Intraluminal Vesicles as Transfection Intermediaries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
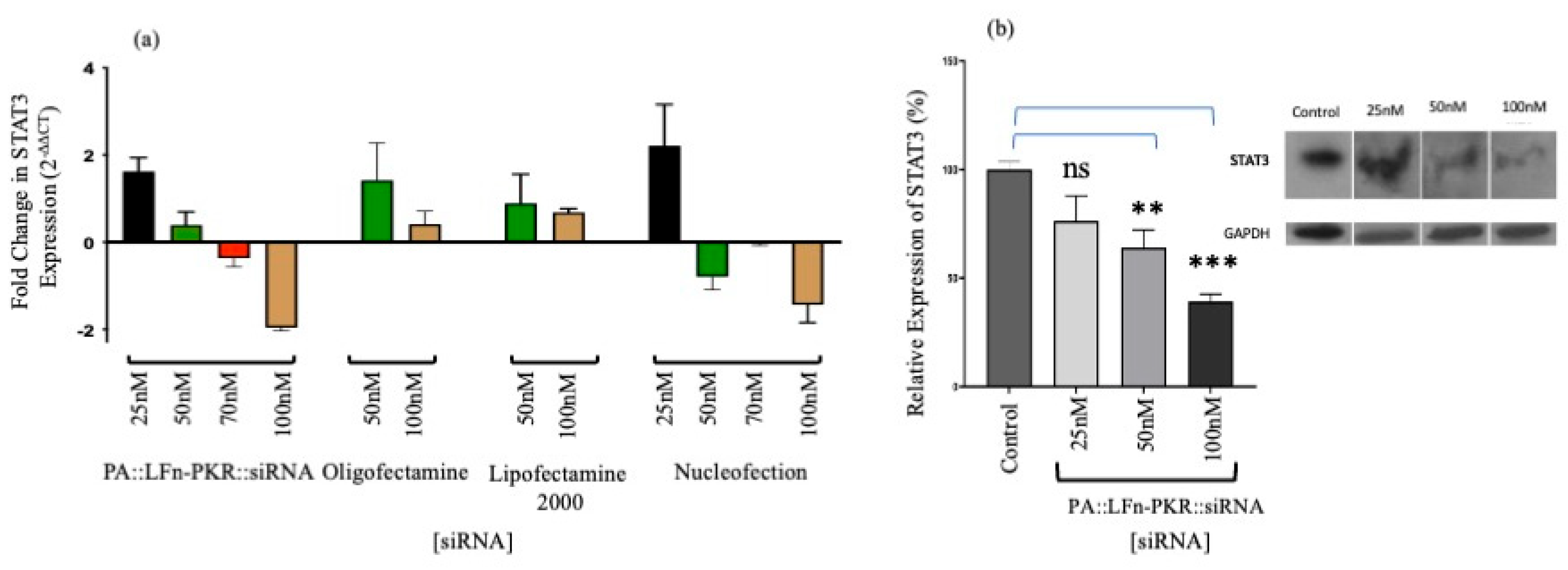
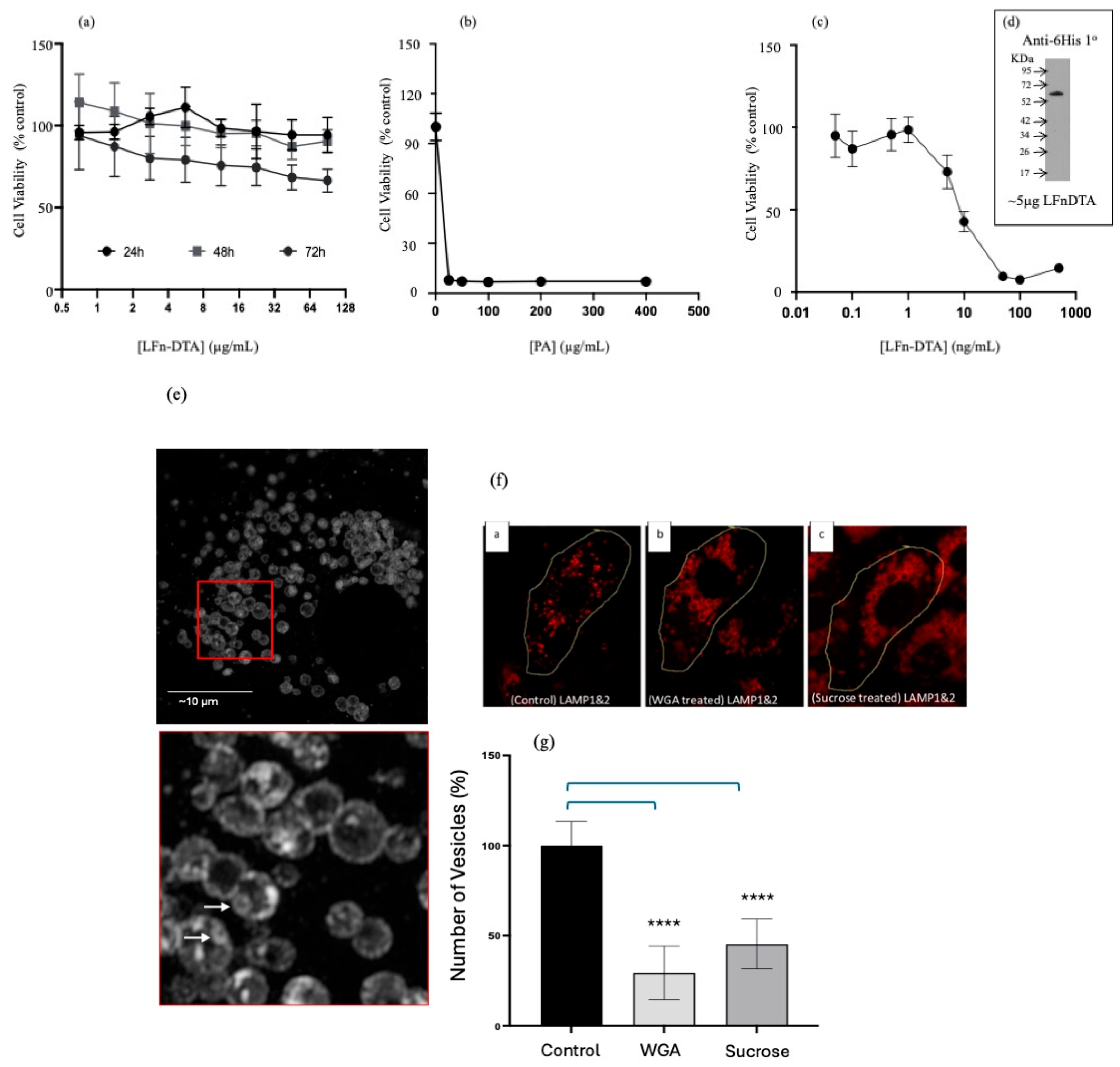
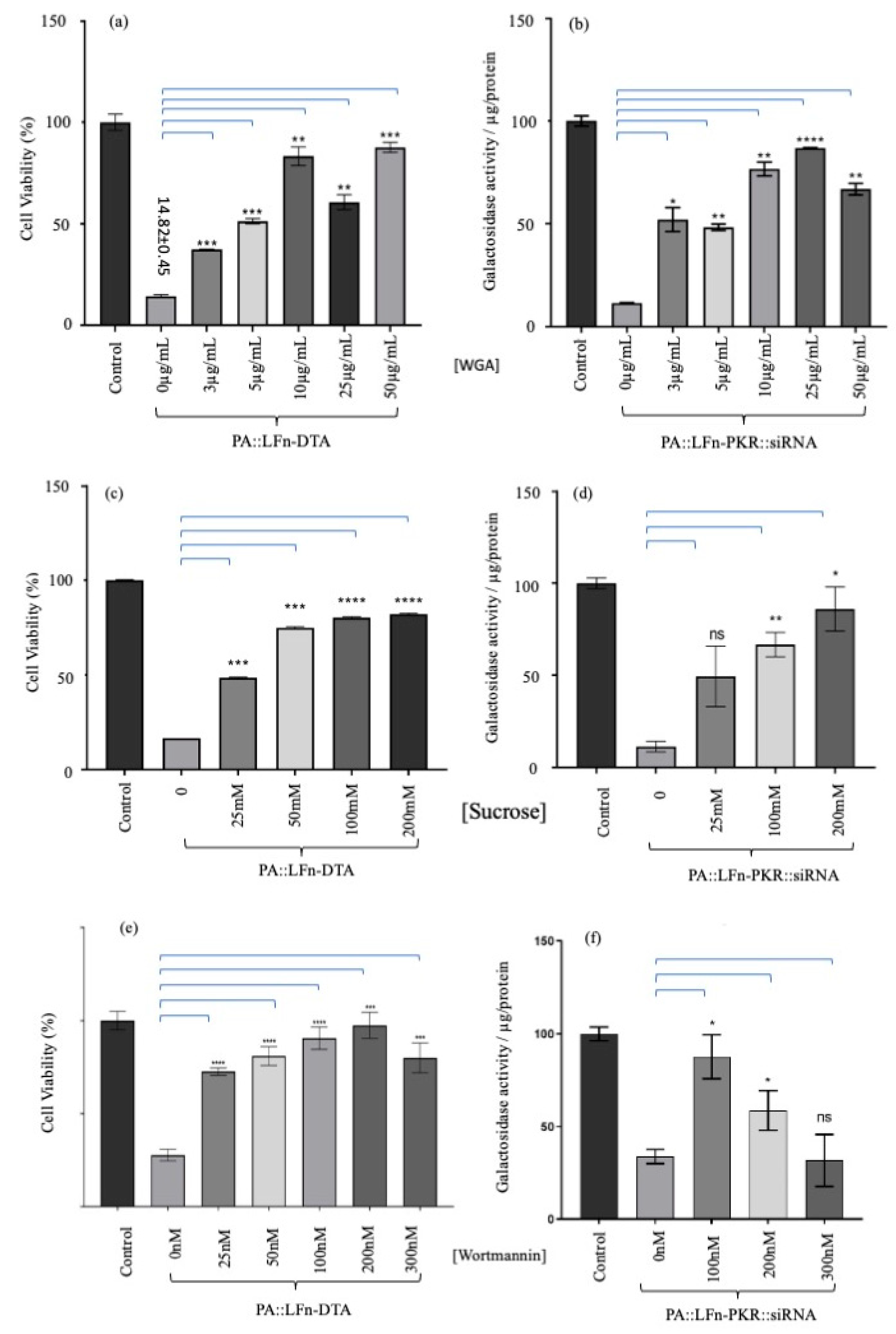
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kerr, J.R.; Schneider, C.R.; Recchia, G.; Dryhurst, S.; Sahlin, U.; Dufouil, C.; Arwidson, P.; Freeman, A.L.; van der Linden, S. Correlates of intended COVID-19 vaccine acceptance across time and countries: Results from a series of cross-sectional surveys. BMJ Open 2021, 11, e048025. [Google Scholar] [CrossRef]
- Jo, S.J.; Chae, S.U.; Bin Lee, C.; Bae, S.K. Clinical Pharmacokinetics of Approved RNA Therapeutics. Int. J. Mol. Sci. 2023, 24, 746. [Google Scholar] [CrossRef]
- Lorenzer, C.; Dirin, M.; Winkler, A.-M.; Baumann, V.; Winkler, J. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J. Control. Release 2015, 203, 1–15. [Google Scholar] [CrossRef]
- Dyer, P.D.; Shepherd, T.R.; Gollings, A.S.; Shorter, S.A.; Gorringe-Pattrick, M.A.; Tang, C.-K.; Cattoz, B.N.; Baillie, L.; Griffiths, P.C.; Richardson, S.C. Disarmed anthrax toxin delivers antisense oligonucleotides and siRNA with high efficiency and low toxicity. J. Control. Release 2015, 220, 316–328. [Google Scholar] [CrossRef]
- Hirschenberger, M.; Stadler, N.; Fellermann, M.; Sparrer, K.M.J.; Kirchhoff, F.; Barth, H.; Papatheodorou, P. CRISPA: A Non-viral, Transient Cas9 Delivery System Based on Reengineered Anthrax Toxin. Front. Pharmacol. 2021, 12, 770283. [Google Scholar] [CrossRef]
