Smart Vesicle Therapeutics: Engineering Precision at the Nanoscale
Abstract
1. Introduction
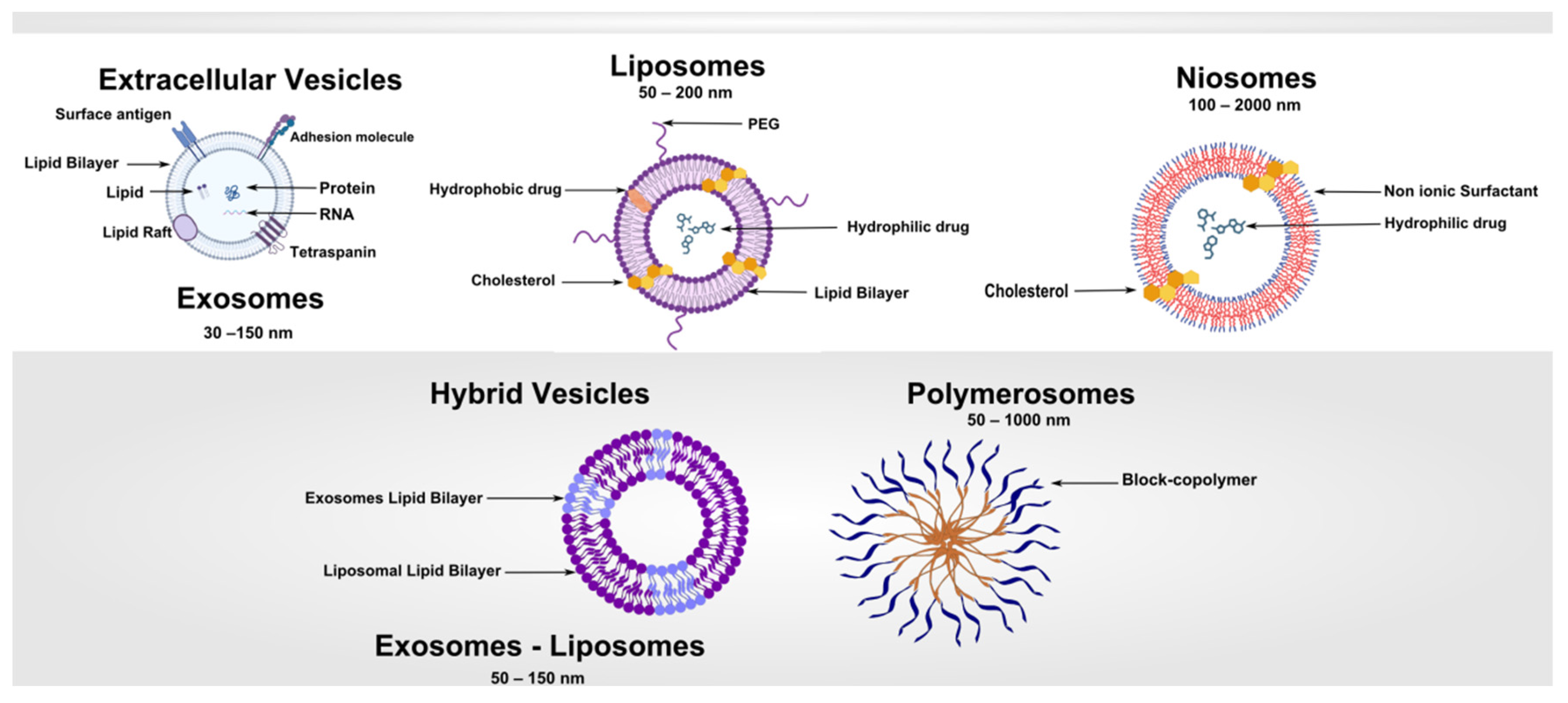
2. Fundamentals of Vesicle-Based Systems
2.1. Vesicle Morphology and Stability
2.2. Drug Encapsulation and Release Mechanisms
2.3. Design Considerations and the Nano–Bio Interface
- Active targeting: Adding antibodies, peptides, aptamers, or small molecules to improve how cells recognize and take in the treatment, regardless of blood vessel permeability.
- Vascular modulation: Using vasodilators, anti-fibrotic agents, or normalization strategies to temporarily increase blood flow and help nanoparticles exit blood vessels.
- Externally triggered delivery: Using ultrasound, heat, magnetic fields, or light to locally increase permeability or trigger the release of treatment when needed.
- Tumor microenvironment remodeling: Lowering interstitial fluid pressure or reducing the density of the extracellular matrix to allow deeper penetration of nanoparticles.
- Patient stratification approaches: Using pre-treatment imaging or functional biomarkers to find individuals with favorable EPR profiles and tailor nanotherapeutic interventions for them.
3. Liposomes: Design, Preparation, and Clinical Applications
4. Niosomes: Composition, Preparation, and Applications
5. Polymersomes: Design, Stimuli-Responsiveness, and Applications
6. Extracellular Vesicles and Exosomes: Biology, Isolation, and Therapeutic Potential
7. Hybrid and Specialized Vesicles
8. Functionalization, Targeting, and Modified Release
8.1. Surface Functionalization and Targeting Strategies
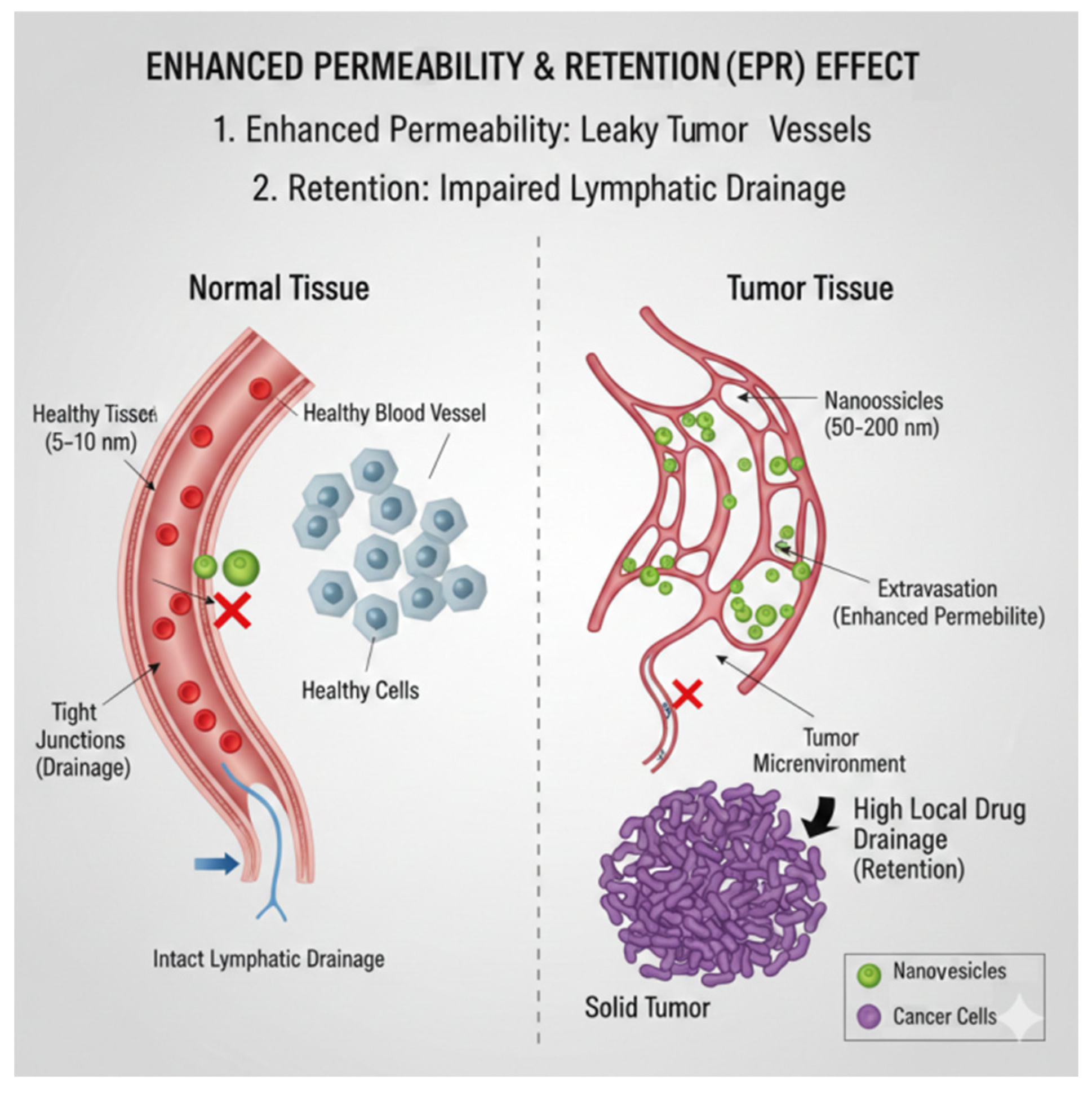
8.2. Passive Targeting
8.3. Stimuli-Responsive and Modified Release Vesicles
8.4. Dual and Multi-Stimuli Vesicles, Clinical Applications, and Design Challenges
| Vesicle Type | Composition/Structural Features | Stimuli-Responsive Mechanisms (Single, Dual, Multi) | Representative Clinical/Biomedical Applications | Key Design Challenges |
|---|---|---|---|---|
| Liposomes |
|
|
|
|
| Niosomes |
|
|
|
|
| Polymersomes |
|
|
|
|
| Exosomes/Extracellular Vesicles |
|
|
|
|
| Hybrid Vesicles (Lipid–Polymer or Cell Membrane–Coated) |
|
|
|
|
| Micelles |
|
|
|
|
| MOF- or Silica-Based Vesicle-Like Nanocarriers |
|
|
|
|
9. Pharmacokinetics and Biodistribution of Vesicle-Based Drug Delivery Systems
10. Applications in Biomedicine and Personalized Care
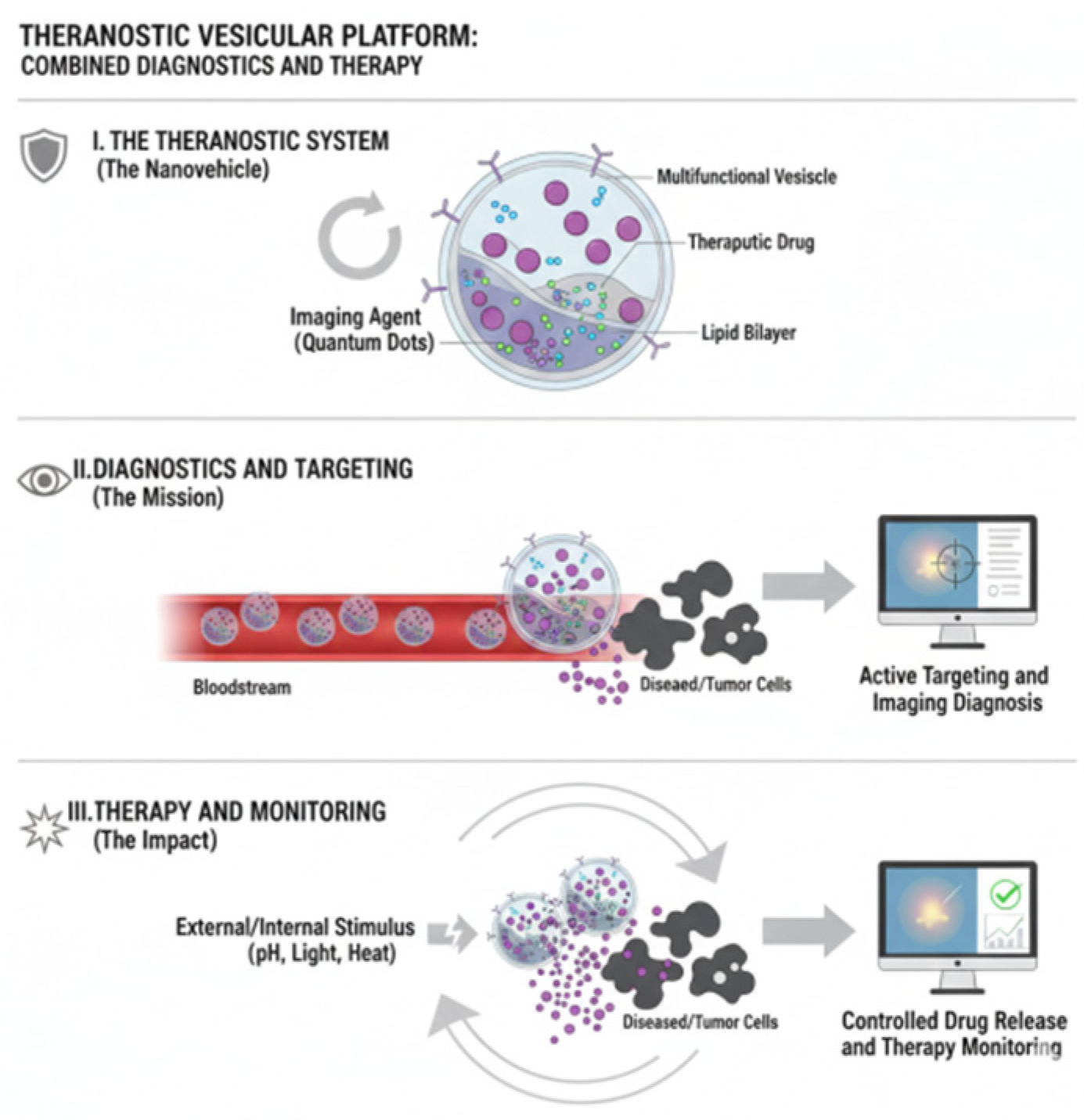
11. Theranostic Vesicles: Integrating Therapy and Diagnosis
12. Regulatory, Safety, and Manufacturing
13. Translational Landscape and Technology Readiness of Smart Vesicles
14. Future Perspectives and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Douanne, N.; Wu, T.; Kaur, I.; Tsering, T.; Erzingatzian, A.; Nadeau, A.; Juncker, D.; Nerguizian, V.; Burnier, J.V. Leveraging nature’s nanocarriers: Translating insights from extracellular vesicles to biomimetic synthetic vesicles for biomedical applications. Sci. Adv. 2025, 11, eads5249. [Google Scholar] [CrossRef]
- Abosheasha, M.A.; Ueda, M.; Ito, Y. Fabrication of Polymersomes with Liposome-Extracted Lipid Membrane Preserving Original Leaflet Asymmetry. Nanoscale 2025, 17, 22529–22539. [Google Scholar] [CrossRef]
- Huang, P.; Li, W.; Guan, J.; Jia, Y.; Wang, D.; Chen, Y.; Xiao, N.; Ou, S.; Wang, Y.; Yang, B. Synthetic vesicle-based drug delivery systems for oral disease therapy: Current applications and future directions. J. Funct. Biomater. 2025, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, Y.; Duan, Y.; Mao, C.; Wan, M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J. Control. Release 2024, 365, 1089–1123. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of extracellular vesicles. Adv. Mater. 2024, 36, 2403199. [Google Scholar] [CrossRef]
- Stiepel, R.T.; Duggan, E.; Batty, C.J.; Ainslie, K.M. Micro and nanotechnologies: The little formulations that could. Bioeng. Transl. Med. 2023, 8, e10421. [Google Scholar] [CrossRef]
- Senjab, R.M.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Advances in liposomal nanotechnology: From concept to clinics. RSC Pharm. 2024, 1, 928–948. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, S. Overcoming the dilemma of permeability and stability of polymersomes through traceless cross-linking. Acc. Chem. Res. 2022, 55, 3404–3416. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Wang, C.-K.; Tsai, T.-H.; Lee, C.-H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Azimizonuzi, H.; Ghayourvahdat, A.; Ahmed, M.H.; Kareem, R.A.; Zrzor, A.J.; Mansoor, A.S.; Athab, Z.H.; Kalavi, S. A state-of-the-art review of the recent advances of theranostic liposome hybrid nanoparticles in cancer treatment and diagnosis. Cancer Cell Int. 2025, 25, 26. [Google Scholar] [CrossRef]
- Abbas, M.; Liang, Z.; Chen, M.; Qu, W.; Khan, S.; Ashaq, M.S.; Chen, D.; Xie, S. Toxicity Challenges and Current Advancement in Metal–Organic Frameworks (MOFs) for Biomedical Applications. Biol. Trace Elem. Res. 2025, 1–17. [Google Scholar] [CrossRef]
- Yang, H.; Luan, Q.; Guan, W.; Nong, W. Oral Delivery Systems of Dietary Bioactive Compounds Based on Metal–Organic Frameworks: A Review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70272. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Benedini, L.; Messina, P. Chapter 8—Proniosomes and niosomes for enhanced drug delivery. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: New York, NY, USA, 2022; pp. 115–128. [Google Scholar]
- Kontogiannis, O.; Selianitis, D.; Lagopati, N.; Pippa, N.; Pispas, S.; Gazouli, M. Surfactant and Block Copolymer Nanostructures: From Design and Development to Nanomedicine Preclinical Studies. Pharmaceutics 2023, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as New Diagnostic Tools in CNS Diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 403–410. [Google Scholar] [CrossRef]
- Chen, J.; Hu, S.; Liu, J.; Jiang, H.; Wang, S.; Yang, Z. Exosomes: A double-edged sword in cancer immunotherapy. MedComm 2025, 6, e70095. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S. Lipid based vesicular drug delivery systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef]
