Bridging Literature and Real-World Evidence: External Evaluation and Development of Fluoxetine Population Pharmacokinetics Model
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Data Extraction and Quality Assessment
2.3. Dataset Preparation
2.4. External Evaluation Process
2.5. Development of Fluoxetine PopPK Model
2.6. Dose Optimization via Monte-Carlo Simulations
3. Results
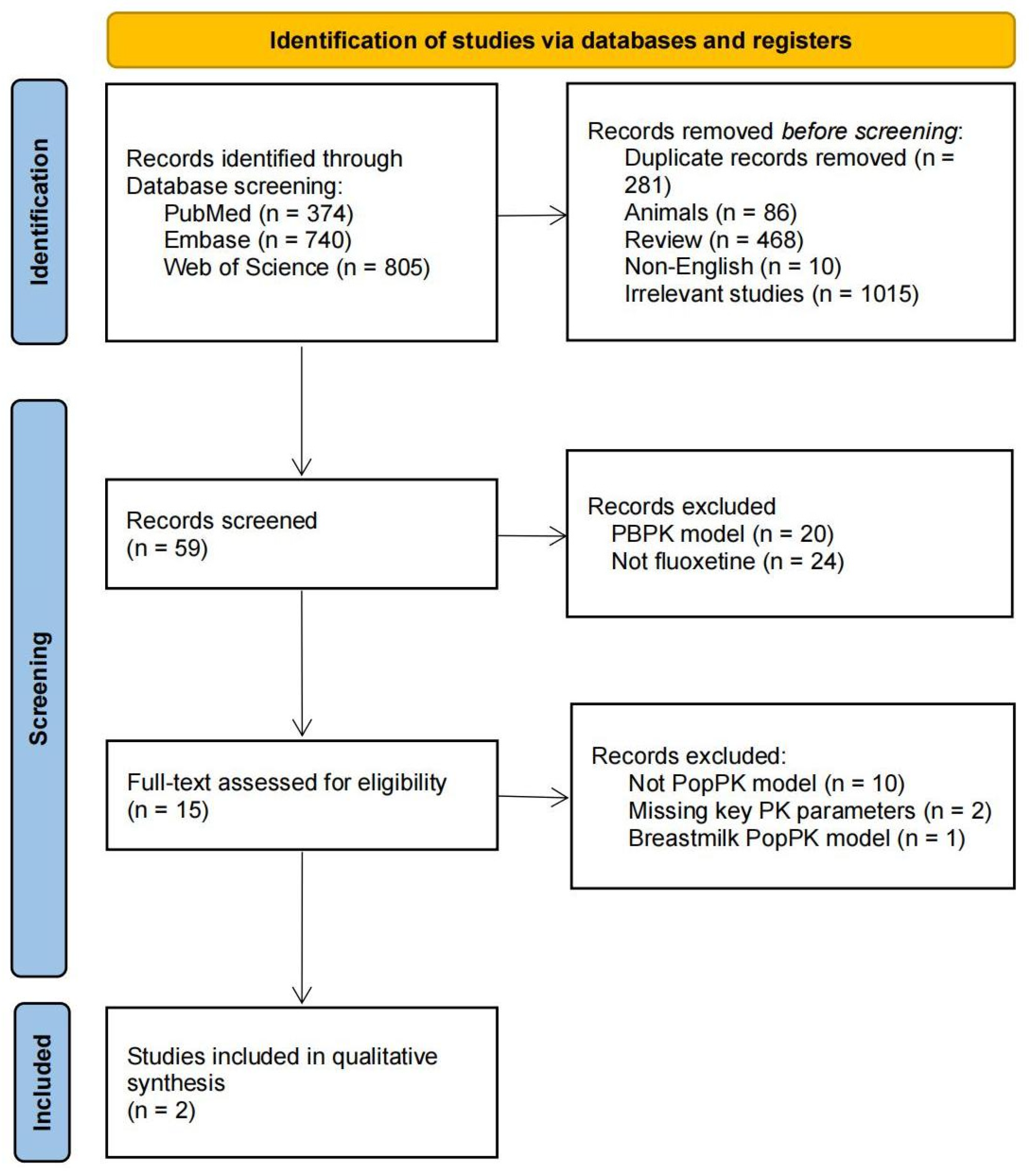
3.1. Study Identification
3.2. Literature Characteristics
3.3. Clinical Dataset Characteristics
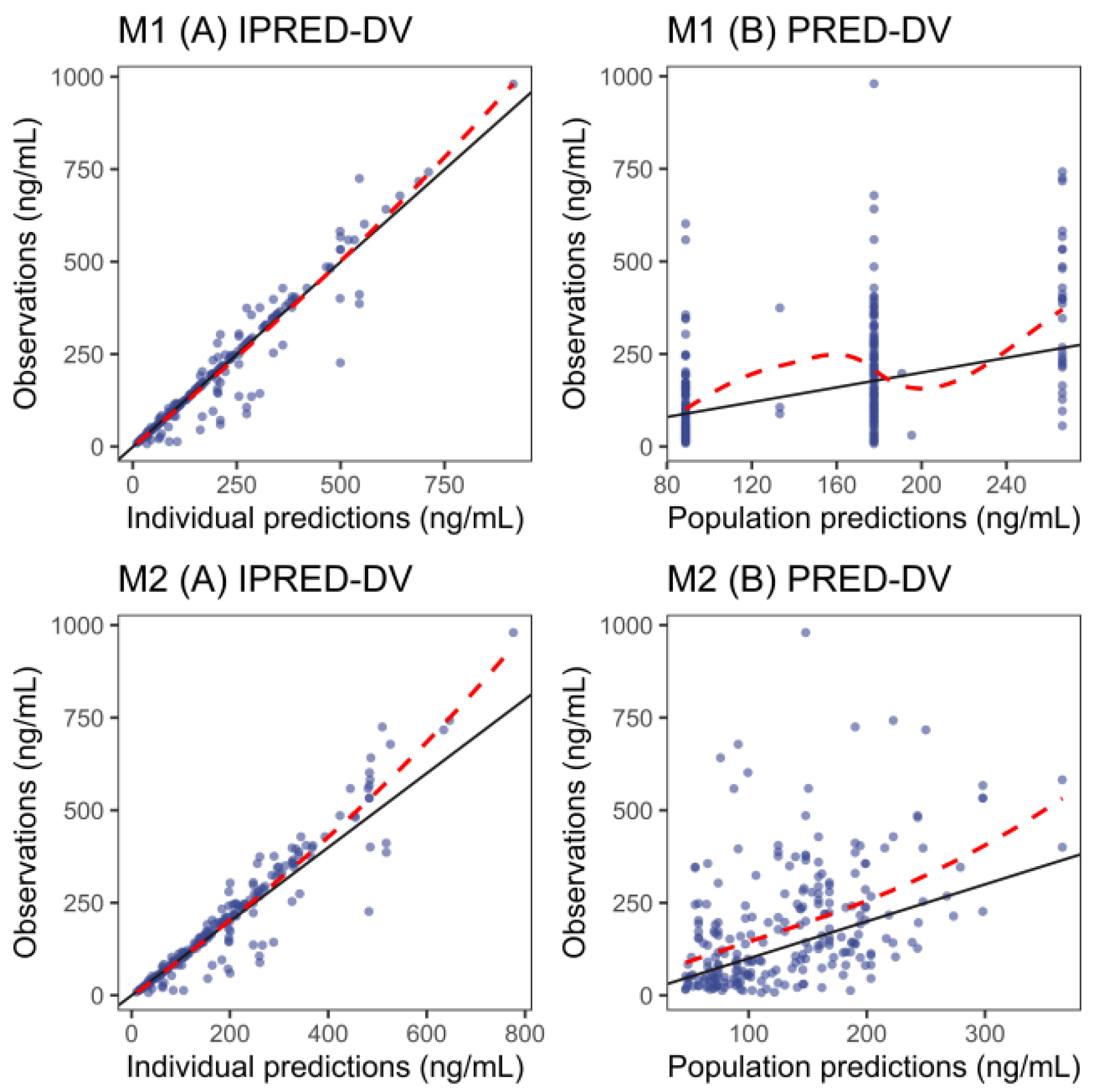
