Development and Characterization of Magnoliae Flos Essential-Oil-Loaded Nanoemulsion: A Spatiotemporal Nose-to-Brain Delivery Enhancer for Solution and Gel-Based Pharmaceutical Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Chemical Characterization of MEO
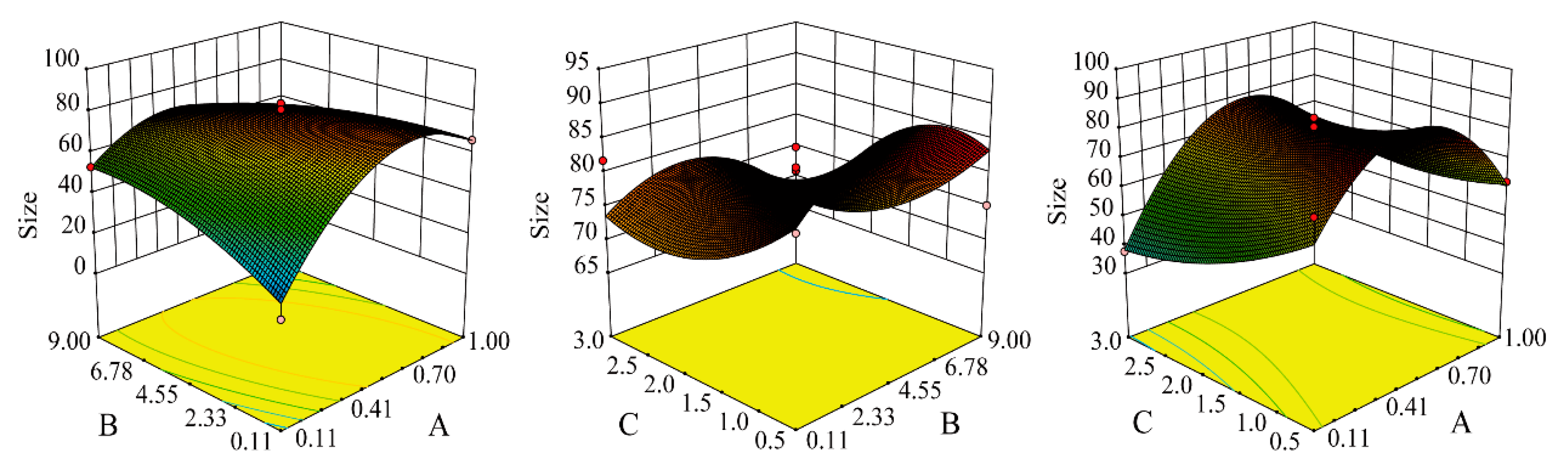
2.3. Preparation and Optimization of MEO-NE
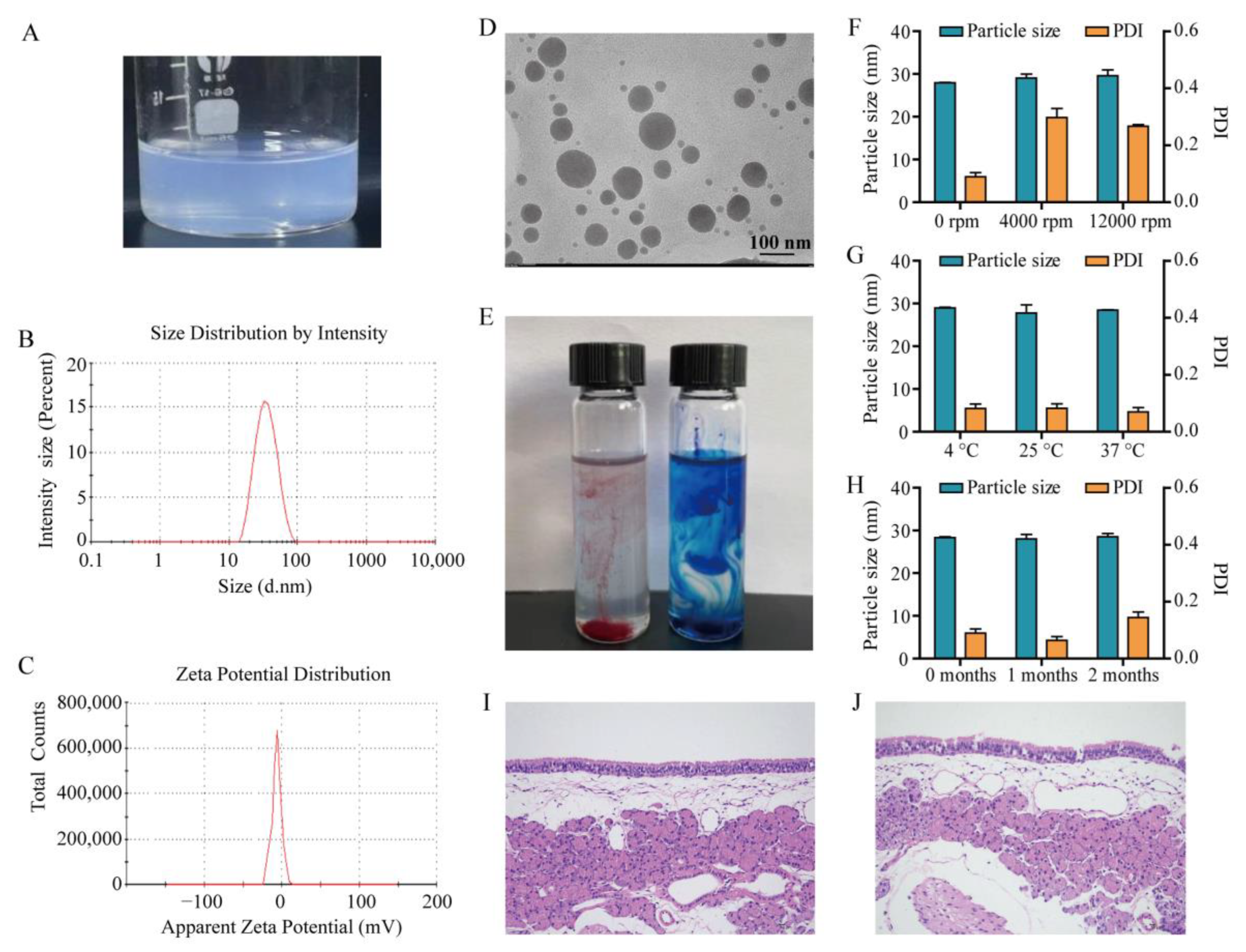
2.4. Characterization of MEO-NE
2.5. Quantification of MEO Content
2.6. Stability Study
2.7. Nasal Mucosal Irritation Test
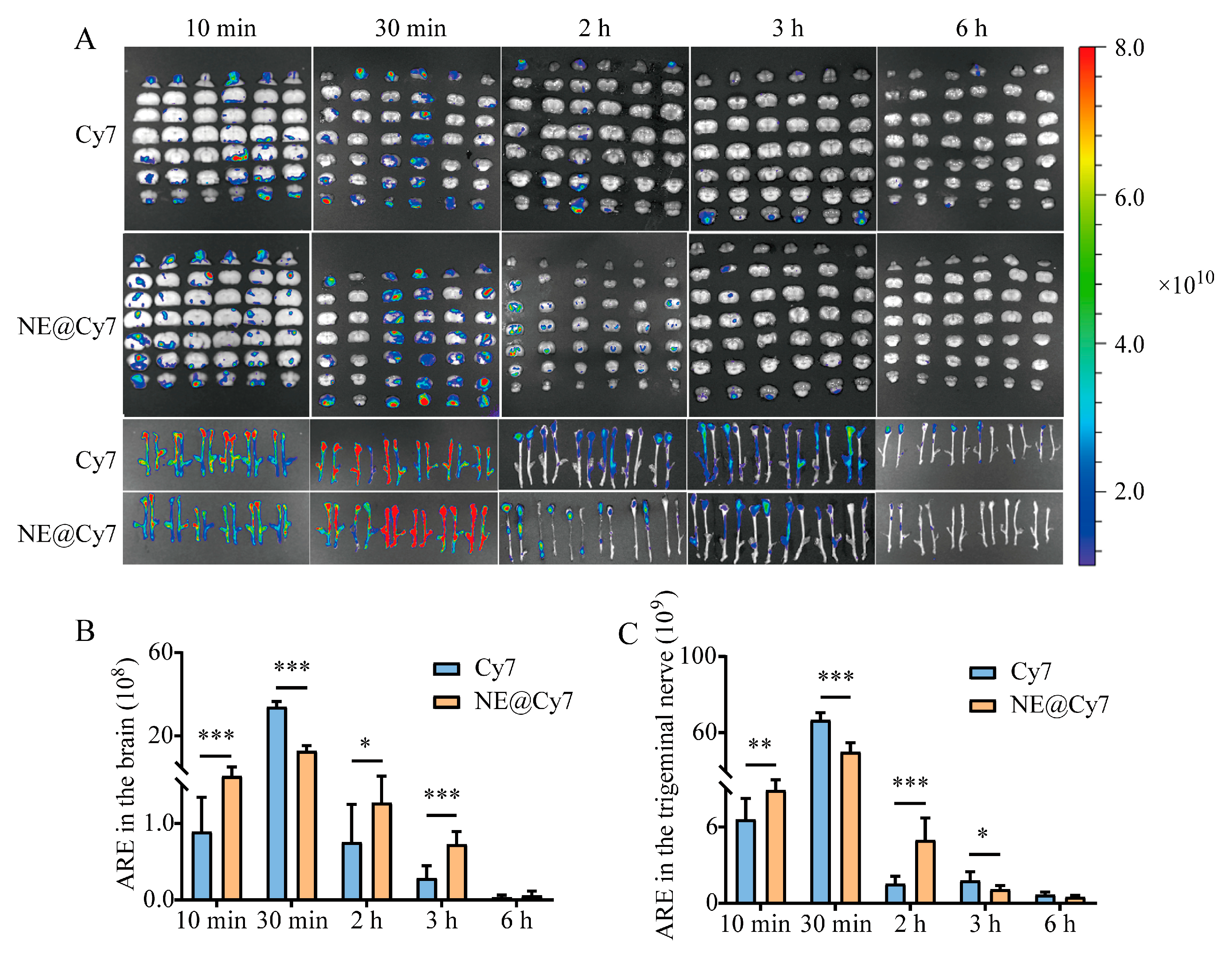
2.8. Effect of MEO-NE on Intranasal Brain Delivery of Solution-Formulated Drug
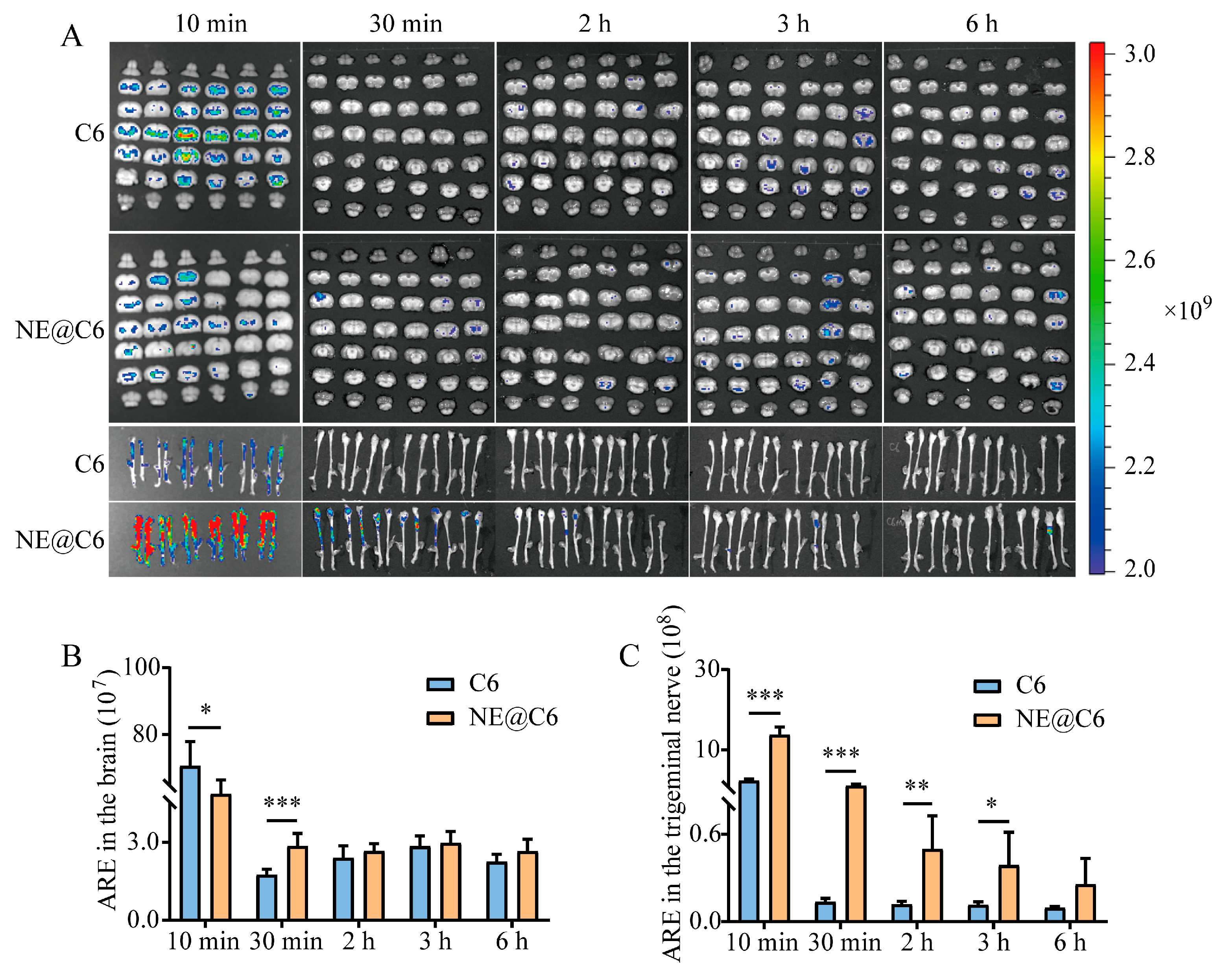
2.9. Effect of MEO-NE on Intranasal Brain Delivery of Gel-Formulated Drugs
2.10. Statistical Analysis
3. Results
3.1. Chemical Composition of MEO
3.2. Preparation of MEO-NE
3.3. Characterization of MEO-NE
3.4. Determination of MEO Content
3.5. Stability Evaluation
3.6. Evaluation of Nasal Mucosal Irritation
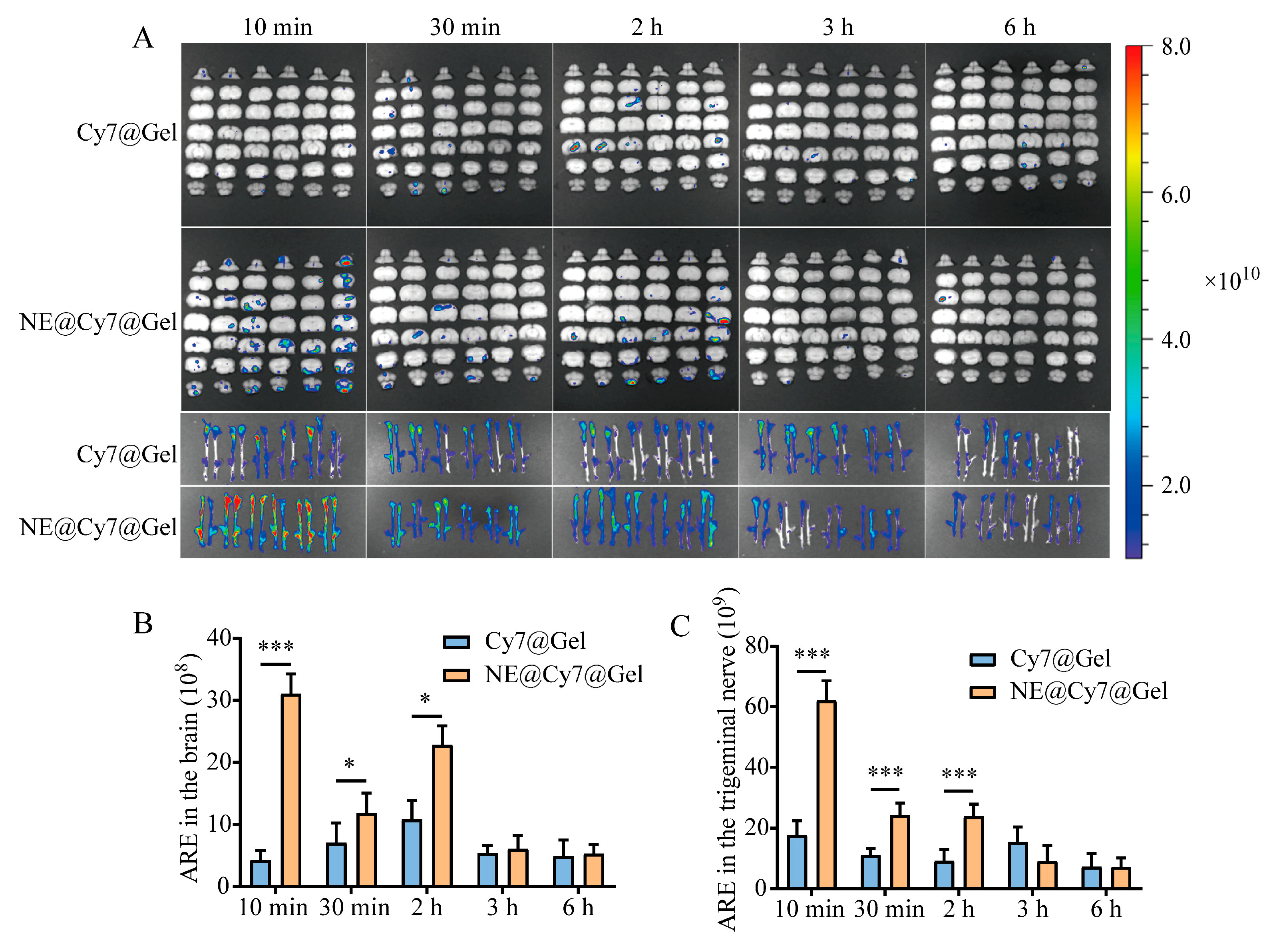
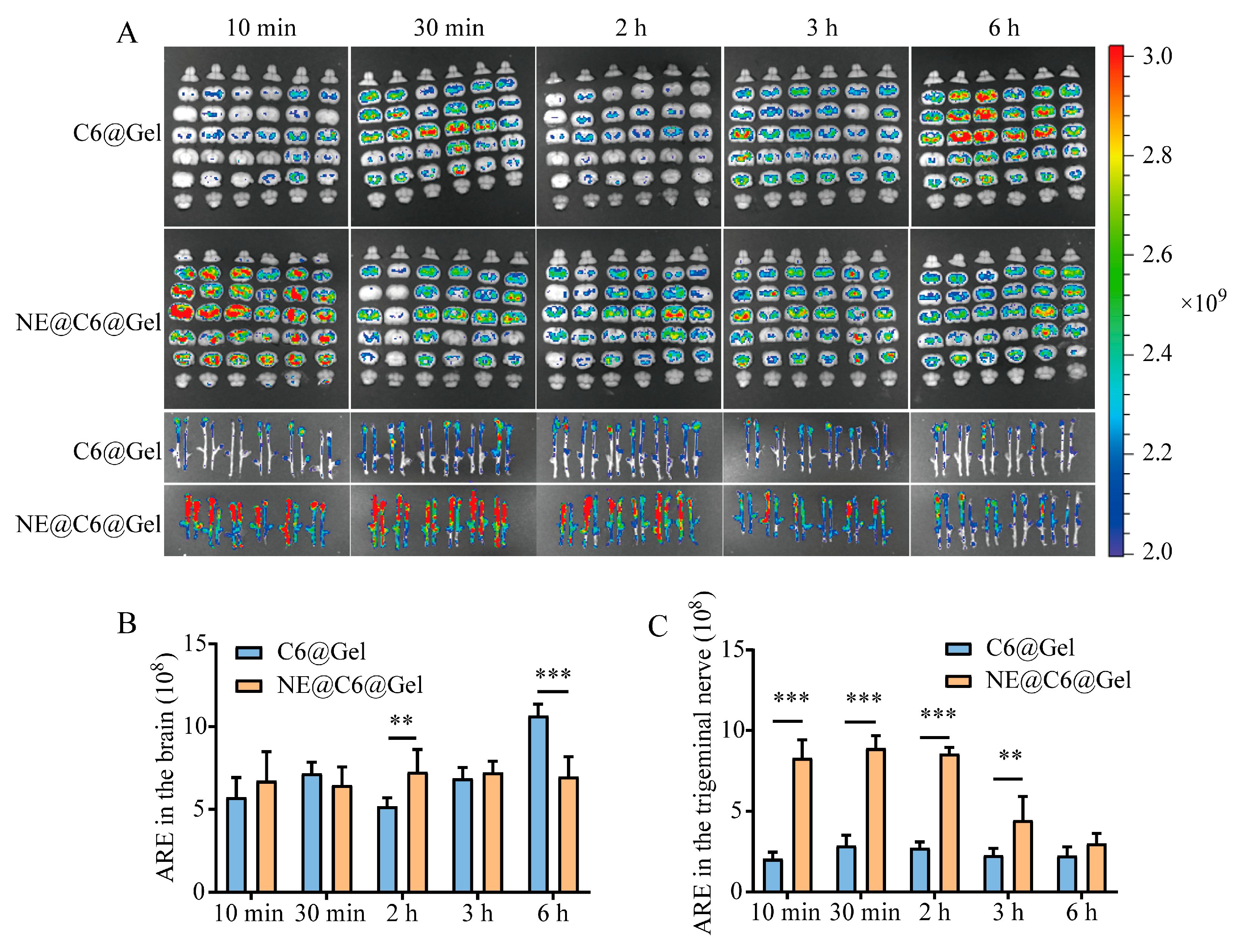
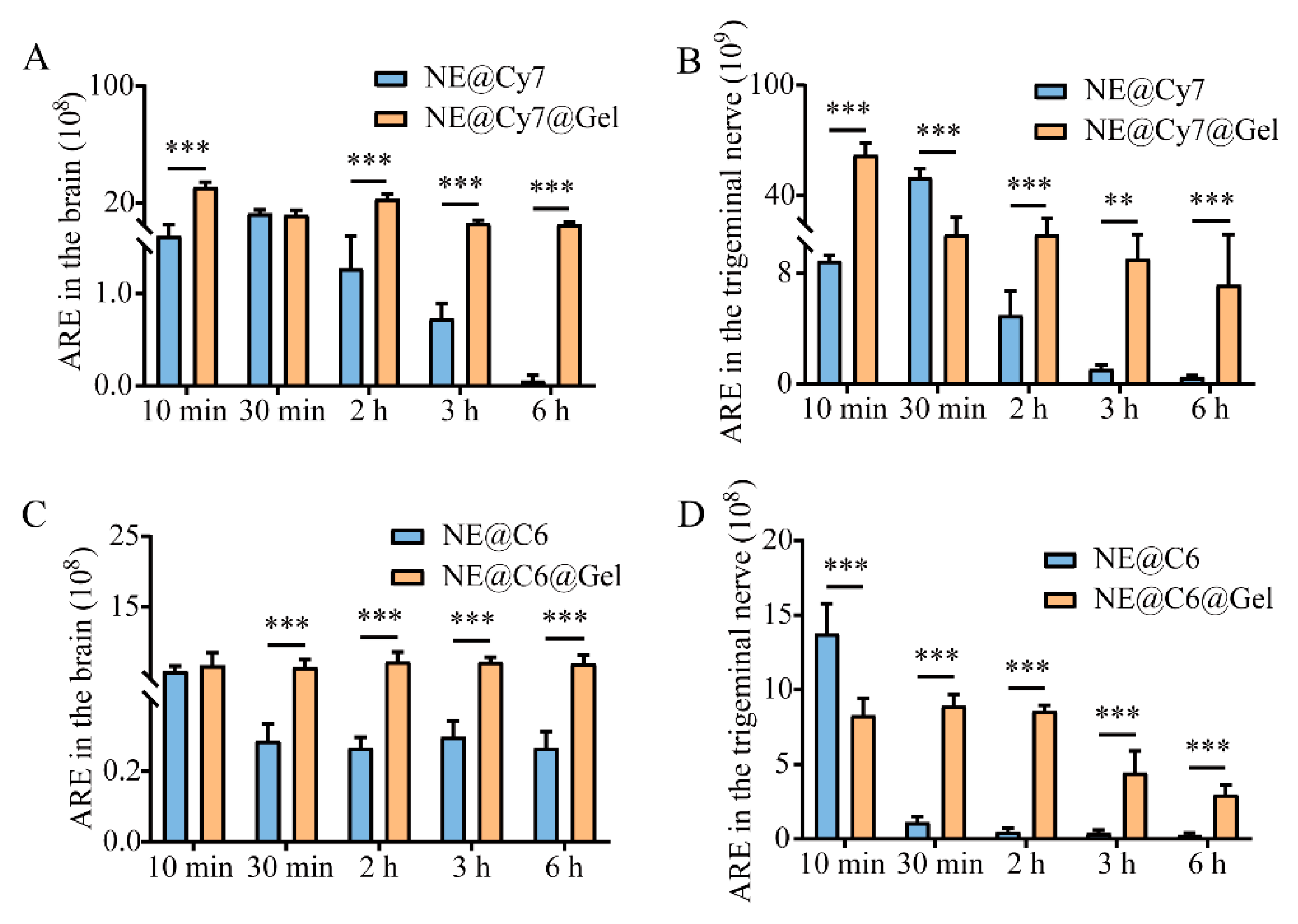
3.7. Effect of MEO-NE on the Nasal-to-Brain Distribution of Solution and Gel Formulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Chen, L.; Liu, F.; Wang, W.; Huang, H. Nose-to-brain drug delivery for the treatment of CNS disease: New development and strategies. Int. Rev. Neurobiol. 2023, 171, 255–297. [Google Scholar]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Song, H.; Yang, A.; Wang, Y.; Xu, R.; Hu, W. Potential roles of inhalation aromatherapy on stress-induced depression by inhibiting inflammation in the peripheral olfactory system. Neurochem. Int. 2025, 186, 105967. [Google Scholar] [CrossRef]