- Chelliah, R.; Rubab, M.; Vijayalakshmi, S.; Karuvelan, M.; Barathikannan, K.; Oh, D.-H. Liposomes for drug delivery: Classification, therapeutic applications, and limitations. Next Nanotechnol. 2025, 8, 100209. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Pistonesi, D.B.; Fernández-Leyes, M.D.; Ritacco, H.; Rivero, P.S.; Sica, M.G.; Benedini, L.A.; Centurión, M.E.; Messina, P.V. Lipid Membrane-Selective Interactions Driven by Nanosilver Anisotropy: Insights from Prokaryotic and Erythrocyte Models. Langmuir 2025, 41, 21509–21524. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive Liposomes for Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef]
- Athulya, K.; Varughese, T.; Kumar, A.C. Polymersomes: Beyond Basics-Synthesis, Stimuli Response and Biomedical Applications. ChemistrySelect 2024, 9, e202402040. [Google Scholar] [CrossRef]
- Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the next attractive generation of drug delivery systems: Definition, synthesis and applications. Materials 2024, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Shi, J.; Zhang, F.; Lv, M.; Ge, Z.; Feng, M.; Fan, Z.; Liu, D.; Du, J.; Sun, Y. Ginsenoside Rd-loaded antioxidant polymersomes to regulate mitochondrial homeostasis for bone defect healing in periodontitis. Adv. Healthc. Mater. 2025, 14, 2403817. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, R.; Mell, M.; López-Montero, I.; Netzel, J.; Hellweg, T.; Monroy, F. Polymersomes: Smart vesicles of tunable rigidity and permeability. Soft Matter 2011, 7, 1532–1542. [Google Scholar] [CrossRef]
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021, 45, 1807–1831. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yu, L.; Ran, M.; Zhong, X.; Sun, M.; Xu, M.; Wang, Y.; Yan, X.; Lee, R.J.; Tang, Y. Harnessing the potential of exosomes in therapeutic interventions for brain disorders. Int. J. Mol. Sci. 2025, 26, 2491. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, M.A. Overview and update on extracellular vesicles: Considerations on exosomes and their application in modern medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, H.; Liu, H.; Kim, S.; Lee, Y.-k.; Kim, Y.-C. Hybrid Nanoparticles of Extracellular Vesicles and Gemcitabine Prodrug-loaded Liposomes with Enhanced Targeting Ability for Effective PDAC Treatment. ACS Appl. Bio Mater. 2024, 7, 6025–6033. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, C.; Li, Y.; Zhao, F.; Feng, Q.; Liu, S.; Jia, S.; Ji, J.; Ye, L.; Zhai, G. Exosome/liposome hybrid nanovesicles for enhanced phototherapy and boosted anti-tumor immunity against melanoma. Eur. J. Med. Chem. 2025, 289, 117485. [Google Scholar] [CrossRef]
- Xu, P.; He, J.; Xu, T.; Wang, W.; Wu, B.; Chen, R.; Wang, H.; Yang, Q.; Wu, W.; Sun, D. Synergistic integration of extracellular vesicles and metal-organic frameworks: Unlocking new opportunities in disease diagnosis and therapy. Theranostics 2025, 15, 8609. [Google Scholar] [CrossRef]
- Ruso, J.M.; Messina, P.V. Application of natural, semi-synthetic, and synthetic biopolymers used in drug delivery systems design. In Biopolymers for Medical Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 46–73. [Google Scholar]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z. Recent advancements in stimuli responsive drug delivery platforms for active and passive cancer targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Lipid systems for the delivery of amphotericin B in antifungal therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Gabizon-Peretz, S.; Modaresahmadi, S.; La-Beck, N.M. Thirty years from FDA approval of pegylated liposomal doxorubicin (Doxil/Caelyx): An updated analysis and future perspective. BMJ Oncol. 2025, 4, e000573. [Google Scholar] [CrossRef]
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53. [Google Scholar] [CrossRef]
- Wang, X.; Allen, C. Synergistic effects of thermosensitive liposomal doxorubicin, mild hyperthermia, and radiotherapy in breast cancer management: An orthotopic mouse model study. Drug Deliv. Transl. Res. 2025, 15, 1011–1022. [Google Scholar] [CrossRef]
- Van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2022, 11, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Sheffey, V.V.; Siew, E.B.; Tanner, E.E.; Eniola-Adefeso, O. PLGA’s plight and the role of stealth surface modification strategies in its use for intravenous particulate drug delivery. Adv. Healthc. Mater. 2022, 11, 2101536. [Google Scholar] [CrossRef] [PubMed]
- Besse, H.C.; Barten-van Rijbroek, A.D.; van der Wurff-Jacobs, K.M.; Bos, C.; Moonen, C.T.; Deckers, R. Tumor drug distribution after local drug delivery by hyperthermia, in vivo. Cancers 2019, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Wakade, V.S. Biointerface: A nano-modulated way for biological transportation. J. Drug Target. 2020, 28, 456–467. [Google Scholar] [CrossRef]
- Wheeler, K.E.; Chetwynd, A.J.; Fahy, K.M.; Hong, B.S.; Tochihuitl, J.A.; Foster, L.A.; Lynch, I. Environmental dimensions of the protein corona. Nat. Nanotechnol. 2021, 16, 617–629. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; Ruso, J.M.; Messina, P.V.; Salgado, F.J.; Nogueira, M.; Costas, M.; Sarmiento, F. Interactions between DMPC liposomes and the serum blood proteins HSA and IgG. J. Phys. Chem. B 2009, 113, 1655–1661. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81. [Google Scholar] [CrossRef]
- Rama, E.; May, J.-N.; Rix, A.; Lammers, T.; Kiessling, F. Image-guided strategies to improve drug delivery to tumors beyond using the enhanced permeability and retention (EPR) effect. Biochem. Biophys. Res. Commun. 2025, 778, 152346. [Google Scholar] [CrossRef]
- Vagena, I.-A.; Malapani, C.; Gatou, M.-A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR effect for passive tumor targeting: Current status and future perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, X.; Huang, F.; Chen, X. Anti-PEG antibodies and their biological impact on PEGylated drugs: Challenges and strategies for optimization. Pharmaceutics 2025, 17, 1074. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kalluri, R. Exosomes as a Multicomponent Biomarker Platform in Cancer. Trends Cancer 2020, 6, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Jin, J.; Fu, Z.; Wang, G.; Lei, X.; Xu, J.; Wang, J. Extracellular vesicle-based drug overview: Research landscape, quality control and nonclinical evaluation strategies. Signal Transduct. Target. Ther. 2025, 10, 255. [Google Scholar] [CrossRef]
- Fernandes, E.; Cardoso, V.F.; Lanceros-Méndez, S.; Lúcio, M. Lipid microfluidic biomimetic models for drug screening: A comprehensive review. Adv. Funct. Mater. 2024, 34, 2315166. [Google Scholar] [CrossRef]