3.4. External Evaluation
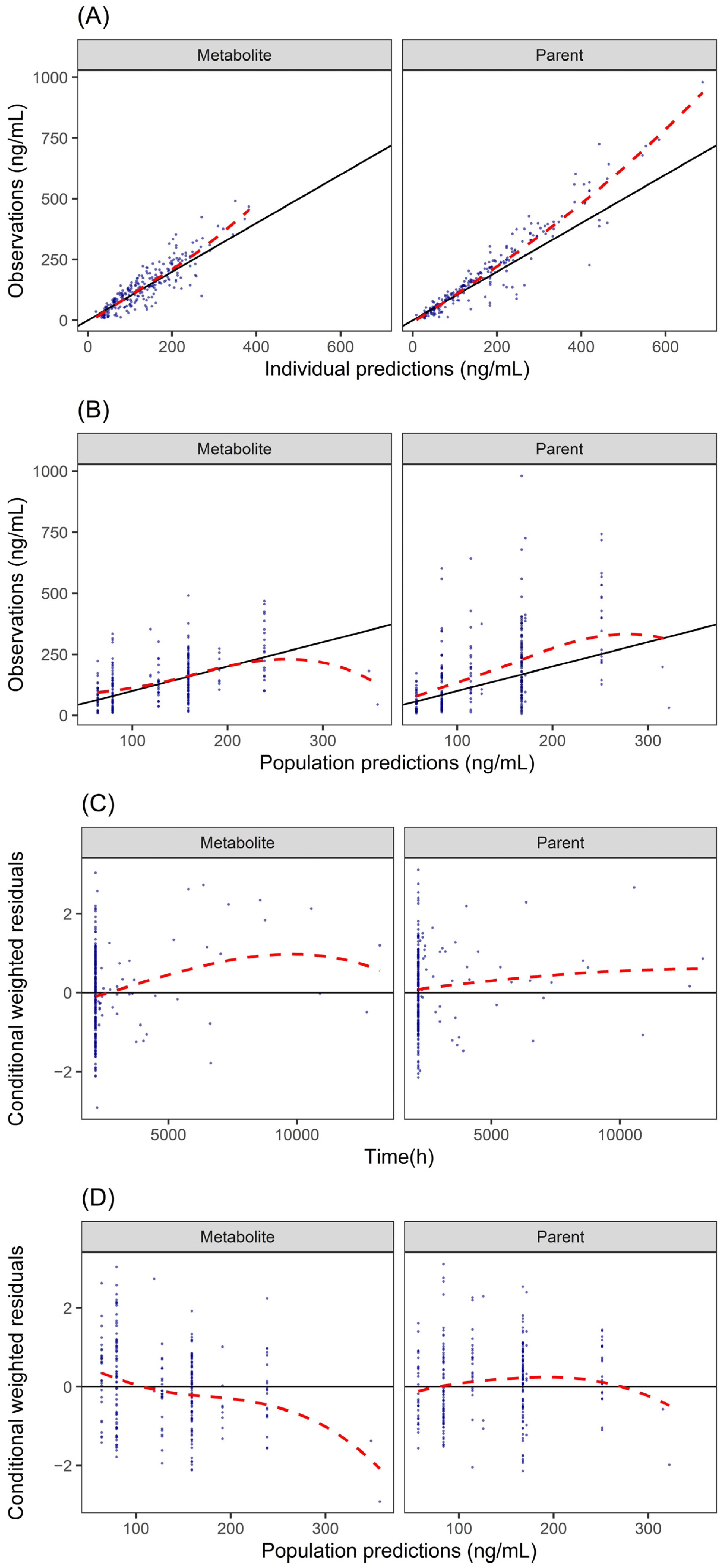
3.5. PopPK Model
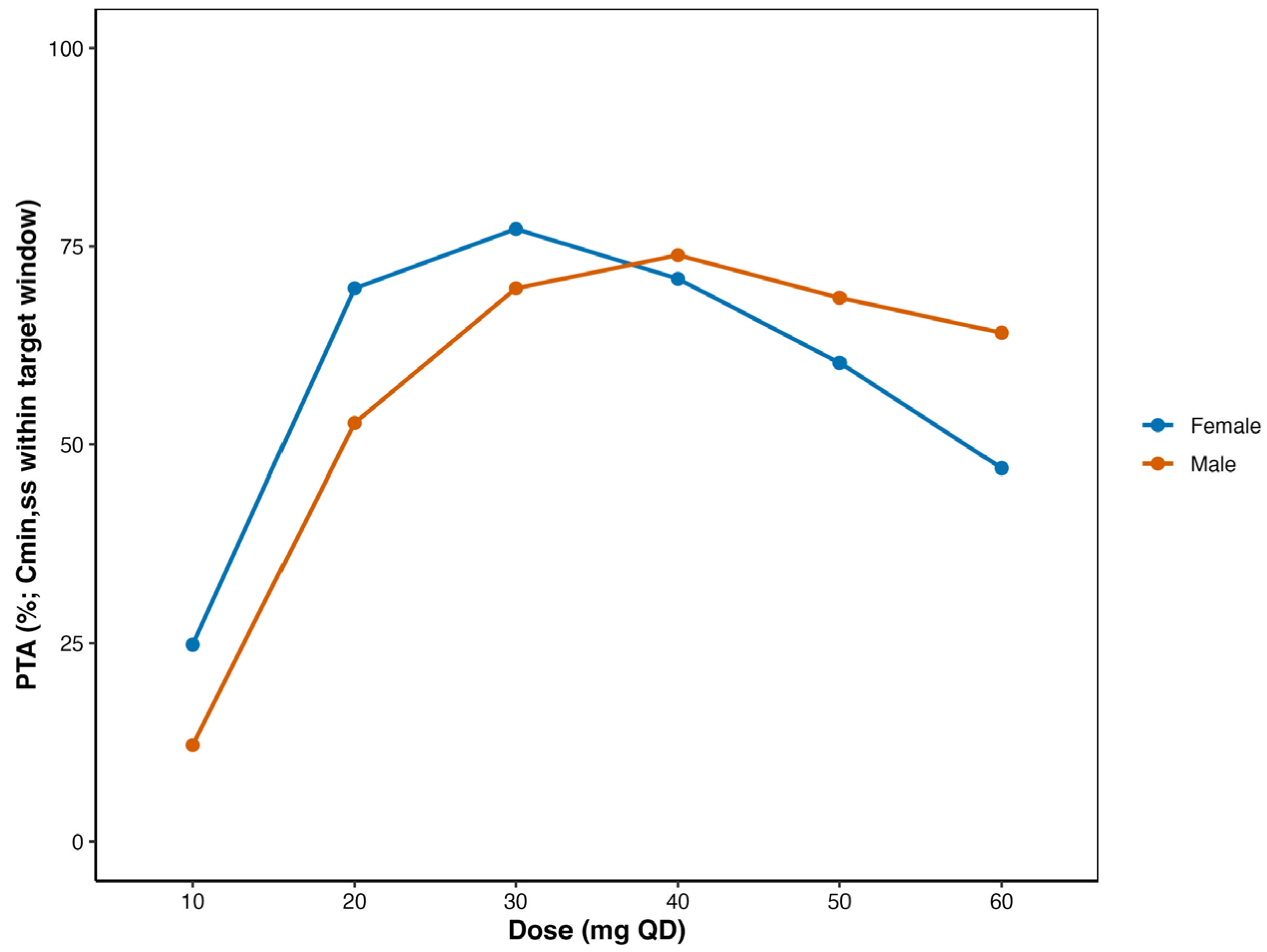
3.6. Optimized Dosage Regimens for Chinese Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Benfield, P.; Heel, R.C.; Lewis, S.P. Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs 1986, 32, 481–508. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Pigott, H.E. The STAR*D Trial: It Is Time to Reexamine the Clinical Beliefs That Guide the Treatment of Major Depression. Can. J. Psychiatry 2015, 60, 9–13. [Google Scholar] [CrossRef]
- Pigott, H.E.; Kim, T.; Xu, C.; Kirsch, I.; Amsterdam, J. What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study’s patient-level data with fidelity to the original research protocol. BMJ Open 2023, 13, e063095. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef]
- Frey, M.; Smigielski, L.; Tini, E.; Fekete, S.; Fleischhaker, C.; Wewetzer, C.; Karwautz, A.; Correll, C.U.; Gerlach, M.; Taurines, R.; et al. Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial. Pharmaceutics 2023, 15, 2202. [Google Scholar] [CrossRef]
- Sagahón-Azúa, J.; Medellín-Garibay, S.E.; Chávez-Castillo, C.E.; González-Salinas, C.G.; Milán-Segovia, R.D.C.; Romano-Moreno, S. Factors associated with fluoxetine and norfluoxetine plasma concentrations and clinical response in Mexican patients with mental disorders. Pharmacol. Res. Perspect. 2021, 9, e00864. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). PROZAC (fluoxetine) Prescribing Information. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/018936%20s112lbl.pdf (accessed on 14 November 2025).
