Effective Oral Delivery of Teriparatide Using Organoclay—Polymethacrylate Nanocomposites for Osteoporosis Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
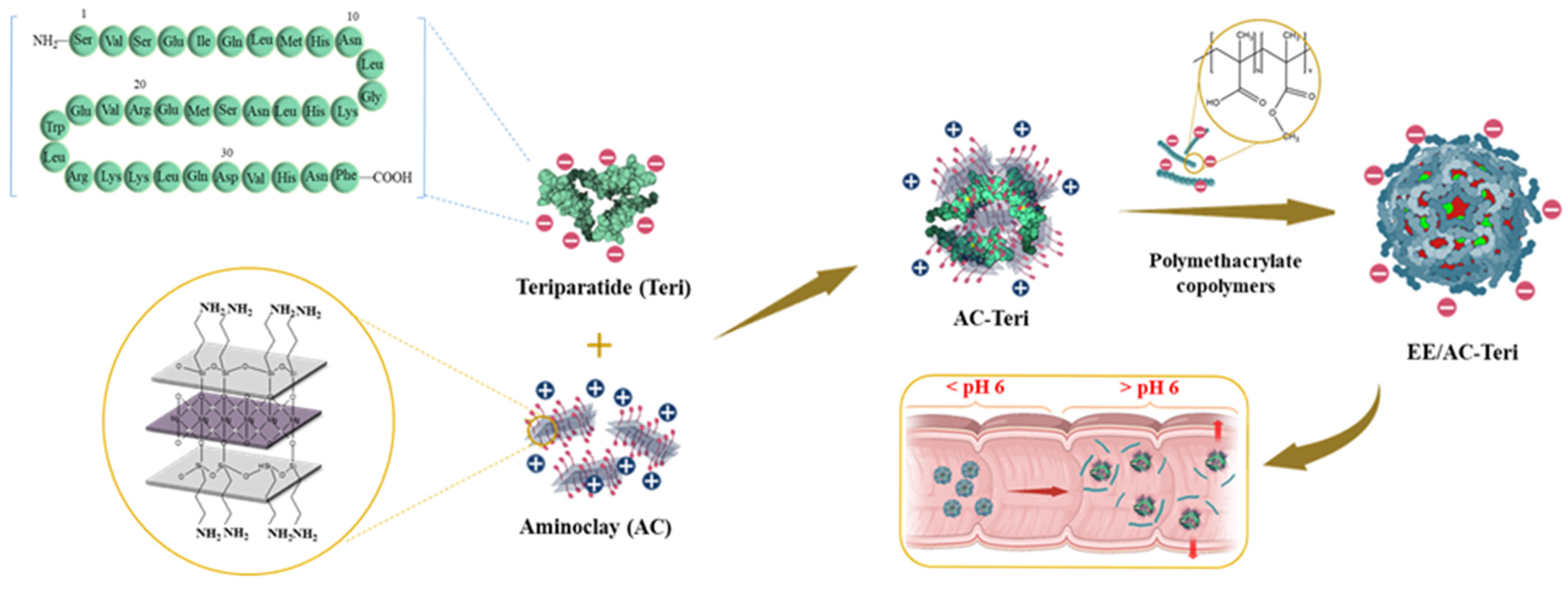
2.2. Preparation of AC-Based Nanocomplex
2.3. Structural Characterization
2.4. In Vitro Drug Release Studies
2.5. Gastrointestinal Stability
2.6. MTS Assay
2.7. Transport Studies
2.8. Efficacy Studies in Dexamethasone-Induced Osteoporosis Rats
2.9. HPLC Assay
3. Results and Discussion
3.1. Fabrication and Characterization of Nanoparticles
3.2. In Vitro Drug Release Profiles
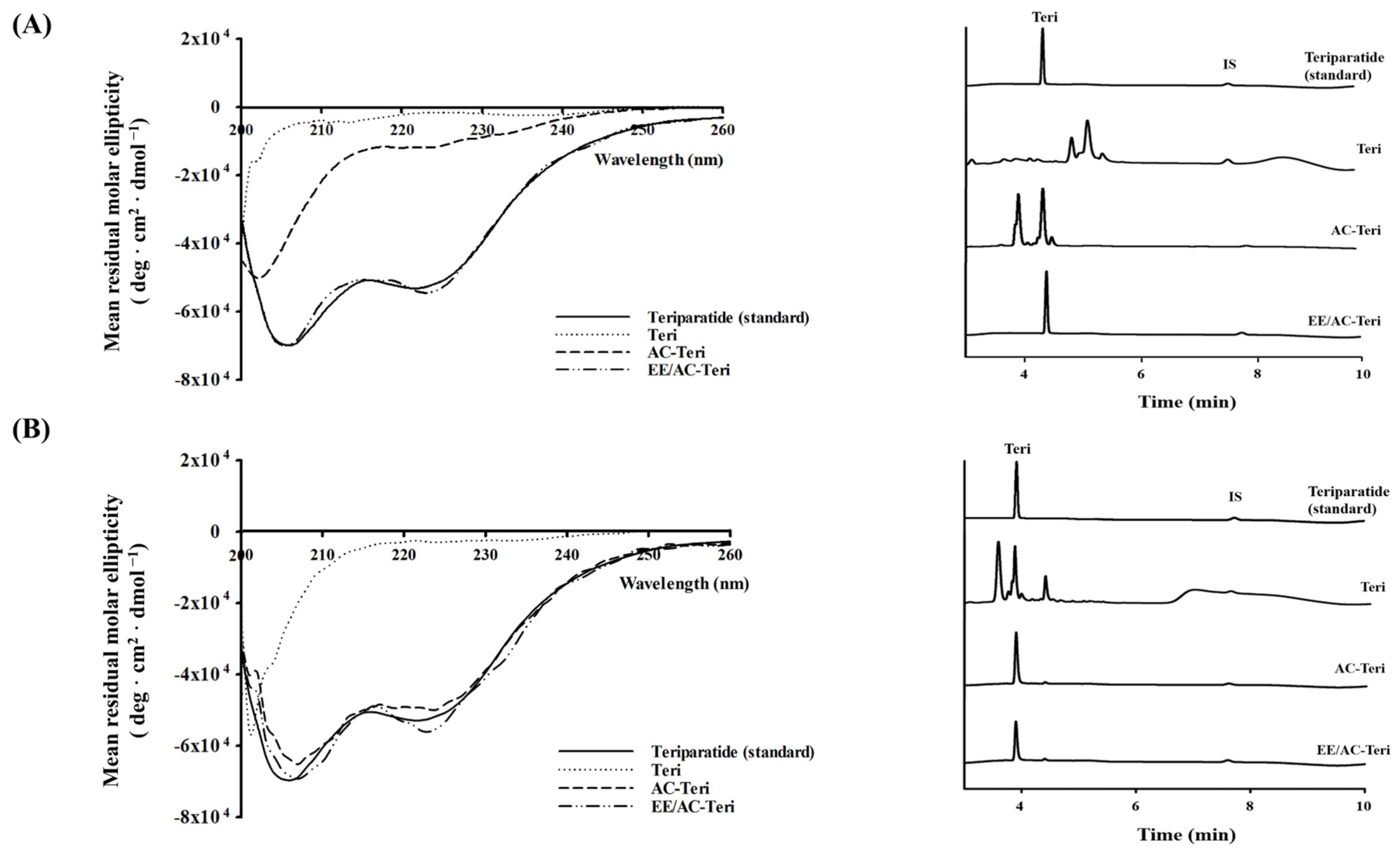
3.3. Protection Against Enzymatic Degradation
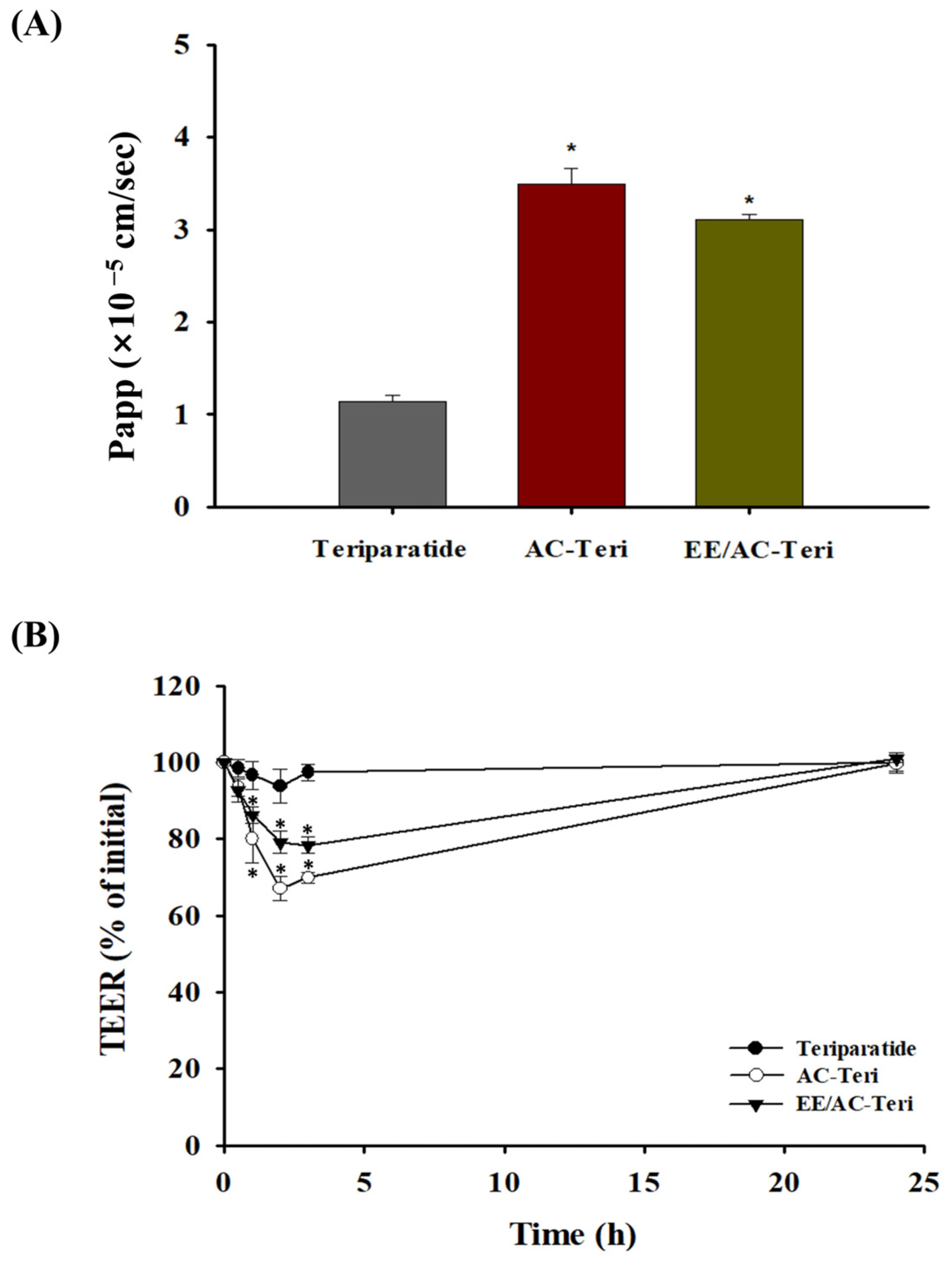
3.4. Cellular Permeability Studies
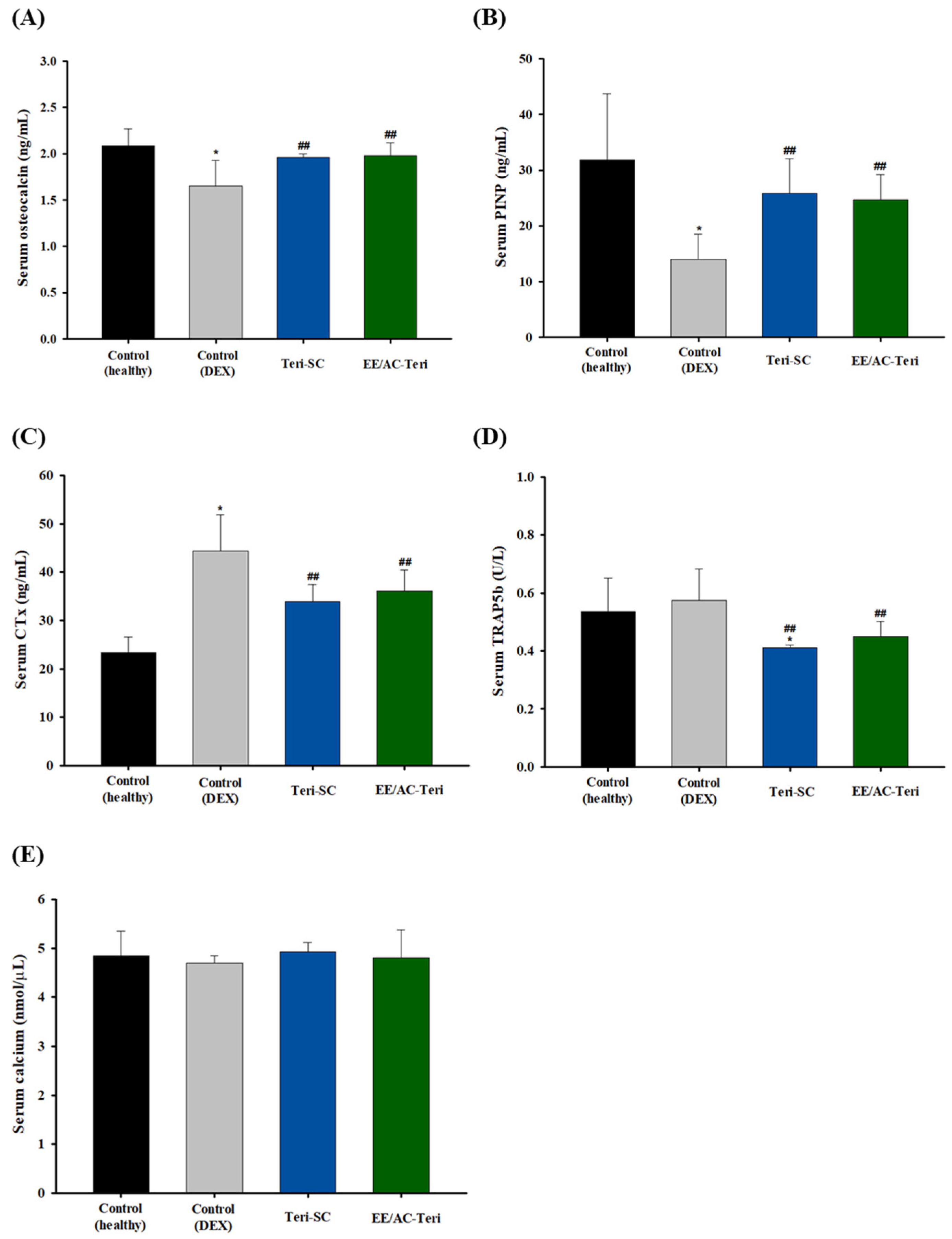
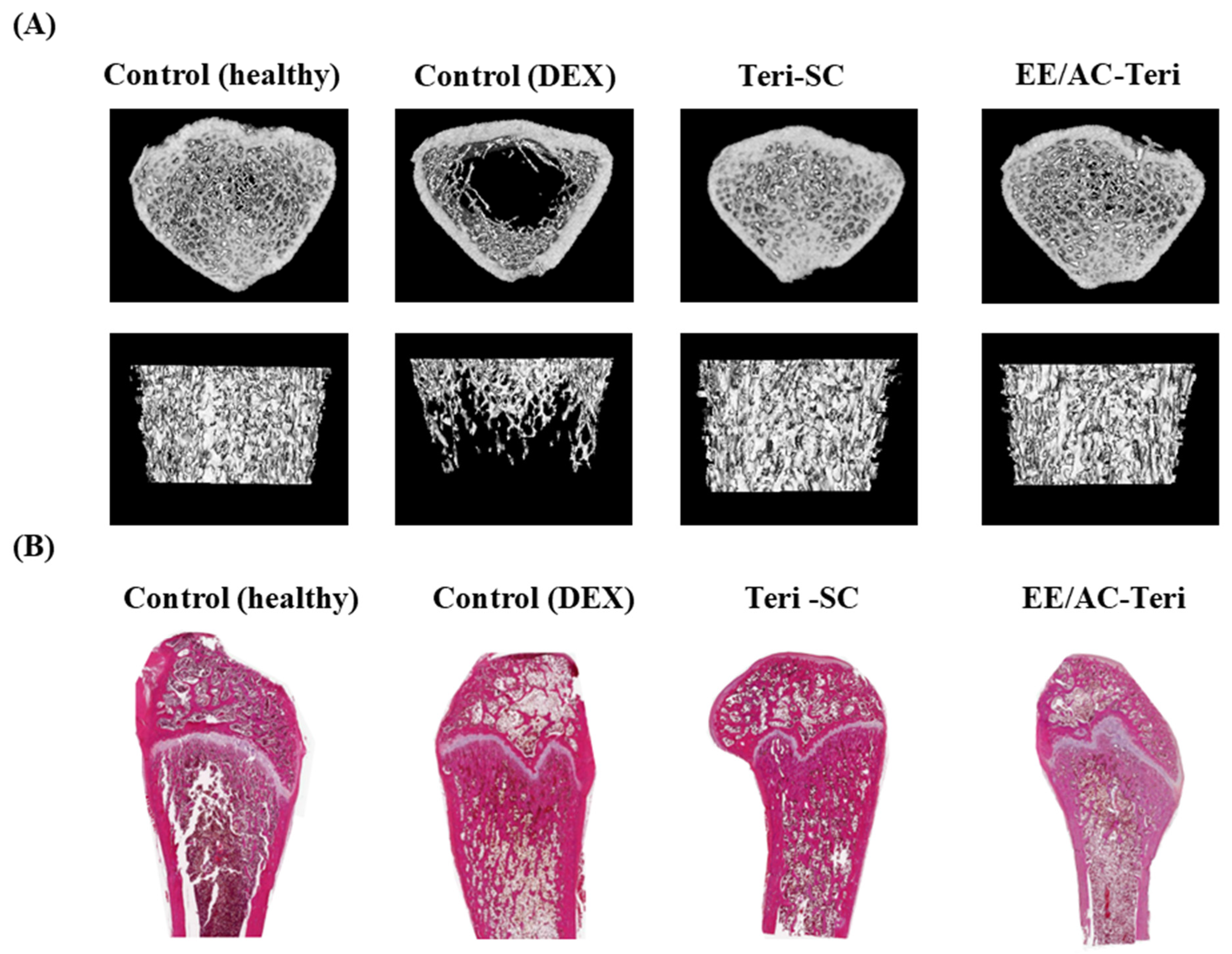
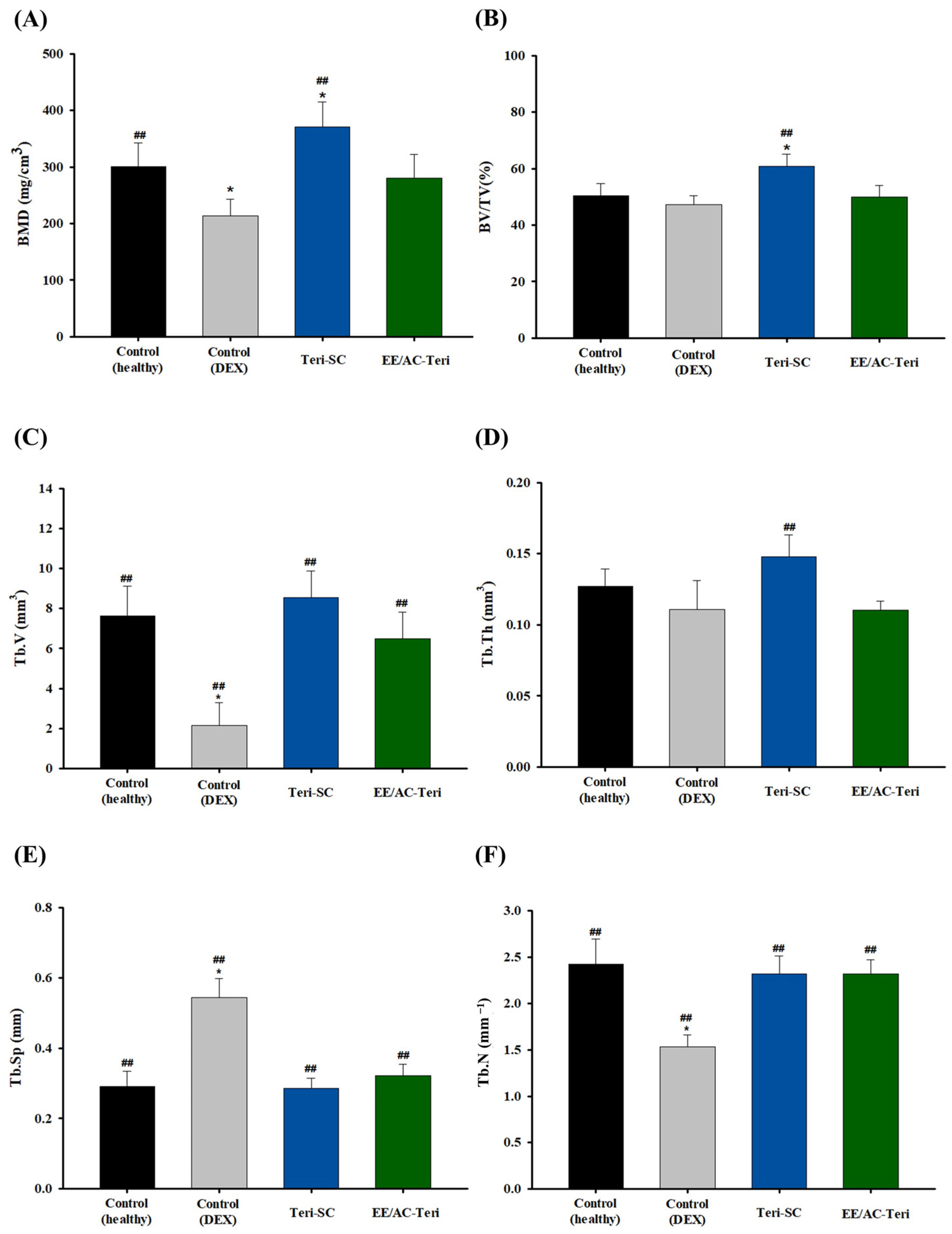
3.5. In Vivo Efficacy Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyazaki, T.; Zhao, Z.; Ichihara, Y.; Yoshino, D.; Sawada, Y. Mechanical regulation of bone homeostasis through p130Cas-mediated alleviation of NF-κB activity. Sci. Adv. 2019, 5, eaau7802. [Google Scholar] [CrossRef]
- Bartolozzi, E. The natural approach to osteoporosis. Clin. Cases Miner. Bone Metab. 2015, 12, 111. [Google Scholar] [CrossRef]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Drug carriers in osteoporosis: Preparation, drug encapsulation and applications. Int. J. Pharm. 