Augmented Wound-Healing Effect of Sodium Thiosulfate-Infused Cosmetic Creams in Frostbite
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
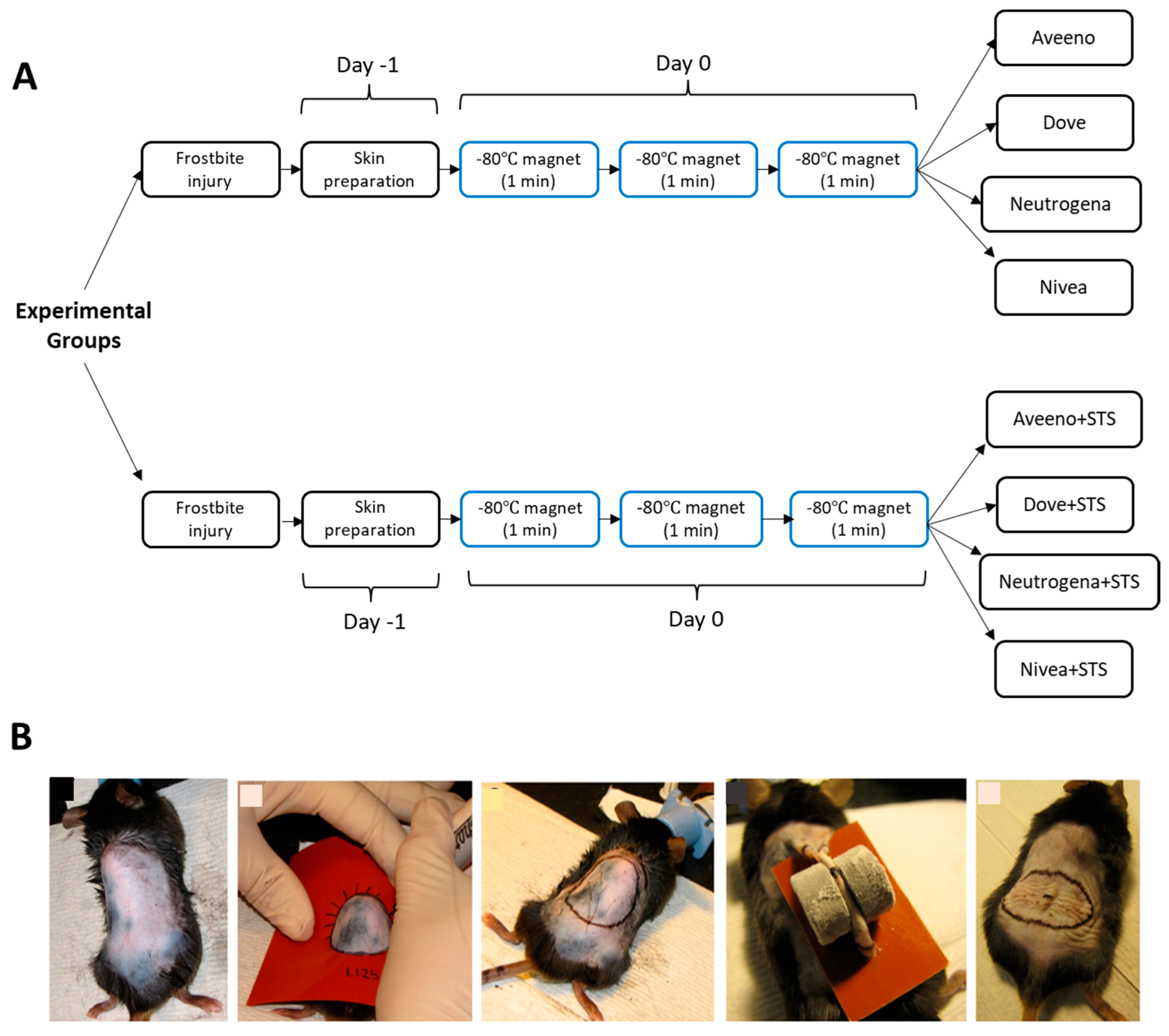
2.2. Animal Grouping
2.3. Skin Preparation
2.4. Preparation of Creams and Their Topical Application
2.5. Induction of Frostbite Injury in Mice
2.6. Histological Examination
2.7. Immunofluorescence Assessment
2.8. Statistical Analysis
3. Results
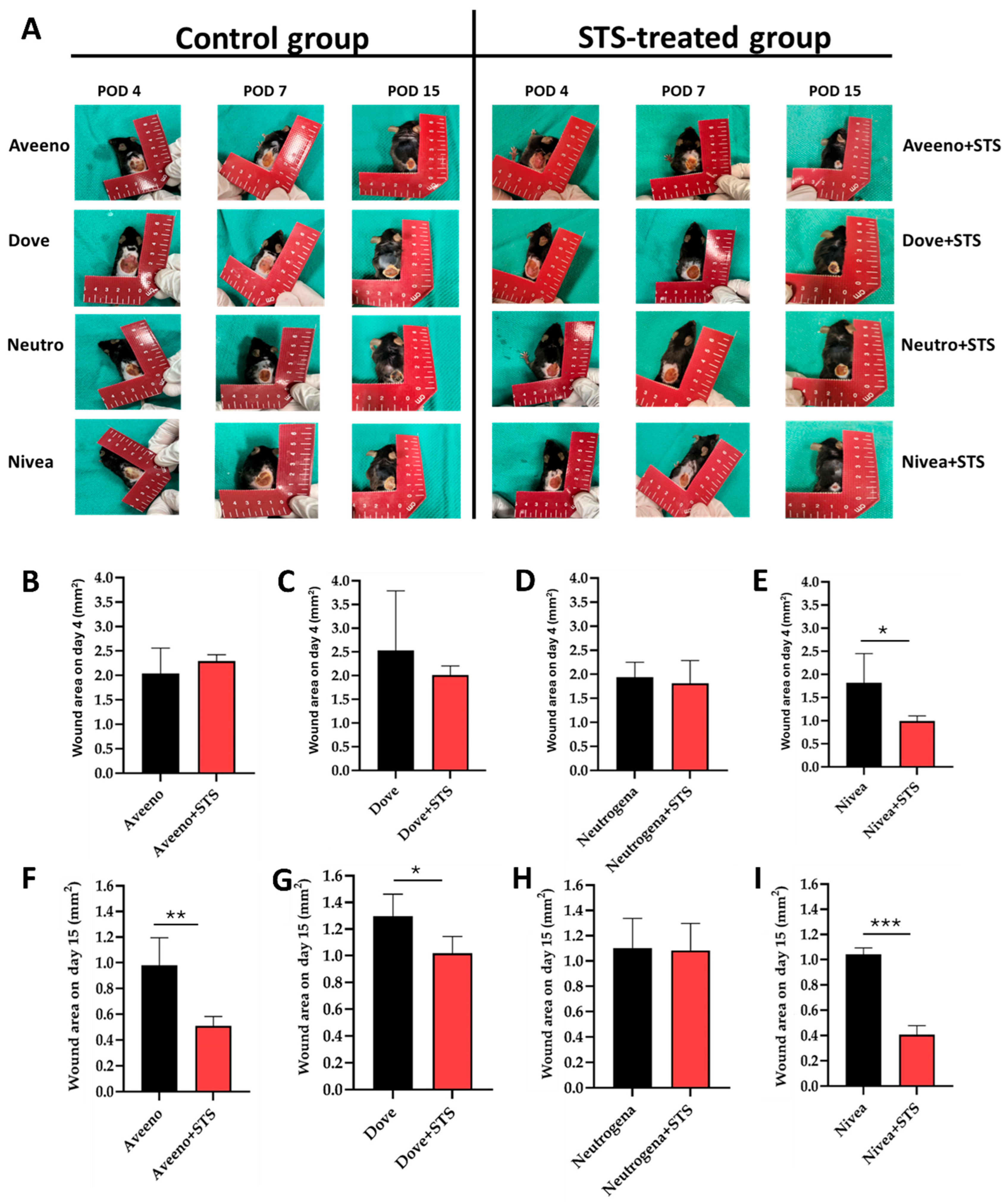
3.1. Effect of Supplementation of Aveeno, Dove, Neutrogena, and Nivea with STS in Frostbite Wound Healing
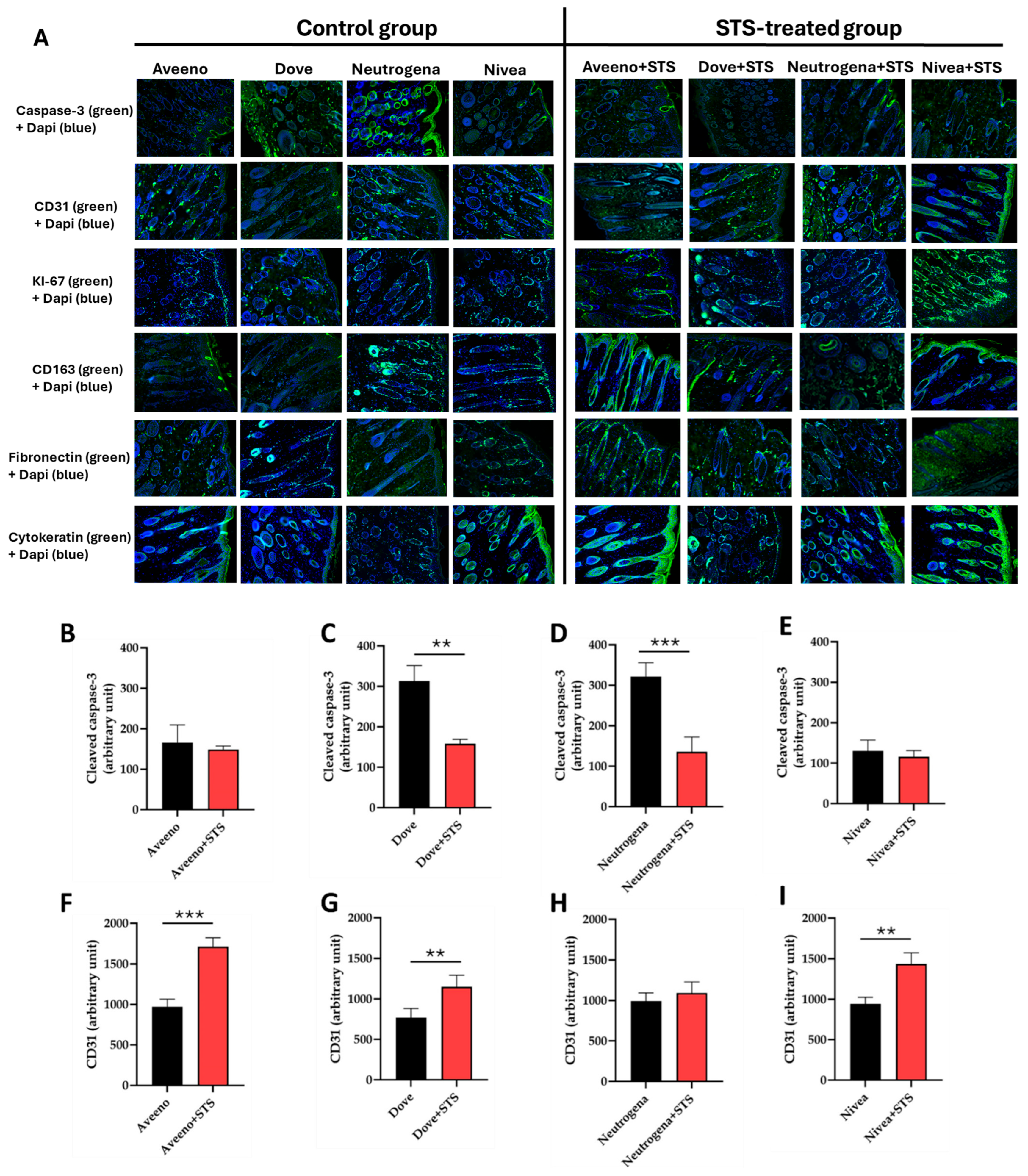
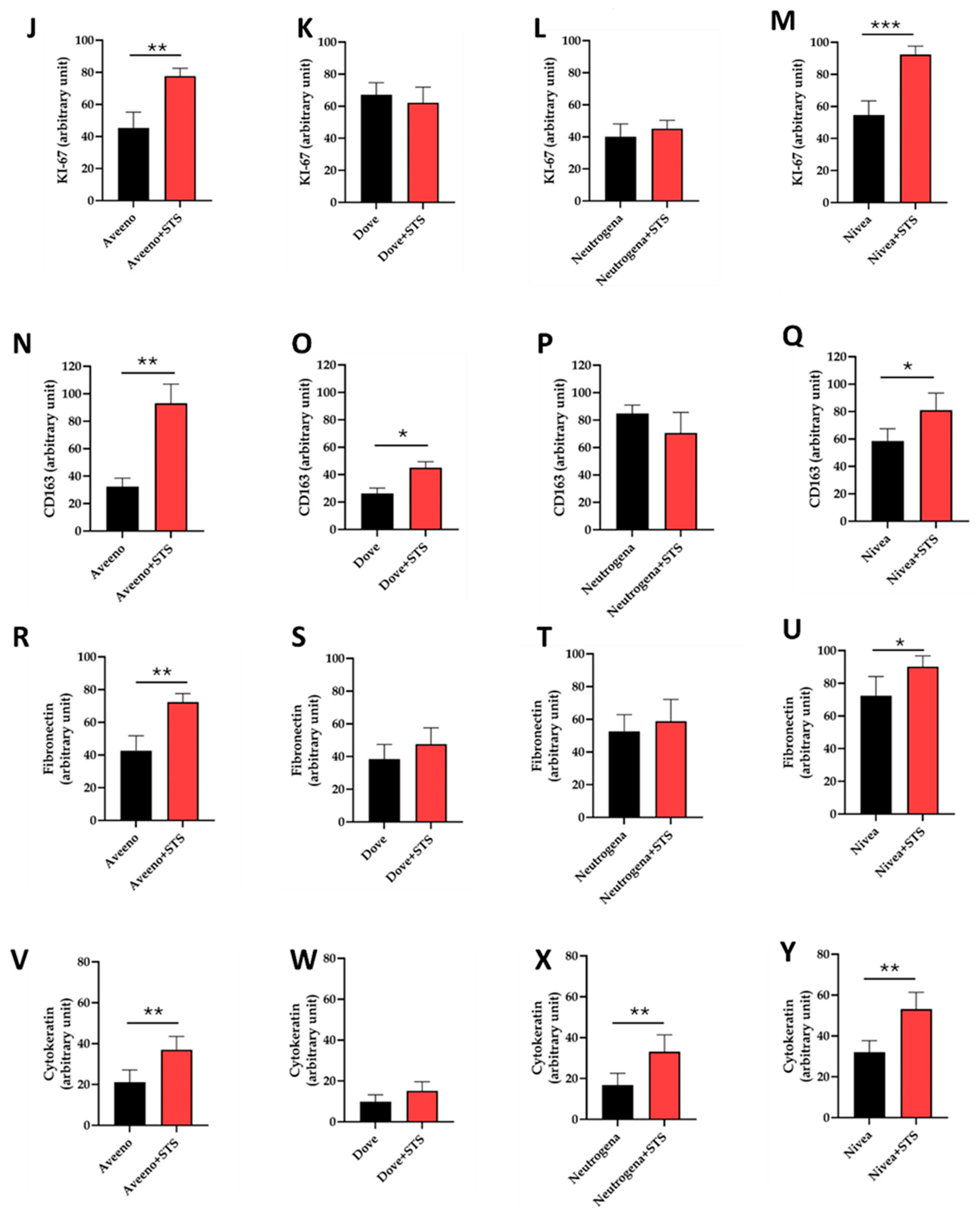
3.2. Effect of STS Addition to Aveeno, Dove, Neutrogena, and Nivea on Angiogenesis, Cell Proliferation, Inflammation, and Granulation Tissue Formation in Frostbite Wound Healing
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, E.A.; Moore, J.C. Using thrombolytics in frostbite injury. J. Emerg. Trauma. Shock. 2012, 5, 267–271. [Google Scholar] [CrossRef]
- Detanac, D.; Marovac, S.; Sengul, I.; Detanac, D.; Sengul, D.; Cinar, E.; Muratovic, S. Severe Frostbite on Both Hands and Feet in a Vignette Case: From Physics to Clinics. Cureus 2022, 14, e29085. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Fang, Y.; Shu, Q.; Ma, R.; Wu, D. Construction and validation of a risk prediction model for soldiers with frostbite in northeast China: A cross-sectional study. BMC Public. Health 2024, 24, 2493. [Google Scholar] [CrossRef]
- Reamy, B.V. Frostbite: Review and current concepts. J. Am. Board. Fam. Pract. 1998, 11, 34–40. [Google Scholar] [CrossRef]