- Yash, J.; Maharsh, J.; Kamble, S.S.; Guldhe, A.; Bastikar, V. In silico techniques, artificial intelligence, and machine learning for enhanced efficacy of extracellular vesicle–based diagnosis and therapeutics. In Extracellular Vesicles for Therapeutic and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2025; pp. 495–521. [Google Scholar]
- Ochoa-Sánchez, C.; Rodríguez-León, E.; Iñiguez-Palomares, R.; Rodríguez-Beas, C. Brief Comparison of the Efficacy of Cationic and Anionic Liposomes as Nonviral Delivery Systems. ACS Omega 2024, 9, 46664–46678. [Google Scholar] [CrossRef]
- Cullis, P.; Felgner, P. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, X.; Lin, X.; Wu, C. Mesenchymal stem cell-derived extracellular vesicles: A regulator and carrier for targeting bone-related diseases. Cell Death Discov. 2024, 10, 212. [Google Scholar] [CrossRef]
- Kumar, R.; Mishra, N.; Tran, T.; Kumar, M.; Vijayaraghavalu, S.; Gurusamy, N. Emerging Strategies in Mesenchymal Stem Cell-based Cardiovascular Therapeutics. Cells 2024, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Li, Y.; Cheng, S.; Zeng, Y. Advances in analytical technologies for extracellular vesicles. Anal. Chem. 2021, 93, 4739–4774. [Google Scholar] [CrossRef]
- Williams, S.; Fernandez-Rhodes, M.; Law, A.; Peacock, B.; Lewis, M.P.; Davies, O.G. Comparison of extracellular vesicle isolation processes for therapeutic applications. J. Tissue Eng. 2023, 14, 20417314231174609. [Google Scholar] [CrossRef]
- Fusco, C.; De Rosa, G.; Spatocco, I.; Vitiello, E.; Procaccini, C.; Frigè, C.; Pellegrini, V.; La Grotta, R.; Furlan, R.; Matarese, G. Extracellular vesicles as human therapeutics: A scoping review of the literature. J. Extracell. Vesicles 2024, 13, e12433. [Google Scholar] [CrossRef]
- Thakur, A.; Rai, D. Global requirements for manufacturing and validation of clinical grade extracellular vesicles. J. Liq. Biopsy 2024, 6, 100278. [Google Scholar] [CrossRef]
- Roerig, J.; Schulz-Siegmund, M. Standardization approaches for extracellular vesicle loading with oligonucleotides and biologics. Small 2023, 19, 2301763. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Moon, J.J.; Suh, H.; Bershteyn, A.; Stephan, M.T.; Liu, H.; Huang, B.; Sohail, M.; Luo, S.; Ho Um, S.; Khant, H. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. [Google Scholar] [CrossRef]
- Benedini, L.; Antollini, S.; Fanani, M.L.; Palma, S.; Messina, P.; Schulz, P. Study of the influence of ascorbyl palmitate and amiodarone in the stability of unilamellar liposomes. Mol. Membr. Biol. 2014, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Yu, J.; Yang, L.; Kuang, X.; Wang, Z.; Wang, Y.; Liu, H.; Lin, G.; He, Z. A facile and universal method to achieve liposomal remote loading of non-ionizable drugs with outstanding safety profiles and therapeutic effect. Acta Pharm. Sin. B 2021, 11, 258–270. [Google Scholar] [CrossRef]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic liposomal lipids: From gene carriers to cell signaling. Prog. Lipid Res. 2008, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.; Stefanick, J.; Ashley, J.; Kiziltepe, T.; Bilgicer, B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol. 2013, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Adler-Moore, J.; Proffitt, R.T. AmBisome: Liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002, 49, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Ahad, A.; Aftab, F.; Michel, A.; Lewis, J.S.; Contel, M. Development of immunoliposomes containing cytotoxic gold payloads against HER2-positive breast cancers. RSC Med. Chem. 2024, 15, 139–150. [Google Scholar] [CrossRef]
- Kumari, M.; Chen, K.-C.; Ke, F.-Y.; Pan, P.-L.; Gusti Ngurah Putu, E.P.; Chen, W.-Y.; Wu, H.-C. Developing a Delivery Strategy for Combined Drug Treatment with Multi-targeting Immunoliposomes. J. Drug Deliv. Sci. Technol. 2024, 101, 106283. [Google Scholar] [CrossRef]
- Nematollahi, M.H.; Pardakhty, A.; Torkzadeh-Mahanai, M.; Mehrabani, M.; Asadikaram, G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: A promising approach for niosome bilayer intervention. RSC Adv. 2017, 7, 49463–49472. [Google Scholar] [CrossRef]
- Mazzotta, E.; Orlando, C.; Muzzalupo, R. New nanomaterials with intrinsic antioxidant activity by surface functionalization of niosomes with natural phenolic acids. Pharmaceutics 2021, 13, 766. [Google Scholar] [CrossRef]
- Bautista-Solano, A.A.; Dávila-Ortiz, G.; Perea-Flores, M.d.J.; Martínez-Ayala, A.L. A Comprehensive Review of Niosomes: Composition, Structure, Formation, Characterization, and Applications in Bioactive Molecule Delivery Systems. Molecules 2025, 30, 3467. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Tambuwala, M.M.; Obeid, M.A. Microfluidic manufacturing of niosomes. In Microfluidics in Pharmaceutical Sciences: Formulation, Drug Delivery, Screening, and Diagnostics; Springer: London, UK, 2024; pp. 77–108. [Google Scholar]
- Safari Sharafshadeh, M.; Tafvizi, F.; Khodarahmi, P.; Ehtesham, S. Folic acid-functionalized PEGylated niosomes co-encapsulated cisplatin and doxoribicin exhibit enhanced anticancer efficacy. Cancer Nanotechnol. 2024, 15, 14. [Google Scholar] [CrossRef]
- Kamble, S.; Rasala, T.; Chikhale, S.; Khandare, K.; Babhulkar, S.; Bhadre, Y. Niosome-Based Vaccines for Enhanced Immunogenic Response. J. Pharm. Res. Integr. Med. Sci. 2025, 2, 107–120. [Google Scholar] [CrossRef]
- Meng, X.; Shen, Y.; Zhao, H.; Lu, X.; Wang, Z.; Zhao, Y. Redox-manipulating nanocarriers for anticancer drug delivery: A systematic review. J. Nanobiotechnol. 2024, 22, 587. [Google Scholar] [CrossRef]
- Hernández Becerra, E.; Quinchia, J.; Castro, C.; Orozco, J. Light-triggered polymersome-based anticancer therapeutics delivery. Nanomaterials 2022, 12, 836. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, J.; Yu, D.; Liu, Y.; Song, D.; Tian, K.; Chen, H.; Ye, Q.; Wang, X.; Xu, T. Polymer-locking fusogenic liposomes for glioblastoma-targeted siRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2024, 19, 1869–1879. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Ni, Q.; Chen, X. Materials engineering strategies for cancer vaccine adjuvant development. Chem. Soc. Rev. 2023, 52, 2886–2910. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Han, H.; Wang, J.; Pu, Y.; Shao, J.; Xie, J.; Che, H.; van Hest, J.C.; Cao, S. Polymersome-based nanomotors: Preparation, motion control, and biomedical applications. Chem. Sci. 2025, 16, 7106–7129. [Google Scholar] [CrossRef]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The ins and outs of microvesicles. FASEB BioAdv. 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Prakash, A.; Gates, T.; Zhao, X.; Wangmo, D.; Subramanian, S. Tumor-derived extracellular vesicles in the colorectal cancer immune environment and immunotherapy. Pharmacol. Ther. 2023, 241, 108332. [Google Scholar] [CrossRef]
- Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular-vesicle-based drug delivery systems for enhanced antitumor therapies through modulating the cancer-immunity cycle. Adv. Mater. 2022, 34, 2201054. [Google Scholar] [CrossRef]
- Torrecillas-Baena, B.; Pulido-Escribano, V.; Dorado, G.; Gálvez-Moreno, M.Á.; Camacho-Cardenosa, M.; Casado-Díaz, A. Clinical potential of mesenchymal stem cell-derived exosomes in bone regeneration. J. Clin. Med. 2023, 12, 4385. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Regulation of extracellular vesicle-mediated immune responses against antigen-specific presentation. Vaccines 2022, 10, 1691. [Google Scholar] [CrossRef] [PubMed]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical translation of extracellular vesicles. Adv. Healthc. Mater. 2023, 12, 2301010. [Google Scholar] [CrossRef]
- Sun, M.; Yang, J.; Fan, Y.; Zhang, Y.; Sun, J.; Hu, M.; Sun, K.; Zhang, J. Beyond extracellular vesicles: Hybrid membrane nanovesicles as emerging advanced tools for biomedical applications. Adv. Sci. 2023, 10, 2303617. [Google Scholar] [CrossRef]
- Sato, Y.; Zhang, W.; Baba, T.; Chung, U.-i.; Teramura, Y. Extracellular vesicle-liposome hybrids via membrane fusion using cell-penetrating peptide-conjugated lipids. Regener. Ther. 2024, 26, 533–540. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Stahl, F.; Scheper, T. Tumor homing and penetrating peptide-conjugated niosomes as multi-drug carriers for tumor-targeted drug delivery. RSC Adv. 2017, 7, 33378–33384. [Google Scholar] [CrossRef]
- Dufes, C.; Schätzlein, A.G.; Tetley, L.; Gray, A.I.; Watson, D.G.; Olivier, J.-C.; Couet, W.; Uchegbu, I.F. Niosomes and polymeric chitosan based vesicles bearing transferrin and glucose ligands for drug targeting. Pharm. Res. 2000, 17, 1250–1258. [Google Scholar] [CrossRef]
- Salvatore, A.; Montis, C.; Berti, D.; Baglioni, P. Multifunctional magnetoliposomes for sequential controlled release. ACS Nano 2016, 10, 7749–7760. [Google Scholar] [CrossRef]
- Wang, C.; Lan, X.; Zhu, L.; Wang, Y.; Gao, X.; Li, J.; Tian, H.; Liang, Z.; Xu, W. Construction strategy of functionalized liposomes and multidimensional application. Small 2024, 20, 2309031. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Valenzuela Oses, J.K.; Rabasco-Álvarez, A.M.; González-Rodríguez, M.L.; García, M.C. Innovations in Cancer Therapy: Endogenous Stimuli-Responsive Liposomes as Advanced Nanocarriers. Pharmaceutics 2025, 17, 245. [Google Scholar] [CrossRef]
- Placente, D.; Benedini, L.A.; Baldini, M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Multi-drug delivery system based on lipid membrane mimetic coated nano-hydroxyapatite formulations. Int. J. Pharm. 2018, 548, 559–570. [Google Scholar] [CrossRef]
- Rodríguez, C.F.; Guzmán-Sastoque, P.; Santacruz-Belalcazar, A.; Rodriguez, C.; Villamarin, P.; Reyes, L.H.; Cruz, J.C. Magnetoliposomes for nanomedicine: Synthesis, characterization, and applications in drug, gene, and peptide delivery. Expert. Opin. Drug Deliv. 2025, 22, 1069–1098. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, T.; Pal, S. Extracellular vesicles for drug delivery and theranostics in vivo. JACS Au 2024, 4, 318–327. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Rai, A.; Suwakulsiri, W.; Chen, M.; Simpson, R.J. Clinical relevance of extracellular vesicles in cancer—Therapeutic and diagnostic potential. Nat. Rev. Clin. Oncol. 2025, 22, 924–952. [Google Scholar] [CrossRef]
- Su, Y.; Feng, H.; Yuan, X.; Du, Q.; Jiang, H.; Han, R.; Yang, Y.; Gao, C.; Fan, R. Progress of Micro/Nanomotors for In Vivo Biomedical Applications. Adv. Healthc. Mater. 2025, e03935. [Google Scholar] [CrossRef]
- Tripathi, D.; Pandey, P.; Sharma, S.; Rai, A.K.; BH, M.P. Advances in nanomaterials for precision drug delivery: Insights into pharmacokinetics and toxicity. BioImpacts BI 2024, 15, 30573. [Google Scholar] [CrossRef]
- Ahmed, W.; Kuniyan, M.S.; Jawed, A.M.; Chen, L. Engineered extracellular vesicles for drug delivery in therapy of stroke. Pharmaceutics 2023, 15, 2173. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Das, T.K. Liposome-based drug delivery systems: From laboratory research to industrial production—Instruments and challenges. ChemEngineering 2025, 9, 56. [Google Scholar]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome formation by amphiphilic polyglycerol-b-polydisulfide-b-polyglycerol and glutathione-triggered intracellular drug delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef]
- Benedini, L.A.; Messina, P.V. Nanodevices for facing new challenges of medical treatments: Stimuli-responsive drug delivery systems. Syst. Rev. Pharm. 2021, 12, 1–23. [Google Scholar]
- Bishal, A.; Paul, P.; Bera, S.; Patra, D.; Bandyopadhyay, B.; Debnath, B.; Ali, K.A. Targeted Drug Delivery for Breast Cancer using Functionalized Liposomes: Preparation Methods, Challenges, and Clinical Translation. AAPS PharmSciTech 2025, 26, 207. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Zahariev, N.; Simeonov, P.; Penkov, D.; Katsarov, P. Next-Generation Drug Delivery for Neurotherapeutics: The Promise of Stimuli-Triggered Nanocarriers. Biomedicines 2025, 13, 1464. [Google Scholar] [CrossRef] [PubMed]
- D’Elía, N.L.; Gravina, N.; Benedini, L.A.; Messina, P.V. A commentary: Harnessing vesicles power with new scenes of membrane-based devices for drug delivery. Biocell 2024, 48, 1401–1403. [Google Scholar] [CrossRef]
- Guo, C.; Lin, L.; Wang, Y.; Jing, J.; Gong, Q.; Luo, K. Nano drug delivery systems for advanced immune checkpoint blockade therapy. Theranostics 2025, 15, 5440. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.S.; Slika, H.; Sattari, S.A.; Malla, A.P.; Xia, Y.; Antar, A.; Ran, K.; Tyler, B. Overcoming the Blood-Brain Barrier for Drug Delivery to the Brain. ACS Omega 2025, 10, 32544–32563. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Xu, M.; Zhang, H.; Tu, C.; Yang, J.; Chen, X.; Yao, Q.; Lan, P.; Xie, M. Engineered exosome for NIR-triggered drug delivery and superior synergistic chemo-phototherapy in a glioma model. Appl. Mater. Today 2020, 20, 100723. [Google Scholar] [CrossRef]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef]
