Preparation, Characterization, and In Vivo Evaluation of an Oral Triptolide Nanomatrix System for Rheumatoid Arthritis Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TP Nanomatrix System
2.3. Determination of Production Yield and Drug Loading
2.3.1. High-Performance Liquid Chromatography Analysis
2.3.2. Production Yield
2.3.3. Drug Loading
2.4. In Vitro Dissolution Study
2.5. Physicochemical Characterization
2.6. Stability Study
2.7. In Vivo Pharmacokinetics Study
2.7.1. Design and Sampling
2.7.2. LC-MS/MS Analysis
2.8. Construction of Collagen-Induced Arthritis Rats
2.9. In Vivo Pharmacodynamics Study
2.9.1. Experimental Protocols and Evaluation
2.9.2. Arthritis Incidence
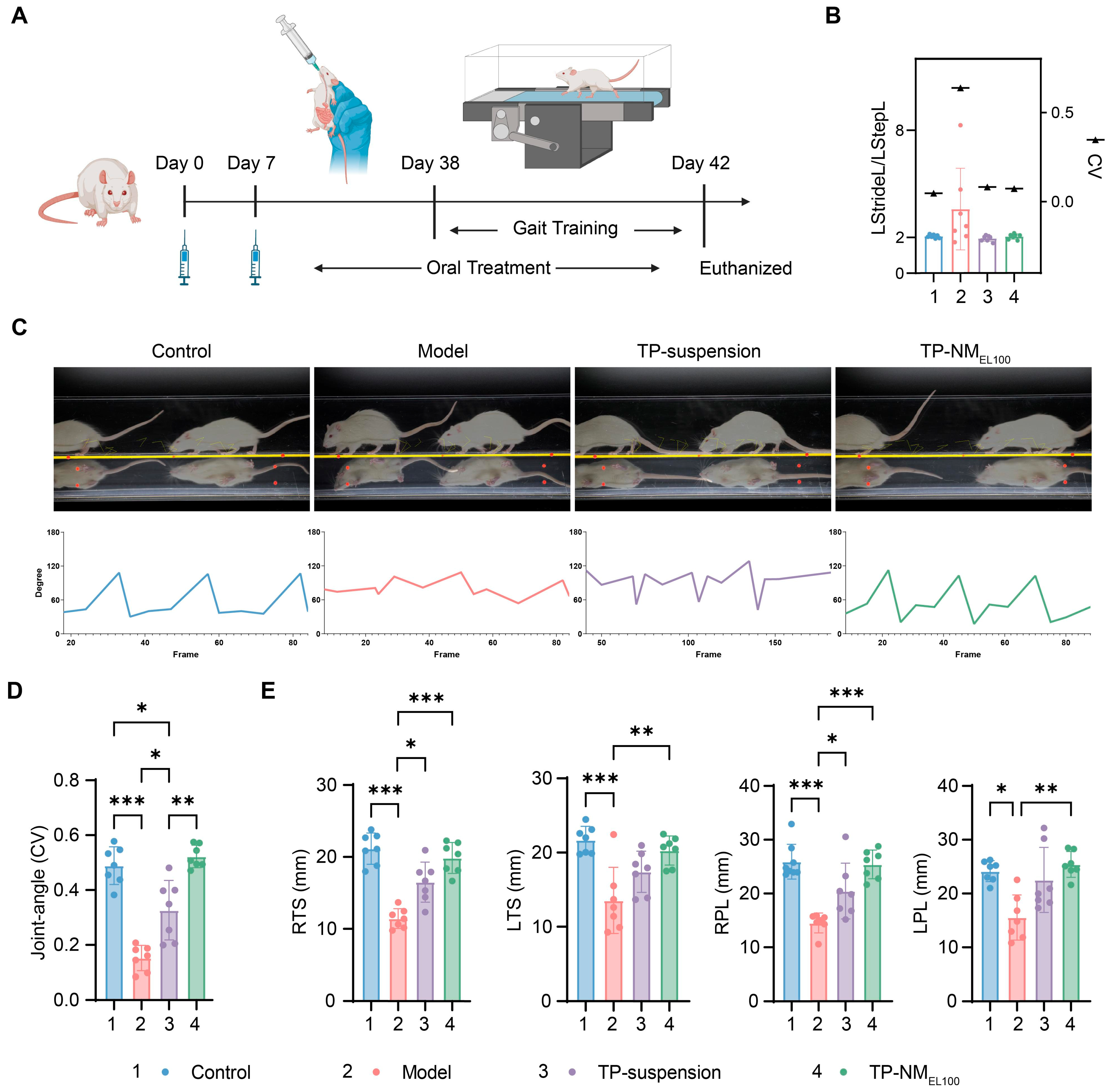
2.9.3. Motion Gait Analysis
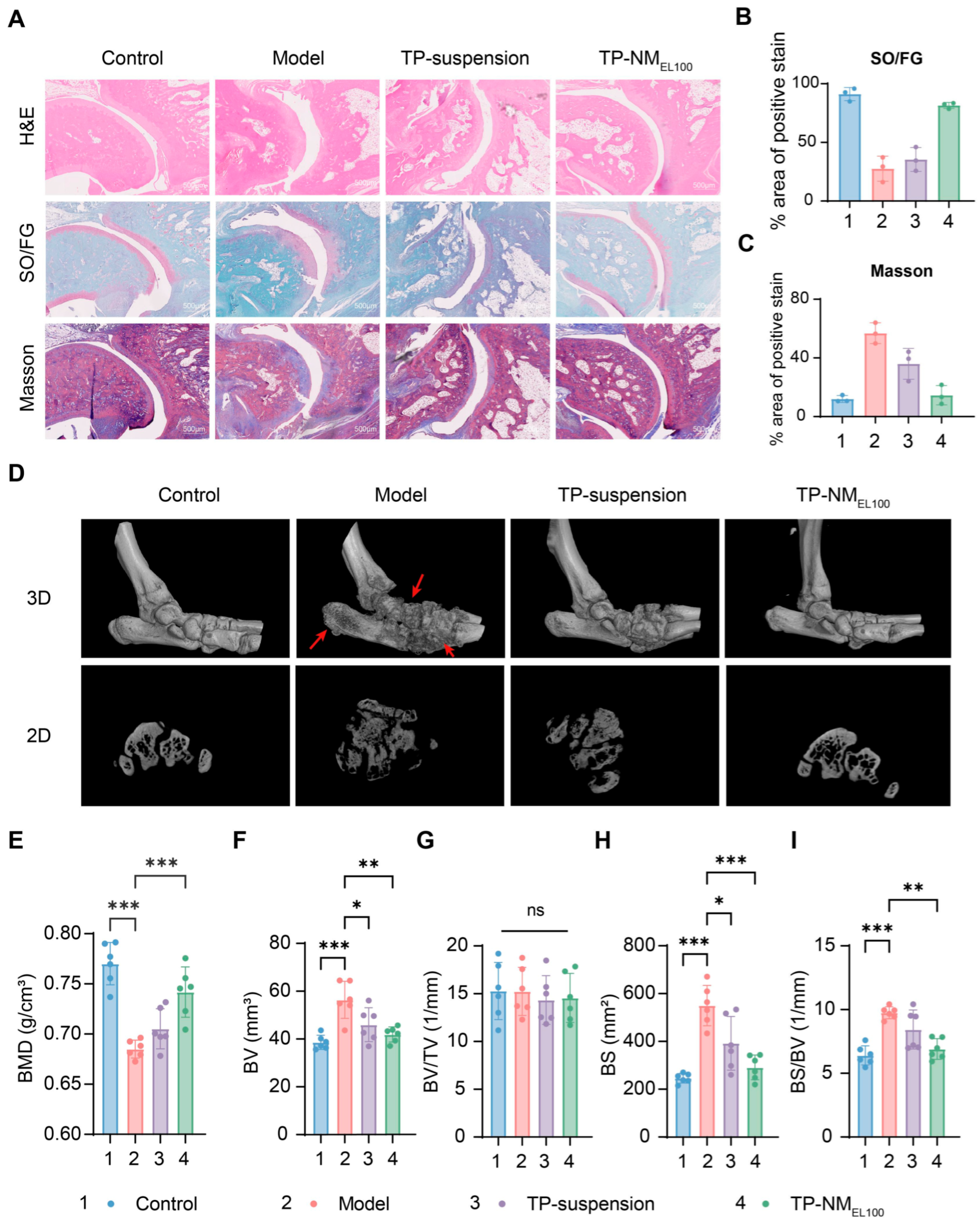
2.9.4. Micro-CT Analysis
2.10. Safety Evaluation
2.10.1. Biochemistry Analysis
2.10.2. Complete Blood Count Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preparation of TP-NMEL100
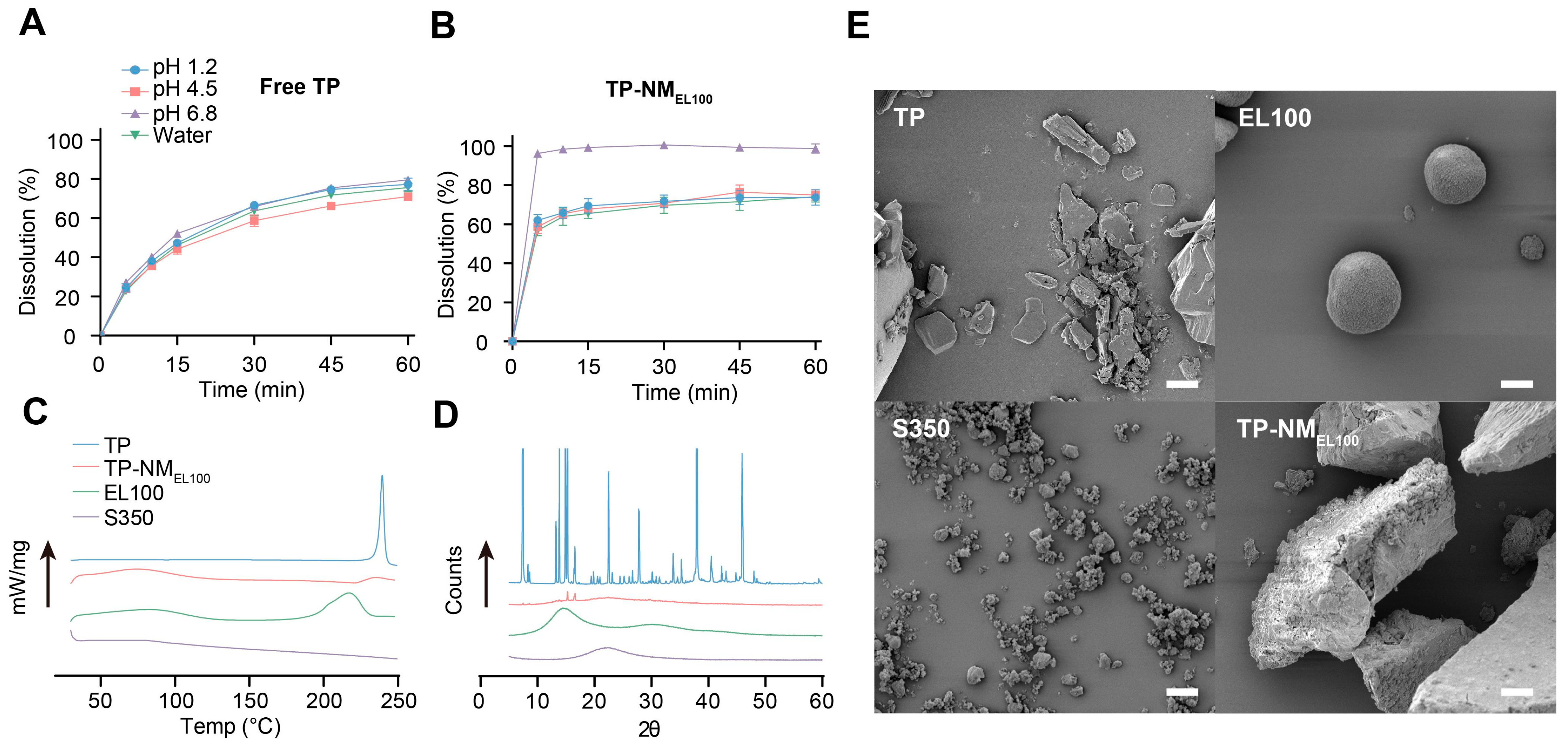
3.2. Characterization of TP-NMEL100
3.3. Stability Study
3.4. In Vivo Pharmacokinetics Study
3.5. Anti-Arthritis Efficacy of TP-NMEL100
3.6. Safety Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TP | Triptolide |
| CMC-Na | Sodium Carboxymethyl Cellulose |
| SEM | Scanning Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| XRD | X-Ray Diffraction |
| Micro-CT | Micro-Computed Tomography |
References
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zuo, Z.; Zhao, H.; Tan, Y.; Xiao, C. Immunoregulatory effects of Tripterygium wilfordii Hook F and its extracts in clinical practice. Front. Med. 2019, 13, 556–563. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.J.; Xia, S.; Wang, M.M.; Ji, W. Tripterygium Glycosides for Treating Late-onset Rheumatoid Arthritis: A Systematic Review and Meta-analysis. Altern. Ther. Health Med. 2016, 22, 32–39. [Google Scholar]
