1. Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder, impacting approximately 0.3% of the population in Western countries. Its prevalence escalates with age, affecting about 1% of individuals aged 60 and reaching around 4% by age 80 [
1]. It is estimated that PD cases will double within the next two decades [
2,
3].
PD is a progressive condition marked by a variety of motor and non-motor symptoms (NMSs). Its pathological hallmark is the accumulation of misfolded, fibrillar alpha-synuclein (aSyn) in neurons. Key motor symptoms include bradykinesia, rigidity, and resting tremor. NMSs also significantly impact those with PD. These include sleep disturbances, dysphagia, constipation, apathy, depression, pain, and autonomic dysfunctions, which collectively can severely affect the quality of life of patients and lead to disease-related complications. The severity of these symptoms often dictates the overall impact of the disease on the individual [
4]. The clinical presentation and progression of PD can vary greatly among patients [
5].
Levodopa (LD) is the most potent antiparkinsonian drug available. Ever since pioneering reports in the 1960s, it has represented the gold standard for the treatment of PD. It increases dopamine levels in the brain, alleviating motor symptoms like bradykinesia and rigidity. LD is combined with either a decarboxylase inhibitor (Carbidopa (CD), Benserazide) or a COMT inhibitor (Entacapone, Tolcapone) to prevent its peripheral degradation (after crossing the blood–brain barrier, Levodopa is transformed into dopamine by cerebral decarboxylases). These agents increase LD’s bioavailability and duration of action. Fixed combinations include LD/CD (added to the WHO list of essential medicines in 1977), LD/Benserazide, and LD/CD/Entacapone. Newer formulations such as Rytary® and Crexont® combine immediate-release (IR) LD/CD and extended-release (ER) LD/CD-containing beads (Rytary®) or ER LD pellets (Crexont®) in capsules. This keeps LD blood levels steady for longer periods of time. However, the LD bioavailability of Rytary® (70–80%) and Crexont® (88–99%) is reduced compared with IR LD/CD preparations (e.g., Sinemet®) necessitating higher doses. Of note, both the conventional and these newer LD dosage forms result in the concomitant release of LD and the decarboxylase inhibitor. To the best of our knowledge, there is currently no LD-containing dosage form available that would have been produced using additive manufacturing.
Despite its effectiveness, the chronic use of conventional oral Levodopa formulations is associated with significant long-term motor complications, notably motor fluctuations and dyskinesias. Within 5 years of conventional LD treatment, 40–50% of patients develop dyskinesias and motor fluctuations, increasing to 70–80% after 10 years [
6]. Furthermore, LD’s effectiveness declines in advanced PD due to fluctuating medication periods and its short half-life. Patients often need to increase the dose and frequency of dosing, which in turn increases the incidence of dyskinesias [
7]. These adverse effects are strongly correlated with pulsatile dopaminergic stimulation resulting from the pharmacokinetic profile of immediate-release levodopa, which leads to non-physiological oscillations in LD plasma and synaptic dopamine concentrations. They contribute to a 41% increase in direct medical costs [
8].
Emerging evidence from both clinical and preclinical studies supports the hypothesis that the pattern of dopaminergic delivery, rather than the dose alone, plays a critical role in the development of these complications [
9]. Non-pulsatile (continuous or sustained) dopaminergic stimulation (CDS) aims to more closely approximate endogenous dopaminergic neurotransmission by maintaining relatively stable plasma Levodopa levels and thereby achieving more uniform receptor activation in the striatum. Therapeutic strategies designed to implement CDS include Levodopa–Carbidopa intestinal gel (LCIG) infusion [
10], controlled-release oral formulations, and the adjunctive use of enzyme inhibitors (e.g., COMT and MAO-B inhibitors) to extend the half-life of Levodopa. These approaches have demonstrated efficacy in reducing both motor fluctuations and dyskinesias, supporting the pathophysiological rationale underlying CDS [
10].
Currently, 3D screen printing is a cutting-edge additive manufacturing technology [
11,
12]. It enables the production of pharmaceutical dosage forms of unprecedented complexity in large quantities. A single production run can produce units of different shapes and sizes [
12]. The release of the active ingredient can be precisely controlled by defining the internal architecture, the size and geometry of the units, and the carrier materials used. As the 3D screen printing process does not involve high temperatures or significant pressure, it can be used with a wide range of substrates and drug substances.