- Wang, P.; Lin, H.; Nguyen, T.T.T.; Hu, C.; Huang, L.; Tam, K.; Kuan, Y.-C. Efficacy of Aromatherapy Against Behavioral and Psychological Disturbances in People With Dementia: A Meta-Analysis of Randomized Controlled Trials. J. Am. Med. Dir. Assoc. 2024, 25, 105199. [Google Scholar] [CrossRef]
- Feng, P.; Chen, J.; Chen, X.; Tang, M.; Song, N.; Zhang, L.; He, T. Comparing Effects of Aromatherapy with Five Herbs Essential Oils on PCPA-induced Insomnia Mice. J. Microbiol. Biotechnol. 2024, 35, e2409021. [Google Scholar] [CrossRef]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS PharmSciTech 2021, 22, 145. [Google Scholar] [CrossRef]
- Malloggi, E.; Menicucci, D.; Cesari, V.; Frumento, S.; Gemignani, A.; Bertoli, A. Lavender aromatherapy: A systematic review from essential oil quality and administration methods to cognitive enhancing effects. Appl. Psychol. Health Well-Being 2021, 14, 663–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Kim, D.-I.; Park, S.Y.; Kang, H.J.; Park, S.-D.; Lee, J.-H. The Protective Role of Magnoliae Flos in Preventing Ovotoxicity and Managing Ovarian Function: An In Vitro and In Vivo Study. Int. J. Mol. Sci. 2024, 25, 6456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Feng, Y.X.; Zheng, Y.; Wang, C.F.; Du, S.S. Essential Oils from Different Parts of Magnolia laevifolia: Chemical Constituents and Insecticidal Activities against Liposcelis bostrychophila. Chem. Biodivers. 2023, 21, e202300935. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Dong, H.; Wu, J.; Wang, W.; Duan, Y.; Chen, J.; Zhang, J. Essential oil of Magnolia denudata is an effective anesthetic for spotted seabass (Lateolabrax maculatus): A test of its effect on blood biochemistry, physiology, and gill morphology. Fish Physiol. Biochem. 2022, 48, 1349–1363. [Google Scholar] [CrossRef]
