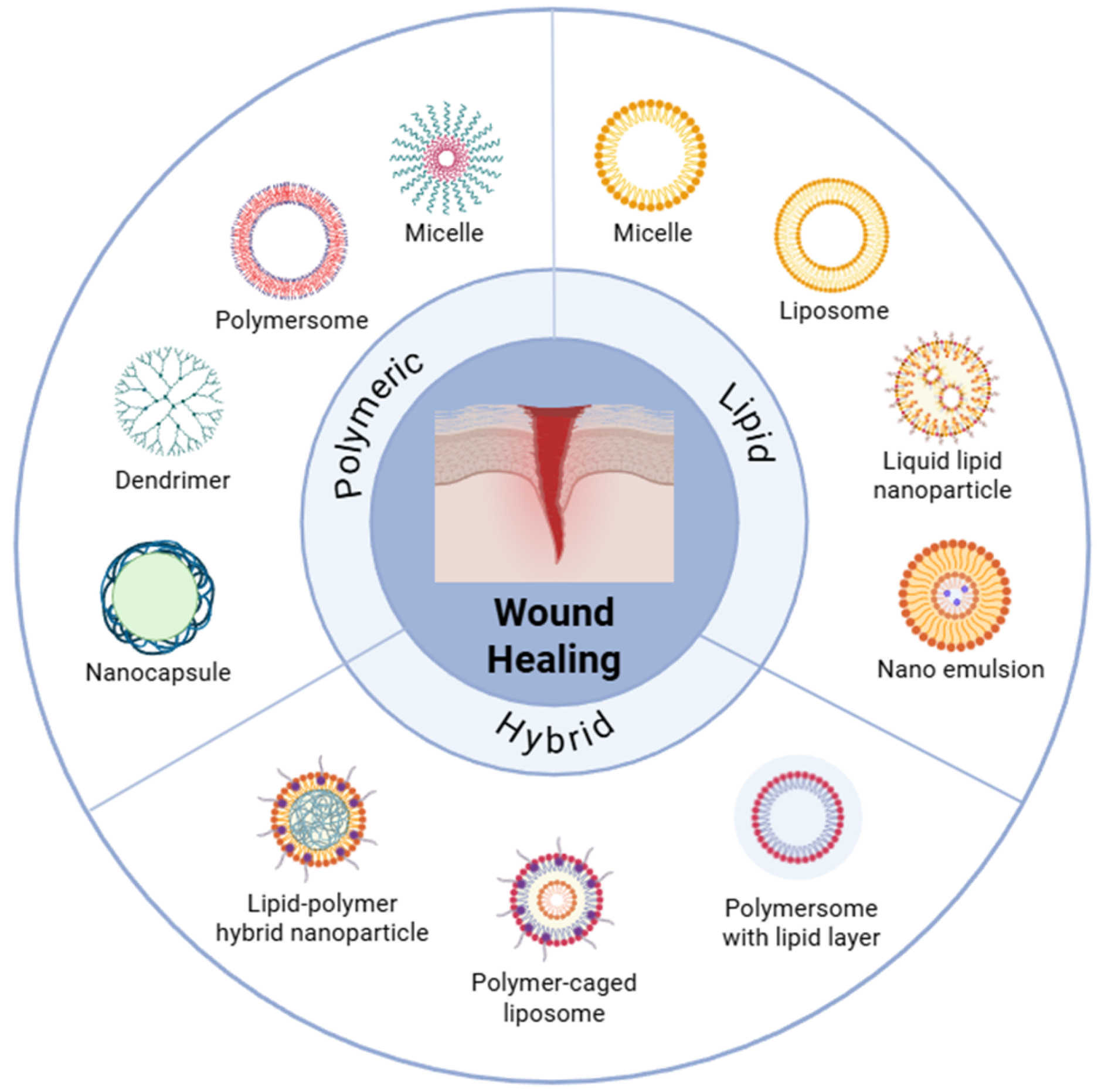
Polymer- and Lipid-Based Nanostructures for Wound Healing with Barrier-Resolved Design
Abstract
1. Introduction
2. Determinants and Barrier Interactions of Polymer- and Lipid-Based Nanostructures in Wound
2.1. Physicochemical Determinants
2.2. Biocompatibility and Tissue Adhesion Profiles
2.3. Degradation and Controlled-Release Profiles
2.4. Dimensional and Interfacial Parameters
3. Fabrication and Surface Engineering of Polymer- and Lipid-Based Nanostructures
3.1. Polymer Nanoparticle Fabrication Techniques
3.1.1. Emulsion–Solvent Evaporation
3.1.2. Nanoprecipitation
3.1.3. Electrospinning Nanofiber Fabrication
3.2. Lipid Nanostructure Engineering
3.2.1. Liposome Fabrication Methods
3.2.2. Solid Lipid Nanoparticle (SLN) Engineering
3.2.3. Nanostructured Lipid Carrier (NLC) Engineering
3.3. Polymer–Lipid Hybrid Platforms (PLHN)
3.4. Surface Functionalization and Bioactive Loading
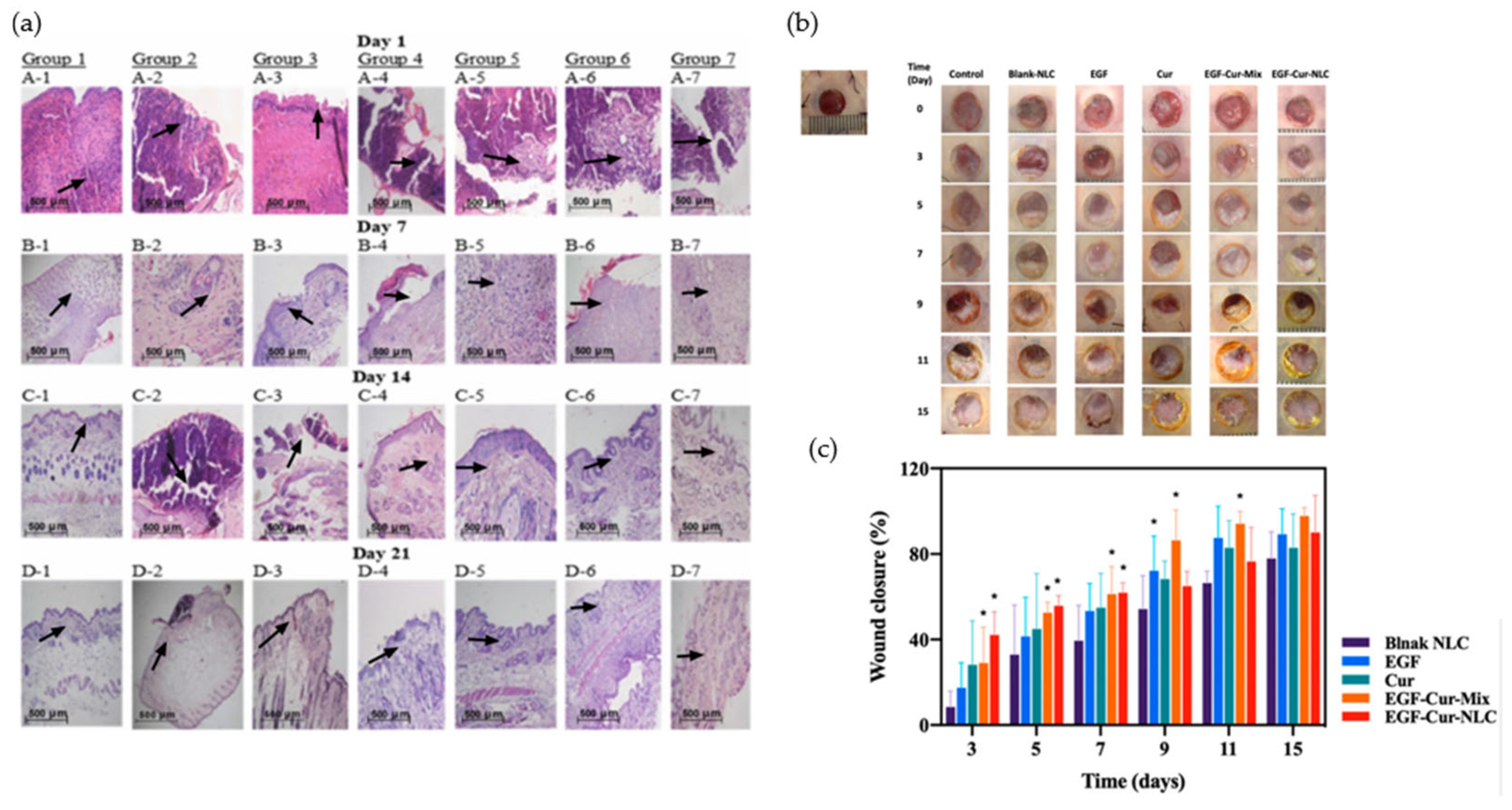
4. Therapeutic Functions of Polymer- and Lipid-Based Nanostructures in Wound Healing
4.1. Antimicrobial Activity and Anti-Inflammatory Modulation
4.2. Angiogenesis Promotion
4.3. Targeted and Stimuli-Responsive Growth Factor Delivery
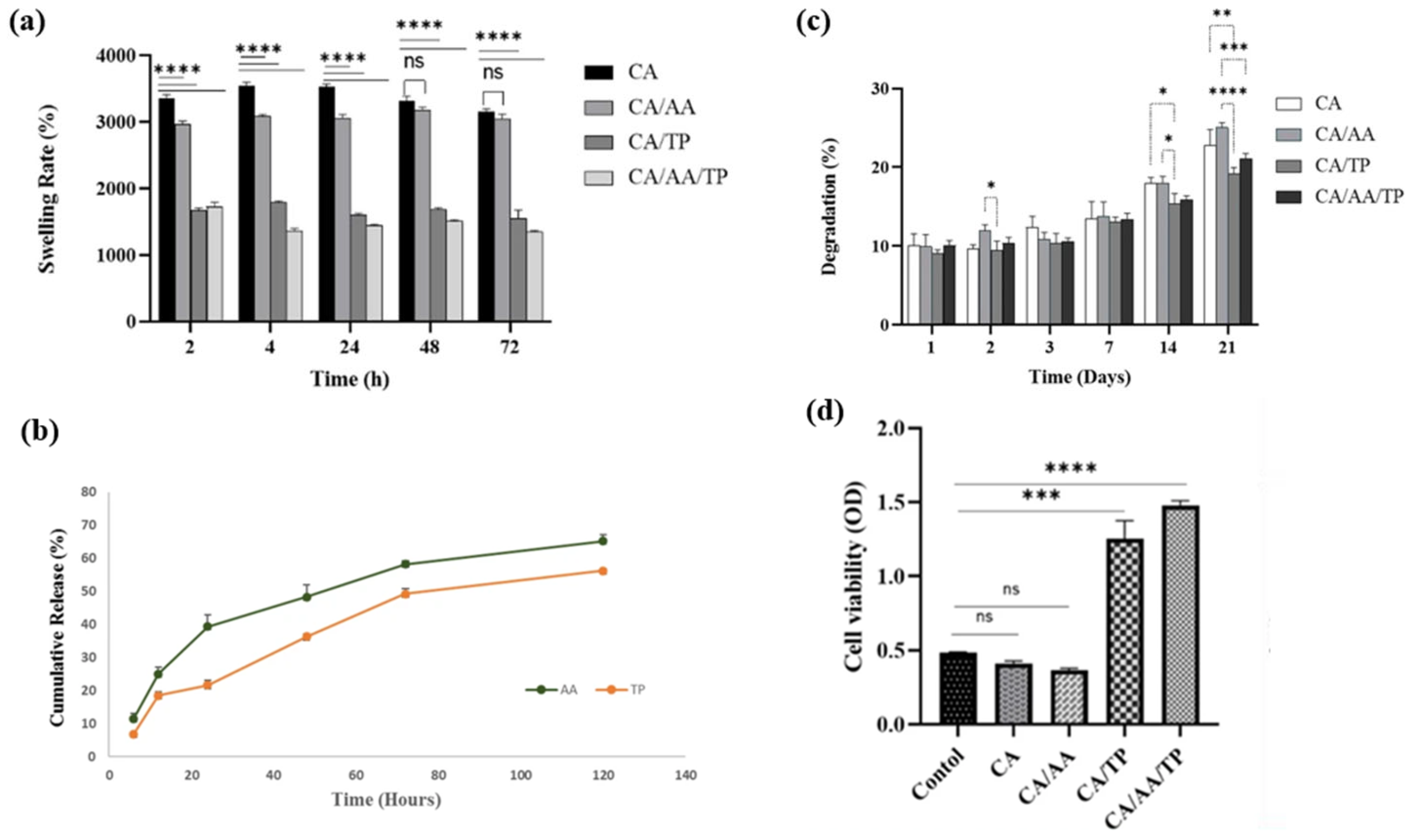
5. Polymeric Platforms and Hydrogel Composites for Wound Healing
5.1. Natural Polymer Platforms
5.2. Synthetic Biodegradable Polymer Platforms
5.3. Hydrogel Nanocomposites and Films
6. Lipid-Based Nanostructure Platforms
6.1. Conventional and Deformable Liposome Platforms
6.2. Solid Lipid Nanoparticles and Nanostructured Lipid Carrier Platforms
6.3. Nonlamellar Lipid Mesophase Platforms
6.4. Lipid–Polymer Hybrid Vesicle Platforms
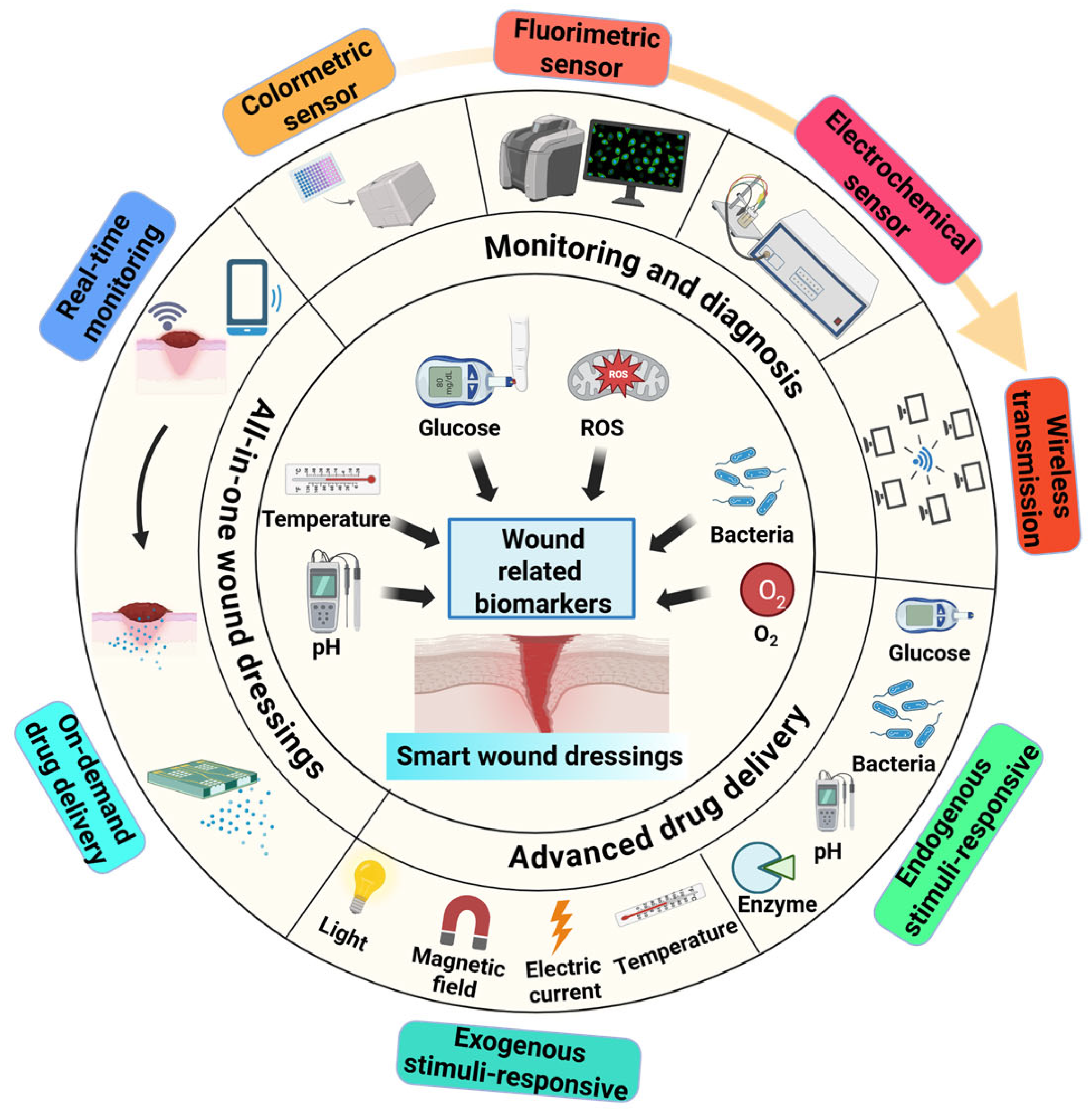
7. Preclinical-to-Clinical Translation and Scalable Production of Smart Wound Dressings
7.1. Preclinical Model Systems for Wound Healing Nanostructure Evaluation
7.2. Manufacturing, Sterilization, and Scalability
7.3. Smart and Personalized Wound-Dressing Systems
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNPs | Polymer nanoparticles |
| RESS | rapid expansion of supercritical solutions |
| RESOLV | rapid expansion of supercritical solution into liquid solvent |
| RAFT | reversible addition fragmentation chain transfer |
| ATRP | atom transfer radical polymerization |
| PLGA | Polylactide-co-glycolide |
| PEG | Polyethylene Glycol |
| PLA | Polylactic acid |
| PCL | Polycaprolactone |
| EA | Ethyl Acetate |
| DCM | Dichloromethane |
| PVA | Poly(vinyl alcohol) |
| NAs | Nucleic Acids |
| AC | Acetonitrile |
| EE | Encapsulation Efficiency |
| W/O/W | Oil-in-Water |
| PEO | Polyethtlene oxide |
| LNPs | Nanostructured Lipid Carriers |
| SLNs | Solid Lipid Nanoparticle |
| ROS | Reactive Oxygen Species |
| TFH | Thin Film Hydration |
| EI | Ethanol Injection |
| REV | Reverse-Phase Evaporation Vesicle |
| DR | Dehydration–Rehydration |
| HPH | High-Pressure Homogenization |
| MLV | Multilamellar Vesicle |
| LUV | Large Unilamellar Vesicle |
| SCF | Supercritical Fluid |
| PCS | Photon Correlation Spectroscopy |
| US | Ultrasound |
| DLS | Dynamic Light Scattering |
| XRD | X-ray Diffraction |
| PLHN | Polymer–Lipid Hybrid Platforms |
| LbL | Layer-by-Layer assembly |
| NLCs | Nanostructured lipid carriers |
| RNA | Ribonucleic Acid |
References
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Bhupendra, G.P. Advances in chitosan based nanocarriers for targetted wound healing therapies: A review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]
- Hao, G.; Qi, Z.; Li, L.; Xu, Z.P. Investigation of the mucin-nanoparticle interactions via real-time monitoring by microbalance and kinetic model simulation. J. Colloid Interface Sci. 2024, 661, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef]
- Gowda, B.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.; Ghosh, S.; Ansari, M.; Pramanik, S. Nanoparticle-based therapeutic approaches for wound healing: A review of the state-of-the-art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Mo, Y.; Zhou, T.; Li, W.; Niu, Y.; Sheu, C. Advances in Nanohybrid Hydrogels for Wound Healing: From Functional Mechanisms to Translational Prospects. Gels 2025, 11, 483. [Google Scholar] [CrossRef]
- Ardhi, A.; Raharjo, S.; Sudjarwo, W.A.A.; Schreiner, M. Oxidative stability of optimized nanostructured lipid carriers containing thymoquinone-rich oil. J. Am. Oil Chem. Soc. 2025, 102, 793–810. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Tsung, T.-H.; Tsai, Y.-C.; Lee, H.-P.; Chen, Y.-H.; Lu, D.-W. Biodegradable polymer-based drug-delivery systems for ocular diseases. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, L.; Li, B.; Reis, R.L.; Kundu, S.C.; Duan, L.; Xiao, B.; Yang, X. Tissue adhesives based on chitosan for skin wound healing: Where do we stand in this era? A review. Int. J. Biol. Macromol. 2024, 258, 129115. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Holz, E.; Darwish, M.; Tesar, D.B.; Shatz-Binder, W. A review of protein-and peptide-based chemical conjugates: Past, present, and future. Pharmaceutics 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sun, Z.; Wang, Q.; Ju, Y.; Sun, N.; Yue, Q.; Deng, Y.; Liu, S.; Yang, S.; Wang, Z. Core–Shell Magnetic Particles: Tailored Synthesis and Applications. Chem. Rev. 2024, 125, 972–1048. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Ali, A.A.; Abdelaziz, A.E.; Nada, D.; Abdelraouf, S.M.; Fouad, S.A.; Bishr, A.; Radwan, R.A. Newly designed curcumin-loaded hybrid nanoparticles: A multifunctional strategy for combating oxidative stress, inflammation, and infections to accelerate wound healing and tissue regeneration. BMC Biotechnol. 2025, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.K.; Javed, Y.; Shad, N.A.; Shahid, M.; Akhtar, B.; Yasin, E.; Sharma, S.K.; Thanh, N.T.K. Polymer coated magnesium hydroxide nanoparticles for enhanced wound healing. N. J. Chem. 2024, 48, 17396–17410. [Google Scholar] [CrossRef]
- Lok, K.-H.; Loo, H.L.; Chuah, L.-H. Topical and transdermal lipid-polymer hybrid nanoparticles (LPN): An integration in advancing dermatological treatments. Drug Deliv. Transl. Res. 2025, 15, 4277–4313. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Wang, Y.; Li, F.; Man, J.; Li, J.; Zhang, C.; Peng, S.; Wang, S. Advances in chitosan-based wound dressings: Modifications, fabrications, applications and prospects. Carbohydr. Polym. 2022, 297, 120058. [Google Scholar] [CrossRef]
- Del Olmo, J.A.; Alonso, J.M.; Sáez-Martínez, V.; Benito-Cid, S.; Moreno-Benítez, I.; Bengoa-Larrauri, M.; Pérez-González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Self-healing, antibacterial and anti-inflammatory chitosan-PEG hydrogels for ulcerated skin wound healing and drug delivery. Biomater. Adv. 2022, 139, 212992. [Google Scholar]
