Targeted Biocatalyst Design for Asymmetric Citalopram Conversion in a Membrane Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
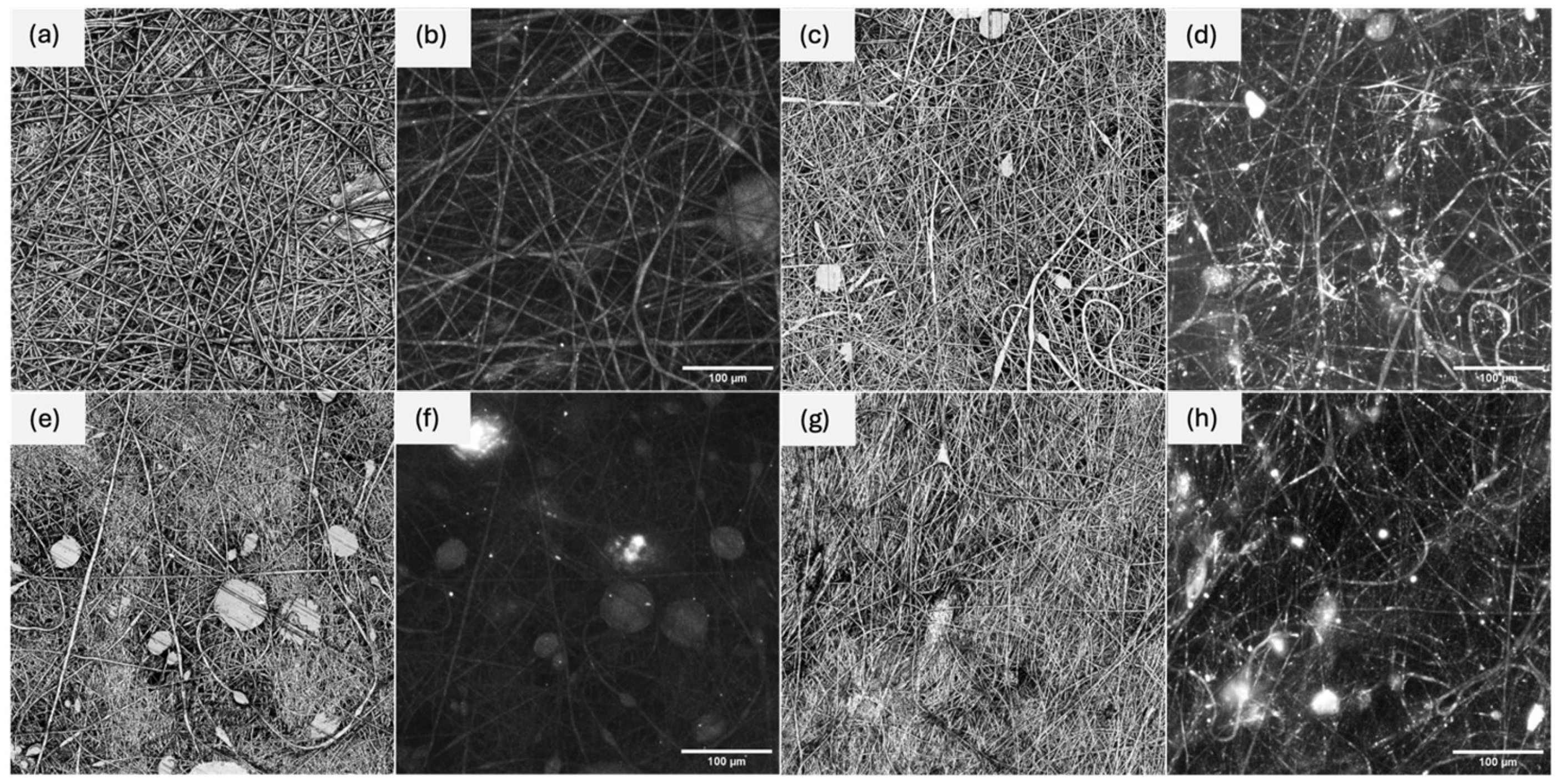
2.2. Analytical Methods for the Morphology Evaluation
2.3. Fabrication of the Electrospun Nanofibers
2.4. Evaluation of MOF Incorporation in the Nanofiber Structure
2.5. Immobilization Procedure
2.6. Immobilization Efficiency
2.7. Evaluation of Fabricated Biocatalysts’ Activity
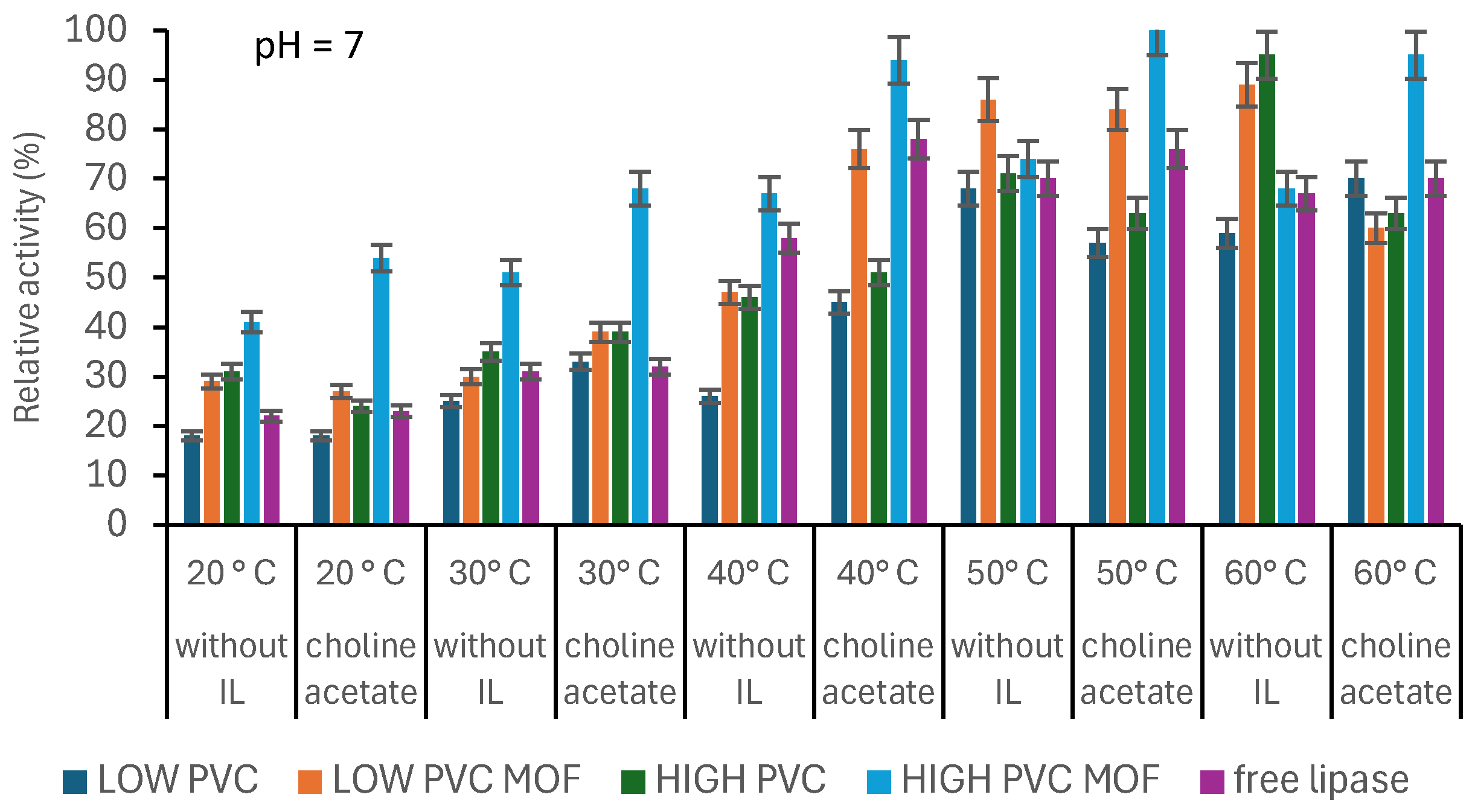
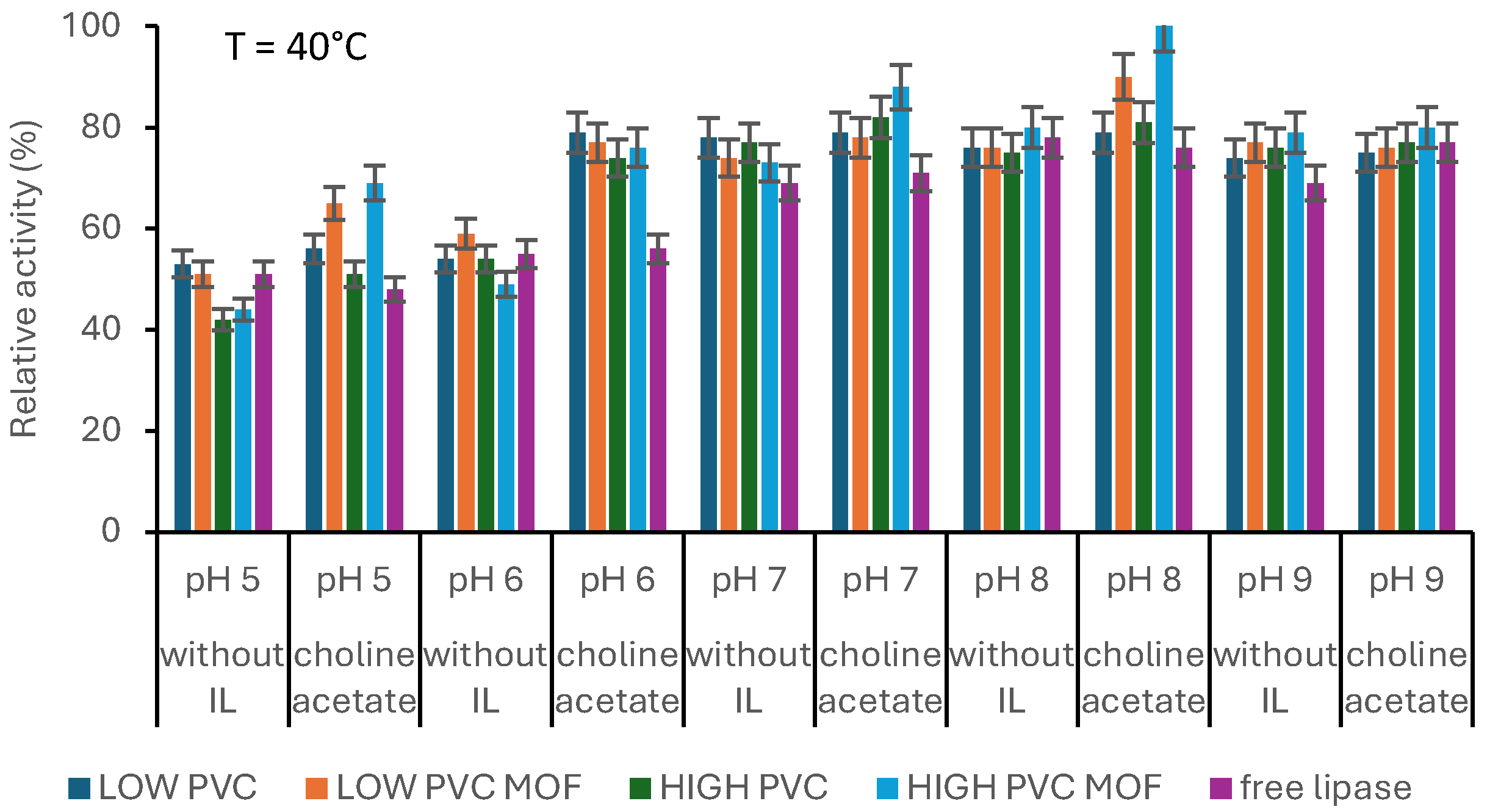
2.8. Influence of Process Parameters on Activity of Produced Systems
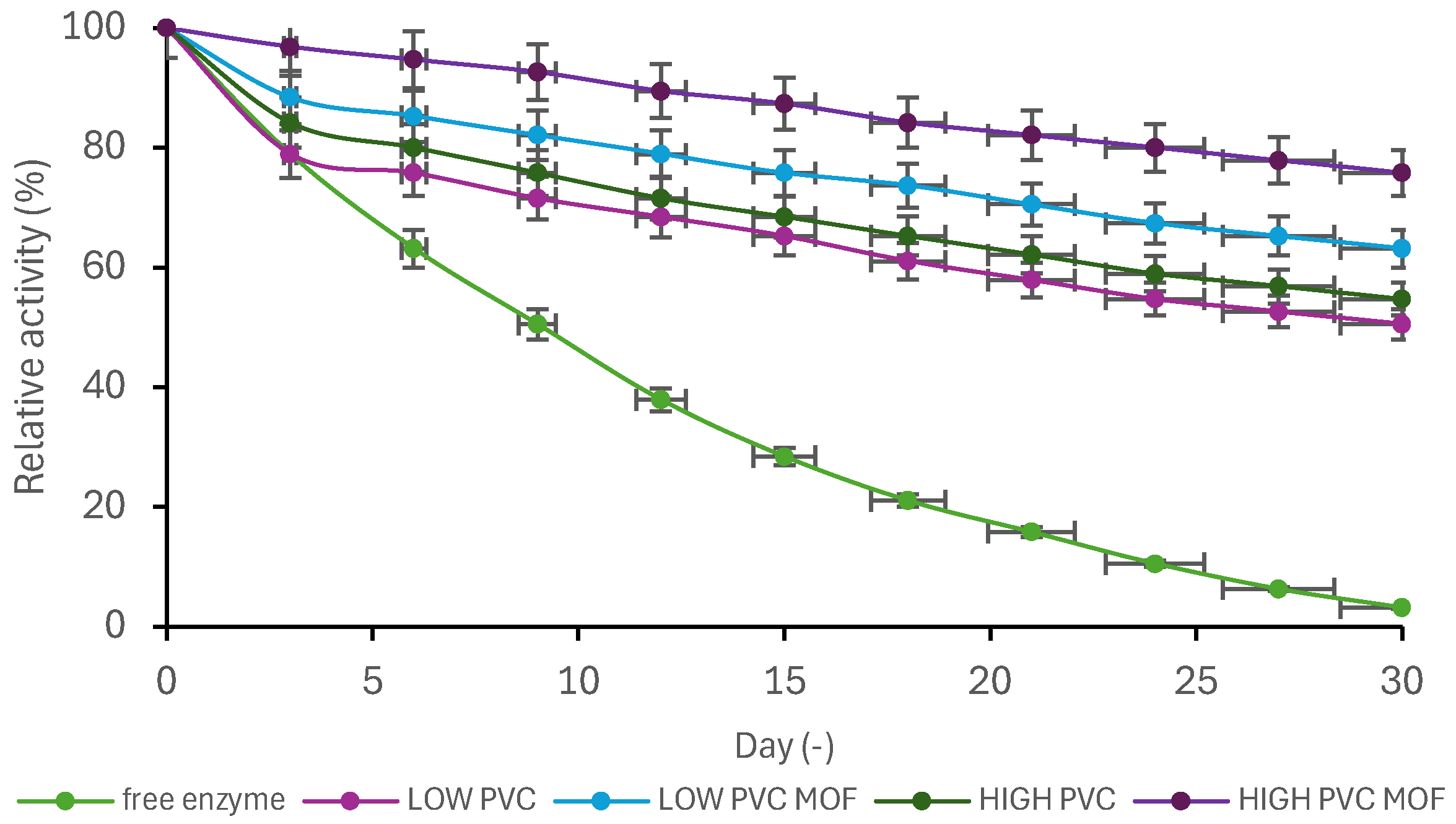
2.9. Storage Stability
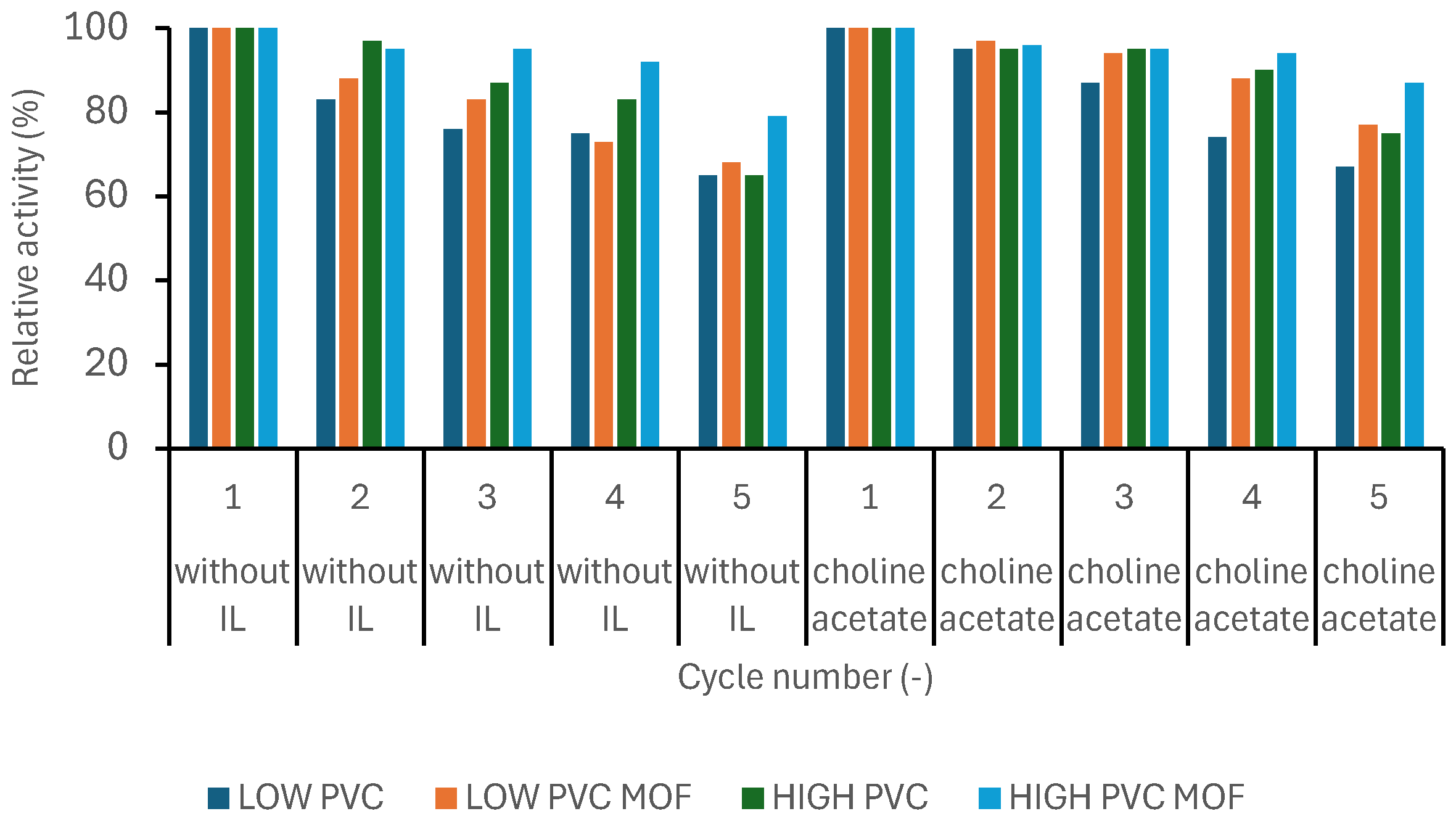
2.10. Reusability
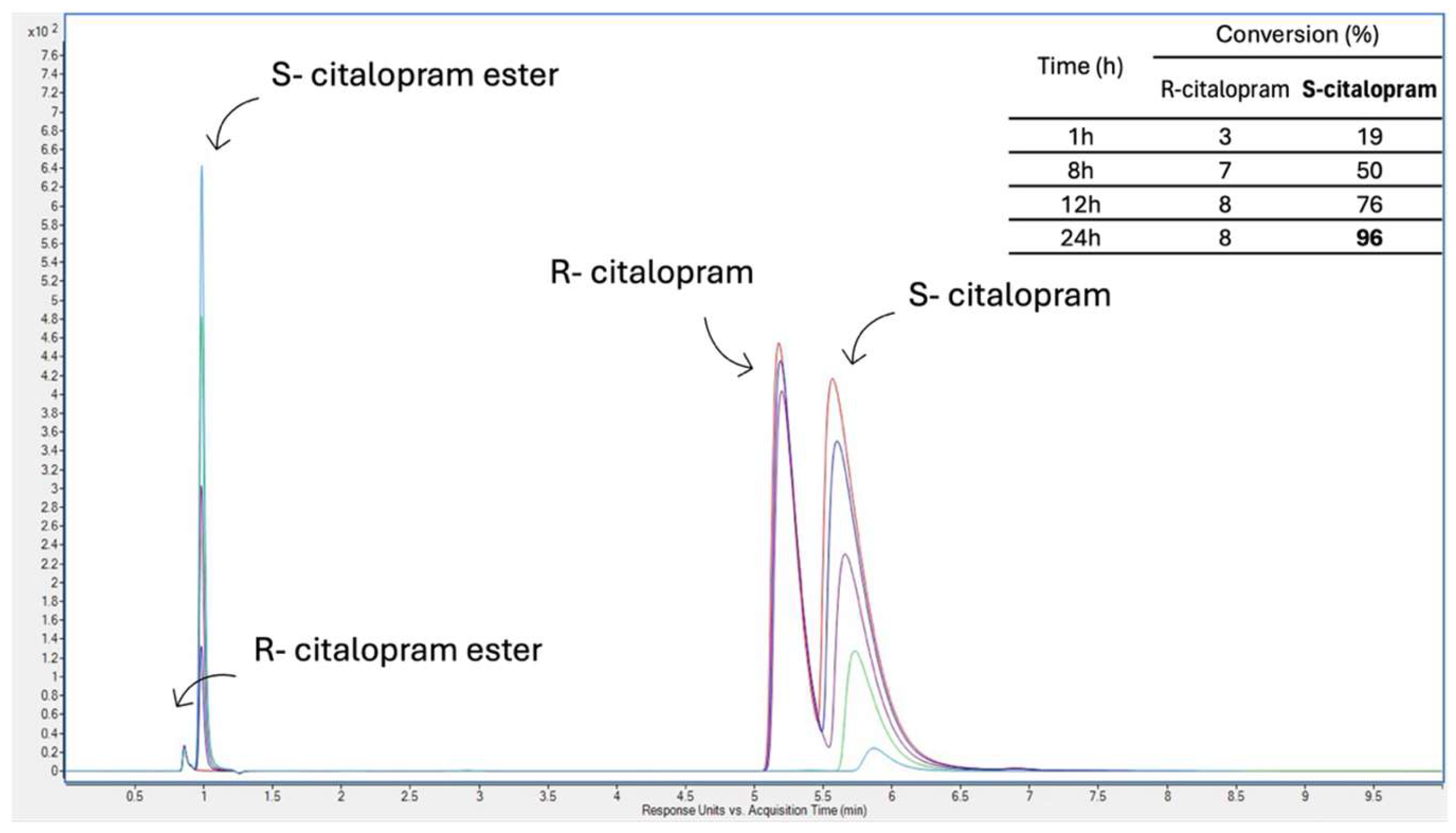
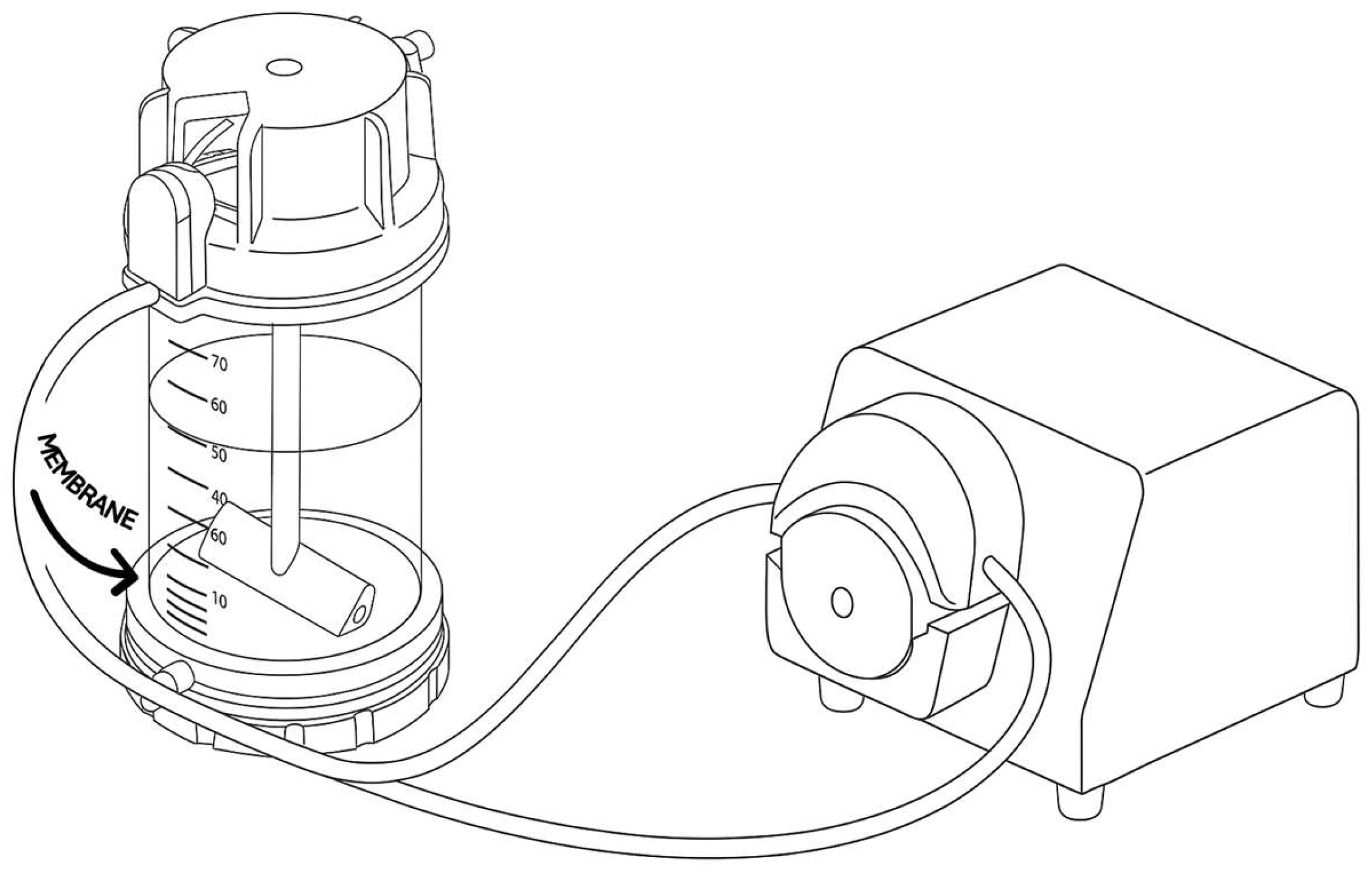
2.11. Enzymatic Resolution of Racemic Citalopram
3. Results and Discussion
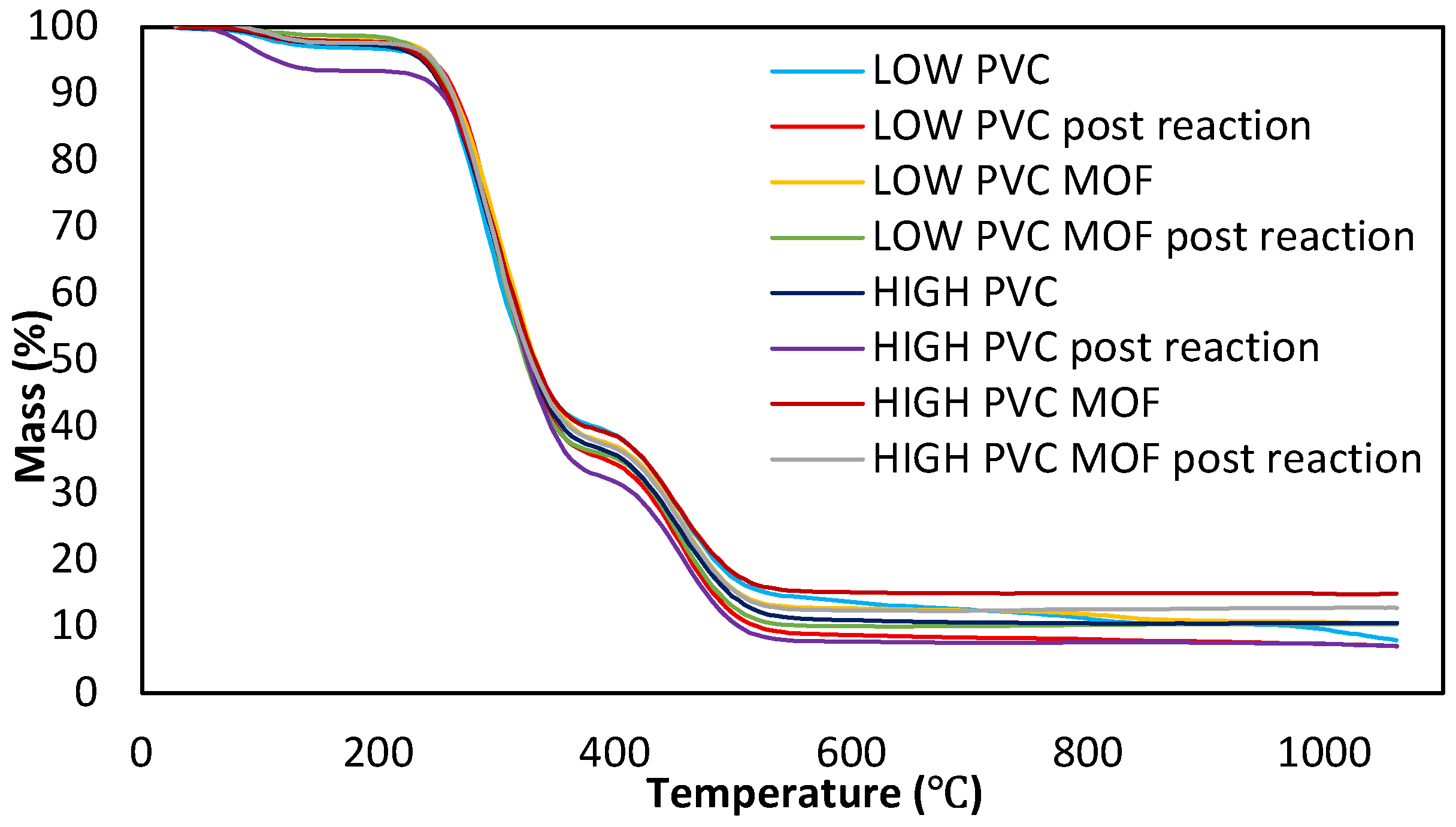
3.1. Physicochemical Characterization
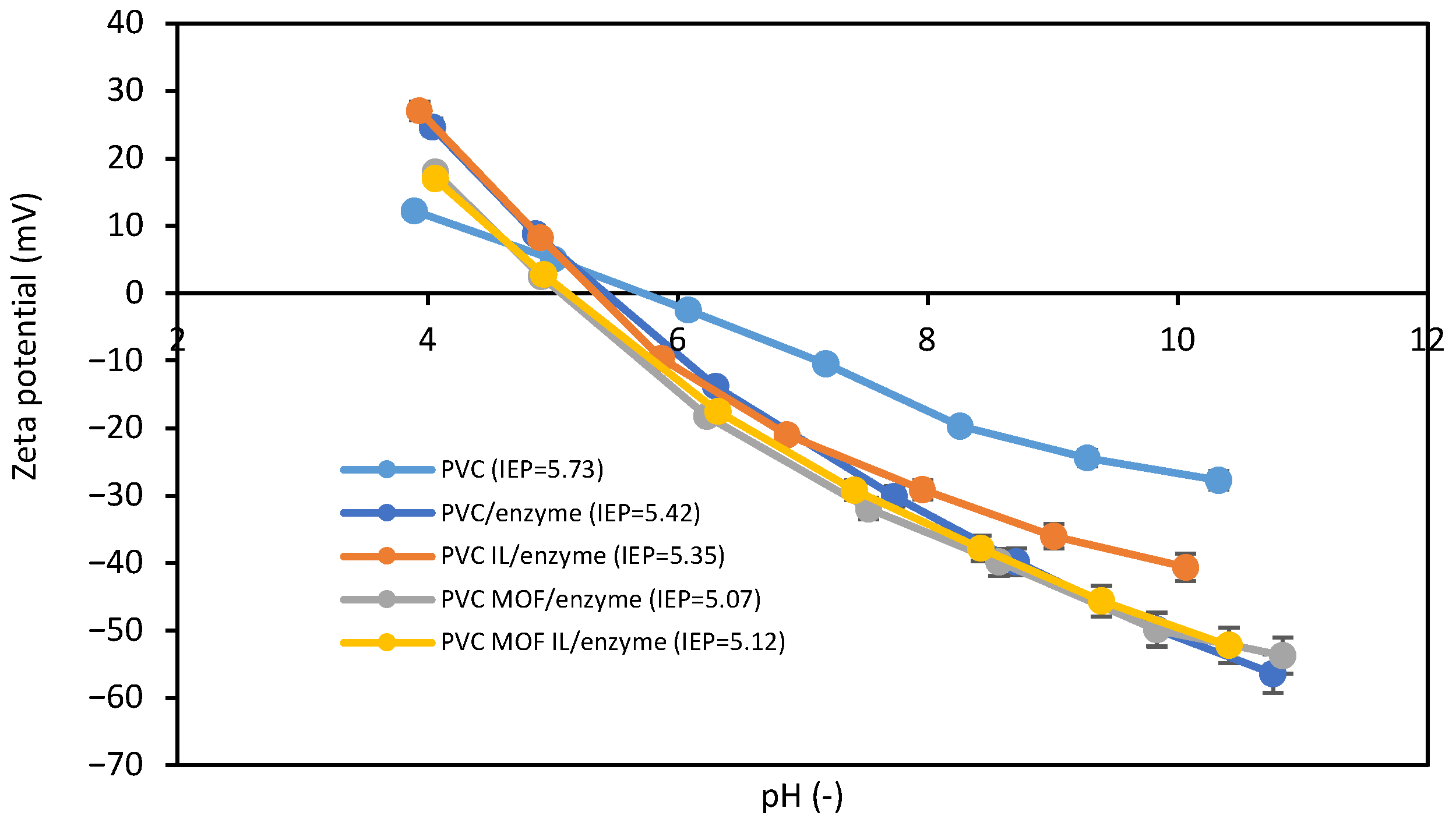
3.2. Immobilization Characterization
3.3. Biocatalysts’ Evaluation
3.4. Enzymatic Resolution of Racemic Citalopram
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active pharmaceutical ingredient |
| CA | Choline acetate |
| CC | Choline chloride |
| DCM | Dichloromethane |
| DMF | N,N-dimethylformamide |
| IL | Ionic liquid |
| MOF | Metal–organic framework |
| pNPP | Paranitrophenyl palmitate |
| PVC | Poly(vinyl chloride) |
References