- Lv, Q.W.; Zhang, W.; Shi, Q.; Zheng, W.J.; Li, X.; Chen, H.; Wu, Q.J.; Jiang, W.L.; Li, H.B.; Gong, L.; et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): A randomised, controlled clinical trial. Ann. Rheum. Dis. 2015, 74, 1078–1086. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, J.; Jiang, Y.; Pisetsky, D.S.; Smolen, J.S.; Mu, R.; Dai, S.; Weinblatt, M.E.; Kvien, T.K.; Li, J.; et al. 2023 International Consensus Guidance for the use of Tripterygium wilfordii Hook F in the treatment of active rheumatoid arthritis. J. Autoimmun. 2024, 142, 103148. [Google Scholar] [CrossRef]
- Xi, C.; Peng, S.; Wu, Z.; Zhou, Q.; Zhou, J. Toxicity of triptolide and the molecular mechanisms involved. Biomed. Pharmacother. 2017, 90, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ni, T.; Miao, J.; Huang, X.; Feng, Z. The role and mechanism of triptolide, a potential new DMARD, in the treatment of rheumatoid arthritis. Ageing Res. Rev. 2025, 104, 102643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Gao, Y.; Wang, M.; Gao, Y.; Li, H.; Sun, J.; Wen, C.; Xie, Z. Triptolide in the treatment of systemic lupus erythematosus—Regulatory effects on miR-146a in B cell TLR7 signaling pathway in mice. Front. Pharmacol. 2022, 13, 952775. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Jiang, J.; Li, Q.; Gao, C.; Li, W.; Zhang, B.; Xin, Y.; Li, H.; Zhao, M.; et al. A rationally designed CD19 monoclonal antibody-triptolide conjugate for the treatment of systemic lupus erythematosus. Acta Pharm. Sin. B 2024, 14, 4560–4576. [Google Scholar] [CrossRef]
- AbdulHussein, A.H.; Al-Taee, M.M.; Radih, Z.A.; Aljuboory, D.S.; Mohammed, Z.Q.; Hashesh, T.S.; Riadi, Y.; Hadrawi, S.K.; Najafi, M. Mechanisms of cancer cell death induction by triptolide. BioFactors 2023, 49, 718–735. [Google Scholar] [CrossRef]
- Gu, Y.; Li, A.; Zeng, Y.; He, M.; Qi, F.; Liu, R.; Cai, H.; Li, D.; Tang, X.; Fu, Z.; et al. Engineering hybrid nanoparticles for targeted codelivery of triptolide and CYP3A4-siRNA against pulmonary metastatic melanoma. Sci. Adv. 2025, 11, eadv6990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, C.; Peng, F.; Liu, W.; Wan, J.; Xu, H.; Lam, C.W.; Yang, X. Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules 2013, 18, 13340–13356. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, Y.; Zheng, Y. Therapeutic potential of triptolide in autoimmune diseases and strategies to reduce its toxicity. Chin. Med. 2021, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, B.; Li, L.; Lu, P.; Yan, L.; Lu, C. Detoxification strategies of triptolide based on drug combinations and targeted delivery methods. Toxicology 2022, 469, 153134. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Song, W.; Peng, Z. Pro-drug approaches to overcome poor solubility and toxicity of triptolide. Phytochem. Rev. 2025, 1–16. [Google Scholar] [CrossRef]
- Kong, L.L.; Zhuang, X.M.; Yang, H.Y.; Yuan, M.; Xu, L.; Li, H. Inhibition of P-glycoprotein Gene Expression and Function Enhances Triptolide-induced Hepatotoxicity in Mice. Sci. Rep. 2015, 5, 11747. [Google Scholar] [CrossRef]
- Zheng, N.; Wei, A.; Wu, T.; Long, L.; Yang, H.; Li, H.; Wang, L. Triptolide and atorvastatin synergistically promote hepatotoxicity in cultured hepatocytes and female Sprague-Dawley rats by inhibiting pregnane X receptor-mediated transcriptional activation of CYP3A4. Toxicol. Lett. 2021, 342, 85–94. [Google Scholar] [CrossRef]
- Veiga-Matos, J.; Morales, A.I.; Prieto, M.; Remião, F.; Silva, R. Study Models of Drug–Drug Interactions Involving P-Glycoprotein: The Potential Benefit of P-Glycoprotein Modulation at the Kidney and Intestinal Levels. Molecules 2023, 28, 7532. [Google Scholar] [CrossRef]
- Tai, T.; Huang, X.; Su, Y.; Ji, J.; Su, Y.; Jiang, Z.; Zhang, L. Glycyrrhizin accelerates the metabolism of triptolide through induction of CYP3A in rats. J. Ethnopharmacol. 2014, 152, 358–363. [Google Scholar] [CrossRef]
- Wei, C.B.; Tao, K.; Jiang, R.; Zhou, L.D.; Zhang, Q.H.; Yuan, C.S. Quercetin protects mouse liver against triptolide-induced hepatic injury by restoring Th17/Treg balance through Tim-3 and TLR4-MyD88-NF-κB pathway. Int. Immunopharmacol. 2017, 53, 73–82. [Google Scholar] [CrossRef]