- Go, Y.K.; Leal, C. Polymer–lipid hybrid materials. Chem. Rev. 2021, 121, 13996–14030. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric lipid hybrid nanoparticles (plns) as emerging drug delivery platform—A comprehensive review of their properties, preparation methods, and therapeutic applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Kim, B.; Yang, J.; Hwang, M.; Choi, J.; Kim, H.-O.; Jang, E.; Lee, J.H.; Ryu, S.-H.; Suh, J.-S.; Huh, Y.-M. Aptamer-modified magnetic nanoprobe for molecular MR imaging of VEGFR2 on angiogenic vasculature. Nanoscale Res. Lett. 2013, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMaterials 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-O.; Son, H.-Y.; Lee, S.-B.; Jang, E.; Kang, B.; Haam, S.; Lim, E.-K.; Huh, Y.-M. Magnetic nanovector enabling miRNA-34a delivery for CD44 suppression with concurrent MR imaging. J. Nanosci. Nanotechnol. 2016, 16, 12939–12946. [Google Scholar] [CrossRef]
- Zhang, Q.; Heuchel, M.; Thüneman, A.F.; Machatschek, R. The role of diffusion in the hydrolytic degradation of poly (lactic-co-glycolic acid): A molecular perspective. Polym. Degrad. Stab. 2025, 232, 111119. [Google Scholar] [CrossRef]
- Doole, F.T.; Kumarage, T.; Ashkar, R.; Brown, M.F. Cholesterol stiffening of lipid membranes. J. Membr. Biol. 2022, 255, 385–405. [Google Scholar] [CrossRef]
- Chen, J.; Mu, Z.; Chen, D.; Huang, C.; Jin, T.; Li, L.; Zeng, Y.; Zhou, Q.; Zhang, Y.; Mao, H. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem. Eng. J. 2023, 469, 143985. [Google Scholar] [CrossRef]
- Varela-Moreira, A.; van Leur, H.; Krijgsman, D.; Ecker, V.; Braun, M.; Buchner, M.; Fens, M.H.; Hennink, W.E.; Schiffelers, R.M. Utilizing in vitro drug release assays to predict in vivo drug retention in micelles. Int. J. Pharm. 2022, 618, 121638. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Wang, Y.; Chen, W.; Zhu, Z.; Wang, S. Hydrogels and hydrogel-based drug delivery systems for promoting refractory wound healing: Applications and prospects. Int. J. Biol. Macromol. 2024, 285, 138098. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Li, J.; Pan, S.; Miao, X. Particle size effect of curcumin nanocrystals on transdermal and transfollicular penetration by hyaluronic acid-dissolving microneedle delivery. Pharmaceuticals 2022, 15, 206. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart pH-responsive nanomedicines for disease therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising nanostructured materials in fluid management, healing and regeneration of wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.-i.; Sakai-Kato, K. Current status and challenges of analytical methods for evaluation of size and surface modification of nanoparticle-based drug formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Haque, M.A.; Shrestha, A.; Mikelis, C.M.; Mattheolabakis, G. Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery. Int. J. Pharm. X 2024, 8, 100283. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126610. [Google Scholar] [CrossRef]
- Wilson, D.R.; Green, J.J. Nanoparticle tracking analysis for determination of hydrodynamic diameter, concentration, and zeta-potential of polyplex nanoparticles. In Biomedical Nanotechnology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 31–46. [Google Scholar]
- Koliqi, R.; Uskoković, V.; Selmani, P.B.; Grapci, A.D.; Mahapatra, C. Polymeric nanoparticles for wound healing. Pharm. Nanotechnol. 2025, 13, 775–793. [Google Scholar] [CrossRef]
- Park, C.; Lim, J.-W.; Park, G.; Kim, H.-O.; Lee, S.; Kwon, Y.H.; Kim, S.-E.; Yeom, M.; Na, W.; Song, D. Kinetic stability modulation of polymeric nanoparticles for enhanced detection of influenza virus via penetration of viral fusion peptides. J. Mater. Chem. B 2021, 9, 9658–9669. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical antisolvent process for pharmaceutical applications: A review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Cunningham, M.F. Controlled/living radical polymerization in aqueous dispersed systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Araujo, P.H.H.d.; Sayer, C.; Giudici, R.; Poço, J. Techniques for reducing residual monomer content in polymers: A review. Polym. Eng. Sci. 2002, 42, 1442–1468. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- McCormack, A.; Stone, V.; McQuat, J.; Johnston, H. Investigating the impact of the dispersion protocol on the physico-chemical identity and toxicity of nanomaterials: A review of the literature with focus on TiO2 particles. Part. Fibre Toxicol. 2025, 22, 11. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.-O.; Kang, J.-T. Optimization of nano-encapsulation on neonatal porcine islet-like cell clusters using polymersomes. Nanoscale Res. Lett. 2021, 16, 53. [Google Scholar] [CrossRef]
- Li, M.; McClements, D.J.; Liu, X.; Liu, F. Design principles of oil-in-water emulsions with functionalized interfaces: Mixed, multilayer, and covalent complex structures. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3159–3190. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidon—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2015, 6, 1. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A. Long-term stability of different kinds of gas nanobubbles in deionized and salt water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Prado-Prone, G.; Díaz-Ballesteros, A.; González-Torres, M.; Silva-Bermudez, P.; Sánchez-Sánchez, R. Nanoparticle and nanomaterial involvement during the wound healing process: An update in the field. J. Nanoparticle Res. 2023, 25, 27. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for application in wound Healing: Current state-of-the-art and future perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- Kozalak, G.; Heyat Davoudian, S.; Natsaridis, E.; Gogniat, N.; Koşar, A.; Tagit, O. Optimization of PLGA nanoparticle formulation via microfluidic and batch nanoprecipitation techniques. Micromachines 2025, 16, 972. [Google Scholar] [CrossRef]
- Bovone, G.; Cousin, L.; Steiner, F.; Tibbitt, M.W. Solvent controls nanoparticle size during nanoprecipitation by limiting block copolymer assembly. Macromolecules 2022, 55, 8040–8048. [Google Scholar] [CrossRef]
- Seegobin, N.; Abdalla, Y.; Li, G.; Murdan, S.; Shorthouse, D.; Basit, A.W. Optimising the production of PLGA nanoparticles by combining design of experiment and machine learning. Int. J. Pharm. 2024, 667, 124905. [Google Scholar] [CrossRef]
- Kuddushi, M.; Kanike, C.; Xu, B.B.; Zhang, X. Recent advances in nanoprecipitation: From mechanistic insights to applications in nanomaterial synthesis. Soft Matter 2025, 21, 2759–2781. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- El Amri, N.; McKinstry, A.; Pollard, R.E.; Lewis, P.K.; Pinkerton, N.M. A Comparative Study of Flash Nanoprecipitation and Sequential Nanoprecipitation: Impact of Formulation Parameters on Drug-Loaded Nanoparticle Formation. Mol. Pharm. 2025, 22, 6108–6119. [Google Scholar] [CrossRef]
