Abstract
RNA interference (RNAi) offers programmable, sequence-specific silencing via small interfering RNA (siRNA) and microRNA (miRNA), but clinical translation hinges on overcoming instability, immunogenicity, and inefficient endosomal escape. This review synthesizes advances in non-viral nanocarriers—liposomes, polymeric nanoparticles, and extracellular vesicles (EVs)—that stabilize nucleic acids, tune biodistribution, and enable organ- and cell-selective delivery. We highlight design levers that now define the field: ligand-guided targeting, stimuli-responsive release, biomimicry and endogenous carriers, and rational co-delivery with small molecules. Across major disease areas—cancer and cardiovascular, respiratory, and urological disorders—these platforms achieve tissue-selective uptake (e.g., macrophages, endothelium, and myocardium), traverse physiological barriers (including the blood–brain barrier and fibrotic stroma), and remodel hostile microenvironments or immune programs to enhance efficacy while maintaining favorable safety profiles. Early clinical studies reflect this diversity, spanning targeted nanoparticles, local drug depots, exosome and cellular carriers, and inhaled formulations, e.g., and converge on core phase-I endpoints (safety, maximum tolerated dose, pharmacokinetics/pharmacodynamics, and early activity). Looking ahead, priorities include good manufacturing practice scale, consistent manufacture—especially for EVs; more efficient loading and cargo control; improved endosomal escape and biodistribution; and rigorous, long-term safety evaluation with standardized, head-to-head benchmarking. Emerging directions such as in vivo EVs biogenesis, theragnostic integration, and data-driven formulation discovery are poised to accelerate translation. Collectively, nanoparticle-enabled RNAi has matured into a versatile, clinically relevant toolkit for precise gene silencing, positioning the field to deliver next-generation therapies across diverse indications.
1. Introduction
RNA interference (RNAi) was first discovered by Fire et al. in 1998 [1] and is an evolutionarily conserved gene-regulatory mechanism mediated by exogenous double-stranded RNA (dsRNA) that triggers sequence-specific, post-transcriptional gene silencing. It works through the degradation of specific mRNA, thereby preventing its translation into proteins [2]. The mechanism relies on two classes of small RNA molecules: microRNA (miRNA) and small interfering RNA (siRNA). These RNA molecules achieve gene silencing by binding to specific mRNAs through complementary base pairing, either by degrading the mRNA or inhibiting its translation. miRNAs are endogenous, non-coding small RNAs of about 18–25 nucleotides in length that act as molecular switches to regulate gene expression post-transcriptionally. By specifically base-pairing with the mRNAs of protein-coding genes in animals and plants, they induce translational repression or promote mRNA degradation, thereby precisely modulating gene-expression networks in recipient cells [3,4]. siRNA is a double-stranded RNA molecule of approximately 20–25 nucleotides in length, with two unpaired nucleotides at the 3′ end of each strand. It plays a central role through RNAi pathway by hybridizing with complementary mRNA molecules to guide the RNA-induced silencing complex (RISC) to specifically degrade target mRNAs, thereby silencing the expression of specific genes [5]. Therefore, miRNAs and siRNAs share the same core RNAi mechanism but differ in origin and targeting breadth, enabling both physiological regulation and experimental/therapeutic manipulation. Exogenous siRNA duplexes and miRNA mimics/inhibitors harness RNAi to probe gene function and offer clinical potential for precise, programmable gene silencing.
However, RNA delivery, particularly for siRNA and miRNA, still faces multiple challenges that limit their therapeutic potential. The main obstacles include stability, immunogenicity and delivery efficiency. Firstly, naked nucleic acids have poor in vivo stability and are rapidly degraded in the bloodstream. Its relatively high molecular weight, negative charge, and hydrophilicity hinder translocation across cell membranes. They tend to accumulate in the kidneys and be excreted in urine, or be captured by the reticuloendothelial system, preventing effective binding to target sequences and thus limiting its functional activity [6]. Secondly, the introduction of foreign nanomaterials into the bloodstream can trigger a cascade of innate immune responses, potentially compromising both safety and efficacy. Two primary concerns are complement activation and the induction of inflammatory cytokine responses, which can be triggered by both the nanoparticle carrier and its nucleic acid payload. Complement activation is an immediate, antibody-independent response often initiated upon intravenous administration. The surfaces of many nanoparticles can activate the complement system, leading to opsonization with fragments like C3b, which marks the particle for rapid clearance by the reticuloendothelial system (RES) [7]. This not only curtails circulation half-life but can also generate potent anaphylatoxins (C3a, C5a), causing infusion-related reactions, sometimes termed complement activation-related pseudoallergy (CARPA) [8]. A widely adopted strategy to mitigate this is PEGylation, which creates a “stealth” layer to hinder protein adsorption and prolong circulation [9]. Another major concern is the pro-inflammatory cytokine response, which is largely driven by the recognition of the RNAi payload itself. The innate immune system can recognize exogenous RNA via pattern recognition receptors (PRRs) like endosomal Toll-like receptors (TLR3, TLR7, TLR8) and cytosolic sensors like retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) [10]. Activation of these pathways triggers a “cytokine storm” (e.g., Type I interferons, TNF-α), leading to systemic inflammation and potential off-target effects. Significant progress has been made to abrogate this response through chemical modifications of the RNA backbone (e.g., 2′-O-methyl), which effectively shield it from PRR recognition without compromising silencing activity [11]. Thirdly, delivery efficiency of RNA-based therapeutics is limited by intracellular trafficking, especially endosomal escape [12]. After endocytic uptake, non-viral oligonucleotides are sequestered in early endosomes that mature into late endosomes and ultimately fuse with lysosomes enriched with degradative enzymes. Unless these cargos escape to the cytosol before lysosomal fusion, they are degraded and cannot engage the RNAi machinery, resulting in reduced therapeutic efficacy and elevated off-target toxicity [13,14]. Due to these challenges of stability, immunogenicity, and cellular delivery, precise delivery to target tissues has become a central objective in the research and development of nucleic acid drugs (NADs). Consequently, substantial advances in nanoparticle technologies and delivery vehicles, designed to overcome these specific hurdles, have markedly improved delivery efficiency and form the basis of this review.
2. Methods: Search Strategy and Selection Criteria
This narrative review was compiled following a structured literature search of the PubMed database for relevant articles published between 1 January 2021, and 31 August 2025. The search strategy was designed to identify studies on nanoparticle-mediated delivery of RNAi-based therapeutics. Specific keywords were combined to retrieve articles for distinct nanoparticle platforms. For liposomal systems, the search query was: (liposomes [Title]) AND ((miRNA [Title/Abstract]) OR (siRNA [Title/Abstract])). For polymeric nanoparticles, the query was: (Polymeric Nanoparticles [Title/Abstract]) AND ((siRNA [Title/Abstract]) OR (miRNA [Title/Abstract])). For exosomes and extracellular vesicles, the search was conducted using: ((Exosomes [Title]) OR (extracellular vesicles [Title])) AND ((miRNA [Title]) OR (siRNA [Title])). Inclusion criteria: (1) Studies involving the treatment of solid tumors/neoplasms, and clinical or animal studies related to cardiovascular diseases, respiratory diseases, or urinary system diseases; (2) Publications in journals with an impact factor of ≥5. Exclusion criteria: Antisense oligonucleotides (ASOs), aptamers, mRNA-based therapeutics, and RNA editing (CRISPR).
3. Nanocarriers for Delivering RNAi-Based Therapeutics
Currently, a broad range of nanocarriers have been developed for siRNA, miRNA deliver into tissues, addressing key in vivo barriers. The principal platforms are lipid-based nanoparticles (e.g., liposomes, micelles, solid lipid nanoparticles), polymer-based nanoparticles (e.g., chitosan, dendrimers, protein-based carriers) and extracellular vesicles (e.g., exosomes, micro vesicles, apoptotic bodies). This review focuses on organ-targeted therapeutic advances achieved with three representative platforms—liposomes, polymeric nanoparticles, and exosomes. Because siRNA and miRNA are structurally similar, they often employ the same formulations to enhance stability and improve in vivo pharmacokinetics. siRNAs, being rigid, double-stranded duplexes (≈21–23 bp), possess a high charge density, which facilitates more efficient loading (typically 10–30% w/w in lipid nanoparticles) and greater stability. In contrast, miRNA mimics, which are often single-stranded and more flexible (≈19–25 nt), have a lower charge density, resulting in lower loading efficiency (often 5–15% w/w in lipid nanoparticles) and a higher propensity for premature release. This divergence necessitates tailored nanocarrier design, where formulations for miRNAs may require chemical modifications to improve loading, while the high efficiency of siRNA loading allows for optimization strategies to minimize potential off-target effects. Accordingly, this section focuses on the characteristics of these non-viral RNAi delivery systems, with particular emphasis on organ-specific targeting strategies.
3.1. Liposomes
While “lipid nanoparticles” (LNPs) is a broad term that technically includes conventional liposomes, in modern scientific literature, LNPs refer to a distinct class of delivery vehicles optimized for nucleic acids. Conventional liposomes are colloidal, spherical vesicles formed by self-assembly of amphiphilic lipids in aqueous media, with polar headgroups facing the inner and outer aqueous phases and hydrophobic tails forming bilayers around an aqueous core [15]. Their classification is based on lamellarity and size, including small unilamellar vesicles (SUVs, <100 nm), large unilamellar vesicles (LUVs, 100–500 nm), giant unilamellar vesicles (GUVs, >500 nm), oligolamellar vesicles (OLVs, 2–5 bilayers), multilamellar vesicles (MLVs, >5 bilayers), and multivesicular vesicles (MVVs) with internal compartments [16,17]. However, recent advancements have pushed design principles far beyond simple modulation. Modern strategies now focus on creating “smart” liposomes with sophisticated functionalities. These include stimulus-responsive systems engineered to release payloads upon exposure to physical triggers like ultrasound for site-specific delivery [18], advanced surface modifications such as one-step self-assembly of chimeric nanobodies to create immunoliposomes with high targeting efficiency [19], and multi-functional biomimetic designs. For example, liposomes can be coated with red blood cell membranes for immune evasion and conjugated with ligands like hyaluronic acid to specifically target pro-inflammatory macrophages in atherosclerotic plaques [20]. Furthermore, highly complex, dual-functionalized liposomes are being developed that combine neuron-targeting peptides (e.g., RVG29) with reactive oxygen species (ROS)-responsive elements to cross the blood–brain barrier (BBB) and achieve precise, stimulus-gated drug release in ischemic brain tissue [21]. In contrast, modern LNPs, which dominate the RNAi and mRNA delivery landscape, possess a different structure and composition. Instead of a distinct aqueous core, LNPs feature a dense, electron-rich core where nucleic acids are complexed with ionizable cationic lipids. This LNP framework allows for sophisticated chemical innovation. For instance, advanced LNPs have been engineered with phenylboronic acid (PBA)-modified lipids that target sialic acid on melanoma cells and trigger adenosine triphosphate (ATP)-responsive intracellular siRNA release, significantly boosting antitumor effects [22]. Furthermore, to overcome off-target liver accumulation, novel peptide-ionizable lipids have been rationally designed, enabling the creation of LNPs with tissue-specific tropism for organs like the lungs, spleen, and bone, and even facilitating in vivo prime editing [23]. Liposomes can encapsulate and protect RNAi molecules, achieve targeting via structural modification to enhance their cellular uptake and improve their biodistribution, and also reduce off-target toxicity, making them efficient carriers suitable for the delivery of RNAi-based therapeutics. While both platforms protect RNAi molecules from degradation and can be engineered for targeted delivery, their primary applications diverge. Conventional liposomes remain a versatile platform, particularly for approved drugs carrying small-molecule payloads. However, for RNAi-based therapeutics, LNPs are the preferred and more advanced system due to their specialized structure that efficiently encapsulates, protects, and facilitates the intracellular delivery of siRNA and miRNA [24].
3.2. Polymeric Nanoparticles
Polymeric nanoparticles are nanoscale carriers (from 1 nm to 1000 nm) constructed from natural or synthetic polymers. They differ from inorganic and lipid systems by offering finely tunable size, shape, surface charge, and a broad range of chemical and biological functionalities [25]. These features support targeted delivery to tissues and subcellular compartments and enable stimulus-responsive release, improving efficacy across diverse cargos [26]. Within this class, functional categories include drug-loaded systems (e.g., albumin-bound formulations, polymeric micelles) and optical probes such as dye-loaded polymeric nanoparticles and semiconducting polymeric nanoparticles, which provide high brightness, photostability, and spectral tunability [27]. Owing to the advantages of polymeric nanoparticles, nucleic acids (including RNAi, the focus of this review) can achieve enhanced stability and site-specific intracellular delivery.
