Development of a 3D Printing Liquid Crystal Display (LCD)-Assisted Micromolding Methodology for Custom Fabrication of Polymeric Microneedles Using Experimental Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Performance Liquid Chromatography (HPLC) Method for Quantitative Analysis of RopHCl
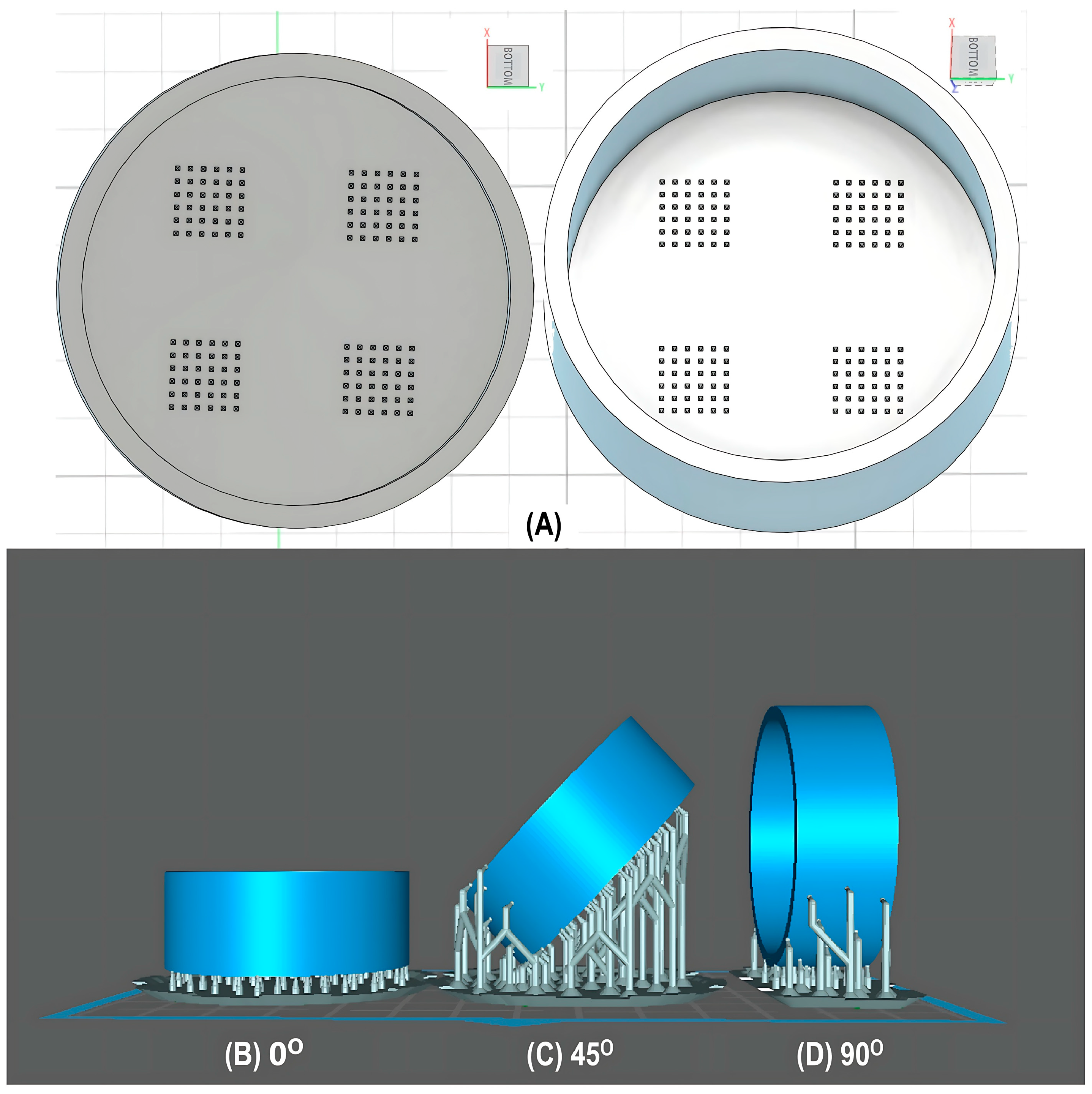
2.3. Optimization of Microneedles’ Mold Fabrication Process via Experimental Design
2.4. Microneedle Mold Preparation Process
2.5. Microneedle Preparation Process
2.6. Performance Evaluation of Printing Process Across Multiple Shapes and Dimensions
| Geometry | Dimensions | 3D Design | Reference(s) |
|---|---|---|---|
| Cone |
|  | [20,21,22] |
|  | ||
| Inclined cone (tip at the edge) |
|  | Modification of conical ones—no references found |
| Triangular pyramid |
| 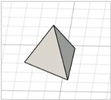 | [22,23] |
| 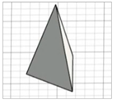 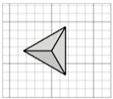 | ||
| Inclined triangular pyramid (tip at the edge) |
| 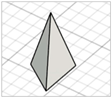 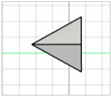 | Modification of triangular pyramid ones—no references found |
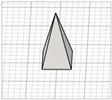
| Square pyramid |
| 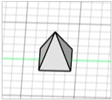 | [20,24,25,26] |
| |||
|  | ||
| Square pyramid with angled edges |
| 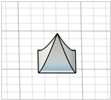 | Modification of square pyramid ones—no references found |
| 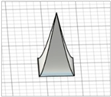 | ||
| Hexagonal-based pyramid |
| 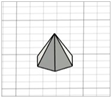 | [22] |
|  | ||
| Beveled-tip |
| 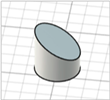 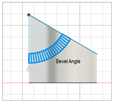 | [20,22,27] |
| 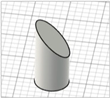 | ||
| Tapered-cone |
|  | [20] |
|  | ||
| Obelisk |
| 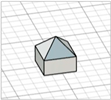 | [23,28] |
| 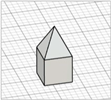 | ||
| Modified pyramid |
| 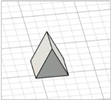 | [29] |
|  | ||
| Arrow-like |
|  | [30] |
|  |
2.7. Performance Study of Microneedle Designs for Adjusting Transdermal Permeation
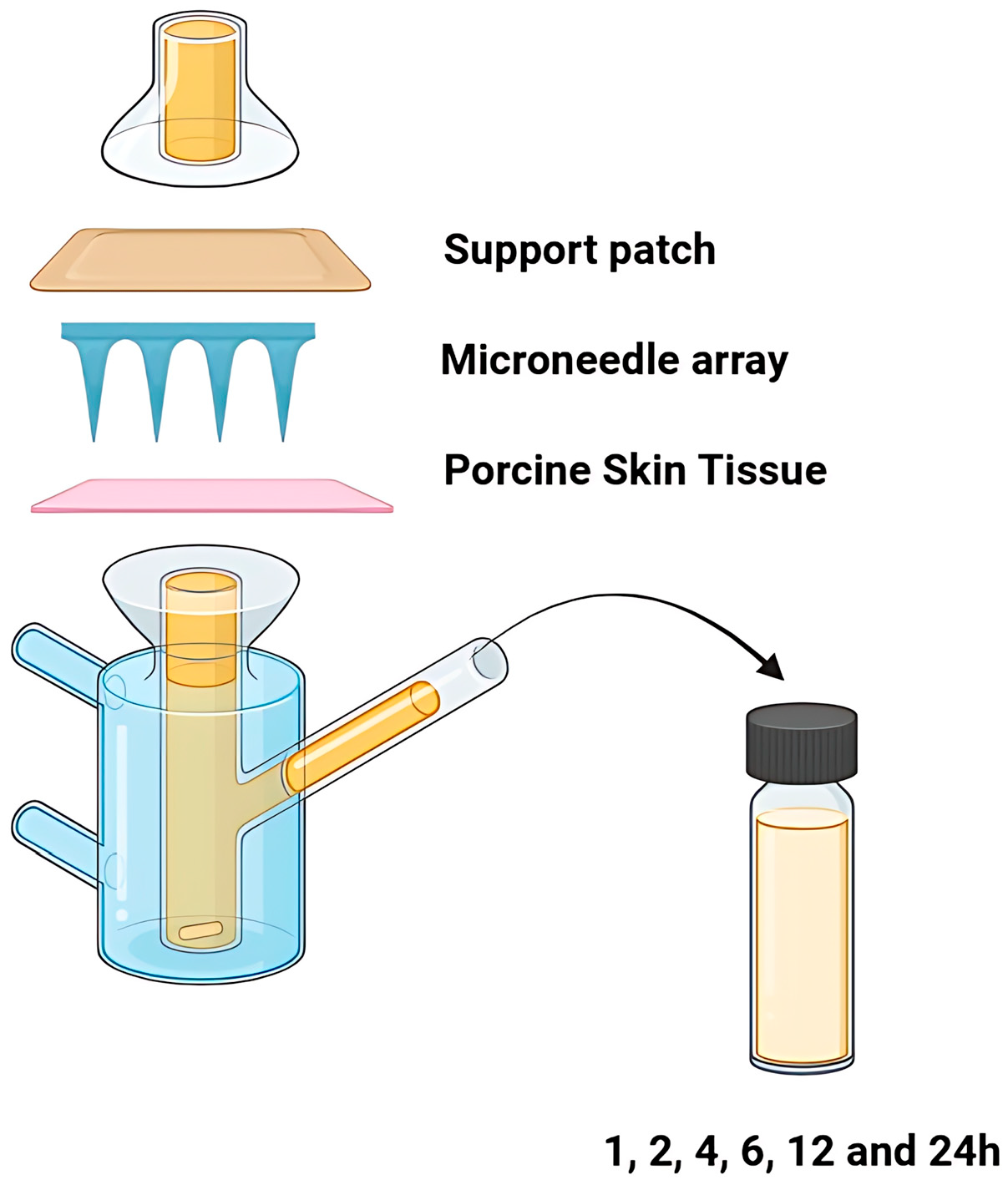
2.8. Preparation of the Support Patch for the Diffusion Studies
2.9. Microneedle Array Characterization
2.9.1. Microscopic Inspection of Microneedle Arrays Through Stereomicroscope
2.9.2. Insertion Depth Determination Studies
2.9.3. Quantitative Determination of Ropinirole Hydrochloride in Microneedle Arrays
2.9.4. Ex Vivo Permeation Studies
