Development, Characterization, and Evaluation of Chi-Tn mAb-Functionalized DOTAP-PLGA Hybrid Nanoparticles Loaded with Docetaxel for Lung Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells Lines and Animals
2.3. Preparation of LPHNps
2.4. Functionalization of LPHNp with Chi-Tn mAb
2.5. Nanoparticle Characterization
2.5.1. Particle Size and Zeta Potential
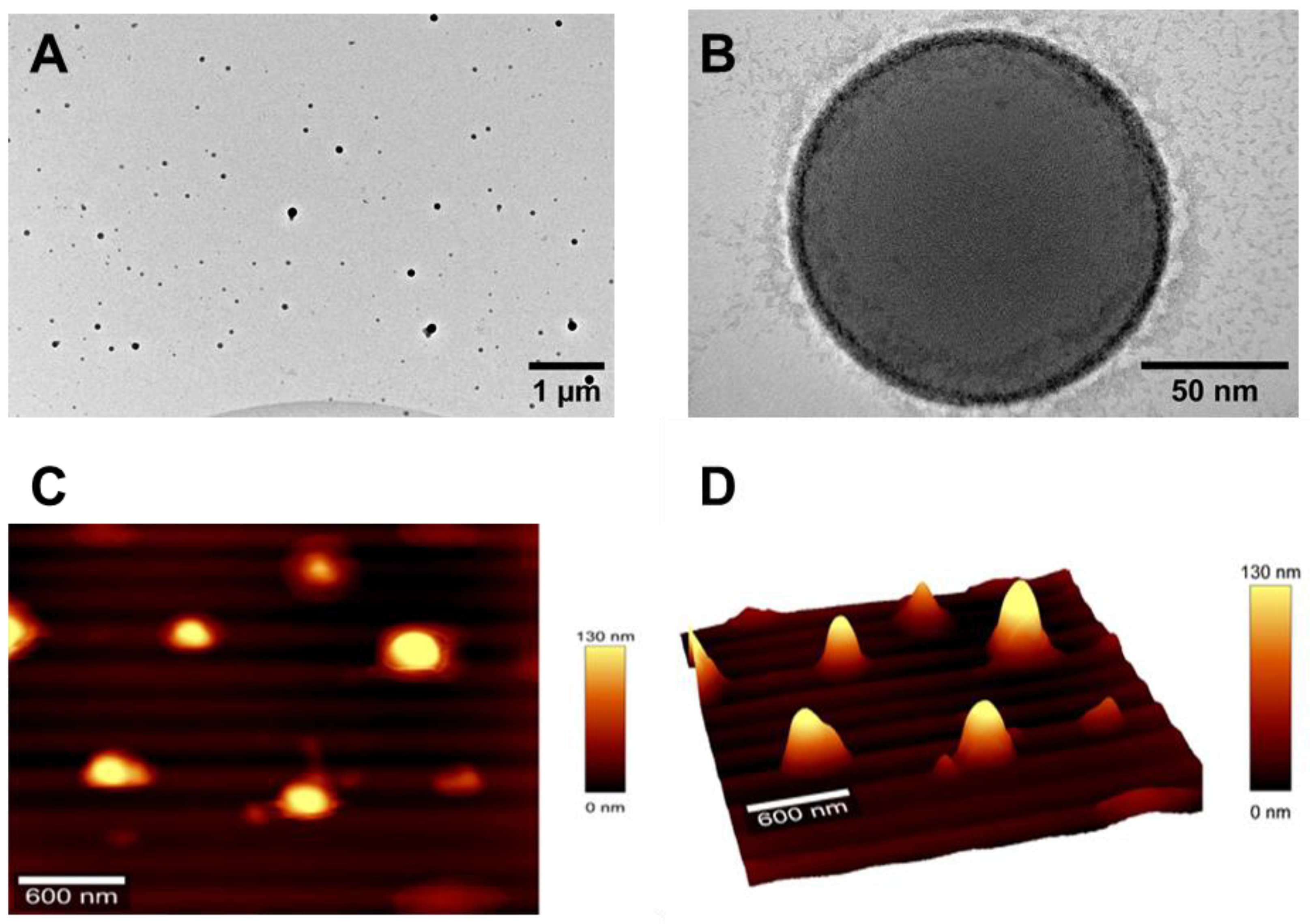
2.5.2. Surface Morphology
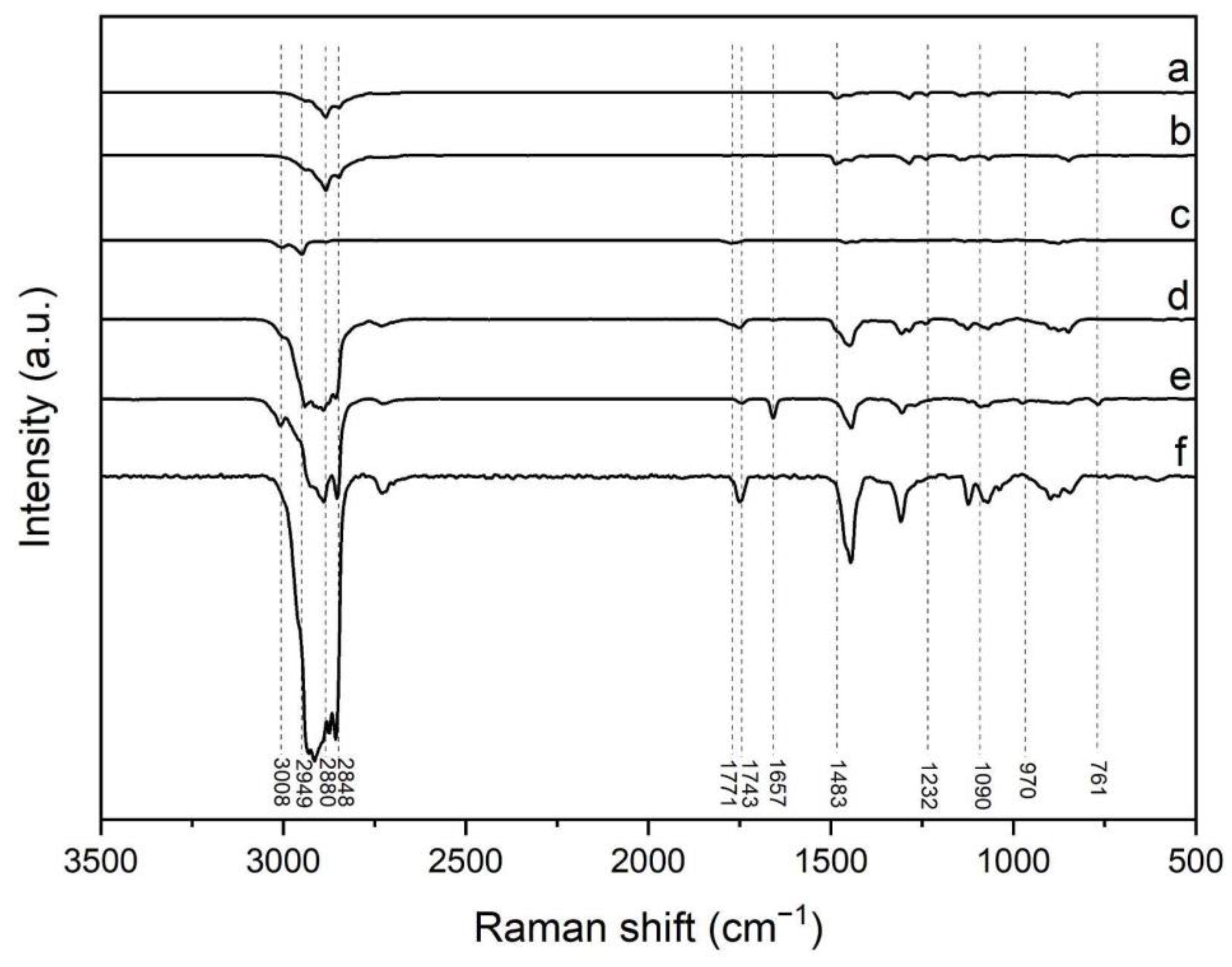
2.5.3. Raman Spectroscopy
2.5.4. Atomic Force Microscopy
2.5.5. Encapsulation Efficiency of DCX-Loaded Nanoparticles
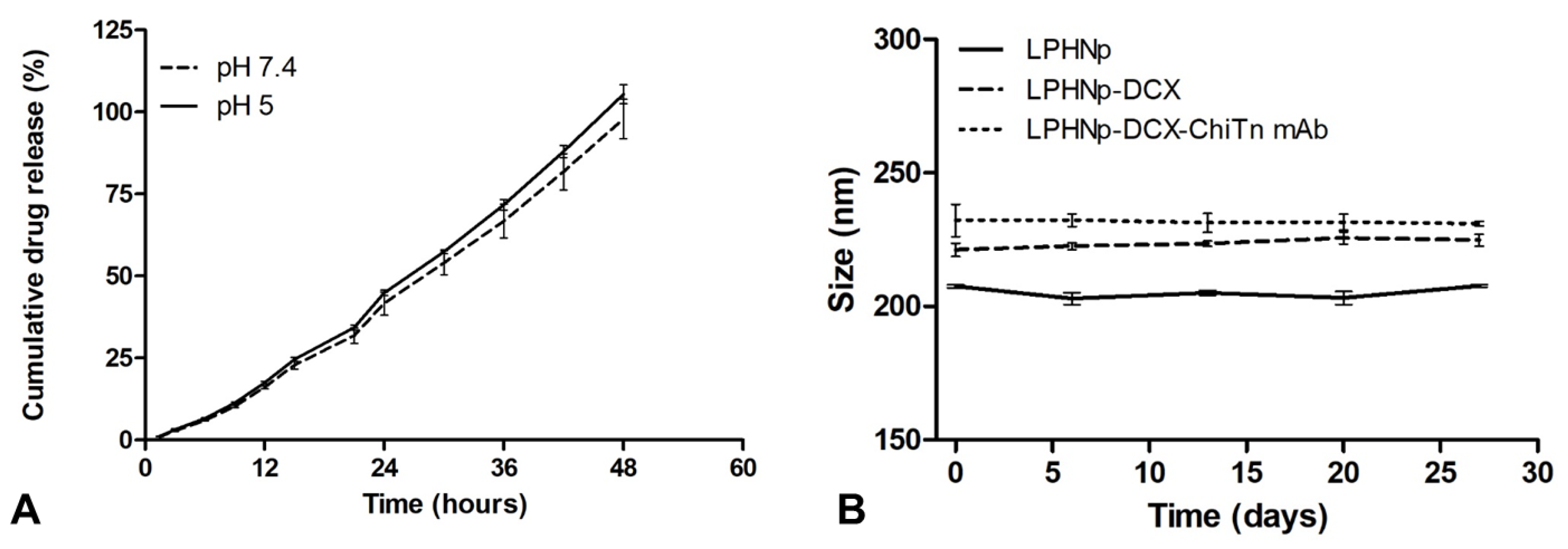
2.5.6. In Vitro Drug Release Study
2.5.7. Short-Term Stability Testing
2.6. In Vitro Cell Test
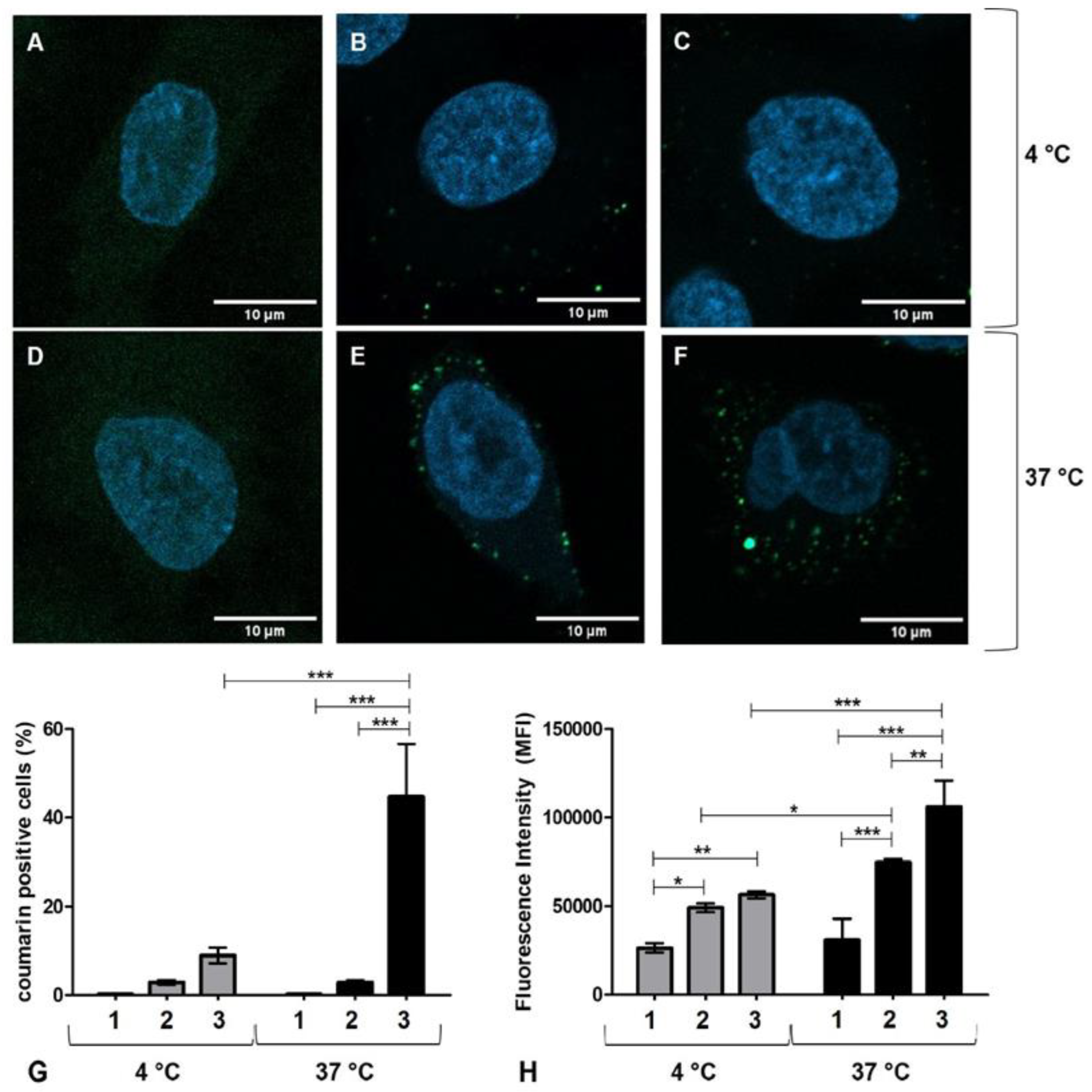
2.6.1. Cell Uptake Studies
2.6.2. Cell Viability Assay
2.7. In Vivo Studies
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of Nanoparticles
3.2. In Vitro Drug Release and Storage Stability Test
3.3. Cell Uptake Study
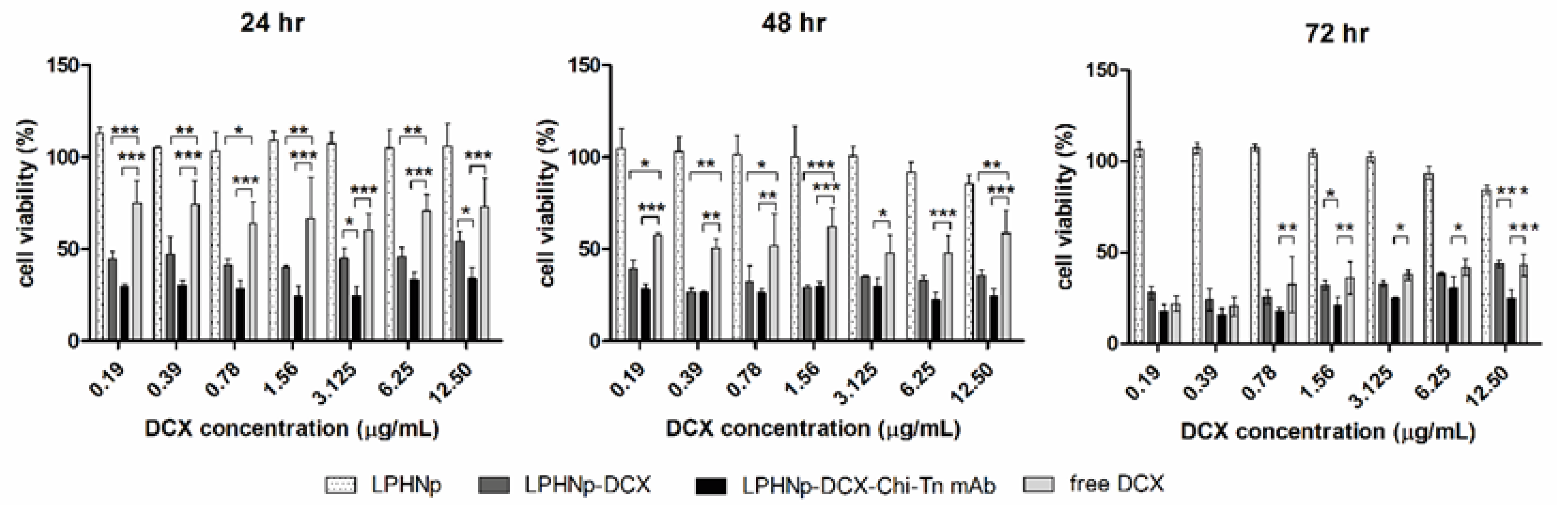
3.4. Cell Viability Assay
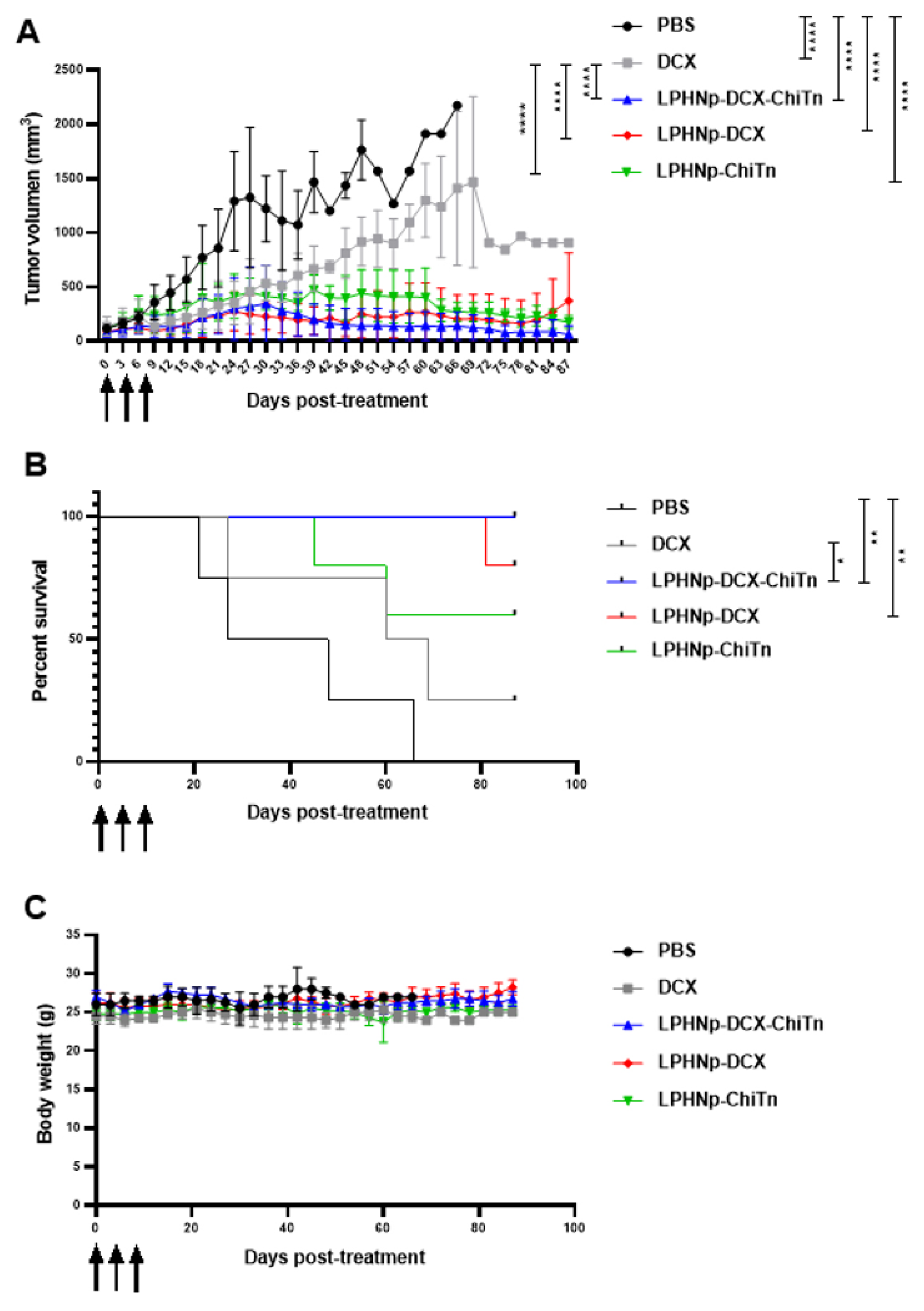