- Tan, Q.-Y.; Hu, Q.; Zhu, S.-N.; Jia, L.-L.; Xiao, J.; Su, H.-Z.; Huang, S.-Y.; Zhang, J.; Jin, J. Licorice root extract and magnesium isoglycyrrhizinate protect against triptolide-induced hepatotoxicity via up-regulation of the Nrf2 pathway. Drug Deliv. 2018, 25, 1213–1223. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, L.; Yao, W.; Han, J.; Wang, G.; Kalafatis, M. Arctiin Antagonizes Triptolide-Induced Hepatotoxicity via Activation of Nrf2 Pathway. BioMed Res. Int. 2020, 2020, 2508952. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, H.; Zhu, X.; Xu, D.; Tian, Q.; Wang, C.; Zhao, L.; Zhao, J.; Miao, M.; Wu, X. TP-CSO: A Triptolide Prodrug for Pancreatic Cancer Treatment. Molecules 2022, 27, 3686. [Google Scholar] [CrossRef]
- Datan, E.; Minn, I.; Xu, P.; He, Q.L.; Ahn, H.H.; Yu, B.; Pomper, M.G.; Liu, J.O. A Glucose-Triptolide Conjugate Selectively Targets Cancer Cells under Hypoxia. iScience 2020, 23, 101536. [Google Scholar] [CrossRef]
- Akhtar, M.; Jamshaid, M.; Zaman, M.; Mirza, A.Z. Bilayer tablets: A developing novel drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 60, 102079. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, H. Preparation of double-deck tablets of Radix Tripterygjun wil fordii. Chin. J. Hosp. Pharm. 2006, 26, 812. [Google Scholar]
- Zhang, Y.; Mao, X.; Li, W.; Chen, W.; Wang, X.; Ma, Z.; Lin, N. Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev. 2021, 41, 1337–1374. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Chen, Y.; Wu, Y. Absorption and Metabolism Characteristics of Triptolide as Determined by a Sensitive and Reliable LC-MS/MS Method. Molecules 2015, 20, 8928–8940. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Sun, J.; Ma, Y.; Cui, X.; Kou, S.; Jiang, Z.; Zhang, L.; Wang, X.; Wang, T.; et al. The intestinal absorption of triptolide for the treatment of rheumatoid arthritis is mediated by transporters. Int. Immunopharmacol. 2024, 143, 113440. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Lin, B.; Lin, L.; Zhou, G.; Song, H. Study on the intestinal absorption of Tripterygium wilfordii solid dispersion in rats. Zhong Cao Yao 2019, 50, 462–470. [Google Scholar]