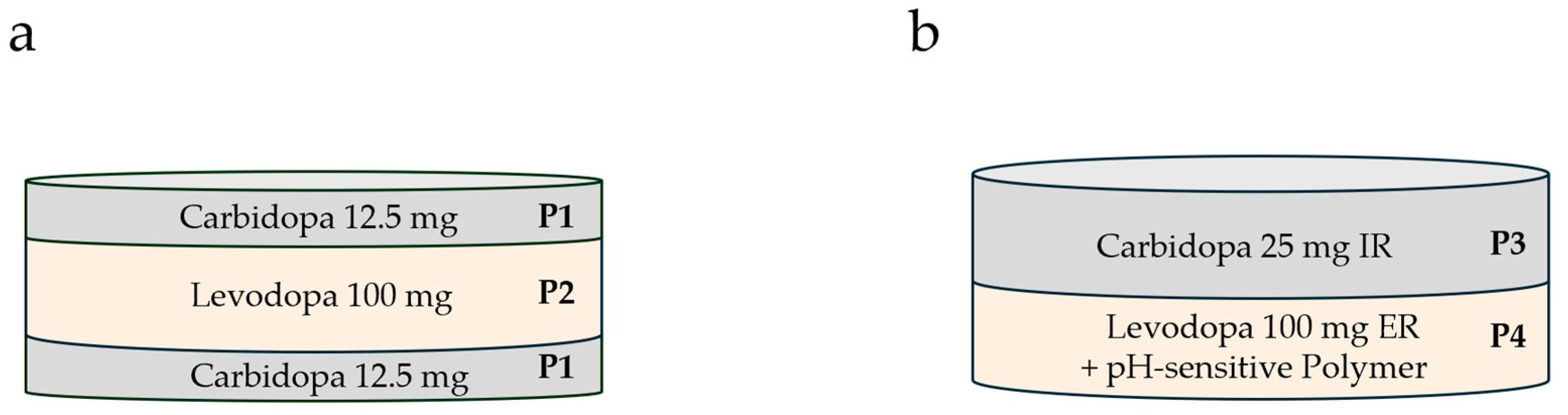
Unlike other manufacturing technologies, 3D screen printing offers the possibility of separating drugs into different compartments of a given oral dosage form. This has the potential to differentially regulate the release of the different drugs. This is of particular interest to oral LD-containing dosage forms, as LD is combined with CD to increase its bioavailability. We hypothesized that the bioavailability of LD could be increased further if the CD were released before the LD, based on its inhibitory action against LD degradation. To this end, we harnessed the potential of additive manufacturing to design two novel dosage forms containing CD and LD in different compartments. Two principal strategies were followed, namely, controlled drug release by the architecture of the dosage form and controlled drug release as a function of the composition of the different compartments. In LXM.5-1 (LXM.5 is an internal project code referring to Laxxon’s novel LD/CD dosage forms; LXM.5-1/2 refers to specific prototypes as described in the manuscript), the release of both drugs was tailored through the geometry of the dosage form whereas in LXM.5-2 the release of the two drugs was controlled by the composition of the compartments, a key component being a pH-sensitive polymer built into the LD compartment. Their evaluation confirmed the concept of increasing the bioavailability of LD by sequentially releasing CD and LD, with CD preceding LD. Furthermore, not only were LD blood levels higher, but they also persisted for longer. These studies have several potential implications which range from practical consequences, such as reducing the number of daily intakes, to the potential to slow the progression of the disease.
2. Materials and Methods
2.1. Chemicals
CD and LD were purchased from Divi’s Laboratories, Hyderabad, India. The various excipients were purchased from established providers: Klucel LF (Ashland, Covington, KY, USA), Avicel PH 105 (Dupont, Wilmington, DE, USA), starch 1500 (Colorcon, Harleysville, PA, USA), glycerol and triactin (AppliChem, Darmstadt, Germany), talc (Imerys, Paris, France), Silfar 350 and Silfar SE4 (Wacker Chemie, Munich, Germany), L-ascorbic acid and aqueous HCl (Carl Roth GmbH, Karlsruhe, Germany), citric acid (Honeywell FlukaTM, Charlotte, NC, USA), Ac-Di-Sol (IFF, Roquette, Lestrem, France), Mannogem Emerald (SPI Pharma, Wilmington, DE, USA), and Miglyol 812 N (IOI Oleo, Hamburg, Germany). Some materials were kindly provided by the respective manufacturers. This includes Shin-Etsu AQOAT® AS-LF provided by SE Tylose GmbH & Co.KG, Wiesbaden, Germany and RxCIPIENTS® FM 1000 by Evonik, Darmstadt, Germany. Demineralized water was used for all formulations and solutions. For HPLC measurements, sodium dihydrogen phosphate and HPLC-grade solvent Chemsolute (water and acetonitrile) from TH.Geyer, Renningen, Germany were used.
2.2. Preparation of Printing Pastes
All pastes were prepared using an HRV-S 2DP vacuum dissolver planetary mixer with 3 agitator tools from Herbst Maschinenfabrik GmbH (Buxtehude, Germany), in a 2-L glass container.