3.5. In Vivo Anti-Tumor Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2018, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly(lactic-co-glycolic acid) and Progress of Poly(lactic-co-glycolic acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Uthaman, S.; Park, I.-K. Utilization of polymer-lipid hybrid nanoparticles for targeted anti-cancer therapy. Molecules 2020, 25, 4377. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Vaiyapuri, R.; Zhang, L.; Chan, J.M. Lipid-coated polymeric nanoparticles for cancer drug delivery. Biomater. Sci. 2015, 3, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Berois, N.; Pittini, A.; Osinaga, E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers 2022, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Otto, V.I.; Cummings, R.D. The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 2011, 50, 1770–1791. [Google Scholar] [CrossRef]
- Ju, T.; Wang, Y.; Aryal, R.P.; Lehoux, S.D.; Ding, X.; Kudelka, M.R.; Cutler, C.; Zeng, J.; Wang, J.; Sun, X.; et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteom. Clin. Appl. 2013, 7, 618–631. [Google Scholar] [CrossRef]
- Osinaga, E.; Bay, S.; Tello, D.; Babino, A.; Pritsch, O.; Assemat, K.; Cantacuzene, D.; Nakada, H.; Alzari, P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett. 2000, 469, 24–28. [Google Scholar] [CrossRef]