3. Results
3.1. Liquid Crystal Display (LCD) 3D Process Design
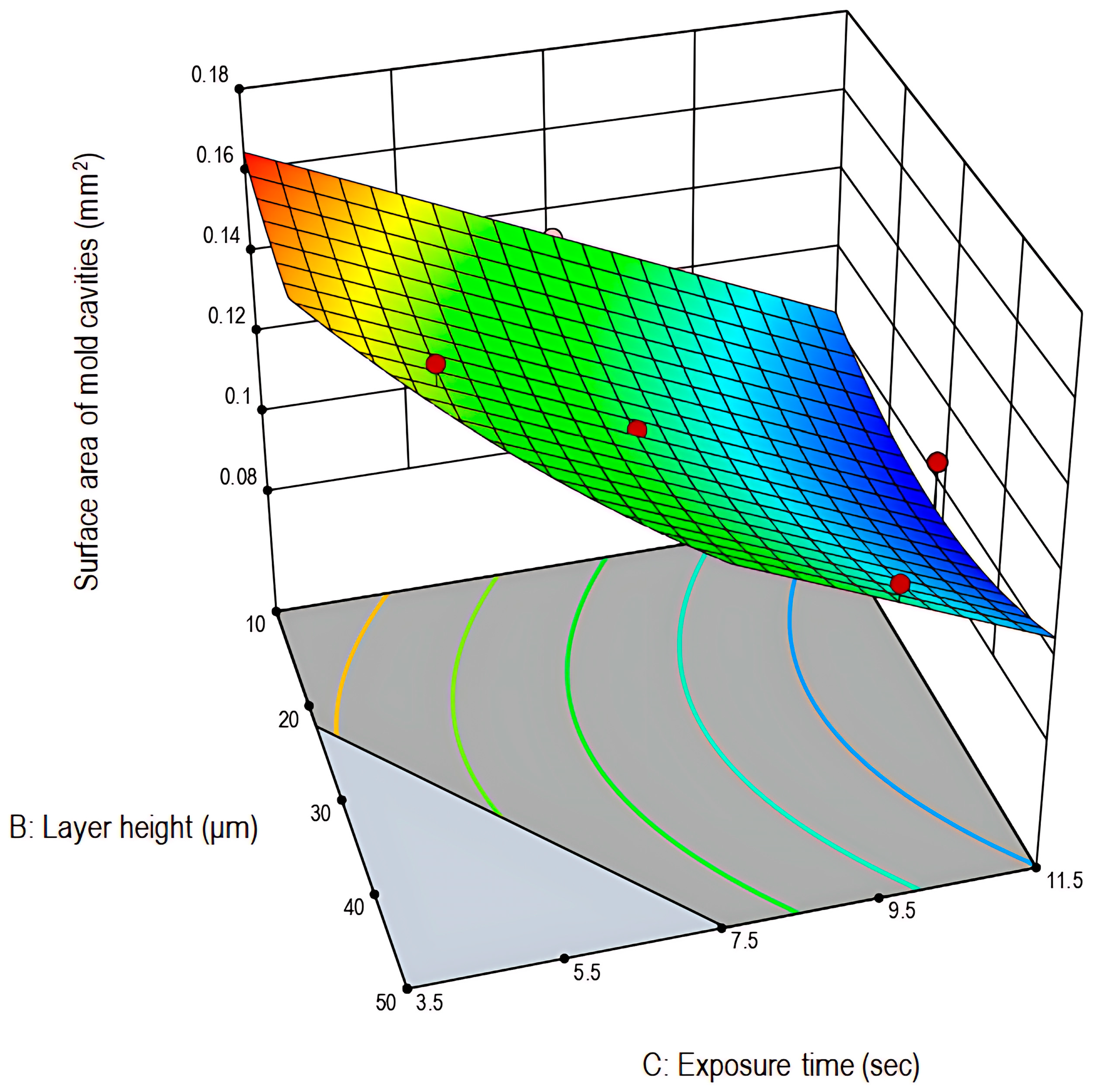
3.1.1. Analysis of Response 1: Surface Area of Mold Cavities (mm2)
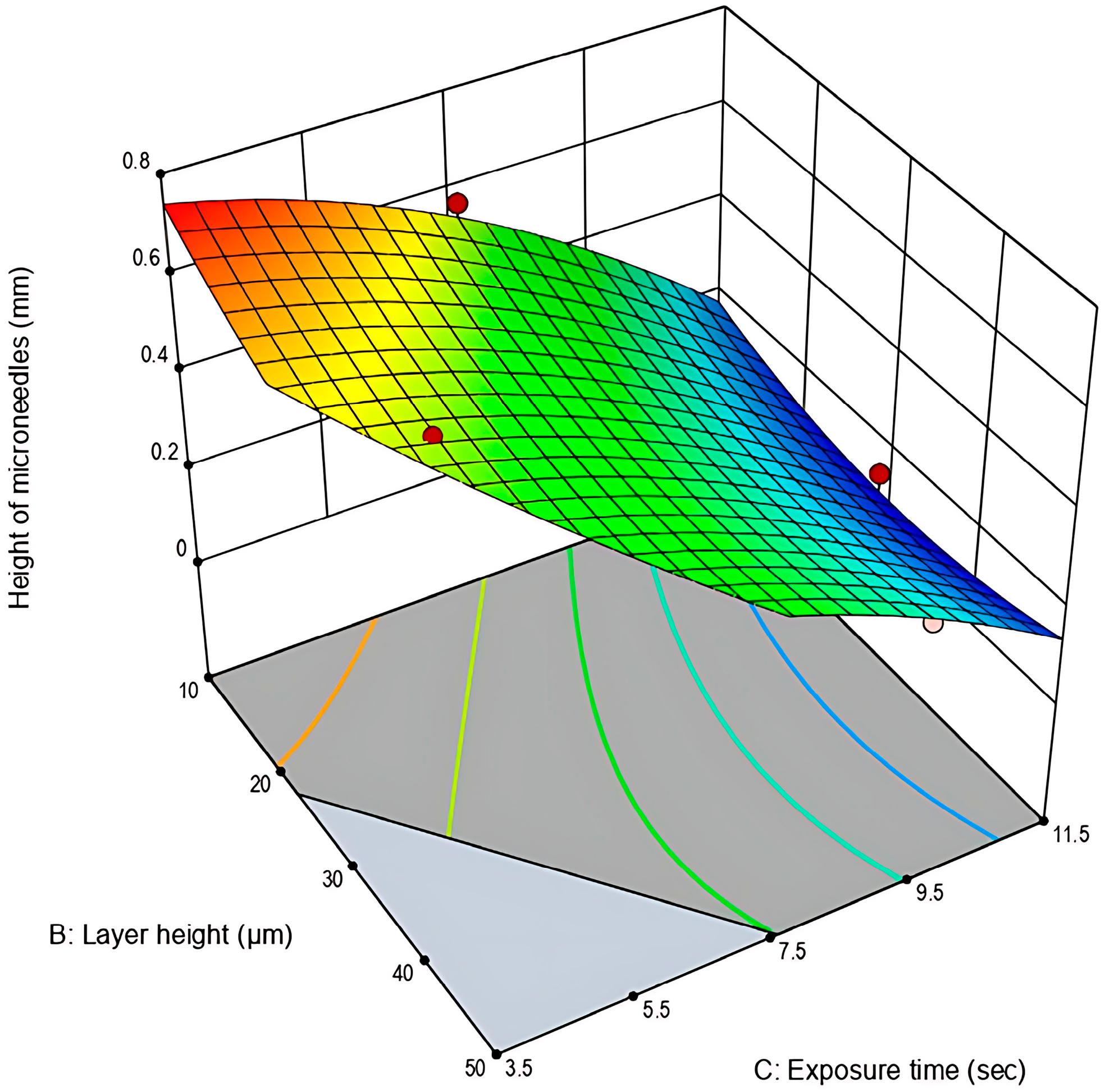
3.1.2. Analysis of Response 2: Height of Microneedles (mm)
3.1.3. Optimization of the 3D Printing Parameters
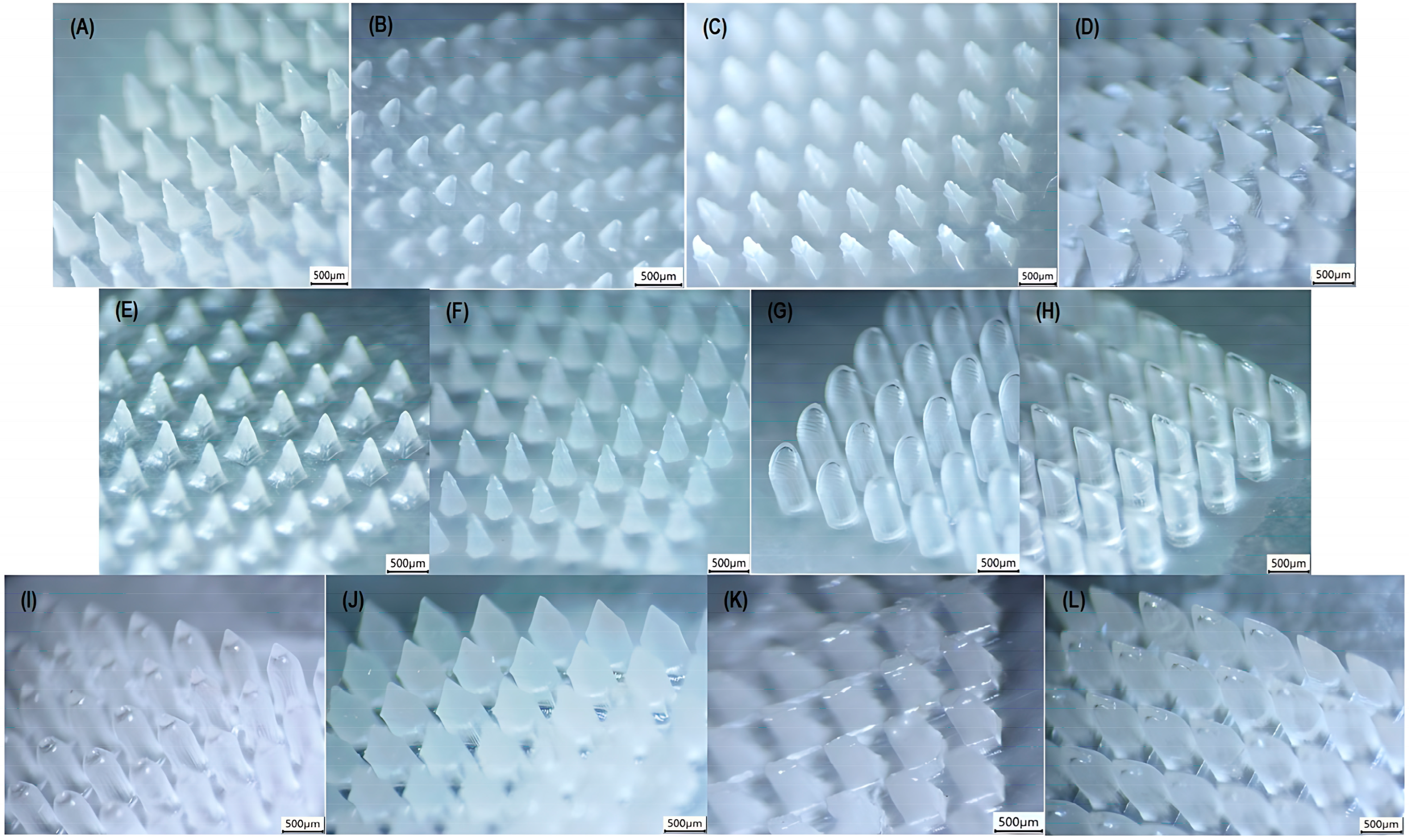
3.2. Performance Analysis of the 3D Printing Process Across Multiple Geometries
3.3. Performance Study of Microneedle Designs for Adjusting Transdermal Permeation
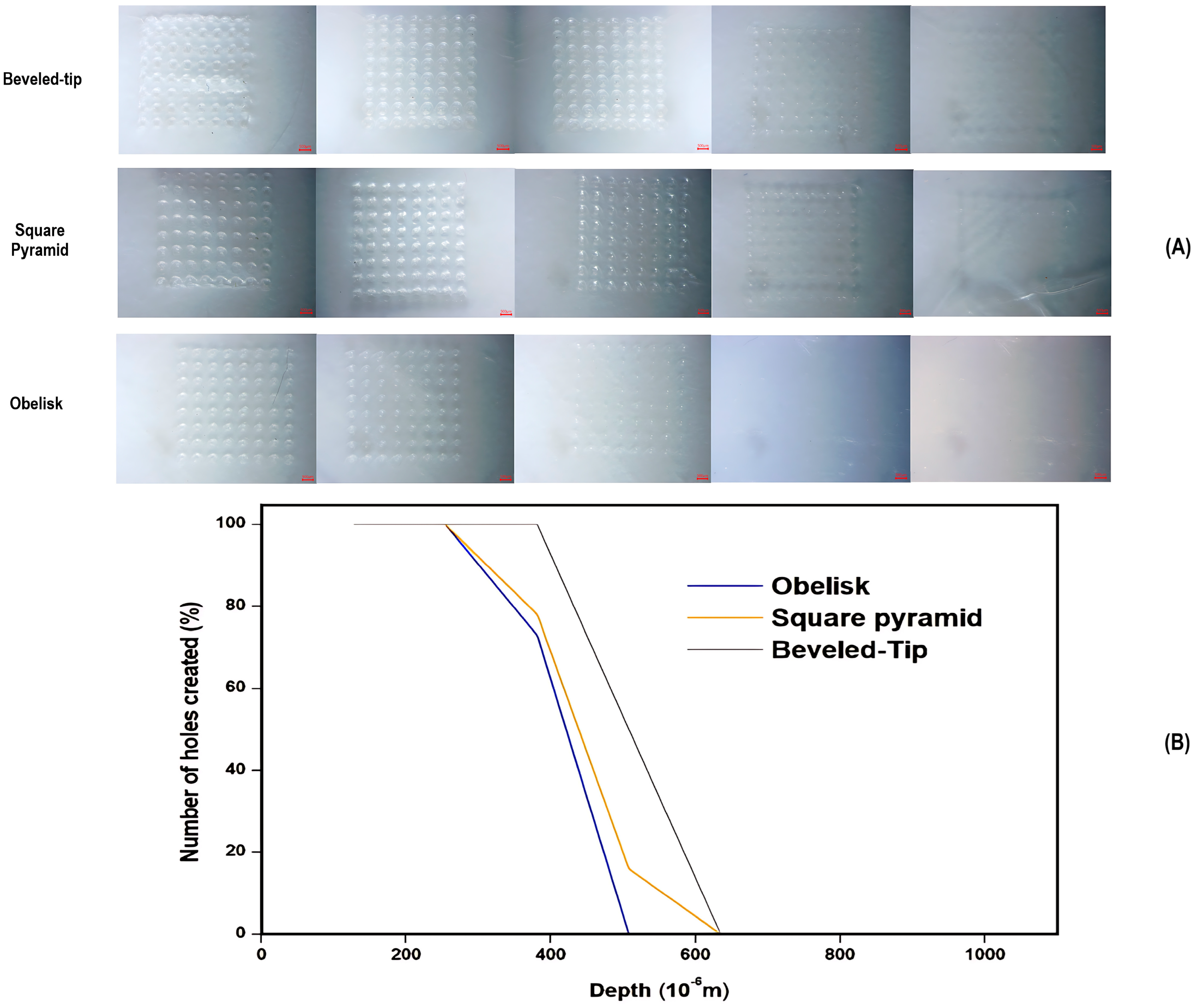
3.3.1. Microscopic Inspection of Microneedle Arrays
3.3.2. Quantitative Determination of Ropinirole Hydrochloride in Microneedle Arrays
3.3.3. Insertion Depth Determination Studies
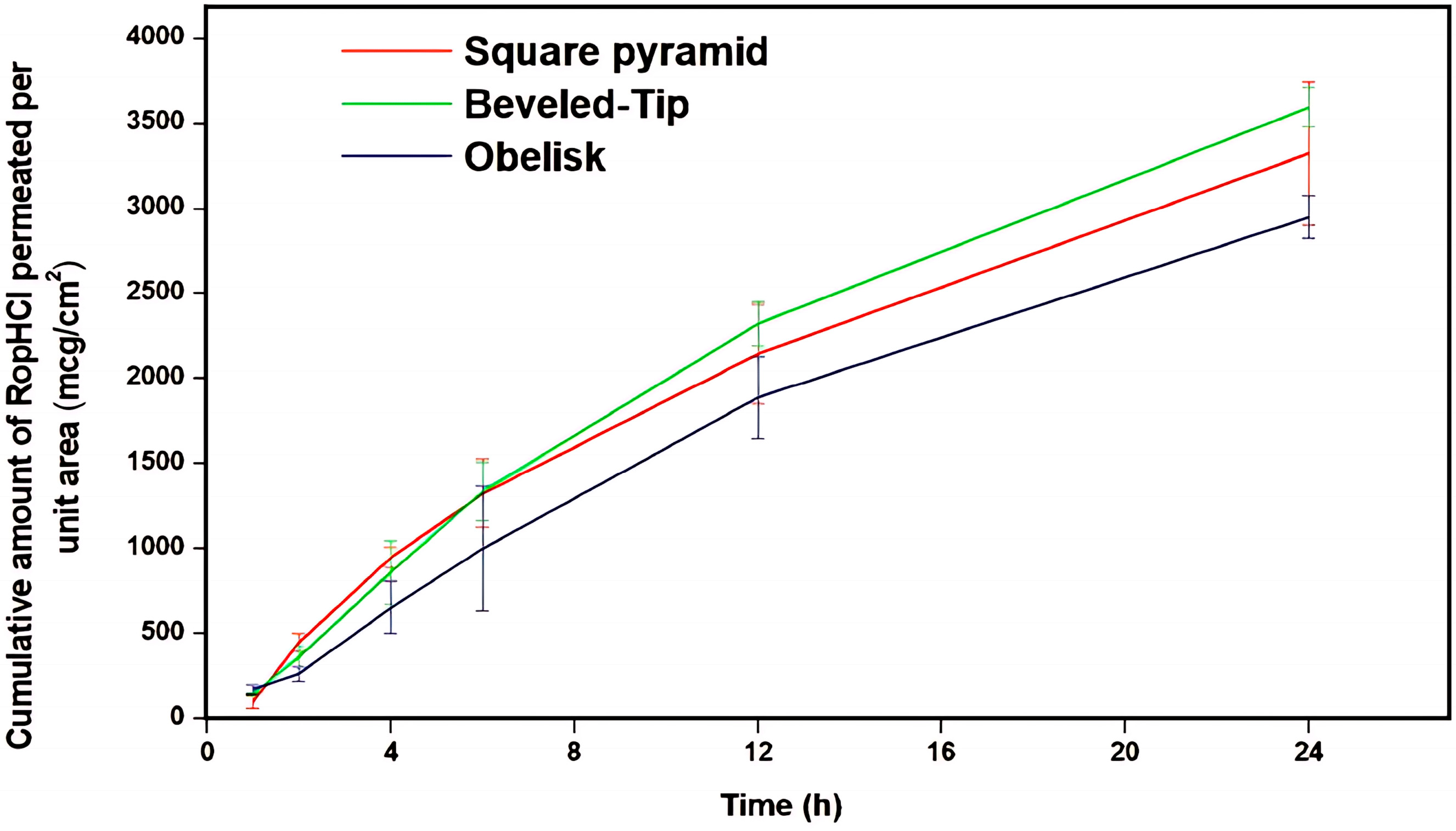
3.3.4. Ex Vivo Permeation Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API(s) | Active Pharmaceutical Ingredient(s) |
| 3D | Three-Dimensional |
| CAD | Computer-Aided Design |
| DoE | Design of Experiments |
| RopHCl | Ropinirole Hydrochloride |
| HPLC | High-Performance Liquid Chromatography |
| LCD | Liquid Crystal Display |
| FCCD | Face-Centered Central Composite Design |
| PVA | Polyvinyl alcohol |
References
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving Microneedles: Applications and Growing Therapeutic Potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef]
- Rajput, A.; Kulkarni, M.; Deshmukh, P.; Pingale, P.; Garkal, A.; Gandhi, S.; Butani, S. A Key Role by Polymers in Microneedle Technology: A New Era. Drug Dev. Ind. Pharm. 2021, 47, 1713–1732. [Google Scholar] [CrossRef]
- Sanjanwala, D.; Shinde, A.; Patravale, V. Formulation, Sterilization, and Clinical Evaluation of Microneedles for Vaccine and Biologic Delivery: A Review. Int. J. Pharm. 2025, 682, 125874. [Google Scholar] [CrossRef] [PubMed]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.F.; Machado, P.; Rodrigues, R.O.; Faustino, V.; Schütte, H.; Gassmann, S.; Lima, R.A.; Minas, G. Recent Advances and Perspectives of Microneedles for Biomedical Applications. Biophys. Rev. 2025, 17, 909–928. [Google Scholar] [CrossRef]
- Chen, Y.; Xian, Y.; Carrier, A.J.; Youden, B.; Servos, M.; Cui, S.; Luan, T.; Lin, S.; Zhang, X. A simple and cost-effective approach to fabricate tunable length polymeric microneedle patches for controllable transdermal drug delivery. RSC Adv. 2020, 10, 15541–15546. [Google Scholar] [CrossRef]