- Hyeon, H.; Hyun, H.B.; Go, B.; Kim, S.C.; Jung, Y.H.; Ham, Y.M. Profiles of Essential Oils and Correlations with Phenolic Acids and Primary Metabolites in Flower Buds of Magnolia heptapeta and Magnolia denudata var. purpurascens. Molecules 2021, 27, 221. [Google Scholar]
- Liang, Y.; Zhang, X.; Zou, J.; Shi, Y.; Wang, Y.; Tai, J.; Yang, Y.; Zhou, X.; Guo, D.; Wang, J.; et al. Pharmacology mechanism of Flos magnoliae and Centipeda minima for treating allergic rhinitis based on pharmacology network. Drug Dev. Ind. Pharm. 2019, 45, 1547–1555. [Google Scholar] [CrossRef]
- Doghish, A.; Shehabeldine, A.; El-Mahdy, H.; Hassanin, M.; Al-Askar, A.; Marey, S.; AbdElgawad, H.; Hashem, A.H. Thymus Vulgaris Oil Nanoemulsion: Synthesis, Characterization, Antimicrobial and Anticancer Activities. Molecules 2023, 28, 6910. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, P.; Zhao, P.; Cheng, W.; Fu, H.; Zheng, X.; Chen, L.; Huang, W.; Xu, J.; Fu, C.; et al. ROS/Thermo dual-sensitive hydrogel loaded with a nanoemulsion of patchouli essential oil for ulcerative colitis. Int. J. Biol. Macromol. 2024, 281, 136542. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Das, P.; Rustagi, S.; Sharma, M.; Sridhar, N.; Hazarika, T.K.; Rana, P.; Nayak, P.K.; Sridhar, K. Essential oil-nanoemulsion based edible coating: Innovative sustainable preservation method for fresh/fresh-cut fruits and vegetables. Food Chem. 2024, 460, 140545. [Google Scholar]
- Tan, M.S.A.; Pandey, P.; Lohman, R.-J.; Falconer, J.R.; Siskind, D.J.; Parekh, H.S. Fabrication and Characterization of Clozapine Nanoemulsion Sol–Gel for Intranasal Administration. Mol. Pharm. 2022, 19, 4055–4066. [Google Scholar] [CrossRef]
- Scherließ, R.A.-O. Nasal formulations for drug administration and characterization of nasal preparations in drug delivery. Ther. Deliv. 2020, 11, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar]
