Abstract
Chronic and hard-to-heal wounds remain burdensome because microbial contamination, dysregulated inflammation, and fragile tissue regeneration slow closure, while passive dressings often injure new tissue during removal. This review synthesizes polymer- and lipid-based nanostructures through a barrier-resolved lens that links composition, architecture, and processing to performance in protease- and salt-rich exudate across topical and transdermal routes. Quantitative trends include effective diameters of approximately 50–300 nm, practical constraints of sterile filtration at 0.2 μm, and therapeutic windows that prioritize contamination control on the first day, support proliferation around day three, and sustain remodeling beyond one week. Mechanistic evidence indicates that interfacial charge and the protein corona govern residence and uptake, lipid bilayers enable dual loading, degradable polymer matrices provide depot-like behavior, and hybrid constructs temper the early burst while improving storage stability.
1. Introduction
Chronic wounds that are hard to heal put a lot of stress on the healthcare system and the economy because they cause a lot of problems with microbial load, inflammation that is not working correctly, and tissue regeneration that is not working correctly. Conventional dressings only provide passive coverage and can hurt fragile granulation when they are taken off [1]. Polymer- and lipid-based nanostructures have arisen as promising wound care carriers capable of actively modulating local antimicrobial delivery, moisture retention, and regenerative signaling within the wound bed. However, many formulations are mechanically fragile, do not stay hydrated for long, and break down when they come into contact with enzyme- and salt-rich exudates. This makes them less effective in clinical settings over time [2]. Scheme 1 organizes the principal carrier families into polymeric, lipid-based, and hybrid classes to frame how architectural and interfacial design choices align with the needs of the wound bed.
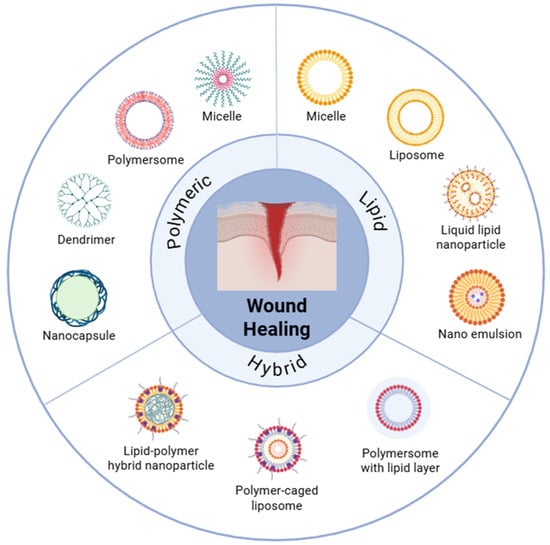
Scheme 1.
Nanostructures for wound healing can be categorized into three groups: polymeric, lipid-based, and hybrid. Some examples of representative systems are polymersome, micelle, dendrimer, nanocapsule, liposome, solid lipid nanoparticle, nanostructured lipid carrier, and polymer–lipid hybrid vesicle. The plan stresses how each structure is built to help the skin heal, control inflammation, and make it easier for drugs to enter and stay in.
Many studies report a single hydrodynamic size and a single surface charge measured in water, which obscures behavior in albumin- and mucin-rich media and complicates dose planning and cross-platform comparisons [3]. In addition, animal models that close by contraction underrepresent ischemia, neuropathy, and polymicrobial biofilm [4]. Figure 1 situates delivery within the staged biology of hemostasis, inflammation, proliferation, and maturation, underscoring the need for time-matched exposure that suppresses early contamination while supporting re-epithelialization, angiogenesis, and matrix organization [5].
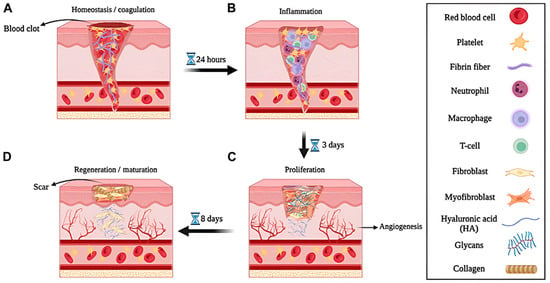
Figure 1.
Multiscale illustration and experimental validation of the wound healing process. Schematic representation of four consecutive wound healing phases: (A) Hemostasis and coagulation, (B) inflammation, (C) proliferation, and (D) regeneration/maturation, with major cellular and extracellular components indicated. Reprintied with permission from Ref. [5]. 2024, Biotechnology.
This review advances a barrier-resolved framework grounded in the premise that particle size distributions, interfacial charge in wound-like media, and internal architecture can be tuned to synchronize early antimicrobial availability with later pro-regenerative signaling, while preserving comfort on removal [6]. We outline design principles for polymer matrices, lipid vesicles, and polymer–lipid hybrids, and we propose a validation path that begins in human exudate surrogates, proceeds through ex vivo human skin and porcine full-thickness models, and links analytical attributes to outcomes including time to closure, reduction in biofilm burden, re-epithelialization, angiogenesis, collagen organization, and pain during dressing changes [7]. To facilitate reliable clinical translation, this approach emphasizes the transparent reporting of number-based size and shape distributions, surface charge measured in protein- and salt-rich media, and chemical integrity indicators such as oxidation markers and residual solvent content, alongside post-sterilization stability tests to ensure reproducibility and regulatory compliance [8].
2. Determinants and Barrier Interactions of Polymer- and Lipid-Based Nanostructures in Wound
2.1. Physicochemical Determinants
Polymer-, lipid-, and hybrid-based nanostructures are the three main types of trans-porters that help wounds heal (Scheme 1). Nanoparticles made of polymers, like PLGA, PEG, and chitosan, make matrices that can be solid or gel-like. You can change these matrices so that they break down at different rates for mechanical support, controlling moisture, and releasing their contents over time [9,10]. Lipid-based nanoparticles, such as liposomes, solid lipid nanoparticles, and nanostructured lipid carriers, resemble biological membranes. They are very biocompatible, can make contact with wet wounds in a way that is not too rough, and can hold two drugs at the same time in both aqueous and lipid domains [11,12]. Polymer–lipid hybrids combine the structural strength of polymers with the biomimetic interfaces of lipids to give you balanced stability, permeability, and explosive control [13,14]. These architectures establish the basis for understanding how physical–chemical determinants such as size, charge, and internal structure regulate performance in protease and salt-rich wound environments.
Polymer and lipid-based nanostructures for wound healing exhibit size, charge, and structure, physicochemical features that control hydration, tissue penetration, and stability in proteases and salt-rich exudates. The 50–300 nm size generally favors follicle entry and interstitial transport. A near-neutral surface reduces irritation, and a cationic surface improves adhesion but increases the risk of opsonization. Lipid vesicles guarantee contact with moist wounds and enable dual drug loading. Biodegradable polymers provide mechanical strength and continuous release, and the hybrid system combines the polymer core and lipid shell to bind for explosion control and ligand attachment, but changes in batch and sterile stability. Recent studies show that this size range can change depending on the situation. Hybrid and inorganic polymer systems can be effectively maintained outside of the usual 50–300 nm range, depending on the carrier composition and the microenvironment of the wound. For example, curcumin–cyclodextrin hybrid nanoparticles (about 150 nm) help the skin cells grow back and speed up the healing process [15]. PEG-coated magnesium hydroxide nanoparticles also helped cells absorb more of the drug and speed up the healing of wounds [16]. These results show that particle size should be adjusted along with surface charge, corona behavior, and payload chemistry, rather than being treated as a constant.
Table 1 shows that nanoparticles made of polymers, lipids, and hybrids have different physical and chemical properties that affect how well they heal wounds. Polymer-immersed carriers can be made larger or smaller by changing the polymer composition and processing. This ensures the strong adhesion to mucus and long-lasting release, but they can only hold a limited amount of hydrophilic material and may produce byproducts that break down in acid. Lipid-based systems are biocompatible and can mimic membranes and make contact with wet wound surfaces, but they can also change phases and become unstable in serum.

Table 1.
Comparative physicochemical and biological features of polymer-, lipid-, and polymer–lipid hybrid-based nanocarriers for wound healing. Summarized properties include particle size control, biocompatibility, tissue adhesion, degradation and release profile, surface functionalization options, morphology and stability, and wound healing performance.
2.2. Biocompatibility and Tissue Adhesion Profiles
Lipid carriers that include liposomes, solid lipid nanoparticles, and nanostructured lipid carriers often display high tolerability on skin because their membrane-mimetic composition aligns with native barriers and supports interaction with keratinocytes and macrophages while limiting disruption of the stratum corneum, although stability can decline in protease- and salt-rich exudate, and repeated application can amplify complement signals and irritation. PLGA, PEG, and chitosan are examples of degradable polymer matrices that can be adjusted to lower inflammation and balance moisture by controlling the flow of water vapor. Chitosan is a strong adhesive that keeps the matrix on exudative wounds longer, but it also makes it harder for the matrix to penetrate dense extracellular matrices and makes it harder to remove without causing trauma [18,19]. Polymer–lipid hybrids have a soft lipid interface that helps cells connect with them and a supportive polymer core that keeps them from leaking. But because the architecture is more complicated, it is harder to keep batches consistent, sterilize them, and store them safely. This means that early process controls are necessary [20,21,22]. Many studies continue to employ simplified media and short exposures that fail to accurately depict cumulative irritation, cytokine release, complement activation, and the interference caused by mucins and reactive oxygen species in wound fluid. Consequently, decision-ready evidence must encompass hemolysis, complement, cytotoxicity, irritation, and transport assays conducted in wound-like media, alongside barrier-resolved outcomes that accurately reflect biofilm penetration, re-epithelialization kinetics, macrophage polarization, and scar quality under equivalent dose and exposure conditions [23,24,25]. The in vivo performance of Cur/CD-HNPs corroborates the aforementioned biocompatibility and adhesion properties (Figure 2). In a burn wound model, Cur/CD-HNPs significantly expedited the healing process in comparison to the untreated, hydrogel, and Cur/CD-PM groups. The series of pictures in Figure 2a clearly shows that the Cur/CD-HNPs group closed their wounds and re-epithelialized quickly by day 14. Quantitative analyses (Figure 2b,c) demonstrated a reduction in the wound area and an increase in the contraction ratios, thereby confirming the acceleration of the healing process. Histopathological evaluation (Figure 2d) further confirmed the tissue’s compatibility and regenerative capacity. The H&E staining showed that the epidermal layers were still there and that the swelling had gone down. Masson’s Trichrome staining, on the other hand, showed that the dermis’s structure was restored and that the collagen fibers were dense and organized. These results show that hybrid nanoparticles are not harmful to cells, reduce irritation, and encourage tissue adhesion and regeneration at the same time. This shows that they are good at transporting medicine to wounds.
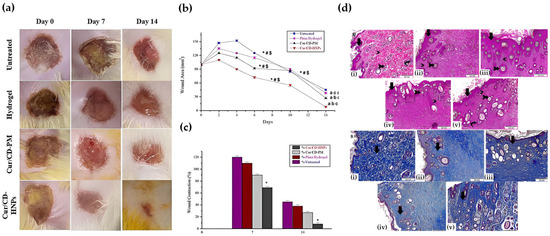
Figure 2.
In vivo and histological evaluation of Cur/CD-HNPs demonstrating biocompatibility and tissue adhesion in burn wound healing. (a) Representative wound photographs taken on days 0, 7, and 14 for the untreated, hydrogel, Cur/CD-PM, and Cur/CD-HNP groups. (b) Quantitative analysis of wound area reduction over 14 days. Statistical symbols indicate significant differences at each time point: * p < 0.05 vs. untreated; # p < 0.05 vs. Cur/CD-PM; $ p < 0.05 vs. hydrogel. (c) Wound contraction (%) on days 7 and 14. (d) Histopathological evaluation of skin tissues: (i–v) H&E staining showing re-epithelialization, reduced edema, and regeneration of dermal and adnexal structures—including newly formed epidermal layers, restored hair follicles, and diminished inflammatory cell infiltration—highlighted by black arrows; (i–v) Masson’s Trichrome staining indicating organized collagen deposition and restored connective tissue architecture in the Cur/CD-HNP treatment group. Reprinted with permission from Ref. [15]. 2025, BMC Biotechnology.
2.3. Degradation and Controlled-Release Profiles
Diffusion through lipid assemblies and hydrolytic erosion of polymer matrices determine when and where therapeutic payloads are accessible within the wound bed. The lactic-to-glycolic ratio, molecular weight, crystallinity, hydration, pH, and temperature of the exudate all affect the ester cleavage of PLGA and related copolymers. But if buffering is not used, acidic byproducts can lower the pH locally and irritate tissue around wounds [26]. The permeability of lipid carriers varies according to the presence of ionizable headgroups, cholesterol content, and acyl chain order. Their integrity can deteriorate in pro-tease- and salt-rich fluid or under dehydration and shear during dressing changes [27]. Hybrid polymer–lipid constructs often temper early burst through a bilayer shell while a polymer core functions as a reservoir. But interfacial defects, drying steps, and sterilization can cause batch variability and leaks that cannot be predicted [21]. The release profile must align with the temporal requirements necessary for healing. During the initial control of contamination, it is important to have quick access to antimicrobials. During granulation and remodeling, on the other hand, it is important to have ongoing signals that promote anti-inflammation or regeneration. But if exposed to the sun for too long, fibroblast migration can be slowed down, and reepithelialization can take longer [28]. Numerous studies continue to define release in uncomplicated buffers under static conditions. These conditions do not include enzymes, mucins, albumin, or reactive oxygen species, and they do not take into account cyclic hydration or shear. As a result, the apparent stability can be inflated, obscuring dosing intervals and dressing replacement plans. Therefore, evaluation should adopt exudate-mimicking media with proteolytic and oxidative challenges, report burst fraction and half-life at a matched dose and surface area-to-volume ratio, include testing under hydration–dehydration cycles, and link in vitro kinetics to barrier-resolved outcomes such as biofilm reduction, macrophage polarization, collagen organization, and rate of re-epithelialization [29,30].
2.4. Dimensional and Interfacial Parameters
Size, surface charge, and morphology govern how polymer- and lipid-based nanostructures distribute within wound tissue, interact with exudate, and deliver cargo to keratinocytes, fibroblasts, and macrophages, with sub-100 nm fractions more capable of traversing follicular routes and biofilm channels, and larger particles favoring local depot behavior with reduced spread [31,32]. Near-neutral or slightly negative interfaces tend to limit nonspecific protein adsorption, complement binding, and irritation, while the controlled inclusion of ionizable lipids or cationic polymers can improve endosomal release through pH-responsive interactions at the expense of stronger mucus trapping and opsonization [33,34]. Spherical and smooth morphologies generally support storage stability and predictable transport, whereas anisotropic or rough surfaces increase contact with irregular wound beds and can heighten phagocytic clearance [35]. These design levers are often constrained because many studies report only a single hydrodynamic diameter in water and a single zeta potential without ionic strength, temperature, or exudate composition, with sparse disclosure of number-based distributions and electron microscopy descriptors, and with comparisons performed at unequal nucleic acid dose and N-to-P ratio, which leaves platform selection uncertain for outcomes such as biofilm penetration, re-epithelialization, and scar quality [36,37]. Decision-ready characterization should include full size and shape distributions with polydispersity, zeta potential measured in exudate-mimicking media that contain albumin, mucins, salts, and reactive oxygen species, explicit buffer and temperature reporting, and performance under cyclic hydration and shear that reflects dressing changes while holding nucleic acid dose and N-to-P ratio constant across formulations [38,39].