- Antonara, L.; Dallas, P.P.; Rekkas, D.M. A novel 3D printing enabled method for fast and reliable construction of polymeric microneedles using experimental design. J. Drug Deliv. Sci. Technol. 2021, 68, 102888. [Google Scholar]
- Azeem, A.; Talegaonkar, S.; Negi, L.M.; Ahmad, F.J.; Khar, R.K.; Iqbal, Z. Oil Based Nanocarrier System for Transdermal Delivery of Ropinirole: A Mechanistic, Pharmacokinetic and Biochemical Investigation. Int. J. Pharm. 2012, 422, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Como, K.; Zhu, X.; Schildt, J. Ophthalmic Ropinirole Is an Equally Effective Emetic Agent in Healthy Dogs Compared to Intravenous Apomorphine. Front. Vet. Sci. 2025, 12, 1554107. [Google Scholar] [CrossRef]
- Saitani, E.-M.; Pippa, N.; Perinelli, D.R.; Forys, A.; Papakyriakopoulou, P.; Lagopati, N.; Bonacucina, G.; Trzebicka, B.; Gazouli, M.; Pispas, S.; et al. PEO-b-PCL/Tween 80/Cyclodextrin Systems: From Bioinspired Fabrication to Possible Nasal Administration of Ropinirole Hydrochloride. J. Mater. Chem. B 2024, 12, 6587–6604. [Google Scholar] [CrossRef]
- Saitani, E.-M.; Pippa, N.; Perinelli, D.R.; Forys, A.; Papakyriakopoulou, P.; Lagopati, N.; Bonacucina, G.; Trzebicka, B.; Gazouli, M.; Pispas, S.; et al. Fabricating Polymer/Surfactant/Cyclodextrin Hybrid Particles for Possible Nose-to-Brain Delivery of Ropinirole Hydrochloride: In Vitro and Ex Vivo Evaluation. Int. J. Mol. Sci. 2024, 25, 1162. [Google Scholar] [CrossRef]
- Kale, M.; Kipping, T.; Banga, A.K. Modulated Delivery of Donepezil Using a Combination of Skin Microporation and Iontophoresis. Int. J. Pharm. 2020, 589, 119853. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Alghauli, M.A.; Almuzaini, S.A.; Aljohani, R.; Alqutaibi, A.Y. Impact of 3D printing orientation on accuracy, properties, cost, and time efficiency of additively manufactured dental models: A systematic review. BMC Oral Health 2024, 24, 1550. [Google Scholar] [CrossRef]
- Kordyl, O.; Styrna, Z.; Wojtyłko, M.; Dlugaszewska, J.; Kaminska, D.; Murias, M.; Mlynarczyk, D.T.; Jadach, B.; Skotnicka, A.; Michniak-Kohn, B.; et al. Optimization of LCD-Based 3D Printing for the Development of Clotrimazole-Coated Microneedle Systems. Materials 2025, 18, 1580. [Google Scholar] [CrossRef]
- Baykara, D.; Bedir, T.; Ilhan, E.; Mutlu, M.E.; Gunduz, O.; Narayan, R.; Ustundag, C.B. Fabrication and optimization of 3D printed gelatin methacryloyl microneedle arrays based on vat photopolymerization. Front. Bioeng. Biotechnol. 2023, 11, 1157541. [Google Scholar] [CrossRef]
