Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives
Abstract
1. Introduction
2. Chemical Structure of Polymeric Nucleotide Fragments
3. Biological and Physiological Role of PN and PDRN
3.1. Role and Function of PN
3.2. Role and Function of PDRN
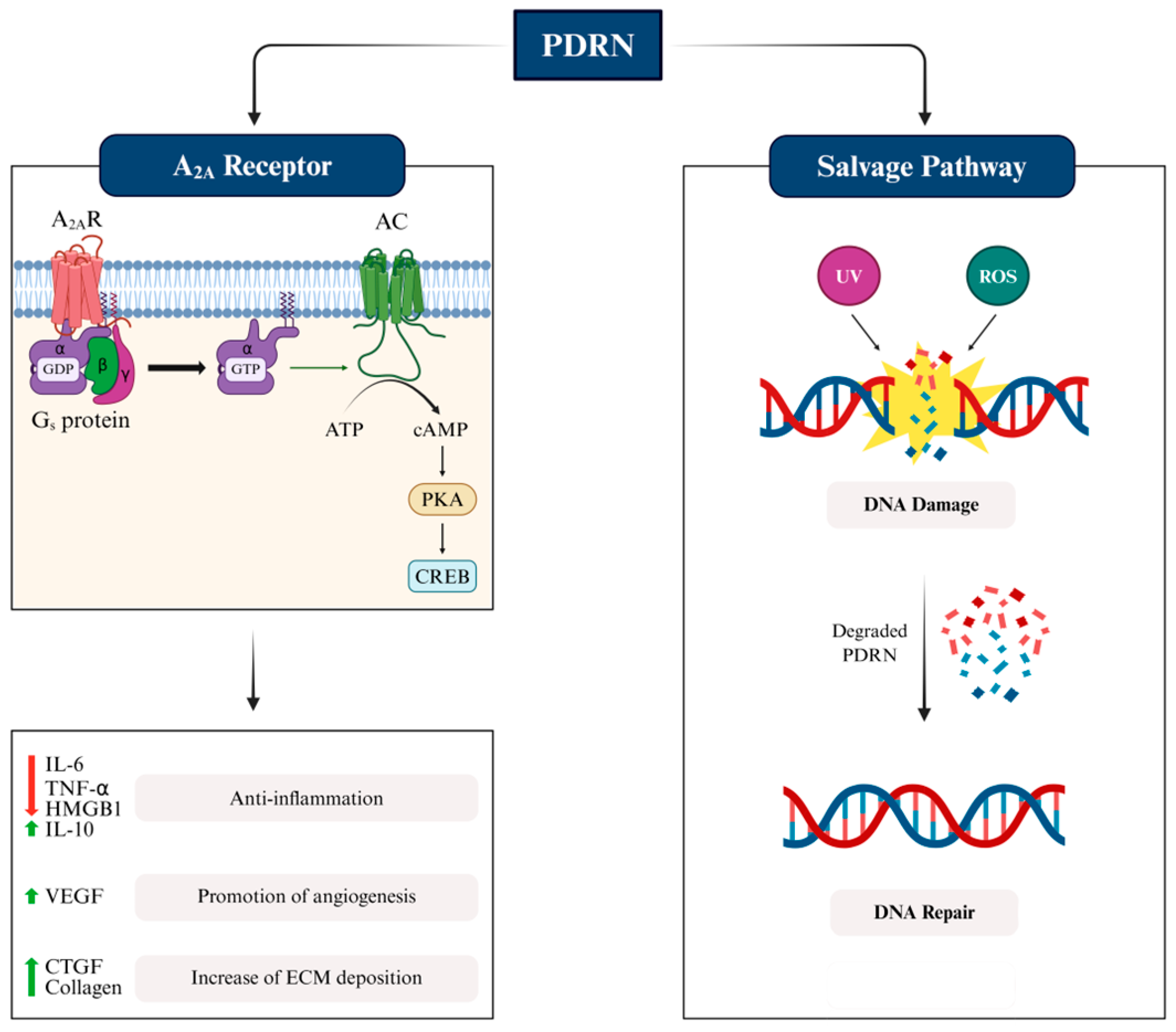
3.2.1. Mode of Action of PDRN: Activation of Adenosine Receptor
3.2.2. Mode of Action of PDRN: Degradation by Salvage Pathway
3.3. Dermatological Effect of PDRN on Skin Health
3.3.1. Skin Regeneration and Rejuvenation
3.3.2. Skin Hypopigmentation
3.3.3. Skin Wound Healing
3.3.4. Hair Regeneration
3.3.5. Anti-Inflammation in Skin
3.3.6. Antioxidant Effect on Skin
3.4. Comparison of PN and PDRN
4. Clinical Uses of PN and PDRN
4.1. Safety of PN and PDRN
4.2. Isolation and Purification of PN and PDRN for Clinical Uses
4.3. Current Status of Clinical Applications of PN
4.4. Current Status of Clinical Use of PDRN
5. Discussion
6. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, M. Human skin through the ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef]
- Menon, G.; Kligman, A. Barrier Functions of Human Skin: A Holistic View. Ski. Pharmacol. Physiol. 2009, 22, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin homeostasis: Mechanism and influencing factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef]
- Wilhelm, K.P. Skin aging. Effect on transepidermal water loss, stratum corneum hydration, skin surface pH, and casual sebum content. Arch. Dermatol. 1991, 127, 1806–1809. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Ak, M. Skin Aging & Modern Age Anti-Aging Strategies. Int. J. Clin. Dermatol. Res. 2019, 209–240. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.H.; Kim, B.R.; Seo, H.-M.; Kwon, S.-H.; Choi, H.; Lee, H.; Na, J.-I.; Choi, C.P.; Ko, J.Y.; et al. Real-World Clinical Practice on Skin Rejuvenation Among Korean Board-Certified Dermatologists: Survey-Based Results. Ann. Dermatol. 2025, 37, 123–130. [Google Scholar] [CrossRef]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Wan, J.; Oksana, L.; Yuliia, L.; Chugay, O.; Platonova, O.; Sydorchuk, O.; Yi, K.-H. Polynucleotide-based treatments for various facial scars including combat injuries. J. Dermatol. Treat. 2024, 35, 2426626. [Google Scholar] [CrossRef]
- Polynucleotides Injectables Market Size-By Application, By End Use, Forecast, 2025–2034. Available online: https://www.gminsights.com/industry-analysis/polynucleotides-injectables-market (accessed on 19 June 2025).
