Enhanced Ocular Delivery of Epalrestat Using Nanostructured Lipid Carrier Laden Soft Contact Lens
Abstract
1. Introduction
2. Materials and Methods
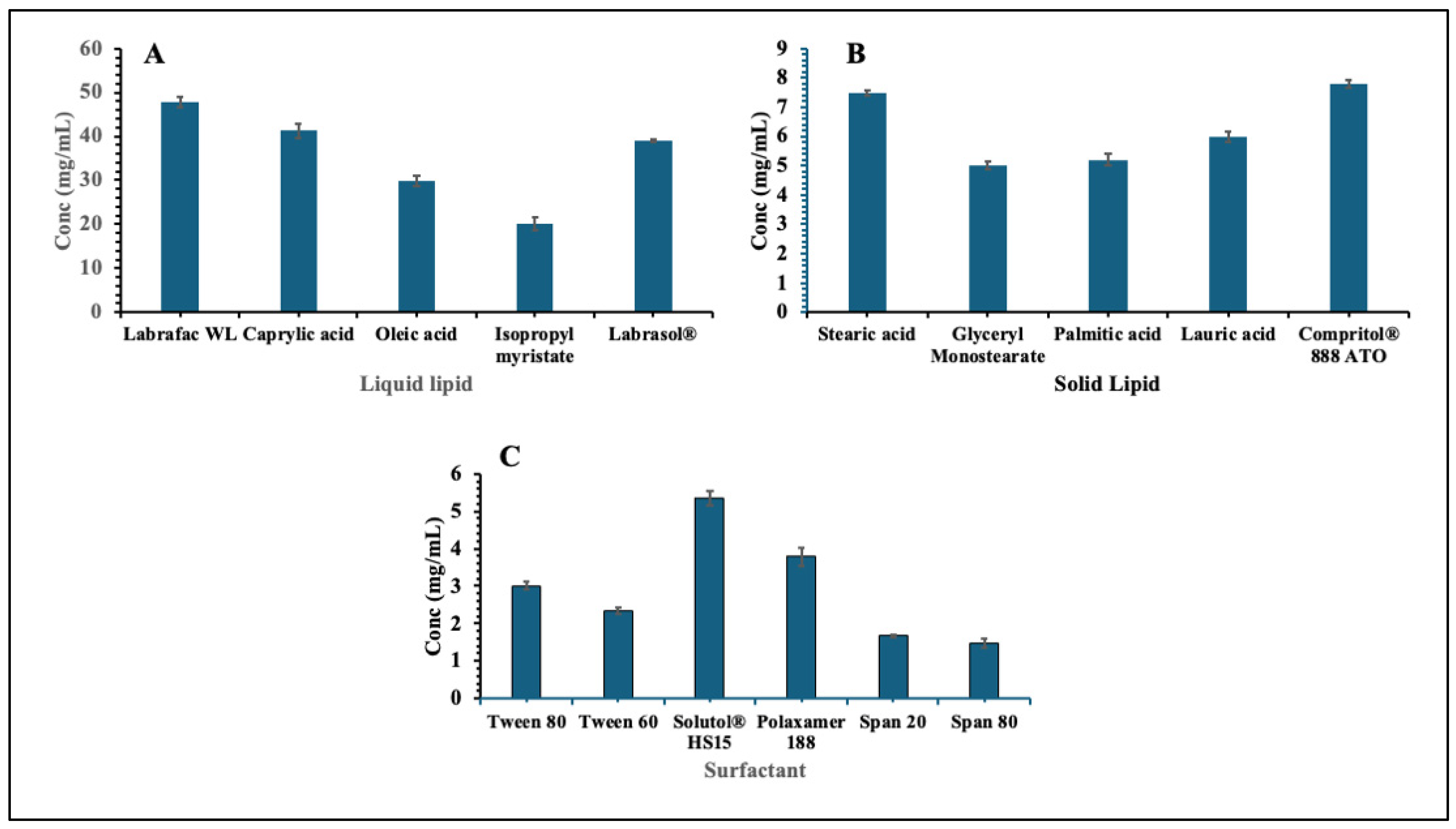
2.1. Solubility Study of EPL in Solid Lipid, Liquid Lipid and Surfactant
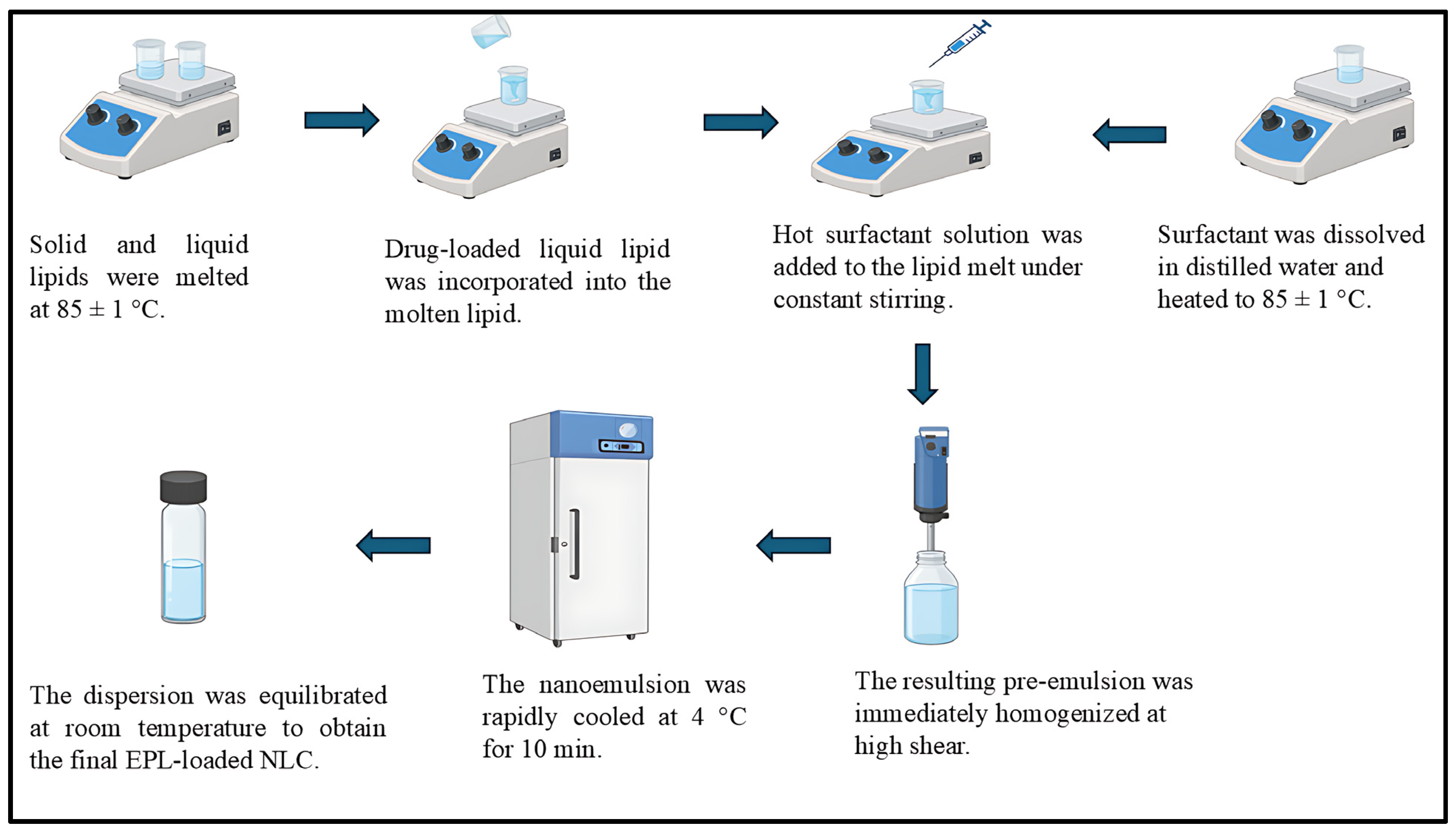
2.2. Preparation of EPL Loaded Nanostructured Lipid Carrier
2.3. Optimization of NLC Using Optimal Mixture Design
2.4. Characterization of Batches of EPL Loaded Nanostructured Lipid Carrier
2.4.1. Globule Size, Polydispersity Index, and Zeta Potential Analysis
2.4.2. Entrapment Efficiency
2.5. Fabrication of Contact Lenses
2.6. Preparation of EPL-Loaded NLC-Laden Contact Lenses
2.7. Characterization of EPL Loaded NLC-Laden Contact Lens
2.7.1. Swelling Study
2.7.2. Optical Clarity Study
2.7.3. Determination of Drug Content in Contact Lenses
2.8. In Vitro Release
2.9. Ocular Irritancy Test
2.10. Ex Vivo Study
2.11. Cytocompatibility and Stability Test
2.12. Statistical Analysis
3. Results and Discussion
3.1. Solubility Study of EPL in Solid Lipid, Liquid Lipid and Surfactant
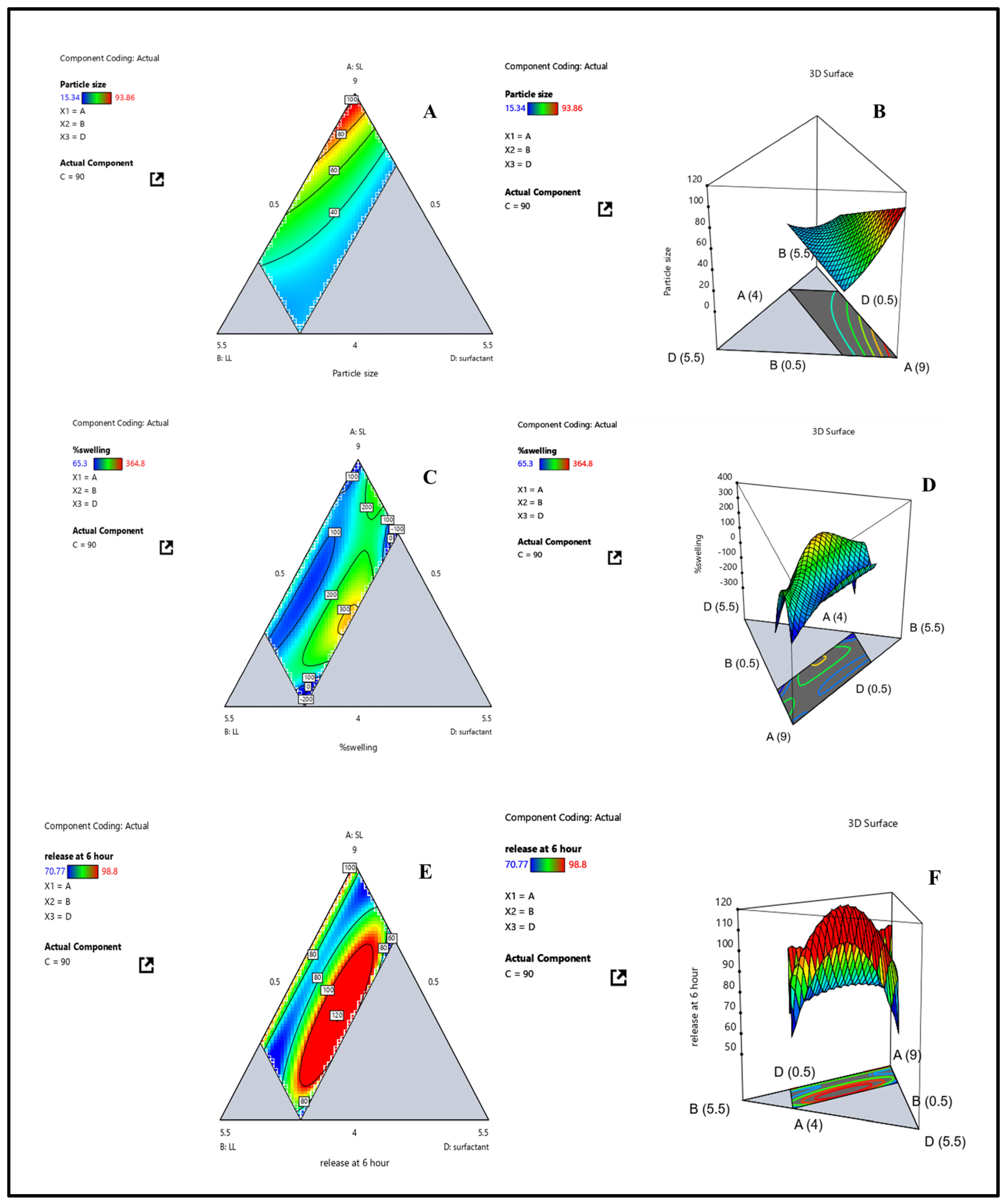
3.2. Data Analysis of Response (Y1): Globule Size
3.3. Data Analysis of Response (Y2): Swelling Index
3.4. Data Analysis of Response (Y3) Drug Release at 6 h
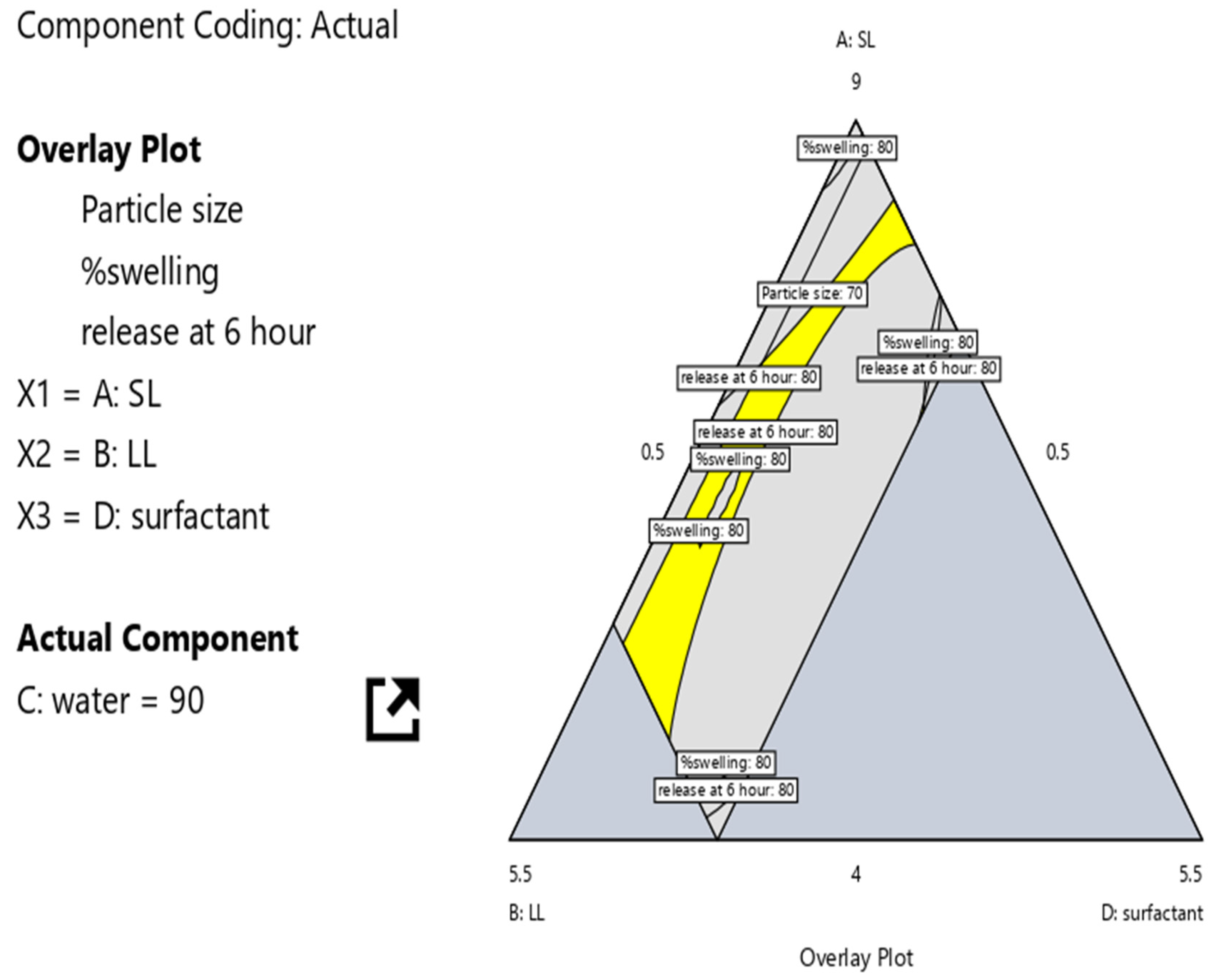
3.5. Optimization of NLC-Laden Contact Lens Using D-Optimal Design
3.6. Characterization of Batches of EPL Loaded Nanostructured Lipid Carrier
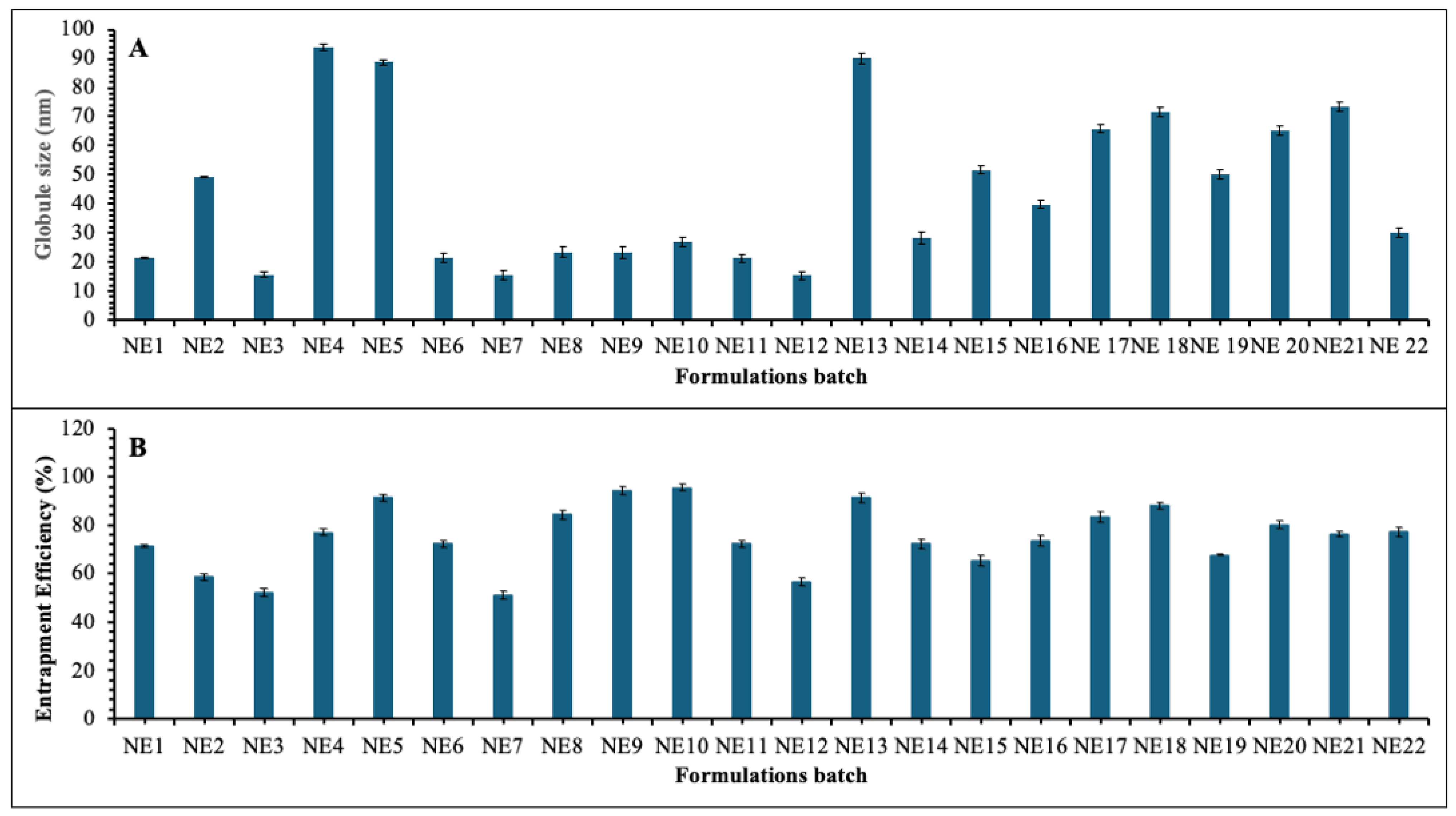
3.6.1. Globule Size and Polydispersity Index Analysis
3.6.2. Entrapment Efficiency