- Verdurmen, W.P.R.; Luginbühl, M.; Honegger, A.; Plückthun, A. Efficient cell-specific uptake of binding proteins into the cytoplasm through engineered modular transport systems. J. Control. Release 2015, 200, 13–22. [Google Scholar] [CrossRef]
- Liao, X.; Rabideau, A.E.; Pentelute, B.L. Delivery of antibody mimics into mammalian cells via anthrax toxin protective antigen. ChemBioChem 2014, 15, 2458–2466. [Google Scholar] [CrossRef]
- Feron, B.K.L.; Gomez, T.; Youens, N.C.; Mahmoud, N.A.M.; Abdelrahman, H.K.S.; Bugert, J.J.; Richardson, S.C.W. Antiviral siRNA Delivered Using Attenuated, Anthrax Toxin Protects Cells from the Cytopathic Effects of Zika virus. Virus Genes 2025, 61, 342–354. [Google Scholar] [CrossRef]
- Eden, E.R.; Huang, F.; Sorkin, A.; Futter, C.E. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic 2011, 13, 329–337. [Google Scholar] [CrossRef]
- Abrami, L.; Lindsay, M.; Parton, R.G.; Leppla, S.H.; van der Goot, F.G. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 2004, 166, 645–651. [Google Scholar] [CrossRef]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic Structure of Anthrax protective antigen pore elucidates toxin translocation. Nature 2015, 521, 545–549. [Google Scholar] [CrossRef]
- Milne, J.C.; Blanket, S.R.; Hanna, P.C.; Collier, R.J. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 1995, 15, 661–666. [Google Scholar] [CrossRef]
