Pharmacokinetics of CYP2C19- and CYP3A4-Metabolized Drugs in Cirrhosis Using a Whole-Body PBPK Approach
Abstract
1. Introduction
2. Materials and Methods
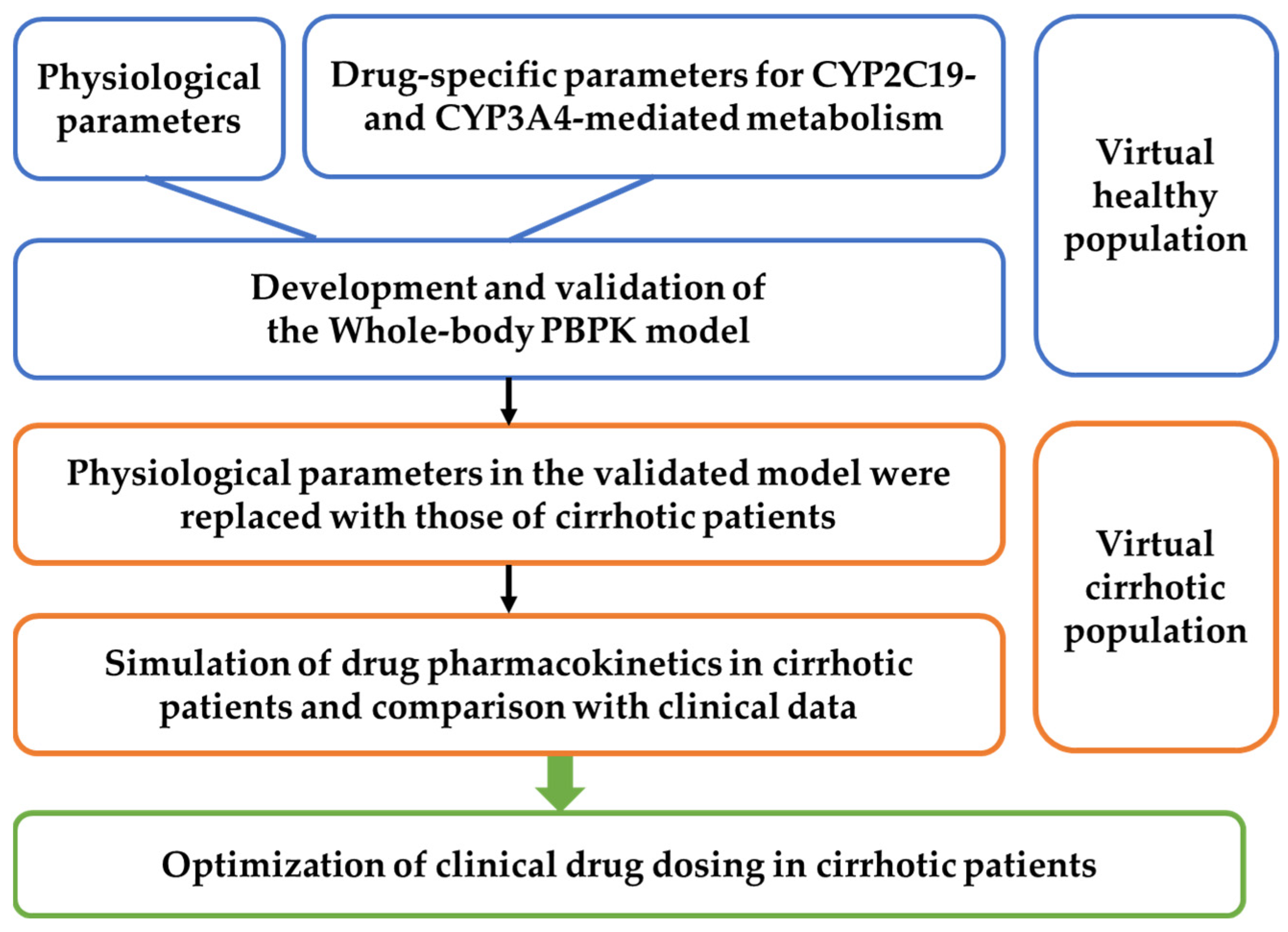
2.1. General Workflow
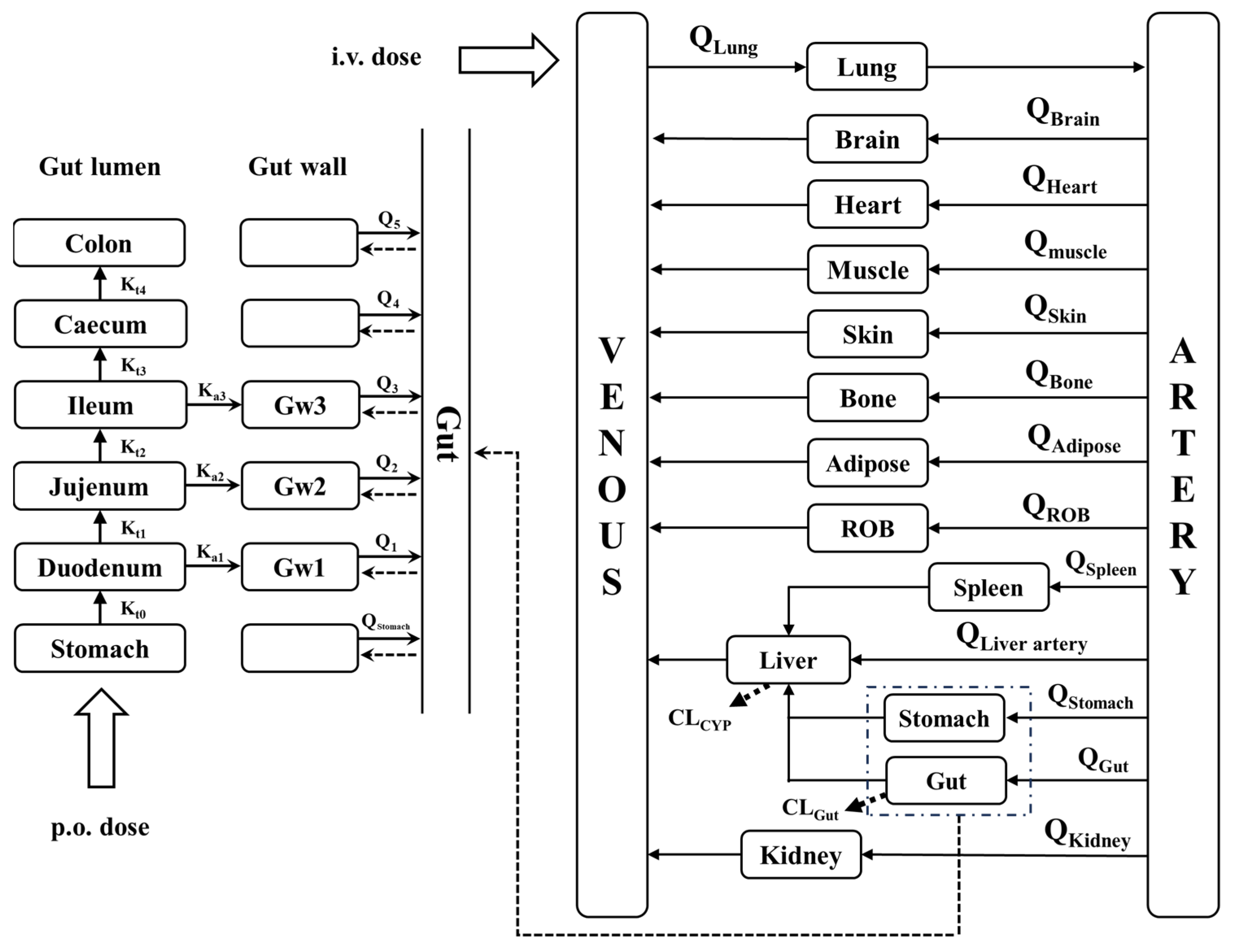
2.2. Model Development
2.3. PBPK Model Development for LC Patients
2.4. Model Validation
| Organs | Volume (mL) | Blood Flow (mL/min) |
|---|---|---|
| Stomach | 160 | 38 |
| Lungs | 1170 | 5600 |
| Muscle | 35,000 | 750 |
| Heart | 310 | 240 |
| Brain | 1450 | 700 |
| Adipose | 10,000 | 260 |
| Skin | 7800 | 300 |
| Liver | 1690 | 300 |
| Kidneys | 280 | 1240 |
| Spleen | 190 | 80 |
| Rest of body | 5100 | 592 |
| Artery blood | 1730 | / |
| Venous blood | 3470 | / |
| Duodenum | 70 | 118 |
| Jejunum | 209 | 413 |
| Ileum | 139 | 244 |
| Cecum | 116 | 44 |
| Colon | 1116 | 281 |
| r1(cm) | 2.00 | / |
| r2(cm) | 1.63 | / |
| r3(cm) | 1.45 | / |
| K0 (min−1) | 0.08 | / |
| K1 (min−1) | 0.07 | / |
| K2 (min−1) | 0.03 | / |
| K3 (min−1) | 0.04 | / |
| K4 (min−1) | 0.003 | / |
| K5 (min−1) | 0.001 | / |
| Parameters | Units | Child–Pugh Class | |||
|---|---|---|---|---|---|
| Healthy | CP-A | CP-B | CP-C | ||
| Qtotal [18] | mL/min | 5600 | 6496 | 7392 | 7896 |
| Hepatic arterial blood flow [33] | mL/min | 300 | 376 | 416 | 509 |
| Liver volume fraction [18] | / | 1.0 | 0.81 | 0.65 | 0.53 |
| Functional liver size [28] | / | 1 | 0.91 | 0.81 | 0.64 |
| Albumin [18] | g/L | 44.7 | 41.1 | 33.9 | 26.3 |
| α1-acid glycoprotein [18] | g/L | 0.8 | 0.57 | 0.52 | 0.46 |
| Hematocrit [18] | % | 40.9 | 36.6 | 32.9 | 31.9 |