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef]
- Ionova, Y.; Ashenhurst, J.; Zhan, J.; Nhan, H.; Kosinski, C.; Tamraz, B.; Chubb, A. CYP2C19 Allele Frequencies in Over 2.2 Million Direct-to-Consumer Genetics Research Participants and the Potential Implication for Prescriptions in a Large Health System. Clin. Transl. Sci. 2020, 13, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ingelman-Sundberg, M.; Lauschke, V.M. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-analysis of Population-scale Sequencing Projects. Clin. Pharmacol. Ther. 2017, 102, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Li, M.S.; Sundaram, S.K.; Tomlinson, B.; Cheung, P.Y.; Tzang, C.H. CYP2D6 allele frequencies, copy number variants, and tandems in the population of Hong Kong. J. Clin. Lab. Anal. 2019, 33, e22634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Cheng, Z.N.; Huang, S.L.; Chen, X.P.; Ou-Yang, D.S.; Jiang, C.H.; Zhou, H.H. Effect of the CYP2C19 oxidation polymorphism on fluoxetine metabolism in Chinese healthy subjects. Br. J. Clin. Pharmacol. 2001, 52, 96–99. [Google Scholar] [CrossRef]
- Del Tredici, A.L.; Malhotra, A.; Dedek, M.; Espin, F.; Roach, D.; Zhu, G.D.; Voland, J.; Moreno, T.A. Frequency of CYP2D6 Alleles Including Structural Variants in the United States. Front. Pharmacol. 2018, 9, 305. [Google Scholar] [CrossRef]
- Dai, H.R.; Guo, H.L.; Hu, Y.H.; Liu, Y.; Lu, K.Y.; Zhang, Y.Y.; Wang, J.; Ding, X.S.; Jiao, Z.; Cheng, R.; et al. Development and application of a population pharmacokinetic model repository for caffeine dose tailoring in preterm infants. Expert. Opin. Drug Metab. Toxicol. 2024, 20, 923–938. [Google Scholar] [CrossRef]
- Xu, N.; Shi, Y.; Wang, Y.; Mak, W.; Yang, W.; Ng, K.W.; Wu, Y.; Tang, Z.; He, Q.; Yan, G.; et al. Development and Quality Control of a Population Pharmacokinetic Model Library for Caspofungin. Pharmaceutics 2024, 16, 819. [Google Scholar] [CrossRef]
- Jeong, H.C.; Chae, Y.J.; Lee, S.; Kang, W.; Yun, H.Y.; Shin, K.H. Prediction of Fluoxetine and Norfluoxetine Pharmacokinetic Profiles Using Physiologically Based Pharmacokinetic Modeling. J. Clin. Pharmacol. 2021, 61, 1505–1513. [Google Scholar] [CrossRef]
- Tanoshima, R.; Bournissen, F.G.; Tanigawara, Y.; Kristensen, J.H.; Taddio, A.; Ilett, K.F.; Begg, E.J.; Wallach, I.; Ito, S. Population PK modelling and simulation based on fluoxetine and norfluoxetine concentrations in milk: A milk concentration-based prediction model. Br. J. Clin. Pharmacol. 2014, 78, 918–928. [Google Scholar] [CrossRef]
- Biso, L.; Aringhieri, S.; Carli, M.; Scarselli, M.; Longoni, B. Therapeutic Drug Monitoring in Psychiatry: Enhancing Treatment Precision and Patient Outcomes. Pharmaceuticals 2024, 17, 642. [Google Scholar] [CrossRef]
- Liu, X.; Ju, G.; Huang, X.; Yang, W.; Chen, L.; Li, C.; He, Q.; Xu, N.; Zhu, X.; Ouyang, D. Escitalopram population pharmacokinetics and remedial strategies based on CYP2C19 phenotype. J. Affect. Disord. 2024, 346, 64–74. [Google Scholar] [CrossRef]