- Zander, A.J.; Ehrlich, M.-S.; ur Rehman, S.; Schneider, M. Evaporation-triggered nanoprecipitation for PLGA nanoparticle formation using a spinning-disc system. J. Drug Deliv. Sci. Technol. 2025, 108, 106901. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Ma, A.; Bai, X.; Zeng, Y.; Liu, D.; Liu, B.; Zhang, W.; Tang, S. Recent advances in coaxial electrospun nanofibers for wound healing. Mater. Today Bio 2024, 29, 101309. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, L.; Song, W. Recent progress of electrospun nanofiber dressing in the promotion of wound healing. Polymers 2024, 16, 2596. [Google Scholar] [CrossRef]
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrins, M.; Laidmae, I.; Tenson, T.; Kogermann, K. Antibacterial porous electrospun fibers as skin scaffolds for wound healing applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Silva, T.J.; da Silva, M.G.; Hashimoto, J.C.; Ribeiro, A.P.B. Lipid nanoparticles: Formulation, production methods and characterization protocols. Foods 2025, 14, 973. [Google Scholar] [CrossRef]
- Mehraji, S.; DeVoe, D.L. Microfluidic synthesis of lipid-based nanoparticles for drug delivery: Recent advances and opportunities. Lab A Chip 2024, 24, 1154–1174. [Google Scholar] [CrossRef]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Mueller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Sakr, O.S.; Borchard, G. Encapsulation of enzymes in Layer-by-Layer (LbL) structures: Latest advances and applications. Biomacromolecules 2013, 14, 2117–2135. [Google Scholar] [CrossRef] [PubMed]
- Patrulea, V.; Borchard, G.; Jordan, O. An update on antimicrobial peptides (AMPs) and their delivery strategies for wound infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent progress of lipid nanoparticles-based lipophilic drug delivery: Focus on surface modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef] [PubMed]
- Farzamfar, S.; Chabaud, S.; Fradette, J.; Rioux, Y.; Bolduc, S. Electrospun Polycaprolactone/Collagen Scaffolds Enhance Manipulability and Influence the Composition of Self-Assembled Extracellular Matrix. Bioengineering 2025, 12, 1077. [Google Scholar] [CrossRef]
- Xiang, B.; Cao, D.-Y. Preparation of drug liposomes by thin-film hydration and homogenization. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–11. [Google Scholar]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- M Rabanel, J.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Farasati Far, B.; Naimi-Jamal, M.R.; Sedaghat, M.; Hoseini, A.; Mohammadi, N.; Bodaghi, M. Combinational system of lipid-based nanocarriers and biodegradable polymers for wound healing: An updated review. J. Funct. Biomater. 2023, 14, 115. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, P.; Poovizhi, P.; Ng, W.; Tan, R. Amorphous formulations for dissolution and bioavailability enhancement of poorly soluble APIs. Powder Technol. 2015, 285, 2–15. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Patel, D.M.; Patel, J.K.; Patel, D.H. Solvent emulsification evaporation and solvent emulsification diffusion techniques for nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 287–300. [Google Scholar]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Kumar, S.; Chand, P.; Chauhan, A.S.; Jakhmola, V. Nanostructured lipid carriers (NLCs): A comprehensive review of drug delivery advancements. J. Appl. Pharm. Res. 2025, 13, 20–38. [Google Scholar] [CrossRef]
- Sahraee, S.; Ghanbarzadeh, B.; Pezeshki, A. Development of heat-stable gelatin-coated nanostructured lipid carriers (NLC): Colloidal and stability properties. LWT 2022, 160, 113265. [Google Scholar] [CrossRef]
- Rahdar, A.; Amini, N.; Askari, F.; Susan, M.A.B.H. Dynamic light scattering and zeta potential measurements: Effective techniques to characterize therapeutic nanoparticles. J. Nanoanal. 2019, 6, 80–89. [Google Scholar]
- Tetyczka, C.; Hodzic, A.; Kriechbaum, M.; Juraić, K.; Spirk, C.; Hartl, S.; Pritz, E.; Leitinger, G.; Roblegg, E. Comprehensive characterization of nanostructured lipid carriers using laboratory and synchrotron X-ray scattering and diffraction. Eur. J. Pharm. Biopharm. 2019, 139, 153–160. [Google Scholar] [CrossRef]
- de Almeida Campos, L.A.; de Souza, J.B.; de Queiroz Macêdo, H.L.R.; Borges, J.C.; de Oliveira, D.N.; Cavalcanti, I.M.F. Synthesis of polymeric nanoparticles by double emulsion and pH-driven: Encapsulation of antibiotics and natural products for combating Escherichia coli infections. Appl. Microbiol. Biotechnol. 2024, 108, 351. [Google Scholar] [CrossRef]
- Rajabifar, N.; Rostami, A.; Afshar, S.; Mosallanezhad, P.; Zarrintaj, P.; Shahrousvand, M.; Nazockdast, H. Wound dressing with electrospun Core-Shell nanofibers: From material selection to synthesis. Polymers 2024, 16, 2526. [Google Scholar] [CrossRef]
- Fathi, F.; Machado, T.O.; de AC Kodel, H.; Portugal, I.; Ferreira, I.O.; Zielinska, A.; Oliveira, M.B.P.; Souto, E.B. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the delivery of bioactives sourced from plants: Part I—Composition and production methods. Expert Opin. Drug Deliv. 2024, 21, 1479–1490. [Google Scholar] [CrossRef]
- Deol, P.K.; Kaur, I.P.; Dhiman, R.; Kaur, H.; Sharma, G.; Rishi, P.; Ghosh, D. Investigating wound healing potential of sesamol loaded solid lipid nanoparticles: Ex-vivo, in vitro and in-vivo proof of concept. Int. J. Pharm. 2024, 654, 123974. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Aqil, M.; Ahad, A.; Imam, S.S.; Waheed, A.; Qadir, A.; Ali, A. Nanostructured lipid carrier for transdermal gliclazide delivery: Development and optimization by Box-Behnken design. Inorg. Nano-Met. Chem. 2024, 54, 474–487. [Google Scholar] [CrossRef]
- Wathoni, N.; Suhandi, C.; Elamin, K.M.; Lesmana, R.; Hasan, N.; Mohammed, A.F.A.; El-Rayyes, A.; Wilar, G. Advancements and challenges of nanostructured lipid carriers for wound healing applications. Int. J. Nanomed. 2024, 19, 8091–8113. [Google Scholar] [CrossRef] [PubMed]
- Godase, S.S.; Kulkarni, N.S.; Dhole, S.N. A comprehensive review on novel lipid-based nano drug delivery. Adv. Pharm. Bull. 2023, 14, 34. [Google Scholar] [CrossRef]
- Verma, J.; Singh, N.K.; Bansal, K.K. Recent patents in polymer–lipid hybrid nanoparticles technology. Ther. Deliv. 2024, 15, 489–493. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Ávila-Orta, C.A.; González-Morones, P.; Agüero-Valdez, D.; González-Sánchez, A.; Martínez-Colunga, J.G.; Mata-Padilla, J.M.; Cruz-Delgado, V.J. Ultrasound-Assisted Melt Extrusion of Polymer Nanocomposites. Recent Evol. 2019, 163. [Google Scholar] [CrossRef]
- Lebreton, V.; Legeay, S.; Vasylaki, A.; Lagarce, F.; Saulnier, P. Protein corona formation on lipidic nanocapsules: Influence of the interfacial PEG repartition. Eur. J. Pharm. Sci. 2023, 189, 106537. [Google Scholar] [CrossRef]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Giraldo-Gomez, D.M.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D.; González-Del Carmen, M.; González-Torres, M.; Cortés, H.; Leyva-Gómez, G. Insights into terminal sterilization processes of nanoparticles for biomedical applications. Molecules 2021, 26, 2068. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; You, W.; Huang, G.; Tian, W.; Huselstein, C.; Wu, C.-L.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial and angiogenic wound dressings for chronic persistent skin injury. Chem. Eng. J. 2021, 404, 126525. [Google Scholar] [CrossRef]