| CYP3A4liver content [34] | pmol/mg protein | 137 | 107 | 70.2 | 42.8 |
| CYP3A4gut content [34] | pmol/mg protein | 65.4 | 65.4 | 39.9 | 31.7 |
| Duodenal CYP3A4 abundance | nmol | 9.7 | 9.7 | 5.92 | 4.70 |
| Jejunal CYP3A4 abundance | nmol | 38.4 | 38.4 | 23.42 | 18.62 |
| Ileal CYP3A4 abundance | nmol | 22.4 | 22.4 | 13.67 | 10.86 |
| CYP2C19liver content [18] | pmol/mg protein | 14 | 4.50 | 3.60 | 1.70 |
| CYP1A2liver content [18] | pmol/mg protein | 52 | 32.9 | 13.6 | 6.10 |
| CYP2D6liver content [18] | pmol/mg protein | 8.0 | 6.10 | 2.60 | 0.84 |
| PBSF | mg protein | 82,472 | 66,802.32 | 53,606.8 | 43,710.16 |
| K0 | min−1 | 0.08 | 0.1 | 0.1 | 0.1 |
| K1 | min−1 | 0.07 | 0.088 | 0.088 | 0.088 |
| K2 | min−1 | 0.03 | 0.038 | 0.038 | 0.038 |
| K3 | min−1 | 0.04 | 0.050 | 0.050 | 0.050 |
| K4 | min−1 | 0.003 | 0.004 | 0.004 | 0.004 |
| K5 | min−1 | 0.001 | 0.001 | 0.001 | 0.001 |
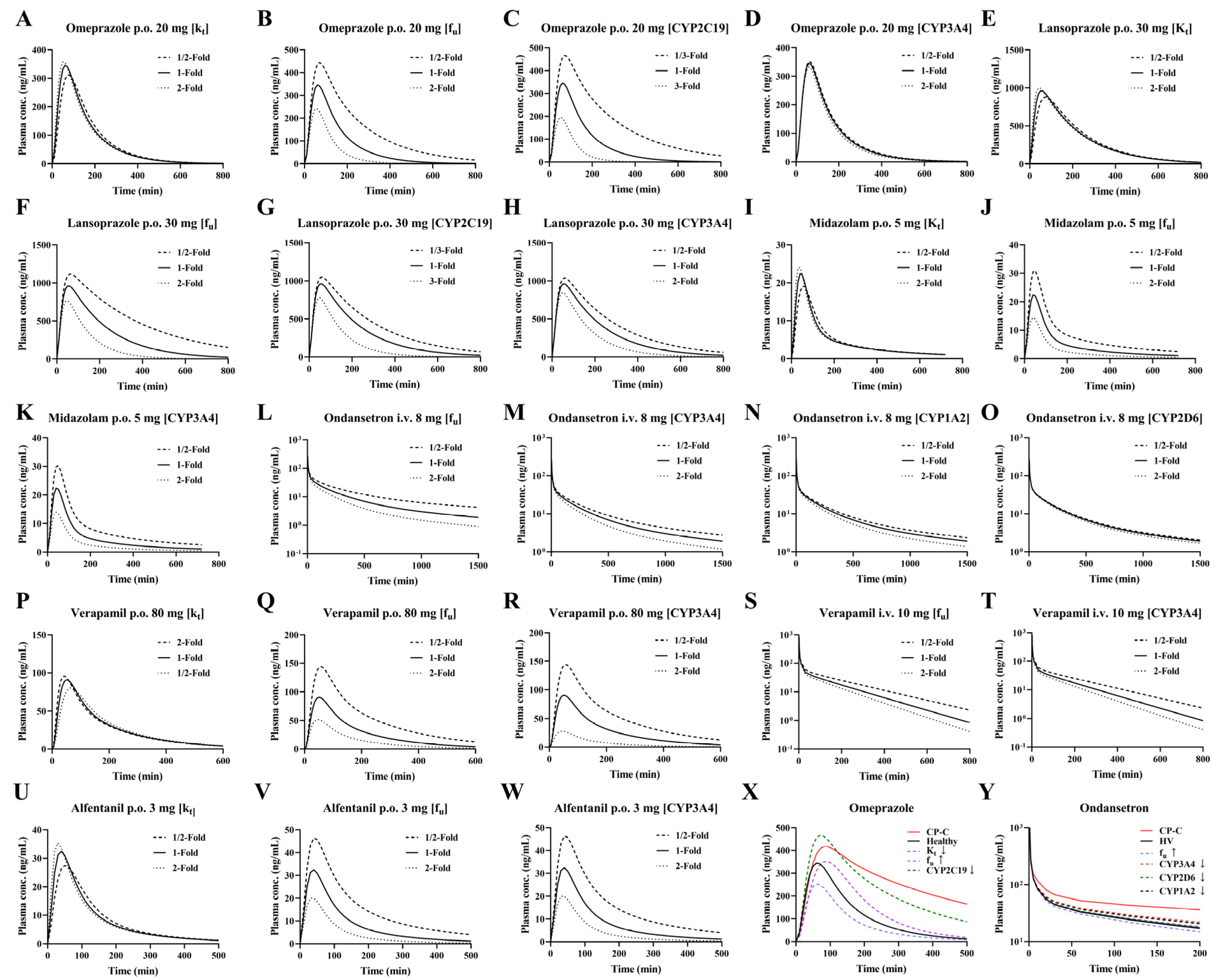
2.5. Sensitivity Analysis
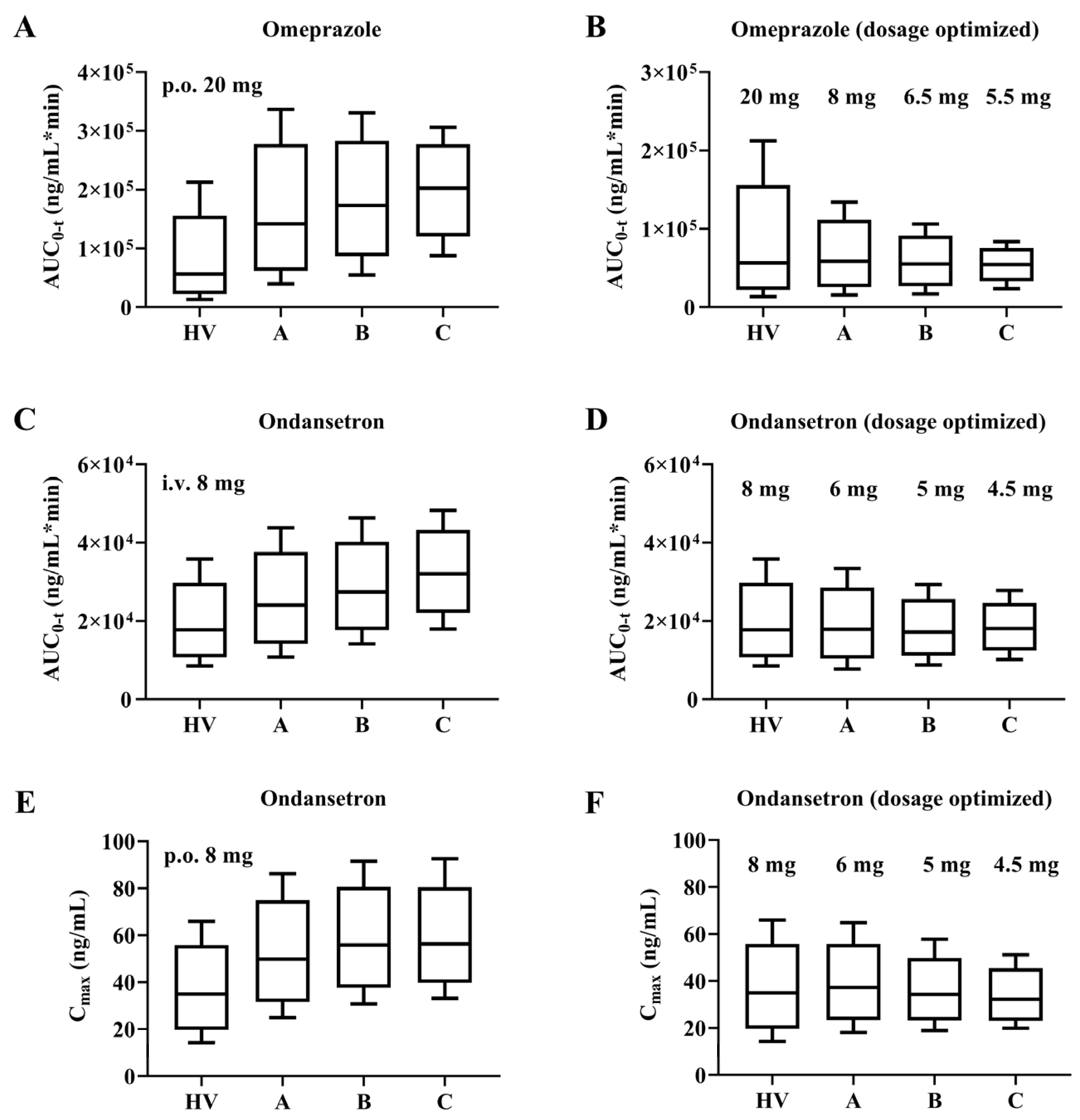
2.6. Dose Optimization
3. Results
3.1. Drug Data Set
- (1)
- Pharmacokinetic parameters (e.g., AUC or plasma drug concentration) following intravenous and/or oral administration in patients with cirrhosis were available.
- (2)
- Clinical pharmacokinetic data could be obtained from different reports. The collected clinical reports are summarized in Table 3.
- (3)
- The pharmacokinetic data were collected from multiple published studies using different analytical methods, which may introduce variability in the validation dataset and influence the comparison between model predictions and observed values.