- Muraki, M.; Ishimura, M.; Harata, K. Interactions of wheat-germ agglutinin with GlcNAcβ, 6Gal sequence. Biochim. Biophys. Acta 2002, 1569, 10–20. [Google Scholar] [CrossRef]
- Shorter, S.A.; Pettit, M.W.; Dyer, P.D.R.; Youngs, E.C.; Gorringe-Pattrick, M.A.M.; El-Daher, S.; Richardson, S. Green Fluorescent Protein (GFP): Is seeing believing and is that enough? J. Drug Target. 2017, 25, 809–817. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Winistorfer, S.C.; Poupon, V.; Luzio, J.P.; Piper, R.C. Mammalian Late Vacuole Protein Sorting Orthologues Participate in Early Endosomal Fusion and Interact with the Cytoskeleton. Mol. Biol. Cell. 2004, 15, 1197–1210. [Google Scholar] [CrossRef]
- Patki, V.; Virbasius, J.; Lane, W.S.; Toh, B.-H.; Shpetner, H.S.; Corvera, S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase (wortmannin). Proc. Natl. Acad. Sci. USA 1997, 94, 7326–7330. [Google Scholar] [CrossRef]
- Bright, N.A.; Reaves, B.J.; Mullock, B.M.; Luzio, J.P. Dense core lysosomes can fuse with late endosomes and are re-formed from the resultant hybrid organelles. J. Cell Sci. 1997, 110, 2027–2040. [Google Scholar] [CrossRef]
- Sava, I.; Davis, L.J.; Gray, S.R.; Bright, N.A.; Luzio, J.P. Reversible assembly and disassembly of V-ATPase during the lysosome regeneration cycle. Mol. Biol. Cell 2024, 35, ar63. [Google Scholar] [CrossRef]