3. Fabrication and Surface Engineering of Polymer- and Lipid-Based Nanostructures
3.1. Polymer Nanoparticle Fabrication Techniques
Polymer nanoparticles (PNPs) for wound healing are produced from preformed polymers or through the in situ polymerization of monomers, with selection guided by the target size profiles, payload compatibility, residual and endotoxin limits, sterilization plan, and intended scale [40,41]. Preformed routes include solvent evaporation, nanoprecipitation, single or double emulsion, dialysis, salting out, and supercritical methods such as RESS (rapid expansion of supercritical solutions) and RESOLV (rapid expansion of supercritical solution into liquid solvent) [42]. These methods enable broad material choice and tunable loading but require the stringent removal of solvent and surfactant to avoid cytotoxicity in protease- and salt-rich exudate, as well as requiring careful control of polydispersity and batch variability [33]. Emulsion, mini or microemulsion, surfactant-free emulsion, interfacial polymerization, and controlled radical polymerization using RAFT (reversible addition fragmentation chain transfer) and ATRP (atom transfer radical polymerization) are all examples of in situ methods [43]. These methods make sure that the chain structure and depot are correct, but they also come with risks from leftover monomers and initiators; they need to follow strict purification and release standards [44]. PLGA and PEG allow for an adjustable release design. On the other hand, microfluidic mixing with continuous manufacturing may help with size dispersion, but it will slow down throughput [45]. Many studies still test particles in deionized water under static conditions that omit enzymes, albumin, mucins, and reactive oxygen species; therefore, method reports should include number-based size and shape distributions, zeta potential in exudate-like media, residual and endotoxin limits, and post-sterilization stability linked to re-epithelialization, biofilm control, and scar quality [46,47].
3.1.1. Emulsion–Solvent Evaporation
PNPs for wound healing are frequently prepared by emulsion–solvent evaporation, in which PLGA, PLA, or PCL with payload is dissolved in a volatile organic phase such as EA or DCM, dispersed into an aqueous phase containing PVA by high shear or ultrasound, and then solidified by solvent removal through stirring, mild heating, or vacuum [47,48]. O/W emulsions suit hydrophobic cargos, while W/O/W double emulsions enable proteins and NAs [49]. The solvent choice, polymer and PVA concentration, energy input, and temperature govern size distribution, porosity, and EE, with implications for burst and depot behavior in exudate [50]. The strengths include broad material compatibility and simple equipment. The limitations include residual solvent and PVA, batch variability, shear-induced protein loss at interfaces, and modest loading of highly water-soluble agents [51]. Numerous studies purify and characterize particles in deionized water, which exaggerates perceived stability and minimizes irritation risk in environments rich in proteases and salts [52]. Development must validate mass balance and residuals in accordance with wound-specific thresholds, regulate endotoxin levels, ensure stability post-terminal sterilization, and be evaluated in exudate-like media containing enzymes, mucins, albumin, and reactive oxygen species (ROS) to correlate process outputs with outcomes including biofilm management, re-epithelialization, and scar quality [53,54].
3.1.2. Nanoprecipitation
Nanoprecipitation produces PNPs by injecting an organic solution of PLA, PLGA, or PCL with a hydrophobic payload into an aqueous non-solvent under rapid mixing, where solvent–water diffusion drives polymer desolvation, nucleation, and development into nanospheres, while the inclusion of an oil phase yields nanocapsules. Water-miscible solvents such as ACN or EA and stabilizers such as TPGS or PVA shape interfacial tension and suppress aggregation. A number of controllable factors have a clear effect on the final particle size and polydispersity. For example, lower concentrations lead to more nucleation events and smaller, more uniform particles, while higher concentrations of polymers lead to more local supersaturation during growth-dominated regimes, which results in a larger and broader PDI [55]. Supersaturation kinetics are significantly influenced by the solvent-to-water ratio; a higher aqueous fraction promotes growth or aggregation and slows desolvation, while a higher solvent content speeds up the diffusion and nucleation and produces smaller particles [56]. Another important factor is the injection or flow rate: slow injection promotes diffusion-limited growth and wider distributions, whereas rapid injection produces steep concentration gradients and a large number of nuclei, which reduce particle size [57]. Elevated temperature increases diffusion and decreases viscosity, generally favoring smaller particles, though excessive heating can destabilize emulsions or induce secondary aggregation [58]. Likewise, high-energy mixing or shear—through turbulent stirring or microfluidic confinement—shrinks diffusion boundary layers, producing smaller and more uniform particles, whereas low-energy mixing often yields multi-modal size distributions [59].
Because nanoprecipitation outcomes reflect the coupled kinetics of nucleation and growth, parameter sweeps (polymer concentration, solvent–water ratio, flow rate, temperature, and mixing intensity) should be explicitly linked to particle size, polydispersity, and encapsulation efficiency. Protease- and salt-rich exudate can be irritated by residual solvent and surfactant, and stability in DI water under static conditions is rarely a reliable indicator of performance under cyclic hydration and shear. Therefore, the zeta potential and release profiles in exudate-like media, mass balance for loading and recovery, and post-sterilization stability should all be included in decision-ready reports [60]. A representative process and parameter effects are illustrated in Figure 3. Evaporation-triggered nanoprecipitation involves injecting a polymer–solvent/antisolvent mixture onto a rotating substrate, where rapid solvent diffusion induces polymer desolvation and nucleation (Figure 3a). Dynamic light scattering (Figure 3b) shows that the type of stabilizer and the concentration of the polymer have a big effect on the hydrodynamic diameter and the polydispersity index. As the concentration of polymers rises, regimes dominated by growth emerge, resulting in a higher PDI, which aligns with the previously discussed supersaturation kinetics. Scanning electron microscopy shows different shapes of nanoparticles and how they stay the same under different polymer and solvent conditions (Figure 3c). This confirms that the best compositions make spherical and monodisperse PLGA nanoparticles [61].
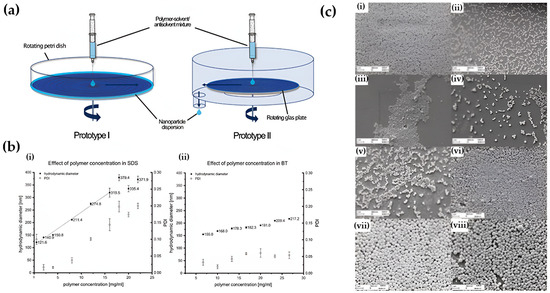
Figure 3.
Mechanism and parameter effects in nanoprecipitation of PLGA nanoparticles. (a) Schematic of evaporation-triggered nanoprecipitation illustrating polymer–solvent/antisolvent mixing and nucleation on a rotating substrate. (b) Influence of polymer concentration and stabilizer type on hydrodynamic diameter and polydispersity index (DLS), demonstrating the shift from nucleation-dominated to growth-dominated regimes. (c) Representative SEM images showing how polymer molecular weight, lactic/glycolic ratio, and solvent/antisolvent combinations modulate nanoparticle morphology and size distribution: (i) Low–molecular-weight PLGA (7–17 kDa, 50:50) in ACE/H2O producing larger, irregular particles. (ii) Medium–molecular-weight PLGA (24–38 kDa, 50:50) forming smaller and more uniform nanospheres. (iii) High–molecular-weight PLGA (38–54 kDa, 50:50) yielding highly uniform and smaller particles due to reduced chain mobility. (iv) Increased LA:GA ratio (65:35) resulting in larger and less monodisperse particles. (v) Higher LA content (75:25) further enlarging particle size and broadening PDI. (vi) ACE/EtOH mixtures producing larger, less uniform nanoparticles due to slower desolvation kinetics. (vii) ACN/H2O conditions yielding the largest particles owing to rapid solvent diffusion and reduced nucleation density. (viii) Optimized ACE/H2O conditions producing the smallest and most spherical particles, reflecting efficient nucleation and narrow size distributions. Reprinted with permission from Ref. [61]. 2025, Drug Delivery Science and Technology.
3.1.3. Electrospinning Nanofiber Fabrication
Electrospinning makes meshes that help wounds heal by pulling a charged polymer jet from a syringe needle to a grounded collector while the solvent evaporates. This makes fibers with a large surface area that can be changed in diameter, alignment, and porosity [62]. When making solutions of PCL, PLA, PLGA, PEO, or mixtures that contain antimicrobials or growth factors, the voltage, flow rate, and tip-to-collector distance must be just right. The temperature and humidity also affect the whipping, crystallinity, and web integrity [63]. Coaxial, triaxial, melt, multi-jet, and needleless formats allow for core–shell loading, solvent-free operation, and higher throughput. Rotating or patterned collectors set alignments that can help keratinocyte and fibroblast migration [64]. The benefits include conformal contact with irregular wounds and continuous delivery from a mechanically compliant depot [65]. Constraints remain, including jet instability, limited industrial yield, and residual solvent that can irritate protease- and salt-rich exudate, while early hydration can trigger bursting when crystallinity or crosslinking is inadequate [66]. Many reports present mean fiber diameter from dry images and elution in DI media, which overstates mechanical and release stability. Decision-ready method sections should disclose diameter distributions and pore fraction, surface charge, drug localization, residuals and endotoxins, wet tensile behavior, post-sterilization stability, and burst and antimicrobial performance in exudate-like media under cyclic hydration [67,68].
3.2. Lipid Nanostructure Engineering
LNP platforms that have SLN and NLC are made with HPH, emulsion, or microfluidics. The size, PDI, loading, and release depend on the composition and process [69,70]. When you mix solid and liquid lipids, you obtain an NLC with lattice defects that change the crystallinity and increase the capacity [71]. PEG makes colloids more stable, lowers the amount of protein that sticks to them, and lets ligands be added after insertion. But thicker coronas can make it harder for cells to touch each other and lower the amount of viscous exudate that cells take up [72]. LbL shells help control charge and protect enzymes, but they have some problems. For example, they do not let enough light through, they cannot dry out, and they cannot kill bacteria at the end [73]. Many researchers believe that sugars, peptides, or Ab fragments can actively target cells. But proteases, ROS, and biofilm matrices in wounds can break down ligands and hide epitopes, making them less useful [74]. Numerous studies demonstrate that DLS sizes in water and zeta function merely as fundamental buffers. They have not been tested with shear, albumin, or mucin yet. This makes it harder to compare reports and figure out how much to use [75]. PEG-LNPs help keep the interface stable, activate the immune system, and stop ions from crossing the epithelial barrier, as seen in Figure 3. This shows how important interfacial engineering is and how important it is to try using it in places that are like wounds and have changing levels of hydration and shear [76]. Figure 4 does not show us much about how PEG changes the way that lipid nanostructures work. Figure 4a shows a diagram with solid lipid cores and a PEG corona around them. This keeps the colloid from breaking down and keeps proteins from sticking to particles in biological fluids. PEGylation lowers immune activation and neuroinflammation, as shown by the drop in microglial activation and the restoration of neurovascular integrity (Figure 4b,c). The immunostaining of ED1-positive cells indicated that PEGylated LNPs inhibited microglial activation and inflammatory signaling. In contrast, immunoblotting showed that MMP-9, caspase-1, and phospho-CaMKII levels went down, which reduced neuroinflammation and kept synaptic signal transmission going. Furthermore, absorption studies employing an Everdead intestinal pocket model (Figure 4d) revealed that PEG-modified SLNs demonstrated enhanced and more consistent absorption in the duodenum, jejunum, and ileum compared to non-PEGylated SLNs. These results all show that modifying the interface of polymers makes lipid nanoparticles (LNPs) more stable, changes immune responses, and maintains epithelial transport, which shows how important it is to design lipid–polymer interfaces for use in wound healing [76].
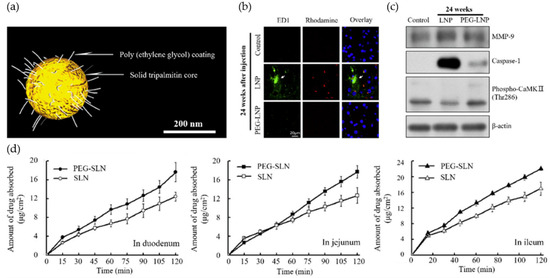
Figure 4.
LNP functionality modulated by polymer-based surface modification. (a) Schematic illustration of PEG-stabilized LNPs composed of a solid tripalmitin core and a PEG coating. (b) Immunofluorescence images showing mitigation of neurovascular damage following PEG-LNP administration. FD1 (green) visualizes fluorescein–dextran leakage across the vascular barrier, Rhodamine (red) labels vascular structures, and DAPI (blue) stains nuclei. PEGylation reduces FD1 extravasation, indicating improved neurovascular integrity. (c) Immunoblot analysis of mouse brain tissue 24 h after injection, probed for MMP-9 (blood–brain barrier integrity), caspase-1 (inflammation), and phospho-CaMKII (synaptic signaling), with β-actin as a loading control. (d) Absorptive characteristics of SLN and PEG-SLN in the everted rat gut sac system across the duodenum, jejunum, and ileum, showing enhanced and more consistent absorption for PEG-SLN. Reproduced with permission from Ref. [77]; Copyright 2023, Bioengineering.
3.2.1. Liposome Fabrication Methods
TFH, EI, REV, DR, extrusion, HPH, microfluidic mixing, and SCF routes make liposomes for LNP platforms for wound care. The lipid mix, hydration conditions, and post-sizing define MLV or LUV populations, EE, and stability. TFH makes a dry film that is hydrated and shrunk by extrusion or HPH. EI injects a lipid solution into a buffer to quickly vesiculate it. REV makes W/O emulsions that collapse into large unilamellar vesicles with high aqueous loading. Finally, DR takes detergents out of mixed micelles to make homogeneous liposomes [78,79]. Adding PEG after insertion makes colloids more stable and less likely to stick to exudate that is high in protein. But thick coronas can make it harder for cells to touch each other and slow down uptake [80]. Microfluidics gives you a lot of control over mixing and size, but throughput and fouling limit the size. Previous studies often reported DLS size in water with insufficient details on oxidation markers, residual solvent, and batch variability, complicating the understanding of its functionality in enzyme- and salt-rich media [81]. Figure 4 illustrates the various pathways through which membrane-derived lipid sources, polymer-modified liposomes, and hydrogel-assisted delivery can reach recipient membranes. Development focused on wound environments should elucidate numerical size distributions, zeta potential in exudate-like media, lipid oxidation, and residual limits, alongside post-sterilization stability related to re-epithelialization and biofilm management [82]. Figure 5 shows how the mechanistic framework of membrane-derived lipid assembly and hydrogel-assisted delivery works. This figure shows that fragmented cell membranes with cholesterol and phospholipids can turn into lipid vesicles through thin-film hydration (TFH) or ethanol injection (EI). After that, the vesicles can be made smaller by extrusion or high-pressure homogenization (HPH). Adding PEG makes the colloidal stability even better and stops the protein-rich fluid that comes out of wounds from sticking together. Changing the polymer makes it stronger and easier to control how bioactive substances are released. The left side of Figure 4 shows how liposomes and liposomes that have been changed by polymers are made from natural membrane pieces. This means that they are very similar to biological barriers in terms of their makeup. The right panel shows hydrogel-assisted delivery, which is when a liposome and a recipient membrane come together to make ion channels. This improves the balance of hydration and ionic communication across the wound interface. These processes show that lipid–polymer–hydrogel platforms that work together can speed up the delivery of drugs and help tissues heal. They show how nanoscale assembly principles can be used to improve wound treatments.
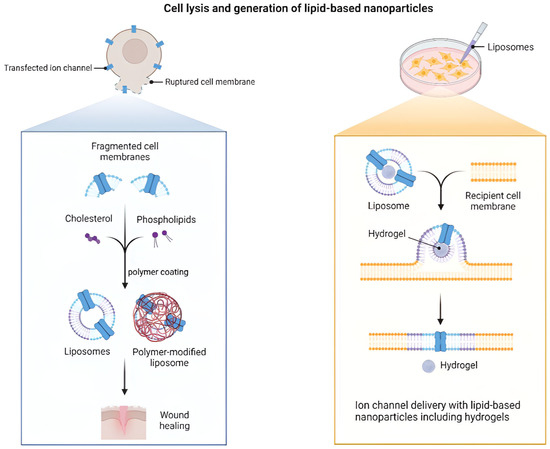
Figure 5.
Cell lysis and LNP generation with hydrogel-assisted delivery. Panels show the following elements: TFH and EI production with size tuning by extrusion or HPH, optional PEG post-insertion membrane fragments providing cholesterol, and PL that assemble into liposomes or polymer-modified liposomes for wound healing, using hydrogel-assisted landing of a liposome on a recipient membrane with insertion of an ion channel [82]. Reproduced with permission from Ref. [82]; Copyright 2023, Functional Biomaterials.