3.2. CYP2C19 Substrate Drugs
Omeprazole and Lansoprazole
3.3. CYP3A4 Substrate Drugs
3.3.1. Midazolam and Ondansetron
3.3.2. Verapamil and Alfentanil
3.4. Development of PBPK Model and Validation Using Pharmacokinetic Parameters from Healthy Subjects Following i.v. or Oral Administrations
3.5. Prediction of Pharmacokinetic Profiles of Intravenous or Oral CYP3A4 and CYP2C19 Substrates in Cirrhosis Patients Using the Developed PBPK Model
3.6. Sensitivity Analysis of Model Parameters
3.7. Dosage Optimization Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | the area under the curve |
| AUC0–t | the area under the curve from 0 to the last measurable concentration |
| Cmax | the peak concentration |
| Tmax | time to peak drug concentration |
| CYP2C19 | Cytochrome P450 2C19 |
| CYP3A4 | Cytochrome P450 3A4 |
| LC | liver cirrhosis |
| fu | the unbound fraction of drug |
| CL | clearance |
| Rb | the ratio of drug concentration in blood to plasma |
| PBSF | the physiologically based scaling factor |
| Kt | the transit rate constant |
| Kt:p | the ratio of drug concentration in tissue to plasma |
| PBPK | physiologically based pharmacokinetic |
References
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global epidemiology of cirrhosis—Aaetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Global Hepatitis Programme. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hepatitis (accessed on 4 December 2025).
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y. Liver Cirrhosis: Evaluation, Nutritional Status, and Prognosis. Mediat. Inflamm. 2015, 2015, 872152. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75 (Suppl. S1), S49–S66. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Guidelines for the Clinical Use of Proton Pump Inhibitors. Available online: https://www.nhc.gov.cn/yzygj/c100068/202012/b59f1401666645009b64aee17185c87b.shtml (accessed on 4 December 2025).
- El Rouby, N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 447–460. [Google Scholar] [CrossRef]
- Desta, Z.; Zhao, X.; Shin, J.G.; Flockhart, D.A. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 2002, 41, 913–958. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.; Braunwald, E.; et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009, 360, 354–362. [Google Scholar] [CrossRef]
- Hamdy, N.A.; Kennedy, H.J.; Nicholl, J.; Triger, D.R. Sedation for gastroscopy: A comparative study of midazolam and Diazemuls in patients with and without cirrhosis. Br. J. Clin. Pharmacol. 1986, 22, 643–647. [Google Scholar] [CrossRef]
- Duthaler, U.; Bachmann, F.; Suenderhauf, C.; Grandinetti, T.; Pfefferkorn, F.; Haschke, M.; Hruz, P.; Bouitbir, J.; Krähenbühl, S. Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel Phenotyping Cocktail Differently. Clin. Pharmacokinet. 2022, 61, 1039–1055. [Google Scholar] [CrossRef]
- Delcò, F.; Tchambaz, L.; Schlienger, R.; Drewe, J.; Krähenbühl, S. Dose adjustment in patients with liver disease. Drug Saf. 2005, 28, 529–545. [Google Scholar] [CrossRef]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 2010, 49, 189–206. [Google Scholar] [CrossRef]
- Zhao, H.; Wei, Y.; He, K.; Zhao, X.; Mu, H.; Wen, Q. Prediction of janagliflozin pharmacokinetics in type 2 diabetes mellitus patients with liver cirrhosis or renal impairment using a physiologically based pharmacokinetic model. Eur. J. Pharm. Sci. 2022, 179, 106298. [Google Scholar] [CrossRef]
- Kalam, M.N.; Rasool, M.F.; Alqahtani, F.; Imran, I.; Rehman, A.U.; Ahmed, N. Development and Evaluation of a Physiologically Based Pharmacokinetic Drug-Disease Model of Propranolol for Suggesting Model Informed Dosing in Liver Cirrhosis Patients. Drug Des. Dev. Ther. 2021, 15, 1195–1211. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Z.; Mu, R.; Hu, G.; Liu, L.; Liu, X. Simultaneously Predicting the Pharmacokinetics of CES1-Metabolized Drugs and Their Metabolites Using Physiologically Based Pharmacokinetic Model in Cirrhosis Subjects. Pharmaceutics 2024, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- McGowan, F.X.; Reiter, M.J.; Pritchett, E.L.; Shand, D.G. Verapamil plasma binding: Relationship to alpha 1-acid glycoprotein and drug efficacy. Clin. Pharmacol. Ther. 1983, 33, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, F.M.; Bogaert, M.G. Binding of alfentanil to human alpha 1-acid glycoprotein, albumin and serum. Int. J. Clin. Pharmacol. Ther. Toxicol. 1991, 29, 96–102. [Google Scholar]
- Cartee, N.M.P.; Wang, M.M. Binding of omeprazole to protein targets identified by monoclonal antibodies. PLoS ONE 2020, 15, e0239464. [Google Scholar] [CrossRef]
- Landes, B.D.; Petite, J.P.; Flouvat, B. Clinical pharmacokinetics of lansoprazole. Clin. Pharmacokinet. 1995, 28, 458–470. [Google Scholar] [CrossRef]
- Hamaoka, N.; Oda, Y.; Hase, I.; Mizutani, K.; Nakamoto, T.; Ishizaki, T.; Asada, A. Propofol decreases the clearance of midazolam by inhibiting CYP3A4: An in vivo and in vitro study. Clin. Pharmacol. Ther. 1999, 66, 110–117. [Google Scholar] [CrossRef]
- Simpson, K.H.; Hicks, F.M. Clinical pharmacokinetics of ondansetron. A review. J. Pharm. Pharmacol. 1996, 48, 774–781. [Google Scholar] [CrossRef]
- Li, R.; Barton, H.A.; Maurer, T.S. A Mechanistic Pharmacokinetic Model for Liver Transporter Substrates Under Liver Cirrhosis Conditions. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, S.; Fynne, L.; Grønbæk, H.; Krogh, K. Small intestinal transit in patients with liver cirrhosis and portal hypertension: A descriptive study. BMC Gastroenterol. 2012, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Pepin, X.J.H.; Johansson Soares Medeiros, J.; Deris Prado, L.; Suarez Sharp, S. The Development of an Age-Appropriate Fixed Dose Combination for Tuberculosis Using Physiologically-Based Pharmacokinetic Modeling (PBBM) and Risk Assessment. Pharmaceutics 2024, 16, 1587. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Liu, X.; Yang, H.; Liu, L. Prediction of Pharmacokinetics for CYP3A4-Metabolized Drugs in Pediatrics and Geriatrics Using Dynamic Age-Dependent Physiologically Based Pharmacokinetic Models. Pharmaceutics 2025, 17, 214. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.M.; Sun, B.B.; Wang, Z.J.; Zheng, X.K.; Zhao, K.J.; Chen, Y.; Zhang, J.X.; Liu, P.H.; Zhu, L.; Xu, R.J.; et al. Physiologically based pharmacokinetic-pharmacodynamic modeling for prediction of vonoprazan pharmacokinetics and its inhibition on gastric acid secretion following intravenous/oral administration to rats, dogs and humans. Acta Pharmacol. Sin. 2020, 41, 852–865. [Google Scholar] [CrossRef]