3.3. Extracellular Vesicle
Exosomes are small extracellular vesicles (40–160 nm) of endosomal origin that carry DNA, RNA, lipids, metabolites, and proteins reflective of their parent cells [28]. EVs are broadly classified into ectosomes, plasma membrane, derived vesicles (~50 nm to 1 μm), and exosomes, which arise from multivesicular bodies [29]. Exosomes have emerged as versatile delivery platforms capable of transporting functional cargo (e.g., miRNA, siRNA) that can promote or restrain disease processes across immunology, infectious diseases, pregnancy-related conditions, cardiovascular, kidney repair [30,31,32], and cancer [33,34]. As drug carriers, exosomes offer high biocompatibility, low toxicity, deep tissue penetration, broad dissemination via the bloodstream, the ability to cross the blood–brain barrier, and the capacity to bypass P-glycoprotein efflux [35]. Key design strategies involve engineering exosomes to package and deliver defined payloads to specific cell types or tissues. Mechanistically, their lipid bilayer and tetraspanin-rich membranes shield RNA cargos from serum nucleases and complement, while endogenous surface cues (e.g., integrins, CD47) support immune evasion, prolonged circulation, and organ tropism [36]. Taken together, these attributes explain why EVs are effective carriers for RNAi: they provide nuclease protection, targeted biodistribution, and improved cytosolic access, resulting in enhanced stability and site-specific intracellular delivery of nucleic acids, including the RNAi therapeutics central to this review.
4. Nanoparticles Used for the Delivery of RNAi-Based Therapeutics Against Cancer
4.1. siRNA-Based Cancer Therapeutics
4.1.1. siRNA-Based Cancer Therapeutics via Liposomes
Liposomal platforms are accelerating siRNA therapeutics across diverse malignancies by uniting ligand-guided targeting, biomimicry, stimuli-responsiveness, and combinatorial payloading to address circulation, penetration, and intracellular release barriers. In lung cancer, an alginate hydrogel embedding endoplasmic reticulum (ER)-modified liposomes co-delivering STAT3 siRNA and lidocaine achieved single-application tumor control, tumor-associated macrophage (TAM) repolarization, natural killer cell (NK) activation, pleural effusion reduction, and analgesia in postoperative non-small cell lung cancer (NSCLC) models, exemplifying local immunoanalgesic management [37], while a focused review delineates formulation advances and translational hurdles specific to siRNA-loaded liposomes in this disease [17]. In hepatocellular carcinoma, tLyP-1–modified liposomes co-delivering SN38 and MEF2D siRNA activated cGAS–STING yet reversed PD-L1 upregulation, reduced M2/MDSCs, expanded intratumoral CD4+ T cells, and suppressed tumor growth [38]; sorafenib plus KIAA1199 siRNA co-loaded liposomes synergistically curtailed proliferation, migration, and invasion with favorable biodistribution and safety [39]; ultrasound-sensitive liposomes released sonosensitizer and siBcl-2 upon insonation, using ROS to rupture vesicles and enable lysosomal escape for gene silencing [40]; and a macrophage-membrane–cloaked, cRGD/CPP-decorated thermosensitive system achieved HepG2 selectivity, strong Bcl-2 knockdown, minimal RES uptake, and robust antitumor effects under mild hyperthermia [41]. For brain tumors, polymer-locking fusogenic liposomes (Plofsomes) crossed the BBB and fused in ROS-rich glioblastoma (GBM) to deliver siRNA or CRISPR ribonucleoprotein complexes (RNPs), suppressing midkine to reduce temozolomide resistance with tumor-restricted activity [42], while echogenic liposomes served as cavitation nuclei for sonoporation in vivo, supporting a physical-trigger route adaptable to siRNA delivery [43]. In breast cancer, brown rice bran powder1 (BRBP1)-modified liposomes traversed the BBB to brain metastases, co-delivering paclitaxel and TWF1 siRNA to reverse epithelial-mesenchymal transition (EMT)-linked taxane resistance and inhibit metastatic growth [44]; cationic liposomes co-loaded crizotinib, paclitaxel, and Bcl-xL siRNA provided coordinated release, synergistic cytotoxicity, and gene knockdown in MCF-7 cells [45]; and Herceptin-conjugated liposomes carrying Lipocalin-2 (LCN2) siRNA internalized efficiently into HER2+ inflammatory breast cancer cells, reduced LCN2 and disrupted tumor emboli, reshaping oncogenic pathways [46]. In pancreatic ductal adenocarcinoma, macrophage-mimetic, LFC131-targeted, CaP-cored liposomes silencing glutamine-fructose-6-phosphate aminotransferase 1 (GFAT1) lowered hyaluronan, decompressed vasculature, and weakened stroma; combined with Doxil, they enhanced penetration and antitumor efficacy while reducing invasiveness versus hyaluronidase [47]. When combining siRNA with other modalities, sequence/schedule dependencies and toxicity management become critical design considerations. For example, cationic liposomes co-encapsulating hydrophobic proteolysis targeting chimeras (PROTACs (DT2216)) and siRNA leveraged lipid solubilization and polymeric condensation to amplify protein degradation and tumor inhibition, showcasing dual modality payloading of hydrophilic and hydrophobic agents [48]. Crucially, the toxicity of such dual-payload systems was rigorously evaluated; in vivo studies using H&E staining of major organs (heart, liver, spleen, lungs, and kidneys) from treated subjects confirmed that the liposomal co-delivery vehicle did not induce significant histopathological abnormalities, demonstrating effective toxicity management. Similarly, in combinations of chemotherapeutics and siRNA, schedule dependence is a key determinant of efficacy. Studies pairing paclitaxel (PTX) and a survivin-targeting siRNA within a single cationic liposome formulation revealed a clear synergistic effect. This synergy was schedule-dependent, observed only when the two agents were co-loaded and delivered simultaneously, which produced significantly higher cytotoxicity (p < 0.01) compared to their administration as separate solutions. This highlights the necessity of synchronized delivery to distinct intracellular targets to maximize the therapeutic outcome. Collectively, these studies converge on five design levers—ligands (tLyP-1, BRBP1, folate, Herceptin), biomimicry (macrophage membranes), stimuli (thermal, ultrasound, ROS), rational combinations (chemotherapeutics, PROTACs), and microenvironment remodeling (HA downregulation)—to advance precise, safe, and effective liposomal siRNA therapeutics toward clinical translation across cancer types.
4.1.2. siRNA-Based Cancer Therapeutics via Polymeric Nanoparticles
Polymeric nanoparticles (PNPs) are redefining siRNA oncology by coupling modular chemistries, ligand display, and stimuli-responsiveness to stabilize cargo, enable tumor-specific uptake, and promote endosomal escape, complementing lipid systems and expanding delivery beyond the liver [25,49,50,51]. Platform advances include high-throughput poly (ethylene oxide) (PEO)–poly (ε-caprolactone) (PCL)–amine triblock libraries that identify carriers with favorable circulation and tumor accumulation in vivo [52], and Photocrosslinked, Bioreducible designs with ester/disulfide linkages that improve serum stability and cytosolic release to achieve potent knockdown in glioma and melanoma, including lung-colonized lesions after systemic dosing [53]. In lung cancer, anisamide-functionalized oligopeptide end-modified poly (beta aminoester) (OM-pBAE) nanoparticles selectively deliver mTOR siRNA to NSCLC, maintaining stability in serum, achieving efficient transfection, and suppressing tumor growth in vitro and in vivo with tumor selectivity [26]. For hepatocellular carcinoma, expert consensus emphasizes nanoparticle engineering to cross vessel walls and extracellular matrix (ECM) and target hepatocellular carcinoma (HCC) receptors [54], while AFP-siRNA-loaded PLGA (PLGA-AFP) platforms synergize with epigallocatechin gallate to amplify apoptosis [55] and with low-dose sorafenib/sunitinib to deepen AFP silencing and inhibit proliferation, supporting dose-sparing combinations [56]. In breast cancer, especially triple-negative breast cancer (TNBC), dermatan sulfate/chitosan “double self-assembled” NPs leverage CD44 targeting to deliver BIRC5 (survivin) siRNA and curb proliferation, migration, and spheroid formation [57]; PLGA nanovectors decorated with a TNBC-specific aptamer enable rapid, selective programmed cell death-ligand1 (PD-L1) silencing and endosomal escape in MDA-MB-231/BT-549 cells [58]; smart pH-responsive, PEG-detachable co-delivery of paclitaxel and vascular endothelial growth factor (VEGF) siRNA inhibits angiogenesis and outperforms monotherapies in 4T1 models [59]; and immune-centric mPEG-PLGA-PLL (PEAL) nanoparticles that carry siCD155 and are coated with anti-PD-L1 antibodies orchestrate asynchronous checkpoint blockade, eliciting CD8+ TIL-dominant immunity to suppress TNBC growth and metastasis with safety [60]. In ovarian cancer, an intraperitoneal, thermosensitive hydrogel reservoir provides sustained release of STAT3-siRNA polyplexes, enriches nodules, and significantly delays tumor growth in advanced models, aligning with reviews positioning PNPs to overcome systemic instability and chemoresistance [61,62]. Pancreatic cancer efforts exploit HA-displaying chitosan/siRNA NPs to target CD44 and knock down HIF-1α under hypoxia, with chitosan molecular weight tuning uptake versus silencing and in vivo gene suppression [63], complemented by broader evidence for siRNA-NP co-therapies and translational momentum [64], and by cholesterol-modified PNPs delivering Inhba siRNA to suppress activin A, reducing Pancreatic ductal adenocarcinoma (PDAC) growth/metastasis and cachexia while reshaping the tumor microenvironment (TME) [65]. Negative design lessons emerge in bladder cancer, where survivin siRNA plus paclitaxel lacked synergy due to survivin-induced cell-cycle arrest antagonizing taxanes, underscoring schedule/biology-aware co-delivery [66]. Beyond tumor cells, tyrosine-modified polyethylenimine/polypropylenimine (PEI/PPI) systems efficiently transfect macrophages; siSTAT3/siSTAT6 reprograms tumor-associated macrophages (TAMs) toward phagocytic M1 phenotypes and enhances tumor cell clearance [67]. Finally, lipid-assisted mPEG-PLGA hybrids optimized for anti-CD47 siRNA demonstrate potent antitumor immunity in melanoma, illustrating immunogene targets enabled by polymer–lipid synergy [68]. Collectively, ligand-guided targeting, redox/pH/Photo-crosslinkable architectures, rational co-delivery, immune reprogramming, and locoregional depots are converging to deliver tumor- and context-tailored siRNA therapies across cancer types.
4.1.3. siRNA-Based Cancer Therapeutics via Exosomes and Other Extracellular Vesicles
EVs are emerging as biocompatible, targetable carriers that overcome key barriers to siRNA therapy by improving stability, biodistribution, immune tolerance, and tumor penetration. Scalable sources such as bovine milk/colostrum exosomes enable high loading, ligand functionalization, and reduced toxicity, positioning them as cost-effective platforms for systemic delivery, including siRNA-based therapeutics [34]. In lung cancer, edible kiwi-derived vesicles loaded with siSTAT3 and decorated with an epidermal growth factor receptor (EGFR) aptamer safely suppressed growth of multidrug-resistant EGFR-mutant NSCLC, highlighting charge-free, plant-derived EVs for precision targeting [69] while an engineered hybrid of EVs with gold–siRNA nanocomplexes silenced the B7-H4 checkpoint, curtailed proliferation/invasion, and achieved antitumor efficacy in NSCLC xenografts with limited effects in normal cells [70]. For brain tumors, blood exosomes co-delivering cytoplasmic phospholipase A2 (cPLA2) siRNA and metformin crossed the BBB and reprogrammed glioblastoma energy metabolism in patient-derived xenograft (PDX) models, prolonging survival and enabling biomarker-guided personalization via polymerase 1 and transcript release factor (PTRF)-mediated uptake [71]; macrophage exosomes co-loaded with panobinostat and mutation of p53-induced protein phosphatase 1(PPM1D) siRNA similarly achieved BBB transit and extended survival in diffuse intrinsic pontine gliomas (DIPG) [72]. Tumor and immune microenvironments can be remodeled with EV–siRNA: M1 macrophage–derived exosomes carrying siSIRPα repolarize M2 macrophages and, with anti-PD-L1, enhance phagocytosis and suppress metastatic traits [73]; M1 EVs delivering CX3CR1 siRNA inhibit PDAC proliferation and migration and suppress tumors in vivo [74]. In ovarian cancer, patient-derived exosomes electroporated with c-Met siRNA selectively accumulated in peritoneal lesions and extended survival after intraperitoneal dosing, supporting autologous, surgery-integrated production [75]. In triple-negative breast cancer, EGFR-aptamer-modified exosomes co-delivering delivery of aspartyl-tRNA synthetase-antisense RNA 1 (DARS-AS1) siRNA and doxorubicin overcame autophagy-mediated chemoresistance via TGF-β/Smad3 inhibition [76], while MEK1-siRNA exosomes downregulated mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling and reduced angiogenesis [77]. Collectively, natural-source, engineered, and immune-cell–derived EVs are enabling targeted siRNA therapies across cancer types, with remaining priorities in scalable manufacture, loading efficiency, and long-term safety benchmarking versus synthetic nanocarriers.