The pastes were prepared according to the compositions in
Table 1 and
Table 2 at room temperature. Briefly, the binder solution (Klucel LF, 10%) was prepared. The appropriate amount of binder gel was then added into the 2-L glass container of the planetary mixer. Then half of the desired water was added. In the next step, all solid excipients were added starting with the highest in mass. Subsequently, all liquid excipients were added. Before mixing, the API and the residual water were added. The final paste was mixed for 20 min at 1000 rpm under 70 mbar vacuum.
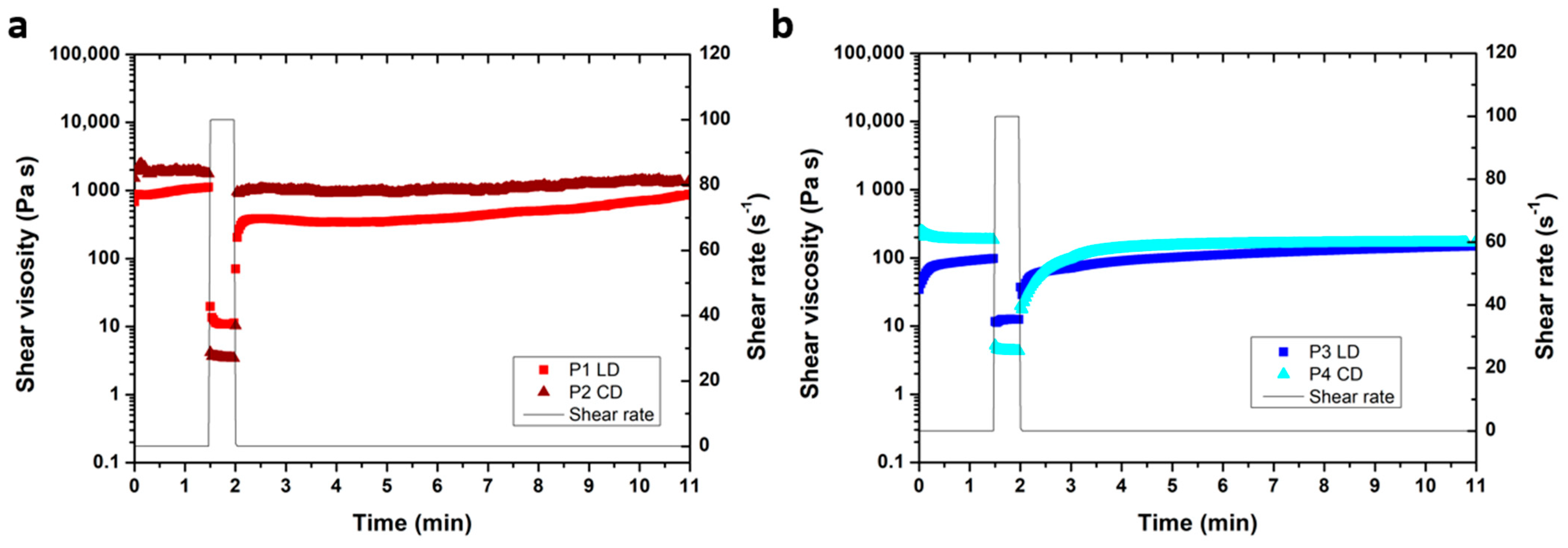
2.3. Rheology Measurements
Rheology measurements of paste P1 to P4 were performed on a Kinexus Rheometer (Netzsch, Selb, Germany) equipped with a plate–plate geometry and a passive solvent trap at 25 °C. A 500 µm gap was selected. In the 3-step shear rate test, shear rates of 0.1 s−1 (for 1.5 min), 100 s−1 (for 0.5 min), and 0.1 s−1 (for 10 min) were applied. The amplitude sweep was conducted at 1 Hz, and the frequency sweep at 0.05% strain.
2.4. Three-Dimensional Screen Printing of Tablets
LXM.5-1 and LXM.5-2 were manufactured on a prototype lab-scale 3D screen printing unit, XHS STS 3D (Exentis Group AG, Stetten, Switzerland), in accordance with the previously delineated methodology [
12]. Printer settings were as follows: flooding and printing squeegee speed 100 mm/s, off-contact distance 2 mm, height increment for screen elevation 15 µm, dry power 65%, and drying time per layer 15 s.
2.5. Tablet Hardness and Friability Testing
The fracture resistance of tablets was detected using tablet hardness testing instrument PTB 51 1E by Pharma Test (Apparatebau AG, Hainburg, Germany), according to Ph. Eur. 2.9.8. The average and standard deviation of 5 randomly selected tablets per batch were calculated.
Friability was tested as described in Ph. Eur. 2.9.7. Deviating from the method, 5 g tablets of LXM.5-2 and 5 g tablets of LXM.5-1 were dedusted by airflow and weighed, then rotated 100 times at 25 rpm on a single drum tablet friability test instrument PTF 100 by Pharma Test (Apparatebau AG, Hainburg, Germany). Friability was determined as the percentage of weight loss after a further dedusting of the tablets and should be less than 1%.