2013, 445, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-R.V.; Liu, M.; Kwon, S.K.; Iida, M. Dysregulation of ectonucleotidase-mediated extracellular adenosine during postmenopausal bone loss. Sci. Adv. 2019, 5, eaax1387. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2016, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Scala, R.; Maqoud, F.; Antonacci, M.; Dibenedetto, J.R.; Perrone, M.G.; Scilimati, A.; Castillo, K.; Latorre, R.; Conte, D.; Bendahhou, S. Bisphosphonates targeting ion channels and musculoskeletal effects. Front. Pharmacol. 2022, 13, 837534. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Beaudart, C.; Bruyère, O.; Abrahamsen, B.; Al-Daghri, N.; Burlet, N.; Chandran, M.; Rosa, M.M.; Cortet, B.; Demonceau, C. Evidence-Based Guideline for the management of osteoporosis in men. Nat. Rev. Rheumatol. 2024, 20, 241–251. [Google Scholar] [CrossRef]
- Bandeira, L.; Lewiecki, E.M. Anabolic therapy for osteoporosis: Update on efficacy and safety. Arch. Endocrinol. Metab. 2022, 66, 707–716. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Bayat, Z.; Sagharidouz, S. Overexpression of recombinant human teriparatide, rhPTH (1–34) in Escherichia coli: An innovative gene fusion approach. Avicenna J. Med. Biotechnol. 2017, 9, 19. [Google Scholar]