- Nadar, S.S.; Vaidya, L.; Rathod, V.K. Enzyme Embedded Metal Organic Framework (Enzyme–MOF): De Novo Approaches for Immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & Prospect of Metal-Organic Frameworks (MOFs) for Enzyme Immobilization (Enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Rogacka, J.; Labus, K. Metal–Organic Frameworks as Highly Effective Platforms for Enzyme Immobilization–Current Developments and Future Perspectives. Braz. J. Chem. Eng. 2024, 42, 1273–1301. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent Advances in Enzyme Immobilization Techniques: Metal-Organic Frameworks as Novel Substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Patil, P.D.; Kelkar, R.K.; Patil, N.P.; Pise, P.V.; Patil, S.P.; Patil, A.S.; Kulkarni, N.S.; Tiwari, M.S.; Phirke, A.N.; Nadar, S.S. Magnetic Nanoflowers: A Hybrid Platform for Enzyme Immobilization. Crit. Rev. Biotechnol. 2024, 44, 795–816. [Google Scholar] [CrossRef]
- Li, S.; Han, W.; An, Q.-F.; Yong, K.-T.; Yin, M.-J. Defect Engineering of MOF-Based Membrane for Gas Separation. Adv. Funct. Mater. 2023, 33, 2303447. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Nakashima, K.; Kamiya, N.; Goto, M. Recent Advances of Enzymatic Reactions in Ionic Liquids. Biochem. Eng. J. 2010, 48, 295–314. [Google Scholar] [CrossRef]
- Wolny, A.; Siewniak, A.; Zdarta, J.; Ciesielczyk, F.; Latos, P.; Jurczyk, S.; Nghiem, L.D.; Jesionowski, T.; Chrobok, A. Supported Ionic Liquid Phase Facilitated Catalysis with Lipase from Aspergillus oryzae for Enhance Enantiomeric Resolution of Racemic Ibuprofen. Environ. Technol. Innov. 2022, 28, 102936. [Google Scholar] [CrossRef]