2.6. Mass Uniformity
Mass uniformity, a pharmacopeial requirement (see Ph. Eur. 2.9.5), was determined by means of weighing 20 tablets from each batch, followed by the calculation of the means and standard deviations. Semi-micro balance SartoriusTM Secura 125-1S G2324665 (Sartorius, Göttingen, Germany) was used.
2.7. Dimension Uniformity
In order to ascertain the uniformity of the dimensions of the tablets, a total of 20 tablets from each formulation were meticulously measured (diameter, height) using a Digimatic Caliper CD-15APX by Mitutoyo, Kawasaki, Japan (certified). The mean values and standard deviations for tablet dimensions and volumes were then calculated.
2.8. Disintegration Testing
Disintegration was tested according to Ph. Eur. 2.9.1 with a Pharmatest Auto1EZ by Pharma Test Apparatebau AG, Hainburg, Germany, an automatic disintegration time tester using disks. Demineralized water (750 mL, 37 °C) was used as test media. The mean value and standard deviation were subsequently calculated.
2.9. In Vitro Dissolution Testing
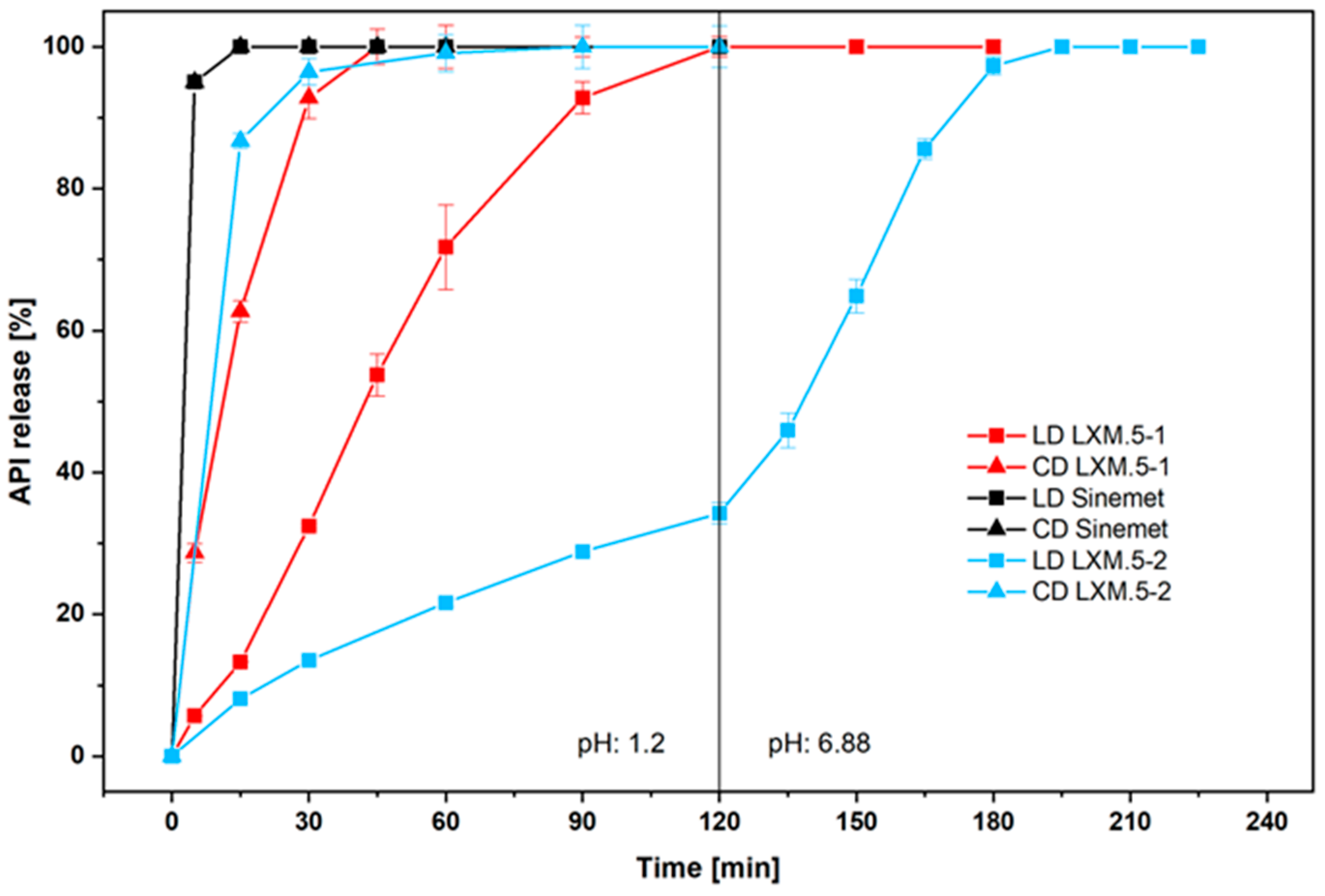
The in vitro dissolution test was performed according to USP <711> in a paddle apparatus (USP apparatus 2) in 900 mL 0.1 M HCl. The number of each type of tablet tested in the dissolution apparatus was six tablets. However, only two tablets of the Sinemet tablet were investigated because it is a well-known tablet on the market. As the solubility products for levodopa and carbidopa are 5000 mg/l and 3.8 mg/L, respectively [
13], to achieve sink conditions, concentrations V/V
sat ≥ 3 [
14] were used in the vessel being 100 mg/900 mL for Levodopa and 25 mg/900mL. To comply with USP43-NF38-753 dissolution test 1 the rotation speed was set to 75 rpm for the dissolution tests in formulation development Testing the influence of stirring speed by varying it between 50, 75, 100, and 150 rpm revealed that there was no further influence on the dissolution beyond 100 rpm. Thus, this speed was chosen for further experiments.