4.2. miRNA-Based Cancer Therapeutics
4.2.1. miRNA-Based Cancer Therapeutics via Liposomes
Liposomal carriers are enabling cancer type–specific miRNA therapeutics by integrating active targeting, organelle tropism, and theranostic design. In a novel theoretical model, optically guided liposomes are proposed for gene delivery to inhibit enzymes involved in drug metabolism. This strategy aims to overcome chemoresistance, allowing for the effective use of inexpensive, existing chemotherapeutics and potentially reducing treatment costs while improving patient outcomes [78]. In lung cancer, mitochondria-targeted, pH-responsive polymeric liposomes (R@DA-TPP-SA) enabled pre-miR-34a imaging via intracellular hybridization chain reaction (HCR) and mitochondrial delivery of therapeutic miR-34a, achieving lysosomal escape, triphenylphosphine (TPP)-mediated organelle accumulation, loss of membrane potential, apoptosis, and in vivo antitumor efficacy with low toxicity—showcasing an organelle-precise, imaging-guided therapy paradigm [79]. For hepatocellular carcinoma, a focused review underscores lipid nanoparticles as leading non-viral vectors for miRNA/siRNA, detailing advances in tumor-specific targeting and transfection alongside formulation challenges (stability, specificity, immunogenicity) and encouraging preclinical outcomes [80]. Broadly across solid tumors, a theoretical framework proposes optically guided, layered liposomes packaging miRNAs to inhibit drug-metabolizing enzymes, thereby resensitizing resistant cancers to affordable chemotherapies and potentially lowering costs while improving outcomes [78]. Collectively, targeted, stimuli-responsive, and organelle-directed liposomal systems are maturing from concept to translational platforms that enhance delivery fidelity, enable real-time monitoring, and potentiate existing therapies across cancer types.
4.2.2. miRNA-Based Cancer Therapeutics via Polymeric Nanoparticles
Polymeric nanoparticles are accelerating miRNA therapeutics across solid and hematologic malignancies by protecting labile cargos, enabling ligand-guided uptake, and programming on-demand release (for example via redox-responsive chemistries) [25]. In multiple myeloma, reviews highlight polymeric platforms among non-viral nanocarriers that improve tumor targeting and reduce systemic toxicity for miRNA delivery [81]. In glioblastoma, Angiopep-2-decorated PNPs co-deliver anti-miR-21 and miR-124 across the blood–brain barrier to co-regulate RAS/PI3K/PTEN/AKT signaling, suppress invasion/angiogenesis, and extend survival in orthotopic models [82]. For cutaneous squamous cell carcinoma, dual cell-penetrating peptide–conjugated PNPs transporting miR-205-5p achieve superior internalization, G0/G1 arrest, apoptosis, and in vivo tumor volume reduction with minimal off-target toxicity [83]. In pancreatic cancer, PNP-mediated miR-24-3p replacement triggers apoptosis and autophagy, yielding robust tumor inhibition in xenografts [84]. While hepatocellular carcinoma efforts have focused on lipid systems, non-viral vectors including polymeric nanoparticles remain promising for miRNA delivery as cell-specific targeting and efficient transfection are optimized [80]. Beyond direct miRNA carriage, zein PNPs potentiate metformin’s antitumor activity while favorably reprogramming miRNA networks and the adenosine monophosphate-activated protein kinase (AMPK) axis in a breast cancer model, underscoring carrier-driven miRNA modulation in vivo [85]. Collectively, functionalized, stimuli-responsive PNPs offer a versatile miRNA delivery toolbox, with translational priorities centered on durability, biodistribution, and safety.
4.2.3. miRNA-Based Cancer Therapeutics via Exosomes and Other Extracellular Vesicles
Exosomes and other small extracellular vesicles are advancing miRNA therapeutics by combining intrinsic biocompatibility, immune evasion, and tissue tropism with engineerable surface ligands and payload control, thereby improving delivery across challenging barriers such as the BBB and hostile tumor microenvironments [33,86,87]. In lung cancer, LXY30 peptide–modified bone marrow mesenchymal stromal cells (MSCs) exosomes loaded with miR-30c, miR-181b, or miR-613 selectively targeted NSCLC, suppressing proliferation/migration and inducing apoptosis with in vivo safety, while underscoring the value of integrin-directed targeting [88]; conversely, Schwann cell–derived exosome miR-21-5p drives lung cancer growth and metastasis by repressing RECK, rationalizing anti–miR-21 or source-targeted interception strategies [89]. For glioblastoma, engineered exosomes packaging a polycistronic cassette encoding miR-124-2/miR-135a-2/let-7i achieved pan-subtype GSC inhibition and prolonged survival, outperforming single-miR or cocktail controls [90]. Immune engineering of T cell small extracellular vesicles (sEVs) with surface IL-2 reprograms vesicular miRNA content (e.g., miR-181a-3p/miR-223-3p) to downregulate tumor PD-L1, augment CD8+ T cell cytotoxicity, and synergize with anticancer drugs in melanoma [91]. In colorectal cancer, TM4SF5-targeted exosomes delivering miR-143 diminish metastasis-associated in colon cancer 1 (MACC1) signaling and curb invasion and tumor growth, whereas macrophage EVs enriched in NEAT1 promote colorectal cancer (CRC) stemness by sponging miR-34a-5p and restoring PEA15, highlighting both therapeutic cargo and decoy axes for intervention [92,93]. Prostate cancer bone pathology is shaped by PCa exosomal miR-92a-1-5p, which suppresses COL1A1 to favor osteoclastogenesis, revealing a tractable EV-miRNA target in metastasis [94]. Translational infrastructure is coalescing: epithelial cell adhesion molecule (EpCAM)-aptamer nanoarrays with CRISPR/Cas13a quantify tumor-EV miR-21/23a in plasma, and urinary exosomal miR-16 supports rapid, low-cost prostate cancer screening—tools that can guide miRNA therapy selection and monitoring [87,95]. Finally, liver-programmed in vivo sEV biogenesis offers a scalable blueprint for RNA loading applicable to miRNA therapeutics [96], while TNBC- and MSC-EV-focused reviews map priorities in loading efficiency, ligand-guided targeting, GMP manufacture, and long-term safety [33,86]. The tumor microenvironment for RNAi therapy is shown in Figure 1. The details of RNAi-based therapeutic in various cancer are shown in Table 1.
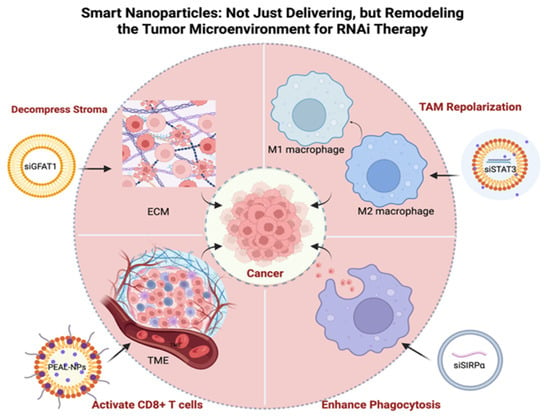
Figure 1.
Smart Nanoparticles Enhance RNAi Therapy by Remodeling the Tumor Microenvironment. This schematic illustrates four key mechanisms by which nanoparticles overcome biological barriers, including: (1) Reprogramming TAMs from a pro-tumoral M2 phenotype to an anti-tumoral M1 phenotype; (2) Enhancing phagocytosis of cancer cells by blocking “don’t eat me” signals like SIRPα; (3) Activating adaptive immunity by promoting CD8+ T cell infiltration and anti-tumor activity; and (4) Decompressing the dense ECM to improve drug penetration. ECM, Extracellular Matrix; EV, Extracellular Vesicle; GFAT1, Glutamine-Fructose-6-Phosphate Aminotransferase 1; NP, Nanoparticle; PEAL, PEG-sheddable and Amino-Lipid-assisted; PNP, Polymer-based Nanoparticle; SIRPα, Signal Regulatory Protein Alpha; STAT3, Signal Transducer and Activator of Transcription 3; TAM, Tumor-Associated Macrophage; TME, Tumor Microenvironment. Created in BioRender. Zhang, C. (2026) https://BioRender.com/h9wt5g8, accessed on 30 September 2025.

Table 1.
RNAi-based therapeutic delivery technologies in various cancer.
5. Nanoparticles Used for the Delivery of RNAi-Based Therapeutics Against Cardiovascular Disease
5.1. siRNA-Based Therapeutics for Cardiovascular Disease
Liposomes and exosomes/extracellular vesicles (EVs) are propelling siRNA therapeutics for cardiovascular disease through precise, ligand-guided delivery to vascular and cardiac targets. In atherosclerosis, dual-targeted liposomes bearing anti-F4/80 and anti-CD68 antibodies concentrate Dll4 siRNA in M1 macrophages, suppressing Notch signaling, limiting vascular smooth muscle cell (VSMC) phenotypic switching/senescence, and reducing plaque vulnerability with favorable pharmacokinetics and safety [101]. Complementarily, VCAM-1-binding cationic liposomes deliver methylated NLRP3 siRNA to inflamed endothelium, blocking TNF-α-driven inflammasome/IL-1β signaling and LDL transcytosis; local and systemic dosing lowered endothelial LDL deposition and plaque burden in rodent models [102]. For ischemic heart disease, PEGylated cationic liposomes co-delivering apigenin and receptor for advanced glycation end products (RAGE) siRNA attenuated oxidative stress and inflammation via the RAGE/NF-κB axis, reducing arrhythmias, apoptosis, and infarct necrosis in vivo [103]. EVs add cardiac selectivity: small EVs engineered with a cardiac-targeting peptide (CTP-LAMP2b) enhanced myocardial uptake >2-fold and delivered siRAGE to dampen myocarditis inflammation in cells and mice [104]. Human serum–derived EVs functionalized with a fibroblast activation protein aptamer homed to injured myocardium; siTGFβ1 cargo reduced cardiac TGFβ1, fibrosis, and hypertrophy, improving function without systemic toxicity [105]. Together, liposomes (modular chemistry, co-delivery) and EVs (biocompatibility, peptide/aptamer retargeting) converge on macrophages, endothelium, and injured tissue to silence nodal pathways with promising translational profiles.
5.2. miRNA-Based Therapeutics for Cardiovascular Disease
Liposomes, extracellular vesicles and polymeric nanoparticles are advancing miRNA-based cardiotherapy by pairing disease-relevant targets with selective delivery and, when needed, small-molecule co-therapy. For atherosclerosis, multifunctional liposomes co-encapsulating EGCG and miR-223 simultaneously suppress oxidative/inflammatory pathways and upregulate ABCA1-driven lipid efflux, accumulate within plaques, and reduce foam cell formation in vivo [44]. Cardiac-targeted liposomes carrying an exosome-derived miR-185-5p inhibitor mitigate apoptosis and cuproptosis, improving left ventricular ejection fraction (LVEF) and limiting fibrosis in doxorubicin-induced dilated cardiomyopathy, illustrating organ-tropic formulation benefits [106]. Across pulmonary arterial hypertension, nanosystems—including liposomes and other nanocarriers—are highlighted for miRNA delivery to modulate pathogenic signaling beyond symptomatic vasodilation, underscoring translational potential and formulation choices by target biology [107]. EVs offer innate biocompatibility and cell-programmable cargo: cardiac-homing peptide–engineered bone marrow mesenchymal stem cell (BMSC) exosomes delivering miR-499a-5p blunt anthracycline cardiotoxicity via CD38/MAPK/NF-κB suppression [108]; human amniotic mesenchymal stem cell (hAMSC) exosomes transfer novel miRNA N-194 to endothelial cells, targeting ING5 to promote angiogenesis [109]; macrophage exosomal miR-204 mediates AT2 receptor–driven improvement of vascular calcification by targeting RUNX2 and dampening Wnt/β-catenin signaling [110]. Therapeutic EV reprogramming can enhance efficacy, as depletion of deleterious miR-192-5p/miR-432-5p from progenitor cell sEVs augments post-infarction repair [111], whereas pathogenic adipose tissue–derived exosomal miR-132/212 accelerates atherogenesis but is mitigable (e.g., by melatonin), informing antimiR strategies [112]. Cardiac matrix–resident vesicles further reveal sex- and age-dependent miRNA synergies that shape anti-fibrotic responses and motivate rational miRNA combinations [113], while exosome RNA atlases in cardiac myxoma–related stroke provide diagnostic scaffolds for patient stratification [114]. Complementing EVs, polymeric nanoparticle–formulated antagomiR-21a-5p attenuates autoimmune myocarditis, reducing inflammation, fibrosis, and hypertrophy in vivo [115]. Collectively, carrier selection—liposomes for modular co-delivery, EVs for biocompatible, tissue-tropic transport, and polymeric nanoparticles for scalable miRNA delivery—enables precise miRNA modulation across cardiovascular indications. The details of RNAi-based therapeutic in cardiovascular disease are shown in Table 2.

Table 2.
RNAi-based therapeutic delivery technologies in cardiovascular disease.