- Lindsay, R.; Krege, J.H.; Marin, F.; Jin, L.; Stepan, J.J. Teriparatide for osteoporosis: Importance of the full course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef]
- Gardner, M.J.; Collinge, C. Management principles of osteoporotic fractures. Injury 2016, 47, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Altaani, B.M.; Almaaytah, A.M.; Dadou, S.; Alkhamis, K.; Daradka, M.H.; Hananeh, W. Oral delivery of teriparatide using a nanoemulsion system: Design, in vitro and in vivo evaluation. Pharm. Res. 2020, 37, 80. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, L.; Gulanski, B.; Wittink, D. Patient treatment preferences for osteoporosis. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2006, 55, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.; Sanchez-Dengra, B.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I. Oral controlled release dosage forms: Dissolution versus diffusion. Expert Opin. Drug. Deliv. 2020, 17, 791–803. [Google Scholar] [CrossRef]
- Subedi, L.; Pandey, P.; Kang, S.H.; Kim, K.-T.; Cho, S.-S.; Chang, K.-Y. Enhanced oral absorption of teriparatide with therapeutic potential for management of osteoporosis. J. Control Release. 2022, 349, 502–519. [Google Scholar] [CrossRef]
- Myers, J.T.; Yamaguchi, A.; Vo, A.T.; Dasari, A.; Battiwala, A.; Patel, N.; Fung, L.C. An oral robotic pill reliably and safely delivers teriparatide with high bioavailability in healthy volunteers: A phase 1 study. Br. J. Clin. Pharmacol. 2025, 91, 2447–2457. [Google Scholar] [CrossRef]