For LXM.5-2 the dissolution method was extended due to the prolonged release. Therefore, Method A of the chapter Extended-Release Dosage Forms in USP <711> were utilized.
2.10. High Performance Liquid Chromatography (HPLC) for LXM.5-1 and LXM.5-2
The liquid chromatograph was equipped with a Diode Array Detector detector adjusted to 280 nm and a 150 mm × 4.6 mm 5 µm ODS C-18 column (Agilent Zorbax Eclipse Plus C18 by Agilent Technologies, Santa Clara, CA, USA) at 40 °C. Injection volume: 5 µL.
Procedure: Protect all solutions from sunlight and maintain them at 10 °C until they are injected into the chromatograph.
Mobile phase: 50 mM NaH2PO4; adjust with Phosphoric acid and Acetonitrile to a pH of 2.7 in 95:5 ratio.
Stock solution: 1 mg/mL of Levodopa in 0.1 M HCl, 1 mg/mL of Carbidopa in 0.1 M HCl.
Standard solution 100 µg/mL of LEV and 30 µg/mL CAR in 0.1 M HCl.
Calibration solution: 100, 50, 25, 12.5, and 6.25 µg/mL in 0.1 M HCl.
Sample solution: 1 mL of the dissolution test solution at the specific time point.
The method was not validated but is in accordance with the USP method [
15].
2.11. In Vivo Pig Study
The pharmacokinetic study was achieved in collaboration with the University of Veterinary Medicine Vienna, University Clinic for Swine, Vienna, Austria, for the in vivo part. The allowance of the study was granted by the respective Animal Welfare Committee (GZ: 2023-0.529.291 Approved on 23 July 2023). For the application of the tablet using a gastroscope (Olympus, Shinjuku, Japan), the study animals (n = 4/group) fasted overnight and were anesthetized (0.5 mg/kg bw azaperone and 10 mg/kg bw ketamine intravenously or 2 mg/kg bw azaperone and 25 mg/kg bw intramuscularly). To determine the pharmacokinetic profile of the levodopa/carbidopa dosage forms, blood samples (maximum blood volume per time point: 2 mL) were taken shortly before application of the tablets (time point 0 h) and after application of the tablet (30, 60, 90, 180, 240, 360, 480 min, and 24 h) by puncturing the vena cava cranialis or vena jugularis with 21G needles. Plasma samples were frozen and stored at −80 °C until analysis. Plasma concentrations of carbidopa and levodopa were determined by HPLC at each sampling time. All study animals are euthanized under general anesthesia at the earliest time after the end of the experiment (24 h after application of the tablets). Pigs had to be older than 6 weeks and weigh less than 25 kg. Mean weight was 15.8 ± 2.4 kg.
2.12. LS-MS Analytics of Levodopa and Carbidopa in Porcine Plasma
The method from [
16] was used. The HPLC system consisted of a Vanquish U-HPLC pump and a Vanquish Autosampler (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectrometry was performed on a Q-Exactive mass spectrometer (Orbitrap
TM technology with accurate mass) equipped with an H-ESI (heated electrospray interface) (Thermo Fisher Scientific, Waltham, MA, USA) connected to a PC running the standard software Chromeleon 7.2.10. The quantitative determination of Levodopa and Carbidopa in plasma was carried out by reversed phase HPLC employing High-Resolution Mass Spectrometry (HRMS) as the detection method. All analytical samples were stabilized by adding 4% of a 10% sodium metabisulfite solution to protect the analytes from degradation.
The validated calibration ranges for measuring Levodopa and Carbidopa in porcine plasma were 75–5000 ng/mL and 3–600 ng/mL, respectively. Sample preparation was performed by protein precipitation.