- Bilardo, R.; Traldi, F.; Vdovchenko, A.; Resmini, M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1788. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Chilkoti, A. From composition to cure: A systems engineering approach to anticancer drug carriers. Angew. Chem. Int. Ed. 2017, 56, 6712–6733. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Larrota, J.S.; Eckhard, U. An introduction to bacterial biofilms and their proteases, and their roles in host infection and immune evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.M.; Chen, W.J.; Qin, Y.; Xu, D.; Lai, Y.K.; He, S.H. Innovative hydrogel design: Tailoring immunomodulation for optimal chronic wound recovery. Adv. Sci. 2025, 12, 2412360. [Google Scholar] [CrossRef]
- Zhou, R.; Xue, S.; Cheng, Y.; Chen, Y.; Wang, Y.; Xing, J.; Liu, H.; Xu, Y.; Lin, Y.; Pei, Z. Macrophage membrane-camouflaged biomimetic nanoparticles for rheumatoid arthritis treatment via modulating macrophage polarization. J. Nanobiotechnol. 2024, 22, 578. [Google Scholar] [CrossRef]
- Tronchin, S.; Forster, J.C.; Hickson, K.; Bezak, E. Dosimetry in targeted alpha therapy. A systematic review: Current findings and what is needed. Phys. Med. Biol. 2022, 67, 09TR01. [Google Scholar] [CrossRef]
- Smith, A.M.; Johnston, K.A.; Crawford, S.E.; Marbella, L.E.; Millstone, J.E. Ligand density quantification on colloidal inorganic nanoparticles. Analyst 2017, 142, 11–29. [Google Scholar] [CrossRef]
- Marden, S.; Campbell, J.M.; Adams, N.; Coelho, R.; Foti, C.; Franca, J.R.; Hostyn, S.; Huang, Z.; Ultramari, M.; Zelesky, T. Mass balance in pharmaceutical stress testing: A review of principles and practical applications. AAPS J. 2024, 26, 96. [Google Scholar] [CrossRef]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) prepared by microwave and ultrasound-assisted synthesis: Promising green strategies for the nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-coated PLGA nanoparticles loaded with peganum harmala alkaloids with promising antibacterial and wound healing activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Ouyang, L.; Lin, C.; Zeng, R.; Liu, G.; Zhou, W. Lipid nanoparticle: Advanced drug delivery systems for promotion of angiogenesis in diabetic wounds. J. Liposome Res. 2025, 35, 76–85. [Google Scholar] [CrossRef]
- Yang, H.; Lv, D.; Qu, S.; Xu, H.; Li, S.; Wang, Z.; Cao, X.; Rong, Y.; Li, X.; Wu, H. A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing. Adv. Sci. 2024, 11, 2403219. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef]
- Rodríguez, D.A.; Vader, P. Extracellular vesicle-based hybrid systems for advanced drug delivery. Pharmaceutics 2022, 14, 267. [Google Scholar] [CrossRef]
- Cao, W.; Peng, S.; Yao, Y.; Xie, J.; Li, S.; Tu, C.; Gao, C. A nanofibrous membrane loaded with doxycycline and printed with conductive hydrogel strips promotes diabetic wound healing in vivo. Acta Biomater. 2022, 152, 60–73. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Li, P.; Hao, X.; Yang, X.; Xi, G.; Liu, W.; Feng, Y.; He, H.; Shi, C. Polysaccharide based hemostatic strategy for ultrarapid hemostasis. Macromol. Biosci. 2020, 20, 1900370. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Wang, Y.; Sun, L.; Fan, X.; Wang, X.; Bai, J. Research Progress on the Application of Novel Wound Healing Dressings in Different Stages of Wound Healing. Pharmaceutics 2025, 17, 976. [Google Scholar] [CrossRef]
- Haririan, Y.; Elahi, A.; Shadman-Manesh, V.; Rezaei, H.; Mohammadi, M.; Asefnejad, A. Advanced nanostructured biomaterials for accelerated wound healing: Insights into biological interactions and therapeutic innovations: A comprehensive review. Mater. Des. 2025, 258, 114698. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef]
- Baghirova, L.; Kaya Tilki, E.; Ozturk, A.A. Evaluation of cell proliferation and wound healing effects of vitamin A palmitate-loaded PLGA/chitosan-coated plga nanoparticles: Preparation, characterization, release, and release kinetics. ACS Omega 2023, 8, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Aghmiuni, A.I.; Keshel, S.H.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–polymer hybrid nanosystems: A rational fusion for advanced therapeutic delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Chen, R.; Wang, D.; Mai, H.; Zhong, Q.; Ning, Y.; Fu, J.; Tang, Z.; Xu, Y. NIR-activated electrospun nanodetonator dressing enhances infected diabetic wound healing with combined photothermal and nitric oxide-based gas therapy. J. Nanobiotechnol. 2024, 22, 232. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.-O.; Park, K.-H.; Wie, M.-B.; Choi, S.-E.; Yun, J.-H. A 60% edible ethanolic extract of ulmus davidiana inhibits vascular endothelial growth factor-induced angiogenesis. Molecules 2021, 26, 781. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, H.; Li, H.; Feng, Y.; Zhao, X.; Zheng, X.; Xu, J.; Cai, X.; Zhou, X.; Wen, J. ROS-responsive nanocomposite hydrogel dressing accelerates diabetic wound healing through modulation of the inflammatory microenvironment. Int. J. Pharm. 2025, 678, 125727. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Luo, Y.; Feng, M.; Yang, Q.; Tang, Y.; Tang, Z.; Xiao, W.; Zheng, Y.; Li, L. Design strategies and application potential of multifunctional hydrogels for promoting angiogenesis. Int. J. Nanomed. 2024, 19, 12719–12742. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Wang, G.; Wang, J.; Wu, D.; Wang, Y. Dual-functional 3D-printed porous bioactive scaffold enhanced bone repair by promoting osteogenesis and angiogenesis. Mater. Today Bio 2024, 24, 100943. [Google Scholar] [CrossRef] [PubMed]
- Heo, T.-H.; Jang, H.-J.; Jeong, G.-J.; Yoon, J.-K. Hydrogel-based nitric oxide delivery systems for enhanced wound healing. Gels 2025, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xu, J.; Wang, X.; Jiang, S.; Zheng, Y.; Liu, Z.; Jia, Z.; Jia, Z.; Lu, X. Smart hydrogels for tissue regeneration. Macromol. Biosci. 2024, 24, 2300339. [Google Scholar] [CrossRef]
- Thangudu, S.; Su, C.-H. Review of light activated antibacterial nanomaterials in the second biological window. J. Nanobiotechnol. 2025, 23, 293. [Google Scholar] [CrossRef]
- Moya, S.E.; Hernández, R.R.; Angelomé, P.C. Degradation of Mesoporous Silica Materials in Biological Milieu: The Gateway for Therapeutic Applications. Adv. NanoBiomed Res. 2024, 4, 2400005. [Google Scholar] [CrossRef]