- Pritzl, S.D.; Morstein, J.; Pritzl, N.A.; Lipfert, J.; Lohmüller, T.; Trauner, D.H. Photoswitchable phospholipids for the optical control of membrane processes, protein function, and drug delivery. Commun. Mater. 2025, 6, 59. [Google Scholar] [CrossRef]
- Zi, Y.; Yang, K.; He, J.; Wu, Z.; Liu, J.; Zhang, W. Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms. Adv. Drug Delivery Rev. 2022, 188, 114449. [Google Scholar] [CrossRef]
- Saha, R.N.; Vasanthakumar, S.; Bende, G.; Snehalatha, M. Nanoparticulate drug delivery systems for cancer chemotherapy. Mol. Membr. Biol. 2010, 27, 215–231. [Google Scholar] [CrossRef]
- Sentoukas, T.; Walach, W.; Filipek, K.; Trzebicka, B. Zwitterionic Poly (Carboxybetaine Methacrylate) s in Drug Delivery, Antifouling Coatings, and Regenerative Tissue Platforms. Materials 2025, 18, 4514. [Google Scholar] [CrossRef]
- Shinde, S.; Shah, S.; Famta, P.; Wagh, S.; Pandey, G.; Sharma, A.; Vambhurkar, G.; Jain, A.; Srivastava, S. Next-Generation Transformable Nanomedicines: Revolutionizing Cancer Drug Delivery and Theranostics. Mol. Pharm. 2025, 22, 2783–2806. [Google Scholar] [CrossRef]
- Sonali; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef]
- Villani, C.; Murugan, P.; George, A. Exosome-Laden hydrogels as promising carriers for oral and bone tissue engineering: Insight into cell-free drug delivery. Int. J. Mol. Sci. 2024, 25, 11092. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Zhang, Y.; Hu, J.; Li, C.; Liu, S.; Li, R.; Du, R. In vivo fate of targeted drug delivery carriers. Int. J. Nanomed. 2024, 19, 6895–6929. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Woo, S. Subcellular drug distribution: Exploring organelle-specific characteristics for enhanced therapeutic efficacy. Pharmaceutics 2024, 16, 1167. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.M.; De Breuck, J.; Salehi, S.; Leiske, M.N. Smart, Bio-Inspired Polymers and Bio-Based Molecules Modified by Zwitterionic Motifs to Design Next-Generation Materials for Medical Applications. Adv. Funct. Mater. 2025, e13765. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Esparham, A.; Tajaldini, M.; Karimi-Shahri, M. RGD peptide in cancer targeting: Benefits, challenges, solutions, and possible integrin–RGD interactions. Cancer Med. 2024, 13, e6800. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Mun, D.; Park, M.; Yoo, G.; Kim, H.; Yun, N.; Joung, B. Injured Cardiac Tissue-Targeted Delivery of TGFβ1 siRNA by FAP Aptamer-Functionalized Extracellular Vesicles Promotes Cardiac Repair. Int. J. Nanomed. 2025, 20, 2575–2592. [Google Scholar] [CrossRef]
- Yoo, D.; Jung, S.Y.; Go, D.; Park, J.Y.; You, D.G.; Jung, W.-K.; Li, Y.; Ding, J.; Park, J.H.; Um, W. Functionalized extracellular vesicles of mesenchymal stem cells for regenerative medicine. J. Nanobiotechnol. 2025, 23, 219. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, R.; He, Y.; Zhu, Y.; Kang, Y.; Ji, X. Genetically engineered cellular nanovesicles: Theories, design and perspective. Adv. Funct. Mater. 2024, 34, 2407842. [Google Scholar] [CrossRef]
- Lopez Baltazar, J.M.; Gu, W.; Yu, Q. Enhancing extracellular vesicle detection via cotargeting tetraspanin biomarkers. Anal. Chem. 2024, 96, 16406–16414. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Cheng, C.; Sun, C.; Cheng, X. Harnessing engineered extracellular vesicles for enhanced therapeutic efficacy: Advancements in cancer immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.; Jiang, W. Engineering therapeutical extracellular vesicles for clinical translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Gabizon, A.A. Liposomal drug carriers in cancer therapy. In Nanoparticulates as Drug Carriers; Torchilin, V.P., Ed.; Imperial College Press: London, UK, 2006; pp. 437–462. [Google Scholar]
- Sharifi, M.; Cho, W.C.; Ansariesfahani, A.; Tarharoudi, R.; Malekisarvar, H.; Sari, S.; Bloukh, S.H.; Edis, Z.; Amin, M.; Gleghorn, J.P. An updated review on EPR-based solid tumor targeting nanocarriers for cancer treatment. Cancers 2022, 14, 2868. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Ohar, H.; Budkowski, A.; Lazzara, G. Molecular design and role of the dynamic hydrogen bonds and hydrophobic interactions in temperature-switchable polymers: From understanding to applications. Polymers 2025, 17, 1580. [Google Scholar] [CrossRef]
- Bao, L.; Hu, S.; Wang, T.; Song, W.; Zhou, L.; Bi, Y.; Wei, J. Glutathione-responsiveness of copolymer vesicles adjusted by the acid degradation of ketals for enhanced drug-loaded stability and controlled release. J. Drug Deliv. Sci. Technol. 2025, 109, 106994. [Google Scholar] [CrossRef]
- Machado, N.; Araujo, D.; Ruano, L.; Palmisano, V.F.; Anguita-Ortiz, N.; Bandeira, C.C.S.; Borges, R.; Nogueira, J.J.; Martinho, H. Enhanced transdermal permeation of caffeine through a skin model using electric field-induced lipid vesicles: A novel approach for drug transport. Phys. Chem. Chem. Phys. 2025, 27, 8824–8832. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Wang, W. Photopharmacology and photoresponsive drug delivery. Chem. Soc. Rev. 2025, 54, 5792–5835. [Google Scholar] [CrossRef]
- Leite, D.; Ribeiro, A.; Espinosa de Oliveira, T.; Pesce da Silveira, N. Interaction of thermoresponsive polymers with hydrophobic compounds: From phase transition to design strategies. J. Phys. Condens. Matter 2025, 37, 47. [Google Scholar] [CrossRef]
- Ridgway-Brown, D.; Leathard, A.S.; France, O.; Muench, S.P.; Webb, M.E.; Jeuken, L.J.; Henderson, P.J.; Taylor, A.F.; Beales, P.A. Membrane transport modulates the pH-regulated feedback of an enzyme reaction confined within lipid vesicles. ACS Nano 2025, 19, 9814–9825. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Hong, Z.; Mu, H.; Liu, N.; Zhou, G.; Miao, L.; Li, S. Encapsulation of doxorubicin in magnesium acetate liposomes as a pH-sensitive drug carrier for tumor therapy. iScience 2025, 28, 113336. [Google Scholar] [CrossRef]
- Sadeghi Jam, Z.; Tafvizi, F.; Khodarahmi, P.; Jafari, P.; Baghbani-Arani, F. PEG-functionalized UiO-66 MOFs for targeted vincristine delivery: Enhanced cytotoxicity in breast and ovarian cancer cell lines. Cancer Nanotechnol. 2025, 16, 10. [Google Scholar] [CrossRef]