3.7. Characterization of EPL Loaded Nanostructured Lipid Carrier Contact Lens
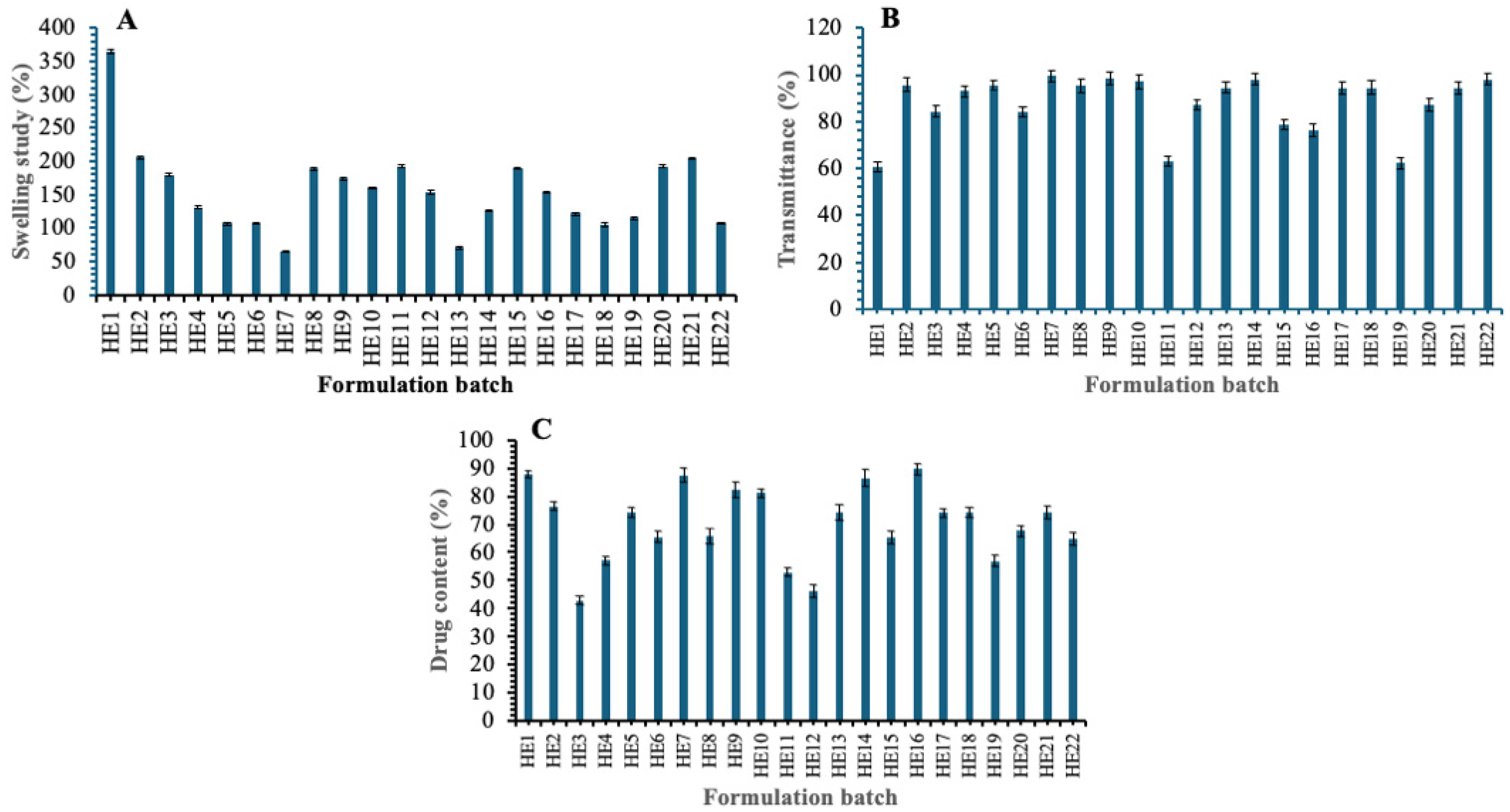
3.7.1. Swelling Study
3.7.2. Transmittance Study
3.7.3. Drug Content
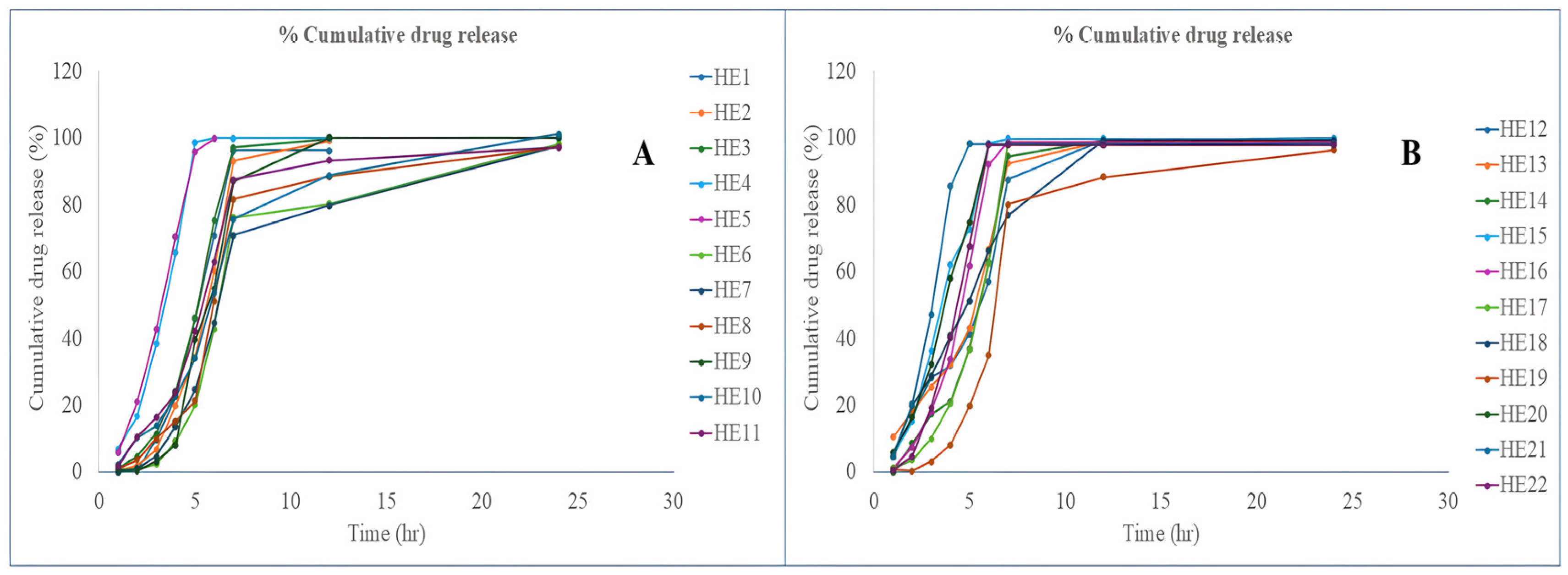
3.7.4. In Vitro Release
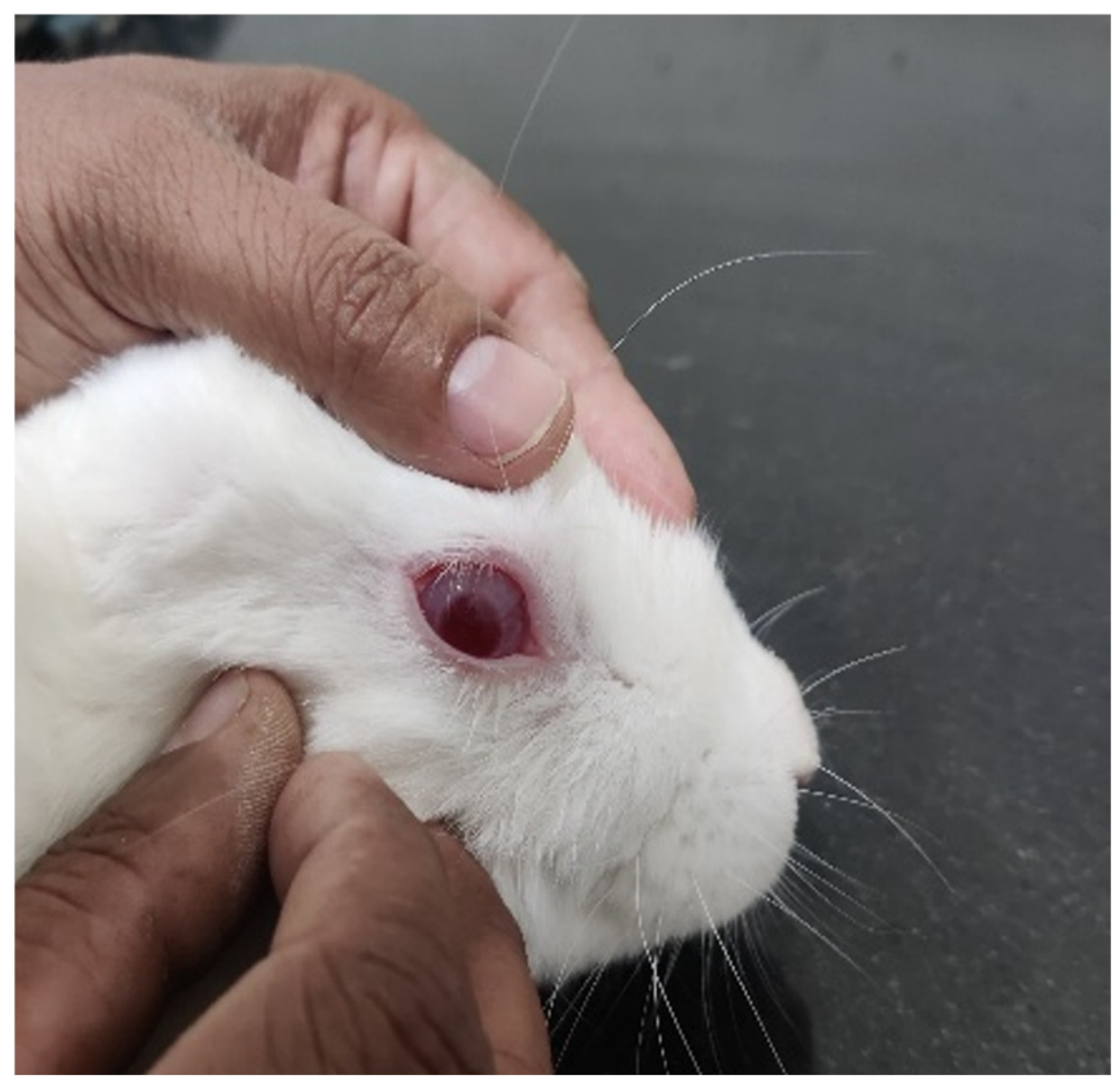
3.8. In Vivo Ocular Irritancy Test
3.9. Ex Vivo Permeability Study
3.10. Cytocompatibility and Stability Test
3.11. Future Prospective
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic Retinopathy: Global Prevalence, Major Risk Factors, Screening Practices and Public Health Challenges: A Review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Ruta, L.M.; Magliano, D.J.; LeMesurier, R.; Taylor, H.R.; Zimmet, P.Z.; Shaw, J.E. Prevalence of Diabetic Retinopathy in Type 2 Diabetes in Developing and Developed Countries. Diabet. Med. 2013, 30, 387–398. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chhikara, R. Diabetic Retinopathy: Present and Past. Procedia Comput. Sci. 2018, 132, 1432–1440. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef]
- Yao, K.; Mou, Q.; Jiang, Z.; Zhao, Y. Posttranslational Modifications in Retinal Degeneration Diseases: An Update on the Molecular Basis and Treatment. Brain-X 2024, 2, e70005. [Google Scholar] [CrossRef]
- Tan, T.E.; Wong, T.Y. Diabetic Retinopathy: Looking Forward to 2030. Front. Endocrinol. 2023, 13, 1077669. [Google Scholar] [CrossRef]
- Kawai, T.; Takei, I.; Tokui, M.; Funae, O.; Miyamoto, K.; Tabata, M.; Hirata, T.; Saruta, T.; Shimada, A.; Itoh, H. Effects of Epalrestat, an Aldose Reductase Inhibitor, on Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes, in Relation to Suppression of Nε-Carboxymethyl Lysine. J. Diabetes Complicat. 2010, 24, 424–432. [Google Scholar] [CrossRef]