- Lazarus, E.; Barnum, L.; Ramesh, S.; Quint, J.; Samandari, M.; Laflamme, S.; Secord, T.W.; Schmidt, T.; Tamayol, A.; Rivero, I.V. Engineering tools for stimulating wound healing. Appl. Phys. Rev. 2024, 11, 021304. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, G.; Li, H. The role of extracellular matrix in wound healing. Dermatol. Surg. 2023, 49, S41–S48. [Google Scholar] [CrossRef]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of electrospun PLGA/collagen scaffolds on cell adhesion, viability, and collagen release: Potential applications in tissue engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef]
- Bogdanović, B.; Fagret, D.; Ghezzi, C.; Montemagno, C. Integrin targeting and beyond: Enhancing cancer treatment with dual-targeting RGD (arginine–glycine–aspartate) strategies. Pharmaceuticals 2024, 17, 1556. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local drug delivery strategies towards wound healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef] [PubMed]
- Hora, S.; Wuestefeld, T. Liver injury and regeneration: Current understanding, new approaches, and future perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Bhagia, A.; Lerman, A. Optimization of polycaprolactone fibrous scaffold for heart valve tissue engineering. Biomed. Mater. 2019, 14, 065014. [Google Scholar] [CrossRef]
- Penoy, N.; Grignard, B.; Evrard, B.; Piel, G. A supercritical fluid technology for liposome production and comparison with the film hydration method. Int. J. Pharm. 2021, 592, 120093. [Google Scholar] [CrossRef]
- Weng, T.; Yang, M.; Zhang, W.; Jin, R.; Xia, S.; Zhang, M.; Wu, P.; He, X.; Han, C.; Zhao, X. Dual gene-activated dermal scaffolds regulate angiogenesis and wound healing by mediating the coexpression of VEGF and angiopoietin-1. Bioeng. Transl. Med. 2023, 8, e10562. [Google Scholar] [CrossRef]
- Li, Z.; Tan, G.; Xie, H.; Lu, S. The application of regenerated silk fibroin in tissue repair. Materials 2024, 17, 3924. [Google Scholar] [CrossRef]
- Han, Y.; Shao, Z.; Zhang, Y.; Zhao, H.; Sun, Z.; Yang, C.; Tang, H.; Han, Y.; Gao, C. 3D matrix stiffness modulation unveils cardiac fibroblast phenotypic switching. Sci. Rep. 2024, 14, 17015. [Google Scholar] [CrossRef]
- Ferraz, M.P. Wound dressing materials: Bridging material science and clinical practice. Appl. Sci. 2025, 15, 1725. [Google Scholar] [CrossRef]
- Giardina, F.; Padrón, R.S.; Stocker, B.D.; Schumacher, D.L.; Seneviratne, S.I. Large biases in the frequency of water limitation across Earth system models. Commun. Earth Environ. 2025, 6, 469. [Google Scholar] [CrossRef]
- Boase, N.R.; Gillies, E.R.; Goh, R.; Kieltyka, R.E.; Matson, J.B.; Meng, F.; Sanyal, A.; Sedlacek, O. Stimuli-responsive polymers at the interface with biology. Biomacromolecules 2024, 25, 5417–5436. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, J.; Tian, Y.; Su, B.; Zeng, M.; Wu, C.; Wei, D.; Sun, J.; Luo, H.; Fan, H. Temperature-responsive hydrogel system integrating wound temperature monitoring and on-demand drug release for sequentially inflammatory process regulation of wound healing. ACS Appl. Mater. Interfaces 2024, 16, 67444–67457. [Google Scholar] [CrossRef]
- Kim, H.O.; Yeom, M.; Kim, J.; Kukreja, A.; Na, W.; Choi, J.; Kang, A.; Yun, D.; Lim, J.W.; Song, D. Reactive Oxygen Species-Regulating Polymersome as an Antiviral Agent against Influenza Virus. Small 2017, 13, 1700818. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, M.-Y.; Lee, S.; Park, J.; Kang, J.H.; Cho, H.; Kim, K.-B.; Son, S.J.; Cheng, X.W.; Lee, Y.J. Nitric oxide releasing nanofiber stimulates revascularization in response to ischemia via cGMP-dependent protein kinase. PLoS ONE 2024, 19, e0303758. [Google Scholar]
- Gao, Y.; Chen, X.; He, C.; Zhang, Z.; Yu, J. Stimulus-responsive hydrogels for diabetic wound management via microenvironment modulation. Biomater. Sci. 2025, 13, 3192–3212. [Google Scholar] [CrossRef] [PubMed]
- Bei, Z.; Ye, L.; Tong, Q.; Ming, Y.; Yang, T.; Zhu, Y.; Zhang, L.; Li, X.; Deng, H.; Liu, J. Thermostimulated shrinking and adhesive hydrogel dressing for treating chronic diabetic wounds. Cell Rep. Phys. Sci. 2024, 5, 102289. [Google Scholar] [CrossRef]
- Hasan, N.; Jiafu, C.; Mustopa, A.Z.; Himawan, A.; Umami, R.N.; Ullah, M.; Wathoni, N.; Yoo, J.-W. Recent advancements of nitric oxide-releasing hydrogels for wound dressing applications. J. Pharm. Investig. 2023, 53, 781–801. [Google Scholar] [CrossRef]
- Le Thi, P.; Tran, D.L.; Park, K.M.; Lee, S.; Oh, D.H.; Park, K.D. Biocatalytic nitric oxide generating hydrogels with enhanced anti-inflammatory, cell migration, and angiogenic capabilities for wound healing applications. J. Mater. Chem. B 2024, 12, 1538–1549. [Google Scholar] [CrossRef]
- Younes, S.; Ahmad, S.M.; Thirabowonkitphithan, P.; Abunasser, S.H.; Zein, N.; Elhadad, A.; Leelahavanichkul, A.; Laiwattanapaisal, W.; Al-Otoom, A.; Mahmoud, K.A. Two-Dimensional Magnesium Phosphate Nanosheets Promote Antibacterial Effects and Wound Closure. Int. J. Nanomed. 2025, 20, 12103–12115. [Google Scholar] [CrossRef]
- Kolahreez, D.; Ghasemi-Mobarakeh, L.; Liebner, F.; Alihosseini, F.; Quartinello, F.; Guebitz, G.M.; Ribitsch, D. Approaches to control and monitor protease levels in chronic wounds. Adv. Ther. 2024, 7, 2300396. [Google Scholar] [CrossRef]
- Ghahremani-Nasab, M.; Akbari-Gharalari, N.; Rahmani Del Bakhshayesh, A.; Ghotaslou, A.; Ebrahimi-Kalan, A.; Mahdipour, M.; Mehdipour, A. Synergistic effect of chitosan-alginate composite hydrogel enriched with ascorbic acid and alpha-tocopherol under hypoxic conditions on the behavior of mesenchymal stem cells for wound healing. Stem Cell Res. Ther. 2023, 14, 326. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O. A short review on chitosan and gelatin-based hydrogel composite polymers for wound healing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1595–1622. [Google Scholar] [CrossRef]
- Mirhaji, S.S.; Soleimanpour, M.; Derakhshankhah, H.; Jafari, S.; Mamashli, F.; Rooki, M.; Karimi, M.R.; Nedaei, H.; Pirhaghi, M.; Motasadizadeh, H. Design, optimization and characterization of a novel antibacterial chitosan-based hydrogel dressing for promoting blood coagulation and full-thickness wound healing: A biochemical and biophysical study. Int. J. Biol. Macromol. 2023, 241, 124529. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent advances in honey-based hydrogels for wound healing applications: Towards natural therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Hu, C.; Li, P.; Zhao, M.; Lu, J.; Xia, G. A fucoidan-gelatin wound dressing accelerates wound healing by enhancing antibacterial and anti-inflammatory activities. Int. J. Biol. Macromol. 2022, 223, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef]
- Chegu Krishnamurthi, M.; Tiwari, S.; Veera Bramhachari, P.; Swarnalatha, G. Exploitation of Marine-Derived Multifunctional Biomaterials in Biomedical Engineering and Drug Delivery. In Marine Bioactive Molecules for Biomedical and Pharmacotherapeutic Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 231–250. [Google Scholar]
- Cortesi, R.; Sguizzato, M.; Ferrara, F. Lipid-based nanosystems for wound healing. Expert Opin. Drug Deliv. 2024, 21, 1191–1211. [Google Scholar] [CrossRef]