3.2.2. Solid Lipid Nanoparticle (SLN) Engineering
SLNs are lipid nanosystems made from natural lipids that are less likely to be harmful and can be made without the use of solvents. They also help active ingredients that do not dissolve well in water enter the skin better and become more available to the body. [83]. Some of their strengths are that they can keep labile cargos safe, are more stable in storage than liposomes, and can handle high drug concentrations and lyophilization [79]. There are still problems, like APIs that take a long time to load, being ejected during polymorphic transitions in storage, and dispersions with a lot of water [84]. Some ways to produce SLNs are HPH, microemulsion, ultrasound, solvent emulsion-evaporation, emulsion-diffusion, membrane contact methods, and SCF extraction with CO2. You can use W/O/W to package hydrophilic substances, but it does not stay stable in media that have a lot of enzymes and proteins [85]. When you are ready to decide on characterization, you should include size and PDI by PCS and LD, zeta to estimate storage stability, crystallinity with polymorphic mapping, the presence of secondary colloids, drug content, release in exudate-like media, and surface morphology. Many studies exclusively utilize water-based PCS and singular zeta values. This makes things look more stable than they really are and makes it harder to figure out how much to provide to wounds that have proteases, salts, and reactive oxygen species (ROS) [38]. Batch disclosure, strict limits on residual and endotoxin levels, and changing the product’s form to dry powders by spray-drying or lyophilization all help make translation easier [86].
3.2.3. Nanostructured Lipid Carrier (NLC) Engineering
NLCs blend solid and liquid lipids to create imperfect lattices that raise loading, suppress expulsion during storage, and enable tunable release for cutaneous use. A typical process heats the lipid blend above Tm with cargo, forms a pre-emulsion with aqueous surfactant, then applies HPH or US and controlled cooling to obtain a solid dispersion [87]. The ratios of solid to liquid lipid, surfactant level, cooling rate, and post-processing with extrusion or microfluidics set size, PDI, and EE, while shaping crystallinity and long-term robustness [88]. The advantages over SLNs include higher capacity and better control of matrix order, although polymorphic transitions, oil leakage under shear, and dehydration during dressing changes remain practical risks [82]. Many reports provide DLS size in water and a single zeta value without testing in media that contain albumin, mucin, enzymes, and ROS, which inflates apparent stability and clouds dose planning [89]. As summarized in Table 2, method descriptions that support translation should add number-based size distributions, DSC or XRD for polymorphism, mass balance for loading and recovery, residual and endotoxin limits, and release in exudate-like media linked to re-epithelialization, biofilm control, and comfortable removal [90].

Table 2.
Fabrication strategies for polymer, lipid, and hybrid nanostructures used in wound healing. Columns list category, technique, principle, advantages, and limitations. Rows include PNP via nanoprecipitation and emulsion–solvent evaporation, SLN via HPH, microemulsion, ultrasound, NLC via melt emulsification with controlled cooling, and PLHN via one-step assembly and two-step coating with microfluidics or HPH.
3.3. Polymer–Lipid Hybrid Platforms (PLHN)
PLHNs combine a polymer core with a lipid shell to make wound delivery stable and allow the polymer to interact with the membrane [21]. Two-step assembly makes polymer NPs and lipid vesicles separately and then combines them by extrusion, mixing, or US. This lets you tune them separately, but it can lead to uneven coating, uneven surface coverage, and limited scale [99,100]. One-step assembly uses solvent diffusion or emulsion–evaporation to form PLHN directly, and HPH or microfluidics can narrow dispersity, while residual solvent and interfacial defects may trigger the leakage of protease- and salt-rich exudate [85]. Post-insertion of PEG and LbL coatings mitigates aggregation and modifies charge; however, increased corona thickness may impair cell interaction and reduce ligand efficacy [101]. Active targeting often overlooks the effects of ligand orientation loss and the damage inflicted by enzymes and reactive oxygen species in wounds, thereby reducing the advantages in the absence of appropriate controls [102]. Many studies report DLS size in aqueous solutions alongside a single zeta value, providing insufficient information on oxidation, residues, or sterilization effects, leading to uncertainty in dose planning and dressing replacement [103]. Figure 6 schematically compares major lipid nanoparticle systems—liposomes, nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers, and lipid–polymer hybrids—highlighting how PLHNs merge the structural stability of polymers with the biological adaptability of lipids. Panels (b) and (c) further illustrate a representative polymer–lipid–hydrogel hybrid and its wound healing mechanism, showing sustained release, antioxidative protection, and enhanced tissue regeneration in vivo. Figure 6 contrasts LNP families and locates PLHN among them, emphasizing the need for number-based size distributions, zeta in media, mass balance for drugs and lipids, oxidation and residual limits, and linkage to re-epithelialization, biofilm control, and comfortable removal [9].
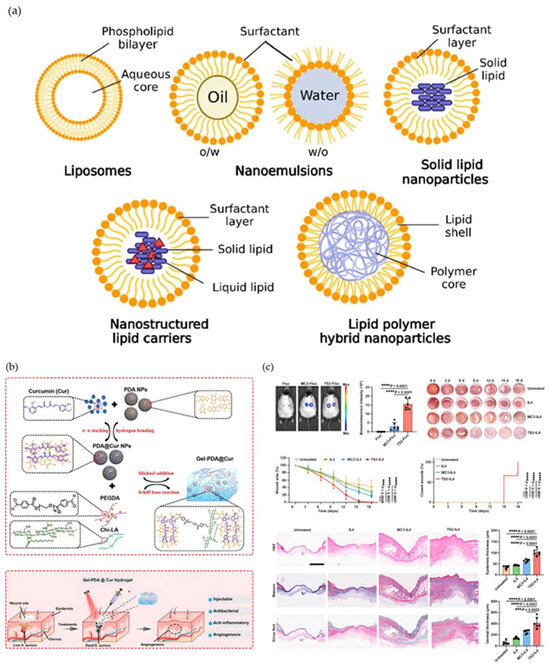
Figure 6.
Comparative illustration of lipid nanoparticle-based platforms and their applications in wound repair. (a) Structural schematics of major lipid nanoparticle formulations, including liposomes, nanoemulsions, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and lipid–polymer hybrid nanoparticles. (b) Representative polymer–lipid–hydrogel hybrid assembly and wound-targeted delivery mechanism illustrating pH-activated PDA–Cur release, PEGDA–CHLA crosslinking, and tissue adhesion properties. (c) In vivo wound-healing efficacy and histological analysis demonstrating accelerated wound closure and enhanced regeneration following NLC-based treatment. Black dots indicate wound boundary markers used to delineate the region of interest. Graphs include statistical comparisons. Reproduced with permission from ref. [9]; Copyright 2023, ACS Materials Au.
3.4. Surface Functionalization and Bioactive Loading
PLHNs make it easier to deliver drugs directly to wounds by using surface engineering and making loading faster [104]. When you put amphiphilic blocks together, like PLA–PEG, the PEG chains stick out. This makes hydrated coronas that proteins can’t stick to, but ligands can. Once lipid shells are added and covalently linked to polymer cores, peptides, sugars, antibody fragments, or nucleic acids can fit inside. The charge and hydration properties of PLHNs make it easier to deliver drugs directly to wounds by using surface engineering and speeding up the loading process [104]. Changes in PEG’s length and density affect how it holds a charge and how much water it can hold [105]. Double-emulsion and ionizable parts make it easier to put hydrophilic cargo inside endosomes and then release it. On the other hand, mobile bilayers could move ligands around, and dense PEG could keep cells from moving around and interacting with each other inside cells. Exudate has reactive oxygen species (ROS) and proteases that can break apart motifs or hide albumin and mucin epitopes [106,107]. Recent studies (2024–2025) have broadened these concepts to encompass immune-modulatory and stimuli-responsive surface designs. PEG coronas and lipid anchors are progressively integrating ROS- or enzyme-cleavable linkers to coordinate drug release with oxidative or proteolytic stress, thereby improving infection management and wound healing in diabetic individuals [108]. The interfacial chemistry of PLHNs can greatly affect how the immune system works. For example, surfaces that have been changed with arginine-rich or zwitterionic peptides can help M2 macrophages polarize and reduce chronic inflammation [109]. Biomimetic coatings that use macrophage or platelet membranes are even better at moving through the immune system and building up in certain areas [110]. In the method sections, there should be measurements of ligand density and stability, a mass balance for the payload, a list of any leftover reactants, and an analysis of zeta potential and release kinetics in exudate-like media after sterilization and drying. You need this to make a decision [111,112,113]. Figure 7 shows how these strategies for surfaces and formulations affect biological performance. The in vivo wound healing images and quantitative closure data demonstrate that EGF–Cur-loaded NLCs accelerate tissue repair compared to blank or single-agent controls. Histological analyses indicate enhancements in re-epithelialization, collagen deposition, and angiogenesis. These results show that controlled bioactive loading and improved lipid–polymer interfaces have a direct effect on how quickly wounds heal and tissues regenerate [114].
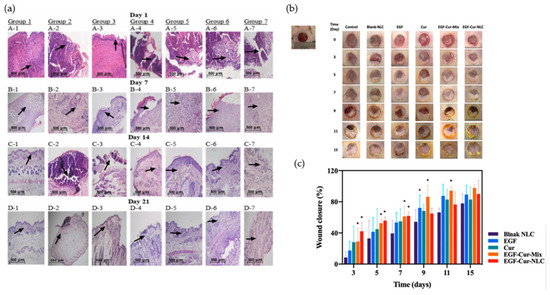
Figure 7.
In vivo assessment of wound healing efficacy and histological analysis of EGF–Cur–NLC treatment. (a) Typical wound images of diabetic rats treated with Blank NLC, EGF, Cur, EGF–Cur–Mix, and EGF–Cur–NLC formulations over 15 days, showing progressive closure and scab reduction. Arrows indicate key histopathological features including re-epithelialization fronts, granulation tissue formation, and collagen deposition areas. (b) Quantitative wound-closure analysis (%) showing that EGF–Cur–NLC had a significantly higher healing rate than other formulations over 15 days. Asterisks (*) denote statistically significant differences compared with the Blank NLC group (* p < 0.05). (c) Histopathological analysis of skin sections from days 1 to 21 post-treatment, demonstrating re-epithelialization, collagen deposition, and angiogenesis in the EGF–Cur–NLC group. Arrows highlight regenerated dermal structures and newly deposited collagen fibers. Reprinted with permission from Ref. [114]. Copyright 2023, Pharmaceutics.
4. Therapeutic Functions of Polymer- and Lipid-Based Nanostructures in Wound Healing
4.1. Antimicrobial Activity and Anti-Inflammatory Modulation
Microbial loads and persistent cytokine signaling impede the healing process of chronic wounds [115]. Polymer- and lipid-based nanostructures can avoid these problems by keeping the level of treatment the same and making their antimicrobial activity stronger than that of regular dressings. Chitosan–PLGA nanoparticles maintained fibroblast viability by more than 85% while inhibiting the growth of S. aureus and E. coli within 12 h, demonstrating a strong selectivity between bacterial virulence and cytocompatibility [116]. Chitosan promotes cationic hemostasis and attaches to microbial membranes, enhancing bacterial elimination and thrombus stabilization [117]. SLN, NLC, and lipid–polymer hybrid nanoparticles (LPHN) protect unstable drugs and make it possible to deliver antimicrobial and anti-inflammatory agents in phases during the inflammatory phase [118]. ROS-responsive lipid nanoparticle hydrogels exhibited approximately 85% bacteriostatic efficacy in infected diabetic wounds and decreased TNF-α levels by 70%, facilitating accelerated granulation tissue formation and re-epithelialization by day 7, in contrast to roughly 45% in untreated controls [119]. When fibroblasts are in the proliferator phase, too much exposure to silver or zinc oxide nanoparticles can kill 55–65% of them. This shows how important it is to test with different doses [120]. Cryogels made of gelatin–silver nanoparticles reduced the number of Pseudomonas aeruginosa and Staphylococcus aureus by more than 4 log CFU. However, releasing more than 1 µg mL of silver in vitro caused fibroblast toxicity by 20–30%, which shows that controlled release is necessary. In an albumin-rich medium, hybrid PLGA-lipid nanoparticles kept an effective load of more than 90% for 48 h. They also had a ζ-potential between −10 and 20 mV, which shows that they were stable in colloidal form under conditions similar to exudate [121]. Nanostructures containing both doxycycline and curcumin facilitated M2 macrophage differentiation (M1/M2 ≈ 0.4) and concurrently decreased TNF-α levels by 70% and IL-6 levels by 65%, leading to significant enhancement in granuloma formation and epithelial obstruction in diabetic wounds [122]. Table 3 shows the results of a quantitative comparison of the antibacterial and anti-inflammatory effects of the above treatments at different stages of wound healing. It shows that the polymer- and lipid-based nanostructures work better than regular dressings when it comes to clearing bacteria, lowering cytokines, and speeding up tissue recovery.

Table 3.
Quantitative performance of polymer- and lipid-based nanostructures in antibacterial and anti-inflammatory wound healing.
4.2. Angiogenesis Promotion
The creation of new microvessels is what makes granulation tissue form and provides nutrients during skin regeneration. Nanostructures made of polymers and lipids can control this process locally by keeping angiogenesis-promoting signals coming in while limiting their spread throughout the body [129]. Liposomes, solid lipid nanoparticles, polymersomes, and polymer–lipid hybrid nanoparticles can deliver vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) within a time frame that promotes endothelial cell proliferation, migration, and lumen formation. They can also be used with nitric oxide (NO) donors to improve perfusion in people with diabetes [130,131,132]. The benefits include weak protein protection, co-mounting anti-inflammatory signals, and being close to wet wound surfaces [133]. Recent studies show that VEGF-integrated polymer–lipid and ROS-responsive hydrogel systems greatly improve neovascularization and tissue perfusion in models of chronic and diabetic wounds. By controlling oxidative stress and inflammatory signaling, ROS-removed lipid nanoparticle-based hydrogels help micro-vascularization and re-epithelialization [134]. Dual-growth factor delivery systems, like hydrogels with VEGF/bFGF, release substances that promote angiogenesis for several days. This makes endothelial formation and wound healing much faster than with single-factor systems [135,136]. Figure 8c,d show that lipid nanoparticle hydrogels are controlled by expansion and react with ROS release signals that promote angiogenesis when there is a constant need for them in wound simulation conditions. This constant transmission helps the blood vessels grow back, raises the levels of CD31/α-SMA, and keeps the first capillary network stable [119,134]. Hybrid nitric oxide-releasing liposome hydrogels stimulate HIF-1α and VEGFR2 signaling, promoting angiogenesis and mitigating oxidative stress-induced vascular collapse in diabetic wounds, resulting in increased vascular density and functional recovery [137]. Important caveats remain. Proteases and reactive oxygen species exudate degrade growth factors and destabilize carriers. An uncontrolled burst may lead to the formation of leaky immature vessels [138]. Excess nitric oxide can harm cells through the formation of peroxynitrite, and light-activated donors may be ineffective in penetrating dressings [139]. Many studies persist in performing tests in water at single time points, resulting in an overestimation of stability and obscuring dosing intervals [140]. Study designs must quantify spatial and temporal release in wound-mimicking media and correlate these profiles with capillary density, perfusion imaging, re-epithelialization, and scar quality [141].
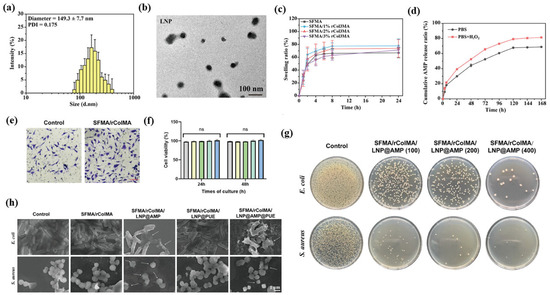
Figure 8.