- Small, B.G.; Hatley, O.; Jamei, M.; Gardner, I.; Johnson, T.N. Incorporation and Performance Verification of Hepatic Portal Blood Flow Shunting in Minimal and Full PBPK Models of Liver Cirrhosis. Clin. Pharmacol. Ther. 2023, 114, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Ladumor, M.K.; Storelli, F.; Liang, X.; Lai, Y.; Enogieru, O.J.; Chothe, P.P.; Evers, R.; Unadkat, J.D. Predicting changes in the pharmacokinetics of CYP3A-metabolized drugs in hepatic impairment and insights into factors driving these changes. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 261–273. [Google Scholar] [CrossRef]
- Andersson, T.; Regårdh, C.G.; Lou, Y.C.; Zhang, Y.; Dahl, M.L.; Bertilsson, L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 1992, 2, 25–31. [Google Scholar] [CrossRef]
- Noubarani, M.; Kobarfard, F.; Motevalian, M.; Keyhanfar, F. Variation in omeprazole pharmacokinetics in a random Iranian population: A pilot study. Biopharm. Drug Dispos. 2012, 33, 324–331. [Google Scholar] [CrossRef]
- Hu, X.P.; Xu, J.M.; Hu, Y.M.; Mei, Q.; Xu, X.H. Effects of CYP2C19 genetic polymorphism on the pharmacokinetics and pharmacodynamics of omeprazole in Chinese people. J. Clin. Pharm. Ther. 2007, 32, 517–524. [Google Scholar] [CrossRef]
- Sakai, T.; Aoyama, N.; Kita, T.; Sakaeda, T.; Nishiguchi, K.; Nishitora, Y.; Hohda, T.; Sirasaka, D.; Tamura, T.; Tanigawara, Y.; et al. CYP2C19 genotype and pharmacokinetics of three proton pump inhibitors in healthy subjects. Pharm. Res. 2001, 18, 721–727. [Google Scholar] [CrossRef]
- Furuta, T.; Ohashi, K.; Kobayashi, K.; Iida, I.; Yoshida, H.; Shirai, N.; Takashima, M.; Kosuge, K.; Hanai, H.; Chiba, K.; et al. Effects of clarithromycin on the metabolism of omeprazole in relation to CYP2C19 genotype status in humans. Clin. Pharmacol. Ther. 1999, 66, 265–274. [Google Scholar] [CrossRef]
- Román, M.; Ochoa, D.; Sánchez-Rojas, S.D.; Talegón, M.; Prieto-Pérez, R.; Rivas, Â.; Abad-Santos, F.; Cabaleiro, T. Evaluation of the relationship between polymorphisms in CYP2C19 and the pharmacokinetics of omeprazole, pantoprazole and rabeprazole. Pharmacogenomics 2014, 15, 1893–1901. [Google Scholar] [CrossRef]
- Cawello, W.; Mueller-Voessing, C.; Fichtner, A. Pharmacokinetics of lacosamide and omeprazole coadministration in healthy volunteers: Results from a phase I, randomized, crossover trial. Clin. Drug Investig. 2014, 34, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Kang, T.S.; Eom, S.O.; Kim, J.I.; Lee, H.J.; Roh, J. CYP2C19 haplotypes in Koreans as a marker of enzyme activity evaluated with omeprazole. J. Clin. Pharm. Ther. 2009, 34, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Yu, K.S.; Jang, I.J.; Yang, B.H.; Shin, S.G.; Yim, D.S. Omeprazole hydroxylation is inhibited by a single dose of moclobemide in homozygotic EM genotype for CYP2C19. Br. J. Clin. Pharmacol. 2002, 53, 393–397. [Google Scholar] [CrossRef]
- Yasui-Furukori, N.; Takahata, T.; Nakagami, T.; Yoshiya, G.; Inoue, Y.; Kaneko, S.; Tateishi, T. Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br. J. Clin. Pharmacol. 2004, 57, 487–494. [Google Scholar] [CrossRef]
- Hussein, Z.; Granneman, G.R.; Mukherjee, D.; Samara, E.; Hogan, D.L.; Koss, M.A.; Isenberg, J.I. Age-related differences in the pharmacokinetics and pharmacodynamics of lansoprazole. Br. J. Clin. Pharmacol. 1993, 36, 391–398. [Google Scholar] [CrossRef]
- Amer, F.; Karol, M.D.; Pan, W.J.; Griffin, J.S.; Lukasik, N.L.; Locke, C.S.; Chiu, Y.L. Comparison of the pharmacokinetics of lansoprazole 15- and 30-mg sachets for suspension versus intact capsules. Clin. Ther. 2004, 26, 2076–2083. [Google Scholar] [CrossRef]
- Doan, T.T.; Wang, Q.; Griffin, J.S.; Lukasik, N.L.; O’Dea, R.F.; Pan, W.J. Comparative pharmacokinetics and pharmacodynamics of lansoprazole oral capsules and suspension in healthy subjects. Am. J. Health Syst. Pharm. 2001, 58, 1512–1519. [Google Scholar] [CrossRef]
- Miura, M. Enantioselective disposition of lansoprazole and rabeprazole in human plasma. Yakugaku Zasshi 2006, 126, 395–402. [Google Scholar] [CrossRef]
- Flouvat, B.; Delhotal-Landes, B.; Cournot, A.; Dellatolas, F. Single and multiple dose pharmacokinetics of lansoprazole in elderly subjects. Br. J. Clin. Pharmacol. 1993, 36, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Delhotal-Landes, B.; Flouvat, B.; Duchier, J.; Molinie, P.; Dellatolas, F.; Lemaire, M. Pharmacokinetics of lansoprazole in patients with renal or liver disease of varying severity. Eur. J. Clin. Pharmacol. 1993, 45, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.D.; Leonowens, C.; Cox, E.J.; González-Pérez, V.; Frederick, K.S.; Scarlett, Y.V.; Fisher, M.B.; Paine, M.F. Indinavir Increases Midazolam N-Glucuronidation in Humans: Identification of an Alternate CYP3A Inhibitor Using an In Vitro to In Vivo Approach. Drug Metab. Dispos. 2019, 47, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.S.; Huang, J.D. Pharmacokinetics of midazolam and 1’-hydroxymidazolam in Chinese with different CYP3A5 genotypes. Drug Metab. Dispos. 2002, 30, 1491–1496. [Google Scholar] [CrossRef]
- Kupferschmidt, H.H.; Ha, H.R.; Ziegler, W.H.; Meier, P.J.; Krähenbühl, S. Interaction between grapefruit juice and midazolam in humans. Clin. Pharmacol. Ther. 1995, 58, 20–28. [Google Scholar] [CrossRef]
- Teng, R.; Butler, K. The effect of ticagrelor on the metabolism of midazolam in healthy volunteers. Clin. Ther. 2013, 35, 1025–1037. [Google Scholar] [CrossRef]