- Wang, M.; Ma, X.; Zong, S.; Su, Y.; Su, R.; Zhang, H.; Liu, Y.; Wang, C.; Li, Y. The prescription design and key properties of nasal gel for CNS drug delivery: A review. Eur. J. Pharm. Sci. 2024, 192, 106623. [Google Scholar]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Rodrigo, A.C.; Patterson, A.K.; Hawkins, K.; Aly, M.M.S.; Sun, J.; Al Jamal, K.T.; Smith, D.K. Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel. Adv. Sci. 2021, 8, e2101058. [Google Scholar] [CrossRef]
- Chávez-Zamudio, R.; Ochoa-Flores, A.A.; Soto-Rodríguez, I.; Garcia-Varela, R.; García, H.S. Preparation, characterization and bioavailability by oral administration of O/W curcumin nanoemulsions stabilized with lysophosphatidylcholine. Food Funct. 2017, 8, 3346–3354. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, C.; Zhou, W.; Zhao, L.; Li, Y.; Hu, H. Construction, characterization and evaluation of Angelica Sinensis Radix essential oil-loaded nanoemulsion drug delivery system based on concept of “combination of drugs and adjuvants”. Chin. Tradit. Herb. Drugs 2023, 54, 1783–1792. [Google Scholar]
- Li, Y.; Wang, C.; Zong, S.; Qi, J.; Dong, X.; Zhao, W.; Wu, W.; Fu, Q.; Lu, Y.; Chen, Z. The Trigeminal Pathway Dominates the Nose-to-Brain Transportation of Intact Polymeric Nanoparticles: Evidence from Aggregation-Caused Quenching Probes. J. Biomed. Nanotechnol. 2019, 15, 686–702. [Google Scholar] [CrossRef]
- Wu, X.; Yin, W.; Li, Y.; Wu, H.; Cheng, Q.; He, Q.; Wu, H.; Hu, M. Assessment of quality in volatile oil from three basic sources of Xinyi from Hubei by anatomy, GC-MS, and chemometric methods. Sci. Rep. 2025, 15, 6857. [Google Scholar] [CrossRef] [PubMed]
- El Hazzam, K.; Mhada, M.; Metougui, M.L.; El Kacimi, K.; Sobeh, M.; Taourirte, M.; Yasri, A. Box–Behnken Design: Wet Process Optimization for Saponins Removal From Chenopodium quinoa Seeds and the Study of Its Effect on Nutritional Properties. Front. Nutr. 2022, 9, 906592. [Google Scholar] [CrossRef]
