; Yi, C.; Pu, Y.; Yin, T.; Na, F.; Zhang, L.; Fu, P.; et al. Incidence and risk factors of acute kidney injury in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1173952. [Google Scholar] [CrossRef]
- Libório, A.B.; Abreu, K.L.; Silva, G.B., Jr.; Lima, R.S.; Barreto, A.G.; Barbosa, O.A.; Daher, E.F. Predicting hospital mortality in critically ill cancer patients according to acute kidney injury severity. Oncology 2011, 80, 160–166. [Google Scholar] [CrossRef]
- Wei, M.; Huang, M.; Duan, Y.; Wang, D.; Xing, X.; Quan, R.; Zhang, G.; Liu, K.; Zhu, B.; Ye, Y.; et al. Prognostic and risk factor analysis of cancer patients after unplanned ICU admission: A real-world multicenter study. Sci. Rep. 2023, 13, 22340. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Flombaum, C.D.; Moreau, D.; Ronco, C. Acute renal failure in cancer patients. Ann. Med. 2005, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Suzuki, M.; Usuki, K.; Kato, T. Amikacin Pharmacokinetics in Terminal Stage of Hematological Malignancy. Ther. Drug Monit. 2019, 41, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Aquino, M.; Tinoco, M.; Bicker, J.; Falcão, A.; Rocha, M.; Fortuna, A. Therapeutic Drug Monitoring of Amikacin in Neutropenic Oncology Patients. Antibiotics 2023, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Bertino, J.S.; Booker, L.A.; Franck, P.A.; Jenkins, P.L.; Franck, K.R.; Nafziger, A.N. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J. Infect. Dis. 1993, 167, 173–179. [Google Scholar] [CrossRef]

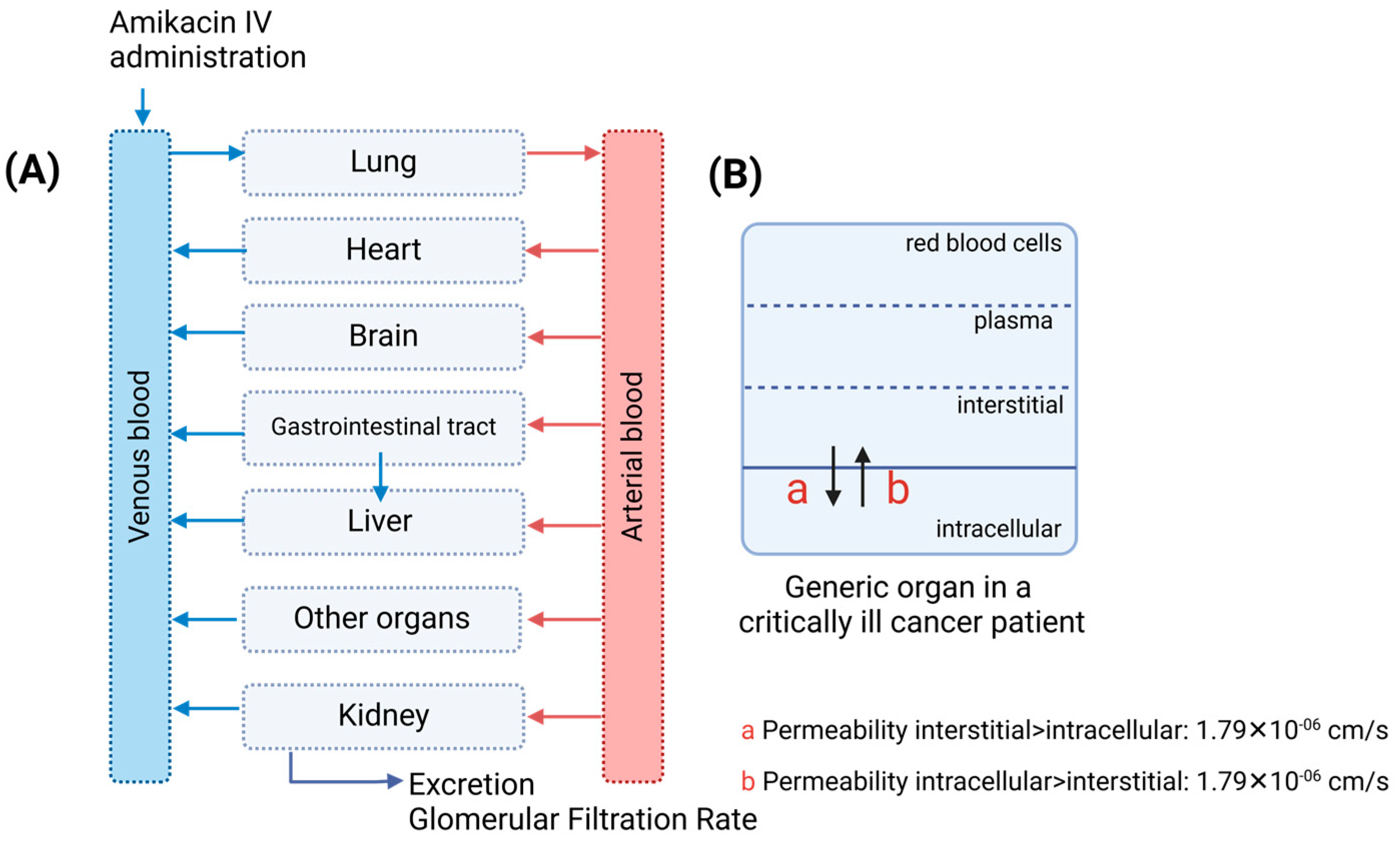
| Physiological Parameters | Digital Cancer Patients | Source | |
|---|---|---|---|
| Cancer Patients with Mild Renal Impairment | Cancer Patients with Severe Renal Impairment | ||
| Plasma protein ratio to HV | 0.99 | 0.85 | Heimbach [23]; Wu [22] |
| Hematocrit | 0.47 | 0.40 | Heimbach [23]; Wu [22] |
| eGFR (mL/min/1.73 m2) | 63.12 * | 18.35 * | Parameter Identification |
| Hepatic blood flow ratio to HV | 1.00 | 0.37 | Heimbach [23]; Wu [22] |
| Kidney volume (L) | 0.37 | 0.16 | Heimbach [23]; Wu [22] |
| Albumin ratio to HV | 1.00 | 0.85 | Heimbach [23]; Wu [22] |