- Mäkinen, T.M.; Jokelainen, J.; Näyhä, S.; Laatikainen, T.; Jousilahti, P.; Hassi, J. Occurrence of frostbite in the general population--work-related and individual factors. Scand. J. Work. Environ. Health 2009, 35, 384–393. [Google Scholar] [CrossRef]
- Valnicek, S.M.; Chasmar, L.R.; Clapson, J.B. Frostbite in the prairies: A 12-year review. Plast. Reconstr. Surg. 1993, 92, 633–641. [Google Scholar] [CrossRef]
- Zhao, J.C.; Fan, X.; Yu, J.A.; Zhang, X.H.; Shi, K.; Hong, L. Deep frostbite: Clinical characteristics and outcomes in northeastern China. J. Tissue Viability 2020, 29, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://health-infobase.canada.ca/injuries/cold-related/ (accessed on 15 October 2025).
- Imray, C.; Grieve, A.; Dhillon, S.; Caudwell Xtreme Everest Research Group. Cold damage to the extremities: Frostbite non-freezing cold injuries. Postgrad. Med. J. 2009, 85, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pirozynski, W.J.; Webster, D.R. Muscle tissue changes in experimental frostbite. Ann. Surg. 1952, 136, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Shakirov, B.M. Frostbite injuries and our experience treatment in the Samarkand area Uzbekistan. Int. J. Burns Trauma. 2020, 10, 156–161. [Google Scholar]
- Shakirov, B.M. Frostbite in hot climates of Central Asia: Retrospective analysis of the microflora of wound and antibiotic therapy. Int. J. Burns Trauma. 2022, 12, 93–97. [Google Scholar]
- Bayley, J.S.; Winther, C.B.; Andersen, M.K.; Gronkjaer, C.; Nielsen, O.B.; Pedersen, T.H.; Overgaard, J. Cold exposure causes cell death by depolarization-mediated Ca(2+) overload in a chill-susceptible insect. Proc. Natl. Acad. Sci. USA 2018, 115, E9737–E9744. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Goverman, J.; Sarhane, K.A.; Donofrio, J.; Walker, T.G.; Fagan, S.P. The emerging role of tissue plasminogen activator in the management of severe frostbite. J. Burn. Care Res. 2015, 36, e62–e66. [Google Scholar] [CrossRef]