- Cetin, F.N.; Mignon, A.; Van Vlierberghe, S.; Kolouchova, K. Polymer-and Lipid-Based Nanostructures Serving Wound Healing Applications: A Review. Adv. Healthc. Mater. 2025, 14, 2402699. [Google Scholar] [CrossRef]
- Motsoene, F.; Abrahamse, H.; Kumar, S.S.D. Multifunctional lipid-based nanoparticles for wound healing and antibacterial applications: A review. Adv. Colloid Interface Sci. 2023, 321, 103002. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhong, Y.; Xue, Y.; Liu, Z.; Wang, C.; Kang, D.D.; Li, H.; Hou, X.; Tian, M. Accelerating diabetic wound healing by ROS-scavenging lipid nanoparticle–mRNA formulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2322935121. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Ghahtan, N.; Dehghan, N.; Ullah, M.; Khoradmehr, A.; Habibi, H.; Nabipour, I.; Baghban, N. From seaweed to healing: The potential of fucoidan in wound therapy. Nat. Prod. Res. 2025, 39, 1345–1358. [Google Scholar] [CrossRef]
- Shafeeq, Z.F.; Al-Saedi, F.; Rajab, E.S.; Al-Musawi, M.H.; Ismaeel, F.E.; Najafi, S.; Vesal, M.; Esfahani, S.N.; Sharifianjazi, F.; Tavamaishvili, K. 3D printed antibacterial and anti-inflammatory scaffold containing vanillin-loaded Soluplus nanomicelles for healing of infected wounds. Sci. Rep. 2025, 15, 32244. [Google Scholar] [CrossRef] [PubMed]
- Hamrun, N.; Herdianto, N.; Gustiono, D.; Oktawati, S.; Kamil, K.; Marlina, E.; Ibriana, I.; Nurfaizah, T.; Arif, A.R.; Azalia, F. Synthesis, physical characteristics, and biocompatibility test of chitosan-alginate-fucoidan scaffold as an alternative material for alveolar bone substitution. BMC Oral. Health 2025, 25, 1199. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.J.; Yunus, M.H.M.; Fauzi, M.B.; Tabata, Y.; Hiraoka, Y.; Phang, S.J.; Chia, M.R.; Buyong, M.R.; Yazid, M.D. Gelatin–chitosan–cellulose nanocrystals as an acellular scaffold for wound healing application: Fabrication, characterisation and cytocompatibility towards primary human skin cells. Cellulose 2023, 30, 5071–5092. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Toumaj, N.; Salehi, M.; Zamani, S.; Arabpour, Z.; Djalian, A.R.; Rahmati, M. Development of alginate/chitosan hydrogel loaded with obestatin and evaluation of collagen type I, III, VEGF and TGF-β1 gene expression for skin repair in a rat model (in vitro and in vitro study). Ski. Res. Technol. 2024, 30, e70018. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, D.-Y.; Hsieh, M.-J.; Hung, K.-C.; Huang, S.-C.; Cho, C.-J.; Liu, S.-J. Nanofibrous insulin/vildagliptin core-shell PLGA scaffold promotes diabetic wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1075720. [Google Scholar] [CrossRef]
- Nasrullah, M.Z. Caffeic acid phenethyl ester loaded PEG–PLGA nanoparticles enhance wound healing in diabetic rats. Antioxidants 2022, 12, 60. [Google Scholar] [CrossRef]
- Teo, Y.C.; Abbas, A.; Park, E.J.; Barbut, C.; Guo, J.; Goh, D.; Yeong, J.P.S.; Mok, W.L.J.; Teo, P. 3D printed bioactive PLGA dermal scaffold for burn wound treatment. ACS Mater. Au 2023, 3, 265–272. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Larijani, G.; Lotfi, A.; Barati, S.; Janzadeh, A.; Abediankenari, S.; Faghihi, F.; Amini, N. The effect of chitosan/alginate hydrogel loaded quercetin on wound healing in diabetic rat model. J. Mol. Histol. 2025, 56, 225. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ming, Y.; Wang, M.; Huang, M.; Liu, H.; Huang, Y.; Huang, Z.; Qing, L.; Wang, Q.; Jia, B. Nanocomposite hydrogels in regenerative medicine: Applications and challenges. Macromol. Rapid Commun. 2023, 44, 2300128. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Z.; Xue, J.; Shang, J.; Ding, D.; Zhang, W.; Liu, Z.; Yan, F.; Cheng, N. Hybrid Ag nanoparticles/polyoxometalate-polydopamine nano-flowers loaded chitosan/gelatin hydrogel scaffolds with synergistic photothermal/chemodynamic/Ag+ anti-bacterial action for accelerated wound healing. Int. J. Biol. Macromol. 2022, 221, 135–148. [Google Scholar] [CrossRef]
- Yang, A.-L.; Sun, S.-B.; Qu, L.-Y.; Li, X.-Y.; Liu, J.-L.; Zhou, F.; Xu, Y.-J. Polysaccharide hydrogel containing silver nanoparticle@ catechol microspheres with photothermal, antibacterial and anti-inflammatory activities for infected-wounds repair. Int. J. Biol. Macromol. 2024, 265, 130898. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, Q.; Liu, C.; Wang, H.; Zhu, C.; Qian, J.; Hu, H.; Li, B.; Guo, Q.; Shi, J. Integrated GelMA and liposome composite hydrogel with effective coupling of angiogenesis and osteogenesis for promoting bone regeneration. Int. J. Biol. Macromol. 2025, 297, 139835. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Pich, A.; He, C. Advanced bioresponsive drug delivery systems for promoting diabetic vascularized bone regeneration. ACS Biomater. Sci. Eng. 2024, 11, 182–207. [Google Scholar] [CrossRef]
- Duan, W.; Liu, X.; Zhao, J.; Zheng, Y.; Wu, J. Porous silicon carrier endowed with photothermal and therapeutic effects for synergistic wound disinfection. ACS Appl. Mater. Interfaces 2022, 14, 48368–48383. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Akl, M.A.; Eldeen, M.A.; Kassem, A.M. Beyond skin deep: Phospholipid-based nanovesicles as game-changers in transdermal drug delivery. AAPS PharmSciTech 2024, 25, 184. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-based antibacterial delivery: An emergent approach to combat bacterial infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Schlich, M.; Musazzi, U.M.; Campani, V.; Biondi, M.; Franzé, S.; Lai, F.; De Rosa, G.; Sinico, C.; Cilurzo, F. Design and development of topical liposomal formulations in a regulatory perspective. Drug Deliv. Transl. Res. 2022, 12, 1811–1828. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Hameed, H.; Riaz, T. A comprehensive review on transethosomes as a novel vesicular approach for drug delivery through transdermal route. J. Liposome Res. 2024, 34, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Seenivasan, R.; Halagali, P.; Nayak, D.; Tippavajhala, V.K. Transethosomes: A comprehensive review of ultra-deformable vesicular systems for enhanced transdermal drug delivery. AAPS PharmSciTech 2025, 26, 41. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as food-grade nanovehicles for hydrophobic nutraceuticals or bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid nanoparticles as carriers for bioactive delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Elkhateeb, O.; Badawy, M.E.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-infused nanostructured lipid carriers: A promising strategy for enhancing skin regeneration and combating microbial infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Razif, R.; Fadilah, N.I.M.; Ahmad, H.; Looi Qi Hao, D.; Maarof, M.; Fauzi, M.B. Asiaticoside-Loaded multifunctional bioscaffolds for enhanced hyperglycemic wound healing. Biomedicines 2025, 13, 277. [Google Scholar] [CrossRef]