- Sturmer, A.; Mehta, N.; Giacchi, J.; Cagatay, T.; Tavakkol, R.; Mitta, S.; Fitzpatrick, L.; Wald, J.; Trang, J.; Stern, W. Pharmacokinetics of oral recombinant human parathyroid hormone [rhPTH(1-31)NH(2)] in postmenopausal women with osteoporosis. Clin. Pharmacokinet. 2013, 52, 995–1004. [Google Scholar] [CrossRef]
- Patil, A.; Mann, S. Self-assembly of bio–inorganic nanohybrids using organoclay building blocks. J. Mater. Chem. 2008, 18, 4605–4615. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, J.G.; Han, H.-K. Development of pH-responsive organic-inorganic hybrid nanocomposites as an effective oral delivery system of protein drugs. J. Control Release 2019, 311, 74–84. [Google Scholar] [CrossRef]
- Yang, L.; Choi, S.-K.; Shin, H.-J.; Han, H.-K. 3-aminopropyl functionalized magnesium phyllosilicate as an organoclay based drug carrier for improving the bioavailability of flurbiprofen. Int. J. Nanomed. 2013, 8, 4147–4155. [Google Scholar] [CrossRef]
- Song, J.G.; Kim, D.H.; Han, H.-K. Fabrication and evaluation of a pH-responsive nanocomposite-based colonic delivery system for improving the oral efficacy of liraglutide. Int. J. Nanomed. 2023, 18, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Song, J.G.; Lee, S.H.; Bajracharya, R.; Ifekpolugo, N.L.; Kim, G.