6. Nanoparticles Used for the Delivery of RNAi-Based Therapeutics Against Respiratory Disease
6.1. siRNA-Based Therapeutics for Respiratory Diseases
Liposomes, polymeric nanoparticles, and extracellular vesicle-based systems are enabling organ- and cell-selective siRNA therapy in lung disease. In pulmonary fibrosis, intravenously administered liposomes passively accumulate in fibrotic foci to silence mechanosensitive ZNF416, dampen p-Smad2/3 signaling, and synergize with TGF-β receptor blockade to reduce injury and fibrosis [116], while Sart1 siRNA liposomes reprogram macrophage polarization by limiting M2 infiltration and attenuate bleomycin-induced pathology [117]. For NSCLC perioperative care, an alginate hydrogel loaded with endoplasmic reticulum–modified liposomes co-delivering STAT3 siRNA and lidocaine induces tumor apoptosis, promotes M1 macrophages, decreases malignant pleural effusion, and relieves pain after a single application [37]. Polymeric oligopeptide-modified pBAE nanoparticles, anisamide-functionalized for tumor selectivity, protect and deliver mTOR siRNA with serum stability, achieving specific NSCLC knockdown and antitumor efficacy in vitro and in vivo [26]. EV-derived platforms broaden safety and targeting: edible, cation-free kiwi EVs carrying STAT3 siRNA and an EGFR aptamer suppress drug-resistant EGFR-mutant NSCLC with superior tolerability to cationic liposomes [69]; engineered EV–gold nanohybrids deliver B7-H4 siRNA to NSCLC cells and spheroids, sparing normal fibroblasts and inhibiting xenografts [70].
6.2. miRNA-Based Therapeutics for Respiratory Disease
Diverse nanocarriers are enabling precise miRNA therapeutics across respiratory indications. In idiopathic pulmonary fibrosis, inhalable liposomes delivering hsa-miR-30a-3p reverse myofibroblast programs and improve lung function, mirroring—but mechanistically focusing—the broader TGF-β attenuation seen with exosome therapy [118]. For chronic obstructive pulmonary disease (COPD), a technology landscape review highlights liposomes, polymeric and inorganic nanoparticles, dendrimers, and micelles as fit-for-purpose vehicles to deliver miRNA/antagomirs for immuno-inflammatory control and epithelial repair, emphasizing carrier–disease matching for biodistribution and compatibility [119]. In lung cancer, α3β1-integrin–targeted BMSC exosomes ferry miR-30c/181b/613 to suppress NSCLC growth, illustrating ligand-guided EV tropism [88], while Schwann cell exosomal miR-21-5p accelerates tumor progression, nominating antimiR-21 or EV-source interception as countermeasures [89]. Beyond oncology, dental pulp stem cell sEVs overexpressing miR-486 mitigate high-altitude pulmonary edema by modulating PTEN/PI3K/Akt/eNOS signaling [120], and neutrophil membrane–engineered Panax ginseng exosomes loaded with miR-182-5p alleviate septic acute lung injury via NOX4/Drp-1/NLRP3 pathway inhibition [121]. Notably, a synthetic-biology strategy that reprograms hepatocytes to self-assemble and export therapeutic siRNA within sEVs outperforms standard therapy for lung-metastatic osteosarcoma, providing a scalable in vivo EV-manufacturing blueprint readily adaptable to miRNA payloads for pulmonary delivery [96]. Together, inhalable liposomes and engineered EVs deliver disease-specific miRNA modulation with growing efficacy and translatability. The details of RNAi-based therapeutic in respiratory disease are shown in Table 3.

Table 3.
RNAi-based therapeutic delivery technologies in respiratory disease.
7. Nanoparticles Used for the Delivery of RNAi-Based Therapeutics Against Urological Disease
Synthetic nanoparticles and extracellular vesicles are enabling precise RNA therapeutics across the urologic spectrum. For siRNA delivery, tailored nanocarriers overcome nuclease instability and tissue penetration barriers in urological cancers—spanning polymeric and lipid nanoparticles, nanobubbles, and magnetic platforms—thereby supporting tumor-selective silencing strategies [123]. A representative therapeutic implementation uses layer-by-layer renal-targeted polymeric nanoparticles (PLGA core; chitosan/hyaluronan shells with a kidney-targeting peptide) to concentrate Arg-2 siRNA in proximal tubules after systemic dosing, attenuating oxidative/nitrosative stress, rescuing mitochondrial dysfunction, and reducing apoptosis in contrast-induced acute kidney injury models [27]. For miRNA modalities, EVs function as both carriers and disease informants: urinary exosomal miR-16-5p differentiates prostate cancer stages with practical, low-cost extraction, positioning uEVs for screening and treatment planning [95], while uEV miRNA signatures in diabetic kidney disease implicate apoptosis/inflammation pathways that can guide target selection and response monitoring [124]. Exosome cargo also reveals actionable biology—prostate cancer–derived exosomes transfer miR-92a-1-5p to suppress COL1A1, tipping bone homeostasis toward osteoclastogenesis and suggesting antimiR or EV-interception strategies against skeletal metastasis [94]. In crystal nephropathy, tubular cell–derived exosomes reprogram macrophage polarization via miRNA networks (e.g., miR-146a-5p, miR-200a-3p), reducing oxidative stress and calcium oxalate deposition in vivo, thereby nominating engineered EVs or miRNA cocktails for renoprotective therapy [125]. Together, advances in kidney-tropic polymeric systems and EV-based miRNA delivery/diagnostics converge on organ-specific biodistribution, pathway-level precision, and translational readiness in urologic disease. The details of RNAi-based therapeutic in urological disease are shown in Table 4.

Table 4.
RNAi-based therapeutic delivery technologies in urological disease.
8. Clinical Translation and Application
8.1. siRNA Therapeutics in Clinical Trials
Across early clinical development, siRNA therapeutics are being advanced with diverse delivery platforms that tailor biodistribution, exposure, and on-target engagement. A ligand-targeted cyclodextrin polymer nanocomplex (CALAA-01) packages an anti-R2 siRNA with PEG stabilization and transferrin targeting to transferrin-receptor–overexpressing tumors, with a first-in-human phase I defining safety, PK, immune readouts, and preliminary antitumor activity after IV dosing in refractory solid tumors [126]. For pancreatic adenocarcinoma, siG12D LODER uses an implantable local drug-eluting depot to maximize intratumoral exposure; an open-label phase 0/phase I program assesses distribution, DLTs/MTD, RP2D, and progression-free follow-up [127]. An intravenous siRNA regimen (TKM-080301) in advanced hepatocellular carcinoma employs a 3 + 3 dose-escalation with an expansion at the MTD, evaluating safety, PK, and preliminary efficacy with weekly infusions in 28-day cycles [128]. Biological nanoparticles are represented by mesenchymal stromal cell–derived exosomes loaded with KRASG12D siRNA (iExosomes), delivered IV on days 1, 4, and 10 in 14-day cycles, with endpoints spanning MTD/DLTs, PK, response, disease control, PFS, OS, and exploratory liquid/tissue biomarker assessments [129]. A cell-based approach infuses siRNA-transfected peripheral blood mononuclear cells (APN401) on days 1, 29, and 57 to characterize safety, immunologic effects, and clinical outcomes [130]. Extending beyond oncology, inhaled MBS-COV (SNS812) tests single and multiple ascending doses in healthy participants in a randomized, double-blind, placebo-controlled design to establish safety, tolerability, and PK for pulmonary delivery [131]. Collectively, these studies highlight the translational breadth of siRNA delivery—targeted nanoparticles, local depots, exosomes, cellular carriers, and inhalation—while converging on core phase I objectives: safety, MTD, PK, and early activity signals. A summary of siRNA therapeutics in clinical trials is shown in Table 5.

Table 5.
Organ-targeted siRNA drug delivery studied in clinical trials.
8.2. miRNA Therapeutics in Clinical Trials
Early clinical studies of miRNA therapeutics highlight diverse delivery strategies spanning nanoparticle carriers. In MesomiR-1, “TargomiRs”—EGFR-targeted, nonliving bacterial minicell nanoparticles encapsulating a miR-16 mimic—are administered intravenously in a phase I dose-escalation for recurrent malignant pleural mesothelioma and NSCLC to establish safety and the MTD via cohort-based escalation [132]. Cobomarsen (MRG-106), an inhibitor of oncogenic miR-155, uses flexible routes (intratumoral for cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF); subcutaneous or intravenous systemically) across MF, chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL), and adult T-cell leukemia/lymphoma (ATLL), with a multi-part design emphasizing safety, tolerability, pharmacokinetics, immunologic pharmacodynamics, and preliminary activity, employing a Days 1/3/5 lead-in followed by weekly dosing [133,134]. Beyond oncology, TenoMiR—a chemically synthesized, non-immunogenic miR-29a mimic engineered for improved stability, activity, and cellular uptake—targets tendon collagen remodeling in lateral epicondylitis in a global study building on a safe phase 1b, enabling treatment at initial diagnosis without invasive biopsies. MRX34, a liposomal microRNA product, is delivered intravenously in a phase I open-label dose-escalation (5 days on, 2 weeks off per 21-day cycle) to assess safety, pharmacokinetics, and pharmacodynamics in primary liver cancer and other advanced malignancies [135,136]. Collectively, these trials demonstrate clinical translation of miRNA therapy via distinct carriers—EGFR-targeted bacterial minicell nanoparticles, liposomal formulations, and chemically stabilized mimics/antagonists—tailored to disease biology while converging on core early-phase objectives: safety, dose finding, PK/PD characterization, and preliminary efficacy signals. Clinical applications of nanoparticle organ targeting are shown in Figure 2. A summary of miRNA therapeutics in clinical trials is shown in Table 6.
Figure 2.
Clinical Application of Nanoparticles Organ Targeting. Created in BioRender. Zhang, C. (2026) https://BioRender.com/h9wt5g8, accessed on 30 September 2025.

Table 6.
Organ-targeted miRNA drug delivery studied in clinical trials.
9. Approved siRNA-Based Therapies
A critical analysis of the current therapeutic landscape reveals that seven siRNA-based drugs have successfully transitioned from clinical trials to regulatory approval, validating RNAi as a powerful therapeutic modality. These approvals uniformly highlight the maturation of two key and distinct delivery platforms. The first, a LNP system for intravenous administration, is exemplified by Patisiran, which targets transthyretin (TTR) mRNA to treat hereditary transthyretin amyloidosis (hATTR) [137]. In contrast, the subsequent approvals have all leveraged a successful carrier-free strategy: the direct conjugation of siRNA to a targeting ligand. This approach bypasses the need for a nanoparticle formulation for specific tissues. The most prominent example is the N-acetylgalactosamine (GalNAc)-siRNA conjugate platform. In this design, the siRNA payload is directly conjugated to a GalNAc ligand, which binds with high affinity to the asialoglycoprotein receptor (ASGPR) abundantly expressed on hepatocytes [138]. This interaction triggers rapid receptor-mediated endocytosis, leading to efficient delivery and potent gene silencing within the liver [139]. This highly effective GalNAc-conjugate platform has proven remarkably versatile, leading to the approval of Vutrisiran (targeting TTR for hATTR) [140], Givosiran (targeting ALAS1 for acute hepatic porphyria) [141], Lumasiran and Nedosiran (targeting HAO1 and LDHA, respectively, for primary hyperoxaluria) [142,143], Inclisiran (targeting PCSK9 for hypercholesterolemia) [144], and Fitusiran (targeting antithrombin for hemophilia A and B) [145]. The consistent success of these therapies underscores a common theme: all currently approved RNAi drugs target genes expressed in the liver, a direct consequence of the tropism of both LNP and GalNAc-based delivery systems. While no miRNA-based therapeutics have yet received approval, the clinical success of these seven siRNA drugs establishes a clear and effective blueprint for future RNAi drug development, demonstrating that targeted nanoparticle and conjugate systems can safely and effectively silence disease-causing genes in humans. The details of approved drugs are shown in Table 7.

Table 7.
Approved siRNA-based therapeutics.
10. Conclusions and Prospects
The convergence of RNAi-based mechanisms and advanced nanoparticle engineering has catalyzed a paradigm shift in precision medicine. As this review has detailed, the journey from the discovery of RNAi to its clinical application has been critically dependent on overcoming the formidable barriers of in vivo delivery. Liposomes, polymeric nanoparticles, and extracellular vesicles have emerged as the vanguard of this effort, each offering a unique combination of stability, biocompatibility, and targetability that has proven essential for translating the potential of siRNA and miRNA into tangible therapeutic strategies across oncology, cardiovascular disease, and beyond.
The studies highlighted herein demonstrate a clear trend toward sophisticated, multi-functional systems. We are moving beyond simple encapsulation to “smart” nanocarriers that feature ligand-guided targeting, stimuli-responsive release, and the capacity for rational co-delivery of both RNAi agents and small-molecule drugs. The use of biomimetic coatings (e.g., macrophage membranes) and endogenous carriers (e.g., exosomes) signifies a deeper integration with host biology, promising enhanced immune evasion and superior biodistribution. Furthermore, the ability to engineer these platforms to remodel the tumor microenvironment, reprogram immune cells, or navigate the blood–brain barrier illustrates the remarkable precision now within reach.