- Vileigas, D.F.; da Silva, R.P.; Dempsey, B.; Massafera, M.P.; Pinz, M.P.; Meotti, F.C. Redox proteomics workflow to unveil extracellular targets of oxidation in vascular endothelial cells. J. Proteom. 2025, 321, 105506. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox Regulation: Mechanisms, Biology and Therapeutic Targets in Diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Pistonesi, D.B.; Belén, F.; Centurión, M.E.; Sica, M.G.; Ruso, J.M.; Messina, P.V. {111}—Faceted Silver Nanoplates: An Automated and Customized Design for Functionality. ChemNanoMat 2023, 9, e202300354. [Google Scholar] [CrossRef]
- Pistonesi, D.B.; Belén, F.; Ruso, J.M.; Centurión, M.E.; Sica, M.G.; Pistonesi, M.F.; Messina, P.V. NIR-responsive nano-holed titanium alloy surfaces: A photothermally activated antimicrobial biointerface. J. Mater. Chem. B 2024, 12, 8993–9004. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, D. Programmable Injectable Dual-Responsive Hydrogels with NIR Ratiometric Self-Monitoring of pH/Ca2+ for Sensing and Actuation. Biomacromolecules 2025, 26, 6129–6139. [Google Scholar] [CrossRef]
- Singhai, H.; Rathee, S.; Sahu, A.; Patil, U.K.; Jain, S.K. Enzyme-responsive vesicular carriers as an emerging approach for tumor targeting. In Tumor-Targeting with Stimuli-Responsive Vesicular Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2025; pp. 111–135. [Google Scholar]
- Jain, A.; Mody, N.; Palakurthi, S. Tumor-Targeting with Stimuli-Responsive Vesicular Nanocarriers: Basics to Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Mohamed, R.R.G.A.; Ali, S.M.; Ahmed, I.S.; Rawas-Qalaji, M.; Hussain, Z. Next-generation nanocarriers for colorectal cancer: Passive, active, and stimuli-responsive strategies for precision therapy. Biomater. Sci. 2025, 13, 5626–5664. [Google Scholar] [CrossRef]
- Lyon, P.C.; Griffiths, L.F.; Lee, J.; Chung, D.; Carlisle, R.; Wu, F.; Middleton, M.R.; Gleeson, F.V.; Coussios, C.C. Clinical trial protocol for TARDOX: A phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J. Ther. Ultrasound 2017, 5, 28. [Google Scholar] [CrossRef]
- Edmans, J.G.; El-Howati, A.; Slowik, K.M.; Colley, H.E.; Murdoch, C.; Hatton, P.V.; Armes, S.P. pH-responsive diblock copolymer vesicles via polymerization-induced self-assembly in aqueous media: Synthesis, loading, and potential biological applications. ACS Appl. Mater. Interfaces 2025, 17, 35140–35154. [Google Scholar] [CrossRef]
- Ma, P.; Wang, Q.; Luo, X.; Mao, L.; Wang, Z.; Ye, E.; Loh, X.J.; Li, Z.; Wu, Y.-L. Recent advances in stimuli-responsive polymeric carriers for controllable CRISPR/Cas9 gene editing system delivery. Biomater. Sci. 2023, 11, 5078–5094. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Zarepour, A.; Zarrabi, A. A new theranostic pH-responsive niosome formulation for doxorubicin delivery and bio-imaging against breast cancer. Int. J. Pharm. 2023, 637, 122845. [Google Scholar] [CrossRef]
- Sun, S.; Sun, Y.; Wang, P.; Zhang, J.; Du, W.; Wang, S.; Liang, X. Bubble-Manipulated Local Drug Release from a Smart Thermosensitive Cerasome for Dual-Mode Imaging Guided Tumor Chemo-Photothermal Therapy. Theranostics 2019, 9, 8138–8154. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular vesicles: A review of their therapeutic potentials, sources, biodistribution, and administration routes. Int. J. Nanomed. 2025, 20, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, C.; Su, Y.; Luo, X.; Liu, X.; Song, Y.; Deng, Y. Enhanced opsonization-independent phagocytosis and high response ability to opsonized antigen–antibody complexes: A new role of kupffer cells in the accelerated blood clearance phenomenon upon repeated injection of PEGylated emulsions. Mol. Pharm. 2018, 15, 3755–3766. [Google Scholar] [CrossRef]
- Kowkabany, G.; Bao, Y. Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles. Molecules 2024, 29, 4672. [Google Scholar] [CrossRef]
- Wardhani, K.; Levina, A.; Grau, G.E.; Lay, P.A. Fluorescent, phosphorescent, magnetic resonance contrast and radioactive tracer labelling of extracellular vesicles. Chem. Soc. Rev. 2024, 53, 6779–6829. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lattekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Dietz, L.; Oberländer, J.; Mateos-Maroto, A.; Schunke, J.; Fichter, M.; Krämer-Albers, E.-M.; Landfester, K.; Mailänder, V. Uptake of extracellular vesicles into immune cells is enhanced by the protein corona. J. Extracell. Vesicles 2023, 12, e12399. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, C.; Airò, G.; Pratticò, F.; Testi, I.; Corianò, M.; Pellegrino, B.; Denaro, N.; Demurtas, L.; Dessì, M.; Murgia, S. Hormone receptor-positive/HER2-Positive Breast Cancer: Hormone Therapy and Anti-HER2 treatment: An update on treatment strategies. J. Clin. Med. 2024, 13, 1873. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Du, S.; Yang, Y.; Zhu, Y.; McKeague, N.; Lin, B.; Bu, W.; Cheng, G.; Liu, Y. Molecular Effects of Zwitterionic Peptide on Monolayer Lipid Membranes upon Enzyme-Catalyzed Degradation. Langmuir 2025, 41, 3402–3412. [Google Scholar] [CrossRef]
- Kianinejad, N.; Razeghifard, R.; Omidian, H.H.; Omidi, Y.; Kwon, Y.M. Preparation and Characterization of Niosomes for the Delivery of a Lipophilic Model Drug: Comparative Stability Study with Liposomes Against Phospholipase-A2. J. Liposome Res. 2025, 35, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.C.; Reyes, L.H. Polymer-Based Nanocarriers: Dendrimers, Polymeric Micelles, Hydrogels. In Nanocarriers for Nucleic Acids and Proteins; Reyes, L.H., Cruz, J.C., Pathak, Y.V., Eds.; CRC Press: Boca Raton, FL, USA, 2025; p. 424. [Google Scholar]
- Sabatke, B.; Rossi, I.V.; Sana, A.; Bonato, L.B.; Ramirez, M.I. Extracellular vesicles biogenesis and uptake concepts: A comprehensive guide to studying host–pathogen communication. Mol. Microbiol. 2024, 122, 613–629. [Google Scholar] [CrossRef]
- Kubeil, M.; Martínez, I.I.S.; Bachmann, M.; Kopka, K.; Tuck, K.L.; Stephan, H. Dual-labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 432. [Google Scholar] [CrossRef]
- Pearson, I. Vesicle Immunotherapy: A Synthetic Upgrade on T-Cell Logic for Rapid Disease and Cancer Treatment. 2025. Available online: https://www.researchgate.net/publication/395720514_Vesicle_Immunotherapy_A_Synthetic_Upgrade_on_T-Cell_Logic_for_Rapid_Disease_and_Cancer_Treatment (accessed on 22 September 2025).