- Julius, A.; Malakondaiah, S.; Subbarayalu, R.; Renuka, R.R.; Pothireddy, R.B. Multi-Target Drug Strategies in the Treatment of Diabetic Retinopathy. SN Compr. Clin. Med. 2025, 7, 708–715. [Google Scholar] [CrossRef]
- Dănilă, A.I.; Ghenciu, L.A.; Stoicescu, E.R.; Bolintineanu, S.L.; Iacob, R.; Săndesc, M.A.; Faur, A.C. Aldose Reductase as a Key Target in the Prevention and Treatment of Diabetic Retinopathy: A Comprehensive Review. Biomedicines 2024, 12, 747. [Google Scholar] [CrossRef]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose Reductase, Oxidative Stress, and Diabetic Mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Ottanà, R. Targeting Aldose Reductase for the Treatment of Diabetes Complications and Inflammatory Diseases: New Insights and Future Directions. J. Med. Chem. 2015, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.A.; Borja, N.L. Epalrestat: An Aldose Reductase Inhibitor for the Treatment of Diabetic Neuropathy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2008, 28, 646–655. [Google Scholar] [CrossRef]
- Jagdale, A.D.; Bavkar, L.N.; More, T.A.; Joglekar, M.M.; Arvindekar, A.U. Strong Inhibition of the Polyol Pathway Diverts Glucose Flux to Protein Glycation Leading to Rapid Establishment of Secondary Complications in Diabetes Mellitus. J. Diabetes Complicat. 2016, 30, 398–405. [Google Scholar] [CrossRef]