| Parameter | Data Internal Validation Cohort #1 (n = 46) | Data External Validation Cohort #2 (n = 98) |
|---|---|---|
| Female ratio (%) | 37 | 40 |
| Age (years) | 65 (20–89) | 65.5 (20–89) |
| Body Weight (kg) | 75.8 (44.8–100.0) | 74.3 (41.5–132) |
| Serum creatinine | 1.36 (0.19–3.56) | 1.21 (0.19–4.82) |
| Creatinine Clearance * (mL/min) | 50.6 (13.9–302) | 57.72 (14.00–304.5) |
| CRP mg/dL (24–48 h) | N.C. | 16.13 (0.50–49.74) |
| Amikacin Dose (mg) | 1400 (300–3000) | 1225 (300–3000) |
| Cmin (mg/L) | 14.6 (0.8–39.4) | 15.5 (0.8–65.4) |
| Cmax (mg/L) | 53.5 (12–125) | 51.9 (9.9–172.5) |
| SOFA score | 5 (0–17) | 5 (0–17) |
| Arterial Hypertension (%) | 47.8 | 39.0 |
| Diabetes Melittus (%) | 26.0 | 21.0 |
| COPD (%) | 10.9 | 5.0 |
| Hearth failure (%) | 2.2 | 4.0 |
| Mechanical ventilation (%) | 57.4 | 41.0 |
| Dialysis (%) | 13 | 11.0 |
| Meropenem (%) | 100 | 79.0 |
| Vancomycin (%) | 76.1 | 65.0 |
| Polymyxin (%) | 21.7 | 20.0 |
| Furosemide (%) | 87.0 | 77.0 |
| Noradrenaline (%) | 45.7 | 43.0 |
| Patient ID | Gender | Age (Years Old) | Body Weight (kg) | Height (cm) | CLcr (mL/min) | Meropenem | Vancomycin | Polymixin | Furosemide | In-Hospital Mortality | Hypertension | Diabetes | Congestive Heart Failure | Surgery Last 15 Days | Noradrenaline | Mechanical Ventilation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer patients with Severe renal dysfunction | ||||||||||||||||
| 1 | M | 67 | 110 | 175 | 21.0 | 1 | 1 | 0 | 1 | Y | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | M | 75 | 87.5 | 182 | 21.7 | 1 | 1 | 0 | 1 | Y | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | W | 20 | 43.6 | 153 | 22.3 | 1 | 1 | 0 | 1 | Y | 0 | 1 | 0 | 1 | 0 | 0 |
| 4 | M | 78 | 67.55 | 166 | 22.5 | 0 | 0 | 0 | 0 | N | 0 | 0 | 0 | 1 | 0 | 0 |
| 5 | M | 25 | 65.65 | 172 | 23.2 | 1 | 1 | 0 | 1 | N | 0 | 0 | 0 | 1 | 0 | 0 |
| 6 | M | 76 | 68.1 | 173 | 31.5 | 1 | 0 | 0 | 1 | Y | 1 | 0 | 0 | 1 | 0 | 0 |
| 7 | W | 79 | 57 | 162 | 33.6 | 0 | 0 | 0 | 1 | Y | 1 | 0 | 0 | 1 | 0 | 0 |
| 8 | M | 71 | 66.2 | 162 | 39.9 | 1 | 1 | 0 | 1 | Y | 1 | 0 | 0 | 0 | 1 | 1 |