- Cisneros, E.P.; Morse, B.A.; Savk, A.; Malik, K.; Peppas, N.A.; Lanier, O.L. The role of patient-specific variables in protein corona formation and therapeutic efficacy in nanomedicine. J. Nanobiotechnol. 2024, 22, 714. [Google Scholar] [CrossRef]
- Azoidis, I.; Cox, S.C.; Davies, O.G. The role of extracellular vesicles in biomineralisation: Current perspective and application in regenerative medicine. J. Tissue Eng. 2018, 9, 2041731418810130. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered extracellular vesicles: Tailored-made nanomaterials for medical applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef]
- Tahover, E.; Patil, Y.P.; Gabizon, A.A. Emerging delivery systems to reduce doxorubicin cardiotoxicity and improve therapeutic index: Focus on liposomes. Anti-Cancer Drugs 2015, 26, 241–258. [Google Scholar] [CrossRef]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Cao, Y.; Liu, K.; Shi, H.; Guo, X.; Liu, W.; Hao, R.; Song, H.; Zhao, R. Nanocarriers for the delivery of antibiotics into cells against intracellular bacterial infection. Biomater. Sci. 2023, 11, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xiong, M.; Bi, Q.; Wang, Y.; Li, C. Self-enhanced targeted delivery of a cell wall–and membrane-active antibiotics, daptomycin, against staphylococcal pneumonia. Acta Pharm. Sin. B 2016, 6, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.R.; Bardeskar, P.; Bhor, V.M. Opportunities and challenges in harnessing the immunomodulatory potential of bacterial extracellular vesicles as vaccine candidates. Discov. Immunol. 2025, 2, 2. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Mohanty, D.; Choudhury, A.; Imam, S.S. Theranostic Applications of Functionalized Vesicular Carriers: Theranostic Applications of Functionalized Vesicular Carriers (Liposomes, Niosomes, Virosomes, Ethosomes, Phytosomes). In Multifunctional and Targeted Theranostic Nanomedicines: Formulation, Design and Applications; Springer: London, UK, 2023; pp. 49–76. [Google Scholar]
- Liu, H.; Huang, L.; Mao, M.; Ding, J.; Wu, G.; Fan, W.; Yang, T.; Zhang, M.; Huang, Y.; Xie, H.Y. Viral Protein-Pseudotyped and siRNA-Electroporated Extracellular Vesicles for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 2006515. [Google Scholar] [CrossRef]
- Akbar, N.; Razzaq, S.S.; Salim, A.; Haneef, K. Mesenchymal stem cell-derived exosomes and their MicroRNAs in heart repair and regeneration. J. Cardiovasc. Transl. Res. 2024, 17, 505–522. [Google Scholar] [CrossRef]
- Parsa, M.B.; Tafvizi, F.; Chaleshi, V.; Ebadi, M. Preparation, characterization, and Co-delivery of cisplatin and doxorubicin-loaded liposomes to enhance anticancer Activities. Heliyon 2023, 9, e20657. [Google Scholar] [CrossRef]
- Verma, N.; Arora, S. Navigating the global regulatory landscape for exosome-based therapeutics: Challenges, strategies, and future directions. Pharmaceutics 2025, 17, 990. [Google Scholar] [CrossRef]
- Jeyamogan, S.; Khan, N.A.; Siddiqui, R. Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer. Arch. Med. Res. 2021, 52, 131–142. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Tannous, B.A. Nanotechnology-inspired extracellular vesicles theranostics for diagnosis and therapy of central nervous system diseases. ACS Appl. Mater. Interfaces 2022, 15, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Xiang, D.; Nguyen, T.N.; Tran, T.T.; Chen, Q.; Yin, W.; Zhang, Y.; Kong, L.; Duan, A.; Chen, K. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics 2020, 10, 3849. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.R.; Port, J.D.; Pandey, M.K. Use of superparamagnetic iron oxide nanoparticles (SPIONs) via multiple imaging modalities and modifications to reduce cytotoxicity: An educational review. J. Nanotheranostics 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, D.; Szigeti, K. Radiolabeling of extracellular vesicles with 99mTc for quantitative in vivo imaging studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef]
- Lazaro-Ibanez, E.; Faruqu, F.N.; Saleh, A.F.; Silva, A.M.; Tzu-Wen Wang, J.; Rak, J.; Al-Jamal, K.T.; Dekker, N. Selection of Fluorescent, Bioluminescent, and Radioactive Tracers to Accurately Reflect Extracellular Vesicle Biodistribution in vivo. ACS Nano 2021, 15, 3212–3227. [Google Scholar] [CrossRef]
- Phua, V.J.; Yang, C.-T.; Xia, B.; Yan, S.X.; Liu, J.; Aw, S.E.; He, T.; Ng, D.C. Nanomaterial Probes for Nuclear Imaging. Nanomaterials 2022, 12, 582. [Google Scholar] [CrossRef]
- Zelger-Paulus, S.; Hadzic, M.C.; Sigel, R.K.; Börner, R. Encapsulation of fluorescently labeled RNAs into surface-tethered vesicles for single-molecule FRET studies in TIRF microscopy. In RNA Spectroscopy: Methods and Protocols; Springer: London, UK, 2020; pp. 1–16. [Google Scholar]
- Arranja, A.G.; Pathak, V.; Lammers, T.; Shi, Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol. Res. 2017, 115, 87–95. [Google Scholar] [CrossRef]
- Clemons, T.D.; Singh, R.; Sorolla, A.; Chaudhari, N.; Hubbard, A.; Iyer, K.S. Distinction between active and passive targeting of nanoparticles dictate their overall therapeutic efficacy. Langmuir 2018, 34, 15343–15349. [Google Scholar] [CrossRef]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic resonance imaging and iron-oxide nanoparticles in the era of personalized medicine. Nanotheranostics 2023, 7, 424. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579. [Google Scholar] [CrossRef]
- Yu, D.; Ding, Q.; Xiang, C.; Wang, D.; Hu, L.; Wang, J.; Qian, K.; Cheng, Z.; Li, Z. NIR-II Engineered Exosome Nanotheranostic Probes for “Oriented Blasting” in Orthotopic Glioblastoma. ACS Nano 2025, 19, 22900–22913. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Zhang, Y.; Li, J.; Zhang, K. Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches. Nanomaterials 2025, 15, 1262. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kim, Y.; Rha, H.; Kim, D.; Debnath, S.; Pu, K.; Kang, H.; Kim, J.S. Site-Specific Transformable Nanostructures for Cancer Therapy and Diagnosis. Chem. Rev. 2025, 125, 9012–9052. [Google Scholar] [CrossRef]
- Stawarska, A.; Bamburowicz-Klimkowska, M.; Runden-Pran, E.; Dusinska, M.; Cimpan, M.R.; Rios-Mondragon, I.; Grudzinski, I.P. Extracellular vesicles as next-generation diagnostics and advanced therapy medicinal products. Int. J. Mol. Sci. 2024, 25, 6533. [Google Scholar] [CrossRef]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory considerations, challenges and risk-based approach in nanomedicine development. Curr. Med. Chem. 2021, 28, 7461–7476. [Google Scholar] [CrossRef]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—Regulatory overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mittal, A.; Kulkarni, M.P. Quality by Design in Pharmaceutical Development. In Computer Aided Pharmaceutics and Drug Delivery; Saharan, V.A., Ed.; Springer: Singapore, 2024; p. 80. [Google Scholar]
- Kapadia, R.; Shevalkar, G.; Das, U.; Singhai, V.; Bari, D.; Pardeshi, C.V. Introduction to Quality by Design. In Introduction to Quality by Design (QbD) From Theory to Practice; Springer: London, UK, 2024; pp. 1–33. [Google Scholar]
- Wigman, L.; Ooi, D. ICH Q10 Quality Systems. In ICH Quality Guidelines: An Implementation Guide; Teasdale, A., Elder, D., Nims, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Chapter 22; pp. 611–637. [Google Scholar] [CrossRef]