- Gralino, B.J.; Porter, J.M.; Rosch, J. Angiography in the diagnosis and therapy of frostbite. Radiology 1976, 119, 301–305. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.E.; Freer, L.; Grissom, C.K.; Rodway, G.W.; Giesbrecht, G.G.; McDevitt, M.; Imray, C.H.; Johnson, E.L.; Pandey, P.; Dow, J.; et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Frostbite: 2024 Update. Wilderness Environ. Med. 2024, 35, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.S.; Daanen, H.A.; McClintock, R.J.; Roberts, N.A.; Dunn, T.L.; Jones, D.M. Cold-induced vasodilation during sequential immersions of the hand. Eur. J. Appl. Physiol. 2024, 124, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.L. Frostbite of the hand. J. Hand Surg. Am. 2014, 39, 1863–1868. [Google Scholar] [CrossRef]
- Fossati, A.; Ruijs, A.C.J. Changes in Fingertip Cold-Induced Vasodilatation (Hunting Reaction) on Acute Exposure to Altitude. High. Alt. Med. Biol. 2024, 25, 212–217. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Penney, L.N.; Mills, L.; Zhang, M.Y.; Juriasingani, S.; Major, S.; McLeod, P.; Liu, W.; Haig, A.; Wood, M.E.; et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, promotes cutaneous wound healing in an in vivo murine model of acute frostbite injury. Biomed. Pharmacother. 2025, 183, 117869. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; An, T.; Xian, M.; Luckanagul, J.A.; Su, Z.; Lin, Y.; Wang, Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. [Google Scholar] [CrossRef]