- Xue, J.; Jia, X.; Tan, X.; Jia, D.; Jiang, J.; Zhang, L. Determination of apparent oil/water partition coefficient and absorption prediction of triptolide. Chin. Pharm. J. 2009, 44, 1560–1563. [Google Scholar]
- Xue, J.; Jia, X.; Tan, X.; Wang, J.; Chen, Y.; Zhang, L. Studies on rat intestinal absorption of triptolide in situ. Chin. Tradit. Herb. Drugs 2010, 41, 86–89. [Google Scholar]
- Li, Y.; Zhang, B.; Liu, M.; Zhang, X.; Shi, D.; Guo, L.; Duan, J.; Zhou, X.; Zhu, H.; Zhang, Q. Further Study of Influence of Panax notoginseng on Intestinal Absorption Characteristics of Triptolide and Tripterine in Rats with Tripterygium wilfordii. Pharmacogn. Mag. 2018, 14, 95. [Google Scholar] [CrossRef]
- Xie, M.; Wu, J.; Ji, L.; Jiang, X.; Zhang, J.; Ge, M.; Cai, X. Development of Triptolide Self-Microemulsifying Drug Delivery System and Its Anti-tumor Effect on Gastric Cancer Xenografts. Front. Oncol. 2019, 9, 978. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, T.-t.; Yuan, Z.-x.; Lu, Y. Self-assembled casein nanoparticles loading triptolide for the enhancement of oral bioavailability. Nat. Prod. Commun. 2020, 15, 1934578X20948352. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, Q.; Chen, Y.; Xie, M.; Li, J.; Yao, S.; Li, M.; Lou, Z.; Cai, Y.; Sun, X. Mechanochemical preparation of triptolide-loaded self-micelle solid dispersion with enhanced oral bioavailability and improved anti-tumor activity. Drug Deliv. 2022, 29, 1398–1408. [Google Scholar] [CrossRef]
- Jia, Z.; Lin, P.; Xiang, Y.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. A novel nanomatrix system consisted of colloidal silica and pH-sensitive polymethylacrylate improves the oral bioavailability of fenofibrate. Eur. J. Pharm. Biopharm. 2011, 79, 126–134. [Google Scholar] [CrossRef]