- Babst, M. MVB Vesicle Formation: ESCRT-Dependent, ESCRT-Independent and Everything in Between. Curr. Opin. Cell Biol. 2011, 23, 452–457. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Wallom, K.L.; Ferguson, E.L.; Deacon, S.P.E.; Davies, M.W.; Powell, A.J.; Piper, R.C.; Duncan, R. The use of fluorescence microscopy to define polymer localisation to the late endocytic compartments in cells that are targets for drug delivery. J. Control. Release 2008, 127, 1–11. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Pattrick, N.G.; Lavignac, N.; Ferruti, P.; Duncan, R. Intracellular fate of bioresponsive poly(amidoamine)s in vitro and in vivo. J. Control. Release 2010, 142, 78–88. [Google Scholar] [CrossRef][Green Version]
- Corrotte, M.; Fernandes, M.C.; Tam, C.; Andrews, N.W. Toxin Pores Endocytosed During Plasma Membrane Repair Traffic into the Lumen of MVBs for Degradation. Traffic 2011, 13, 483–494. [Google Scholar] [CrossRef]
- Abrami, L.; Brandi, L.; Moayeri, M.; Brown, M.J.; Krantz, B.A.; Leppla, S.H.; van der Goot, F.G. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013, 5, 986–996. [Google Scholar] [CrossRef]
- Sergeeva, O.; van der Goot, F. Converging physiological roles of the anthrax toxin receptors. F1000research 2019, 8, 1415. [Google Scholar] [CrossRef]
- Abrami, L.; Leppla, S.H.; van der Goot, F.G. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J. Cell Biol. 2006, 172, 309–320. [Google Scholar] [CrossRef]
- Richardson, S.; Feron, B. WO2020030923—Method for Preparing Liposomes PCT/GB2019/052239, 9 August 2019.