- Ji, L.; Chen, M.; Zhang, W.; Nian, B.; Hu, Y. Comprehensive Applications of Ionic Liquids in Enzyme Immobilization: Current Status and Prospects. Mol. Catal. 2024, 552, 113675. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Khan, A.B.; Ali, M. Effect of Ionic Liquid on Activity, Stability, and Structure of Enzymes: A Review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, J.; Sun, B.; Sun, C.; Xu, W.; Tang, K. Enhancement of the Catalytic Efficiency of Candida Antarctica Lipase A in Enantioselective Hydrolysis through Immobilization onto a Hydrophobic MOF Support. Biochem. Eng. J. 2021, 173, 108066. [Google Scholar] [CrossRef]
- Xin, J.; Zhao, Y.; Zhao, G.; Zheng, Y.; Ma, X.; Xia, C.; Li, S. Enzymatic Resolution of (R, S)-Naproxen in Water-Saturated Ionic Liquid. Biocatal. Biotransform. 2005, 23, 353–361. [Google Scholar] [CrossRef]
- Swain, S.P.; Khanra, M. Lipase Enzymes for Sustainable Synthesis of Chiral Active Pharmaceutical Ingredients (API) and Key Starting Materials. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Sikora, A.; Siódmiak, T.; Marszałł, M.P. Kinetic Resolution of Profens by EnantioselectiveEsterification Catalyzed by Candida Antarctica and Candida Rugosa Lipases. Chirality 2014, 26, 663–669. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Y.; Sun, C.; Sun, F.; Zhou, E.; Lei, C.; Zhang, D. UiO-66-NH2 Incorporated Nanofibrous Membranes by Direct Electrospinning/in-Situ Growth for Toluene Adsorption. J. Environ. Chem. Eng. 2025, 13, 115198. [Google Scholar] [CrossRef]