- Lian, J.; Ju, G.; Cai, X.; Cai, Y.; Li, C.; Ma, S.; Cao, Y. Nanofibrous Membrane Dressings Loaded with Sodium Hydrogen Sulfide/Endothelial Progenitor Cells Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 657549. [Google Scholar] [CrossRef]
- Wu, J.; Chen, A.; Zhou, Y.; Zheng, S.; Yang, Y.; An, Y.; Xu, K.; He, H.; Kang, J.; Luckanagul, J.A.; et al. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials 2019, 222, 119398. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef] [PubMed]
- Abou Taka, M.; Dugbartey, G.J.; Richard-Mohamed, M.; McLeod, P.; Jiang, J.; Major, S.; Arp, J.; O'Neil, C.; Liu, W.; Gabril, M.; et al. Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 2210. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Dugbartey, G.J.; McFarlane, L.; McLeod, P.; Major, S.; Jiang, J.; O'Neil, C.; Haig, A.; Sener, A. Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 9529. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. Cellular and molecular mechanisms of cell damage and cell death in ischemia–reperfusion injury in organ transplantation. Mol. Biol. Rep. 2024, 51, 473. [Google Scholar] [CrossRef]
- Auerbach, L.J.; Galvez, M.G.; De Clerck, B.K.; Glotzbach, J.; Wehner, M.R.; Chang, E.I.; Gurtner, G.C.; Auerbach, P.S. A novel mouse model for frostbite injury. Wilderness Environ. Med. 2013, 24, 94–104. [Google Scholar] [CrossRef]
- Stout, E.I.; McKessor, A. Glycerin-Based Hydrogel for Infection Control. Adv. Wound Care 2012, 1, 48–51. [Google Scholar] [CrossRef]
- Roehrs, H.; Stocco, J.G.; Pott, F.; Blanc, G.; Meier, M.J.; Dias, F.A. Dressings and topical agents containing hyluronic acid for chronic wound healing. Cochrane Database Syst. Rev. 2023, 7, CD012215. [Google Scholar]