- Rho, N.K.; Han, K.H.; Cho, M.; Kim, H.S. A survey on the cosmetic use of injectable polynucleotide: The pattern of practice among Korean Dermatologists. J. Cosmet. Dermatol. 2023, 23, 1243–1252. [Google Scholar] [CrossRef]
- Yi, K.; Winayanuwattikun, W.; Kim, S.; Wan, J.; Vachatimanont, V.; Putri, A.I.; Hidajat, I.J.; Yogya, Y.; Pamela, R. Skin boosters: Definitions and varied classifications. Ski. Res. Technol. 2024, 30, e13627. [Google Scholar] [CrossRef]
- Webb, W.R.; Rahman, E.; Rao, P.; Abu-Farsakh, H.N.; Yu, N.; Garcia, P.E.; Ioannidis, S.; Sayed, K.; Tam, E.; Philipp-Dormston, W.G.; et al. Points to ponder on the role of polynucleotides in regenerative and aesthetic medicine: A systematic review. Eur. J. Plast. Surg. 2024, 47, 1–14. [Google Scholar] [CrossRef]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Samizadeh, S. Polynucleotides: The crucial biomolecules bridging therapeutics and aesthetics. J. Aesthetic Nurs. 2023, 12, 391–399. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Baek, E.J.; Kim, K.J.; Park, E.J. Polydeoxyribonucleotide exerts opposing effects on ERK activity in human skin keratinocytes and fibroblasts. Mol. Med. Rep. 2023, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abuyousif, H.S.; Porcello, A.; Cerrano, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Chemali, M.; Raffoul, W.; Hirt-Burri, N.; et al. In Vitro Evaluation and Clinical Effects of a Regenerative Complex with Non-Cross-Linked Hyaluronic Acid and a High-Molecular-Weight Polynucleotide for Periorbital Treatment. Polymers 2025, 17, 638. [Google Scholar] [CrossRef]
- Park, K.Y.; Seok, J.; Rho, N.K.; Kim, B.J.; Kim, M.N. Long-chain polynucleotide filler for skin rejuvenation: Efficacy and complications in five patients. Dermatol. Ther. 2015, 29, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R.; et al. Polydeoxyribonucleotide: A Promising Biological Platform to Accelerate Impaired Skin Wound Healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, H.; Jung, R.; Won, C.; Ohk, S.; Kim, H.; Roh, N.; Yi, K. High-resolution 3-D scanning electron microscopy (SEM) images of DOT™ polynucleotides (PN): Unique scaffold characteristics and potential applications in biomedicine. Ski. Res. Technol. 2024, 30. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.; Oh, S.M.; Yi, K. Polynucleotide injection treatment for iatrogenic fat atrophy in two patients: Potential for safe volumization in aesthetic medicine. Ski. Res. Technol. 2023, 29, e13439. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes Wound Healing in a Primary Gingival Fibroblast Model. Appl. Sci. 2021, 11, 4405. [Google Scholar] [CrossRef]
- Pitassi, L.H.U.; Pearson, K.; de Assis, L.A.; Biesman, B.; Calomeni, M.; Bay-Aguilera, S.D.; Wyles, S.P. Polynucleotides in Skin Regeneration: Targeting the Adenosine A2A Receptor and Salvage Pathway. Dermatol. Surg. 2024, 50, S131–S134. [Google Scholar] [CrossRef]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Palmieri, I.P.; Papagni, M.; Priori, M.; Trocchi, G. Value and Benefits of the Polynucleotides HPT™ Dermal Priming Paradigm A Consensus on Practice Guidance for Aesthetic Medicine Practitioners and Future Research. Clin. Exp. Dermatol. Ther. 2024, 9, 224. [Google Scholar] [CrossRef]
- Lee, D.; Choi, H.; Yoo, K.; Park, Y.J.; Park, H.J.; Oh, S.M.; Ji, G.H.; Rah, G.C.; Shin, D.W. Assessment of current practices and perceived effectiveness of injectable polynucleotide for enlarged facial pores among cosmetic physicians: A survey-based evaluation. Ski. Res. Technol. 2024, 30, e13738. [Google Scholar] [CrossRef]
- Oh, N.; Hwang, J.; Kang, M.S.; Yoo, C.-Y.; Kwak, M.; Han, D.-W. Versatile and Marvelous Potentials of Polydeoxyribonucleotide for Tissue Engineering and Regeneration. Biomater. Res. 2025, 29, 0183. [Google Scholar] [CrossRef]
- Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M.; Won, C.H. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J. Dermatol. Treat. 2020, 33, 254–260. [Google Scholar] [CrossRef]
- Ha, Y.J.; Tak, K.H.; Jung, J.; Lee, J.L.; Kim, C.W.; Ah, Y.; Kim, S.; Moon, I.J.; Yoon, Y.S. The Effect of Polynucleotide-Hyaluronic Acid Hydrogel in the Recovery After Mechanical Skin Barrier Disruption. Ski. Res. Technol. 2024, 30, e70068. [Google Scholar] [CrossRef]
- Titcomb, L. A literature review on polynucleotide efficacy on skin rejuvenation, and review of the regulatory status and guidelines around polynucleotides. J. Aesthetic Nurs. 2025, 14, 98–126. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, H.C.; Jang, S.G.; Lee, Y.J.; Heo, J.Y.; Kweon, G.R.; Ryu, M.J. Effects of a Combination of Polynucleotide and Hyaluronic Acid for Treating Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 1714. [Google Scholar] [CrossRef]
- Heo, T.-H.; Gu, B.K.; Ohk, K.; Yoon, J.-K.; Son, Y.H.; Chun, H.J.; Yang, D.-H.; Jeong, G.-J. Polynucleotide and Hyaluronic Acid Mixture for Skin Wound Dressing for Accelerated Wound Healing. Tissue Eng. Regen. Med. 2025, 22, 515–526. [Google Scholar] [CrossRef]