- Guo, T.; Mao, G.F.; Xia, D.Y.; Su, X.Y.; Zhao, L.S. Pharmacokinetics of midazolam tablet in different Chinese ethnic groups. J. Clin. Pharm. Ther. 2011, 36, 406–411. [Google Scholar] [CrossRef]
- Lepper, E.R.; Hicks, J.K.; Verweij, J.; Zhai, S.; Figg, W.D.; Sparreboom, A. Determination of midazolam in human plasma by liquid chromatography with mass-spectrometric detection. J. Chromatogr. B 2004, 806, 305–310. [Google Scholar] [CrossRef]
- Dale, O.; Nilsen, T.; Loftsson, T.; Hjorth Tønnesen, H.; Klepstad, P.; Kaasa, S.; Holand, T.; Djupesland, P.G. Intranasal midazolam: A comparison of two delivery devices in human volunteers. J. Pharm. Pharmacol. 2006, 58, 1311–1318. [Google Scholar] [CrossRef]
- VanDenBerg, C.M.; Kazmi, Y.; Stewart, J.; Weidler, D.J.; Tenjarla, S.N.; Ward, E.S.; Jann, M.W. Pharmacokinetics of three formulations of ondansetron hydrochloride in healthy volunteers: 24-mg oral tablet, rectal suppository, and i.v. infusion. Am. J. Health Syst. Pharm. 2000, 57, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Hsyu, P.H.; Pritchard, J.F.; Bozigian, H.P.; Lloyd, T.L.; Griffin, R.H.; Shamburek, R.; Krishna, G.; Barr, W.H. Comparison of the pharmacokinetics of an ondansetron solution (8 mg) when administered intravenously, orally, to the colon, and to the rectum. Pharm. Res. 1994, 11, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.F.; Bryson, J.C.; Kernodle, A.E.; Benedetti, T.L.; Powell, J.R. Age and gender effects on ondansetron pharmacokinetics: Evaluation of healthy aged volunteers. Clin. Pharmacol. Ther. 1992, 51, 51–55. [Google Scholar] [CrossRef]
- Ashforth, E.I.; Palmer, J.L.; Bye, A.; Bedding, A. The pharmacokinetics of ondansetron after intravenous injection in healthy volunteers phenotyped as poor or extensive metabolisers of debrisoquine. Br. J. Clin. Pharmacol. 1994, 37, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.C.; Palmer, J.L.; Minton, N.A.; Burroughs, A.K. The pharmacokinetics of intravenous ondansetron in patients with hepatic impairment. Br. J. Clin. Pharmacol. 1993, 35, 441–443. [Google Scholar] [CrossRef]
- Dominic, J.A.; Bourne, D.W.; Tan, T.G.; Kirsten, E.B.; McAllister, R.G., Jr. The pharmacology of verapamil. III. Pharmacokinetics in normal subjects after intravenous drug administration. J. Cardiovasc. Pharmacol. 1981, 3, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Hla, K.K.; Henry, J.A.; Latham, A.N. Pharmacokinetics and pharmacodynamics of two formulations of verapamil. Br. J. Clin. Pharmacol. 1987, 24, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, S.; Javadian, B.; Sadeghian, S. The effect of gender on the pharmacokinetics of verapamil and norverapamil in human. Biopharm. Drug Dispos. 2006, 27, 329–334. [Google Scholar] [CrossRef]
- Somogyi, A.; Albrecht, M.; Kliems, G.; Schäfer, K.; Eichelbaum, M. Pharmacokinetics, bioavailability and ECG response of verapamil in patients with liver cirrhosis. Br. J. Clin. Pharmacol. 1981, 12, 51–60. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Hoffer, C.; Walker, A.; Sheffels, P. Disposition and miotic effects of oral alfentanil: A potential noninvasive probe for first-pass cytochrome P4503A activity. Clin. Pharmacol. Ther. 2003, 73, 199–208. [Google Scholar] [CrossRef]
- Schwagmeier, R.; Boerger, N.; Meissner, W.; Striebel, H.W. Pharmacokinetics of intranasal alfentanil. J. Clin. Anesth. 1995, 7, 109–113. [Google Scholar] [CrossRef]
- Ferrier, C.; Marty, J.; Bouffard, Y.; Haberer, J.P.; Levron, J.C.; Duvaldestin, P. Alfentanil pharmacokinetics in patients with cirrhosis. Anesthesiology 1985, 62, 480–484. [Google Scholar] [CrossRef]
- Li, S.; Xie, L.; Yang, L.; Jiang, L.; Yang, Y.; Zhi, H.; Liu, X.; Yang, H.; Liu, L. Prediction of Omeprazole Pharmacokinetics and its Inhibition on Gastric Acid Secretion in Humans Using Physiologically Based Pharmacokinetic-Pharmacodynamic Model Characterizing CYP2C19 Polymorphisms. Pharm. Res. 2023, 40, 1735–1750. [Google Scholar] [CrossRef]
- Nies, A.S.; Shand, D.G.; Wilkinson, G.R. Altered hepatic blood flow and drug disposition. Clin. Pharmacokinet. 1976, 1, 135–155. [Google Scholar] [CrossRef]
- Rowland, M. Protein binding and drug clearance. Clin. Pharmacokinet. 1984, 9 (Suppl. S1), 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA 2023, 329, 1589–1602. [Google Scholar] [CrossRef]
- Zardi, E.M.; Abbate, A.; Zardi, D.M.; Dobrina, A.; Margiotta, D.; Van Tassell, B.W.; Afeltra, A.; Sanyal, A.J. Cirrhotic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Acalovschi, M.; Dumitraşcu, D.L.; Csakany, I. Gastric and gall bladder emptying of a mixed meal are not coordinated in liver cirrhosis--a simultaneous sonographic study. Gut 1997, 40, 412–417. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Unadkat, J.D.; Zhang, X.; Wu, D.; Heimbach, T. Applications, Challenges, and Outlook for PBPK Modeling and Simulation: A Regulatory, Industrial and Academic Perspective. Pharm. Res. 2022, 39, 1701–1731. [Google Scholar] [CrossRef]
- Loisios-Konstantinidis, I.; Dressman, J. Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling to Support Waivers of In Vivo Clinical Studies: Current Status, Challenges, and Opportunities. Mol. Pharm. 2021, 18, 1–17. [Google Scholar] [CrossRef]
- Upton, R.N.; Foster, D.J.; Abuhelwa, A.Y. An introduction to physiologically-based pharmacokinetic models. Paediatr. Anaesth. 2016, 26, 1036–1046. [Google Scholar] [CrossRef]
- Reddy, M.B.; Cabalu, T.D.; de Zwart, L.; Ramsden, D.; Dowty, M.E.; Taskar, K.S.; Badée, J.; Bolleddula, J.; Boulu, L.; Fu, Q.; et al. Building Confidence in Physiologically Based Pharmacokinetic Modeling of CYP3A Induction Mediated by Rifampin: An Industry Perspective. Clin. Pharmacol. Ther. 2025, 117, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, Z.; Yao, X.; Lei, Z.; Zhao, K.; Wang, W.; Zhang, X.; Chen, X.; Liu, D. Prediction of pyrotinib exposure based on physiologically-based pharmacokinetic model and endogenous biomarker. Front. Pharmacol. 2022, 13, 972411. [Google Scholar] [CrossRef]