- Milani, M.; Sparavigna, A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Arabshahi, B.; Silverman, R.A.; Jones, O.Y.; Rider, L.G. Abatacept and sodium thiosulfate for treatment of recalcitrant juvenile dermatomyositis complicated by ulceration and calcinosis. J. Pediatr. 2012, 160, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Eleryan, M.G.; Awosika, O.; Akhiyat, S.; Qureshi, A.; Rengifo-Pardo, M.; Curiel, R.; Rider, L.G.; Ehrlich, A. Treatment of calcinosis associated with adult and juvenile dermatomyositis using topical sodium thiosulfate via fractionated CO2 laser treatment. Clin. Exp. Rheumatol. 2019, 37, 1092–1093. [Google Scholar]
- Wang, D.; Liu, Y.; Zong, X.; Li, X.; Yang, S.; Zeng, Y.; Lu, J. Sodium thiosulfate ameliorates atopic dermatitis via inhibiting the activation of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2023, 673, 160–168. [Google Scholar] [CrossRef]
- Karasawa, T.; Komada, T.; Yamada, N.; Aizawa, E.; Mizushina, Y.; Watanabe, S.; Baatarjav, C.; Matsumura, T.; Takahashi, M. Cryo-sensitive aggregation triggers NLRP3 inflammasome assembly in cryopyrin-associated periodic syndrome. Elife 2022, 11, e75166. [Google Scholar] [CrossRef]
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Liu, Y.; Liu, P.P.; Liu, L.; Zheng, X.S.; Zheng, H.; Yang, C.C.; Luobu, C.R.; Liu, Y. Triptolide inhibits TGF-beta-induced matrix contraction and fibronectin production mediated by human Tenon fibroblasts. Int. J. Ophthalmol. 2018, 11, 1108–1113. [Google Scholar]
- Yang, Z.G.; Awan, F.M.; Du, W.W.; Zeng, Y.; Lyu, J.; Wu, D.; Gupta, S.; Yang, W.; Yang, B.B. The Circular RNA Interacts with STAT3, Increasing Its Nuclear Translocation and Wound Repair by Modulating Dnmt3a and miR-17 Function. Mol. Ther. 2017, 25, 2062–2074. [Google Scholar] [CrossRef]