- Dallari, D.; Sabbioni, G.; Del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Fini, M. Efficacy of Intra-Articular Polynucleotides Associated With Hyaluronic Acid Versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Clinical Trial. Am. J. Ther. 2020, 30, 1–7. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Segreto, F.; Carotti, S.; Marangi, G.F.; Francesconi, M.; Scaramuzzino, L.; Gratteri, M.; Caldaria, E.; Morini, S.; Persichetti, P. The use of acellular porcine dermis, hyaluronic acid and polynucleotides in the treatment of cutaneous ulcers: Single blind randomised clinical trial. Int. Wound J. 2020, 17, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; Del Piccolo, N.; Rani, N.; Govoni, M.; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, double-blind comparison of a fixed co-formulation of intra-articular polynucleotides and hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: Two-year follow-up. BMC Musculoskelet. Disord. 2021, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwon, T.-R.; Lee, S.E.; Na Jang, Y.; Han, H.S.; Mun, S.K.; Kim, B.J. Comparative Evaluation of the Effectiveness of Novel Hyaluronic Acid-Polynucleotide Complex Dermal Filler. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Draelos, Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012, 30, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A. Effectiveness and utility of hyaluronic acid in osteoarthritis. Bone Abstr. 2015, 12, 31–33. [Google Scholar] [CrossRef]
- Kuppa, S.S.; Kim, H.-K.; Kang, J.-Y.; Lee, S.-C.; Yang, H.-Y.; Sankaranarayanan, J.; Seon, J.-K. Polynucleotides Suppress Inflammation and Stimulate Matrix Synthesis in an In Vitro Cell-Based Osteoarthritis Model. Int. J. Mol. Sci. 2023, 24, 12282. [Google Scholar] [CrossRef]
- Kim, T.W.; Chang, M.J.; Shin, C.Y.; Chang, C.B.; Kang, S.-B. A randomized controlled trial for comparing efficacy and safety between intraarticular polynucleotide and hyaluronic acid for knee osteoarthritis treatment. Sci. Rep. 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kim, J.; Lee, J.Y.; Ko, Y.; Park, H.J.; Jeon, Y.H. Comparison of Polynucleotide, Sodium Hyaluronate, and Crosslinked Sodium Hyaluronate for the Management of Painful Knee Osteoarthritis: A Multi-Center, Randomized, Double-Blind, Parallel-Group Study. Pain Med. 2022, 24, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, W.-H.; Ha, J.H.; Kim, H.; Kim, J.; Shin, D.W. Current Practices and Perceived Effectiveness of Clinicians Regarding Polynucleotide Injection for Knee Osteoarthritis: A Survey-Based Evaluation. Healthcare 2025, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Heo, S.-J.; Jung, W.-K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Mar. Drugs 2021, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Kim, Y.-T.; Hwang, J.-T. Clinical Updates in Polydeoxyribonucleotide Injection. J. Korean Orthop. Assoc. 2024, 59, 386–394. [Google Scholar] [CrossRef]
- Shin, D.Y.; Park, J.-U.; Choi, M.-H.; Kim, S.; Kim, H.-E.; Jeong, S.-H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Akaberi, S.M.; Sharma, K.; Ahmadi-Ashtiani, H.R.; Hedayati, M. Polydeoxyribonucleotide in Skincare and Cosmetics: Mechanisms, Therapeutic Applications, and Advancements Beyond Wound Healing and Anti-aging. J. Ski. Stem Cell 2025, 12, e159728. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Yun, Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS). Mater. Sci. Eng. C 2016, 69, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Stefano, V.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A Safe Approach to Induce Therapeutic Angiogenesis in Peripheral Artery Occlusive Disease and in Diabetic Foot Ulcers. Cardiovasc. Hematol. Agents Med. Chem. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Kim, T.; Heo, S.; Han, J.S.; Jung, W. Anti-inflammatory effect of polydeoxyribonucleotides (PDRN) extracted from red alga (Porphyra sp.) (Ps-PDRN) in RAW 264.7 macrophages stimulated with Escherichia coli lipopolysaccharides: A comparative study with commercial PDRN. Cell Biochem. Funct. 2023, 41, 889–897. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Sebastiã£O, A. Fine-tuning neuromodulation by adenosine. Trends Pharmacol. Sci. 2000, 21, 341–346. [Google Scholar] [CrossRef]
- Cunha, R. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: Different roles, different sources and different receptors. Neurochem. Int. 2001, 38, 107–125. [Google Scholar] [CrossRef]
- de Mendonça, A.; Ribeiro, J.A. Adenosine and synaptic plasticity. Drug Dev. Res. 2001, 52, 283–290. [Google Scholar] [CrossRef]
- Li, J.-M.; Fenton, R.A.; Wheeler, H.; Powell, C.C.; Peyton, B.D.; Cutler, B.S.; Dobson, J.G. Adenosine A2aReceptors Increase Arterial Endothelial Cell Nitric Oxide. J. Surg. Res. 1998, 80, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. A2A receptors in inflammation and injury: Lessons learned from transgenic animals. J. Leukoc. Biol. 2007, 83, 447–455. [Google Scholar] [CrossRef]