4. Discussion
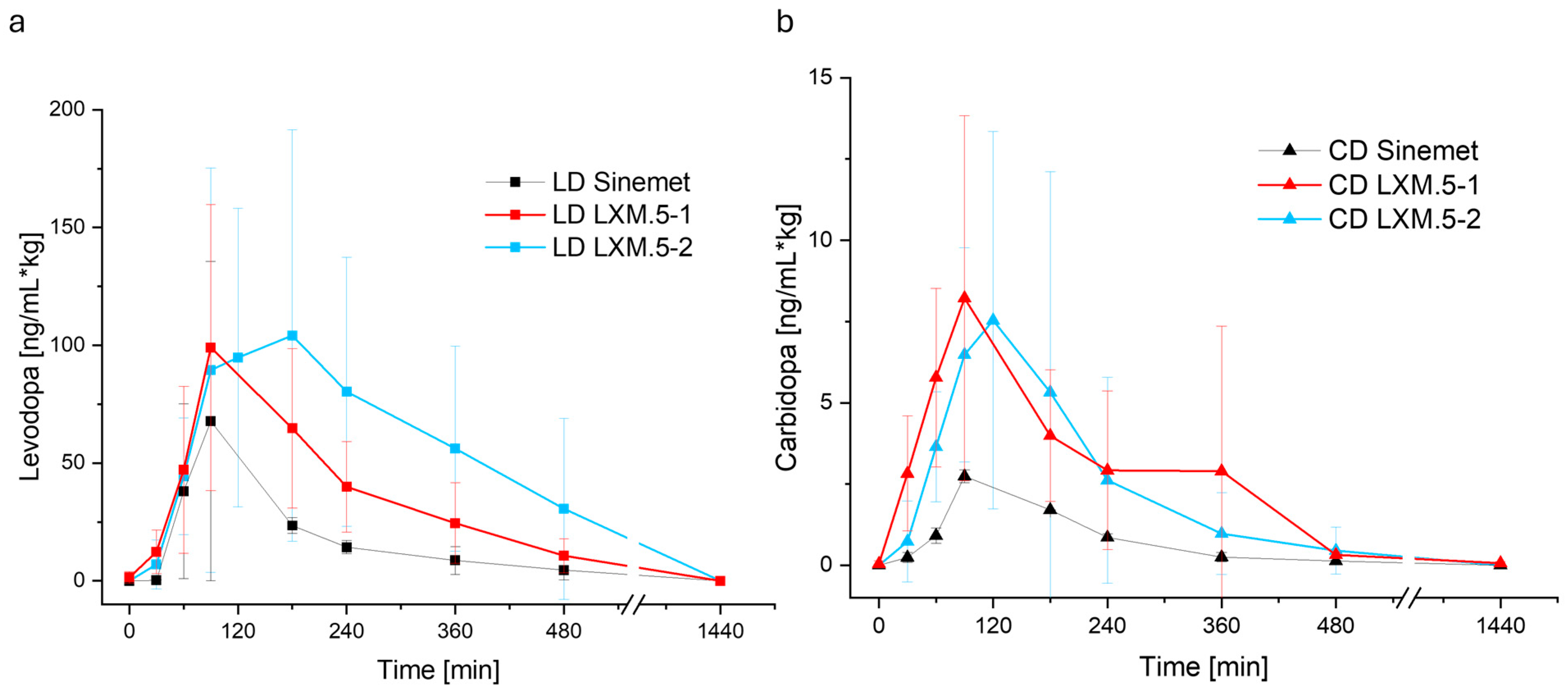
We hypothesized that the bioavailability of LD from solid oral dosage forms could be increased through the sequential release of CD and LD, with the decarboxylase inhibitor being released first. To this end, we used 3D screen printing technology to create tablets with different compartments containing either CD or LD. We evaluated two approaches. The first approach (realized in LXM.5-1) was based on the geometry of the tablet to control the drug release. The second approach (LXM.5-2) used different formulations to achieve immediate (for CD) or extended (for LD) drug release. We further tested whether the addition of a pH-sensitive polymer would enhance the bioavailability of LXM.5-2 by tailoring the LD release to the small intestine. Three-dimensional screen-printing technology was found to be well suited to realize both product candidates. The resulting tablets fulfilled the requirements of EU and US pharmacopeia. Both approaches were shown to increase the bioavailability of LD and to extend its blood levels.