| 9 | W | 69 | 81 | 168 | 39.9 | 1 | 1 | 0 | 0 | Y | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | W | 74 | 103 | 160 | 42.9 | 0 | 1 | 0 | 1 | Y | 0 | 0 | 0 | 1 | 1 | 0 |
| 11 | M | 25 | 72.5 | 172 | 43.7 | 1 | 1 | 0 | 1 | Y | 0 | 0 | 0 | 1 | 1 | 1 |
| 12 | W | 66 | 77.6 | 154 | 45.5 | 1 | 1 | 1 | 1 | Y | 1 | 0 | 0 | 1 | 0 | 0 |
| 13 | W | 84 | 57.5 | 167 | 52.1 | 1 | 1 | 0 | 1 | Y | 1 | 0 | 0 | 0 | 0 | 0 |
| 14 | M | 66 | 98 | 169 | 54.4 | 1 | 0 | 0 | 1 | Y | 1 | 0 | 0 | 0 | 1 | 0 |
| 15 | W | 57 | 96.8 | 158 | 54.8 | 1 | 1 | 0 | 1 | N | 1 | 1 | 0 | 1 | 0 | 0 |
| 16 | W | 69 | 48.3 | 160 | 56.2 | 1 | 1 | 0 | 1 | N | 0 | 0 | 0 | 1 | 1 | 0 |
| 17 | W | 68 | 97.5 | 165 | 56.4 | 1 | 1 | 0 | 1 | Y | 0 | 0 | 0 | 0 | 1 | 0 |
| 18 | W | 54 | 58.5 | 163 | 57.1 | 0 | 0 | 0 | 1 | Y | 0 | 0 | 0 | 0 | 0 | 1 |
| 19 | M | 68 | 63.55 | 170 | 58.3 | 1 | 1 | 0 | 1 | N | 1 | 1 | 0 | 0 | 0 | 1 |
| 20 | W | 44 | 92 | 167 | 58.9 | 1 | 1 | 1 | 1 | Y | 0 | 0 | 0 | 1 | 0 | 1 |
| Cancer patients with Mild renal dysfunction | ||||||||||||||||
| 21 | W | 68 | 43.95 | 169 | 63.3 | 0 | 0 | 0 | 0 | N | 0 | 0 | 0 | 0 | 0 | 0 |
| 22 | W | 68 | 45.65 | 169 | 68.1 | 0 | 0 | 0 | 1 | N | 0 | 0 | 0 | 0 | 0 | 0 |
| 23 | W | 73 | 78.1 | 156 | 71.8 | 0 | 1 | 0 | 1 | Y | 1 | 1 | 0 | 1 | 1 | 1 |
| 24 | M | 60 | 65.6 | 150 | 80.1 | 1 | 1 | 0 | 0 | N | 0 | 0 | 0 | 1 | 0 | 0 |
| 25 | M | 80 | 68.7 | 178 | 83.0 | 1 | 0 | 0 | 0 | Y | 0 | 0 | 0 | 1 | 1 | 1 |
| 26 | M | 77 | 51 | 153 | 85.8 | 0 | 0 | 0 | 1 | N | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 | W | 81 | 69 | 149 | 90.7 | 1 | 0 | 0 | 1 | Y | 0 | 0 | 0 | 1 | 0 | 0 |
| 28 | W | 55 | 71.9 | 159 | 91.3 | 1 | 1 | 0 | 0 | N | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | W | 53 | 50.1 | 150 | 105.0 | 0 | 0 | 0 | 0 | N | 0 | 0 | 0 | 1 | 0 | 0 |
| 30 | M | 54 | 100.5 | 185 | 105.3 | 1 | 0 | 0 | 1 | Y | 1 | 0 | 0 | 1 | 0 | 0 |
| 31 | M | 64 | 78 | 169 | 111.3 | 1 | 1 | 0 | 0 | Y | 0 | 0 | 0 | 1 | 1 | 1 |
| 32 | M | 65 | 51.5 | 178 | 111.8 | 1 | 0 | 0 | 1 | N | 0 | 0 | 0 | 0 | 1 | 0 |
| 33 | M | 56 | 83 | 178 | 112.6 | 1 | 1 | 0 | 1 | N | 0 | 0 | 0 | 0 | 0 | 0 |