- Polo-Castellano, C.; Álvarez, J.Á.; Palma, M.; Barbero, G.F.; Ayuso, J.; Ferreiro-González, M. Optimization through a Box–Behnken Experimental Design of the Microwave-Assisted Extraction of the Psychoactive Compounds in Hallucinogenic Fungi (Psylocibe cubensis). J. Fungi 2022, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Alghaith, A.F.; Alshehri, S.; Alhakamy, N.A.; Hosny, K.M. Development, optimization and characterization of nanoemulsion loaded with clove oil-naftifine antifungal for the management of tinea. Drug Deliv. 2021, 28, 343–356. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Ortis, A.; Musumeci, T.; Carbone, C.; Hussain, M.; Di Salvatore, V.; Battiato, S.; Pappalardo, F.; Pignatello, R. Nose-to-Brain Drug Delivery and Physico-Chemical Properties of Nanosystems: Analysis and Correlation Studies of Data from Scientific Literature. Int. J. Nanomed. 2024, 19, 5619–5636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, J.; Cui, Z.; Binks, B.P. Influence of positively charged nanoparticles on the stability of oil-in-water emulsions stabilized by a cationic surfactant at extremely low concentration. J. Colloid Interface Sci. 2025, 687, 449–460. [Google Scholar]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Lu, T.; Yang, Y.; Yang, Z.; Liu, Z.; Li, M.; Lu, Z.; Gong, T.; Zhang, J. Mechanisms of the Compound of Magnoliae Flos and Xanthii Fructus Essential Oils for the Treatment of Allergic Rhinitis based on the Integration of Network Pharmacology, Molecular Docking, and Animal Experiment. Comb. Chem. High Throughput Screen. 2025; ahead of print. [Google Scholar] [CrossRef]