- Dai, W.; Guo, Y.; Zhang, H.; Wang, X.; Zhang, Q. Sylysia 350/Eudragit S100 solid nanomatrix as a promising system for oral delivery of cyclosporine A. Int. J. Pharm. 2015, 478, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhong, T.; Duan, X.C.; Zhang, S.; Yao, X.; Yin, Y.F.; Huang, D.; Ren, W.; Zhang, Q.; Zhang, X. Improving anti-tumor activity of sorafenib tosylate by lipid- and polymer-coated nanomatrix. Drug Deliv. 2017, 24, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-F.; Guo, Y.; Song, W.-D.; Duan, X.-C.; Zheng, X.-C.; Zhong, T.; Zhang, S.; Yao, X.; Xu, M.-Q.; Zhang, Q.; et al. Improving Solubility and Oral Bioavailability of Febuxostat by Polymer-Coated Nanomatrix. AAPS PharmSciTech 2017, 19, 934–940. [Google Scholar] [CrossRef]
- Baumgartner, A.; Planinšek, O. Application of commercially available mesoporous silica for drug dissolution enhancement in oral drug delivery. Eur. J. Pharm. Sci. 2021, 167, 106015. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Li, Y.; Zhou, L.; Liu, Z.; Wei, Y.; Dong, W.; Wu, X. R-Q@P-D nanoparticle for improving the oral bioavailability and therapeutic efficacy: A novel reversible dual-target-mild-inhibition intestinal metabolic enzymes strategy for resveratrol delivery. Chem. Eng. J. 2024, 497, 154662. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, H.; Sun, S.; Yang, Y.; Fan, D.; Yang, Y.; Zhao, Y.; Song, Z.; Chen, Y.; Zhu, R.; et al. Adipose-derived stem cell exosomes loaded with icariin alleviates rheumatoid arthritis by modulating macrophage polarization in rats. J. Nanobiotechnol. 2024, 22, 423. [Google Scholar] [CrossRef]
- Malengier-Devlies, B.; Bernaerts, E.; Ahmadzadeh, K.; Filtjens, J.; Vandenhaute, J.; Boeckx, B.; Burton, O.; De Visscher, A.; Mitera, T.; Berghmans, N.; et al. Role for Granulocyte Colony-Stimulating Factor in Neutrophilic Extramedullary Myelopoiesis in a Murine Model of Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022, 74, 1257–1270. [Google Scholar] [CrossRef]
- Watanabe, T.; Wakiyama, N.; Usui, F.; Ikeda, M.; Isobe, T.; Senna, M. Stability of amorphous indomethacin compounded with silica. Int. J. Pharm. 2001, 226, 81–91. [Google Scholar] [CrossRef]