| 34 | W | 51 | 53.5 | 158 | 127.8 | 1 | 0 | 0 | 1 | Y | 0 | 0 | 0 | 1 | 0 | 0 |
| 35 | M | 29 | 106.5 | 182 | 129.3 | 0 | 0 | 0 | 0 | N | 1 | 0 | 0 | 1 | 0 | 0 |
| 36 | M | 59 | 114 | 180 | 135.0 | 1 | 1 | 0 | 1 | N | 1 | 0 | 0 | 1 | 0 | 0 |
| 37 | M | 26 | 56.5 | 179 | 149.1 | 1 | 1 | 1 | 0 | Y | 0 | 0 | 0 | 0 | 0 | 0 |
| 38 | W | 55 | 61.4 | 160 | 157.6 | 1 | 1 | 0 | 1 | N | 0 | 0 | 0 | 0 | 0 | 1 |
| 39 | M | 51 | 66.5 | 173 | 228.3 | 0 | 0 | 0 | 0 | N | 0 | 0 | 0 | 1 | 0 | 0 |
| 40 | M | 72 | 115 | 122 | 236.1 | 1 | 0 | 0 | 1 | Y | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.Q.d.; Moraes, N.V.d.; Estrela, R.; Coelho, D., Jr.; Feriani, D.; Migotto, K.; Caruso, P.; Silva, I.L.F.e.; Oliveira, D.d.A.; Telles, J.P.; et al. Amikacin Dosing Adjustment in Critically Ill Oncologic Patients: A Study with Real-World Patients, PBPK Analysis, and Digital Twins. Pharmaceutics 2025, 17, 297. https://doi.org/10.3390/pharmaceutics17030297
Silva JQd, Moraes NVd, Estrela R, Coelho D Jr., Feriani D, Migotto K, Caruso P, Silva ILFe, Oliveira DdA, Telles JP, et al. Amikacin Dosing Adjustment in Critically Ill Oncologic Patients: A Study with Real-World Patients, PBPK Analysis, and Digital Twins. Pharmaceutics. 2025; 17(3):297. https://doi.org/10.3390/pharmaceutics17030297
Chicago/Turabian StyleSilva, Juliana Queiroz da, Natália Valadares de Moraes, Rita Estrela, Diogenes Coelho, Jr., Diego Feriani, Karen Migotto, Pedro Caruso, Ivan Leonardo França e Silva, Daiane de Araujo Oliveira, João Paulo Telles, and et al. 2025. "Amikacin Dosing Adjustment in Critically Ill Oncologic Patients: A Study with Real-World Patients, PBPK Analysis, and Digital Twins" Pharmaceutics 17, no. 3: 297. https://doi.org/10.3390/pharmaceutics17030297
APA StyleSilva, J. Q. d., Moraes, N. V. d., Estrela, R., Coelho, D., Jr., Feriani, D., Migotto, K., Caruso, P., Silva, I. L. F. e., Oliveira, D. d. A., Telles, J. P., & Moreira, F. d. L. (2025). Amikacin Dosing Adjustment in Critically Ill Oncologic Patients: A Study with Real-World Patients, PBPK Analysis, and Digital Twins. Pharmaceutics, 17(3), 297. https://doi.org/10.3390/pharmaceutics17030297