- Liu, Y.; Han, N.; Meng, F. Magnolia essential oil: A preliminary exploration of chemical composition and its antimicrobial and antioxidant potential. Front. Microbiol. 2025, 16, 1509796. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dai, L.; Lin, J.; Cao, W.; Lv, M.; Jiang, Y.; Wang, Q.; Guo, Y.; Yao, Z.; Shen, S.; et al. Supramolecular gel with enhanced immunomodulatory effects presents a minimally invasive treatment strategy for eosinophilic chronic rhinosinusitis. J. Control. Release 2025, 378, 503–516. [Google Scholar] [CrossRef]
- Teng, C.; Lv, W.; Chen, Y.; Liu, L.; Yin, J.; Li, S.; Min, Z.; Zhang, Q.; He, W.; Ma, K.; et al. Enhanced the treatment of ischemic stroke through intranasal temperature-sensitive hydrogels of edaravone and borneol inclusion complex. Int. J. Pharm. 2024, 651, 123748. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.; Rai, V.K.; Halder, J.; Kar, D.; Prusty, S.K.; Rout, S.K.; Manoharadas, S.; Palanisamy, S.; Dash, P.; Das, C.; et al. Development and Characterization of Chitosan Nanoparticles Containing Quercetin-β-Cyclodextrin Inclusion Complex for Improved Solubility, Brain Targeting, and Neuroprotective Potential Against Epilepsy. AAPS PharmSciTech 2025, 26, 124. [Google Scholar] [CrossRef]
- Cama, E.S.; Catenacci, L.; Perteghella, S.; Sorrenti, M.; Caira, M.R.; Rassu, G.; Gavini, E.; Giunchedi, P.; Bonferoni, M.C. Design and development of a chitosan-based nasal powder of dimethyl fumarate-cyclodextrin binary systems aimed at nose-to-brain administration. A stability study. Int. J. Pharm. 2024, 659, 124216. [Google Scholar] [PubMed]
- Mathure, D.; Ranpise, H.; Awasthi, R.; Pawar, A. Formulation and Characterization of Nanostructured Lipid Carriers of Rizatriptan Benzoate-Loaded In Situ Nasal Gel for Brain Targeting. ASSAY Drug Dev. Technol. 2022, 20, 211–224. [Google Scholar] [CrossRef]
| No. | Retention Time (min) | Compound | Peak Area | Relative Percentage (%) | CAS Registry No. |