-L.; Park, S.J.; Jeong, S.H.; Lee, C.H.; Han, H.-K. Layered silicate nanoparticles as a non-injectable drug delivery system for biomacromolecules. J. Pharm. Investig. 2024, 54, 593–604. [Google Scholar] [CrossRef]
- Datta, K.K.R.; Achari, A.; Eswaramoorthy, M. Aminoclay: A functional layered material with multifaceted applications. J. Mater. Chem. A 2013, 1, 6707–6718. [Google Scholar] [CrossRef]
- Song, J.G.; Lee, S.H.; Han, H.K. Biophysical evaluation of aminoclay as an effective protectant for protein stabilization during freeze-drying and storage. Int. J. Nanomed. 2016, 11, 6609–6619. [Google Scholar] [CrossRef]
- Patil, A.J.; Muthusamy, E.; Mann, S. Synthesis and self-assembly of organoclay-wrapped biomolecules. Angew. Chem. Int. Ed. 2004, 43, 4928–4933. [Google Scholar] [CrossRef]
- Wang, S.; Cao, H.; Zhong, Y. A novel aminoclay-curcumin hybrid for enhanced chemotherapy. J. Mater. Chem. B 2016, 4, 4295–4301. [Google Scholar] [CrossRef]
- Tran, V.V.; Moon, J.Y.; Lee, Y.C. Novel magnesium aminoclay-vitamin C hybrid for enhanced stability and bioactivity in cosmeceutical applications. J. Nanosci. Nanotechnol. 2020, 20, 4257–4262. [Google Scholar] [CrossRef]
- Kim, G.L.; Song, J.G.; Han, H.-K. Enhanced oral efficacy of semaglutide via an ionic nanocomplex with organometallic phyllosilicate in type 2 diabetic rats. Pharmaceutics 2024, 16, 886. [Google Scholar] [CrossRef]