- Odashima, M.; Otaka, M.; Jin, M.; Komatsu, K.; Wada, I.; Matsuhashi, T.; Horikawa, Y.; Hatakeyama, N.; Oyake, J.; Ohba, R.; et al. Selective adenosine A2A receptor agonist, ATL-146e, attenuates stress-induced gastric lesions in rats. J. Gastroenterol. Hepatol. 2005, 20, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Biaggioni, I.; Paul, S.; Puckett, A.; Arzubiaga, C. Caffeine and theophylline as adenosine receptor antagonists in humans. J. Pharmacol. Exp. Ther. 1991, 258, 588–593. [Google Scholar] [CrossRef]
- Saini, A.; Patel, R.; Gaba, S.; Singh, G.; Gupta, G.; Monga, V. Adenosine receptor antagonists: Recent advances and therapeutic perspective. Eur. J. Med. Chem. 2022, 227, 113907. [Google Scholar] [CrossRef]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory Effect of DNA Polymeric Molecules in a Cell Model of Osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Säve, S.; Persson, K. Effects of Adenosine A2A and A2B Receptor Activation on Signaling Pathways and Cytokine Production in Human Uroepithelial Cells. Pharmacology 2010, 86, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Irrera, N.; D’AScola, A.; Avenoso, A.; Nastasi, G.; Campo, G.M.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The Effects of Polydeoxyribonucleotide on Wound Healing and Tissue Regeneration: A Systematic Review of the Literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-K.; Jung, J.-W.; Baek, S.-W.; Kim, J.H.; Heo, Y.; Kim, T.-H.; Han, D.K. Promotion of Bone Regeneration Using Bioinspired PLGA/MH/ECM Scaffold Combined with Bioactive PDRN. Materials 2021, 14, 4149. [Google Scholar] [CrossRef] [PubMed]
- Cámara, Y.; González-Vioque, E.; Scarpelli, M.; Torres-Torronteras, J.; Martí, R. Feeding the deoxyribonucleoside salvage pathway to rescue mitochondrial DNA. Drug Discov. Today 2013, 18, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Nyhan, W.L. Nucleotide Synthesis via Salvage Pathway. In Encyclopedia of Life Sciences (eLS); Wiley: Chichester, UK, 2001. [Google Scholar]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef]
- Bitto, A.; Galeano, M.; Squadrito, F.; Minutoli, L.; Polito, F.; Dye, J.F.; Clayton, E.A.F.; Calò, M.; Venuti, F.S.; Vaccaro, M.; et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit. Care Med. 2008, 36, 1594–1602. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, J.; Park, E.Y.; Cha, S. An effective range of polydeoxyribonucleotides is critical for wound healing quality. Mol. Med. Rep. 2018, 18, 5166–5172. [Google Scholar] [CrossRef]
- Bristow, R.G.; Hill, R.P. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Tran, D.H.; Kim, D.; Kesavan, R.; Brown, H.; Dey, T.; Soflaee, M.H.; Vu, H.S.; Tasdogan, A.; Guo, J.; Bezwada, D.; et al. De novo and salvage purine synthesis pathways across tissues and tumors. Cell 2024, 187, 3602–3618.e20. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, F.; Mosella, F. Bioinductive Dressing. In Pearls and Pitfalls in Skin Ulcer Management; Maruccia, M., Papa, G., Ricci, E., Giudice, G., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 215–244. [Google Scholar]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1–9. [Google Scholar] [CrossRef]
- D’eRrico, M.; Lemma, T.; Calcagnile, A.; De Santis, L.P.; Dogliotti, E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat. Res. Mol. Mech. Mutagen. 2007, 614, 37–47. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage—How and why we age? eLife 2021, 10, e62852. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lee, J.Y. Polydeoxyribonucleotide improves wound healing of fractional laser resurfacing in rat model. J. Cosmet. Laser Ther. 2016, 19, 43–48. [Google Scholar] [CrossRef]
- Tharaux, P.-L.; Chatziantoniou, C.; Fakhouri, F.; Dussaule, J.-C. Angiotensin II Activates Collagen I Gene Through a Mechanism Involving the MAP/ER Kinase Pathway. Hypertension 2000, 36, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Belletti, S.; Uggeri, J.; Gatti, R.; Govoni, P.; Guizzardi, S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2007, 23, 242–249. [Google Scholar] [CrossRef]
- Ishii, T.; Asuwa, N. Collagen and elastin degradation by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinase in aortic dissection. Hum. Pathol. 2000, 31, 640–646. [Google Scholar] [CrossRef]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin. J. Photochem. Photobiol. B Biol. 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Kim, H.M.; Byun, K.-A.; Oh, S.; Yang, J.Y.; Park, H.J.; Chung, M.S.; Son, K.H.; Byun, K. A Mixture of Topical Forms of Polydeoxyribonucleotide, Vitamin C, and Niacinamide Attenuated Skin Pigmentation and Increased Skin Elasticity by Modulating Nuclear Factor Erythroid 2-like 2. Molecules 2022, 27, 1276. [Google Scholar] [CrossRef]
- Park, J.M.; Nam, G.B.; Lee, E.-S.; Kim, H.-M.; Kim, H.; Myoung, K.; Lee, J.E.; Baek, H.S.; Ko, J.; Lee, C.S. Effects of Chlorella protothecoides-derived polydeoxyribonucleotides on skin regeneration and wound healing. Arch. Dermatol. Res. 2025, 317, 1–10. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, BSR20210427. [Google Scholar] [CrossRef]