- Arnold, A.E.; Smith, L.J.; Beilhartz, G.L.; Bahlmann, L.C.; Jameson, E.; Melnyk, R.A.; Shoichet, M.S. Attenuated diphtheria toxin mediates siRNA delivery. Sci. Adv. 2020, 6, eaaz4848. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Brown, M.J.; Thoren, K.L.; Krantz, B.A. Role of the α Clamp in the Protein Translocation Mechanism of Anthrax Toxin. J. Mol. Biol. 2015, 427, 3340–3349. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.; Debska, G.; Szewczyk, A.C. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Kolbe, H.V.J.; Duncan, R. Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Sinden, R.R.; Pearson, C.E.; Potaman, V.N.; Ussery, D.W. DNA: Structure and Function. In Advances in Genome Biology; JAI Press: Greenwich, CT, USA, 1998. [Google Scholar] [CrossRef]
- Beaufay, H.; de Duve, C. The hexosephosphatase system. VI. Attempted fractionation of microsomes containing glucose-6-phosphatase. Bull. Soc. Chim. Biol. 1954, 36, 1551–1568. [Google Scholar] [PubMed]
- Zerial, M.; Melancon, P.; Schneider, C.; Garoff, H. The transmembrane segment of the human transferrin receptor functions as a signal peptide. EMBO J. 1986, 5, 1543–1550. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based analysis of lipid nanoparticle–mediated sirNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Yang, M.; Jin, H.; Chen, J.; Ding, L.; Ng, K.K.; Lin, Q.; Lovell, J.F.; Zhang, Z.; Zheng, G. Efficient Cytosolic Delivery of siRNA Using HDL-Mimicking Nanoparticles. Small 2011, 7, 568–573. [Google Scholar] [CrossRef]
- Hedlund, H.; Du Rietz, H.; Johansson, J.; Zedan, W.; Huang, L.; Wallin, J.; Wittrup, A. Absolute quantification and single-cell dose-response of cytosolic siRNA delivery. Nat. Commun. 2021, 14, 1–75. [Google Scholar] [CrossRef]
- Detzer, A.; Overhoff, M.; Wünsche, W.; Rompf, M.; Turner, J.J.; Ivanova, G.D.; Gait, M.J.; Sczakiel, G. Increased RNAi is related to intracellular release of siRNA via a covalently attached signal peptide. RNA 2009, 15, 627–636. [Google Scholar] [CrossRef]

| siRNA | Specific Activity (Ci/mmol) | Labelled siRNA (ng/mL) | Labelling Efficiency (%) | Free 32P (%) |
| 32P-Labelled GFP siRNA | 1679.786 | 40.24 | 65.55 | 14.82 |
| 32P-Labelled STAT3 siRNA | 2808.233 | 75.65 | 79.12 | 10.1 |
| Reagent | Utility | Catalogue Number | Supplier |
| GFP Reporter control siRNA | Stealth siRNA | 12935145 | ThermoFisher (Invitrogen) (Paisley, UK) |
| STAT3 | Silencer siRNA | AM16708 | ThermoFisher (Invitrogen) (Paisley, UK) |
| STAT3 (VIC) | TaqMan Probes set | Hs00374280_m1 | ThermoFisher (Invitrogen) (Paisley, UK) |
| GAPDH (FAM) | TaqMan Probes set | Hs02786624_g1 | ThermoFisher (Invitrogen) (Paisley, UK) |
| High GC scrambled siRNA | Control Stealth siRNA | 12935400 | ThermoFisher (Invitrogen) (Paisley, UK) |
| Antigen | Species | Cat No. | Supplier | Dilution |
| LAMP1 | Mouse MAb | H4A3 | DHSB * (Iowa City, IA, USA) | 1:10 (IF) |
| LAMP2 | Mouse MAb | H4B4 | DHSB * (Iowa City, IA, USA) | 1:10 (IF) |
| STAT3 | Mouse MAb | ab119352 | AbCam (Cambridge, UK) | 1:1000 (IB) |
| GAPDH | Rabbit PAb | Ab2609746 | AbCam (Cambridge, UK) | 1:5000 (IB) |
| TfR | Mouse MAb | 612124 | BD Bioscience (Berkshire UK) | 1:200 (IB) |