Representative experimental data illustrating multifunctional therapeutic actions of polymer- and lipid-based nanostructures in wound healing. (a) Particle size distribution of LNPs (~150 nm, PDI 0.17). (b) TEM image confirming spherical morphology. (c) Swelling behavior of SFMA/rColMA hydrogels with varying compositions. (d) Cumulative AMP release profiles under PBS- and ROS-rich (H2O2) conditions. (e) Fibroblast morphology after incubation on hydrogel surfaces. (f) Cell-viability analysis at 24 h and 48 h showing excellent cytocompatibility, with ‘ns’ indicating no significant difference and the bar colors representing each tested formulation. (g) Antibacterial activity against E. coli and S. aureus after treatment with LNP@AMP hydrogels at different concentrations. (h) SEM micrographs showing membrane disruption of bacteria after exposure to SFMA/rColMA/LNP@AMP or SFMA/rColMA/LNP@PUE composites, with arrows indicating sites of membrane damage. Reprinted with permission from Ref. [119]. 2024, Advanced Science.
For wounds to heal, the formation and remodeling of the extracellular matrix must be coordinated. Polymer and lipid nanostructures give structural cues and control the activity of fibroblasts to make these processes more effective [142,143]. Electrospun nanofiber scaffolds made from biocompatible polymers help fibroblasts stick to each other, move around, and make collagen fibrils. Gelatin nanofibers have arginine–glycine–aspartic acid motifs that help integrins stick to them and integrate with tissue more effectively [144]. Hybrid dressings that mix nanofibers and lipid nanoparticles make it possible to deliver pro-regenerative agents directly to the right place while keeping the area moist, which is good for matrix assembly [145]. Gene-regulatory strategies, such as the administration of small interfering RNA aimed at transforming growth factor beta one, can inhibit excessive collagen accumulation and mitigate hypertrophic characteristics, provided that the dosing is calibrated to prevent the suppression of normal remodeling processes [146]. A recent study has shown that polymer–lipid composite nanofiber scaffolds greatly improve the alignment of collagen and the strength of tissue during remodeling. Electrospun PCL–collagen scaffolds exhibit superior extracellular matrix (ECM) organization and tensile strength compared to single-component systems, promoting expedited substrate maturation and fibroblast infiltration in vivo [147,148]. Double-transmission scaffolds that release TGF-β1 target both small interference RNA (siRNA) and VEGF at the same time have been shown to effectively block signals that cause fibrosis, bring the collagen I/III ratio back to normal, and speed up the rebuilding of the extracellular matrix. This greatly reduces scar thickness and improves tissue structure. These gene-activated constructs integrate anti-fibroblast and angiogenesis-promoting signals within a single platform, enabling time-coordinated regulation of fibroblast activity and vascular regeneration for scarless wound healing [149]. Figure 8e,f show that fibroblasts grown on SFMA/rColMA hybrid hydrogels spread out a lot and had well-defined actin cytoskeletons. They formed thick, straight collagen fibers in less than 48 h. Fluorescence staining for life and death showed that more than 90% of the cells were alive, and confocal imaging showed that the ECM structure was uniform in three dimensions, which meant that the cells were strongly interacting with the substrate and were very biocompatible [150]. Mechanically, these hybrid nanostructures regulate cytoskeletal tension and substrate reconstruction by activating the focal adhesion kinase (FAK) and integrin-associated kinase (ILK) signaling pathways. This regulation encourages fibroblasts to change into a phenotype that promotes regeneration, helps keep collagen I/III levels balanced, and stops excessive crosslinking and stiffness, which are common signs of fibrosis [151]. Important risks remain. Proteinases and reactive oxygen species in wound fluid can degrade bioactive payloads and weaken scaffold mechanics, residual crosslinkers or solvents may irritate the peri-wound margin, and overly adhesive matrices can hinder cell infiltration and removal [152]. Many studies assess fibers and carriers in water at single time points, leading to an overestimation of stability and a lack of clarity regarding dose schedules [153]. Development should quantify release and mechanical recovery in wound-mimicking media and relate these profiles to collagen architecture, re-epithelialization, and scar quality [141].
4.3. Targeted and Stimuli-Responsive Growth Factor Delivery
Polymer and lipid nanostructures enable spatiotemporal control in wound therapy via local sensing and on-demand release, reducing off-site exposure [154]. Smart dressings that monitor acidity or temperature can initiate antibiotic release from temperature-responsive hydrogels, sustaining effective levels during initial contamination without the need for repeated handling of delicate tissue [155,156]. Liposomes, solid lipid nanoparticles, polymersomes, and polymer–lipid hybrid nanoparticles can present vascular endothelial growth factor and basic fibroblast growth factor in sustained or pulsed modes that support endothelial migration and perfusion, and carriers that liberate nitric oxide can improve microcirculatory flow in ischemic beds [12,157]. A recent study has demonstrated that polymeric–lipid hybrid hydrogels responsive to pH and reactive oxygen species (ROS) can sustain VEGF-dependent release profiles and markedly enhance local angiogenesis compared to non-responsive systems [158]. The more responsive chitosan–lipid nanocomposites were also designed to induce antimicrobial or antibiotic release under mild high heat (about 37 to 40 °C), resulting in improved bacteriostatic effects and faster wound sutures compared to passive hydrogels [159]. A dual-stimulation platform incorporating near-infrared (NIR) and redox/ROS triggers enables the sequential release of VEGF and nitric oxide (NO), promoting increased blood flow and stable neovascular network formation in chronic wound models [160,161]. These observations demonstrate the ability to deliver on demand, consistent with the pH- and ROS-induced antimicrobial peptide (AMP) release profiles (Figure 8d) observed in LNP hydrogels. SEM images (Figure 8h) further show bacterial membrane breakdown, including pore formation and surface collapse, which supports catalytic and oxidative bactericidal action [162]. Design tradeoffs require early attention because proteases and reactive oxygen species in exudate degrade proteins and destabilize carriers, uncontrolled bursts can generate immature leaky vessels, excessive nitric oxide forms cytotoxic species, and sensor drift or poor light penetration can misfire triggers [163]. Laboratory protocols that rely on simple buffers and single time points overstate stability and obscure dosing intervals. Future evaluations should quantify release half-life and trigger responsiveness in exudate-mimicking media and correlate these with perfusion index, biofilm suppression rate, and scar thickness reduction to support translational reproducibility.
5. Polymeric Platforms and Hydrogel Composites for Wound Healing
5.1. Natural Polymer Platforms
Chitosan, gelatin, and alginate exhibit hemostatic, antibacterial, moisture-retentive, and extracellular matrix-guiding properties for localized therapeutic use in wound healing polymers [164]. Natural polysaccharides have different charges, molecular structures, and degradation kinetics, which determine their wound-specific applicability and healing performance. Chitosan exhibits strong antibacterial and hemostatic properties thanks to its cationic amino groups, which promote thrombus formation and macrophage activation to achieve wound closure rates of approximately 85 to 90% and bacterial inhibition rates of over 90% within 10 days in infected wound models [165,166]. Alginate creates a hydrogel by exchanging Ca2+ and Na+ ions with wound exudate. It keeps moisture in at a rate of about 85 to 90% and closes at a rate of about 90% after 10 days. But too much crosslinking by calcium can cause too much gelation and make the material weaker [167]. Fucoidan, a sulfurized polysaccharide obtained from brown algae, enhances antioxidant and angiogenic activity by upregulating VEGF and inhibiting ROS. In ischemic or diabetic wound models, the fucoidan-based hydrogel achieves a suture rate of approximately 93% and a reduction of approximately 40% of active oxygen species (ROS) within 10 days [168,169]. Chitosan, alginic, and fucoidan complexes produce porous and compressible scaffolds and exhibit a variety of antimicrobial and angiogenic properties. In anterior wounds, these hybrid materials achieved a closure rate of approximately 95% and a collagen I/III ratio approximately 1.8-fold higher within 10 days, indicating improvements in angiogenesis and extracellular matrix restructuring [170]. Figure 9a–d show that the antioxidant-embedded chitosan–alginate hydrogel rapidly expanded, was continuously released for more than 48 h, degraded in a controlled manner, and kept the cells alive for more than 95% of the time. This indicates that the cells were suitable and stable during the wound healing process [164].
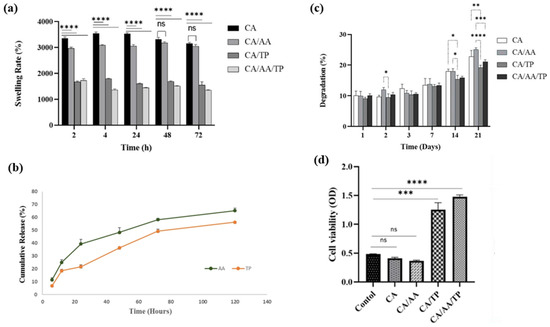
Figure 9.
Quantitative characterization of chitosan–alginate composite hydrogels incorporating ascorbic acid (AA) and tannic acid (TP). (a) Swelling behavior showing rapid hydration within 2 h and reduced water uptake in AA/TP-modified hydrogels. (b) Cumulative release profiles of AA and TP demonstrating sustained release over 120 h. (c) Controlled biodegradation profile indicating moderate mass loss (≤40%) over 21 days. (d) Cell viability (MTT assay) confirming excellent cytocompatibility of AA/TP formulations relative to control groups. Statistical significance indicators: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns = not significant. Reprinted with permission from Ref. [164]. 2023, BMC.
Table 4 further summarizes the representative polymeric materials discussed in this section, highlighting how their structural differences, advantages, limitations, and quantitative healing efficiencies contribute to distinct wound healing mechanisms.

Table 4.
Comparative characteristics and therapeutic efficiencies of natural polymer-based wound healing platforms.
5.2. Synthetic Biodegradable Polymer Platforms
Controlling molecular weight, erosion rate, and mechanical properties through synthetic biodegradable polymers lets you customize wound healing nanostructures for local treatment delivery [82]. The representative synthetic biodegradable polymers poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG) provide adjustable degradation rates, mechanical stability, and controlled drug release properties, making them widely utilized as scaffolding materials for wound healing applications [183]. PLGA effectively encapsulates antibiotics, anti-inflammatory drugs, proteins, and nucleic acids. Its hydrolysis gradually releases glycolic and lactic acids, which help new blood vessels grow and rebuild collagen matrices in diabetic and burn wound models [184]. The improved PLGA scaffold closed more than 90% of wounds in 10 days and also sped up granulation and re-epithelialization. This shows that it could be helpful for treating chronic wounds [185]. But to keep the pH level stable during the process, researchers often use PEG mixing or a calcium carbonate buffer. This is because acid decomposition products can irritate the tissues around the wound or make sensitive biomolecules less stable [184]. PCL is a semi-crystalline polymer that slowly dissolves and provides strength and long-term stability. It makes an electrospinning mesh that sticks to uneven wound sites and keeps its shape for up to four weeks [186]. In vivo studies showed a wound closure rate of about 85–90% and a fibroblast proliferation rate of about twice that of the PLGA scaffold, suggesting the mechanical superiority and bio-compatibility of PCL [180]. However, PCL’s high crystallinity and hydrophobicity can make it harder to break down and cause it to release quickly during the first hydration process. Therefore, surface bonding using hydrophilic PEG chains or adhesive peptides was introduced for the improvement of wettability and cell adhesion [96]. E.g., a hydrophilic polymer and surface modifier improves protein stability, solubility, and bio-compatibility while reducing nonspecific protein adsorption [17]. PEG-based hydrogels maintain a moisture retention rate of 90 to 95% under exudate-like conditions, support fibroblast survival rates of over 90%, and reduce bacterial loads by approximately 70% [187]. However, dense PEG corona can inhibit cell adhesion, and although immune recognition for PEG is rare, molecular weight and purity must be carefully managed [188].
5.3. Hydrogel Nanocomposites and Films
Hydrogel nanocomposites combine water-rich polymer networks with therapeutic nanoparticles to heal wounds, control moisture, and give medications at the right time and place [189]. Crosslinked matrices such as gelatin, chitosan, or alginate facilitate conformal contact and elasticity, whereas embedded carriers, including liposomes, silver polyoxometalate clusters, and plant-derived polyphenols, promote antimicrobial activity, near-infrared photothermal heating, and pro-regenerative signals [190,191]. A representative data integration hydrogel system is the GelMA–liposome hybrid matrix, which double-encapsulates small molecules and growth factors to achieve a phased release of peptides and continuous fluorescence expression in vivo while maintaining expansion ability and degradation stability [192]. This hybrid structure allows for spatial and temporal control of transmission and local regeneration signaling, which improves the balance of tissue integrity and angiogenesis in wound environments [193]. Complementary, multifunctional Ag-polyoxometalate (Ag-POM) hydrogels combine catalytic antimicrobial chemistry with mild photothermal effects, effectively inhibiting methicillin-resistant S.aureus (MRSA) and accelerating collagen deposition and epithelial regeneration in whole-layer wound models (Figure 10a–d) [190]. As shown in Figure 10a, the Ag-POM/cha polyphenol/gelatin hybrid hydrogel possesses a modular design that integrates catalytically active oxygen (ROS) regulation and near-infrared (NIR) reactive photothermal sterilization activity. Figure 10b shows a wide range of antimicrobial effects against E. coli, S. aureus, and P. gingivalis, achieving a bacterial reduction rate of >95% in 6 h. Life-and-death fluorescence imaging (Figure 10c) demonstrates efficient bacterial membrane destruction while minimizing fibroblast toxicity (<10%), confirming the biosafety profile [194]. In vivo wound healing experiments (Figure 10d) demonstrate rapid epithelialization, attaining a minimum of 85% healing by day 7, accompanied by organized collagen alignment. This shows how catalytic free oxygen regulation and local photothermal treatment work together to make things better. These results suggest that the combination of catalytic and photothermal properties in the polymeric hydrogel matrix may improve wound healing while reducing excessive thermal accumulation or cytotoxic stress. For clinical application, subsequent studies are required to validate manufacturing stability and biological safety via reproducible degradation kinetics, quantified release profiles, perfusion recovery mapping, and stability assessment of post-sterilization crosslinking agents [188].
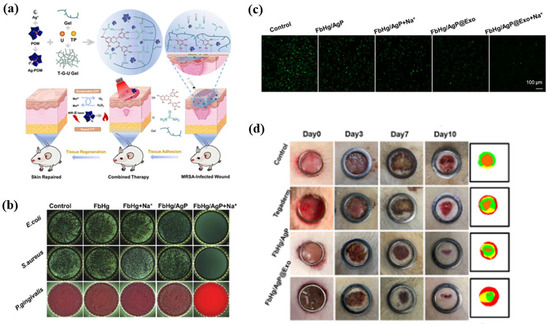
Figure 10.
Multifunctional Ag–polyoxometalate (Ag-POM) hydrogel nanocomposites for infected-wound healing. (a) Schematic of Ag-POM/gelatin–urea hydrogel showing catalytic and photothermal antibacterial effects. (b) Antibacterial activity against E. coli, S. aureus, and P. gingivalis. (c) Live/dead fluorescence images of bacteria after hydrogel treatments. (d) Wound-closure images over 10 days showing markedly accelerated healing in the FbHg/AgP@Exo and FbHg/AgP@Exo + Na⁺ treatment groups. The color-coded maps on the right visualize wound-area quantification, where green represents the initial wound boundary, yellow indicates intermediate healing, and red denotes the remaining unhealed area. Reprinted with permission from Ref. [190]. 2022, Elsevier.