- Zarovni, N.; Loria, F.; Zenatelli, R.; Mladenovic, D.; Paolini, L.; Adamo, G.; Radeghieri, A.; Bongiovanni, A.; Bergese, P. Standardization and commercialization of extracellular vesicles. In Extracellular Vesicles: Applications to Regenerative Medicine, Therapeutics and Diagnostics; Chrzanowski, W., Lim, C.T., Kim, S.Y., Eds.; Biomaterials Science Series; Royal Society of Chemistry (RSC): London, UK, 2021; pp. 303–335. [Google Scholar]
- Takakura, Y.; Hanayama, R.; Akiyoshi, K.; Futaki, S.; Hida, K.; Ichiki, T.; Ishii-Watabe, A.; Kuroda, M.; Maki, K.; Miura, Y. Quality and safety considerations for therapeutic products based on extracellular vesicles. Pharm. Res. 2024, 41, 1573–1594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef]
- Denman, D.S.; Dalhaimer, P. The curious case of anti-PEG antibodies. Nanoscale 2025, 17, 22594–22605. [Google Scholar] [CrossRef]
- Narayanan, P. Examining the Toxicological Landscape of New Molecular Entities and Biotherapeutics. In Approved: The Life Cycle of Drug Development; Chirmule, N., Ghalsasi, V.V., Eds.; Springer: London, UK, 2025; pp. 365–394. [Google Scholar]
- Rudmann, D.G. On-target and off-target-based toxicologic effects. Toxicol. Pathol. 2013, 41, 310–314. [Google Scholar] [CrossRef]
- Nazari-Shafti, T.Z.; Neuber, S.; Duran, A.G.; Exarchos, V.; Beez, C.M.; Meyborg, H.; Krüger, K.; Wolint, P.; Buschmann, J.; Böni, R. MiRNA profiles of extracellular vesicles secreted by mesenchymal stromal cells—Can they predict potential off-target effects? Biomolecules 2020, 10, 1353. [Google Scholar] [CrossRef]
- Levchuk, J.W. Compliance with Good Manufacturing Practice. In Chemists’ Views of Imaging Centers; Springer: London, UK, 1995; pp. 97–103. [Google Scholar]
- Cheng, Y.; Hay, C.D.; Mahuttanatan, S.M.; Hindley, J.W.; Ces, O.; Elani, Y. Microfluidic technologies for lipid vesicle generation. Lab. A Chip 2024, 24, 4679–4716. [Google Scholar] [CrossRef]
- Hendrix, A.; Lippens, L.; Pinheiro, C.; Théry, C.; Martin-Jaular, L.; Lötvall, J.; Lässer, C.; Hill, A.F.; Witwer, K.W. Extracellular vesicle analysis. Nat. Rev. Methods Primers 2023, 3, 56. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical liposomal delivery—Specific considerations of innovation and challenges. Biomater. Sci. 2023, 11, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Zhong, Y.; Shen, J.; An, W. Biomimetic exosomes: A new generation of drug delivery system. Front. Bioeng. Biotechnol. 2022, 10, 865682. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- Kansız, S.; Elçin, Y.M. Advanced liposome and polymersome-based drug delivery systems: Considerations for physicochemical properties, targeting strategies and stimuli-sensitive approaches. Adv. Colloid. Interface Sci. 2023, 317, 29. [Google Scholar] [CrossRef] [PubMed]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
| Vesicle Type | Structural Features | Functional Attributes | Advantages | Limitations | Performance in Delivery |
|---|---|---|---|---|---|
| Liposomes |
|
|
|
|
|
| Polymeric vesicles (polymersomes) |
|
|
|
|
|
| Extracellular vesicles (exosomes) |
|
|
|
|
|
| Hybrid vesicles (lipid–polymer or cell membrane -coated) |
|
|
|
|
|
| Metal—organic framework (MOF) nano-vesicles |
|
|
|
|
|
| Vesicle Type | Composition | Key Properties | Preparation Methods | Applications | Advantages | Challenges |
|---|---|---|---|---|---|---|
| Liposomes | Phospholipid bilayers ± Cholesterol |
|
|
|
|
|
| Niosomes | Non-ionic surfactants (Span, Tween) ± Cholesterol |
|
|
|
|
|
| Polymersomes | Amphiphilic block copolymers |
|
|
|
|
|
| Extracellular Vesicles (EVs)/Exosomes | Naturally secreted nanoscale vesicles (lipids, proteins, nucleic acids) |
|
|
|
|
|
| Hybrid and Specialized Vesicles | Combinations of natural and synthetic systems (liposome–EV, polymersome–lipid, niosome–lipid) Functionalized constructs (magnetoliposomes, immunoliposomes) |
|
|
|
|
|
| Biomedical Area | Vesicle Types | Objectives/Function | Examples/Applications | Advantages | Challenges |
|---|---|---|---|---|---|
| Oncology | Liposomes, Polymersomes, EVs |
|
|
|
|
| Neurology/CNS | EVs, Polymersomes, PEGylated liposomes |
|
|
|
|
| Infectious Diseases | Liposomes, EVs, Niosomes |
|
|
|
|
| Regenerative Medicine | EVs, Liposomes, Polymersomes |
|
|
|
|
| Vaccines | Liposomes, Niosomes, EVs |
|
|
|
|
| Combination Therapies | Liposomes, Polymersomes, EVs |
|
|
|
|
| Vesicle Type | Regulatory Considerations | Safety Concerns | Manufacturing Challenges |
|---|---|---|---|
| Liposomes |
|
|
|
| Polymersomes |
|
|
|
| Niosomes |
|
|
|
| Extracellular Vesicles (EVs) |
|
|
|
| Hybrid Vesicles (lipid–polymer, liposome–EV) |
|
|
|
| Stimuli-Responsive Vesicles |
|
|
|
| Theranostic Vesicles |
|
|
|
| Vesicle Type | Approximate TRL Range | Approved Products (Examples) | Representative Clinical Trials (NCT/Estudio) | Remarks/Considerations |
|---|---|---|---|---|
| Liposomes (conventional/PEGylated/stimuli-sensitive) | TRL 8–9 (clinically established) |
|
| The most mature platform overall, backed by established manufacturing processes and regulatory frameworks, has allowed for the encapsulation of a wide variety of payloads, from classic small-molecule drugs, such as antifungals and chemotherapeutics, to mRNA vaccines. This highlights its versatility and biocompatibility. |
| Extracellular Vesicles (EVs/exosomes/microvesicles) | TRL 3–6 (preclinical/early trials) | There are no approved therapies for EVs yet as a regulated commercial product |
| It shows great promise due to its biological origin and ability to transport complex molecules. However, it lacks production standards (GMP), robust characterization, and solid clinical data. Clinical translation is still in its early stages. |
| Polymersomes (polymer vesicles) | TRL 2–4 (mainly preclinical) | As far as our knowledge extends, no polymersome-based formulation has achieved approval. | To date, there are few, if any, published clinical trials using polymersomes as a regular therapeutic platform. Their use is mostly limited to preclinical studies. | They offer high versatility: different polymers, the possibility of adapting to stimuli (pH, redox, temperature, etc.), but biocompatibility, biodegradability, and regulatory pathways are not yet fully established. Slow clinical translation. |
| Stimuli-responsive/Hybrid/Biomimetic Vesicles (hybrid lipid/polymer vesicles) | TRL 1–3 (concept/in vitro/preclinical) | There are no publicly available reports of major clinical trials. Most developments remain at the research stage. | These platforms combine advanced features (controlled release, stimulus response, targeted delivery, bio-synthetic hybrids), making them conceptually very attractive. However, they face significant challenges: production scale, reproducibility, toxicology, regulation, and a lack of in vivo data. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedini, L.A.; Messina, P.V. Smart Vesicle Therapeutics: Engineering Precision at the Nanoscale. Pharmaceutics 2025, 17, 1588. https://doi.org/10.3390/pharmaceutics17121588
Benedini LA, Messina PV. Smart Vesicle Therapeutics: Engineering Precision at the Nanoscale. Pharmaceutics. 2025; 17(12):1588. https://doi.org/10.3390/pharmaceutics17121588
Chicago/Turabian StyleBenedini, Luciano A., and Paula V. Messina. 2025. "Smart Vesicle Therapeutics: Engineering Precision at the Nanoscale" Pharmaceutics 17, no. 12: 1588. https://doi.org/10.3390/pharmaceutics17121588
APA StyleBenedini, L. A., & Messina, P. V. (2025). Smart Vesicle Therapeutics: Engineering Precision at the Nanoscale. Pharmaceutics, 17(12), 1588. https://doi.org/10.3390/pharmaceutics17121588