- Sideek, S.A.; El-Nassan, H.B.; Fares, A.R.; ElMeshad, A.N.; Elkasabgy, N.A. Different curcumin-loaded delivery systems for wound healing applications: A comprehensive review. Pharmaceutics 2022, 15, 38. [Google Scholar] [CrossRef]
- Cholakova, D.; Glushkova, D.; Tcholakova, S.; Denkov, N. Nanopore and nanoparticle formation with lipids undergoing polymorphic phase transitions. ACS Nano 2020, 14, 8594–8604. [Google Scholar] [CrossRef] [PubMed]
- Sivadasan, D.; Sultan, M.H.; Alqahtani, S.S.; Javed, S. Cubosomes in drug delivery—A comprehensive review on its structural components, preparation techniques and therapeutic applications. Biomedicines 2023, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Björklund, S.; Nilsson, E.J.; Engblom, J. Bicontinuous Cubic Liquid Crystals as Potential Matrices for Non-Invasive Topical Sampling of Low-Molecular-Weight Biomarkers. Pharmaceutics 2023, 15, 2031. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic liquid crystal phases from anisotropic nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Luo, P.; Shu, L.; Huang, Z.; Huang, Y.; Wu, C.; Pan, X.; Hu, P. Utilization of Lyotropic Liquid Crystalline Gels for Chronic Wound Management. Gels 2023, 9, 738. [Google Scholar] [CrossRef]
- Chen, C.-H.; Dierking, I. Nanoparticles in thermotropic and lyotropic liquid crystals. Front. Soft Matter 2025, 4, 1518796. [Google Scholar] [CrossRef]
- Scialabba, C.; Craparo, E.F.; Bonsignore, S.; Cabibbo, M.; Cavallaro, G. Lipid–polymer hybrid nanoparticles in microparticle-based powder: Evaluating the potential of methylprednisolone delivery for future lung disease treatment via inhalation. Pharmaceutics 2024, 16, 1454. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Elmowafy, M.; Shalaby, K.; Alzarea, S.I.; Massoud, D.; Kassem, A.M.; Ibrahim, M.F. Hydrocortisone-loaded lipid–polymer hybrid nanoparticles for controlled topical delivery: Formulation design optimization and in vitro and in vivo appraisal. ACS omega 2023, 8, 18714–18725. [Google Scholar] [CrossRef]
- Yuan, M.; Niu, J.; Li, F.; Ya, H.; Liu, X.; Li, K.; Fan, Y.; Zhang, Q. Dipeptide-1 modified nanostructured lipid carrier-based hydrogel with enhanced skin retention and topical efficacy of curcumin. RSC Adv. 2023, 13, 29152–29162. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in wound dressing materials: Highlighting recent progress in hydrogels, foams, and antimicrobial dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Saeed, S.; Martins-Green, M. Assessing animal models to study impaired and chronic wounds. Int. J. Mol. Sci. 2024, 25, 3837. [Google Scholar] [CrossRef] [PubMed]
- Hölken, J.M.; Friedrich, K.; Merkel, M.; Blasius, N.; Engels, U.; Buhl, T.; Mewes, K.R.; Vierkotten, L.; Teusch, N.E. A human 3D immune competent full-thickness skin model mimicking dermal dendritic cell activation. Front. Immunol. 2023, 14, 1276151. [Google Scholar] [CrossRef] [PubMed]
- Elloso, M.; Hutter, M.F.; Jeschke, N.; Rix, G.; Chen, Y.; Douglas, A.; Jeschke, M.G. Challenges of Porcine Wound Models: A Review. Int. J. Transl. Med. 2025, 5, 4. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Bernardi, T.; Provot, C.; Tasse, J.; Sotto, A.; Lavigne, J.-P. A relevant wound-like in vitro media to study bacterial cooperation and biofilm in chronic wounds. Front. Microbiol. 2022, 13, 705479. [Google Scholar] [CrossRef]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic manufacture of lipid-based nanomedicines. Pharmaceutics 2022, 14, 1940. [Google Scholar] [CrossRef]
- Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Effect of High-Pressure Homogenization and Wall Material Composition on the Encapsulation of Polyunsaturated Fatty Acids from Fish Processing. Molecules 2025, 30, 1434. [Google Scholar] [CrossRef]
- Patel, B.A.; Gospodarek, A.; Larkin, M.; Kenrick, S.A.; Haverick, M.A.; Tugcu, N.; Brower, M.A.; Richardson, D.D. Multi-angle light scattering as a process analytical technology measuring real-time molecular weight for downstream process control. In MAbs; Taylor & Francis: Abingdon, UK, 2018; pp. 945–950. [Google Scholar]
- Walsh, S.E.; Denyer, S.P. Filtration sterilization. In Russell, Hugo & Ayliffe's: Principles and Practice of Disinfection, Preservation and Sterilization; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 343–370. [Google Scholar]
- Pardeshi, S.R.; Deshmukh, N.S.; Telange, D.R.; Nangare, S.N.; Sonar, Y.Y.; Lakade, S.H.; Harde, M.T.; Pardeshi, C.V.; Gholap, A.; Deshmukh, P.K. Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: A state-of-the-art review. Future J. Pharm. Sci. 2023, 9, 99. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-based drug delivery systems: From laboratory research to industrial production—instruments and challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in nanoparticles as non-viral vectors for efficient delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197. [Google Scholar] [CrossRef]
- Vega-Zambrano, C.; Diangelakis, N.A.; Charitopoulos, V.M. Data-driven model predictive control for continuous pharmaceutical manufacturing. Int. J. Pharm. 2025, 672, 125322. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef]
- Kim, M.; Song, C.Y.; Lee, J.S.; Ahn, Y.R.; Choi, J.; Lee, S.H.; Shin, S.; Na, H.J.; Kim, H.O. Exosome isolation using chitosan oligosaccharide lactate-1-pyrenecarboxylic acid-based self-assembled magnetic nanoclusters. Adv. Healthc. Mater. 2024, 13, 2303782. [Google Scholar] [CrossRef]
- Noushin, T.; Hossain, N.I.; Tabassum, S. IoT-enabled integrated smart wound sensor for multiplexed monitoring of inflammatory biomarkers at the wound site. Front. Nanotechnol. 2022, 4, 851041. [Google Scholar] [CrossRef]
- Wu, J.-M.; Liu, Y.; Han, H.-Y.; Song, Z.-Y. Recent advances in endogenous and exogenous stimuli-responsive nanoplatforms for bacterial infection treatment. Biomed. Eng. Commun. 2023, 2, 2–23. [Google Scholar] [CrossRef]
- Taimoor, N.; Rehman, S. Reliable and resilient AI and IoT-based personalised healthcare services: A survey. IEEE Access 2021, 10, 535–563. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Kalji, S.-O.; Movsesian, L. Smartphone-based wound dressings: A mini-review. Heliyon 2022, 8, e09876. [Google Scholar] [CrossRef]











| Property | Polymer-Based | Lipid-Based | Polymer–Lipid Hybrid | Ref. |
|---|---|---|---|---|
| Size control | Broad tunability via polymer type and fabrication process. | Governed by lipid composition and process parameters | Adjustable by polymer–lipid ratio and co-assembly technique | [9,17] |
| Biocompatibility | High with degradable polymers (PLGA, PEG, chitosan) | Excellent due to membrane-mimetic structure | Combined stability and compatibility | [10] |
| Tissue adhesion | Strong with mucoadhesive polymers | Moderate, improvable with ligands or PEG derivatives | Tunable via core–shell chemistry | [11] |
| Degradation and release | Controlled by polymer erosion rate | Diffusion across lipid bilayers | Sequential release from shell and core | [12] |