- Nomair, A.; ElDeeb, M.K.; Maharem, D. Aldose Reductase (-106) C/T Gene Polymorphism and Possibility of Macrovascular Complications in Egyptian Type 2 Diabetic Patients. Indian J. Endocrinol. Metab. 2016, 20, 648. [Google Scholar] [CrossRef]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Furuishi, T.; Nagase, H.; Fukuzawa, K.; Uekusa, H.; Yonemochi, E. Solubility Improvement of Epalrestat by Layered Structure Formation via Cocrystallization. CrystEngComm 2017, 19, 2614–2622. [Google Scholar] [CrossRef]
- Yu, S.; Tan, G.; Liu, D.; Yang, X.; Pan, W. Nanostructured Lipid Carrier (NLC)-Based Novel Hydrogels as Potential Carriers for Nepafenac Applied after Cataract Surgery for the Treatment of Inflammation: Design, Characterization and in Vitro Cellular Inhibition and Uptake Studies. RSC Adv. 2017, 7, 16668–16677. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-Loaded Silicone Hydrogels as Contact Lenses to Address Diabetic-Eye Complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef]
- Kattar, A.; Quelle-Regaldie, A.; Sánchez, L.; Concheiro, A.; Alvarez-Lorenzo, C. Formulation and Characterization of Epalrestat-Loaded Polysorbate 60 Cationic Niosomes for Ocular Delivery. Pharmaceutics 2023, 15, 1247. [Google Scholar] [CrossRef]
- Kattar, A.; Vivero-Lopez, M.; Concheiro, A.; Mudakavi, R.; Chauhan, A.; Alvarez-Lorenzo, C. Oleogels for the Ocular Delivery of Epalrestat: Formulation, in Vitro, in Ovo, Ex Vivo and in Vivo Evaluation. Drug Deliv. Transl. Res. 2024, 14, 3291–3308. [Google Scholar] [CrossRef] [PubMed]