- Davoudinejad, A.; Cai, Y.; Pedersen, D.B.; Luo, X.; Tosello, G. Fabrication of micro-structured surfaces by additive manufacturing, with simulation of dynamic contact angle. Mater. Des. 2019, 176, 107839. [Google Scholar] [CrossRef]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, M.J.; Das, D.B. Translation of Polymeric Microneedles for Treatment of Human Diseases: Recent Trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Al-Qallaf, B.; Das, D.B. Optimizing microneedle arrays to increase skin permeability for transdermal drug delivery. Ann. N. Y. Acad. Sci. 2009, 1161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-T.; Shen, Y.-K.; Fan, F.-Y.; Lin, Y.; Kang, S.-C. Optimal design and fabrication of a microneedle arrays patch. J. Manuf. Process. 2020, 54, 274–285. [Google Scholar] [CrossRef]
- Monou, P.K.; Andriotis, E.G.; Saropoulou, E.; Tzimtzimis, E.; Tzetzis, D.; Komis, G.; Bekiari, C.; Bouropoulos, N.; Demiri, E.; Vizirianakis, I.S.; et al. Fabrication of Hybrid Coated Microneedles with Donepezil Utilizing Digital Light Processing and Semisolid Extrusion Printing for the Management of Alzheimer’s Disease. Mol Pharm. 2024, 21, 4450–4464. [Google Scholar] [CrossRef]
- Sully, R.E.; Moore, C.J.; Garelick, H.; Loizidou, E.; Podoleanu, A.G.; Gubala, V. Nanomedicines and microneedles: A guide to their analysis and application. Anal. Methods 2021, 13, 3326–3347. [Google Scholar] [CrossRef]
- Visscher, M.; Frijlink, H.W.; Hinrichs, W.L.J. What Is the Optimal Geometry of Dissolving Microneedle Arrays? A Literature Review. Pharmaceutics 2025, 17, 124. [Google Scholar] [CrossRef]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Control Release 2017, 265, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Justyna, K.; Patrycja, Ś.; Krzysztof, M.; Rafał, W. Dissolving microneedles fabricated from 3D-printed master molds for application in veterinary medicine. Sci. Rep. 2025, 15, 14102. [Google Scholar] [CrossRef] [PubMed]
- Monou, P.K.; Saropoulou, E.; Junqueira, L.A.; Kolipaka, S.S.; Andriotis, E.G.; Tzimtzimis, E.; Tzetzis, D.; Bekiari, C.; Bouropoulos, N.; Harding, B.; et al. Fabrication and characterization of dissolving microneedles combining digital light processing and vacuum compression molding technique for the transdermal delivery of rivastigmine. Eur. J. Pharm. Biopharm. 2025, 210, 114687. [Google Scholar] [CrossRef]