- Hubert, P.; Heitzmann, A.; Viel, S.; Nicolas, A.; Sastre-Garau, X.; Oppezzo, P.; Pritsch, O.; Osinaga, E.; Amigorena, S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011, 71, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Sedlik, C.; Heitzmann, A.; Viel, S.; Ait Sarkouh, R.; Batisse, C.; Schmidt, F.; De La Rochere, P.; Amzallag, N.; Osinaga, E.; Oppezzo, P.; et al. Effective antitumor therapy based on a novel antibody-drug conjugate targeting the Tn carbohydrate antigen. OncoImmunology 2016, 5, e1171434. [Google Scholar] [CrossRef]
- Castro, A.; Berois, N.; Malanga, A.; Ortega, C.; Oppezzo, P.; Pristch, O.; Mombrú, A.W.; Osinaga, E.; Pardo, H. Docetaxel in chitosan-based nanocapsules conjugated with an anti-Tn antigen mouse/human chimeric antibody as a promising targeting strategy of lung tumors. Int. J. Biol. Macromol. 2021, 182, 806–814. [Google Scholar] [CrossRef]
- Bose, R.J.; Lee, S.-H.; Park, H. Lipid polymer hybrid nanospheres encapsulating antiproliferative agents for stent applications. J. Ind. Eng. Chem. 2016, 36, 284–292. [Google Scholar] [CrossRef]