- Hashem, M.H.; Wehbe, M.; Damacet, P.; El Habbal, R.K.; Ghaddar, N.; Ghali, K.; Ahmad, M.N.; Karam, P.; Hmadeh, M. Electrospun Metal–Organic Framework-Fabric Nanocomposites as Efficient Bactericides. Langmuir 2023, 39, 9503–9513. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, W.; Zhu, X.; Ma, H.; Wang, P.; Shang, J.; Luo, P. In Situ Growth of UIO-66-NH2 on Thermally Stabilized Electrospun Polyacrylonitrile Nanofibers for Visible-Light Driven Cr (VI) Photocatalytic Reduction. J. Solid State Chem. 2022, 307, 122836. [Google Scholar] [CrossRef]
- Peterson, G.W.; Lu, A.X.; Epps, T.H. Tuning the Morphology and Activity of Electrospun Polystyrene/UiO-66-NH2 Metal-Organic Framework Composites to Enhance Chemical Warfare Agent Removal. ACS Appl. Mater. Interfaces 2017, 9, 32248–32254. [Google Scholar] [CrossRef]
- Molco, M.; Laye, F.; Samperio, E.; Ziv Sharabani, S.; Fourman, V.; Sherman, D.; Tsotsalas, M.; Wöll, C.; Lahann, J.; Sitt, A. Performance Fabrics Obtained by In Situ Growth of Metal–Organic Frameworks in Electrospun Fibers. ACS Appl. Mater. Interfaces 2021, 13, 12491–12500. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. Recent Update on Use of Ionic Liquids for Enzyme Immobilization, Activation, and Catalysis: A Partnership for Sustainability. Curr. Opin. Green Sustain. Chem. 2022, 36, 100621. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic Liquids as a Potential Solvent for Lipase-Catalysed Reactions: A Review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Degórska, O.; Szada, D.; Fu, Q.; Nghiem, L.D.; Biadasz, A.; Jesionowski, T.; Zdarta, J. Ionic Liquid Supported Hydrogel–Lipase Biocatalytic Systems in Asymmetric Synthesis of Enantiomerically Pure S-Ibuprofen. Int. J. Biol. Macromol. 2024, 281, 136221. [Google Scholar] [CrossRef]
- de Castro Bizerra, V.; Leandro Fernandes Melo, R.; Simão Neto, F.; Carlos de Castro, E.; Bessa Sales, M.; Gonçalves de Sousa Junior, P.; Nascimento Dari, D.; Izaias da Silva Aires, F.; Moreira dos Santos, K.; de França Serpa, J.; et al. Exploring the Synergy of Ionic Liquids and Lipase in Sustainable Enzymatic Engineering. J. Mol. Liq. 2024, 399, 124373. [Google Scholar] [CrossRef]
- Huang, B.; Wang, C.; Zhang, W.; Fu, C.; Liu, H.; Wang, H. Study on the Stability of Produced Water from Alkali/Surfactant/Polymer Flooding under the Synergetic Effect of Quartz Sand Particles and Oil Displacement Agents. Processes 2020, 8, 315. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, J.; Li, J.; Fan, M.; Han, M.; Liu, Z.; Li, Z.; Kong, F. Metal Organic Framework UiO-66 Incorporated Ultrafiltration Membranes for Simultaneous Natural Organic Matter and Heavy Metal Ions Removal. Environ. Res. 2022, 208, 112651. [Google Scholar] [CrossRef]
- EL Hady, R.H.M.; Gheith; Khalil, M.M.H.; Mubarak, M.F. Development and Characterization of PVC-Based Membranes Embedded with Magnetic Silica Core-Shell Composites for Enhanced Oil-Water Separation. Desalin. Water Treat. 2025, 322, 101127. [Google Scholar] [CrossRef]