- Rowland Yeo, K.; Gil Berglund, E.; Chen, Y. Dose Optimization Informed by PBPK Modeling: State-of-the Art and Future. Clin. Pharmacol. Ther. 2024, 116, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Xenodochidis, C.; Krasteva, N. Old age as a risk factor for liver diseases: Modern therapeutic approaches. Exp. Gerontol. 2023, 184, 112334. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Physiologically Based Pharmacokinetic (PBPK) Modeling of Lornoxicam: Exploration of doses for CYP2C9 Genotypes and Patients with Cirrhosis. J. Pharm. Sci. 2022, 111, 3174–3184. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef]
- Zhou, W.; Johnson, T.N.; Bui, K.H.; Cheung, S.Y.A.; Li, J.; Xu, H.; Al-Huniti, N.; Zhou, D. Predictive Performance of Physiologically Based Pharmacokinetic (PBPK) Modeling of Drugs Extensively Metabolized by Major Cytochrome P450s in Children. Clin. Pharmacol. Ther. 2018, 104, 188–200. [Google Scholar] [CrossRef]
- Wu, F.; Gaohua, L.; Zhao, P.; Jamei, M.; Huang, S.M.; Bashaw, E.D.; Lee, S.C. Predicting nonlinear pharmacokinetics of omeprazole enantiomers and racemic drug using physiologically based pharmacokinetic modeling and simulation: Application to predict drug/genetic interactions. Pharm. Res. 2014, 31, 1919–1929. [Google Scholar] [CrossRef]
- Qi, F.; Zhu, L.; Li, N.; Ge, T.; Xu, G.; Liao, S. Influence of different proton pump inhibitors on the pharmacokinetics of voriconazole. Int. J. Antimicrob. Agents 2017, 49, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Katashima, M.; Yamamoto, K.; Sugiura, M.; Sawada, Y.; Iga, T. Comparative pharmacokinetic/pharmacodynamic study of proton pump inhibitors, omeprazole and lansoprazole in rats. Drug Metab. Dispos. 1995, 23, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Tsuneto, Y.; Saito, Y.; Maekawa, K.; Sawada, J.; Narimatsu, S. Influence of CYP2C19*18 and CYP2C19*19 alleles on omeprazole 5-hydroxylation: In vitro functional analysis of recombinant enzymes expressed in Saccharomyces cerevisiae. Basic. Clin. Pharmacol. Toxicol. 2008, 102, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Kim, M.J.; Park, J.Y.; Shon, J.H.; Yoon, Y.R.; Lee, S.S.; Liu, K.H.; Chun, J.H.; Hyun, M.H.; Shin, J.G. Stereoselective metabolism of lansoprazole by human liver cytochrome P450 enzymes. Drug Metab. Dispos. 2003, 31, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Steere, B.; Baker, J.A.; Hall, S.D.; Guo, Y. Prediction of in vivo clearance and associated variability of CYP2C19 substrates by genotypes in populations utilizing a pharmacogenetics-based mechanistic model. Drug Metab. Dispos. 2015, 43, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Inoue, K.; Shaw, P.M.; Checovich, W.J.; Guengerich, F.P.; Shimada, T. Different contributions of cytochrome P450 2C19 and 3A4 in the oxidation of omeprazole by human liver microsomes: Effects of contents of these two forms in individual human samples. J. Pharmacol. Exp. Ther. 1997, 283, 434–442. [Google Scholar] [CrossRef]
- Hanke, N.; Turk, D.; Selzer, D.; Wiebe, S.; Fernandez, É.; Stopfer, P.; Nock, V.; Lehr, T. A Mechanistic, Enantioselective, Physiologically Based Pharmacokinetic Model of Verapamil and Norverapamil, Built and Evaluated for Drug-Drug Interaction Studies. Pharmaceutics 2020, 12, 556. [Google Scholar] [CrossRef]
- Qian, C.Q.; Zhao, K.J.; Chen, Y.; Liu, L.; Liu, X.D. Simultaneously predict pharmacokinetic interaction of rifampicin with oral versus intravenous substrates of cytochrome P450 3A/P-glycoprotein to healthy human using a semi-physiologically based pharmacokinetic model involving both enzyme and transporter turnover. Eur. J. Pharm. Sci. 2019, 134, 194–204. [Google Scholar] [CrossRef]
- Ezuruike, U.; Zhang, M.; Pansari, A.; De Sousa Mendes, M.; Pan, X.; Neuhoff, S.; Gardner, I. Guide to development of compound files for PBPK modeling in the Simcyp population-based simulator. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 805–821. [Google Scholar] [CrossRef]
- Shum, S.; Shen, D.D.; Isoherranen, N. Predicting Maternal-Fetal Disposition of Fentanyl Following Intravenous and Epidural Administration Using Physiologically Based Pharmacokinetic Modeling. Drug Metab. Dispos. 2021, 49, 1003–1015. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Li, J.; Zhang, M.; Hu, M.; Xu, P.; Liu, L.; Liu, X. A mechanistic physiologically based pharmacokinetic-enzyme turnover model involving both intestine and liver to predict CYP3A induction-mediated drug-drug interactions. J. Pharm. Sci. 2013, 102, 2819–2836. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S.; Lombardo, F.; Waters, N.J. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab. Dispos. 2008, 36, 1385–1405. [Google Scholar] [CrossRef]
- Baneyx, G.; Parrott, N.; Meille, C.; Iliadis, A.; Lave, T. Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: Influence of time between substrate and inducer administration. Eur. J. Pharm. Sci. 2014, 56, 1–15. [Google Scholar] [CrossRef]
- Yerasi, N.; Vurimindi, H.; Devarakonda, K. Frog intestinal perfusion to evaluate drug permeability: Application to p-gp and cyp3a4 substrates. Front. Pharmacol. 2015, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chen, X.; Jiang, J.; Shi, J.; Hu, P. Evaluating a physiologically based pharmacokinetic model for predicting the pharmacokinetics of midazolam in Chinese after oral administration. Acta Pharmacol. Sin. 2016, 37, 276–284. [Google Scholar] [CrossRef] [PubMed]
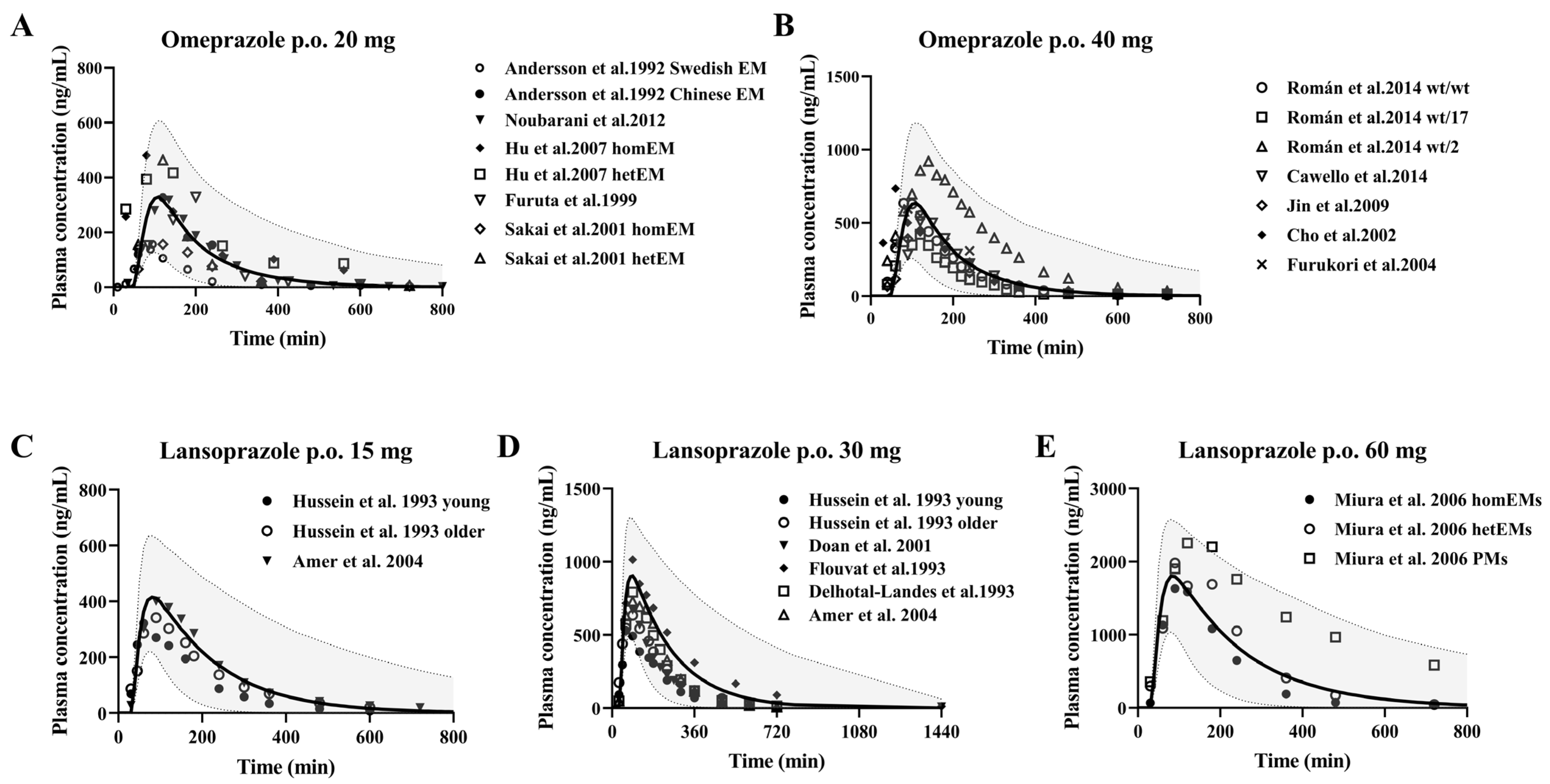
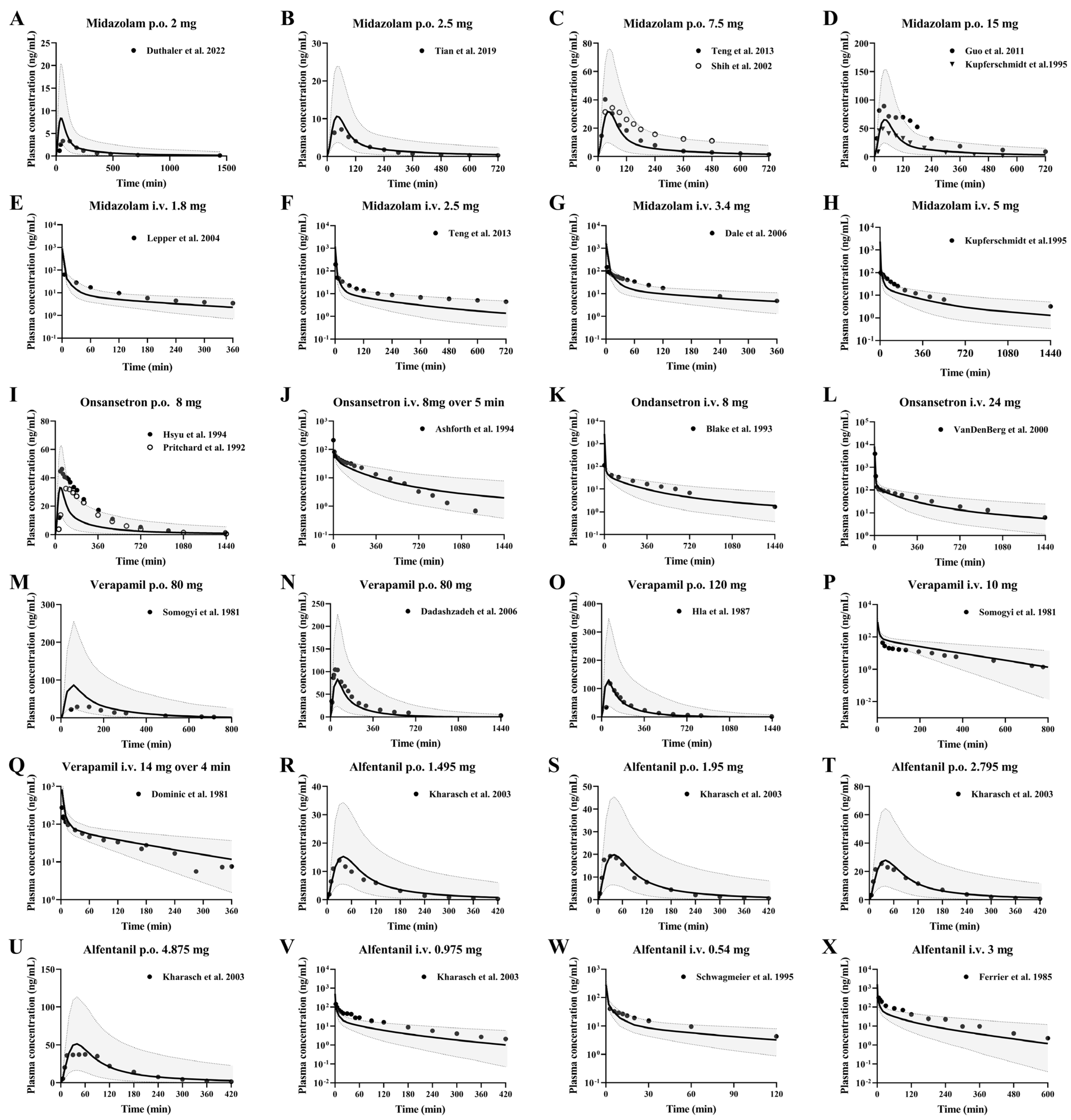
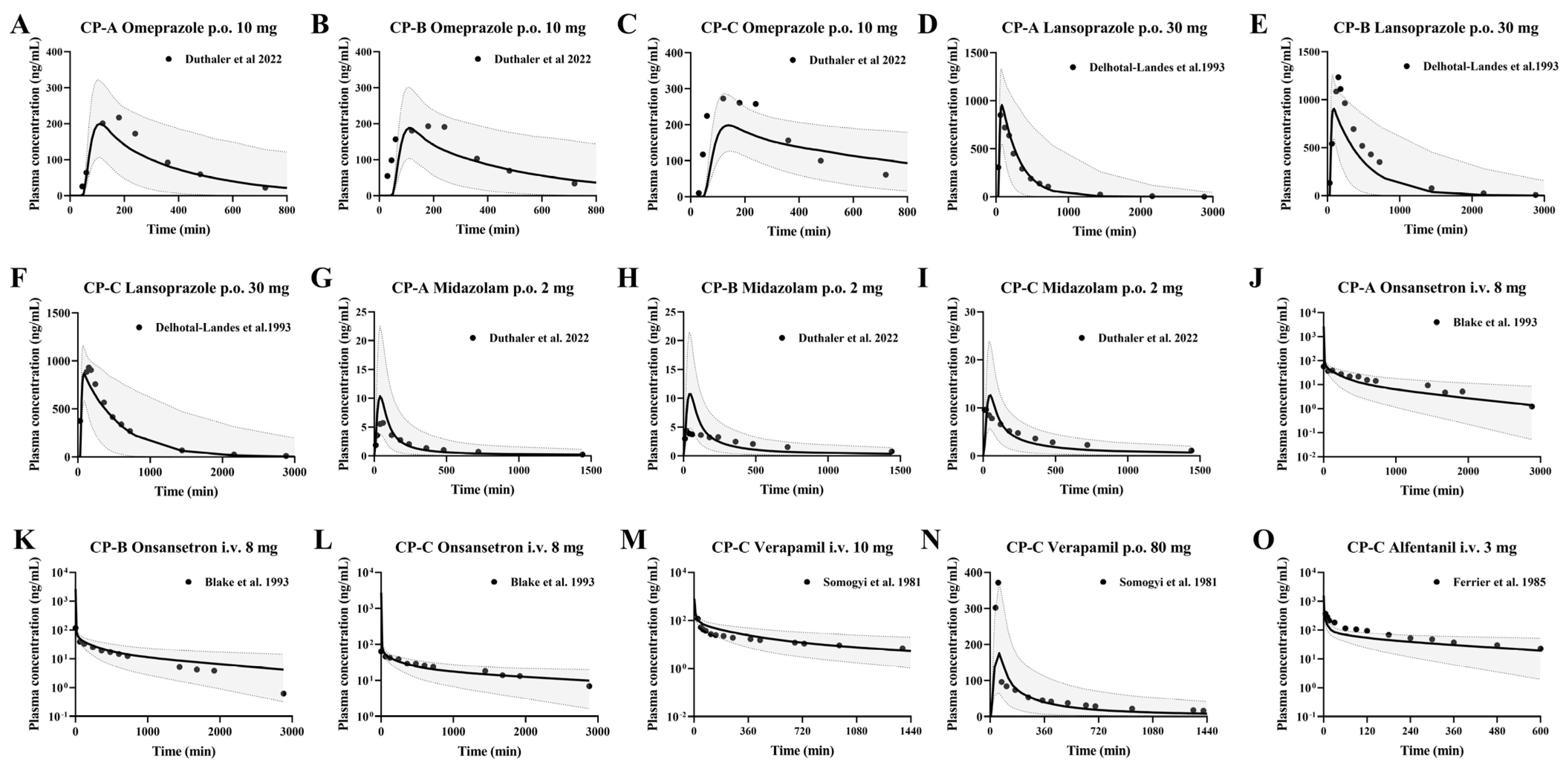
| No | Research | Drug | Dose | Assays | Subjects (n) | Refs. |
|---|---|---|---|---|---|---|
| 1 | Andersson et al., 1992 | Omeprazole | p.o. 20 mg | HPLC | Healthy (14) | [35] |
| 2 | Noubarani et al., 2012 | Omeprazole | p.o. 20 mg | LC-MS/MS | Healthy (30) | [36] |
| 3 | Hu et al., 2007 | Omeprazole | p.o. 20 mg | HPLC-UV | Healthy (18) | [37] |
| 4 | Sakai et al., 2001 | Omeprazole | p.o. 20 mg | HPLC-UV | Healthy (18) | [38] |