- Gade, S.; Vora, L.K.; Thakur, R.R.S. Design and characterization of hollow microneedles for localized intrascleral drug delivery of ocular formulations. Methods 2025, 234, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Yerneni, S.S.; Yalcintas, E.P.; Smith, J.D.; Averick, S.; Campbell, P.G.; Ozdoganlar, O.B. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022, 149, 198–212. [Google Scholar] [CrossRef]
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Siddaramaiah, G.D.V.; Gowda, D.V.; Moin, A. Microneedles drug delivery systems for treatment of cancer: A recent update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering microneedle patches for improved penetration: Analysis, skin models and factors affecting needle insertion. Nano-Micro Lett. 2021, 13, 93. [Google Scholar] [CrossRef]
- Gera, A.K.; Burra, R.K. The Rise of Polymeric Microneedles: Recent Developments, Advances, Challenges, and Applications with Regard to Transdermal Drug Delivery. J. Funct. Biomater. 2022, 13, 81. [Google Scholar] [CrossRef]
- Malek-Khatabi, A.; Sadat Razavi, M.; Abdollahi, A.; Rahimzadeghan, M.; Moammeri, F.; Sheikhi, M.; Tavakoli, M.; Rad-Malekshahi, M.; Faraji Rad, Z. Recent Progress in PLGA-Based Microneedle-Mediated Transdermal Drug and Vaccine Delivery. Biomater. Sci. 2023, 11, 5390–5409. [Google Scholar] [CrossRef]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef]
- Lutton, R.E.; Moore, J.; Larrañeta, E.; Ligett, S.; Woolfson, A.D.; Donnelly, R.F. Microneedle characterisation: The need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv. Transl. Res. 2015, 5, 313–331. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Meira, A.; Battistel, A.; Teixeira, H.; Volpato, N. Evaluation of porcine skin layers separation methods, freezing storage and anatomical site in in vitro percutaneous absorption studies using penciclovir formulations. J. Drug Deliv. Sci. Technol. 2020, 60, 101926. [Google Scholar] [CrossRef]
- Kearney, M.C.; Caffarel-Salvador, S.J.; Fallows, H.O.; McCarthy, E.; Donnelly, R.F. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016, 109, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Turek, P.; Bazan, A.; Kubik, P.; Chlost, M. Development of a calibration procedure of the additive masked stereolithography method for improving the accuracy of model manufacturing. Appl. Sci. 2025, 15, 7412. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Kipping, T.; Banga, A.K. Polymeric microneedles enhance transdermal delivery of therapeutics. Pharmaceutics 2024, 16, 845. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Kim, Y.; Nam, J.; Ahn, H.; Kim, M.; Kang, G.; Jang, M.; Yang, H.; Jung, H. Shape of Dissolving Microneedles Determines Skin Penetration Ability and Efficacy of Drug Delivery. Biomater. Adv. 2023, 145, 213248. [Google Scholar] [CrossRef]