- Rodrigues Sousa, R.; Sant’Ana Silva, A.; Fernandez-Lafuente, R.; Santana Ferreira-Leitão, V. Solvent-Free Esterifications Mediated by Immobilized Lipases: A Review from Thermodynamic and Kinetic Perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J. Understanding Lipase-Deep Eutectic Solvent Interactions Towards Biocatalytic Esterification. Catalysts 2025, 15, 358. [Google Scholar] [CrossRef]
- Palomo, J.M.; Ortiz, C.; Fernández-Lorente, G.; Fuentes, M.; Guisán, J.M.; Fernández-Lafuente, R. Lipase–Lipase Interactions as a New Tool to Immobilize and Modulate the Lipase Properties. Enzym. Microb. Technol. 2005, 36, 447–454. [Google Scholar] [CrossRef]
- Waggett, A.; Pfaendtner, J. Hydrophobic Residues Promote Interfacial Activation of Candida Rugosa Lipase: A Study of Rotational Dynamics. Langmuir 2024, 40, 18262–18271. [Google Scholar] [CrossRef]
- Abellanas-Perez, P.; Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. The Effects of the Chemical Modification on Immobilized Lipase Features Are Affected by the Enzyme Crowding in the Support. Biotechnol. Prog. 2024, 40, e3394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase Catalysis in Organic Solvents: Advantages and Applications. Biol Proced Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ge, M.; Guo, X.; Li, Z.; Huang, A.; Yang, F.; Guo, R. Hydrophobic Poly(Ionic Liquid)s as “Two-Handed Weapons”: Maximizing Lipase Catalytic Efficiency in Transesterification of Soybean Oil toward Biodiesel. Appl. Catal. A Gen. 2021, 626, 118350. [Google Scholar] [CrossRef]