Despite this progress, the path to widespread clinical adoption requires addressing several key challenges. First, manufacturing, scalability, reproducibility and the associated regulatory hurdles remain a primary hurdle, particularly for complex biologic carriers like EVs. This challenge of scalability and reproducibility varies significantly across nanoparticle classes. For instance, lipid-based systems (e.g., LNPs) are the most mature, with scalable microfluidic manufacturing and well-defined release tests (e.g., particle size via DLS, encapsulation efficiency) that enabled the success of COVID-19 vaccines. In contrast, biogenic carriers like EVs present the highest barrier due to inherent variability from cellular sources, demanding distinct batch-to-batch metrics focused on particle concentration (via nanoparticle tracking analysis, NTA) and specific protein markers (e.g., CD9/CD63) to ensure consistency—a major challenge the field is actively working to overcome for potential MSC-derived exosome therapeutics. Establishing robust, GMP-compliant production methods is only the initial step. For regulatory approval and commercial viability, a comprehensive characterization, manufacturing, and controls (CMC) strategy is paramount. This involves defining stringent, quantifiable release criteria for identity, purity, and potency [29], as demanded by agencies like the food and drug administration(FDA) and european medicines agency (EMA). Identity must be rigorously confirmed using a multi-faceted approach, integrating qualitative analysis of specific protein markers (e.g., tetraspanins like CD9/CD63) and the demonstrated absence of co-isolating contaminants (e.g., lipoproteins, cytosolic proteins), alongside quantitative measurements of vesicle size, concentration, and morphology. Purity requires setting and meeting strict thresholds for process- and product-related impurities, including host cell DNA/proteins and endotoxins, often using metrics like the particle-to-protein ratio to ensure product integrity. Finally, a validated, function-based potency assay that reflects the therapeutic’s mechanism of action—such as measuring dose-dependent mRNA knockdown for an siRNA payload—is non-negotiable for ensuring batch-to-batch consistency and predicting clinical efficacy. Second, optimizing the therapeutic window continues to be a delicate balance. Enhancing on-target delivery while minimizing off-target effects and potential immunogenicity is an ongoing pursuit that demands further innovation in both carrier design and RNA sequence chemistry. A prime example of this challenge lies at the subcellular level: ensuring efficient endosomal escape. A crucial determinant of therapeutic success for RNAi nanoparticles is their ability to facilitate endosomal escape, releasing the siRNA payload into the cytoplasm to engage the RNA-induced silencing complex (RISC). Several distinct strategies have been developed to overcome this barrier, each with a growing body of in vivo evidence. The most clinically advanced strategy involves ionizable lipids, which are central to the success of FDA-approved lipid nanoparticles (LNPs) like Patisiran (Onpattro®) [137,147]. These lipids are engineered with an apparent pKa that keeps them neutral at physiological pH (≈7.4), but upon endosomal acidification (pH ≈ 5.0–6.5), they become protonated, disrupting the membrane and leading to payload release. Another widely explored chemical strategy is the “proton sponge” effect, commonly associated with cationic polymers like polyethyleneimine (PEI), which uses osmotic pressure to rupture the endosome [13,148]. More biomimetic approaches include using fusogenic lipids or peptides that merge with the endosomal membrane [149], or even rationally designed “mini-proteins” that unfold at low pH to expose membrane-disrupting domains [12]. Finally, physical triggers like light or ultrasound offer external control over release, with preclinical studies demonstrating significant enhancement of targeted gene silencing in vivo [150,151]. While effective, these strategies must be carefully refined to maximize cytoplasmic delivery without inducing cytotoxicity, illustrating the fine line between efficacy and safety. Third, the long-term safety profile of these nanoparticles, including their accumulation and degradation in vivo, must be rigorously characterized to ensure patient safety over chronic treatment courses.
Looking forward, the future of nanoparticle-mediated RNAi delivery is marked by several promising research trajectories. We anticipate the rise of in vivo-generated therapeutics, where strategies like the hepatocyte-reprogramming system create a “bioreactor” within the patient to produce a sustained supply of therapeutic EVs. The integration of artificial intelligence and machine learning will likely accelerate the discovery of novel nanoparticle formulations and predict their in vivo behavior, offering a potential pathway for high-throughput screening of carrier candidates. Finally, the synergy between diagnostic and therapeutic (theranostic) platforms, where nanoparticles not only deliver a payload but also provide real-time feedback on target engagement and therapeutic response, will pave the way for more precise and personalized RNAi medicine. By continuing to bridge the gap between materials science, molecular biology, and clinical medicine, nanoparticle-based RNAi delivery is poised to unlock a new class of therapeutics with the potential to address humanity’s most challenging diseases.
Author Contributions
Writing—original draft preparation, T.R.; writing—review and editing, C.Z.; visualization, T.R. and C.Z.; supervision, C.Z., L.M. and P.F.; project administration, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the National Natural Science Foundation of China (Grant No. 82200764), Sichuan Science and Technology Program (Grant No. 2025ZNSFSC1602), Postdoctoral Research Fund from West China Hospital of Sichuan University (Grant No. 2023HXB092), Young Elite Scientists Sponsorship Program by CAST (Grant No. 2022QNRC001) and 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (Grant No. ZYGD23015).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RNAi | RNA interference |
| miRNA | microRNA |
| siRNA | small interfering RNA |
| MTD | maximum tolerated dose |
| PK/PD | pharmacokinetics/pharmacodynamics |
| GMP | good manufacturing practice |
| RISC | RNA-induced silencing complex |
| RES | reticuloendothelial system |
| CARPA | complement activation-related pseudoallergy |
| PRRs | pattern recognition receptors |
| RIG-I | retinoic acid-inducible gene I |
| MDA5 | melanoma differentiation-associated gene 5 |
| NADs | nucleic acid drugs |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| LNPs | lipid nanoparticles |
| BBB | blood–brain barrier |
| NSCLC | non-small cell lung cancer |
| ROS | reactive oxygen species |
| RNPs | ribonucleoprotein complexes |
| BRBP1 | brown rice bran powder1 |
| EMT | epithelial-mesenchymal transition |
| PROTACs | proteolysis targeting chimeras |
| PNPs | polymeric nanoparticles |
| ECM | extracellular matrix |
| HCC | hepatocellular carcinoma |
| TNBC | triple-negative breast cancer |
| PEG | polyethylene glycol |
| VEGF | vascular endothelial growth factor |
| PEAL | mPEG-PLGA-PLL |
| PDAC | pancreatic ductal adenocarcinoma |
| TME | tumor microenvironment |
| PEI/PPI | polyethylenimine/polypropylenimine |
| TAMs | tumor-associated macrophages |
| EGFR | epidermal growth factor receptor |
| cPLA2 | cytoplasmic phospholipase A2 |
| PDX | patient-derived xenograft |
| PTRF | polymerase 1 and transcript release factor |
| PPM1D | mutation of p53-induced protein phosphatase 1 |
| DIPG | diffuse intrinsic pontine gliomas |
| DARS-AS1 | delivery of aspartyl-tRNA synthetase-antisense RNA 1 |
| MAPK/ERK | mitogen-activated protein kinase/extracellular signal-regulated kinase |
| HCR | hybridization chain reaction |
| TPP | triphenylphosphine |
| AMPK | 5′-adenosine monophosphate-activated protein kinase |
| MSCs | mesenchymal stromal cells |
| GSC | glioblastoma stem cell |
| MACC1 | metastasis-associated in colon cancer 1 |
| CRC | colorectal cancer |
| EpCAM | epithelial cell adhesion molecule |
| RAGE | receptor for advanced glycation end products |
| EGCG | Co-encapsulating epigallocatechin-3-gallate |
| LVEF | left ventricular ejection fraction |
| BMSC | bone marrow mesenchymal stem cell |
| hAMSC | human Amniotic Mesenchymal Stem Cell |
| VSMC | vascular smooth muscle cell |
| COPD | chronic obstructive pulmonary disease |
| DLTs | dose-limiting toxicities |
| RP2D | recommended phase 2 dose |
| CTCL/MF | cutaneous T-cell lymphoma/mycosis fungoides |
| CLL | chronic lymphocytic leukemia |
| DLBCL | diffuse large B-cell lymphoma |
| ATLL | adult T-cell leukemia/lymphoma |
| NTA | nanoparticle tracking analysis |
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Ambros, V. The Functions of Animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a Bidentate Ribonuclease in the Initiation Step of RNA Interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Zhang, Z.; Conniot, J.; Amorim, J.; Jin, Y.; Prasad, R.; Yan, X.; Fan, K.; Conde, J. Nucleic Acid-Based Therapy for Brain Cancer: Challenges and Strategies. J. Control. Release 2022, 350, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for Imaging and Therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-Dependent Stimulation of the Mammalian Innate Immune Response by Synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Ayadi, L.; Galvanin, A.; Pichot, F.; Marchand, V.; Motorin, Y. RNA Ribose Methylation (2′-O-Methylation): Occurrence, Biosynthesis and Biological Functions. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2019, 1862, 253–269. [Google Scholar] [CrossRef]
- Giudice, J.; Brauer, D.D.; Zoltek, M.; Vázquez-Maldonado, A.L.; Dadina, N.; Kelly, M.; Schepartz, A. The Biophysical Requirements That Govern the Efficient Endosomal Escape of Designed Mini-Proteins. Nat. Chem. 2025, 17, 1227–1235. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal Escape Pathways for Delivery of Biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Vermeulen, L.M.P.; Brans, T.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Methodologies to Investigate Intracellular Barriers for Nucleic Acid Delivery in Non-Viral Gene Therapy. Nano Today 2018, 21, 74–90. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660-IN10. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Abla, K.K.; Zahwi, M.; El-Lakany, S.A.; Karam, M. Advances in siRNA-Loaded Liposomes for Lung Cancer: Challenges and Future Perspectives. Int. J. Biol. Macromol. 2025, 319, 145461. [Google Scholar] [CrossRef]