- Fantini, A.; Delledonne, A.; Casula, L.; Nicoli, S.; Pescina, S.; Cardia, M.C.; Lai, F.; Sissa, C.; Santi, P.; Padula, C. Application of Microneedles for High-Molecular-Weight Dextran Penetration Across the Buccal Mucosa. Pharmaceuticals 2025, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Ullah, A.; Jang, M.J.; Lee, U.S.; Shin, M.C.; An, S.H.; Kim, D.; Kim, G.M. Microneedle Patch Casting Using a Micromachined Carbon Master for Enhanced Drug Delivery. Sci. Rep. 2024, 14, 19228. [Google Scholar] [CrossRef]
- Tamez-Tamez, J.I.; Vázquez-Lepe, E.; Rodriguez, C.A.; Espinosa-Moreno, A.; Peña, A.; Vázquez, J. Assessment of Geometrical Dimensions and Puncture Feasibility of Microneedles Manufactured by Micromilling. Int. J. Adv. Manuf. Technol. 2023, 126, 4983–4996. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. Investigating Laser Ablation Process Parameters for the Fabrication of Customized Microneedle Arrays for Therapeutic Applications. Pharmaceutics 2024, 16, 885. [Google Scholar] [CrossRef] [PubMed]
| Factors | Levels | Responses | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Printing speed (mm/min) (A) | 50 | 100 | 150 |
|
| Layer height (μm) (B) | 10 | 30 | 50 | |
| Exposure time (s) (C) | 3.5 | 7.5 | 11.5 | |
| Design Constraint | Layer height ≤ 6.8 × Exposure time | |||
| Experimental Run (ER) | Factor A: Printing Speed (mm/min) | Factor B: Layer Height (μm) | Factor C: Exposure Time (s) |
|---|---|---|---|
| ER1 | 100 | 30 | 7.5 |
| ER2 | 50 | 10 | 11.5 |
| ER3 | 150 | 50 | 8.0 |
| ER4 | 150 | 30 | 7.5 |
| ER5 | 50 | 30 | 7.5 |
| ER6 | 150 | 10 | 3.5 |
| ER7 | 100 | 30 | 7.5 |
| ER8 | 100 | 30 | 11.5 |
| ER9 | 100 | 10 | 7.5 |
| ER10 | 100 | 30 | 7.5 |
| ER11 | 100 | 30 | 5.0 |
| ER12 | 50 | 10 | 3.5 |
| ER13 | 150 | 10 | 11.5 |
| ER14 | 150 | 50 | 11.5 |
| ER15 | 50 | 50 | 8.0 |
| ER16 | 100 | 50 | 9.5 |
| ER17 | 50 | 50 | 11.5 |
| Printing Parameters | Set Value |
| Bottom Layer Count | 8 |
| Bottom Exposure time (s) | 120 |
| Rest time after retract (s) | 5.0 |
| Lifting Distance (mm) | 5.0 |
| Supports | |
| Support setting | Medium |
| Density (%) | 55.0 |