6. Lipid-Based Nanostructure Platforms
6.1. Conventional and Deformable Liposome Platforms
Liposomes are bilayer vesicles that entrap hydrophilic agents in an aqueous core while accommodating lipophilic agents within the membrane, and the membrane composition echoes the stratum corneum, which supports topical delivery [195]. Conventional liposomes frequently stall within superficial layers and lose integrity in protein-rich exudate, which limits access to the dermis and promotes premature leakage [196]. Existing liposomes allow for controlled release, but they do not always penetrate the skin well and are less stable in protein-rich exudate. This can cause drugs to leak out too soon and settle on the surface of the skin [197]. Albumin adsorption and membrane instability usually hurt the integrity of liposomes in physiological conditions, which makes them less bioavailable [198]. To overcome these limitations, modifiable liposomes have been developed to improve bilayer elasticity and hydration sensitivity by incorporating modification activators like sodium cholate, Tween 80, and Span 80 [199]. This flexibility lets it go deeper into the surviving epidermis and dermis than regular liposomes, which makes it easier for drugs to penetrate the skin and wounds without changing the surface morphology too much. But too much surfactant can cause stimulation and make encapsulated biomolecules unstable. This shows how important it is to have the right formulations. Ethosomes that add ethanol to the phospholipid bilayer make the membrane more fluid and the lipids more soluble, which makes the skin more permeable. Formulations with 20–40% ethanol had drug flow rates that were dozens of times higher than those of regular liposomes. However, they can make sensitive skin dry or irritated for a short time [198]. Niosomes are made of nonionic surfactants instead of phospholipids. This makes them more stable and less expensive, and they keep drugs encapsulated well even after long periods of storage [197]. Epidermal penetration depth generally reaches a viable epidermal layer (100–200 µm) depending on surfactant type and vesicular elasticity, but cytotoxicity and protein adsorption vary with formulation [200]. Overall, modifiable ethanol-based nonionic vesicles provide a versatile strategy for improving local and transdermal delivery of antimicrobial and anti-inflammatory drugs while maintaining biocompatibility and structural stability under exudate-like conditions. Figure 11 summarizes these architectures and highlights the need for decision-ready characterization that reports number-based size distributions and surface charge in exudate-like media, monitors lipid oxidation, verifies stability after sterilization and drying, and links transport to outcomes that include biofilm penetration, re-epithelialization, and comfort on removal.
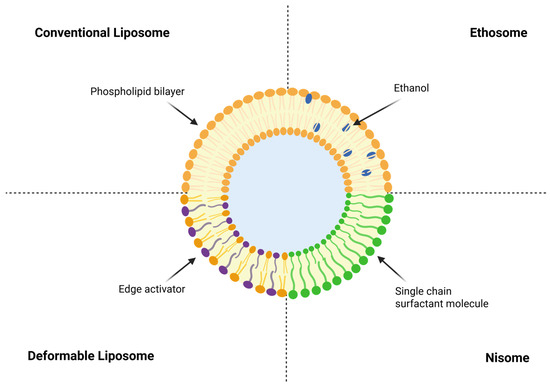
Figure 11.
Schematic representation of liposomal architecture for topical and transdermal delivery. Conventional liposomes consist of a phospholipid bilayer surrounding an aqueous core. Deformable liposomes incorporate edge activators, such as sodium cholate or polysorbates, that enhance bilayer fluidity and facilitate passage through narrow pores. Ethosomes contain ethanol within the bilayer, which increases membrane permeability and improves skin penetration. Niosomes are formed from nonionic surfactants, providing a phospholipid-free and chemically stable alternative.
6.2. Solid Lipid Nanoparticles and Nanostructured Lipid Carrier Platforms
SLNs provide a biocompatible solid lipid matrix that protects unstable active ingredients and allows for long-term use on wound surfaces and follicles [201]. SLN is a matrix that is safe for living things and can be used to deliver drugs through the skin. However, the structure can change when crystals are stored, which can cause internal molecules to leak over time. This can compromise the stability of the encapsulated active ingredient [202]. Hydration cycle and mechanical shear force can also cause rapid release, which leads to a decrease in long-term exposure effects in conditions such as wound exudate [203]. In simulated exudate media containing albumin, standard SLNs typically exhibit significant drug leakage during the first 12 to 24 h, implying limited colloidal stability under physiological stress [204]. NLC addresses certain limitations by generating lattice imperfections through the amalgamation of solid and liquid lipids. This increases drug loading capacity and prevents excretion during the crystallization process. When you change the solid-to-liquid lipid ratio (about 70:30 w/w) and the surfactant concentration, you usually obtain a 1.3 to 1.8 times better encapsulation efficiency and a longer release time (t1/2 ≈ 20 to 30 h) than SLNs [205]. The release kinetics, particle size (100–180 nm), and polydispersity (<0.3) are very dependent on the cooling rate and lipid composition, which affects how stable the material is mechanically and chemically [202]. In a full-layer mouse model, NLC with curcumin and asiaticosides sped up wound healing, with a closure rate of about 85–90% within 12–14 days compared to controls. It also reduced reactive oxygen species by about 30–40% and increased collagen deposition [206]. These therapeutic enhancements stem from the synergies of continuous drug release, antioxidant activity, and the regulation of inflammatory signaling. Nevertheless, key challenges to be addressed in future formulation designs remain emulsion migration, polymorphic conversion, surfactant-associated stimulation, and instability under enzyme- or salt-rich exudate. Figure 12 illustrates a curcumin-loaded nanostructured lipid carrier gel stabilized by acetyl dipeptide-1 cetyl ester that traverses the stratum corneum and distributes through the epidermis into the dermis for sustained delivery, which is promising for antioxidant and anti-inflammatory support but still requires dose and safety verification under wound-relevant conditions. Future development should focus on verifying dose safety and post-sterilization stability under wound-relevant hydration cycles [207].
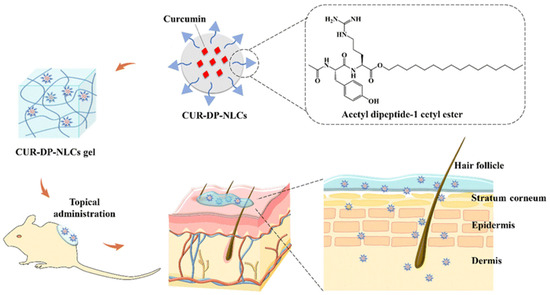
Figure 12.
Schematic representation of curcumin-loaded nanostructured lipid carriers (CUR-DP-NLCs) incorporated into a gel system for topical wound therapy. The CUR-DP-NLCs, stabilized with acetyl dipeptide-1 cetyl ester, enhance curcumin encapsulation and stability. Following topical administration, the formulation penetrates the stratum corneum and distributes through the epidermis into the dermis, enabling sustained curcumin delivery. This process promotes anti-inflammatory and antioxidant effects, reduces bacterial colonization, and accelerates tissue regeneration for effective wound healing. Reprinted with permission from ref. [174]; 2023, RSC Advances.
6.3. Nonlamellar Lipid Mesophase Platforms
Cubosomes and hexosomes are lipid particles that are not lamellar. They have water and lipid channels that connect them, which allows hydrophilic and hydrophobic agents to be loaded at the same time. They also protect fragile peptides from being broken down by enzymes in exudate [208]. Bicontinuous cubic structures promote rapid hydration and possess extensive interfacial areas that enhance cutaneous distribution, whereas inverse hexagonal structures frequently impede outward diffusion and are desirable for prolonged release in dressings [209]. Cubosomes and hexosomes are non-structured lyotropic liquid crystal nanoparticles, suitable for loading various cargoes and protecting unstable active ingredients by forming aqueous–lipid pathways in which double continuous cubic or inverted hexagonal lattices are interconnected [208]. Monoolein-poloxamer cubosome formulations demonstrate the acceleration of wound healing in rodent skin models, supporting their usefulness as skin drug carriers, but the reported closure rates vary with drug loading and model [210]. Hexosome (HII) systems provide more continuous diffusion-restricted release than the cubic phase due to their narrow channel and phase-dependent transport properties, and a cubic ↔ hexagonal phase transition within the physiological range has been reported [211]. In vivo experiments on liquid crystal gels (LLCs) in chronic wound models have demonstrated that the incorporation of bioactive factors expedites wound healing, enhances collagen deposition, and diminishes inflammatory infiltration. Steps like drying, lyophilizing, or sterilizing can change the lattice spacing, which in turn changes the release kinetics and reproducibility [212]. For translation purposes, subsequent reports must encompass particle size, lattice constant, and release performance during the wound-mimicking hydration cycle, which is associated with epithelialization, collagen alignment, and removal efficiency [213]. In general, well-optimized cubic and hexagonal mesophases allow for controllable continuous delivery and are good for the skin, which is pushing this field toward structure–activity-based design instead of qualitative statements.
6.4. Lipid–Polymer Hybrid Vesicle Platforms
Lipid–polymer hybrid vesicles (LPHVs) are the best way to treat local wounds because they combine the biomimetic interfacial behavior of lipids with the mechanical stability and controllable degradation properties of polymers [17]. These hybrid nanostructures create a stable bilayer or core–shell shape, which allows for long-term colloidal stability and controlled drug release (Figure 13a–c). As shown in Figure 10a, the optimized LPHV formulation produces uniform spherical nanoparticles (~150 nm) with ζ-potential in the range of −10 to −25 mV, maintaining structural integrity even under periodic hydration and shear [214]. Drug release analysis (Figure 13b) confirms stable encapsulation and progressive effective load transfer under simulated exudate conditions, showing that they are released without continuous diffusion and burst effects for 24 h [215]. Histological evaluation (Figure 13c(A–D)) indicates that LPHV dressing maintains the epidermis and dermis without inducing inflammation or irritation in vivo, thereby demonstrating enhanced safety and biocompatibility in whole-layer wound models [17]. For clinical applications, manufacturing methods like solvent diffusion, emulsion evaporation, and microfluidic mixing should be improved to ensure that residual solvent removal, sterilization stability, and batch reproducibility are all good [12]. A thorough characteristic analysis must encompass particle size (100–200 nm), ζ-potential (−10–25 mV), encapsulation efficiency, and the determination of half-life of emission in exudate-like media, which should correlate with biofilm inhibition, reepithelialization, and angiogenesis outcomes to establish a solid structure–function relationship [215].
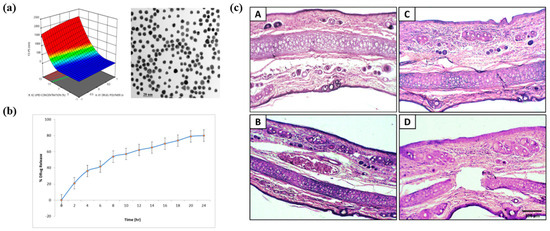
Figure 13.
Characterization, release behavior, and skin tolerance of hydrocortisone-loaded lipid–polymer hybrid nanoparticles (HCL-LPHNs). (a) 3D response surface plot and TEM image showing uniform spherical nanoparticles within the optimized size range. The color gradient in the surface plot represents changes in particle size across the tested formulation variables. (b) Cumulative drug-release profile indicating sustained release of HCL over 24 h. (c) Histological sections (A–D) of rat skin after topical application of L-PHHN formulations showing intact epidermal and dermal layers without significant irritation or inflammatory changes. Reprinted with permission from Ref. [215]. 2023, ACS Publications.
7. Preclinical-to-Clinical Translation and Scalable Production of Smart Wound Dressings
7.1. Preclinical Model Systems for Wound Healing Nanostructure Evaluation
Preclinical model systems help establish the translational value of polymer- and lipid-based nanostructures for wound healing [216,217]. However, common rodent excisional wounds close primarily by contraction and underrepresent ischemia, neuropathy, and polymicrobial biofilm that shape human chronic ulcers, constraining inference [218]. Three-dimensional human skin equivalents with stratified epidermis and dermis, organotypic models that integrate vascular and immune components, ex vivo human skin under controlled perfusion and infection, and porcine full-thickness wounds more faithfully capture epidermal architecture, adnexal structures, and biomechanics [219,220]. Chronic wound surrogates that incorporate protease-rich exudate, reactive oxygen species, recurrent bacterial challenge, impaired perfusion, diabetes, and pressure-related tissue deformation test nanostructure stability, cargo retention, release kinetics, and biofilm control [221].
7.2. Manufacturing, Sterilization, and Scalability
How useful polymer- and lipid-based nanostructures are for treating wounds depends on how scalable they are to make. The size, shape, charge, and release properties of particles are mostly affected by the temperature, the ratio of solvent to solid, the amount of energy added, and the order in which the particles are mixed [222]. Continuous microfluidic mixing gives you precise control and a narrow size range, but you need materials that will not dissolve in the solvent and cleaning methods that have been tested [223]. It is easy to scale up with high-pressure homogenization and wet grinding, but shear and heat need to be controlled to keep the material from breaking down [224]. When combined with automatic feedback control, process analysis techniques (PATs) that use real-time particle size measurements and spectroscopy can help keep quality stable [225]. Sterilization and stability are still big problems. Most nanostructures are ruined by steam and filtration processes. To keep them from clumping together again, freeze-drying with cryoprotective agents like sucrose or trehalose is the best method [226,227]. The cost and purity of the materials also make it hard to sell, which shows how important it is to have an energy-efficient continuous system that follows the rules for solvent recovery and Good Manufacturing Practices (GMP) [228,229,230]. To keep quality high on a large scale, future manufacturing should use closed continuous lines, AI-based supervision, and sensors that have been tested [231].
7.3. Smart and Personalized Wound-Dressing Systems
Smart wound-dressing systems combine polymer and lipid nanostructures with multiple types of sensors, wireless communication, and stimulus-responsive release to make sure that treatment matches the changing biology of the wound [232,233]. Colorimetric, fluorometric, and electrochemical components measure temperature, acidity, glucose, oxygen, bacterial load, reactive oxygen species, and inflammatory cytokines like interleukin-6 and interleukin-10 [234]. Wireless modules send data to patient devices, and heaters, soft electrodes, microneedles, and microfluidic reservoirs give antimicrobials, anti-inflammatory agents, or electrical stimuli when the sensor thresholds are met [234]. Nanostructures that are tuned to internal signals respond to acidity, enzymes, and bacterial metabolites. External signals include light, magnetic fields, electrical current, and controlled warming [235]. Personalization occurs when artificial intelligence analyzes Internet of Things data streams and clinical images to modify dosage and timing. Additionally, three-dimensional printing facilitates the creation of patient-specific geometries using bioinks that incorporate patient-derived cells or factors [236]. The promise is faster closure, less scarring, and fewer visits to the doctor, but calibration in protein-rich and salt-rich exudate, mechanical durability under bending, gentle adhesion that protects periwound skin, reliable power management with safe disposal, and clear data governance for home care are still needed [237]. Figure 14 shows the general idea and functional integration of these smart wound-dressing systems. It shows how polymer- and lipid-based nanostructures can monitor wound-related biomarkers in real time and release drugs in response to stimuli.
Figure 14.
Concept of smart wound dressings that integrate colorimetric, fluorometric, and electrochemical sensors for real-time monitoring of temperature, acidity, glucose, oxygen, bacterial burden, and reactive oxygen species with wireless transmission. Polymer and lipid nanostructures enable endogenous stimulus responsiveness to acidity, enzymes, and bacterial metabolites, and permit exogenous control by light, magnetic field, electric current, and controlled warming for on-demand therapy. The platform illustrates all-in-one wound management that links monitoring and early diagnosis with personalized drug delivery.
8. Conclusions
Polymer- and lipid-based nanostructures present a practical pathway to overcoming the limitations of conventional wound dressing by maintaining local exposure, regulating moisture, reducing microbial load, and inducing angiogenesis and extracellular matrix organization. The evidence summarized in this review highlights the recurring risks that need to be addressed prior to extensive clinical applications, including excessive dependence on aqueous properties, instability in proteases and salt-rich exudates, decomposition of active ingredients during sterilization or drying processes, ligand obstruction in protein body fluids, and dependence on rodent wounds that heal through contraction rather than reepithelialization. Progress will be stronger when development links ex vivo human skin and porcine models with predefined, patient-centered outcomes such as time to closure, pain on removal, quality of the regenerated tissue, and reduction in biofilm burden, while reporting number-based size distributions, surface charge in wound-like media, oxidation markers, residual solvent profiles, and post-sterilization performance. Continuous mixing, process analytical technologies, and verified cleaning and containment can all help with scalable manufacturing. It is also important to think about the cost, supply security, and environmental impact of single-use parts early on. In the future, hybrid nanostructures ought to develop into multipurpose smart dressings that integrate wireless data transmission, adaptive drug release, and real-time biosensing. By combining polymer–lipid platforms with the Internet of Things and AI-driven analytics, it may be possible to remotely monitor infection biomarkers, pH, and temperature, directing on-demand therapeutic release with little patient involvement. Furthermore, in order to balance clinical effectiveness with environmental responsibility and make sure that advanced wound care is in line with the principles of circular healthcare, it will be essential to design sustainable, bio-derived, and recyclable materials. Smart dressings that use nanostructures, the Internet of Things, and artificial intelligence (AI) can personalize care through monitored triggers and responsive release, provided that calibration in complex exudate, gentle adhesion at fragile margins, reliable low-profile power, safe disposal, and strong data governance are in place. In the short term, the focus should be on strong formulations that clearly help with re-epithelialization, infection control, vascularization, and scar quality. After that, targeted clinical trials should be performed to compare performance to current standards while being open about methods and materials.