Based on current knowledge, the faster and greater increase in the CD blood levels of LXM.5-1 and LXM.5-2 compared with Sinemet
® is difficult to reconcile with the in vitro CD release characteristics of the three dosage forms. It is currently widely accepted that there is no interaction between CD and LD during the absorption process in the intestines [
19,
23]. Levodopa has a narrow absorption window in the proximal small intestine (duodenum and jejunum). It is actively transported across the intestinal epithelium via large neutral amino acid transporters (LAT1s). Specifically, the transporter system SLC7A9–SLC3A1 was recently shown to mediate intestinal LD transport, with leucine and arginine acting as competitors. Transport into the central nervous system, however, is mediated by LAT transporter SLC6A19 [
19]. Carbidopa, in contrast, is poorly absorbed and does not rely on LAT1 or similar amino acid transporters [
23]. Its absorption is thought to occur via passive diffusion or other non-saturable mechanisms. Our results challenge these notions. Given that all three dosage forms contain the same amount of CD and their in vitro release profiles are highly comparable, why should CD blood levels of LXM.5-1 and LXM.5-2 be higher than those of Sinemet
®, assuming there is no interaction between CD and LD absorption? The model chosen might offer a potential explanation. The uptake of the two molecules may differ between pigs and humans. However, despite pigs often being used as a model system, there have been no reports on this so far. Additionally, it has to be at least considered that this study used anesthetized piglets fasted for at least 12 h to ensure an empty stomach. While both conditions might impact the transport of the dosage forms and the absorption of CD and LD slightly, this setup was chosen due to several reasons: Primarily, gastro endoscopic application is the only viable method to ensure the structural integrity of the 3D screen-printed dosage form, which was the main variable tested in this study. Secondly, an empty stomach is the closest option to standardized conditions in terms of the pH and the placement of the dosage forms inside the stomach. Additionally, the commercially available Sinemet
® is advised to not be taken together with meals which are rich in proteins, as this can delay the absorption [
22]. As a consequence, if the stomachs of the piglets were filled with various amounts of protein-rich feed, this might impact the absorption rate and speed and therefore disallow comparison between piglets.
On the other hand, our current knowledge of the absorption of CD and LD in humans is certainly limited by the fact that, until now, it has not been possible to administer them sequentially via the oral route. In fact, studies demonstrating that CD augmented the bioavailability were performed in a hybrid fashion: LD was given intravenously, while CD was administered orally [
10,
18,
24]. Although this setting was suitable for observing and assessing the effect of CD on LD bioavailability, it could not address the question of whether the two interact in the intestinal absorption process. Therefore, our data could provide the first experimental evidence of such an interaction. Since the difference between Sinemet
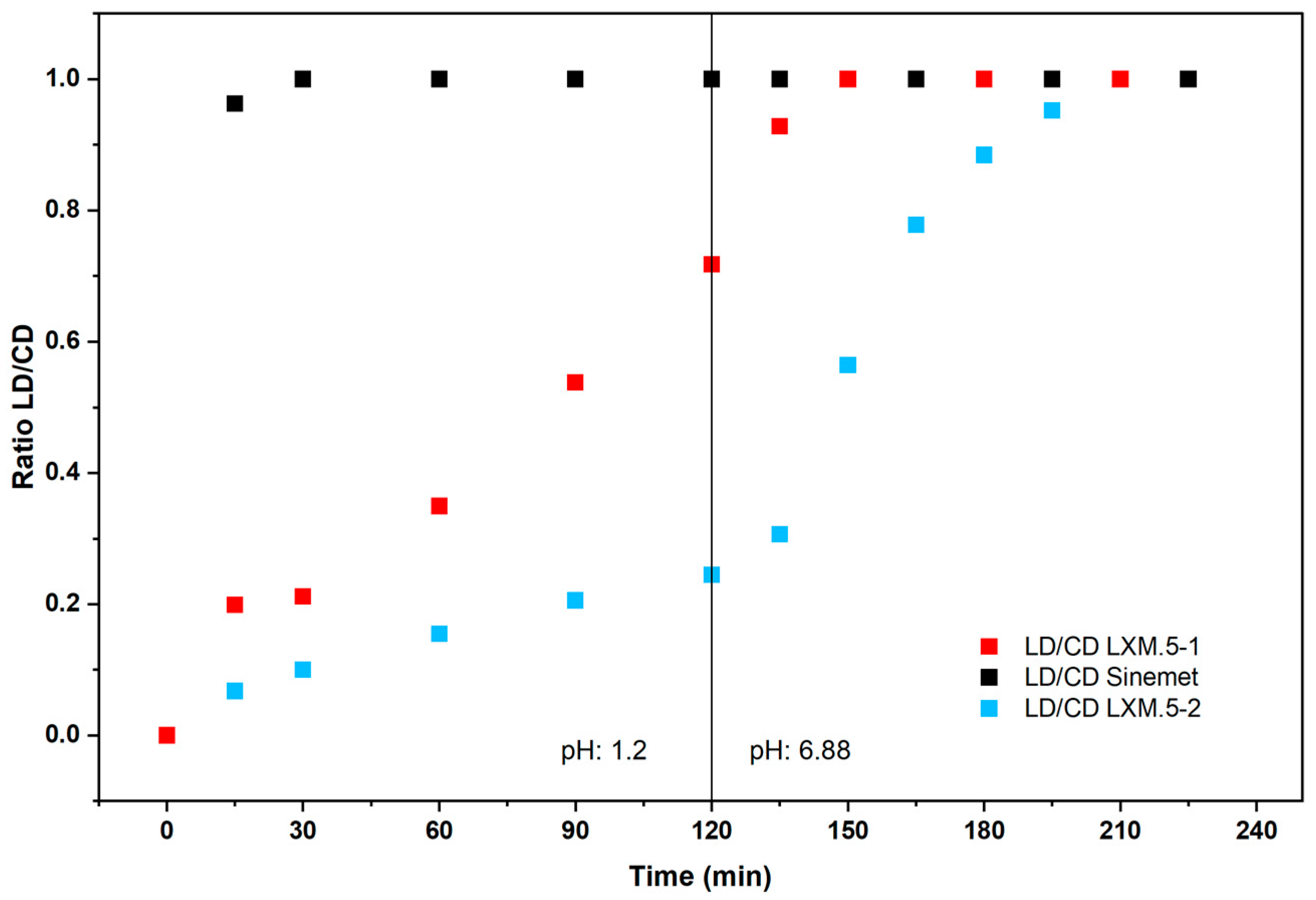
® and LXM.5-1 and LXM.5-2 lies in the initial amount of LD released (as assessed by the ratio of both drugs, see
Figure 3), it is theoretically possible that the absorption of both molecules occurs, at least in part, via overlapping mechanisms.