| Angle (°) | 70.0 |
| Microneedle Designs for Permeation Study | Dimensions |
|---|---|
| Square pyramid |
|
| Beveled-tip |
|
| Obelisk |
|
| Experimental Run | Factor A: Printing Speed (mm/min) | Factor B: Layer Height (μm) | Factor C: Exposure Time (s) | Response 1: Surface Area of Mold Cavities (mm2) | Response 2: Height of Microneedles (mm) |
|---|---|---|---|---|---|
| ER1 | 100 | 30 | 7.5 | 0.122 | 0.385 |
| ER2 | 50 | 10 | 11.5 | 0.098 | 0.132 |
| ER3 | 150 | 50 | 8.0 | 0.141 | 0.392 |
| ER4 | 150 | 30 | 7.5 | 0.127 | 0.437 |
| ER5 | 50 | 30 | 7.5 | 0.107 | 0.358 |
| ER6 | 150 | 10 | 3.5 | 0.163 | 0.731 |
| ER7 | 100 | 30 | 7.5 | 0.120 | 0.375 |
| ER8 | 100 | 30 | 11.5 | 0.105 | 0.131 |
| ER9 | 100 | 10 | 7.5 | 0.134 | 0.570 |
| ER10 | 100 | 30 | 7.5 | 0.125 | 0.396 |
| ER11 | 100 | 30 | 5.0 | 0.148 | 0.540 |
| ER12 | 50 | 10 | 3.5 | 0.167 | 0.701 |
| ER13 | 150 | 10 | 11.5 | 0.111 | 0.163 |
| ER14 | 150 | 50 | 11.5 | 0.104 | 0.120 |
| ER15 | 50 | 50 | 8.0 | 0.132 | 0.362 |
| ER16 | 100 | 50 | 9.5 | 0.125 | 0.288 |
| ER17 | 50 | 50 | 11.5 | 0.094 | 0.102 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Conclusion |
|---|---|---|---|---|---|---|
| Modified Quadratic Model | 0.0066 | 4 | 0.0017 | 35.11 | <0.0001 | Significant |
| A—Printing speed | 0.0002 | 1 | 0.0002 | 4.87 | 0.0476 | Significant |
| B—Layer height | 4.306 × 10−6 | 1 | 4.306 × 10−6 | 0.0910 | 0.7681 | Non-significant |
| C—Exposure time | 0.0057 | 1 | 0.0057 | 120.94 | <0.0001 | Significant |
| B2 | 0.0005 | 1 | 0.0005 | 11.46 | 0.0054 | Significant |
| Residual | 0.0006 | 12 | 0.0000 | - | - | - |
| Lack of fit | 0.0006 | 10 | 0.0001 | 8.76 | 0.1067 | Non-significant |
| Pure Error | 0.0000 | 2 | 6.333 × 10−6 | - | - | - |
| Cor total | 0.0072 | 16 | - | - | - | - |
| R-squared | 0.9213 | Pred R-squared | 0.8280 | |||
| Adj. R-squared | 0.8950 | Adeq precision | 20.59 | |||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Conclusion |
|---|---|---|---|---|---|---|
| Quadratic Model | 0.6055 | 9 | 0.0673 | 73.38 | <0.0001 | Significant |
| B—Layer height | 0.0117 | 1 | 0.0117 | 12.76 | 0.0091 | Significant |
| C-Exposure time | 0.1142 | 1 | 0.1142 | 124.58 | <0.0001 | Significant |
| B2 | 0.0118 | 1 | 0.0118 | 12.85 | 0.0089 | Significant |
| C2 | 0.0080 | 1 | 0.0080 | 8.76 | 0.0211 | Significant |
| Residual | 0.0064 | 7 | 0.0009 | - | - | - |
| Lack of fit | 0.0062 | 5 | 0.0012 | 11.23 | 0.0838 | Not significant |