Author Contributions
Conceptualization, J.-H.Y. and H.-O.K.; investigation, E.C., S.Y., S.L., M.K., J.C., S.E.C., K.S.L. and S.-J.H.; writing—original draft preparation, J.-H.Y., H.-O.K., E.C. and S.Y.; writing—review and editing, J.-H.Y. and H.-O.K.; visualization, S.L., M.K., J.C., S.E.C., K.S.L. and S.-J.H.; supervision, J.-H.Y. and H.-O.K.; funding acquisition, H.-O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea grant funded by the Korean government through the Korea Institute of Planning (RS-2025-00512586). This research was supported by the Regional Innovation System & Education (RISE) Glocal University 30 Project program, funded by the Ministry of Education (MOE) and the Gangwon State (G.S.), Republic of Korea (2025-RISE-10-002).
Data Availability Statement
No new data were created for this study. All data analyzed in this review are available in the publications cited in the references section.
Acknowledgments
The authors would like to thank their colleagues at Kangwon National University for their helpful discussions and advice during the preparation of this manuscript.
Conflicts of Interest
The authors declare no commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PNPs | Polymer nanoparticles |
| RESS | rapid expansion of supercritical solutions |
| RESOLV | rapid expansion of supercritical solution into liquid solvent |
| RAFT | reversible addition fragmentation chain transfer |
| ATRP | atom transfer radical polymerization |
| PLGA | Polylactide-co-glycolide |
| PEG | Polyethylene Glycol |
| PLA | Polylactic acid |
| PCL | Polycaprolactone |
| EA | Ethyl Acetate |
| DCM | Dichloromethane |
| PVA | Poly(vinyl alcohol) |
| NAs | Nucleic Acids |
| AC | Acetonitrile |
| EE | Encapsulation Efficiency |
| W/O/W | Oil-in-Water |
| PEO | Polyethtlene oxide |
| LNPs | Nanostructured Lipid Carriers |
| SLNs | Solid Lipid Nanoparticle |
| ROS | Reactive Oxygen Species |
| TFH | Thin Film Hydration |
| EI | Ethanol Injection |
| REV | Reverse-Phase Evaporation Vesicle |
| DR | Dehydration–Rehydration |
| HPH | High-Pressure Homogenization |
| MLV | Multilamellar Vesicle |
| LUV | Large Unilamellar Vesicle |
| SCF | Supercritical Fluid |
| PCS | Photon Correlation Spectroscopy |
| US | Ultrasound |
| DLS | Dynamic Light Scattering |
| XRD | X-ray Diffraction |
| PLHN | Polymer–Lipid Hybrid Platforms |
| LbL | Layer-by-Layer assembly |
| NLCs | Nanostructured lipid carriers |
| RNA | Ribonucleic Acid |
References
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Bhupendra, G.P. Advances in chitosan based nanocarriers for targetted wound healing therapies: A review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]
- Hao, G.; Qi, Z.; Li, L.; Xu, Z.P. Investigation of the mucin-nanoparticle interactions via real-time monitoring by microbalance and kinetic model simulation. J. Colloid Interface Sci. 2024, 661, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef]
- Gowda, B.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.; Ghosh, S.; Ansari, M.; Pramanik, S. Nanoparticle-based therapeutic approaches for wound healing: A review of the state-of-the-art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Mo, Y.; Zhou, T.; Li, W.; Niu, Y.; Sheu, C. Advances in Nanohybrid Hydrogels for Wound Healing: From Functional Mechanisms to Translational Prospects. Gels 2025, 11, 483. [Google Scholar] [CrossRef]
- Ardhi, A.; Raharjo, S.; Sudjarwo, W.A.A.; Schreiner, M. Oxidative stability of optimized nanostructured lipid carriers containing thymoquinone-rich oil. J. Am. Oil Chem. Soc. 2025, 102, 793–810. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Tsung, T.-H.; Tsai, Y.-C.; Lee, H.-P.; Chen, Y.-H.; Lu, D.-W. Biodegradable polymer-based drug-delivery systems for ocular diseases. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, L.; Li, B.; Reis, R.L.; Kundu, S.C.; Duan, L.; Xiao, B.; Yang, X. Tissue adhesives based on chitosan for skin wound healing: Where do we stand in this era? A review. Int. J. Biol. Macromol. 2024, 258, 129115. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Holz, E.; Darwish, M.; Tesar, D.B.; Shatz-Binder, W. A review of protein-and peptide-based chemical conjugates: Past, present, and future. Pharmaceutics 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sun, Z.; Wang, Q.; Ju, Y.; Sun, N.; Yue, Q.; Deng, Y.; Liu, S.; Yang, S.; Wang, Z. Core–Shell Magnetic Particles: Tailored Synthesis and Applications. Chem. Rev. 2024, 125, 972–1048. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Ali, A.A.; Abdelaziz, A.E.; Nada, D.; Abdelraouf, S.M.; Fouad, S.A.; Bishr, A.; Radwan, R.A. Newly designed curcumin-loaded hybrid nanoparticles: A multifunctional strategy for combating oxidative stress, inflammation, and infections to accelerate wound healing and tissue regeneration. BMC Biotechnol. 2025, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.K.; Javed, Y.; Shad, N.A.; Shahid, M.; Akhtar, B.; Yasin, E.; Sharma, S.K.; Thanh, N.T.K. Polymer coated magnesium hydroxide nanoparticles for enhanced wound healing. N. J. Chem. 2024, 48, 17396–17410. [Google Scholar] [CrossRef]
- Lok, K.-H.; Loo, H.L.; Chuah, L.-H. Topical and transdermal lipid-polymer hybrid nanoparticles (LPN): An integration in advancing dermatological treatments. Drug Deliv. Transl. Res. 2025, 15, 4277–4313. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Wang, Y.; Li, F.; Man, J.; Li, J.; Zhang, C.; Peng, S.; Wang, S. Advances in chitosan-based wound dressings: Modifications, fabrications, applications and prospects. Carbohydr. Polym. 2022, 297, 120058. [Google Scholar] [CrossRef]
- Del Olmo, J.A.; Alonso, J.M.; Sáez-Martínez, V.; Benito-Cid, S.; Moreno-Benítez, I.; Bengoa-Larrauri, M.; Pérez-González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Self-healing, antibacterial and anti-inflammatory chitosan-PEG hydrogels for ulcerated skin wound healing and drug delivery. Biomater. Adv. 2022, 139, 212992. [Google Scholar]
- Go, Y.K.; Leal, C. Polymer–lipid hybrid materials. Chem. Rev. 2021, 121, 13996–14030. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Kim, B.; Yang, J.; Hwang, M.; Choi, J.; Kim, H.-O.; Jang, E.; Lee, J.H.; Ryu, S.-H.; Suh, J.-S.; Huh, Y.-M. Aptamer-modified magnetic nanoprobe for molecular MR imaging of VEGFR2 on angiogenic vasculature. Nanoscale Res. Lett. 2013, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-O.; Son, H.-Y.; Lee, S.-B.; Jang, E.; Kang, B.; Haam, S.; Lim, E.-K.; Huh, Y.-M. Magnetic nanovector enabling miRNA-34a delivery for CD44 suppression with concurrent MR imaging. J. Nanosci. Nanotechnol. 2016, 16, 12939–12946. [Google Scholar] [CrossRef]
- Zhang, Q.; Heuchel, M.; Thüneman, A.F.; Machatschek, R. The role of diffusion in the hydrolytic degradation of poly (lactic-co-glycolic acid): A molecular perspective. Polym. Degrad. Stab. 2025, 232, 111119. [Google Scholar] [CrossRef]
- Doole, F.T.; Kumarage, T.; Ashkar, R.; Brown, M.F. Cholesterol stiffening of lipid membranes. J. Membr. Biol. 2022, 255, 385–405. [Google Scholar] [CrossRef]
- Chen, J.; Mu, Z.; Chen, D.; Huang, C.; Jin, T.; Li, L.; Zeng, Y.; Zhou, Q.; Zhang, Y.; Mao, H. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem. Eng. J. 2023, 469, 143985. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; van Leur, H.; Krijgsman, D.; Ecker, V.; Braun, M.; Buchner, M.; Fens, M.H.; Hennink, W.E.; Schiffelers, R.M. Utilizing in vitro drug release assays to predict in vivo drug retention in micelles. Int. J. Pharm. 2022, 618, 121638. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Wang, Y.; Chen, W.; Zhu, Z.; Wang, S. Hydrogels and hydrogel-based drug delivery systems for promoting refractory wound healing: Applications and prospects. Int. J. Biol. Macromol. 2024, 285, 138098. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Li, J.; Pan, S.; Miao, X. Particle size effect of curcumin nanocrystals on transdermal and transfollicular penetration by hyaluronic acid-dissolving microneedle delivery. Pharmaceuticals 2022, 15, 206. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart pH-responsive nanomedicines for disease therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising nanostructured materials in fluid management, healing and regeneration of wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.-i.; Sakai-Kato, K. Current status and challenges of analytical methods for evaluation of size and surface modification of nanoparticle-based drug formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Haque, M.A.; Shrestha, A.; Mikelis, C.M.; Mattheolabakis, G. Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery. Int. J. Pharm. X 2024, 8, 100283. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Wilson, D.R.; Green, J.J. Nanoparticle tracking analysis for determination of hydrodynamic diameter, concentration, and zeta-potential of polyplex nanoparticles. In Biomedical Nanotechnology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 31–46. [Google Scholar]
- Koliqi, R.; Uskoković, V.; Selmani, P.B.; Grapci, A.D.; Mahapatra, C. Polymeric nanoparticles for wound healing. Pharm. Nanotechnol. 2025, 13, 775–793. [Google Scholar] [CrossRef]
- Park, C.; Lim, J.-W.; Park, G.; Kim, H.-O.; Lee, S.; Kwon, Y.H.; Kim, S.-E.; Yeom, M.; Na, W.; Song, D. Kinetic stability modulation of polymeric nanoparticles for enhanced detection of influenza virus via penetration of viral fusion peptides. J. Mater. Chem. B 2021, 9, 9658–9669. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical antisolvent process for pharmaceutical applications: A review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Cunningham, M.F. Controlled/living radical polymerization in aqueous dispersed systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Araujo, P.H.H.d.; Sayer, C.; Giudici, R.; Poço, J. Techniques for reducing residual monomer content in polymers: A review. Polym. Eng. Sci. 2002, 42, 1442–1468. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- McCormack, A.; Stone, V.; McQuat, J.; Johnston, H. Investigating the impact of the dispersion protocol on the physico-chemical identity and toxicity of nanomaterials: A review of the literature with focus on TiO2 particles. Part. Fibre Toxicol. 2025, 22, 11. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.-O.; Kang, J.-T. Optimization of nano-encapsulation on neonatal porcine islet-like cell clusters using polymersomes. Nanoscale Res. Lett. 2021, 16, 53. [Google Scholar] [CrossRef]
- Li, M.; McClements, D.J.; Liu, X.; Liu, F. Design principles of oil-in-water emulsions with functionalized interfaces: Mixed, multilayer, and covalent complex structures. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3159–3190. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidon—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2015, 6, 1. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A. Long-term stability of different kinds of gas nanobubbles in deionized and salt water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Prado-Prone, G.; Díaz-Ballesteros, A.; González-Torres, M.; Silva-Bermudez, P.; Sánchez-Sánchez, R. Nanoparticle and nanomaterial involvement during the wound healing process: An update in the field. J. Nanoparticle Res. 2023, 25, 27. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Kozalak, G.; Heyat Davoudian, S.; Natsaridis, E.; Gogniat, N.; Koşar, A.; Tagit, O. Optimization of PLGA nanoparticle formulation via microfluidic and batch nanoprecipitation techniques. Micromachines 2025, 16, 972. [Google Scholar] [CrossRef]
- Bovone, G.; Cousin, L.; Steiner, F.; Tibbitt, M.W. Solvent controls nanoparticle size during nanoprecipitation by limiting block copolymer assembly. Macromolecules 2022, 55, 8040–8048. [Google Scholar] [CrossRef]
- Seegobin, N.; Abdalla, Y.; Li, G.; Murdan, S.; Shorthouse, D.; Basit, A.W. Optimising the production of PLGA nanoparticles by combining design of experiment and machine learning. Int. J. Pharm. 2024, 667, 124905. [Google Scholar] [CrossRef]
- Kuddushi, M.; Kanike, C.; Xu, B.B.; Zhang, X. Recent advances in nanoprecipitation: From mechanistic insights to applications in nanomaterial synthesis. Soft Matter 2025, 21, 2759–2781. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- El Amri, N.; McKinstry, A.; Pollard, R.E.; Lewis, P.K.; Pinkerton, N.M. A Comparative Study of Flash Nanoprecipitation and Sequential Nanoprecipitation: Impact of Formulation Parameters on Drug-Loaded Nanoparticle Formation. Mol. Pharm. 2025, 22, 6108–6119. [Google Scholar] [CrossRef]
- Zander, A.J.; Ehrlich, M.-S.; ur Rehman, S.; Schneider, M. Evaporation-triggered nanoprecipitation for PLGA nanoparticle formation using a spinning-disc system. J. Drug Deliv. Sci. Technol. 2025, 108, 106901. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Ma, A.; Bai, X.; Zeng, Y.; Liu, D.; Liu, B.; Zhang, W.; Tang, S. Recent advances in coaxial electrospun nanofibers for wound healing. Mater. Today Bio 2024, 29, 101309. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, L.; Song, W. Recent progress of electrospun nanofiber dressing in the promotion of wound healing. Polymers 2024, 16, 2596. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrins, M.; Laidmae, I.; Tenson, T.; Kogermann, K. Antibacterial porous electrospun fibers as skin scaffolds for wound healing applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Silva, T.J.; da Silva, M.G.; Hashimoto, J.C.; Ribeiro, A.P.B. Lipid nanoparticles: Formulation, production methods and characterization protocols. Foods 2025, 14, 973. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab A Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Mueller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Sakr, O.S.; Borchard, G. Encapsulation of enzymes in Layer-by-Layer (LbL) structures: Latest advances and applications. Biomacromolecules 2013, 14, 2117–2135. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Borchard, G.; Jordan, O. An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent progress of lipid nanoparticles-based lipophilic drug delivery: Focus on surface modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Chabaud, S.; Fradette, J.; Rioux, Y.; Bolduc, S. Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix. Bioengineering 2025, 12, 1077. [Google Scholar] [CrossRef]
- Xiang, B.; Cao, D.-Y. Preparation of drug liposomes by thin-film hydration and homogenization. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–11. [Google Scholar]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- M Rabanel, J.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Sedaghat, M.; Hoseini, A.; Mohammadi, N.; Bodaghi, M. Combinational system of lipid-based nanocarriers and biodegradable polymers for wound healing: An updated review. J. Funct. Biomater. 2023, 14, 115. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, P.; Poovizhi, P.; Ng, W.; Tan, R. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent emulsification evaporation and solvent emulsification diffusion techniques for nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 287–300. [Google Scholar]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Kumar, S.; Chand, P.; Chauhan, A.S.; Jakhmola, V. Nanostructured lipid carriers (NLCs): A comprehensive review of drug delivery advancements. J. Appl. Pharm. Res. 2025, 13, 20–38. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Pezeshki, A. Development of heat-stable gelatin-coated nanostructured lipid carriers (NLC): Colloidal and stability properties. LWT 2022, 160, 113265. [Google Scholar] [CrossRef]