- Purohit, M.P.; Yu, B.J.; Roy, K.S.; Xiang, Y.; Ewbank, S.N.; Azadian, M.M.; Hart, A.R.; Muwanga, G.P.B.; Martinez, P.J.; Wang, J.B.; et al. Acoustically Activatable Liposomes as a Translational Nanotechnology for Site-Targeted Drug Delivery and Noninvasive Neuromodulation. Nat. Nanotechnol. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Wang, J.; Wang, G.; Su, Z.; Li, Y.; Chen, Y.; Meng, J.; Yao, Y.; Wang, L.; Wilkens, S.; et al. Chimeric Nanobody-Decorated Liposomes by Self-Assembly. Nat. Nanotechnol. 2024, 19, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Wang, E.; Yang, H.; Zhao, T.; Wang, Q.; Zhang, Z.; Song, Y.; Liu, X. Reprogramming Mitochondrial Metabolism and Epigenetics of Macrophages via miR-10a Liposomes for Atherosclerosis Therapy. Nat. Commun. 2025, 16, 9117. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, H.; Sun, L.; Nurzat, Y.; Qin, H.; Zhao, Z.; Liu, D.; Zheng, S.; Wang, L.; Fu, Y.; et al. Neuron-Targeted ROS-Responsive Liposomes for Puerarin Delivery Remodel Ischemic Microenvironment via Microglial Modulation and Neurovascular Regeneration. J. Nanobiotechnol. 2025, 23, 677. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, S.; Li, S.; He, D.; Wang, Y.; Zhang, Q.; He, Z.; Li, M.; He, Q. ATP-Responsive Tumor Targeted Lipid Nanoparticle for Enhanced siRNA Delivery and Improved Treatment Efficacy in Melanoma. J. Control. Release 2025, 382, 113622. [Google Scholar] [CrossRef]
- Lin, Y.; Li, M.; Luo, Z.; Meng, Y.; Zong, Y.; Ren, H.; Yu, X.; Tan, X.; Liu, F.; Wei, T.; et al. Tissue-Specific mRNA Delivery and Prime Editing with Peptide-Ionizable Lipid Nanoparticles. Nat. Mater. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, Y.; Xu, Y.; Li, R.; Xu, X. Redox-Responsive Polymeric Nanoparticle for Nucleic Acid Delivery and Cancer Therapy: Progress, Opportunities, and Challenges. Macromol. Biosci. 2024, 24, e2300238. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Torres-Coll, A.; Olmo, L.; Garcia-Fernandez, C.; Guerra-Rebollo, M.; Borrós, S. Engineering Oncogene-Targeted Anisamide-Functionalized pBAE Nanoparticles as Efficient Lung Cancer Antisense Therapies. RSC Adv. 2023, 13, 29986–30001. [Google Scholar] [CrossRef]
- Gu, X.-R.; Tai, Y.-F.; Liu, Z.; Zhang, X.-Y.; Liu, K.; Zhou, L.-Y.; Yin, W.-J.; Deng, Y.-X.; Kong, D.-L.; Midgley, A.C.; et al. Layer-by-Layer Assembly of Renal-Targeted Polymeric Nanoparticles for Robust Arginase-2 Knockdown and Contrast-Induced Acute Kidney Injury Prevention. Adv. Healthc. Mater. 2024, 13, e2304675. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, C.; Xiao, T.; Jiang, B.; Xu, X.; Cui, S.; Shen, W.; Zheng, X.; Guo, X.; Chen, X.; et al. Ultrasound-Guided Functionalized Extracellular Vesicles for Visualized, Controlled, and Efficient Renal Delivery for Enhanced Kidney Repair. Adv. Healthc. Mater. 2025, 14, e2404719. [Google Scholar] [CrossRef]
- Peng, F.; Chen, X.; Wu, L.; He, J.; Li, Z.; Hong, Q.; Zhao, Q.; Qian, M.; Wang, X.; Shen, W.; et al. Nitric Oxide-Primed Engineered Extracellular Vesicles Restore Bioenergetics in Acute Kidney Injury via Mitochondrial Transfer. Theranostics 2025, 15, 5499–5517. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, Y.; Chen, X.; Midgley, A.C.; Wang, Z.; Zhu, D.; Wu, J.; Chen, P.; Wu, L.; Wang, X.; et al. Supramolecular Nanofibers Containing Arginine-Glycine-Aspartate (RGD) Peptides Boost Therapeutic Efficacy of Extracellular Vesicles in Kidney Repair. ACS Nano 2020, 14, 12133–12147. [Google Scholar] [CrossRef]
- Goyal, A.; Afzal, M.; Goyal, K.; Ganesan, S.; Kumari, M.; Sunitha, S.; Dash, A.; Saini, S.; Rana, M.; Gupta, G.; et al. MSC-Derived Extracellular Vesicles: Precision miRNA Delivery for Overcoming Cancer Therapy Resistance. Regen. Ther. 2025, 29, 303–318. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Spencer, W.; Gupta, R.C. Milk/Colostrum Exosomes: A Nanoplatform Advancing Delivery of Cancer Therapeutics. Cancer Lett. 2023, 561, 216141. [Google Scholar] [CrossRef]
- Veider, F.; Haddadzadegan, S.; Sanchez Armengol, E.; Laffleur, F.; Kali, G.; Bernkop-Schnürch, A. Inhibition of P-Glycoprotein-Mediated Efflux by Thiolated Cyclodextrins. Carbohydr. Polym. 2024, 327, 121648. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, Z.; Zhao, Y.; Fan, F.; Xiong, W.; Song, S.; Yin, Y.; Hu, J.; Yang, K.; Yang, L.; et al. Mononuclear Phagocyte System Blockade Using Extracellular Vesicles Modified with CD47 on Membrane Surface for Myocardial Infarction Reperfusion Injury Treatment. Biomaterials 2021, 275, 121000. [Google Scholar] [CrossRef]
- Fu, X.; Shi, Y.; Gu, Z.; Zang, H.; Li, L.; Wang, Q.; Wang, Y.; Zhao, X.; Wu, H.; Qiu, S.; et al. Immunotherapeutic Hydrogel for Co-Delivery of STAT3 siRNA Liposomes and Lidocaine Hydrochloride for Postoperative Comprehensive Management of NSCLC in a Single Application. Asian J. Pharm. Sci. 2024, 19, 100925. [Google Scholar] [CrossRef]
- Du, J.; Que, Z.; Aihaiti, A.; Zhai, M.; Zhang, Z.; Shao, Y.; Zhang, Y.; Miao, F.; Shen, Y.; Chen, X.; et al. Co-Delivery of SN38 and MEF2D-siRNA via tLyp-1-Modified Liposomes Reverses PD-L1 Expression Induced by STING Activation in Hepatocellular Carcinoma. Colloids Surf. B Biointerfaces 2025, 245, 114318. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Q.; Xu, F.; Yao, T. Enhanced Anti-Tumor Therapy for Hepatocellular Carcinoma via Sorafenib and KIAA1199-siRNA Co-Delivery Liposomes. Saudi Pharm. J. 2024, 32, 102153. [Google Scholar] [CrossRef]
- Wang, G.; Lu, H.; Pan, Y.; Qi, Y.; Huang, Y. Ultrasound-Sensitive Targeted Liposomes as a Gene Delivery System for the Synergistic Treatment of Hepatocellular Carcinoma. Small Weinh. Bergstr. Ger. 2024, 20, e2406182. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, J.; Li, J.; Li, H.; Yang, Y.; Yang, M.; Wang, Y.; Gong, W.; Li, Z.; Li, L.; et al. Macrophage Membrane- and cRGD-Functionalized Thermosensitive Liposomes Combined with CPP to Realize Precise siRNA Delivery into Tumor Cells. Mol. Ther. Nucleic Acids 2022, 27, 349–362. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, J.; Yu, D.; Liu, Y.; Song, D.; Tian, K.; Chen, H.; Ye, Q.; Wang, X.; Xu, T.; et al. Polymer-Locking Fusogenic Liposomes for Glioblastoma-Targeted siRNA Delivery and CRISPR-Cas Gene Editing. Nat. Nanotechnol. 2024, 19, 1869–1879. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, Y.-K.; Lee, H.; Choi, D.-H.; Rhee, K.-J.; Kim, H.S.; Seo, J.-B. Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models. Pharmaceutics 2025, 17, 509. [Google Scholar] [CrossRef]
- Li, D.; Liu, D.; Wang, Y.; Sun, Q.; Sun, R.; Zhang, J.; Hong, X.; Huo, R.; Zhang, S.; Cui, C. Multifunctional Liposomes Co-Encapsulating Epigallocatechin-3-Gallate (EGCG) and miRNA for Atherosclerosis Lesion Elimination. Nanoscale Adv. 2023, 6, 221–232. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Li, Y.; Li, X.; Yang, G.; Li, M.; Xie, Y.; Su, W.; Wu, J.; Jia, L.; et al. Cationic Liposomes Co-Deliver Chemotherapeutics and siRNA for the Treatment of Breast Cancer. Eur. J. Med. Chem. 2022, 233, 114198. [Google Scholar] [CrossRef]
- Flores-Colón, M.; Rivera-Serrano, M.; Peterson-Peguero, E.A.; Vivas-Rivera, P.E.; Valiyeva, F.; Vivas-Mejía, P.E. Synthesis and Biological Evaluation of Herceptin-Conjugated Liposomes Loaded with Lipocalin-2 siRNA for the Treatment of Inflammatory Breast Cancer. Pharmaceuticals 2025, 18, 1053. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Qian, K.; Yang, P.; Zhou, L.; Xu, M.; Sheng, D.; Wang, T.; Li, Y.; Yang, X.; et al. siRNA-Encapsulated Biomimetic Liposomes Effectively Inhibit Tumor Cells’ Hexosamine Biosynthesis Pathway for Attenuating Hyaluronan Barriers to Pancreatic Cancer Chemotherapy. ACS Nano 2025, 19, 7928–7947. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Y.; Wang, J.; Gu, M.; Wang, Y.; Zhang, X.; Zhang, Y.; Yu, W.; Liu, Y.; Yuan, W.-E.; et al. Co-Delivery of PROTAC and siRNA via Novel Liposomes for the Treatment of Malignant Tumors. J. Colloid Interface Sci. 2025, 678, 896–907. [Google Scholar] [CrossRef]
- Molinar, C.; Tannous, M.; Meloni, D.; Cavalli, R.; Scomparin, A. Current Status and Trends in Nucleic Acids for Cancer Therapy: A Focus on Polysaccharide-Based Nanomedicines. Macromol. Biosci. 2023, 23, e2300102. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Current Advancements in Self-Assembling Nanocarriers-Based siRNA Delivery for Cancer Therapy. Colloids Surf. B Biointerfaces 2023, 221, 113002. [Google Scholar] [CrossRef]
- Qiao, M.; Zeng, C.; Liu, C.; Lei, Z.; Liu, B.; Xie, H. The Advancement of siRNA-Based Nanomedicine for Tumor Therapy. Nanomedicine 2024, 19, 1841–1862. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, F.; Su, H.; Liu, X.; Ren, Q.; Huang, F.; Ye, W.; Zhao, M.; Zhao, Y.; Zhao, J.; et al. Construction of Degradable and Amphiphilic Triblock Polymer Carriers for Effective Delivery of siRNA. Macromol. Biosci. 2022, 22, e2200232. [Google Scholar] [CrossRef]
- Karlsson, J.; Tzeng, S.Y.; Hemmati, S.; Luly, K.M.; Choi, O.; Rui, Y.; Wilson, D.R.; Kozielski, K.L.; Quiñones-Hinojosa, A.; Green, J.J. Photocrosslinked Bioreducible Polymeric Nanoparticles for Enhanced Systemic siRNA Delivery as Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2009768. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Weiskirchen, S.; Grassi, C.; Scaggiante, B.; Grassi, M.; Tierno, D.; Biasin, A.; Truong, N.H.; Minh, T.D.; Cemazar, M.; et al. Recent Advances in Optimizing siRNA Delivery to Hepatocellular Carcinoma Cells. Expert Opin. Drug Deliv. 2025, 22, 729–745. [Google Scholar] [CrossRef]
- Rodponthukwaji, K.; Pingrajai, P.; Jantana, S.; Taya, S.; Duangchan, K.; Nguyen, K.T.; Srisawat, C.; Punnakitikashem, P. Epigallocatechin Gallate Potentiates the Anticancer Effect of AFP-siRNA-Loaded Polymeric Nanoparticles on Hepatocellular Carcinoma Cells. Nanomaterials 2023, 14, 47. [Google Scholar] [CrossRef]
- Punuch, K.; Wongwan, C.; Jantana, S.; Somboonyosdech, C.; Rodponthukwaji, K.; Kunwong, N.; Nguyen, K.T.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Srisawat, C.; et al. Study of siRNA Delivery via Polymeric Nanoparticles in Combination with Angiogenesis Inhibitor for The Treatment of AFP-Related Liver Cancer. Int. J. Mol. Sci. 2022, 23, 12666. [Google Scholar] [CrossRef]
- Sader, D.; Zlotver, I.; Moya, S.; Calabrese, G.C.; Sosnik, A. Doubly Self-Assembled Dermatan Sulfate/Chitosan Nanoparticles for Targeted siRNA Delivery in Cancer Therapy. J. Colloid Interface Sci. 2025, 680, 763–775. [Google Scholar] [CrossRef]
- Camorani, S.; Tortorella, S.; Agnello, L.; Spanu, C.; d’Argenio, A.; Nilo, R.; Zannetti, A.; Locatelli, E.; Fedele, M.; Comes Franchini, M.; et al. Aptamer-Functionalized Nanoparticles Mediate PD-L1 siRNA Delivery for Effective Gene Silencing in Triple-Negative Breast Cancer Cells. Pharmaceutics 2022, 14, 2225. [Google Scholar] [CrossRef]
- Jin, M.; Hou, Y.; Quan, X.; Chen, L.; Gao, Z.; Huang, W. Smart Polymeric Nanoparticles with pH-Responsive and PEG-Detachable Properties (II): Co-Delivery of Paclitaxel and VEGF siRNA for Synergistic Breast Cancer Therapy in Mice. Int. J. Nanomed. 2021, 16, 5479–5494. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, H.; Yu, J.; Wang, L.; Sun, Y.; Zhang, J.; Duan, Y. Asynchronous Blockade of PD-L1 and CD155 by Polymeric Nanoparticles Inhibits Triple-Negative Breast Cancer Progression and Metastasis. Biomaterials 2021, 275, 120988. [Google Scholar] [CrossRef]
- Casadidio, C.; Fens, M.H.A.M.; Fliervoet, L.A.L.; Censi, R.; Vermonden, T. Injectable Thermosensitive Hydrogel for Local and Controlled Delivery of siRNA-STAT3 Polyplexes to Treat Advanced-Stage Ovarian Cancer. J. Control. Release 2025, 384, 113890. [Google Scholar] [CrossRef] [PubMed]