| 5 | Furuta et al., 1999 | Omeprazole | p.o. 20 mg | HPLC-UV | Healthy (6) | [39] |
| 6 | Román et al., 2014 | Omeprazole | p.o. 40 mg | LC-MS | Healthy (35) | [40] |
| 7 | Cawello et al., 2014 | Omeprazole | p.o. 40 mg | LC-MS | Healthy (36) | [41] |
| 8 | Jin et al., 2009 | Omeprazole | p.o. 40 mg | HPLC-UV | Healthy (9) | [42] |
| 9 | Cho et al., 2002 | Omeprazole | p.o. 40 mg | HPLC-UV | Healthy (8) | [43] |
| 10 | Furukori et al., 2004 | Omeprazole | p.o. 40 mg | HPLC-UV | Healthy (6) | [44] |
| 11 | Duthaler et al., 2022 | Omeprazole | p.o. 10 mg | LC-MS/MS | CP (A-C) (36) | [16] |
| 12 | Hussein et al., 1993 | Lansoprazole | p.o. 15; 30 mg | HPLC-UV | Healthy (24) | [45] |
| 13 | Amer et al., 2004 | Lansoprazole | p.o. 15; 30 mg | LC-MS | Healthy (36) | [46] |
| 14 | Doan et al., 2001 | Lansoprazole | p.o. 30 mg | LC-MS | Healthy (36) | [47] |
| 15 | Miura et al., 2006 | Lansoprazole | p.o. 60 mg | HPLC | Healthy (18) | [48] |
| 16 | Flouvat et al., 1993 | Lansoprazole | p.o. 30 mg | HPLC- | Healthy (18) | [49] |
| 17 | Delhotal-Landes et al., 1993 | Lansoprazole | p.o. 30 mg | HPLC | CP (A-C) (24) | [50] |
| 18 | Tian et al., 2019 | Midazolam | p.o. 2.5 mg | LC-MS/MS | Healthy (8) | [51] |
| 19 | Shih et al., 2002 | Midazolam | p.o. 7.5 mg | HPLC-UV | Healthy (42) | [52] |
| 20 | Kupferschmidt et al., 1995 | Midazolam | p.o. 15 mg | HPLC-UV | Healthy (8) | [53] |
| 21 | Teng et al., 2013 | Midazolam | p.o. 7.5 mg; i.v. 2.5 mg | LC-MS/MS | Healthy (28) | [54] |
| 22 | Guo et al., 2011 | Midazolam | p.o. 15 mg | HPLC | Healthy (49) | [55] |
| 23 | Lepper et al., 2004 | Midazolam | i.v. 1.8 mg | LC-MS | Healthy (35) | [56] |
| 24 | Dale et al., 2006 | Midazolam | i.v. 3.4 mg | GC-MS | Healthy (12) | [57] |
| 25 | Duthaler et al., 2022 | Midazolam | p.o. 2 mg | LC-MS/MS | CP (A-C) (36) | [16] |
| 26 | VanDenBerg et al., 2000 | Ondansetron | p.o. 24 mg | HPLC-UV | Healthy (12) | [58] |
| 27 | Hsyu et al., 1994 | Ondansetron | p.o. 8 mg | HPLC-UV | Healthy (6) | [59] |
| 28 | Pritchard et al., 1992 | Ondansetron | p.o. 8 mg | HPLC-UV | Healthy (11) | [60] |
| 29 | Ashforth et al., 1994 | Ondansetron | i.v. 8 mg over 5 min | GC-UV | Healthy (12) | [61] |
| 30 | Blake et al., 1993 | Ondansetron | i.v. 8 mg over 5 min | HPLC | CP (A-C) (19) | [62] |
| 31 | Dominic et al., 1981 | Verapamil | i.v. 14 mg over 4 min | GC-UV | Healthy (8) | [63] |
| 32 | Hla et al., 1987 | Verapamil | p.o. 120 mg | HPLC | Healthy (10) | [64] |
| 33 | Dadashzadeh et al., 2006 | Verapamil | p.o. 80 mg | HPLC-FLD | Healthy (12) | [65] |
| 34 | Somogyi et al., 1981 | Verapamil | p.o. 80 mg i.v. 10 mg | LC-UV | Healthy (6) CP (C) (7) | [66] |
| 35 | Kharasch et al., 2003 | Alfentanil | i.v. 0.975 mg; p.o. 1.495 mg; 1.95 mg; 2.795 mg; 4.875 mg | LC-MS | Healthy (10) | [67] |
| 36 | Schwagmeier et al., 1995 | Alfentanil | i.v. 0.54 mg | RIA | Healthy (10) | [68] |
| 37 | Ferrier et al., 1985 | Alfentanil | i.v. 3 mg | RIA | Healthy (10) CP (C) (11) | [69] |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| 20 mg [35] | Healthy | 258.37 ± 99.48 | 329.75 | 1.28 | NR | / | / | 324.69 ± 120.9 | 926.13 | 2.85 * |
| 20 mg [35] | Healthy | 203.11 ± 81.17 | 329.75 | 1.62 | NR | / | / | 905.00 ± 614.85 | 926.13 | 1.02 |
| 20 mg [36] | Healthy | 397.2 | 329.75 | 0.83 | 1.9 | 2.3 | 1.21 | 825.1 | 939.37 | 1.14 |
| 20 mg [37] | Healthy | 513.9 ± 294.8 | 329.75 | 0.64 | NR | / | / | 1644.6 ± 745.8 | 920.16 | 0.56 |
| 20 mg [37] | Healthy | 566.8 ± 294.8 | 329.75 | 0.58 | NR | / | / | 1759.4 ± 838.6 | 920.16 | 0.52 |
| 20 mg [38] | Healthy | 251.2 ± 46.2 | 329.75 | 1.31 | 2.8 ± 0.3 | 2.3 | 0.82 | 623.1 ± 149.1 | 936.21 | 1.50 |
| 20 mg [38] | Healthy | 618.3 ± 141.9 | 329.75 | 0.53 | 3.0 ± 0.6 | 2.3 | 0.77 | 1061.8 ± 269.2 | 936.21 | 0.88 |
| 20 mg [39] | Healthy | NR | / | / | NR | / | / | 383.9 ± 26.3 | 926.13 | 2.41 * |
| 20 mg [39] | Healthy | NR | / | / | NR | / | / | 1001.9 ± 160.3 | 926.13 | 0.92 |
| 40 mg [40] | Healthy | 834.37 ± 394.54 | 634.89 | 0.76 | NR | / | / | 1658.69 ± 1271.58 | 1749.81 | 1.05 |
| 40 mg [40] | Healthy | 1266.48 ± 477.05 | 634.89 | 0.50 | NR | / | / | 3887.72 ± 2087.92 | 1749.81 | 0.45 * |
| 40 mg [40] | Healthy | 722.76 ± 346.14 | 634.89 | 0.88 | NR | / | / | 1096.7 ± 646.53 | 1749.81 | 1.60 |
| 40 mg [41] | Healthy | 586 | 634.89 | 1.08 | 2 | 2.3 | 1.15 | 1027 | 1478.09 | 1.44 |
| 40 mg [42] | Healthy | 723.0 ± 67.3 | 634.89 | 0.88 | 2.1 ± 0.2 | 2.3 | 1.10 | 1385.6 ± 183.7 | 1733.32 | 1.25 |
| 40 mg [43] | Healthy | 986.56 | 634.89 | 0.64 | 1.83 | 2.3 | 1.26 | 1834.34 | 1834.34 | 1.00 |
| 40 mg [44] | Healthy | 900 | 634.89 | 0.71 | 1.75 | 2.3 | 1.31 | 1481 | 1691.35 | 1.14 |
| 10 mg [16] | CP-A | 218 | 188.3 | 0.86 | NR | / | / | 986 | 1015.91 | 1.03 |
| 10 mg [16] | CP-B | 273 | 198.67 | 0.73 | NR | / | / | 1327 | 1123.19 | 0.85 |
| 10 mg [16] | CP-C | 343 | 199.34 | 0.58 | NR | / | / | 2111 | 1676.53 | 0.79 |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| 15 mg [45] | Healthy | 413 ± 199 | 415.06 | 1.00 | 1.15 ± 0.39 | 1.5 | 1.30 | 950 ± 593 | 1398.42 | 1.47 |
| 15 mg [45] | Healthy | 449 ± 150 | 415.06 | 0.92 | 1.46 ± 0.53 | 1.5 | 1.03 | 1334 ± 673 | 1398.42 | 1.05 |
| 15 mg [46] | Healthy | 578.6 | 415.06 | 0.72 | 1.7 | 1.5 | 0.88 | 1451 | 1421.86 | 0.98 |
| 30 mg [45] | Healthy | 750 ± 331 | 902.90 | 1.20 | 1.48 ± 0.99 | 1.5 | 1.01 | 1763 ± 1056 | 3449.40 | 1.96 |
| 30 mg [45] | Healthy | 773 ± 248 | 902.90 | 1.17 | 1.56 ± 0.94 | 1.5 | 0.96 | 2678 ± 1144 | 3449.40 | 1.29 |
| 30 mg [46] | Healthy | 1077 | 902.90 | 0.84 | 1.8 | 1.5 | 0.83 | 2641 | 3546.69 | 1.34 |
| 30 mg [47] | Healthy | 810.4 ± 340.5 | 902.90 | 1.11 | 1.6 ± 0.7 | 1.5 | 0.94 | 2157 ± 1933 | 3709.69 | 1.72 |
| 30 mg [49] | Healthy | 1148 ± 536 | 902.90 | 0.79 | 1.5 | 1.5 | 1.00 | 5216 ± 3855 | 3709.69 | 0.71 |
| 30 mg [50] | Healthy | 1033 | 902.90 | 0.87 | 1.5 | 1.5 | 1.00 | 2670 | 3546.69 | 1.33 |
| 60 mg [48] | Healthy | 1957 ± 413 | 1801.38 | 0.92 | 1.9 ± 0.6 | 1.5 | 0.79 | 5009 ± 919 | 6865.35 | 1.37 |