Another theoretical assumption is that the potential competition for uptake mechanisms could impact LD blood levels directly. Specifically, once all the CD has been cleared from the intestine, all the “shared” channels will be available for LD uptake. This could explain the higher peak LD blood levels observed for both LXM.5-1 and LXM.5-2 compared with Sinemet®. As an alternative explanation, the increase in LD peak levels could be solely due to the stronger inhibition of LD degradation based on higher CD blood levels. The LD shoulders seen with LXM.5-1 and LXM.5-2 are likely dependent on several factors: the height of the LD peak, the sustained CD blood levels that reflect LD degradation inhibition capacity, the tailoring of the LD release to the upper intestine (LXM.5-2) through the addition of pH-sensitive polymers to the LD compartment, and the sequential release interval. Additional studies will be needed to determine the specific role of these factors. The current study suggests that the longer interval observed in LXM.5-2 (compared with LXM.5-1) and the boost of LD release enabled and mediated by pH-sensitive polymers both contribute to higher peak levels and a greater shoulder. Further studies will address this by varying (i) the length of the sequential release interval, (ii) the pH-sensitive polymers, (iii) the tablet architecture to gradually increase pH-guided LD release (which can be achieved by adding further layers with pH-sensitive polymers).
The 3D screen-printed novel oral CD/LD dosage forms, LXM.5-1 and LXM.5-2, and their potential variations, could transform the pharmacotherapy of Parkinson’s disease. Their prolonged and smooth LD blood levels would avoid pulsatile dopaminergic stimulation which is correlated with motor complications notably motor fluctuations and dyskinesias [
9]. Compared with intestinal pump systems, which have been shown to effectively reduce dyskinesias and motor fluctuations by maintaining continuous LD blood levels [
25], LXM.5-1 and LXM.5-2 can be easily administered to patients at all stages of the disease. It is tempting to speculate whether this therapy could slow down the progression of the disease itself. Assuming that LXM.5-1 and LXM.5-2 would positively impact the symptoms of the disease—which is highly likely, given the achieved LD blood levels—their use would directly impact patients’ lives. Based on the sustained blood levels, the expected number of daily intakes would be significantly reduced to about 2-3 doses per day. This is life-changing, particularly in the later stages of the disease when patients require 6-8 doses per day, not to mention the fact that they must be taken on an empty stomach.
The safety profile of LXM.5-1 and LXM.5-2 is expected to be comparable to existing LD products. This is because LD peak levels fall well within the range of marketed products. Peak levels of both novel dosage forms were only 30–50% higher than those of 25/100 Sinemet® (which comes at strengths of 25/100 and 50/200), whereas bioavailability was between 211.36% and 383.64%. The situation for CD needs further consideration, as the new dosage forms appear to triple its peak blood levels. Further experiments will be required to determine the amount of CD necessary to increase LD bioavailability.
Finally, after more than 50 years of LD therapy, LXM.5-1 and LXM.5-2, along with their variations, offer the potential for a more personalized approach to Parkinson’s disease treatment. The flexibility of the 3D screen printing technology allows for variations in the content of CD and LD, as well as in the interval between their release. By taking sequential blood level measurements, the most appropriate dosage form could be selected for an individual patient, based on its drug content and release interval.