| Surface functionalization | Wide range of PEGylation/ligand conjugation | Ligand anchoring within bi-layer; ionizable lipids for pH response. | Dual functionalization across core and shell. | [13] |
| Stability and morphology | Rigid, low polymorphism risk | Sensitive to phase transition/serum instability | Core stability with bilayer versatility. | [14] |
| Advantages in wound care | Sustained release, mechanical durability, barrier maintenance | Excellent biocompatibility, conformal contact | Dual loading capacity, balanced penetration, and residence | [17] |
| Practical limitations | Acidic degradation products, burst risk | Structural instability, lipid polymorphism | Manufacturing complexity, sterilization sensitivity | [12] |
| Category | Technique | Principle | Advantages | Limitations | Ref. |
| Polymer based | Nanoprecipitation | Solvent diffusion and polymer nucleation. | Mild, narrow size, scalable. | Low hydrophilic loading, solvent residue. | [12] |
| Emulsion–solvent evaporation (O/W or W/O/W) | Polymer solution in emulsion, solvent removal. | Versatile, tunable size. | Solvent residue, batch variation. | [91] | |
| Electrospinning | High-voltage jet to nanofibers. | High area, sustained release. | Low yield, burst release. | [92] | |
| Lipid based | SLN (HPH/microemulsion) | Melt dispersion and solidification. | Biocompatible, stable, deep delivery. | Drug expulsion, limited load. | [93,94] |
| NLC (melt emulsification) | Solid–liquid lipid lattice formation. | High load, controlled release. | Oil leakage, shear sensitivity. | [95,96] | |
| Liposomes (TFH/EI/REV/DR/HPH) | Self-assembled bilayers. | Dual loading, surface modifiable | Oxidation, leakage. | [97] | |
| Hybrid | PLHN one-step assembly | Polymer–lipid co-assembly. | Single-step, tunable core–shell. | Solvent residue, leakage. | [98] |
| PLHN two-step coating | Polymer NP coated with lipid vesicle. | Independent core/shell control. | Patchy coating, low reproducibility. | [12] |
| Stage of Wound Healing | Key Events | Limitations of Conventional Dressings | Performance of Polymer- and Lipid-Based Nanostructures | Ref. |
| Hemostasis | Platelet activation, fibrin network formation, clot stabilization | Passive protection, delayed clotting, insufficient hemostasis | Rapid hemostasis, reduced clotting time (5 min → 2 min), decreased blood loss (≈45%) | [117,123,124] |
| Inflammation | Neutrophil recruitment, macrophage activation, bacterial clearance | Weak antibacterial activity, excessive cytokine release | Enhanced bacterial inhibition (>95%), fibroblast viability (>85%), cytokine suppression (TNF-α ↓ 70%, IL-6 ↓ 65%), ROS reduction (≈60%) | [119,125,126] |
| Proliferation | Fibroblast migration, angiogenesis, collagen deposition, epithelialization | Poor moisture retention, secondary trauma | Accelerated wound closure (~80% by day 7 vs. 45% control), increased collagen deposition (1.8-fold), enhanced fibroblast migration (2-fold) | [118,127] |
| Maturation/Remodeling | ECM organization, collagen crosslinking, scar remodeling | Incomplete collagen alignment, excessive contraction | Sustained drug release (>14 days), reduced scar index (~40%), improved tensile strength (~30%) | [124,128] |
| Polymer | Distinct Characteristics | Advantages | Limitations | Typical Wound Environment/Application | Quantitative Healing Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Chitosan | Cationic polysaccharide (β-1,4-linked D-glucosamine); electrostatic interaction with cells | Hemostatic activity, antibacterial effect, macrophage activation, collagen alignment | pH-dependent solubility, variability in deacetylation degree, limited neutral stability | Acute bleeding wounds, infected sites | Wound closure 88% (day 10), bacterial inhibition 95%, collagen deposition 1.7× vs. control | [171,172] |
| Gelatin | Denatured collagen with RGD motifs; peptide-based adhesion | Cell adhesion, fibroblast proliferation, angiogenesis promotion | Rapid enzymatic degradation, weak mechanical strength | Deep tissue defects, graft or granulating wounds | Wound closure 90% (day 12), fibroblast proliferation 1.6×, angiogenic index 1.5× | [96,173,174] |
| Alginate | Anionic polysaccharide (M/G blocks); Ca2+ crosslinking, ion-exchange gelation | Moisture retention, exudate absorption, autolytic debridement | Fragility from Na+–Ca2+ exchange, over-gelation risk, low elasticity, limited elasticity | Chronic exudative ulcers, moist environments | Moisture retention 85%, wound closure 90% (day 10), fibroblast density 1.4× | [96,125,172] |
| Fucoidan (composite use) | Sulfated polysaccharide from brown algae; negative charge with angiogenic potential | Antibacterial, antioxidant, VEGF upregulation | Ionic instability, viscosity variation, extraction cost | Ischemic or diabetic wounds | VEGF expression 2.3×, wound closure 93% (day 10), ROS reduction 40% | [175,176] |
| Chitosan–Alginate–Fucoidan Composite | Hybrid ionic network (CS:Alg:Fu = 2:1:0.5); calcium-crosslinked porous structure | Synergistic antibacterial and angiogenic response, high porosity and compressibility | Batch variability, Ca2+ exchange instability | Full-thickness, delayed-healing wounds | Collagen I/III ratio 1.9×, microvessel density 1.8×, wound closure 95% (day 10) | [177,178] |
| Chitosan–Gelatin Blend | Cationic–neutral polymeric matrix | Improved elasticity, moisture balance, adhesion | Thermal instability, degradation rate mismatch | Burn wounds, surgical sites | Wound closure 91% (day 12), fibroblast migration 1.7×, tensile strength 0.35 MPa | [179,180] |
| Alginate–Pectin–Collagen Composite | Triple-polymer hydrogel; collagen-enriched ECM mimic | ECM remodeling, anti-inflammatory activity | Limited mechanical resistance | Chronic diabetic ulcers | Wound closure 92% (day 14), collagen density 1.8×, TNF-α reduction 40% | [181,182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Yun, S.; Lee, S.; Kim, M.; Choi, J.; Choi, S.E.; Lim, K.S.; Ha, S.-J.; Yun, J.-H.; Kim, H.-O. Polymer- and Lipid-Based Nanostructures for Wound Healing with Barrier-Resolved Design. Pharmaceutics 2025, 17, 1501. https://doi.org/10.3390/pharmaceutics17111501
Cho E, Yun S, Lee S, Kim M, Choi J, Choi SE, Lim KS, Ha S-J, Yun J-H, Kim H-O. Polymer- and Lipid-Based Nanostructures for Wound Healing with Barrier-Resolved Design. Pharmaceutics. 2025; 17(11):1501. https://doi.org/10.3390/pharmaceutics17111501
Chicago/Turabian StyleCho, Eunsoo, Soyeon Yun, Subin Lee, Minse Kim, Jaewon Choi, Sun Eun Choi, Kwang Suk Lim, Suk-Jin Ha, Jang-Hyuk Yun, and Hyun-Ouk Kim. 2025. "Polymer- and Lipid-Based Nanostructures for Wound Healing with Barrier-Resolved Design" Pharmaceutics 17, no. 11: 1501. https://doi.org/10.3390/pharmaceutics17111501
APA StyleCho, E., Yun, S., Lee, S., Kim, M., Choi, J., Choi, S. E., Lim, K. S., Ha, S.-J., Yun, J.-H., & Kim, H.-O. (2025). Polymer- and Lipid-Based Nanostructures for Wound Healing with Barrier-Resolved Design. Pharmaceutics, 17(11), 1501. https://doi.org/10.3390/pharmaceutics17111501