|---|---|---|---|---|---|
| 1 | 6.886 | β-Ocimene | 364,011,868 | 2.73 | 13877-91-3 |
| 2 | 7.118 | (−)-Camphene | 377,367,712 | 2.83 | 5794-04-7 |
| 3 | 7.397 | 6-(4-fluorophenyl)-4,5-diazaspiro[2.4]hept-4-ene | 436,562,133 | 3.27 | 920338-72-3 |
| 4 | 7.519 | (+)-Camphene | 341,839,731 | 2.56 | 5794-03-6 |
| 5 | 7.92 | 1,2,3,4,5-Pentamethylcyclopentadiene | 160,909,813 | 1.21 | 4045-44-7 |
| 6 | 7.988 | (3,4-Dimethylphenyl)methanol | 188,819,137 | 1.42 | 6966-10-5 |
| 7 | 8.135 | cis-Chrysanthenyl formate | 635,510,838 | 4.77 | 241123-18-2 |
| 8 | 8.23 | (1R,2R)-1,2-di(prop-1-en-2-yl)cyclobutane | 307,782,589 | 2.31 | 19465-02-2 |
| 9 | 8.295 | 1,8-Cineole | 230,189,679 | 1.73 | 470-82-6 |
| 10 | 8.496 | 3-Methyl-6-propan-2-ylidenecyclohexene | 245,013,511 | 1.84 | 586-63-0 |
| 11 | 8.844 | (+)-4-Carene | 170,050,531 | 1.28 | 29050-33-7 |
| 12 | 8.950 | L-Fenchone | 160,050,083 | 1.20 | 7787-20-4 |
| 13 | 9.036 | Linalool | 397,927,991 | 2.98 | 78-70-6 |
| 14 | 9.882 | (−)-Camphor | 833,156,796 | 6.25 | 464-48-2 |
| 15 | 10.020 | 2,3,3-Trimethylbicyclo(2.2.1)heptan-2-ol | 233,568,079 | 1.75 | 465-31-6 |
| 16 | 10.260 | Terpinen-4-ol | 270,655,589 | 2.03 | 562-74-3 |
| 17 | 10.469 | (−)-α-Terpineol | 450,728,955 | 3.38 | 10482-56-1 |
| 18 | 11.611 | (−)-Bornyl Acetate | 182,898,316 | 1.37 | 5655-61-8 |
| 19 | 13.563 | 2-Methylene-4,8,8-trimethyl-4-vinylbicyclo[5.2.0]nonane | 297,129,756 | 2.23 | 242794-76-9 |
| 20 | 14.180 | γ-Muurolene | 197,781,281 | 1.48 | 30021-74-0 |
| 21 | 14.348 | (1S,4aR,8aS)-1-isopropyl-7-methyl-4-methylene-1,2,3,4,4a,5,6,8a-octahydronaphthalene | 411,098,533 | 3.08 | 6980-46-7 |
| 22 | 14.475 | α-Muurolene | 284,519,322 | 2.13 | 31983-22-9 |
| 23 | 14.744 | (+)-δ-Cadinene | 487,297,194 | 3.65 | 483-76-1 |