- Rehman, U.; Parveen, N.; Sheikh, A.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Polymeric Nanoparticles-siRNA as an Emerging Nano-Polyplexes against Ovarian Cancer. Colloids Surf. B Biointerfaces 2022, 218, 112766. [Google Scholar] [CrossRef]
- Spadea, A.; Tirella, A.; Rios de la Rosa, J.M.; Lallana, E.; Mehibel, M.; Telfer, B.; Tirelli, N.; Lawrence, M.J.; Williams, K.J.; Stratford, I.J.; et al. Targeting Hypoxia-Inducible Factor-1α in Pancreatic Cancer: siRNA Delivery Using Hyaluronic Acid-Displaying Nanoparticles. Pharmaceutics 2024, 16, 1286. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Ang, H.L.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Delfi, M.; Khan, H.; Ashrafizadeh, M.; et al. Pre-Clinical and Clinical Applications of Small Interfering RNAs (siRNA) and Co-Delivery Systems for Pancreatic Cancer Therapy. Cells 2021, 10, 3348. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Luan, Y.; Tang, S.; Abazarikia, A.; Dong, R.; Caffrey, T.C.; Hollingsworth, M.A.; Oupicky, D.; Kim, S.-Y. Uncovering Tumor-Promoting Roles of Activin A in Pancreatic Ductal Adenocarcinoma. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2207010. [Google Scholar] [CrossRef]
- Arista-Romero, M.; Cascante, A.; Fornaguera, C.; Borrós, S. Role of Survivin in Bladder Cancer: Issues to Be Overcome When Designing an Efficient Dual Nano-Therapy. Pharmaceutics 2021, 13, 1959. [Google Scholar] [CrossRef]
- Walther, M.; Jenke, R.; Aigner, A.; Ewe, A. Efficient Polymeric Nanoparticles for RNAi in Macrophage Reveal Complex Effects on Polarization Markers upon Knockdown of STAT3/STAT6. Eur. J. Pharm. Biopharm. 2024, 197, 114232. [Google Scholar] [CrossRef]
- Lin, S.; Jing, H.; Du, X.; Yang, X.; Wang, J. Optimization of Lipid Assisted Polymeric Nanoparticles for siRNA Delivery and Cancer Immunotherapy. Biomater. Sci. 2024, 12, 2057–2066. [Google Scholar] [CrossRef]
- Huang, H.; Yi, X.; Wei, Q.; Li, M.; Cai, X.; Lv, Y.; Weng, L.; Mao, Y.; Fan, W.; Zhao, M.; et al. Edible and Cation-Free Kiwi Fruit Derived Vesicles Mediated EGFR-Targeted siRNA Delivery to Inhibit Multidrug Resistant Lung Cancer. J. Nanobiotechnol. 2023, 21, 41. [Google Scholar] [CrossRef]
- Asfiya, R.; Paramanantham, A.; Premnath, R.; McCully, G.; Yousuf, F.; Goetz, G.; Srivastava, A. Engineered Extracellular Vesicles for Tumor-Targeted Delivery of Therapeutic siRNA for Lung Cancer Therapy. ACS Biomater. Sci. Eng. 2025, 11, 4206–4218. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood Exosomes-Based Targeted Delivery of cPLA2 siRNA and Metformin to Modulate Glioblastoma Energy Metabolism for Tailoring Personalized Therapy. Neuro-Oncology 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2200353. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Taghavi-Farahabadi, M.; Hashemi, S.M.; Ghanbarian, H.; Noorbakhsh, F.; Mousavizadeh, K.; Mojtabavi, N.; Rezaei, N. Dual Checkpoint Inhibition in M2 Macrophages via Anti-PD-L1 and siRNA-Loaded M1-Exosomes: Enhancing Tumor Immunity through RNA-Targeting Strategies. Eur. J. Pharmacol. 2025, 990, 177271. [Google Scholar] [CrossRef]
- Miao, C.; Huang, G.; Wen, Y.; He, Y.; Bai, P.; Yan, W.; Li, L.; Li, C.; Wei, A.; Wen, J. M1 Macrophage-Derived Extracellular Vesicles Loaded with CX3CR1 siRNA for the Treatment of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2025, 17, 40226–40236. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Oi, Y.; Oride, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Patient-Derived Exosomes as siRNA Carriers in Ovarian Cancer Treatment. Cancers 2024, 16, 1482. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Yu, T.; Liu, J.; Chai, X.; Yin, D.; Zhang, C. CL4-Modified Exosomes Deliver lncRNA DARS-AS1 siRNA to Suppress Triple-Negative Breast Cancer Progression and Attenuate Doxorubicin Resistance by Inhibiting Autophagy. Int. J. Biol. Macromol. 2023, 250, 126147. [Google Scholar] [CrossRef]
- Ferreira, D.; Santos-Pereira, C.; Costa, M.; Afonso, J.; Yang, S.; Hensel, J.; McAndrews, K.M.; Longatto-Filho, A.; Fernandes, R.; Melo, J.B.; et al. Exosomes Modified with Anti-MEK1 siRNA Lead to an Effective Silencing of Triple Negative Breast Cancer Cells. Biomater. Adv. 2023, 154, 213643. [Google Scholar] [CrossRef]
- Cipu, R.-I.; Stănişteanu, M.-L.; Andrei, M.-A.; Banciu, D.D.; Banciu, A. Theoretical Model for In Vivo Induction of Chemotherapy Sensitization Using miRNA Packaged in Distinct Layered Liposomes. J. Funct. Biomater. 2024, 15, 298. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Sun, S.; Gao, Y.; Yang, Z.; Dong, H.; Zhang, X. Mitochondria-Targeted Polymeric Liposomes for Pre-miRNA Imaging and Gene Therapy. Anal. Chem. 2025, 97, 17251–17260. [Google Scholar] [CrossRef]
- Dogheim, G.; Chinnam, S.; Amralla, M.T. Lipid Nanoparticles as a Platform for miRNA and siRNA Delivery in Hepatocellular Carcinoma. Curr. Drug Deliv. 2024, 22, 837–861. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Zhu, L.; You, L.; Tong, H.; Meng, H.; Sheng, J.; Jin, J. Harnessing Nanotechnology: Emerging Strategies for Multiple Myeloma Therapy. Biomolecules 2024, 14, 83. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, M.; Jiao, M.; Yan, C.; Xu, S.; Du, Q.; Morsch, M.; Yin, J.; Shi, B. Polymeric Nanoparticle Mediated Inhibition of miR-21 with Enhanced miR-124 Expression for Combinatorial Glioblastoma Therapy. Biomaterials 2021, 276, 121036. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Fang, J.-Y.; Hsiao, C.-Y.; Lee, C.-W.; Alshetaili, A.; Lin, Z.-C. Dual Cell-Penetrating Peptide-Conjugated Polymeric Nanocarriers for miRNA-205-5p Delivery in Gene Therapy of Cutaneous Squamous Cell Carcinoma. Acta Biomater. 2025, 196, 332–349. [Google Scholar] [CrossRef]
- Borchardt, H.; Ewe, A.; Morawski, M.; Weirauch, U.; Aigner, A. miR24-3p Activity after Delivery into Pancreatic Carcinoma Cell Lines Exerts Profound Tumor-Inhibitory Effects through Distinct Pathways of Apoptosis and Autophagy Induction. Cancer Lett. 2021, 503, 174–184. [Google Scholar] [CrossRef]
- Elmahboub, Y.; Albash, R.; Magdy William, M.; Rayan, A.H.; Hamed, N.O.; Ousman, M.S.; Raslan, N.A.; Mosallam, S. Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study. Molecules 2024, 29, 1614. [Google Scholar] [CrossRef]
- de Rezende, C.P.; de Lima Alves, D.; de Almeida Chuffa, L.G.; Pires de Campos Zuccari, D.A. Extracellular Vesicles and miRNA-Based Therapies in Triple-Negative Breast Cancer: Advances and Clinical Perspectives. Extracell. Vesicles Circ. Nucleic Acids 2025, 6, 54–71. [Google Scholar] [CrossRef]
- Chen, M.; Choi, H.K.; Goldston, L.L.; Hou, Y.; Jiang, C.; Lee, K.-B. Advanced Cancer Liquid Biopsy Platform for miRNA Detection in Extracellular Vesicles Using CRISPR/Cas13a and Gold Nanoarrays. ACS Nano 2025, 19, 31438–31456. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, W.; Han, X.; Xu, M.; Wang, Z.; Shi, M.; Shi, Y.; Yu, Y. Modified Bone Marrow Mesenchymal Stem Cells Derived Exosomes Loaded with MiRNA Ameliorates Non-Small Cell Lung Cancer. J. Cell. Mol. Med. 2024, 28, e70115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Yang, Y.; Wang, W.; Gu, A.; Han, B.; Shurin, G.V.; Zhong, R.; et al. Schwann Cell-Derived Exosomes Promote Lung Cancer Progression via miRNA-21-5p. Glia 2024, 72, 692–707. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.F.; Hossain, A.; Momin, E.N.; Hasan, I.; Singh, S.; Adachi, S.; Gumin, J.; Ledbetter, D.; Yang, J.; Long, L.; et al. Tumor-Specific Polycistronic miRNA Delivered by Engineered Exosomes for the Treatment of Glioblastoma. Neuro-Oncology 2024, 26, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Shin, S.; Kang, S.-M.; Jung, I.; Ryu, S.; Noh, S.; Choi, S.-J.; Jeong, J.; Lee, B.Y.; Kim, K.-S.; et al. Reprogramming of T Cell-Derived Small Extracellular Vesicles Using IL2 Surface Engineering Induces Potent Anti-Cancer Effects through miRNA Delivery. J. Extracell. Vesicles 2022, 11, e12287. [Google Scholar] [CrossRef]
- Choi, B.-J.; Lee, D.; Park, J.H.; Hong, T.H.; Kim, O.-H.; Lee, S.C.; Kim, K.-H.; Choi, H.J.; Kim, S.-J. Innovative Therapeutic Delivery of Metastasis-Associated in Colon Cancer 1-Suppressing miRNA Using High Transmembrane 4 L6 Family Member 5-Targeting Exosomes in Colorectal Cancer Mouse Models. Int. J. Mol. Sci. 2024, 25, 9232. [Google Scholar] [CrossRef]
- Liu, F.; Ai, F.; Tang, A.; Yang, Z.; Li, Z.; Liu, S. Macrophage-Derived Exosomes Promoted the Development and Stemness of Inflammatory Bowel Disease-Related Colorectal Cancer via Nuclear Paraspeckle Assembly Transcript 1-Mediated miRNA-34a-5p/Phosphoprotein Enriched in Astrocytes 15 Axis. Inflamm. Bowel Dis. 2025, 31, 524–538. [Google Scholar] [CrossRef]
- Yu, L.; Sui, B.; Fan, W.; Lei, L.; Zhou, L.; Yang, L.; Diao, Y.; Zhang, Y.; Li, Z.; Liu, J.; et al. Exosomes Derived from Osteogenic Tumor Activate Osteoclast Differentiation and Concurrently Inhibit Osteogenesis by Transferring COL1A1-Targeting miRNA-92a-1-5p. J. Extracell. Vesicles 2021, 10, e12056. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.-H.; Chiou, Y.-E.; Zeng, W.-Z.; Chen, M.-H.; Pang, S.-T. Hsa-miRNA-16-5p Of Urinary Exosomes A Reliable Biosignature Effective For Prostate Cancer Screening. Int. J. Nanomed. 2025, 20, 9291–9300. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fan, G.; Wang, Q.; Zhu, Y.; Zhu, H.; Chang, J.; Wang, Z.; Zhan, S.; Hua, X.; She, D.; et al. In Vivo Self-Assembly and Delivery of VEGFR2 siRNA-Encapsulated Small Extracellular Vesicles for Lung Metastatic Osteosarcoma Therapy. Cell Death Dis. 2023, 14, 626. [Google Scholar] [CrossRef]
- Du, J.; Shao, Y.; Hu, Y.; Chen, Y.; Cang, J.; Chen, X.; Pei, W.; Miao, F.; Shen, Y.; Muddassir, M.; et al. Multifunctional Liposomes Enable Active Targeting and Twinfilin 1 Silencing to Reverse Paclitaxel Resistance in Brain Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 23396–23409. [Google Scholar] [CrossRef]
- Sánchez-Meza, L.V.; Bello-Rios, C.; Eloy, J.O.; Gómez-Gómez, Y.; Leyva-Vázquez, M.A.; Petrilli, R.; Bernad-Bernad, M.J.; Lagunas-Martínez, A.; Medina, L.A.; Serrano-Bello, J.; et al. Cationic Liposomes Carrying HPV16 E6-siRNA Inhibit the Proliferation, Migration, and Invasion of Cervical Cancer Cells. Pharmaceutics 2024, 16, 880. [Google Scholar] [CrossRef]
- Tang, M.; Sakasai, S.; Onishi, H.; Kawano, K.; Hattori, Y. Effect of PEG Anchor in PEGylation of Folate-Modified Cationic Liposomes with PEG-Derivatives on Systemic siRNA Delivery into the Tumor. J. Drug Target. 2023, 31, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Shabana, A.M.; Xu, B.; Schneiderman, Z.; Ma, J.; Chen, C.C.; Kokkoli, E. Targeted Liposomes Encapsulating miR-603 Complexes Enhance Radiation Sensitivity of Patient-Derived Glioblastoma Stem-Like Cells. Pharmaceutics 2021, 13, 1115. [Google Scholar] [CrossRef]