- Alvi, Z.; Akhtar, M.; Rahman, N.U.; Hosny, K.M.; Sindi, A.M.; Khan, B.A.; Nazir, I.; Sadaquat, H. Utilization of Gelling Polymer to Formulate Nanoparticles Loaded with Epalrestat-Cyclodextrin Inclusion Complex: Formulation, Characterization, in-Silico Modelling and in-Vivo Toxicity Evaluation. Polymers 2021, 13, 4350. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Y.; Ping, Q.; Xiao, Y.; Huang, X. Mucoadhesive Effect of Thiolated PEG Stearate and Its Modified NLC for Ocular Drug Delivery. J. Control. Release 2009, 137, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, M.; Xing, D.; Zhang, J.; Fang, T.; Zhang, F.; Nie, Z.; Liu, Y.; Yang, L.; Li, J.; et al. Contact Lens as an Emerging Platform for Ophthalmic Drug Delivery: A Systematic Review. Asian J. Pharm. Sci. 2023, 18, 100847. [Google Scholar] [CrossRef]
- Dave, J.; Jani, H.; Patel, Y.; Mohite, P.; Puri, A.; Chidrawar, V.R.; Datta, D.; Bandi, S.P.; Ranch, K.; Singh, S. Polyol-Modified Deformable Liposomes Fortified Contact Lenses for Improved Ocular Permeability. Nanomedicine 2025, 20, 649–662. [Google Scholar] [CrossRef]
- Singh, S.; Supaweera, N.; Nwabor, O.F.; Chaichompoo, W.; Suksamrarn, A.; Chittasupho, C.; Chunglok, W. Poly (Vinyl Alcohol)-Gelatin-Sericin Copolymerized Film Fortified with Vesicle-Entrapped Demethoxycurcumin/Bisdemethoxycurcumin for Improved Stability, Antibacterial, Anti-Inflammatory, and Skin Tissue Regeneration. Int. J. Biol. Macromol. 2024, 258, 129071. [Google Scholar] [CrossRef]
- Gilani, S.J.; Jumah, M.N.b.; Zafar, A.; Imam, S.S.; Yasir, M.; Khalid, M.; Alshehri, S.; Ghuneim, M.M.; Albohairy, F.M. Formulation and Evaluation of Nano Lipid Carrier-Based Ocular Gel System: Optimization to Antibacterial Activity. Gels 2022, 8, 255. [Google Scholar] [CrossRef]