- Kumar, R.; Majumdar, D.; Panda, A. Oral delivery of insulin using composite polymeric microparticles. Int. J. Pharm. Chem. Biol. Sci. 2014, 4, 834. [Google Scholar]
- Soni, M.L.; Namdeo, K.P.; Jain, S.K.; Gupta, M.; Dangi, J.S.; Kumar, M.; Dangi, Y.S. pH-enzyme di-dependent chronotherapeutic drug delivery system of theophylline for nocturnal asthma. Chem. Pharm. Bull. 2011, 59, 191–195. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Song, J.G.; Lee, S.H.; Han, H.-K. Sustained-release solid dispersion of pelubiprofen using the blended mixture of aminoclay and pH independent polymers: Preparation and in vitro/in vivo characterization. Drug Deliv. 2017, 24, 1731–1739. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Contreras, M.d.M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Olivieri, P.H.; Jesus, M.B.; Nader, H.B.; Justo, G.Z.; Sousa, A.A. Cell-surface glycosaminoglycans regulate the cellular uptake of charged polystyrene nanoparticles. Nanoscale 2022, 14, 7350–7363. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone matrix non-collagenous proteins in tissue engineering: Creating new bone by mimicking the extracellular matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.-K.; Risteli, L.; Risteli, J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin. Biochem. 