- Kim, J.C.; Park, T.J.; Kang, H.Y. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J. Differential diagnosis and management of hyperpigmentation. Clin. Exp. Dermatol. 2021, 47, 251–258. [Google Scholar] [CrossRef]
- Ladizinski, B.; Mistry, N.; Kundu, R.V. Widespread Use of Toxic Skin Lightening Compounds: Medical and Psychosocial Aspects. Dermatol. Clin. 2011, 29, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster. Int. J. Mol. Sci. 2016, 17, 1448. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2019, 191, 540–554. [Google Scholar] [CrossRef]
- Lima, T.d.P.d.L.; Passos, M.F. Skin wounds, the healing process, and hydrogel-based wound dressings: A short review. J. Biomater. Sci. Polym. Ed. 2021, 32, 1910–1925. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Eaglstein, W.H. The Wound Healing Process. Dermatol. Clin. 1993, 11, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65, iii35–iii44. [Google Scholar] [CrossRef]
- Lin, X.; Lai, Y. Scarring Skin: Mechanisms and Therapies. Int. J. Mol. Sci. 2024, 25, 1458. [Google Scholar] [CrossRef]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Wells, A.; Nuschke, A.; Yates, C.C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016, 49, 25–36. [Google Scholar] [CrossRef]
- Varano, F.; Catarzi, D.; Vigiani, E.; Calenda, S.; Colotta, V. Adenosine Receptor Ligands as Potential Therapeutic Agents for Impaired Wound Healing and Fibrosis. In Purinergic Receptors and Their Modulators; Springer International Publishing: Cham, Switzerland, 2023; pp. 89–99. [Google Scholar]
- Kwon, T.-R.; Han, S.W.; Kim, J.H.; Lee, B.C.; Kim, J.M.; Hong, J.Y.; Kim, B.J. Polydeoxyribonucleotides Improve Diabetic Wound Healing in Mouse Animal Model for Experimental Validation. Ann. Dermatol. 2019, 31, 403–413. [Google Scholar] [CrossRef]
- Baek, A.; Kim, Y.; Lee, J.W.; Lee, S.C.; Cho, S.-R. Effect of Polydeoxyribonucleotide on Angiogenesis and Wound Healing in an In Vitro Model of Osteoarthritis. Cell Transplant. 2018, 27, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Dallari, D.; Sabbioni, G.; Carubbi, C.; Martini, L.; Fini, M. Polydeoxyribonucleotides (PDRNs) From Skin to Musculoskeletal Tissue Regeneration via Adenosine A2A Receptor Involvement. J. Cell. Physiol. 2017, 232, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2013, 41, 317–320. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Gooren, L.J.G.; Morales, A. Hormone treatment and preventative strategies in aging men: Whom to treat, when to treat and how to treat. In Textbook of Men’s Health and Aging; Informa Healthcare: London, UK, 2008. [Google Scholar]
- Alessandrini, A.; Bruni, F.; Piraccini, B.; Starace, M. Common causes of hair loss–clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 629–640. [Google Scholar] [CrossRef]
- Liu, D.; Xu, Q.; Meng, X.; Liu, X.; Liu, J. Status of research on the development and regeneration of hair follicles. Int. J. Med. Sci. 2024, 21, 80–94. [Google Scholar] [CrossRef]
- Lee, S.; Zheng, Z.; Kang, J.; Kim, D.; Oh, S.H.; Bin Cho, S. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015, 23, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bin Cho, S.; Zheng, Z.; Kang, J.-S.; Kim, H. Therapeutic Efficacy of 1,927-nm Fractionated Thulium Laser Energy and Polydeoxyribonucleotide on Pattern Hair Loss. Med. Lasers 2016, 5, 22–28. [Google Scholar] [CrossRef]
- Choi, Y.J.; Cho, S.; Kim, Y.K.; Kim, D.S. Improvement of Hair Graying during a Treatment of Male Pattern Hair Loss Using 1,927-nm Fractionated Thulium Laser Energy and Polydeoxyribonucleotide Injections. Med. Lasers 2017, 6, 37–40. [Google Scholar] [CrossRef]
- Ma, L.; Chen, M.; Fa, Z.; Pan, W.; Liao, W.; Gao, X.-H.; Huo, W.; Yang, Y.; Chen, H.-D.; Holahan, H.M.; et al. Skin diseases caused by factors from the environment. In Skin Diseases Caused by Factors from the Environment; Springer Science+Business Media: Dordrecht, The Netherlands, 2017; pp. 145–198. [Google Scholar]
- Agrawal, R.; Hu, A.; Bollag, W.B. The Skin and Inflamm-Aging. Biology 2023, 12, 1396. [Google Scholar] [CrossRef]
- Harvanová, G.; Duranková, S.; Bernasovská, J. The role of cytokines and chemokines in the inflammatory response. Alergol. Pol.-Pol. J. Allergol. 2023, 10, 210–219. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. Polydeoxyribonucleotide Regulation of Inflammation. Adv. Wound Care 2020, 9, 576–589. [Google Scholar] [CrossRef]
- Castellini, C.; Belletti, S.; Govoni, P.; Guizzardi, S. Anti Inflammatory Property of PDRN—An in Vitro Study on Cultured Macrophages. Adv. Biosci. Biotechnol. 2017, 08, 13–26. [Google Scholar] [CrossRef]