- Dai, Y.; Xing, H.; Song, F.; Yang, Y.; Qiu, Z.; Lu, X.; Liu, Q.; Ren, S.; Chen, X.; Li, N. Biotin-Conjugated Multilayer Poly [D,L-lactide-co-glycolide]-Lecithin-Polyethylene Glycol Nanoparticles for Targeted Delivery of Doxorubicin. J. Pharm. Sci. 2016, 105, 2949–2958. [Google Scholar] [CrossRef]
- Klippstein, R.; Wang, J.T.-W.; El-Gogary, R.I.; Bai, J.; Mustafa, F.; Rubio, N.; Bansal, S.; Al-Jamal, W.T.; Al-Jamal, K.T. Passively Targeted Curcumin-Loaded PEGylated PLGA Nanocapsules for Colon Cancer Therapy In Vivo. Small 2015, 11, 4704–4722. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; Mendez-Gomez, H.R.; Mitchell, D.A. Cancer vaccine immunotherapy with RNA-loaded liposomes. Int. J. Mol. Sci. 2018, 19, 2890. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Benz, F.; Sigle, D.O.; Bowman, R.W.; Bao, P.; Roth, J.S.; Heath, G.R.; Evans, S.D.; Baumberg, J.J. Watching individual molecules flex within lipid membranes using SERS. Sci. Rep. 2014, 4, 5940. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Shayganpour, A.; Salis, B.; Dante, S. Surface-enhanced Raman scattering of self-assembled thiol monolayers and supported lipid membranes on thin anodic porous alumina. Beilstein J. Nanotechnol. 2017, 8, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Pigorsch, E. Spectroscopic characterisation of cationic quaternary ammonium starches. Starch/Stärke 2009, 61, 129–138. [Google Scholar] [CrossRef]