- Panchal, K.; Patel, Y.; Jani, H.; Dalal, M.; Chidrawar, V.R.; Datta, D.; Mohite, P.; Puri, A.; Ranch, K.; Singh, S. Indomethacin-Incorporated Microemulsion-Laden Contact Lenses for Improved Ocular Drug Delivery and Therapeutic Efficacy. RSC Adv. 2025, 15, 16110–16124. [Google Scholar] [CrossRef]
- Moghddam, S.M.M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Optimization of Nanostructured Lipid Carriers for Topical Delivery of Nimesulide Using Box–Behnken Design Approach. Artif. Cells Nanomed. Biotechnol. 2017, 45, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery. Adv. Pharm. Bull. 2021, 36, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Shanley, C.; Wang, Q.J.; Livingston, B. Approach for Contact Medical Device Development via Integrated Testing, Skeletal Muscle Modeling, and Finite Element Analysis. J. Mech. Behav. Biomed. Mater. 2024, 155, 106541. [Google Scholar] [CrossRef]
- Khan, M.F.A.; Ur.Rehman, A.; Howari, H.; Alhodaib, A.; Ullah, F.; Mustafa, Z.u.; Elaissari, A.; Ahmed, N. Hydrogel Containing Solid Lipid Nanoparticles Loaded with Argan Oil and Simvastatin: Preparation, In Vitro and Ex Vivo Assessment. Gels 2022, 8, 277. [Google Scholar] [CrossRef]
- Fang, C.-L.; Al-Suwayeh, S.A.; Fang, J.-Y. Nanostructured Lipid Carriers (NLCs) for Drug Delivery and Targeting. Recent Pat. Nanotechnol. 2012, 7, 41–55. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and Its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef]