- Kaar, J.L.; Jesionowski, A.M.; Berberich, J.A.; Moulton, R.; Russell, A.J. Impact of Ionic Liquid Physical Properties on Lipase Activity and Stability. J. Am. Chem. Soc. 2003, 125, 4125–4131. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Shahba, A.; Zaare, F.; Sargazi, G.; Seyedalipour, B.; Karami, Z. Lipase Immobilization on a Novel Class of Zr-MOF/Electrospun Nanofibrous Polymers: Biochemical Characterization and Efficient Biodiesel Production. Int. J. Biol. Macromol. 2021, 192, 1292–1303. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of Lipase within Metal-Organic Framework (MOF) with Enhanced Activity Intensified under Ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Wu, J.-P.; Xu, G.; Yang, L.-R. Lipase-Catalyzed Remote Kinetic Resolution of Citalopram Intermediate by Asymmetric Alcoholysis and Thermodynamic Analysis. Bioprocess Biosyst. Eng. 2012, 35, 1043–1048. [Google Scholar] [CrossRef]
| Immobilization Efficiency (%) | |||
|---|---|---|---|
| Without IL | Choline Chloride | Choline Acetate | |
| 1 h | |||
| LOW | 23 ± 0.9 | 29 ± 0.5 | 34 ± 1.1 |
| LOW MOF | 24 ± 1 | 26 ± 0.9 | 37 ± 0.8 |
| HIGH | 21 ± 0.8 | 19 ± 0.1 | 40 ± 0.8 |
| HIGH MOF | 25 ± 0.05 | 27 ± 0.1 | 38 ± 0.2 |
| 24 h | |||
| LOW | 22 ± 0.8 | 21 ± 1 | 42 ± 1.2 |
| LOW MOF | 28 ± 0.2 | 33 ± 0.9 | 35 ± 0.6 |
| HIGH | 27 ± 0.3 | 45 ± 0.7 | 48 ± 0.2 |
| HIGH MOF | 47 ± 0.05 | 45 ± 0.2 | 52 ± 0.1 |
| Activity Recovery (%) | |||