- Ju, G.; Liu, X.; Gu, M.; Chen, L.; Wang, X.; Li, C.; Yang, N.; Zhang, G.; Zhang, C.; Zhu, X.; et al. Parametric Population Pharmacokinetics Model Repository of Rifampicin: Model-Informed Individualized Therapy. Clin. Pharmacol. 2025, 17, 49–78. [Google Scholar] [CrossRef]
- Liu, X.; Ju, G.; Yang, W.; Chen, L.; Xu, N.; He, Q.; Zhu, X.; Ouyang, D. Escitalopram Personalized Dosing: A Population Pharmacokinetics Repository Method. Drug Des. Devel Ther. 2023, 17, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, A.; Garcia-Bournissen, F.; Csajka, C.; Kristensen, J.H.; Taddio, A.; Ilett, K.F.; Begg, E.J.; Ito, S. Prediction of infant drug exposure through breastfeeding: Population PK modeling and simulation of fluoxetine exposure. Clin. Pharmacol. Ther. 2011, 89, 830–836. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Center for Drug Evaluation and Research Clinical Pharmacology and Biopharma ceutics Review(s) for a New Drug Application (NDA); 2003. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20974_Prozac_biopharmr.pdf (accessed on 14 November 2025).
- Wilens, T.E.; Cohen, L.; Biederman, J.; Abrams, A.; Neft, D.; Faird, N.; Sinha, V. Fluoxetine pharmacokinetics in pediatric patients. J. Clin. Psychopharmacol. 2002, 22, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Libby, A.M.; Brent, D.A.; Morrato, E.H.; Orton, H.D.; Allen, R.; Valuck, R.J. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am. J. Psychiatry 2007, 164, 884–891. [Google Scholar] [CrossRef]
- Allegaert, K.; van den Anker, J. Ontogeny of Phase I Metabolism of Drugs. J. Clin. Pharmacol. 2019, 59 (Suppl. S1), S33–S41. [Google Scholar] [CrossRef]
- Upreti, V.V.; Wahlstrom, J.L. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J. Clin. Pharmacol. 2016, 56, 266–283. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, X.; Du, Z.; Zhou, Q.; Jiang, Y.; Zhu, H. Potential Adverse Events of Fluoxetine: A Real-world Study Based on FAERS Database. Braz. J. Psychiatry 2025, 47, e20243879. [Google Scholar] [CrossRef]
- Pritchard, J.F.; Bryson, J.C.; Kernodle, A.E.; Benedetti, T.L.; Powell, J.R. Age and gender effects on ondansetron pharmacokinetics: Evaluation of healthy aged volunteers. Clin. Pharmacol. Ther. 1992, 51, 51–55. [Google Scholar] [CrossRef]
- Gex-Fabry, M.; Balant-Gorgia, A.E.; Balant, L.P.; Garrone, G. Clomipramine metabolism model-based analysis of variability factors from drug monitoring data. Clin. Pharmacokinet 1990, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Laine, K.; Tybring, G.; Bertilsson, L. No sex-related differences but significant inhibition by oral contraceptives of CYP2C19 activity as measured by the probe drugs mephenytoin and omeprazole in healthy Swedish white subjects. Clin. Pharmacol. Ther. 2000, 68, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, W.J.; Wemer, J.; Oosterhuis, B.; Weiling, J.; Wilffert, B.; de Leij, L.F.; de Zeeuw, R.A.; Jonkman, J.H. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: Indications for oral contraceptive-related gender differences. Eur. J. Clin. Pharmacol. 1999, 55, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Fric, M.; Gerlach, M.; Greiner, C.; Gründer, G.; et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsychiatry 2011, 44, 195–235. [Google Scholar] [CrossRef]