- Vanden-Hehir, S.; Tipping, W.J.; Lee, M.; Brunton, V.G.; Williams, A.; Hulme, A.N. Raman imaging of nanocarriers for drug delivery. Nanomaterials 2019, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef]
- Huang, S.-J.; Wang, T.-H.; Chou, Y.-H.; Wang, H.-M.D.; Hsu, T.-C.; Yow, J.-L.; Tzang, B.-S.; Chiang, W.-H. Hybrid PEGylated chitosan/PLGA nanoparticles designed as pH-responsive vehicles to promote intracellular drug delivery and cancer chemotherapy. Int. J. Biol. Macromol. 2022, 210, 565–578. [Google Scholar] [CrossRef]
- Yang, C.; Gao, S.; Dagnæs-Hansen, F.; Jakobsen, M.; Kjems, J. Impact of PEG Chain Length on the Physical Properties and Bioactivity of PEGylated Chitosan/siRNA Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 12203–12216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, A.; Verma, A.K.; Kant, S.; Pandey, A.K.; Khare, P.; Prakash, V. The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up. Vaccines 2022, 10, 1801. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid−polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, J.; Wang, Y.; Ji, H.; Peng, R.; Jin, L.; Wu, W. Advances in refunctionalization of erythrocyte-based nanomedicine for enhancing cancer-targeted drug delivery. Theranostics 2019, 9, 6885–6900. [Google Scholar] [CrossRef]
- Torrecilla, D.; Lozano, M.V.; Lallana, E.; Neissa, J.I.; Novoa-Carballal, R.; Vidal, A.; Fernandez-Megia, E.; Torres, D.; Riguera, R.; Alonso, M.J.; et al. Anti-tumor efficacy of chitosan-g-poly(ethylene glycol) nanocapsules containing docetaxel: Anti-TMEFF-2 functionalized nanocapsules vs. non-functionalized nanocapsules. Eur. J. Pharm. Biopharm. 2013, 83, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological safety and biodistribution of chitosan nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Rueda, F.; Löwik, C.; Ossendorp, F.; Cruz, L.J. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 2016, 83, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Shigdar, S.; Yang, W.; Duan, W.; Li, Q.; Lin, J.; Liu, K.; Li, L. Epithelial cell adhesion molecule aptamer functionalized PLGA-lecithin-curcumin-PEG nanoparticles for targeted drug delivery to human colorectal adenocarcinoma cells. Int. J. Nanomed. 2014, 9, 1083–1096. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, Q.; Li, Y.; Zhou, S. Engineering docetaxel-loaded micelles for non-small cell lung cancer: A comparative study of microfluidic and bulk nanoparticle preparation. RSC Adv. 2018, 8, 31950–31966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-Coated Nanoparticles Loaded with Docetaxel for Breast Cancer Therapy. Dose-Response 2019, 17, 1559325819872583. [Google Scholar] [CrossRef]
- Panaampon, J.; Sasamoto, K.; Kariya, R.; Okada, S. Establishment of Nude Mice Lacking NK Cells and Their Application for Human Tumor Xenografts. Asian Pac. J. Cancer Prev. 2021, 22, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Size ± SD (nm) | IP ± SD | Z Potential ± SD (mV) | EE ± SD (%) |
|---|---|---|---|---|
| LPHNp | 201.9 ± 2.3 | 0.1 ± 0.01 | 32.1 ± 1.2 | - |
| LPHNp-Chi-Tn mAb | 205.5 ± 1.5 | 0.2 ± 0.01 | 36.6 ± 1.6 | - |
| LPHNp-DCX | 222.5 ± 1.3 | 0.1 ± 0.02 | 36.0 ± 0.9 | 99.92 ± 0.01 |
| LPHNp-DCX-Chi-Tn mAb | 232.3 ± 2.4 | 0.2 ± 0.02 | 37.6 ± 0.8 | 99.92 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.; Pittini, Á.; Berois, N.; Faccio, R.; Miranda, P.; Mombrú, Á.W.; Osinaga, E.; Pardo, H. Development, Characterization, and Evaluation of Chi-Tn mAb-Functionalized DOTAP-PLGA Hybrid Nanoparticles Loaded with Docetaxel for Lung Cancer Therapy. Pharmaceutics 2025, 17, 164. https://doi.org/10.3390/pharmaceutics17020164