- Rahdar, A.; Amini, N.; Askari, F.; Susan, M.A.B.H. Dynamic light scattering and zeta potential measurements: Effective techniques to characterize therapeutic nanoparticles. J. Nanoanal. 2019, 6, 80–89. [Google Scholar]
- Tetyczka, C.; Hodzic, A.; Kriechbaum, M.; Juraić, K.; Spirk, C.; Hartl, S.; Pritz, E.; Leitinger, G.; Roblegg, E. Comprehensive characterization of nanostructured lipid carriers using laboratory and synchrotron X-ray scattering and diffraction. Eur. J. Pharm. Biopharm. 2019, 139, 153–160. [Google Scholar] [CrossRef]
- de Almeida Campos, L.A.; de Souza, J.B.; de Queiroz Macêdo, H.L.R.; Borges, J.C.; de Oliveira, D.N.; Cavalcanti, I.M.F. Synthesis of polymeric nanoparticles by double emulsion and pH-driven: Encapsulation of antibiotics and natural products for combating Escherichia coli infections. Appl. Microbiol. Biotechnol. 2024, 108, 351. [Google Scholar] [CrossRef]
- Rajabifar, N.; Rostami, A.; Afshar, S.; Mosallanezhad, P.; Zarrintaj, P.; Shahrousvand, M.; Nazockdast, H. Wound dressing with electrospun Core-Shell nanofibers: From material selection to synthesis. Polymers 2024, 16, 2526. [Google Scholar] [CrossRef]
- Fathi, F.; Machado, T.O.; de AC Kodel, H.; Portugal, I.; Ferreira, I.O.; Zielinska, A.; Oliveira, M.B.P.; Souto, E.B. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the delivery of bioactives sourced from plants: Part I—Composition and production methods. Expert Opin. Drug Deliv. 2024, 21, 1479–1490. [Google Scholar] [CrossRef]
- Deol, P.K.; Kaur, I.P.; Dhiman, R.; Kaur, H.; Sharma, G.; Rishi, P.; Ghosh, D. Investigating wound healing potential of sesamol loaded solid lipid nanoparticles: Ex-vivo, in vitro and in-vivo proof of concept. Int. J. Pharm. 2024, 654, 123974. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Ali, A. Nanostructured lipid carrier for transdermal gliclazide delivery: Development and optimization by Box-Behnken design. Inorg. Nano-Met. Chem. 2024, 54, 474–487. [Google Scholar] [CrossRef]
- Wathoni, N.; Suhandi, C.; Elamin, K.M.; Lesmana, R.; Hasan, N.; Mohammed, A.F.A.; El-Rayyes, A.; Wilar, G. Advancements and challenges of nanostructured lipid carriers for wound healing applications. Int. J. Nanomed. 2024, 19, 8091–8113. [Google Scholar] [CrossRef] [PubMed]
- Godase, S.S.; Kulkarni, N.S.; Dhole, S.N. A comprehensive review on novel lipid-based nano drug delivery. Adv. Pharm. Bull. 2023, 14, 34. [Google Scholar] [CrossRef]
- Verma, J.; Singh, N.K.; Bansal, K.K. Recent patents in polymer–lipid hybrid nanoparticles technology. Ther. Deliv. 2024, 15, 489–493. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Ávila-Orta, C.A.; González-Morones, P.; Agüero-Valdez, D.; González-Sánchez, A.; Martínez-Colunga, J.G.; Mata-Padilla, J.M.; Cruz-Delgado, V.J. Ultrasound-Assisted Melt Extrusion of Polymer Nanocomposites. Recent Evol. 2019, 163. [Google Scholar] [CrossRef]
- Lebreton, V.; Legeay, S.; Vasylaki, A.; Lagarce, F.; Saulnier, P. Protein corona formation on lipidic nanocapsules: Influence of the interfacial PEG repartition. Eur. J. Pharm. Sci. 2023, 189, 106537. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Giraldo-Gomez, D.M.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D.; González-Del Carmen, M.; González-Torres, M.; Cortés, H.; Leyva-Gómez, G. Insights into terminal sterilization processes of nanoparticles for biomedical applications. Molecules 2021, 26, 2068. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; You, W.; Huang, G.; Tian, W.; Huselstein, C.; Wu, C.-L.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial and angiogenic wound dressings for chronic persistent skin injury. Chem. Eng. J. 2021, 404, 126525. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. From composition to cure: A systems engineering approach to anticancer drug carriers. Angew. Chem. Int. Ed. 2017, 56, 6712–6733. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Larrota, J.S.; Eckhard, U. An introduction to bacterial biofilms and their proteases, and their roles in host infection and immune evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.M.; Chen, W.J.; Qin, Y.; Xu, D.; Lai, Y.K.; He, S.H. Innovative hydrogel design: Tailoring immunomodulation for optimal chronic wound recovery. Adv. Sci. 2025, 12, 2412360. [Google Scholar] [CrossRef]
- Zhou, R.; Xue, S.; Cheng, Y.; Chen, Y.; Wang, Y.; Xing, J.; Liu, H.; Xu, Y.; Lin, Y.; Pei, Z. Macrophage membrane-camouflaged biomimetic nanoparticles for rheumatoid arthritis treatment via modulating macrophage polarization. J. Nanobiotechnol. 2024, 22, 578. [Google Scholar] [CrossRef]
- Tronchin, S.; Forster, J.C.; Hickson, K.; Bezak, E. Dosimetry in targeted alpha therapy. A systematic review: Current findings and what is needed. Phys. Med. Biol. 2022, 67, 09TR01. [Google Scholar] [CrossRef]
- Smith, A.M.; Johnston, K.A.; Crawford, S.E.; Marbella, L.E.; Millstone, J.E. Ligand density quantification on colloidal inorganic nanoparticles. Analyst 2017, 142, 11–29. [Google Scholar] [CrossRef]
- Marden, S.; Campbell, J.M.; Adams, N.; Coelho, R.; Foti, C.; Franca, J.R.; Hostyn, S.; Huang, Z.; Ultramari, M.; Zelesky, T. Mass balance in pharmaceutical stress testing: A review of principles and practical applications. AAPS J. 2024, 26, 96. [Google Scholar] [CrossRef]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) prepared by microwave and ultrasound-assisted synthesis: Promising green strategies for the nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-coated PLGA nanoparticles loaded with peganum harmala alkaloids with promising antibacterial and wound healing activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Ouyang, L.; Lin, C.; Zeng, R.; Liu, G.; Zhou, W. Lipid nanoparticle: Advanced drug delivery systems for promotion of angiogenesis in diabetic wounds. J. Liposome Res. 2025, 35, 76–85. [Google Scholar] [CrossRef]
- Yang, H.; Lv, D.; Qu, S.; Xu, H.; Li, S.; Wang, Z.; Cao, X.; Rong, Y.; Li, X.; Wu, H. A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing. Adv. Sci. 2024, 11, 2403219. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef]
- Rodríguez, D.A.; Vader, P. Extracellular vesicle-based hybrid systems for advanced drug delivery. Pharmaceutics 2022, 14, 267. [Google Scholar] [CrossRef]
- Cao, W.; Peng, S.; Yao, Y.; Xie, J.; Li, S.; Tu, C.; Gao, C. A nanofibrous membrane loaded with doxycycline and printed with conductive hydrogel strips promotes diabetic wound healing in vivo. Acta Biomater. 2022, 152, 60–73. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Li, P.; Hao, X.; Yang, X.; Xi, G.; Liu, W.; Feng, Y.; He, H.; Shi, C. Polysaccharide based hemostatic strategy for ultrarapid hemostasis. Macromol. Biosci. 2020, 20, 1900370. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Wang, Y.; Sun, L.; Fan, X.; Wang, X.; Bai, J. Research Progress on the Application of Novel Wound Healing Dressings in Different Stages of Wound Healing. Pharmaceutics 2025, 17, 976. [Google Scholar] [CrossRef]
- Haririan, Y.; Elahi, A.; Shadman-Manesh, V.; Rezaei, H.; Mohammadi, M.; Asefnejad, A. Advanced nanostructured biomaterials for accelerated wound healing: Insights into biological interactions and therapeutic innovations: A comprehensive review. Mater. Des. 2025, 258, 114698. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef]
- Baghirova, L.; Kaya Tilki, E.; Ozturk, A.A. Evaluation of cell proliferation and wound healing effects of vitamin A palmitate-loaded PLGA/chitosan-coated plga nanoparticles: Preparation, characterization, release, and release kinetics. ACS Omega 2023, 8, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Aghmiuni, A.I.; Keshel, S.H.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–polymer hybrid nanosystems: A rational fusion for advanced therapeutic delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Chen, R.; Wang, D.; Mai, H.; Zhong, Q.; Ning, Y.; Fu, J.; Tang, Z.; Xu, Y. NIR-activated electrospun nanodetonator dressing enhances infected diabetic wound healing with combined photothermal and nitric oxide-based gas therapy. J. Nanobiotechnol. 2024, 22, 232. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.-O.; Park, K.-H.; Wie, M.-B.; Choi, S.-E.; Yun, J.-H. A 60% edible ethanolic extract of ulmus davidiana inhibits vascular endothelial growth factor-induced angiogenesis. Molecules 2021, 26, 781. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, H.; Li, H.; Feng, Y.; Zhao, X.; Zheng, X.; Xu, J.; Cai, X.; Zhou, X.; Wen, J. ROS-responsive nanocomposite hydrogel dressing accelerates diabetic wound healing through modulation of the inflammatory microenvironment. Int. J. Pharm. 2025, 678, 125727. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Luo, Y.; Feng, M.; Yang, Q.; Tang, Y.; Tang, Z.; Xiao, W.; Zheng, Y.; Li, L. Design strategies and application potential of multifunctional hydrogels for promoting angiogenesis. Int. J. Nanomed. 2024, 19, 12719–12742. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Wang, G.; Wang, J.; Wu, D.; Wang, Y. Dual-functional 3D-printed porous bioactive scaffold enhanced bone repair by promoting osteogenesis and angiogenesis. Mater. Today Bio 2024, 24, 100943. [Google Scholar] [CrossRef] [PubMed]
- Heo, T.-H.; Jang, H.-J.; Jeong, G.-J.; Yoon, J.-K. Hydrogel-based nitric oxide delivery systems for enhanced wound healing. Gels 2025, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xu, J.; Wang, X.; Jiang, S.; Zheng, Y.; Liu, Z.; Jia, Z.; Jia, Z.; Lu, X. Smart hydrogels for tissue regeneration. Macromol. Biosci. 2024, 24, 2300339. [Google Scholar] [CrossRef]
- Thangudu, S.; Su, C.-H. Review of light activated antibacterial nanomaterials in the second biological window. J. Nanobiotechnol. 2025, 23, 293. [Google Scholar] [CrossRef]
- Moya, S.E.; Hernández, R.R.; Angelomé, P.C. Degradation of Mesoporous Silica Materials in Biological Milieu: The Gateway for Therapeutic Applications. Adv. NanoBiomed Res. 2024, 4, 2400005. [Google Scholar] [CrossRef]
- Lazarus, E.; Barnum, L.; Ramesh, S.; Quint, J.; Samandari, M.; Laflamme, S.; Secord, T.W.; Schmidt, T.; Tamayol, A.; Rivero, I.V. Engineering tools for stimulating wound healing. Appl. Phys. Rev. 2024, 11, 021304. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, G.; Li, H. The role of extracellular matrix in wound healing. Dermatol. Surg. 2023, 49, S41–S48. [Google Scholar] [CrossRef]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of electrospun PLGA/collagen scaffolds on cell adhesion, viability, and collagen release: Potential applications in tissue engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef]
- Bogdanović, B.; Fagret, D.; Ghezzi, C.; Montemagno, C. Integrin targeting and beyond: Enhancing cancer treatment with dual-targeting RGD (arginine–glycine–aspartate) strategies. Pharmaceuticals 2024, 17, 1556. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local drug delivery strategies towards wound healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef] [PubMed]
- Hora, S.; Wuestefeld, T. Liver injury and regeneration: Current understanding, new approaches, and future perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Bhagia, A.; Lerman, A. Optimization of polycaprolactone fibrous scaffold for heart valve tissue engineering. Biomed. Mater. 2019, 14, 065014. [Google Scholar] [CrossRef]
- Penoy, N.; Grignard, B.; Evrard, B.; Piel, G. A supercritical fluid technology for liposome production and comparison with the film hydration method. Int. J. Pharm. 2021, 592, 120093. [Google Scholar] [CrossRef]
- Weng, T.; Yang, M.; Zhang, W.; Jin, R.; Xia, S.; Zhang, M.; Wu, P.; He, X.; Han, C.; Zhao, X. Dual gene-activated dermal scaffolds regulate angiogenesis and wound healing by mediating the coexpression of VEGF and angiopoietin-1. Bioeng. Transl. Med. 2023, 8, e10562. [Google Scholar] [CrossRef]
- Li, Z.; Tan, G.; Xie, H.; Lu, S. The application of regenerated silk fibroin in tissue repair. Materials 2024, 17, 3924. [Google Scholar] [CrossRef]
- Han, Y.; Shao, Z.; Zhang, Y.; Zhao, H.; Sun, Z.; Yang, C.; Tang, H.; Han, Y.; Gao, C. 3D matrix stiffness modulation unveils cardiac fibroblast phenotypic switching. Sci. Rep. 2024, 14, 17015. [Google Scholar] [CrossRef]
- Ferraz, M.P. Wound dressing materials: Bridging material science and clinical practice. Appl. Sci. 2025, 15, 1725. [Google Scholar] [CrossRef]
- Giardina, F.; Padrón, R.S.; Stocker, B.D.; Schumacher, D.L.; Seneviratne, S.I. Large biases in the frequency of water limitation across Earth system models. Commun. Earth Environ. 2025, 6, 469. [Google Scholar] [CrossRef]
- Boase, N.R.; Gillies, E.R.; Goh, R.; Kieltyka, R.E.; Matson, J.B.; Meng, F.; Sanyal, A.; Sedlacek, O. Stimuli-responsive polymers at the interface with biology. Biomacromolecules 2024, 25, 5417–5436. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, J.; Tian, Y.; Su, B.; Zeng, M.; Wu, C.; Wei, D.; Sun, J.; Luo, H.; Fan, H. Temperature-responsive hydrogel system integrating wound temperature monitoring and on-demand drug release for sequentially inflammatory process regulation of wound healing. ACS Appl. Mater. Interfaces 2024, 16, 67444–67457. [Google Scholar] [CrossRef]
- Kim, H.O.; Yeom, M.; Kim, J.; Kukreja, A.; Na, W.; Choi, J.; Kang, A.; Yun, D.; Lim, J.W.; Song, D. Reactive Oxygen Species-Regulating Polymersome as an Antiviral Agent against Influenza Virus. Small 2017, 13, 1700818. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, M.-Y.; Lee, S.; Park, J.; Kang, J.H.; Cho, H.; Kim, K.-B.; Son, S.J.; Cheng, X.W.; Lee, Y.J. Nitric oxide releasing nanofiber stimulates revascularization in response to ischemia via cGMP-dependent protein kinase. PLoS ONE 2024, 19, e0303758. [Google Scholar]
- Gao, Y.; Chen, X.; He, C.; Zhang, Z.; Yu, J. Stimulus-responsive hydrogels for diabetic wound management via microenvironment modulation. Biomater. Sci. 2025, 13, 3192–3212. [Google Scholar] [CrossRef] [PubMed]
- Bei, Z.; Ye, L.; Tong, Q.; Ming, Y.; Yang, T.; Zhu, Y.; Zhang, L.; Li, X.; Deng, H.; Liu, J. Thermostimulated shrinking and adhesive hydrogel dressing for treating chronic diabetic wounds. Cell Rep. Phys. Sci. 2024, 5, 102289. [Google Scholar] [CrossRef]
- Hasan, N.; Jiafu, C.; Mustopa, A.Z.; Himawan, A.; Umami, R.N.; Ullah, M.; Wathoni, N.; Yoo, J.-W. Recent advancements of nitric oxide-releasing hydrogels for wound dressing applications. J. Pharm. Investig. 2023, 53, 781–801. [Google Scholar] [CrossRef]
- Le Thi, P.; Tran, D.L.; Park, K.M.; Lee, S.; Oh, D.H.; Park, K.D. Biocatalytic nitric oxide generating hydrogels with enhanced anti-inflammatory, cell migration, and angiogenic capabilities for wound healing applications. J. Mater. Chem. B 2024, 12, 1538–1549. [Google Scholar] [CrossRef]