| 60 mg [48] | Healthy | 2196 ± 405 | 1801.38 | 0.82 | 2.3 ± 0.8 | 1.5 | 0.65 | 7300 ± 1008 | 6865.35 | 0.94 |
| 60 mg [48] | Healthy | 2516 ± 357 | 1801.38 | 0.72 | 2.4 ± 0.9 | 1.5 | 0.63 | 20,132 ± 3570 | 6865.35 | 0.34 * |
| 30 mg [50] | CP-A | 1080 | 864.29 | 0.80 | 1.4 | 1.5 | 1.07 | 5200 | 4841.99 | 0.93 |
| 30 mg [50] | CP-B | 1440 | 904.69 | 0.63 | 2.1 | 1.5 | 0.71 | 11,700 | 7453.58 | 0.64 |
| 30 mg [50] | CP-C | 1140 | 951.89 | 0.83 | 2.1 | 1.5 | 0.71 | 10,700 | 8305.20 | 0.78 |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| p.o. 2.5 mg [51] | Healthy | 7.56 | 10.62 | 1.40 | 1 | 0.67 | 0.67 | 20.69 | 26.80 | 1.30 |
| p.o. 2 mg [16] | Healthy | 4.44 | 8.33 | 1.88 | NR | / | / | 11.9 | 25.06 | 2.11 * |
| p.o. 7.5 mg [52] | Healthy | NR | / | NR | / | / | 153.95 ± 17.5 | 81.87 | 0.53 | |
| p.o. 7.5 mg [54] | Healthy | 47.3 | 31.61 | 0.67 | 0.75 | 0.67 | 0.89 | 115.4 | 81.87 | 0.71 |
| p.o. 15 mg [53] | Healthy | 54.3 ± 6.4 | 65.08 | 1.20 | 0.62 ± 0.20 | 0.67 | 1.08 | 143 ± 26 | 157.84 | 1.10 |
| p.o. 15 mg [55] | Healthy | 116.2 ± 61.0 | 65.08 | 0.56 | 1.0 ± 0.06 | 0.67 | 0.67 | 330.7 ± 139.6 | 195.93 | 0.59 |
| p.o. 15 mg [55] | Healthy | 130.2 ± 59.4 | 65.08 | 0.50 | 1.0 ± 0.8 | 0.67 | 0.67 | 365.0 ± 103.8 | 195.93 | 0.54 |
| i.v. 1.8 mg [56] | Healthy | NR | / | / | NR | / | / | 96.1 ± 42.7 | 109.00 | 1.13 |
| i.v. 2.5 mg [54] | Healthy | NR | / | / | NR | / | / | 115.7 | 161.33 | 1.39 |
| i.v. 3.4 mg [57] | Healthy | NR | / | / | NR | / | / | 117.03 | 208.16 | 1.78 |
| i.v. 5 mg [53] | Healthy | NR | / | / | NR | / | / | 199 | 351.01 | 1.76 |
| p.o. 2 mg [16] | CP-A | 6.37 | 10.32 | 1.62 | NR | / | / | 20 | 28.95 | 1.45 |
| p.o. 2 mg [16] | CP-B | 5.83 | 10.73 | 1.84 | NR | / | / | 29.7 | 38.49 | 1.30 |
| p.o. 2 mg [16] | CP-C | 11.2 | 12.62 | 1.13 | NR | / | / | 58.1 | 55.42 | 0.95 |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| i.v. 24 mg [58] | Healthy | NR | / | / | NR | / | / | 1276.4 ± 340.4 | 1650.83 | 1.29 |
| p.o. 8 mg [59] | Healthy | 40 ± 22 | 33.05 | 0.83 | 1.3 ± 0.7 | 0.67 | 0.52 | 225 ± 79 | 123.83 | 0.55 |
| p.o. 8 mg | Healthy | NR | / | / | NR | / | / | NR | / | / |
| i.v. 8 mg over 5 min [61] | Healthy | NR | / | / | NR | / | / | 257 | 199.67 | 0.78 |
| i.v. 8 mg over 5 min [61] | / | / | / | / | / | / | / | 247 | 199.67 | 0.81 |
| i.v. 8 mg [62] | Healthy | NR | / | / | NR | / | / | 279 | 536.51 | 1.92 |
| i.v. 8 mg [62] | CP-A | NR | / | / | NR | / | / | 633 | 736.64 | 1.16 |
| i.v. 8 mg [62] | CP-B | NR | / | / | NR | / | / | 446 | 940.72 | 2.11 * |
| i.v. 8 mg [62] | CP-C | NR | / | / | NR | / | / | 1383 | 1226.70 | 0.89 |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| p.o. 120 mg [64] | Healthy | 123 ± 43 | 129.83 | 1.06 | 1.05 ± 0.28 | 1 | 0.95 | 450 ± 130 | 412.53 | 0.92 |
| p.o. 80 mg [65] | Healthy | 130.66 ± 33.58 | 82.89 | 0.63 | 0.54 ± 0.18 | 1 | 1.85 | 383.67 ± 110.54 | 261.50 | 0.68 |
| p.o. 80 mg [65] | Healthy | 139.28 ± 77.88 | 82.89 | 0.60 | 0.69 ± 0.24 | 1 | 1.45 | 344.22 ± 239.90 | 261.50 | 0.76 |
| p.o. 80 mg [66] | Healthy | 38.6 | 82.89 | 0.47 | NR | / | / | NR | / | / |
| i.v. 14 mg over 4 min [63] | Healthy | NR | / | / | NR | / | / | NR | / | / |
| p.o. 80 mg [66] | CP-C | 134.5 | 175.14 | 1.30 | NR | / | / | NR | / | / |
| i.v. 10 mg [66] | CP-C | NR | / | / | NR | / | / | NR | / | / |
| Dose | Subjects | Cmax (ng/mL) | Tmax (Hour) | AUC0–t (ng × Hour/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obs | Pre | Ratio | Obs | Pre | Ratio | Obs | Pre | Ratio | ||
| p.o. 1.495 mg [67] | Healthy | 16 ± 8 | 15.29 | 0.96 | 0.6 ± 0.3 | 0.67 | 1.12 | 26 ± 14 | 32.31 | 1.24 |
| p.o. 1.95 mg [67] | Healthy | 23 ± 16 | 19.80 | 0.86 | 0.7 ± 0.4 | 0.67 | 0.96 | 38 ± 22 | 41.90 | 1.10 |
| p.o. 2.795 mg [67] | Healthy | 31 ± 18 | 27.83 | 0.90 | 0.9 ± 0.8 | 0.67 | 0.74 | 57 ± 31 | 57.87 | 1.02 |
| p.o. 4.895 mg [67] | Healthy | 50 ± 22 | 51.45 | 1.03 | 0.7 ± 0.5 | 0.67 | 0.96 | 105 ± 59 | 107.40 | 1.02 |
| i.v. 0.975 mg [67] | Healthy | NR | / | / | NR | / | / | NR | / | / |
| i.v. 0.54 mg [68] | Healthy | NR | / | / | NR | / | / | 33.10 ± 13.75 | 21.94 | 0.66 |
| i.v. 3 mg [69] | Healthy | NR | / | / | NR | / | / | NR | / | / |
| i.v. 3 mg [69] | CP-C | NR | / | / | NR | / | / | NR | / | / |
| Drugs | Values | Cmax | Tmax | AUC0–t |
|---|---|---|---|---|
| Omeprazole | AFE | 0.80 | 1.07 | 1.06 |
| PE | 26.47% | 19.34% | 22.48% | |
| GMFE | 1.41 | 1.22 | 1.40 | |
| Lansoprazole | AFE | 0.88 | 0.88 | 1.04 |
| PE | 13.50% | 11.79% | 22.84% | |
| GMFE | 1.21 | 1.19 | 1.40 | |
| Midazolam | AFE | 1.08 | 0.78 | 1.09 |
| PE | 41.17% | 20.63% | 32.81% | |
| GMFE | 1.57 | 1.32 | 1.48 | |
| Ondansetron | AFE | 0.83 | 0.52 | 1.08 |
| PE | 17.38% | 48.46% | 31.88% | |
| GMFE | 1.21 | 1.94 | 1.45 | |
| Verapamil | AFE | 0.75 | 1.37 | 0.78 |
| PE | 30.97% | 26.32% | 18.54% | |
| GMFE | 1.51 | 1.41 | 1.28 | |
| Alfentanil | AFE | 0.93 | 0.93 | 0.99 |
| PE | 6.54% | 8.60% | 7.82% | |
| GMFE | 1.09 | 1.13 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, R.; Gao, J.; Wang, X.; Ling, J.; Hu, N.; Yang, H. Pharmacokinetics of CYP2C19- and CYP3A4-Metabolized Drugs in Cirrhosis Using a Whole-Body PBPK Approach. Pharmaceutics 2025, 17, 1582. https://doi.org/10.3390/pharmaceutics17121582
Mu R, Gao J, Wang X, Ling J, Hu N, Yang H. Pharmacokinetics of CYP2C19- and CYP3A4-Metabolized Drugs in Cirrhosis Using a Whole-Body PBPK Approach. Pharmaceutics. 2025; 17(12):1582. https://doi.org/10.3390/pharmaceutics17121582
Chicago/Turabian StyleMu, Ruijing, Jingjing Gao, Xiaoli Wang, Jing Ling, Nan Hu, and Hanyu Yang. 2025. "Pharmacokinetics of CYP2C19- and CYP3A4-Metabolized Drugs in Cirrhosis Using a Whole-Body PBPK Approach" Pharmaceutics 17, no. 12: 1582. https://doi.org/10.3390/pharmaceutics17121582
APA StyleMu, R., Gao, J., Wang, X., Ling, J., Hu, N., & Yang, H. (2025). Pharmacokinetics of CYP2C19- and CYP3A4-Metabolized Drugs in Cirrhosis Using a Whole-Body PBPK Approach. Pharmaceutics, 17(12), 1582. https://doi.org/10.3390/pharmaceutics17121582