- Abdi, B.; Mofidfar, M.; Hassanpour, F.; Kirbas Cilingir, E.; Kalajahi, S.K.; Milani, P.H.; Ghanbarzadeh, M.; Fadel, D.; Barnett, M.; Ta, C.N.; et al. Therapeutic Contact Lenses for the Treatment of Corneal and Ocular Surface Diseases: Advances in Extended and Targeted Drug Delivery. Int. J. Pharm. 2023, 638, 122740. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.; Popa, L.; Anuţa, V.; Arsene, A.-L.; Dinu-Pîrvu, C.-E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Lin, S.; Zheng, Y.; Xue, P.; Wang, L.; Cong, Y.; He, X.; Zhang, C.; Yuan, C. Hydrogel Contact Lenses Modified by Quaternary Chitosan and Epigallocatechin Gallate with Enhancement of Antimicrobial for Bacterial Keratitis. Int. J. Biol. Macromol. 2025, 306, 141787. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, Y.; Wang, J.; Chen, Z.; Xie, J.; Cui, Z.; Xu, D.; Zhang, X.; Yao, W. Advances in Polysaccharide-Based Antibacterial Materials. Int. J. Biol. Macromol. 2025, 308, 142598. [Google Scholar] [CrossRef] [PubMed]
| Independent Variable | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | |||
| Compritol 888 ATO | LabrafacWL1349 | Solutol HS15 | |||
| Low (g) | High (g) | Low (mL) | High (mL) | Low (mL) | High (mL) |
| 0.43 | 0.87 | 0.05 | 0.4 | 0.05 | 0.2 |
| Dependent variable | |||||
| Y1-Globule Size (nm) | Y2-Equilibrium Water content | Y3-Drug release at 6 h | |||
| Batch | Solid Lipid (g) | Liquid Lipid (mL) | Surfactant (mL) | Water (mL) |
|---|---|---|---|---|
| NE1 | 0.58 | 0.22 | 0.2 | 9 |
| NE2 | 0.64 | 0.31 | 0.05 | 9 |
| NE3 | 0.49 | 0.31 | 0.2 | 9 |
| NE4 | 0.77 | 0.18 | 0.05 | 9 |
| NE5 | 0.87 | 0.08 | 0.05 | 9 |
| NE6 | 0.66 | 0.23 | 0.11 | 9 |
| NE7 | 0.43 | 0.4 | 0.17 | 9 |