2012, 45, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Ferreras, M.; Karsdal, M.; Nicamhlaoibh, R.; Risteli, J.; Borel, O.; Qvist, P.; Delmas, P.; Foged, N.; Delaisse, J. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003, 18, 859–867. [Google Scholar] [CrossRef]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Väänänen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef]
- Oikonomou, D.; Laina, A.; Xydaki, A.; Christopoulo, C. Steroid-induced hypocalcaemia with tetany in a patient with hypoparathyroidism. BMJ Case Rep. 2014, 2014, bcr2014207562. [Google Scholar] [CrossRef]
- Satterwhite, J.; Heathman, M.; Miller, P.; Marín, F. Pharmacokinetics of teriparatide (rhPTH [1–34]) and calcium pharmacodynamics in postmenopausal women with osteoporosis. Calcif. Tissue Int. 2010, 87, 485–492. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Y.; Chen, Y.; Gao, X.; Su, Y.; Cui, L. Effects of dexamethasone, celecoxib, and methotrexate on the histology and metabolism of bone tissue in healthy Sprague Dawley rats. Clin. Interv. Aging 2015, 10, 1245–1253. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, J.; Shen, T.; Mu, S.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016, 37, 329–338. [Google Scholar] [CrossRef]
- Wu, Y.; Adeeb, S.; Doschak, M.R. Using micro-CT derived bone microarchitecture to analyze bone stiffness–a case study on osteoporosis rat bone. Front. Endocrinol. 2015, 6, 80. [Google Scholar] [CrossRef]