- Johnson, M.B.; Pang, B.; Gardner, D.J.; Niknam-Benia, S.; Soundarajan, V.; Bramos, A.; Perrault, D.P.; Banks, K.; Lee, G.K.; Baker, R.Y.; et al. Topical Fibronectin Improves Wound Healing of Irradiated Skin. Sci. Rep. 2017, 7, 3876. [Google Scholar] [CrossRef]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair. Regen. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Golin, A.P.; Jalili, R.B.; Ghahary, A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Exp. Dermatol. 2022, 31, 475–484. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Macabrey, D.; Joniová, J.; Gasser, Q.; Bechelli, C.; Longchamp, A.; Urfer, S.; Lambelet, M.; Fu, C.Y.; Schwarz, G.; Wagnières, G.; et al. Sodium thiosulfate, a source of hydrogen sulfide, stimulates endothelial cell proliferation and neovascularization. Front. Cardiovasc. Med. 2022, 9, 965965. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Khanna, K.; Bhatnagar, A.; Purkayastha, J. In vivo-wound healing studies of sodium thiosulfate gel in rats. Biomed. Pharmacother. 2021, 140, 111797. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Li, W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran. J. Basic. Med. Sci. 2019, 22, 1192–1197. [Google Scholar]
- Wu, J.; Li, Y.; He, C.; Kang, J.; Ye, J.; Xiao, Z.; Zhu, J.; Chen, A.; Feng, S.; Li, X.; et al. Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 27474–27481. [Google Scholar] [CrossRef]
- Namiki, A.; Brogi, E.; Kearney, M.; Kim, E.A.; Wu, T.; Couffinhal, T.; Varticovski, L.; Isner, J.M. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J. Biol. Chem. 1995, 270, 31189–31195. [Google Scholar] [CrossRef] [PubMed]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Detmar, M.; Brown, L.F.; Berse, B.; Jackman, R.W.; Elicker, B.M.; Dvorak, H.F.; Claffey, K.P. Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J. Invest. Dermatol. 1997, 108, 263–268. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Morello, S.; Petrella, A. The combination of mesoglycan and VEGF promotes skin wound repair by enhancing the activation of endothelial cells and fibroblasts and their cross-talk. Sci. Rep. 2022, 12, 11041. [Google Scholar] [CrossRef]
- Gersztenkorn, D.; Coletta, C.; Zhu, S.; Ha, Y.; Liu, H.; Tie, H.; Zhou, J.; Szabo, C.; Zhang, W.; Motamedi, M. Hydrogen Sulfide Contributes to Retinal Neovascularization in Ischemia-Induced Retinopathy. Invest. Ophthalmol. Vis. Sci. 2016, 57, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Monti, M.; Vellecco, V.; Bucci, M.; Cirino, G.; Ziche, M.; Morbidelli, L. Characterization of zofenoprilat as an inducer of functional angiogenesis through increased H2S availability. Br. J. Pharmacol. 2015, 172, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, D.D.; Sun, X.; Xie, H.H.; Yuan, H.; Jia, W.; Chen, A.F. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014, 63, 1763–1778. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Chang, L.; Chen, S.; Wei, X.; Du, H.; Cheng, J.; Chen, X.; Yuan, Z.; Zhao, P.; Geng, M.; et al. Multifunctional injectable hydrogel with self-supplied H(2)S release and bacterial inhibition for the wound healing with enhanced macrophages polarization via interfering with PI3K/Akt pathway. Biomaterials 2025, 318, 123144. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Hu, Q.; Xu, J.; Zhang, N.; Chen, Y.; Jiang, X.; Gu, C.; Zhang, K.; Wu, G. Hydrogen sulfide as a therapeutic agent for diabetic wounds: Effects on inflammation and fibroblast pyroptosis. Front. Immunol. 2025, 16, 1558443. [Google Scholar] [CrossRef]