- Irrera, N.; Bitto, A.; Vaccaro, M.; Mannino, F.; Squadrito, V.; Pallio, G.; Arcoraci, V.; Minutoli, L.; Ieni, A.; Lentini, M.; et al. PDRN, a Bioactive Natural Compound, Ameliorates Imiquimod-Induced Psoriasis through NF-κB Pathway Inhibition and Wnt/β-Catenin Signaling Modulation. Int. J. Mol. Sci. 2020, 21, 1215. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, D.-S.; Hwang, G.Y.; Lee, J.-K.; Jung, J.-W.; Hwang, S.Y.; Baek, S.-W.; Yoon, S.L.; Ha, Y.; Kim, K.N.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio 2023, 19, 100611. [Google Scholar] [CrossRef]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Panon. Adriat. 2012, 21, 33–36. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pr. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Ji, Y.; Liu, F.; Jing, Y.; Jiao, C.; Li, Y.; Zhao, Y.; Wang, G.; Zhang, J. Proteomics Analysis of the Protective Effect of Polydeoxyribonucleotide Extracted from Sea Cucumber (Apostichopus japonicus) Sperm in a Hydrogen Peroxide-Induced RAW264.7 Cell Injury Model. Mar. Drugs 2024, 22, 325. [Google Scholar] [CrossRef]
- Chae, D.; Oh, S.-W.; Choi, Y.-S.; Kang, D.-J.; Park, C.-W.; Lee, J.; Seo, W.-S. First Report on Microbial-Derived Polydeoxyribonucleotide: A Sustainable and Enhanced Alternative to Salmon-Based Polydeoxyribonucleotide. Curr. Issues Mol. Biol. 2025, 47, 41. [Google Scholar] [CrossRef] [PubMed]
- Pittala, V.; Vanella, L.; Salerno, L.; Romeo, G.; Marrazzo, A.; Di Giacomo, C.; Sorrenti, V. Effects of Polyphenolic Derivatives on Heme Oxygenase-System in Metabolic Dysfunctions. Curr. Med. Chem. 2018, 25, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Lee, E.-B.; Choi, J.-H.; Jung, J.; Jeong, U.-Y.; Bae, U.-J.; Jang, H.-H.; Park, S.-Y.; Cha, Y.-S.; Lee, S.-H. Antioxidant and Immune Stimulating Effects of Allium cepa Skin in the RAW 264.7 Cells and in the C57BL/6 Mouse Immunosuppressed by Cyclophosphamide. Antioxidants 2023, 12, 892. [Google Scholar] [CrossRef]
- Chung, E.; Choi, H.; Lim, J.E.; Son, Y. Development of skin inflammation test model by co-culture of reconstituted 3D skin and RAW264.7 cells. Tissue Eng. Regen. Med. 2014, 11, 87–92. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, D.; Lee, M.; Lee, J.; Cho, S.; Kim, T.-J.; Kim, H.S. Micro-Current Stimulation Suppresses Inflammatory Responses in Peptidoglycan-Treated Raw 264.7 Macrophages and Propionibacterium acnes-Induced Skin Inflammation via TLR2/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 2508. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Chen, S.-J.; Yeh, J.-T.; Vadivalagan, C.; Chiu, J.-H.; Hseu, J.-H.; Hseu, Y.-C. The anti-melanogenesis, anti-photoaging, and anti-inflammation of coenzyme Q0, a major quinone derivative from Antrodia camphorata, through antioxidant Nrf2 signaling pathways in UVA/B-irradiated keratinocytes. J. Funct. Foods 2024, 116, 106206. [Google Scholar] [CrossRef]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Kim, J.K.; Chung, J.Y. Effectiveness of polydeoxyribonucleotide injection versus normal saline injection for treatment of chronic plantar fasciitis: A prospective randomised clinical trial. Int. Orthop. 2015, 39, 1329–1334. [Google Scholar] [CrossRef]
- Chai, A.C.; Siegwart, D.J.; Wang, R.C. Nucleic Acid Therapy for the Skin. J. Investig. Dermatol. 2024, 145, 780–789. [Google Scholar] [CrossRef]
- Araco, A.; Araco, F. Preliminary Prospective and Randomized Study of Highly Purified Polynucleotide vs Placebo in Treatment of Moderate to Severe Acne Scars. Aesthetic Surg. J. 2021, 41, NP866–NP874. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L.; Nguyen, V.B. Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef]
- Rubegni, P.; De Aloe, G.; Mazzatenta, C.; Cattarini, L.; Fimiani, M. Clinical Evaluation of the Trophic Effect of Polydeoxyribonucleotide (PDRN) in Patients Undergoing Skin Explants. A Pilot Study. Curr. Med. Res. Opin. 2001, 17, 128–131. [Google Scholar] [CrossRef]
- Lampridou, S.; Bassett, S.; Cavallini, M.; Christopoulos, G. The Effectiveness of Polynucleotides in Esthetic Medicine: A Systematic Review. J. Cosmet. Dermatol. 2024, 24, e16721. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, S.; Na, J.; Kim, J.H. Comparative Evaluation of Safety and Efficacy of a Novel Hyaluronic Acid-polynucleotide/Poly-L-lactic Acid Composite Dermal Filler. Aesthetic Plast. Surg. 2021, 45, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Drug Approval by MFDS, Drug Information, Announcement of Drug Master File (DMF) Registration: Search by ingredient-Sodium polydeoxyribonucleotide. Available online: https://nedrug.mfds.go.kr/ (accessed on 23 June 2025).
- EMEDI: Information Search-REJURAN. Available online: https://emedi.mfds.go.kr/search/data/MNU20237#item (accessed on 23 June 2025).
- EMEDI: Information Search-Polydeoxyribonucleotide. Available online: https://emedi.mfds.go.kr/search/data/MNU20237#item (accessed on 23 June 2025).
- Lim, T.S.; Liew, S.; Tee, X.J.; Chong, I.; Lo, F.J.; Ho, M.J.; Ong, K.; Cavallini, M. Polynucleotides HPT for Asian Skin Regeneration and Rejuvenation. Clin. Cosmet. Investig. Dermatol. 2024, 17, 417–431. [Google Scholar] [CrossRef]
- Malaysia Medical Device Register (MMDR)-Public Search: Rejuran. Available online: https://mdar.mda.gov.my/frontend/web/index.php?r=carian%2Findex&CompletedApplicationAllmdrSearch%5BglobalSearch%5D=Rejuran (accessed on 24 June 2025).
- Therapeutic Goods Administration (TGA)-REJURAN. Available online: https://www.tga.gov.au/resources/artg/412630 (accessed on 24 June 2025).