- Younes, S.; Ahmad, S.M.; Thirabowonkitphithan, P.; Abunasser, S.H.; Zein, N.; Elhadad, A.; Leelahavanichkul, A.; Laiwattanapaisal, W.; Al-Otoom, A.; Mahmoud, K.A. Two-Dimensional Magnesium Phosphate Nanosheets Promote Antibacterial Effects and Wound Closure. Int. J. Nanomed. 2025, 20, 12103–12115. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Liebner, F.; Alihosseini, F.; Quartinello, F.; Guebitz, G.M.; Ribitsch, D. Approaches to control and monitor protease levels in chronic wounds. Adv. Ther. 2024, 7, 2300396. [Google Scholar] [CrossRef]
- Ghahremani-Nasab, M.; Akbari-Gharalari, N.; Rahmani Del Bakhshayesh, A.; Ghotaslou, A.; Ebrahimi-Kalan, A.; Mahdipour, M.; Mehdipour, A. Synergistic effect of chitosan-alginate composite hydrogel enriched with ascorbic acid and alpha-tocopherol under hypoxic conditions on the behavior of mesenchymal stem cells for wound healing. Stem Cell Res. Ther. 2023, 14, 326. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1595–1622. [Google Scholar] [CrossRef]
- Mirhaji, S.S.; Soleimanpour, M.; Derakhshankhah, H.; Jafari, S.; Mamashli, F.; Rooki, M.; Karimi, M.R.; Nedaei, H.; Pirhaghi, M.; Motasadizadeh, H. Design, optimization and characterization of a novel antibacterial chitosan-based hydrogel dressing for promoting blood coagulation and full-thickness wound healing: A biochemical and biophysical study. Int. J. Biol. Macromol. 2023, 241, 124529. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Hu, C.; Li, P.; Zhao, M.; Lu, J.; Xia, G. A fucoidan-gelatin wound dressing accelerates wound healing by enhancing antibacterial and anti-inflammatory activities. Int. J. Biol. Macromol. 2022, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef]
- Chegu Krishnamurthi, M.; Tiwari, S.; Veera Bramhachari, P.; Swarnalatha, G. Exploitation of Marine-Derived Multifunctional Biomaterials in Biomedical Engineering and Drug Delivery. In Marine Bioactive Molecules for Biomedical and Pharmacotherapeutic Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 231–250. [Google Scholar]
- Cortesi, R.; Sguizzato, M.; Ferrara, F. Lipid-based nanosystems for wound healing. Expert Opin. Drug Deliv. 2024, 21, 1191–1211. [Google Scholar] [CrossRef]
- Cetin, F.N.; Mignon, A.; Van Vlierberghe, S.; Kolouchova, K. Polymer-and Lipid-Based Nanostructures Serving Wound Healing Applications: A Review. Adv. Healthc. Mater. 2025, 14, 2402699. [Google Scholar] [CrossRef]
- Motsoene, F.; Abrahamse, H.; Kumar, S.S.D. Multifunctional lipid-based nanoparticles for wound healing and antibacterial applications: A review. Adv. Colloid Interface Sci. 2023, 321, 103002. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhong, Y.; Xue, Y.; Liu, Z.; Wang, C.; Kang, D.D.; Li, H.; Hou, X.; Tian, M. Accelerating diabetic wound healing by ROS-scavenging lipid nanoparticle–mRNA formulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2322935121. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Ghahtan, N.; Dehghan, N.; Ullah, M.; Khoradmehr, A.; Habibi, H.; Nabipour, I.; Baghban, N. From seaweed to healing: The potential of fucoidan in wound therapy. Nat. Prod. Res. 2025, 39, 1345–1358. [Google Scholar] [CrossRef]
- Shafeeq, Z.F.; Al-Saedi, F.; Rajab, E.S.; Al-Musawi, M.H.; Ismaeel, F.E.; Najafi, S.; Vesal, M.; Esfahani, S.N.; Sharifianjazi, F.; Tavamaishvili, K. 3D printed antibacterial and anti-inflammatory scaffold containing vanillin-loaded Soluplus nanomicelles for healing of infected wounds. Sci. Rep. 2025, 15, 32244. [Google Scholar] [CrossRef] [PubMed]
- Hamrun, N.; Herdianto, N.; Gustiono, D.; Oktawati, S.; Kamil, K.; Marlina, E.; Ibriana, I.; Nurfaizah, T.; Arif, A.R.; Azalia, F. Synthesis, physical characteristics, and biocompatibility test of chitosan-alginate-fucoidan scaffold as an alternative material for alveolar bone substitution. BMC Oral. Health 2025, 25, 1199. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.J.; Yunus, M.H.M.; Fauzi, M.B.; Tabata, Y.; Hiraoka, Y.; Phang, S.J.; Chia, M.R.; Buyong, M.R.; Yazid, M.D. Gelatin–chitosan–cellulose nanocrystals as an acellular scaffold for wound healing application: Fabrication, characterisation and cytocompatibility towards primary human skin cells. Cellulose 2023, 30, 5071–5092. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Toumaj, N.; Salehi, M.; Zamani, S.; Arabpour, Z.; Djalian, A.R.; Rahmati, M. Development of alginate/chitosan hydrogel loaded with obestatin and evaluation of collagen type I, III, VEGF and TGF-β1 gene expression for skin repair in a rat model (in vitro and in vitro study). Ski. Res. Technol. 2024, 30, e70018. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, D.-Y.; Hsieh, M.-J.; Hung, K.-C.; Huang, S.-C.; Cho, C.-J.; Liu, S.-J. Nanofibrous insulin/vildagliptin core-shell PLGA scaffold promotes diabetic wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1075720. [Google Scholar] [CrossRef]
- Nasrullah, M.Z. Caffeic acid phenethyl ester loaded PEG–PLGA nanoparticles enhance wound healing in diabetic rats. Antioxidants 2022, 12, 60. [Google Scholar] [CrossRef]
- Teo, Y.C.; Abbas, A.; Park, E.J.; Barbut, C.; Guo, J.; Goh, D.; Yeong, J.P.S.; Mok, W.L.J.; Teo, P. 3D printed bioactive PLGA dermal scaffold for burn wound treatment. ACS Mater. Au 2023, 3, 265–272. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Larijani, G.; Lotfi, A.; Barati, S.; Janzadeh, A.; Abediankenari, S.; Faghihi, F.; Amini, N. The effect of chitosan/alginate hydrogel loaded quercetin on wound healing in diabetic rat model. J. Mol. Histol. 2025, 56, 225. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ming, Y.; Wang, M.; Huang, M.; Liu, H.; Huang, Y.; Huang, Z.; Qing, L.; Wang, Q.; Jia, B. Nanocomposite hydrogels in regenerative medicine: Applications and challenges. Macromol. Rapid Commun. 2023, 44, 2300128. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Z.; Xue, J.; Shang, J.; Ding, D.; Zhang, W.; Liu, Z.; Yan, F.; Cheng, N. Hybrid Ag nanoparticles/polyoxometalate-polydopamine nano-flowers loaded chitosan/gelatin hydrogel scaffolds with synergistic photothermal/chemodynamic/Ag+ anti-bacterial action for accelerated wound healing. Int. J. Biol. Macromol. 2022, 221, 135–148. [Google Scholar] [CrossRef]
- Yang, A.-L.; Sun, S.-B.; Qu, L.-Y.; Li, X.-Y.; Liu, J.-L.; Zhou, F.; Xu, Y.-J. Polysaccharide hydrogel containing silver nanoparticle@ catechol microspheres with photothermal, antibacterial and anti-inflammatory activities for infected-wounds repair. Int. J. Biol. Macromol. 2024, 265, 130898. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Q.; Liu, C.; Wang, H.; Zhu, C.; Qian, J.; Hu, H.; Li, B.; Guo, Q.; Shi, J. Integrated GelMA and liposome composite hydrogel with effective coupling of angiogenesis and osteogenesis for promoting bone regeneration. Int. J. Biol. Macromol. 2025, 297, 139835. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Pich, A.; He, C. Advanced bioresponsive drug delivery systems for promoting diabetic vascularized bone regeneration. ACS Biomater. Sci. Eng. 2024, 11, 182–207. [Google Scholar] [CrossRef]
- Duan, W.; Liu, X.; Zhao, J.; Zheng, Y.; Wu, J. Porous silicon carrier endowed with photothermal and therapeutic effects for synergistic wound disinfection. ACS Appl. Mater. Interfaces 2022, 14, 48368–48383. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Akl, M.A.; Eldeen, M.A.; Kassem, A.M. Beyond skin deep: Phospholipid-based nanovesicles as game-changers in transdermal drug delivery. AAPS PharmSciTech 2024, 25, 184. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-based antibacterial delivery: An emergent approach to combat bacterial infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Schlich, M.; Musazzi, U.M.; Campani, V.; Biondi, M.; Franzé, S.; Lai, F.; De Rosa, G.; Sinico, C.; Cilurzo, F. Design and development of topical liposomal formulations in a regulatory perspective. Drug Deliv. Transl. Res. 2022, 12, 1811–1828. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Hameed, H.; Riaz, T. A comprehensive review on transethosomes as a novel vesicular approach for drug delivery through transdermal route. J. Liposome Res. 2024, 34, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, R.; Halagali, P.; Nayak, D.; Tippavajhala, V.K. Transethosomes: A comprehensive review of ultra-deformable vesicular systems for enhanced transdermal drug delivery. AAPS PharmSciTech 2025, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as food-grade nanovehicles for hydrophobic nutraceuticals or bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Elkhateeb, O.; Badawy, M.E.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-infused nanostructured lipid carriers: A promising strategy for enhancing skin regeneration and combating microbial infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Razif, R.; Fadilah, N.I.M.; Ahmad, H.; Looi Qi Hao, D.; Maarof, M.; Fauzi, M.B. Asiaticoside-Loaded multifunctional bioscaffolds for enhanced hyperglycemic wound healing. Biomedicines 2025, 13, 277. [Google Scholar] [CrossRef]
- Sideek, S.A.; El-Nassan, H.B.; Fares, A.R.; ElMeshad, A.N.; Elkasabgy, N.A. Different curcumin-loaded delivery systems for wound healing applications: A comprehensive review. Pharmaceutics 2022, 15, 38. [Google Scholar] [CrossRef]
- Cholakova, D.; Glushkova, D.; Tcholakova, S.; Denkov, N. Nanopore and nanoparticle formation with lipids undergoing polymorphic phase transitions. ACS Nano 2020, 14, 8594–8604. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in drug delivery—A comprehensive review on its structural components, preparation techniques and therapeutic applications. Biomedicines 2023, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Björklund, S.; Nilsson, E.J.; Engblom, J. Bicontinuous Cubic Liquid Crystals as Potential Matrices for Non-Invasive Topical Sampling of Low-Molecular-Weight Biomarkers. Pharmaceutics 2023, 15, 2031. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Luo, P.; Shu, L.; Huang, Z.; Huang, Y.; Wu, C.; Pan, X.; Hu, P. Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management. Gels 2023, 9, 738. [Google Scholar] [CrossRef]
- Chen, C.-H.; Dierking, I. Nanoparticles in thermotropic and lyotropic liquid crystals. Front. Soft Matter 2025, 4, 1518796. [Google Scholar] [CrossRef]
- Scialabba, C.; Craparo, E.F.; Bonsignore, S.; Cabibbo, M.; Cavallaro, G. Lipid–polymer hybrid nanoparticles in microparticle-based powder: Evaluating the potential of methylprednisolone delivery for future lung disease treatment via inhalation. Pharmaceutics 2024, 16, 1454. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Elmowafy, M.; Shalaby, K.; Alzarea, S.I.; Massoud, D.; Kassem, A.M.; Ibrahim, M.F. Hydrocortisone-loaded lipid–polymer hybrid nanoparticles for controlled topical delivery: Formulation design optimization and in vitro and in vivo appraisal. ACS omega 2023, 8, 18714–18725. [Google Scholar] [CrossRef]
- Yuan, M.; Niu, J.; Li, F.; Ya, H.; Liu, X.; Li, K.; Fan, Y.; Zhang, Q. Dipeptide-1 modified nanostructured lipid carrier-based hydrogel with enhanced skin retention and topical efficacy of curcumin. RSC Adv. 2023, 13, 29152–29162. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in wound dressing materials: Highlighting recent progress in hydrogels, foams, and antimicrobial dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Saeed, S.; Martins-Green, M. Assessing animal models to study impaired and chronic wounds. Int. J. Mol. Sci. 2024, 25, 3837. [Google Scholar] [CrossRef] [PubMed]
- Hölken, J.M.; Friedrich, K.; Merkel, M.; Blasius, N.; Engels, U.; Buhl, T.; Mewes, K.R.; Vierkotten, L.; Teusch, N.E. A human 3D immune competent full-thickness skin model mimicking dermal dendritic cell activation. Front. Immunol. 2023, 14, 1276151. [Google Scholar] [CrossRef] [PubMed]
- Elloso, M.; Hutter, M.F.; Jeschke, N.; Rix, G.; Chen, Y.; Douglas, A.; Jeschke, M.G. Challenges of Porcine Wound Models: A Review. Int. J. Transl. Med. 2025, 5, 4. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Bernardi, T.; Provot, C.; Tasse, J.; Sotto, A.; Lavigne, J.-P. A relevant wound-like in vitro media to study bacterial cooperation and biofilm in chronic wounds. Front. Microbiol. 2022, 13, 705479. [Google Scholar] [CrossRef]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic manufacture of lipid-based nanomedicines. Pharmaceutics 2022, 14, 1940. [Google Scholar] [CrossRef]
- Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing. Molecules 2025, 30, 1434. [Google Scholar] [CrossRef]
- Patel, B.A.; Gospodarek, A.; Larkin, M.; Kenrick, S.A.; Haverick, M.A.; Tugcu, N.; Brower, M.A.; Richardson, D.D. Multi-angle light scattering as a process analytical technology measuring real-time molecular weight for downstream process control. In MAbs; Taylor & Francis: Abingdon, UK, 2018; pp. 945–950. [Google Scholar]
- Walsh, S.E.; Denyer, S.P. Filtration sterilization. In Russell, Hugo & Ayliffe's: Principles and Practice of Disinfection, Preservation and Sterilization; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 343–370. [Google Scholar]
- Pardeshi, S.R.; Deshmukh, N.S.; Telange, D.R.; Nangare, S.N.; Sonar, Y.Y.; Lakade, S.H.; Harde, M.T.; Pardeshi, C.V.; Gholap, A.; Deshmukh, P.K. Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: A state-of-the-art review. Future J. Pharm. Sci. 2023, 9, 99. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-based drug delivery systems: From laboratory research to industrial production—instruments and challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in nanoparticles as non-viral vectors for efficient delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197. [Google Scholar] [CrossRef]
- Vega-Zambrano, C.; Diangelakis, N.A.; Charitopoulos, V.M. Data-driven model predictive control for continuous pharmaceutical manufacturing. Int. J. Pharm. 2025, 672, 125322. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef]
- Kim, M.; Song, C.Y.; Lee, J.S.; Ahn, Y.R.; Choi, J.; Lee, S.H.; Shin, S.; Na, H.J.; Kim, H.O. Exosome isolation using chitosan oligosaccharide lactate-1-pyrenecarboxylic acid-based self-assembled magnetic nanoclusters. Adv. Healthc. Mater. 2024, 13, 2303782. [Google Scholar] [CrossRef]
- Noushin, T.; Hossain, N.I.; Tabassum, S. IoT-enabled integrated smart wound sensor for multiplexed monitoring of inflammatory biomarkers at the wound site. Front. Nanotechnol. 2022, 4, 851041. [Google Scholar] [CrossRef]
- Wu, J.-M.; Liu, Y.; Han, H.-Y.; Song, Z.-Y. Recent advances in endogenous and exogenous stimuli-responsive nanoplatforms for bacterial infection treatment. Biomed. Eng. Commun. 2023, 2, 2–23. [Google Scholar] [CrossRef]
- Taimoor, N.; Rehman, S. Reliable and resilient AI and IoT-based personalised healthcare services: A survey. IEEE Access 2021, 10, 535–563. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Kalji, S.-O.; Movsesian, L. Smartphone-based wound dressings: A mini-review. Heliyon 2022, 8, e09876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).