- Kane, M. CYP2D6 Overview: Allele and Phenotype Frequencies. In Medical Genetics Summaries; National Center for Biotechnology Information: Bethesda, MA, USA, 2021. [Google Scholar]
- Koopmans, A.B.; Braakman, M.H.; Vinkers, D.J.; Hoek, H.W.; van Harten, P.N. Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl. Psychiatry 2021, 11, 141. [Google Scholar] [CrossRef]
- Blazquez, A.; Mas, S.; Plana, M.T.; Lafuente, A.; Lázaro, L. Fluoxetine pharmacogenetics in child and adult populations. Eur. Child. Adolesc. Psychiatry 2012, 21, 599–610. [Google Scholar] [CrossRef]
- Muzyk, A.; Syed, F.Z.; Zhou, H.; Cong, J.; Tabuteau, H.; Zhao, Y. Real-world treatment patterns of patients with major depressive disorder treated with Auvelity in the United States. J. Med. Econ. 2024, 27, 1003–1010. [Google Scholar] [CrossRef]

| Study (Publication Year) | Country (Type of Study) | Number of Subjects (M/F) | Number of Observations | Sampling Schedule (h) | Age Mean ± SD Median [Range] | Weight (Kg) Mean ± SD Median [Range] | Dose [Range] | Bioassay [LOQ] |
|---|---|---|---|---|---|---|---|---|
| Panchaud (2011) [25] | Study1: Australia Study2: Canada | Study1: Women: 14 Infants: 14 (7/7) Study2: Women: 10 Infants: 11 | Maternal plasma: 49 Breastmilk: 112 | Study1: Four women: 1, 2, 3, 4, 6, 8, 12, 24 h; ten women: one sample (1.1–23.5 h) to collect milk and plasma Study2: 2, 5, 8, 12, and 24 h to collect milk sample | Maternal: 31.8 [22.7–44.0] years Infant: 6.3 [0.12–25] months | Maternal: 64.5 [31–85] Infant: 5.3 [2.8–10] | Study1: 0.51 [0.24–0.94] mg/kg/day Study2: 0.39 [0.17–0.85] mg/kg/day | Study1: HPLC, 15 ug/L Study2: Gas-liquid chromatography, 1 ng/mL |
| Wilens (2002) [26,27] | America | Pediatrics: 21 (11/10) | Plasma: 168 | 6–10 samples per subject, 8–12 h post-dose | 12.6 [6.0–17.0] years | Children: 39.9 ± 12.8 Adolescents: 67.3 ± 16.1 | 20 mg QD | LC–MS/MS 1.0 ng/mL |
| External dataset | China | 198 (Women: 146; Men: 52) | 241 fluoxetine and 241 norfluoxetine plasma concentrations | All concentration was trough concentration | 17 [12–56] | 59 [35.9–115] | 20–60 mg QD | HPLC–MS/MS [1 ng/mL] |
| Study (Publication Year) | Software (Algorithm) | Model | Fixed Effect Parameters | Between-Subject Variability (%CV) | Residual Unexplained Variability Prop% Add (mg/L) | External Validation | Model Application | |
|---|---|---|---|---|---|---|---|---|
| Panchaud (2011) [25] | NONMEM VI (FOCE-I) | One-compartment model with first-order absorption, fixed ka = 0.3, MPR (milk-to-plasma ratio) applied as a scaling factor for milk compartment | CL/F (L/h) | =8.42 | 38.00% | Prop.err Plasma: 8% Milk: 37% | 59 individuals from 8 external studies (MPR validation) | To predict infant drug exposure through breastfeeding |