| 24 | 15.515 | (2E,4S,7E)-4-Isopropyl-1,7-dimethylcyclodeca-2,7-dienol | 268,300,097 | 2.01 | 198991-79-6 |
| 25 | 16.295 | T-Cadinol | 516,214,127 | 3.87 | 5937-11-1 |
| 26 | 16.461 | α-Cadinol | 549,827,089 | 4.12 | 481-34-5 |
| 27 | 17.043 | 1-(3,3-dimethylcyclohexylidene)ethaol | 679,676,654 | 5.10 | 26532-23-0 |
| 28 | 17.194 | (−)-cis-Isopiperitenol | 1,156,650,161 | 8.67 | 96555-02-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, S.; Wang, M.; Ma, X.; Cheng, Y.; Li, Y.; Zhang, H.; Wang, C. Development and Characterization of Magnoliae Flos Essential-Oil-Loaded Nanoemulsion: A Spatiotemporal Nose-to-Brain Delivery Enhancer for Solution and Gel-Based Pharmaceutical Formulations. Pharmaceutics 2025, 17, 1535. https://doi.org/10.3390/pharmaceutics17121535
Zong S, Wang M, Ma X, Cheng Y, Li Y, Zhang H, Wang C. Development and Characterization of Magnoliae Flos Essential-Oil-Loaded Nanoemulsion: A Spatiotemporal Nose-to-Brain Delivery Enhancer for Solution and Gel-Based Pharmaceutical Formulations. Pharmaceutics. 2025; 17(12):1535. https://doi.org/10.3390/pharmaceutics17121535
Chicago/Turabian StyleZong, Shiyu, Miao Wang, Xinyu Ma, Yunlong Cheng, Ye Li, Hong Zhang, and Chunliu Wang. 2025. "Development and Characterization of Magnoliae Flos Essential-Oil-Loaded Nanoemulsion: A Spatiotemporal Nose-to-Brain Delivery Enhancer for Solution and Gel-Based Pharmaceutical Formulations" Pharmaceutics 17, no. 12: 1535. https://doi.org/10.3390/pharmaceutics17121535
APA StyleZong, S., Wang, M., Ma, X., Cheng, Y., Li, Y., Zhang, H., & Wang, C. (2025). Development and Characterization of Magnoliae Flos Essential-Oil-Loaded Nanoemulsion: A Spatiotemporal Nose-to-Brain Delivery Enhancer for Solution and Gel-Based Pharmaceutical Formulations. Pharmaceutics, 17(12), 1535. https://doi.org/10.3390/pharmaceutics17121535