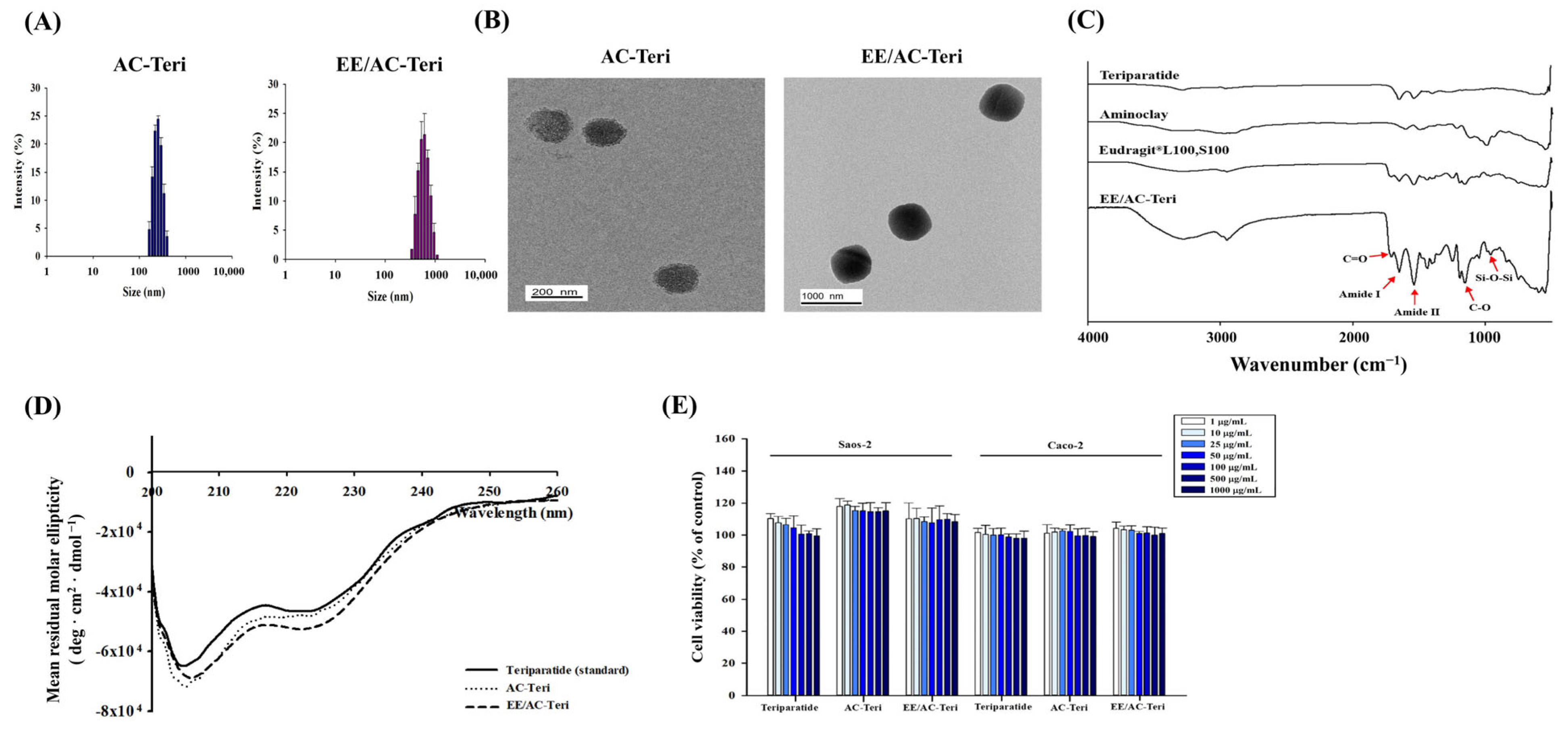

| Formulation | Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| AC-Teri | 264 ± 8.4 | 0.216 ± 0.045 | 8.74 ± 0.19 | 90.1 ± 0.3 |
| EE/AC-Teri | 569 ± 13.1 | 0.240 ± 0.071 | −23.3 ± 1.96 | 97.8 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.L.; Kang, Y.J.; Seo, S.H.; Jeon, J.; Han, H.-K. Effective Oral Delivery of Teriparatide Using Organoclay—Polymethacrylate Nanocomposites for Osteoporosis Therapy. Pharmaceutics 2025, 17, 1450. https://doi.org/10.3390/pharmaceutics17111450
Kim GL, Kang YJ, Seo SH, Jeon J, Han H-K. Effective Oral Delivery of Teriparatide Using Organoclay—Polymethacrylate Nanocomposites for Osteoporosis Therapy. Pharmaceutics. 2025; 17(11):1450. https://doi.org/10.3390/pharmaceutics17111450
Chicago/Turabian StyleKim, Gyu Lin, Yeon Ju Kang, Soo Hwa Seo, Jiwoon Jeon, and Hyo-Kyung Han. 2025. "Effective Oral Delivery of Teriparatide Using Organoclay—Polymethacrylate Nanocomposites for Osteoporosis Therapy" Pharmaceutics 17, no. 11: 1450. https://doi.org/10.3390/pharmaceutics17111450
APA StyleKim, G. L., Kang, Y. J., Seo, S. H., Jeon, J., & Han, H.-K. (2025). Effective Oral Delivery of Teriparatide Using Organoclay—Polymethacrylate Nanocomposites for Osteoporosis Therapy. Pharmaceutics, 17(11), 1450. https://doi.org/10.3390/pharmaceutics17111450