Castro A, Pittini Á, Berois N, Faccio R, Miranda P, Mombrú ÁW, Osinaga E, Pardo H. Development, Characterization, and Evaluation of Chi-Tn mAb-Functionalized DOTAP-PLGA Hybrid Nanoparticles Loaded with Docetaxel for Lung Cancer Therapy. Pharmaceutics. 2025; 17(2):164. https://doi.org/10.3390/pharmaceutics17020164
Chicago/Turabian StyleCastro, Analía, Álvaro Pittini, Nora Berois, Ricardo Faccio, Pablo Miranda, Álvaro W. Mombrú, Eduardo Osinaga, and Helena Pardo. 2025. "Development, Characterization, and Evaluation of Chi-Tn mAb-Functionalized DOTAP-PLGA Hybrid Nanoparticles Loaded with Docetaxel for Lung Cancer Therapy" Pharmaceutics 17, no. 2: 164. https://doi.org/10.3390/pharmaceutics17020164
APA StyleCastro, A., Pittini, Á., Berois, N., Faccio, R., Miranda, P., Mombrú, Á. W., Osinaga, E., & Pardo, H. (2025). Development, Characterization, and Evaluation of Chi-Tn mAb-Functionalized DOTAP-PLGA Hybrid Nanoparticles Loaded with Docetaxel for Lung Cancer Therapy. Pharmaceutics, 17(2), 164. https://doi.org/10.3390/pharmaceutics17020164