- Du, H.; Ma, Y.; Wang, X.; Zhang, J.; Zhu, L.; Guan, G.; Pan, S.; Zhang, Y.; Wang, J.; Liu, Z. Therapeutic Suppression of Atherosclerotic Burden and Vulnerability via Dll4 Inhibition in Plaque Macrophages Using Dual-Targeted Liposomes. ACS Appl. Bio Mater. 2024, 7, 7219–7232. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Bai, X.; Yang, X.; Wang, L.; Lu, Y.; Zhu, L.; Zhao, Y.; Cheng, W.; Shu, M.; Mei, Q.; et al. VCAM-1-Binding Peptide Targeted Cationic Liposomes Containing NLRP3 siRNA to Modulate LDL Transcytosis as a Novel Therapy for Experimental Atherosclerosis. Metabolism 2022, 135, 155274. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Yang, H.; Zhao, M.; Liu, Y.; Zhao, R.; Li, Z.; Sun, M. PEG-Modified Nano Liposomes Co-Deliver Apigenin and RAGE-siRNA to Protect Myocardial Ischemia Injury. Int. J. Pharm. 2024, 649, 123673. [Google Scholar] [CrossRef]
- Kim, H.; Mun, D.; Kang, J.-Y.; Lee, S.-H.; Yun, N.; Joung, B. Improved Cardiac-Specific Delivery of RAGE siRNA Within Small Extracellular Vesicles Engineered to Express Intense Cardiac Targeting Peptide Attenuates Myocarditis. Mol. Ther. Nucleic Acids 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Mun, D.; Park, M.; Yoo, G.; Kim, H.; Yun, N.; Joung, B. Injured Cardiac Tissue-Targeted Delivery of TGFβ1 siRNA by FAP Aptamer-Functionalized Extracellular Vesicles Promotes Cardiac Repair. Int. J. Nanomed. 2025, 20, 2575–2592. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, Y.; Tan, X.; Zhang, G.; Xu, A.; Fan, H.; Yu, F.; Qin, Z.; Song, Y.; Jiang, Y.; et al. Targeted Delivery of Exosome-Derived miRNA-185-5p Inhibitor via Liposomes Alleviates Apoptosis and Cuproptosis in Dilated Cardiomyopathy. Int. J. Nanomed. 2025, 20, 9407–9425. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, S.; Patravale, V.; Weaver, E.; Lamprou, D.A.; Patravale, T. Comprehensive Review on Novel Targets and Emerging Therapeutic Modalities for Pulmonary Arterial Hypertension. Int. J. Pharm. 2022, 621, 121792. [Google Scholar] [CrossRef]
- Ma, C.; Yang, Z.; Wang, J.; She, H.; Tan, L.; Ye, Q.; Wang, F.; Feng, X.; Mo, X.; Liu, K.; et al. Exosomes miRNA-499a-5p Targeted CD38 to Alleviate Anthraquinone Induced Cardiotoxicity: Experimental Research. Int. J. Surg. 2024, 110, 1992–2006. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.; Shi, P.; Gao, Y.; Pang, X. Exosomes Derived from Human Amniotic Mesenchymal Stem Cells Promotes Angiogenesis in hUVECs by Delivering Novel miRNA N-194. Mol. Med. Camb. Mass 2025, 31, 173. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.-Y.; Lv, X.-R.; Gu, H.-B.; Li, H.; Shan, B.-S. Involvement of miRNA-204 Carried by the Exosomes of Macrophages in the AT2 Receptor-Mediated Improvement of Vascular Calcification. Cell. Mol. Life Sci. 2025, 82, 165. [Google Scholar] [CrossRef]
- Park, H.-J.; Hoffman, J.R.; Brown, M.E.; Bheri, S.; Brazhkina, O.; Son, Y.H.; Davis, M.E. Knockdown of Deleterious miRNA in Progenitor Cell-Derived Small Extracellular Vesicles Enhances Tissue Repair in Myocardial Infarction. Sci. Adv. 2023, 9, eabo4616. [Google Scholar] [CrossRef]
- Guo, B.; Zhuang, T.-T.; Li, C.-C.; Li, F.; Shan, S.-K.; Zheng, M.-H.; Xu, Q.-S.; Wang, Y.; Lei, L.-M.; Tang, K.-X.; et al. MiRNA-132/212 Encapsulated by Adipose Tissue-Derived Exosomes Worsen Atherosclerosis Progression. Cardiovasc. Diabetol. 2024, 23, 331. [Google Scholar] [CrossRef]
- Ronan, G.; Bahcecioglu, G.; Yang, J.; Zorlutuna, P. Cardiac Tissue-Resident Vesicles Differentially Modulate Anti-Fibrotic Phenotype by Age and Sex through Synergistic miRNA Effects. Biomaterials 2024, 311, 122671. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.-H.; Liu, C.; Wang, S.-Q.; Ma, J.; Li, X.-Q.; Ren, M.; Yang, T.-T.; Liu, G.-Z. lncRNA, miRNA, and mRNA of Plasma and Tumor-Derived Exosomes of Cardiac Myxoma-Related Ischaemic Stroke. Sci. Data 2025, 12, 91. [Google Scholar] [CrossRef]
- Mirna, M.; Paar, V.; Topf, A.; Kraus, T.; Sotlar, K.; Aigner, A.; Ewe, A.; Watzinger, S.; Podesser, B.K.; Hackl, M.; et al. A New Player in the Game: Treatment with antagomiR-21a-5p Significantly Attenuates Histological and Echocardiographic Effects of Experimental Autoimmune Myocarditis. Cardiovasc. Res. 2022, 118, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, Z.; Wang, Y.; Xiong, H.; Sun, W.; Zhou, S.; Liu, Y.; Ni, C. Targeted Delivery of ZNF416 siRNA-Loaded Liposomes Attenuates Experimental Pulmonary Fibrosis. J. Transl. Med. 2022, 20, 523. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Zhou, Q.; Miao, K.; Zhang, L.; Wu, G.; Yu, J.; Xu, Y.; Xiong, W.; Li, Y.; Wang, Y. Suppressing Sart1 to Modulate Macrophage Polarization by siRNA-Loaded Liposomes: A Promising Therapeutic Strategy for Pulmonary Fibrosis. Theranostics 2021, 11, 1192–1206. [Google Scholar] [CrossRef]
- Liu, S.; Popowski, K.D.; Eckhardt, C.M.; Zhang, W.; Li, J.; Jing, Y.; Silkstone, D.; Belcher, E.; Cislo, M.; Hu, S.; et al. Inhalable Hsa-miR-30a-3p Liposomes Attenuate Pulmonary Fibrosis. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2025, 12, e2405434. [Google Scholar] [CrossRef]
- Khanna, V.; Singh, K. MicroRNAs as Promising Drug Delivery Target to Ameliorate Chronic Obstructive Pulmonary Disease Using Nano-Carriers: A Comprehensive Review. Mol. Cell. Biochem. 2025, 480, 1431–1448. [Google Scholar] [CrossRef]
- Wang, C.; Mao, Z.; Gomchok, D.; Li, X.; Liu, H.; Shao, J.; Cao, H.; Xue, G.; Lv, L.; Duan, J.; et al. Small Extracellular Vesicles Derived from miRNA-486 Overexpressed Dental Pulp Stem Cells Mitigate High Altitude Pulmonary Edema through PTEN/PI3K/AKT/eNOS Pathway. Heliyon 2025, 11, e41960. [Google Scholar] [CrossRef]
- Ma, C.; Liu, K.; Wang, F.; Fei, X.; Niu, C.; Li, T.; Liu, L. Neutrophil Membrane-Engineered Panax Ginseng Root-Derived Exosomes Loaded miRNA 182-5p Targets NOX4/Drp-1/NLRP3 Signal Pathway to Alleviate Acute Lung Injury in Sepsis: Experimental Studies. Int. J. Surg. Lond. Engl. 2024, 110, 72–86. [Google Scholar] [CrossRef]
- Lu, H.; Liu, X.; Zhang, M.; Bera, H.; Xu, W.; Jiang, H.; Zhao, X.; Wu, L.; Cun, D.; Yang, M. Pulmonary Fibroblast-Specific Delivery of siRNA Exploiting Exosomes-Based Nanoscaffolds for IPF Treatment. Asian J. Pharm. Sci. 2024, 19, 100929. [Google Scholar] [CrossRef]
- Halib, N.; Pavan, N.; Trombetta, C.; Dapas, B.; Farra, R.; Scaggiante, B.; Grassi, M.; Grassi, G. An Overview of siRNA Delivery Strategies for Urological Cancers. Pharmaceutics 2022, 14, 718. [Google Scholar] [CrossRef]
- Zapała, B.; Kamińska, A.; Piwowar, M.; Paziewska, A.; Gala-Błądzińska, A.; Stępień, E.Ł. miRNA Signature of Urine Extracellular Vesicles Shows the Involvement of Inflammatory and Apoptotic Processes in Diabetic Chronic Kidney Disease. Pharm. Res. 2023, 40, 817–832. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, L.; Hong, S.; Wang, Q.; Zhang, J.; Wang, S. The Matrix of Exosomes: Decoding the Role of miRNA in Macrophage Polarization and Oxidative Stress. Int. Immunopharmacol. 2025, 159, 114942. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in Humans from Systemically Administered siRNA via Targeted Nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Mitra, R.; McArthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123. [Google Scholar] [CrossRef]
- El Dika, I.; Lim, H.Y.; Yong, W.P.; Lin, C.-C.; Yoon, J.-H.; Modiano, M.; Freilich, B.; Choi, H.J.; Chao, T.-Y.; Kelley, R.K.; et al. An Open-Label, Multicenter, Phase I, Dose Escalation Study with Phase II Expansion Cohort to Determine the Safety, Pharmacokinetics, and Preliminary Antitumor Activity of Intravenous TKM-080301 in Subjects with Advanced Hepatocellular Carcinoma. Oncologist 2019, 24, 747-e218. [Google Scholar] [CrossRef]
- Sall, I.M.; Flaviu, T.A. Plant and Mammalian-Derived Extracellular Vesicles: A New Therapeutic Approach for the Future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef]
- Wolf, D.; Baier, G. IFNγ Helps CBLB-Deficient CD8+ T Cells to Put up Resistance to Tregs. Cancer Immunol. Res. 2022, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, Y.-F.; Yang, C.-F.; Ho, H.-J.; Yang, J.F.; Chou, Y.-L.; Lin, C.-W.; Yang, P.-C. Pharmacokinetics and Safety Profile of SNS812, a First in Human Fully Modified siRNA Targeting Wide-Spectrum SARS-CoV-2, in Healthy Subjects. Clin. Transl. Sci. 2025, 18, e70202. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of microRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Ganguly, K.; Kishore, U.; Madan, T. Interplay between C-Type Lectin Receptors and microRNAs in Cellular Homeostasis and Immune Response. FEBS J. 2021, 288, 4210–4229. [Google Scholar] [CrossRef]
- Valipour, A.; Jäger, M.; Wu, P.; Schmitt, J.; Bunch, C.; Weberschock, T. Interventions for Mycosis Fungoides. Cochrane Database Syst. Rev. 2020, 7, CD008946. [Google Scholar] [CrossRef]
- Lucibello, G.; Mograbi, B.; Milano, G.; Hofman, P.; Brest, P. PD-L1 Regulation Revisited: Impact on Immunotherapeutic Strategies. Trends Mol. Med. 2021, 27, 868–881. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal miR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Qin, Z.-X.; Zuo, L.; Zeng, Z.; Ma, R.; Xie, W.; Zhu, X.; Zhou, X. GalNac-siRNA Conjugate Delivery Technology Promotes the Treatment of Typical Chronic Liver Diseases. Expert Opin. Drug Deliv. 2025, 22, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A. Oligonucleotide Therapeutics—A New Class of Cholesterol-Lowering Drugs. N. Engl. J. Med. 2017, 376, 4–7. [Google Scholar] [CrossRef]
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.-C.; Sekijima, Y.; et al. Efficacy and Safety of Vutrisiran for Patients with Hereditary Transthyretin-Mediated Amyloidosis with Polyneuropathy: A Randomized Clinical Trial. Amyloid 2023, 30, 18–26. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226. [Google Scholar] [CrossRef]
- Baum, M.A.; Langman, C.; Cochat, P.; Lieske, J.C.; Moochhala, S.H.; Hamamoto, S.; Satoh, H.; Mourani, C.; Ariceta, G.; Torres, A.; et al. PHYOX2: A Pivotal Randomized Study of Nedosiran in Primary Hyperoxaluria Type 1 or 2. Kidney Int. 2023, 103, 207–217. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Young, G.; Srivastava, A.; Kavakli, K.; Ross, C.; Sathar, J.; You, C.-W.; Tran, H.; Sun, J.; Wu, R.; Poloskey, S.; et al. Efficacy and Safety of Fitusiran Prophylaxis in People with Haemophilia A or Haemophilia B with Inhibitors (ATLAS-INH): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Lond. Engl. 2023, 401, 1427–1437. [Google Scholar] [CrossRef]
- Srivastava, A.; Rangarajan, S.; Kavakli, K.; Klamroth, R.; Kenet, G.; Khoo, L.; You, C.-W.; Xu, W.; Malan, N.; Frenzel, L.; et al. Fitusiran Prophylaxis in People with Severe Haemophilia A or Haemophilia B without Inhibitors (ATLAS-A/B): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Haematol. 2023, 10, e322–e332. [Google Scholar] [CrossRef]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in Vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Hafez, I.M.; Cullis, P.R. Roles of Lipid Polymorphism in Intracellular Delivery. Adv. Drug Deliv. Rev. 2001, 47, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Høgset, A.; Prasmickaite, L.; Selbo, P.K.; Hellum, M.; Engesaeter, B.Ø.; Bonsted, A.; Berg, K. Photochemical Internalisation in Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2004, 56, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Chen, X.; Zhu, J.; Kopechek, J.; Helfield, B.; Yu, F.; Cyriac, J.; Lavery, L.; Grandis, J.R.; Villanueva, F.S. Ultrasound Enhanced siRNA Delivery Using Cationic Liposome-Microbubble Complexes for the Treatment of Squamous Cell Carcinoma. Nanotheranostics 2024, 8, 285–297. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).