| V/F (L) | =690 | / | ||||||
| Ka (/h) | =0.3 FIX | / | ||||||
| MPR | =0.59 | 32.00% | ||||||
| Wilens (2002) [26,27] | NONMEM V (FOCE) | One-compartment model with first-order absorption and elimination | CL/F (L/h) | =0.181 × BW | 52.00% | Prop.err: 18% | No | To characterize fluoxetine pharmacokinetics in pediatric patients (children and adolescents) with depression or OCD |
| V/F (L) | =37.4 × BW | 20.50% | ||||||
| Ka (/h) | =0.666 FIX | / | ||||||
| IPRED | PRED | |||||
|---|---|---|---|---|---|---|
| Model | Median PE (%) | MPE (%) | RMSE (%) | Median PE (%) | MPE (%) | RMSE (%) |
| M1 | −2.99 | −2.66 | 8.43 | −2.48 | 50.7 | 189.87 |
| M2 | −0.52 | −0.43 | 2.57 | 2.72 | 78.32 | 248.65 |
| Compounds | Parameter | Estimate (RSE%) [Shrinkage] | Bootstrap Median [95% CI] |
|---|---|---|---|
| Fluoxetine | CL/F, L/h | 2.91 (23%) | 2.76 [1.53–4.13] |
| Sex effects on CL/F 1, % | 16.50 (44%) | 16.43 [6.64–27.21] | |
| V/F, L | 24.9 (38%) | 22.76 [9.06–38.48] | |
| Ka (fixed) h−1 | 0.3 | / | |
| FM (fixed) 1 | 1 | / | |
| IIV CL/F, % | 31.6 (27%) [10%] | 30.67 [23.39–37.51] | |
| Prop.err.sd, % | 34.1 (12%) [20.8%] | 34.64 [27.86–40.35] | |
| Add.err.sd, ng/mL | 14.9 (49%) [20.8%] | 14.84 [8.74–21.48] | |
| Norfluoxetine | CL/F, L/h | 3.24 (20%) | 3.06 [1.77–4.53] |
| V/F, L | 1.52 (57%) | 1.17 [0.67–1.98] | |
| IIV CL/F, % | 20.9 (41%) [48%] | 21.01 [4.96–29.46] | |
| Prop.err.sd, % | 30.5 (16%) [37.3%] | 29.38 [19.87–40.01] | |
| Add.err.sd ng/mL | 22.9 (31%) [37.3%] | 23.20 [13.28–34.44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Xu, N.; Ma, C.; Ju, G.; Xi, X.; Qian, C.; Guo, N.; Liu, X.; Zhu, X.; Li, C.; et al. Bridging Literature and Real-World Evidence: External Evaluation and Development of Fluoxetine Population Pharmacokinetics Model. Pharmaceutics 2025, 17, 1516. https://doi.org/10.3390/pharmaceutics17121516
Han B, Xu N, Ma C, Ju G, Xi X, Qian C, Guo N, Liu X, Zhu X, Li C, et al. Bridging Literature and Real-World Evidence: External Evaluation and Development of Fluoxetine Population Pharmacokinetics Model. Pharmaceutics. 2025; 17(12):1516. https://doi.org/10.3390/pharmaceutics17121516
Chicago/Turabian StyleHan, Bing, Nuo Xu, Chen Ma, Gehang Ju, Xie Xi, Cheng Qian, Nan Guo, Xin Liu, Xiao Zhu, Cong Li, and et al. 2025. "Bridging Literature and Real-World Evidence: External Evaluation and Development of Fluoxetine Population Pharmacokinetics Model" Pharmaceutics 17, no. 12: 1516. https://doi.org/10.3390/pharmaceutics17121516
APA StyleHan, B., Xu, N., Ma, C., Ju, G., Xi, X., Qian, C., Guo, N., Liu, X., Zhu, X., Li, C., & Liu, L. (2025). Bridging Literature and Real-World Evidence: External Evaluation and Development of Fluoxetine Population Pharmacokinetics Model. Pharmaceutics, 17(12), 1516. https://doi.org/10.3390/pharmaceutics17121516