- Lee, D.; Kim, M.J.; Park, H.J.; Rah, G.C.; Choi, H.; Anh, S.; Ji, G.H.; Kim, M.S.; Kim, G.; Shin, D.W.; et al. Current practices and perceived effectiveness of polynucleotides for treatment of facial erythema by cosmetic physicians. Ski. Res. Technol. 2023, 29, e13466. [Google Scholar] [CrossRef]
- DeCollibus, D.P.; Searcy, J.; Tivesten, A.; Akhtar, N.; Lindenberg, C.; Abarrou, N.; Pradhan, S.; Fiandaca, M.; Franklin, J.; Govindan, G.; et al. Considerations for the Terminal Sterilization of Oligonucleotide Drug Products. Nucleic Acid Ther. 2023, 33, 159–177. [Google Scholar] [CrossRef]
- Yi, K.-H.; Park, M.-S.; Ree, Y.-S.; Kim, H.M. A Review on “Skin Boosters”: Hyaluronic Acid, Poly-L-lactic Acid and Pol-D-lactic Acid, Polydeoxyribonucleotide, Polynucleotides, Growth Factor, and Exosome. Korean Assoc. Laser Dernatology Trichology 2023, 4, 1–5. [Google Scholar] [CrossRef]
- Rho, N.-K.; Kim, H.-S.; Kim, S.-Y.; Lee, W. Injectable “Skin Boosters” in Aging Skin Rejuvenation: A Current Overview. Arch. Plast. Surg. 2024, 51, 528–541. [Google Scholar] [CrossRef]
- Gupta, N. DNA extraction and polymerase chain reaction. J. Cytol. 2019, 36, 116–117. [Google Scholar] [CrossRef]
- Cheng, L.-Y.; Sun, X.-M.; Tang, M.-Y.; Jin, R.; Cui, W.-G.; Zhang, Y.-G. An update review on recent skin fillers. Plast. Aesthetic Res. 2016, 3, 92–99. [Google Scholar] [CrossRef]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Palmieri, I.P.; Papagni, M.; Priori, M.; Trocchi, G.; members of The Polynucleotides HPT™ Priming Board, Collegio Italiano delle Società Scientifiche di Medicina Estetica (Italian College of the Aesthetic Medicine Scientific Societies)—SIME, AGORÀ, SIES. Consensus report on the use of PN-HPT™ (polynucleotides highly purified technology) in aesthetic medicine. J. Cosmet. Dermatol. 2020, 20, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, J.W.; Byun, J.H.; Lee, W.M.; Kim, M.H.; Wu, W.H. Comparison of wound healing effects between Oncorhynchus keta-derived polydeoxyribonucleotide (PDRN) and Oncorhynchus mykiss-derived PDRN. Arch. Craniofacial Surg. 2018, 19, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, I.P.; Moro, L.; Fraone, N.; De Luca, C.; Prussia, C. An Innovative PN HPT™-based Medical Device for the Therapy of Deteriorated Periocular Skin Quality. Surg. Res. 2023, 5, 1–5. [Google Scholar] [CrossRef]
- Nabila, I. Literature Review Mikroinjeksi Deoxyribonucleic Acid (DNA) Salmon Sebagai Agen Peremajaan Kulit Wajah. Ph.D. Thesis, Universitas Muhammadiyah Surabaya, Surabaya, Republic of Indonesia, 2024. [Google Scholar]
- Araco, A.; Araco, F.; Raichi, M. Clinical efficacy and safety of polynucleotides highly purified technology (PN-HPT®) and cross-linked hyaluronic acid for moderate to severe nasolabial folds: A prospective, randomized, exploratory study. J. Cosmet. Dermatol. 2022, 22, 146–155. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, J.J.; Lee, Y.I.; Lee, W.J.; Lee, C.; Chung, W.Y.; Nam, K.; Lee, J.H. Preventive effect of polynucleotide on post-thyroidectomy scars: A randomized, double-blinded, controlled trial. Lasers Surg. Med. 2018, 50, 755–762. [Google Scholar] [CrossRef]
- Yogya, Y.; Wanitphakdeedecha, R.; Wongdama, S.; Nanchaipruek, Y.; Yan, C.; Rakchart, S. Efficacy and Safety of Using Noninsulated Microneedle Radiofrequency Alone versus in Combination with Polynucleotides for Treatment of Periorbital Wrinkles. Dermatol. Ther. 2022, 12, 1133–1145. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, Y.H.; Kim, H.; Park, K.Y. Therapeutic Performance of Needle Injection Versus Needle-Free Jet Injector System for Polynucleotide Filler in Skin Rejuvenation. J. Cosmet. Dermatol. 2024, 24, e16595. [Google Scholar] [CrossRef]
- Choi, S.Y.; Koh, Y.G.; Yoo, K.H.; Han, H.S.; Seok, J.; Kim, B.J. A Randomized, Participant- and Evaluator-Blinded, Matched-Pair, Prospective Study Comparing the Safety and Efficacy Between Polycaprolactone and Polynucleotide Fillers in the Correction of Crow’s Feet. J. Cosmet. Dermatol. 2024, 24, e16576. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, R.K.; In, Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis. Medicine 2019, 98, e17386. [Google Scholar] [CrossRef]
- Jo, S.; Baek, A.; Cho, Y.; Kim, S.H.; Baek, D.; Hwang, J.; Cho, S.-R.; Kim, H.J. Therapeutic effects of polydeoxyribonucleotide in an in vitro neuronal model of ischemia/reperfusion injury. Sci. Rep. 2023, 13, 1–10. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Dictionary by the Korean Cosmetic Association. Available online: https://kcia.or.kr/cid/search/ingd_view.php?no=1048 (accessed on 23 July 2025).
- Kim, B.R.; Kwon, S.H.; Kim, J.W.; Jeong, W.-J.; Cha, W.; Jung, Y.H.; Na, J.-I.; Huh, C.-H.; Shin, J.-W. Early Postoperative Injections of Polydeoxyribonucleotide Prevent Hypertrophic Scarring After Thyroidectomy: A Randomized Controlled Trial. Adv. Wound Care 2023, 12, 361–370. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, E.S.; Kim, S.W.; Hong, S.P.; Kim, J. Effects of Polynucleotide Dermal Filler in the Correction of Crow’s Feet Using an Antera Three-Dimensional Camera. Aesthetic Plast. Surg. 2022, 46, 1902–1909. [Google Scholar] [CrossRef]
- Korea Pharmaceutical Information Center (KPIC): Drug Information Search—PDRN. Available online: https://www.health.kr/ (accessed on 19 June 2025).