- Stuart, J.M.; Cremer, M.A.; Kang, A.H.; Townes, A.S. Collagen-induced arthritis in rats. Evaluation of early immunologic events. Arthritis Rheumatol. 1979, 22, 1344–1351. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Wang, S.R.; Chen, X.; Ling, S.; Ni, R.Z.; Guo, H.; Xu, J.W. MicroRNA expression, targeting, release dynamics and early-warning biomarkers in acute cardiotoxicity induced by triptolide in rats. Biomed. Pharmacother. 2019, 111, 1467–1477. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Cao, W.; Yuan, Z.; Sun, L.; Zhang, L. Th17/Treg imbalance in triptolide-induced liver injury. Fitoterapia 2014, 93, 245–251. [Google Scholar] [CrossRef] [PubMed]





| Oral Administration | Tmax (min) | T1/2 (min) | Cmax (ng/mL) | AUC0–240 (min∙ng/mL) |
|---|---|---|---|---|
| TP-suspension | 15.83 ± 7.36 | 25.43 ± 12.49 | 7.54 ± 4.45 | 249.03 ± 52.37 |
| TP-NMEL100 | 16.00 ± 7.75 | 20.68 ± 19.86 | 13.91 ± 2.67 | 412.28 ± 109.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Li, M.; Zhou, Q.; Liu, C.; Lin, L.; Yang, L.; Dai, W. Preparation, Characterization, and In Vivo Evaluation of an Oral Triptolide Nanomatrix System for Rheumatoid Arthritis Therapy. Pharmaceutics 2025, 17, 1567. https://doi.org/10.3390/pharmaceutics17121567
Liang Y, Li M, Zhou Q, Liu C, Lin L, Yang L, Dai W. Preparation, Characterization, and In Vivo Evaluation of an Oral Triptolide Nanomatrix System for Rheumatoid Arthritis Therapy. Pharmaceutics. 2025; 17(12):1567. https://doi.org/10.3390/pharmaceutics17121567
Chicago/Turabian StyleLiang, Yujian, Mingyu Li, Qing Zhou, Chenyang Liu, Longfei Lin, Liuqing Yang, and Wenbing Dai. 2025. "Preparation, Characterization, and In Vivo Evaluation of an Oral Triptolide Nanomatrix System for Rheumatoid Arthritis Therapy" Pharmaceutics 17, no. 12: 1567. https://doi.org/10.3390/pharmaceutics17121567
APA StyleLiang, Y., Li, M., Zhou, Q., Liu, C., Lin, L., Yang, L., & Dai, W. (2025). Preparation, Characterization, and In Vivo Evaluation of an Oral Triptolide Nanomatrix System for Rheumatoid Arthritis Therapy. Pharmaceutics, 17(12), 1567. https://doi.org/10.3390/pharmaceutics17121567