|---|---|---|---|
| Without IL | Choline Chloride | Choline Acetate | |
| 1 h | |||
| LOW | 30 ± 1.1 | 37 ± 1 | 40 ± 0.9 |
| LOW MOF | 34 ± 0.6 | 38 ± 1.1 | 44 ± 0.8 |
| HIGH | 32 ± 1.1 | 44 ± 0.5 | 52 ± 1 |
| HIGH MOF | 59 ± 0.2 | 60 ± 0.9 | 74 ± 0.1 |
| 24 h | |||
| LOW | 37 ± 0.4 | 39 ± 0.8 | 49 ± 1 |
| LOW MOF | 45 ± 0.2 | 44 ± 0.4 | 57 ± 1 |
| HIGH | 51 ± 0.2 | 49 ± 0.5 | 58 ± 0.3 |
| HIGH MOF | 76 ± 0.2 | 77 ± 0.4 | 100 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degórska, O.; Zasada, N.; Badzińska, W.; Fu, Q.; Jesionowski, T.; Zdarta, J. Targeted Biocatalyst Design for Asymmetric Citalopram Conversion in a Membrane Reactor. Pharmaceutics 2025, 17, 1497. https://doi.org/10.3390/pharmaceutics17111497
Degórska O, Zasada N, Badzińska W, Fu Q, Jesionowski T, Zdarta J. Targeted Biocatalyst Design for Asymmetric Citalopram Conversion in a Membrane Reactor. Pharmaceutics. 2025; 17(11):1497. https://doi.org/10.3390/pharmaceutics17111497
Chicago/Turabian StyleDegórska, Oliwia, Natalia Zasada, Weronika Badzińska, Qiang Fu, Teofil Jesionowski, and Jakub Zdarta. 2025. "Targeted Biocatalyst Design for Asymmetric Citalopram Conversion in a Membrane Reactor" Pharmaceutics 17, no. 11: 1497. https://doi.org/10.3390/pharmaceutics17111497
APA StyleDegórska, O., Zasada, N., Badzińska, W., Fu, Q., Jesionowski, T., & Zdarta, J. (2025). Targeted Biocatalyst Design for Asymmetric Citalopram Conversion in a Membrane Reactor. Pharmaceutics, 17(11), 1497. https://doi.org/10.3390/pharmaceutics17111497