- Kim, D.; Kim, W.-J.; Lee, H.-K.; Kwon, Y.-S.; Choi, Y.-M. Efficacy in vitro Antioxidation and in vivo Skin Barrier Recovery of Composition Containing Mineral-cation-phyto DNA Extracted from Aloe vera Adventitious Root. Asian J. Beauty Cosmetol. 2023, 21, 231–246. [Google Scholar] [CrossRef]
- Song, M.H.; Choi, M.H.; Jeong, J.H.; Lee, S.S.; Jeong, W.Y. Efficiency of PDNR (Polydeoxyribonucleotide) Extraction from Various Plant Species and Its in Vitro Wound Healing Activity. J. Korea Inst. Inf. Electron. Commun. Technol. 2022, 15, 387–395. [Google Scholar]
- Yang, C.Y.; Han, J.S.; Lee, W.S.; Bae, J.S.; Lee, C.W.; Jeong, E.H.; Kim, G.H.; Park, K.H. The effect of wound healing of Nile tilapia (Oreochromis niloticus) using PDRN (polydeoxyribonucleotide) extracted from seaweed (Porphyra sp.). J. Fish Pathol. 2021, 34, 233–241. [Google Scholar]
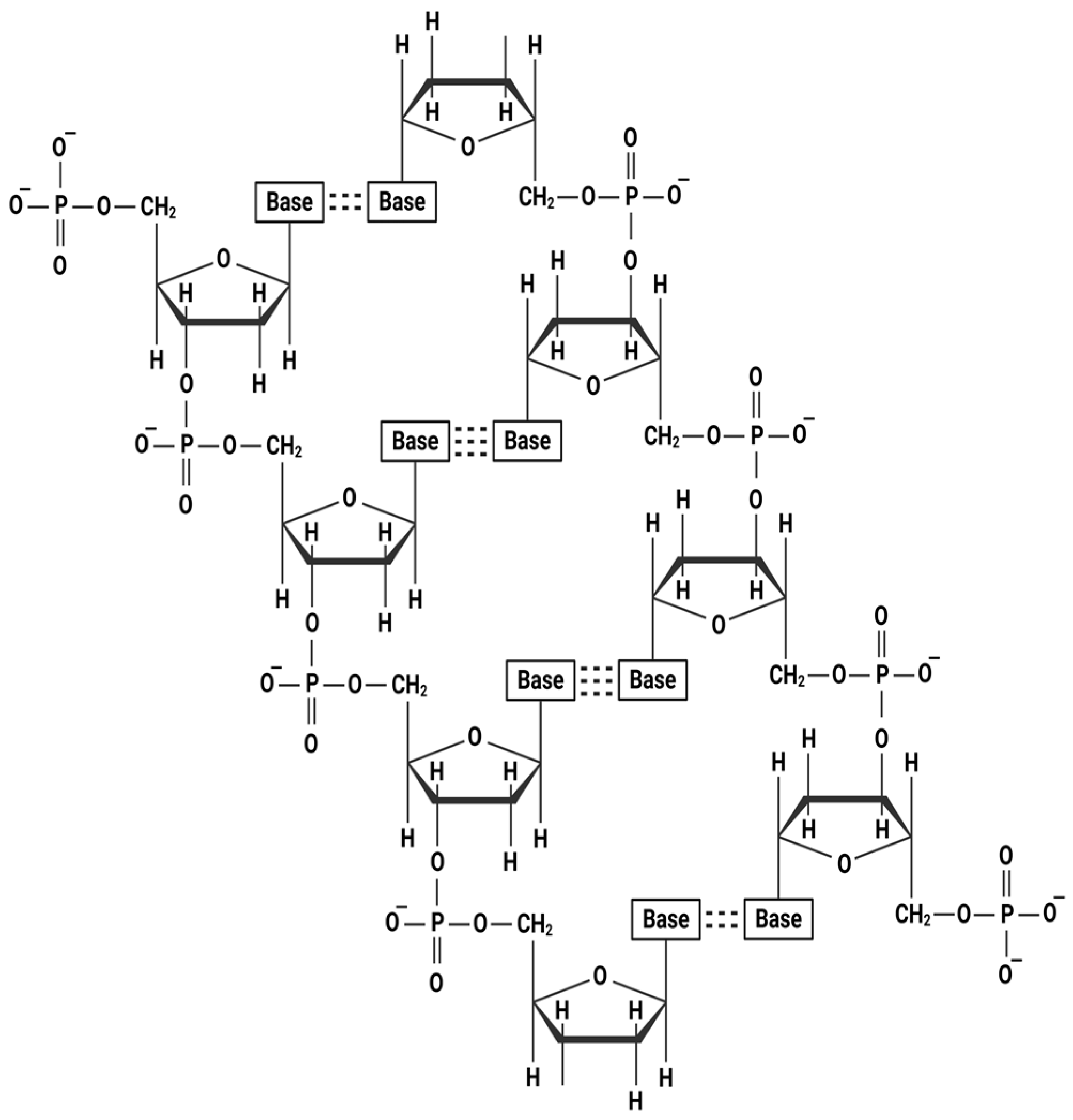
| Feature | PN | PDRN |
|---|---|---|
| Structural Characteristics | Longer chain of DNA fragments; relatively higher molecular weight, clinical use of relatively lower-gauge needle (e.g., generally, 27 to 30-gauge) 1 | Shorter chain of DNA fragments; relatively lower molecular weight, clinical use of relatively higher-gauge needle 2 |
| Water Retention/Viscoelasticity | High water content and viscoelasticity, hydrogel formation | Lower viscoelasticity, more fluidic property |
| Main Mechanism of Action | Hydration, structural support | A2A adenosine receptor activation, nucleotide salvage pathway |
| Clinical Applications | Skin boosters, intravenous-articular injection, etc. | Wound healing, skin regeneration, anti-inflammatory, hypopigmentation, hair regrowth, etc. |
| Regulatory Classification (in South Korea) | Medical device (e.g., Rejuran®) | Pharmaceutical agent (e.g., Placentex®, Newdien Injection, PDRN Injection) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.T. Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives. Pharmaceutics 2025, 17, 1024. https://doi.org/10.3390/pharmaceutics17081024
Kim ST. Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives. Pharmaceutics. 2025; 17(8):1024. https://doi.org/10.3390/pharmaceutics17081024
Chicago/Turabian StyleKim, Sung Tae. 2025. "Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives" Pharmaceutics 17, no. 8: 1024. https://doi.org/10.3390/pharmaceutics17081024
APA StyleKim, S. T. (2025). Comparison of Polynucleotide and Polydeoxyribonucleotide in Dermatology: Molecular Mechanisms and Clinical Perspectives. Pharmaceutics, 17(8), 1024. https://doi.org/10.3390/pharmaceutics17081024