| Cosmetic Cream | Composition (Active Ingredients) |
|---|---|
| Aveeno | Glycerin, water, ceramides, cetyl alcohol, shea butter, dimethicone, and hyaluronic acid |
| Dove | Glycerin, water, dimethicone, stearic acid, mineral oil, niacinamide, and petrolatum |
| Neutrogena | Glycerin, water, dimethicone, cetearyl alcohol, phenoxyethanol, caprylyl glycol, and ceramides |
| Nivea | Glycerin, water, mineral oil, lanolin alcohol, microcrystalline wax, hyaluronic acid, panthenol, aluminum stearates, citronellol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugbartey, G.J.; McFarlane, L.; Ortas, T.S.; Major, S.; Haig, A.; Sener, A. Augmented Wound-Healing Effect of Sodium Thiosulfate-Infused Cosmetic Creams in Frostbite. Pharmaceutics 2025, 17, 1610. https://doi.org/10.3390/pharmaceutics17121610
Dugbartey GJ, McFarlane L, Ortas TS, Major S, Haig A, Sener A. Augmented Wound-Healing Effect of Sodium Thiosulfate-Infused Cosmetic Creams in Frostbite. Pharmaceutics. 2025; 17(12):1610. https://doi.org/10.3390/pharmaceutics17121610
Chicago/Turabian StyleDugbartey, George J., Liam McFarlane, Tamara S. Ortas, Sally Major, Aaron Haig, and Alp Sener. 2025. "Augmented Wound-Healing Effect of Sodium Thiosulfate-Infused Cosmetic Creams in Frostbite" Pharmaceutics 17, no. 12: 1610. https://doi.org/10.3390/pharmaceutics17121610
APA StyleDugbartey, G. J., McFarlane, L., Ortas, T. S., Major, S., Haig, A., & Sener, A. (2025). Augmented Wound-Healing Effect of Sodium Thiosulfate-Infused Cosmetic Creams in Frostbite. Pharmaceutics, 17(12), 1610. https://doi.org/10.3390/pharmaceutics17121610