| NE8 | 0.67 | 0.13 | 0.2 | 9 |
| NE9 | 0.79 | 0.05 | 0.16 | 9 |
| NE10 | 0.79 | 0.05 | 0.16 | 9 |
| NE11 | 0.66 | 0.23 | 0.11 | 9 |
| NE12 | 0.43 | 0.4 | 0.17 | 9 |
| NE13 | 0.87 | 0.08 | 0.05 | 9 |
| NE14 | 0.66 | 0.23 | 0.11 | 9 |
| NE15 | 0.56 | 0.31 | 0.13 | 9 |
| NE16 | 0.56 | 0.39 | 0.05 | 9 |
| NE17 | 0.74 | 0.13 | 0.13 | 9 |
| NE18 | 0.74 | 0.13 | 0.13 | 9 |
| NE19 | 0.5 | 0.39 | 0.11 | 9 |
| NE20 | 0.71 | 0.24 | 0.05 | 9 |
| NE21 | 0.74 | 0.13 | 0.13 | 9 |
| NE22 | 0.61 | 0.27 | 0.12 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranch, K.; Patel, Y.; Acharya, E.; Gupta, P.; Singh, A.K.; Singh, S. Enhanced Ocular Delivery of Epalrestat Using Nanostructured Lipid Carrier Laden Soft Contact Lens. Pharmaceutics 2025, 17, 1515. https://doi.org/10.3390/pharmaceutics17121515
Ranch K, Patel Y, Acharya E, Gupta P, Singh AK, Singh S. Enhanced Ocular Delivery of Epalrestat Using Nanostructured Lipid Carrier Laden Soft Contact Lens. Pharmaceutics. 2025; 17(12):1515. https://doi.org/10.3390/pharmaceutics17121515
Chicago/Turabian StyleRanch, Ketan, Yashkumar Patel, Esha Acharya, Paras Gupta, Anil Kumar Singh, and Sudarshan Singh. 2025. "Enhanced Ocular Delivery of Epalrestat Using Nanostructured Lipid Carrier Laden Soft Contact Lens" Pharmaceutics 17, no. 12: 1515. https://doi.org/10.3390/pharmaceutics17121515
APA StyleRanch, K., Patel, Y., Acharya, E., Gupta, P., Singh, A. K., & Singh, S. (2025). Enhanced Ocular Delivery of Epalrestat Using Nanostructured Lipid Carrier Laden Soft Contact Lens. Pharmaceutics, 17(12), 1515. https://doi.org/10.3390/pharmaceutics17121515