| EEA1 | Mouse MAb | E41120 | BD Bioscience (Berkshire UK) | 1:1000 (IB) |
| LDH | Mouse MAb | L7106 | Sigma (Dorset, UK) | 1:200 (IB) |
| 6His | Mouse MAb | 631212 | ClonTech Ltd. (Oxford, UK) | 1:5000 (IB) |
| RFP | Mouse MAb | 6G6 | Chromotech (Stockport, UK) | 1:500 (IB) |
| GFP | Rabbit PAb | SAB4301138 | Sigma (Dorset, UK) | 1:1000 (IB) |
| Anti-Rabbit HRP conjugate | Donkey | NA934 | GE Healthcare (Hatfield, UK) | 1:1000 (IB) |
| Anti-Mouse HRP conjugate | Sheep | NA931 | GE Healthcare (Hatfield, UK) | 1:1000 (IB) |
| Anti-Rabbit Texas Red conjugate | Goat | T-2767 | ThermoFisher (Invitrogen) (Paisley, UK) | 1:200 (IF) |
| Anti-Mouse Texas Red conjugate | Goat | T-862 | ThermoFisher (Invitrogen) (Paisley, UK) | 1:200 (IF) |
| Anti-Rabbit Alexa fluor 488 conjugate | Goat | A-11008 | ThermoFisher (Invitrogen) (Paisley, UK) | 1:200 (IF) |
| Anti-Mouse Alexa fluor 488 conjugate | Goat | A-11001 | ThermoFisher (Invitrogen) (Paisley, UK) | 1:200 (IF) |
| Cells | Transgene | Supplier | Cat No | Culture Conditions | Media |
| HEK293 | EmGFP-LacZ::RFP | ASMBio (Abingdon, UK) | SC008 | Split 1:20 twice a week | Dulbecco’s Modified Eagle Medium + 10% (v/v) Foetal Bovine Serum, 10 µg/mL blastocydin S, 1× penicillin–streptomycin-glutamine,1× nonessential amino acids |
| HeLa | - | American Type Culture Collection (VA, USA) | CCL2 | Split 1:20 twice a week | Eagle’s Minimum Essential Medium + 10% (v/v) Foetal Bovine Serum, 1× penicillin–streptomycin-glutamine,1× nonessential amino acid |
| MCF-7 | - | American Type Culture Collection (VA, USA) | HTB-22 | Split 1:3 twice a week | Eagle’s Minimum Essential Medium + 10% (v/v) Foetal Bovine Serum, 1× penicillin–streptomycin-glutamine,1× nonessential amino acid |
| Vero E6 | - | American Type Culture Collection (VA, USA) | CRL1568 | Split 1:20 twice a week | Eagle’s Minimum Essential Medium + 10% (v/v) Foetal Bovine Serum, 1× penicillin–streptomycin-glutamine,1× nonessential amino acids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.A.M.; Abdelrahman, H.K.S.; Feron, B.K.L.; Pintilie, A.; Fivaz, M.; Miest-Bray, J.J.; Gomez, T.; Youens, N.; Tripathi, V.; Richardson, S.C.W. Intraluminal Vesicles as Transfection Intermediaries. Pharmaceutics 2025, 17, 1584. https://doi.org/10.3390/pharmaceutics17121584
Mahmoud NAM, Abdelrahman HKS, Feron BKL, Pintilie A, Fivaz M, Miest-Bray JJ, Gomez T, Youens N, Tripathi V, Richardson SCW. Intraluminal Vesicles as Transfection Intermediaries. Pharmaceutics. 2025; 17(12):1584. https://doi.org/10.3390/pharmaceutics17121584
Chicago/Turabian StyleMahmoud, Nourhan A. M., Hadeer K. S. Abdelrahman, Benedita K. L. Feron, Andra Pintilie, Marc Fivaz, Joanna J. Miest-Bray, Timothy Gomez, Natalie Youens, Vineeta Tripathi, and Simon C. W. Richardson. 2025. "Intraluminal Vesicles as Transfection Intermediaries" Pharmaceutics 17, no. 12: 1584. https://doi.org/10.3390/pharmaceutics17121584
APA StyleMahmoud, N. A. M., Abdelrahman, H. K. S., Feron, B. K. L., Pintilie, A., Fivaz, M., Miest-Bray, J. J., Gomez, T., Youens, N., Tripathi, V., & Richardson, S. C. W. (2025). Intraluminal Vesicles as Transfection Intermediaries. Pharmaceutics, 17(12), 1584. https://doi.org/10.3390/pharmaceutics17121584