| Pure Error | 0.0002 | 2 | 0.001 | - | - | - |
| Cor total | 0.6119 | 16 | - | - | - | - |
| R-squared | 0.9895 | Pred R-squared | 0.8615 | |||
| Adj. R-squared | 0.9760 | Adeq precision | 28.02 | |||
| Geometry | Dimensional Evaluation | ||
|---|---|---|---|
| Cone | Lower Limit |
|
|
| Upper Limit |
|
| |
| Inclined cone (tip at the edge) | Medium Level |
|
|
| Triangular pyramid | Lower Limit |
|
|
| Upper Limit |
|
| |
| Inclined triangular pyramid (tip at the edge) | Medium Level |
|
|
| Square pyramid | Minimum Interspacing Distance |
| |
| Lower Limit |
|
| |
| Upper Limit |
|
| |
| Square pyramid with angled edges | Lower Limit |
|
|
| Upper Limit |
|
| |
| Hexagonal-based pyramid | Lower Limit |
|
|
| Upper Limit |
|
| |
| Beveled-tip | Lower Limit |
|
|
| Upper Limit |
|
| |
| Tapered-cone | Lower Limit |
|
|
| Upper Limit |
|
| |
| Obelisk | Lower Limit |
|
|
| Upper Limit |
|
Average: 489.5 ± 6.4 μm (97.9%) | |
| Modified pyramid | Lower Limit |
|
|
| Upper Limit |
|
| |
| Arrow-like | Lower Limit |
|
|
| Upper Limit |
|
| |
| Geometry | Dimensional Evaluation | |
|---|---|---|
| Square pyramid |
|
|
| Beveled-tip |
|
|
| Obelisk |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonara, L.; Rekkas, D.M.; Pippa, N.; Dallas, P.P. Development of a 3D Printing Liquid Crystal Display (LCD)-Assisted Micromolding Methodology for Custom Fabrication of Polymeric Microneedles Using Experimental Design. Pharmaceutics 2025, 17, 1571. https://doi.org/10.3390/pharmaceutics17121571
Antonara L, Rekkas DM, Pippa N, Dallas PP. Development of a 3D Printing Liquid Crystal Display (LCD)-Assisted Micromolding Methodology for Custom Fabrication of Polymeric Microneedles Using Experimental Design. Pharmaceutics. 2025; 17(12):1571. https://doi.org/10.3390/pharmaceutics17121571
Chicago/Turabian StyleAntonara, Lefkothea, Dimitrios M. Rekkas, Natassa Pippa, and Paraskevas P. Dallas. 2025. "Development of a 3D Printing Liquid Crystal Display (LCD)-Assisted Micromolding Methodology for Custom Fabrication of Polymeric Microneedles Using Experimental Design" Pharmaceutics 17, no. 12: 1571. https://doi.org/10.3390/pharmaceutics17121571
APA StyleAntonara, L., Rekkas, D. M., Pippa, N., & Dallas, P. P. (2025). Development of a 3D Printing Liquid Crystal Display (LCD)-Assisted Micromolding Methodology for Custom Fabrication of Polymeric Microneedles Using Experimental Design. Pharmaceutics, 17(12), 1571. https://doi.org/10.3390/pharmaceutics17121571









