Comprehensive Analytical Studies on the Solubility and Dissolution Rate Enhancement of Tadalafil with Type IV Lipid Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Studies
2.3. Solubility Studies
2.4. Preformulation Studies
2.5. Characterization and Evaluation of Type IV Formulations
2.5.1. Droplet Size and Polydispersity Index (PDI) Analysis
2.5.2. Transmittance Determination (T, %)
2.5.3. Robustness to Dilution
2.6. Preparation of TDL-Loaded Type IV Formulations
2.7. Thermodynamic Stability Studies
2.8. Preparation of Solid TDL-Loaded Type IV Lipid Formulations
2.9. Dissolution Studies
2.10. In Vitro Lipolysis Test
2.11. Cell Culture and Cytotoxicity Assay
3. Results
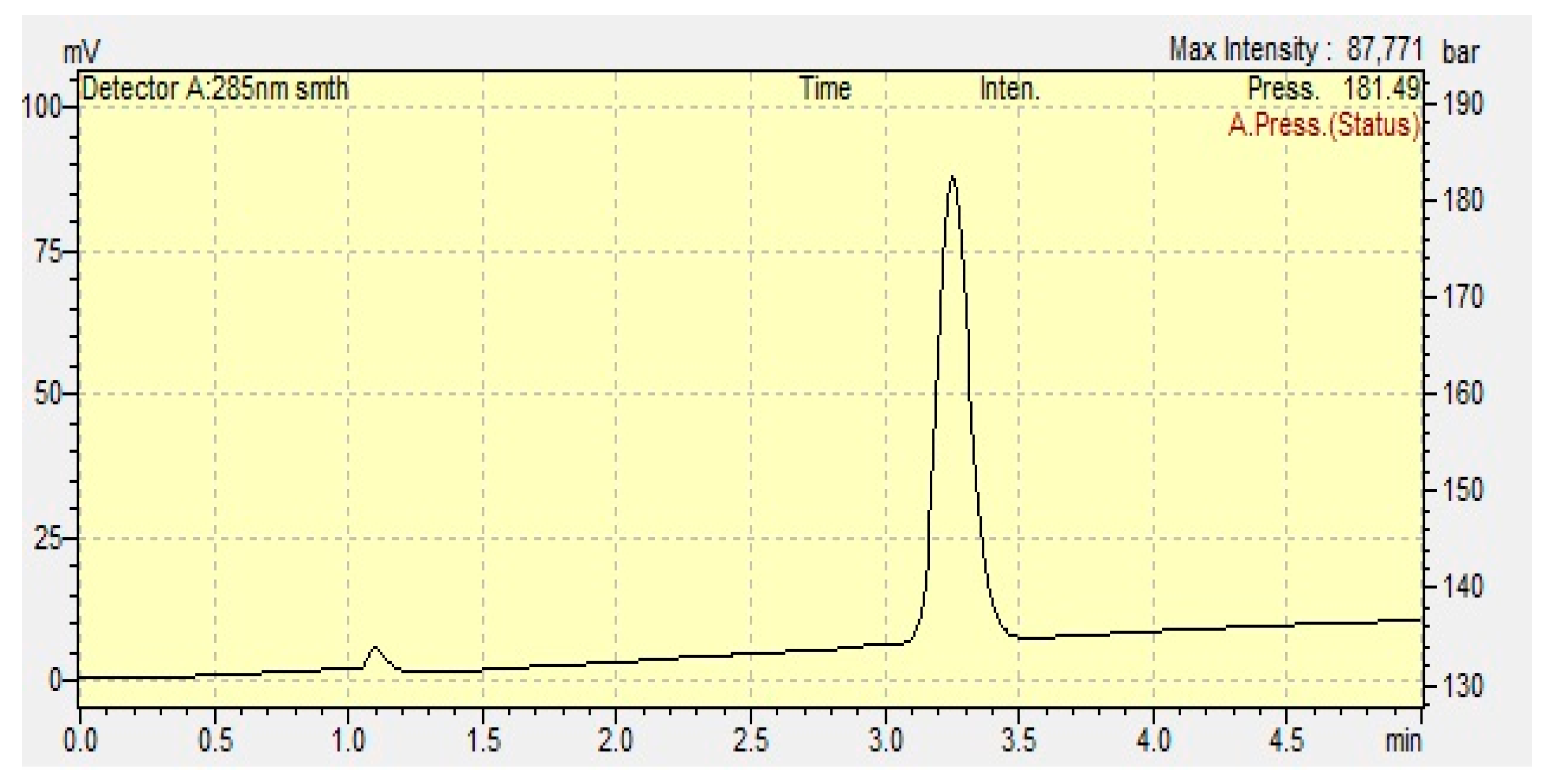
3.1. Analytical Studies
3.2. Solubility Studies
3.3. Characterization and Evaluation of TDL-Unloaded Type IV Formulations
3.3.1. Droplet Size and PDI Analysis
3.3.2. Transmittance Determination (T, %)
3.3.3. Robustness to Dilution
3.3.4. Preparation of TDL-Loaded Type IV Formulations
3.3.5. Thermodynamic Stability Studies
3.4. Solidification of the Selected LBFs
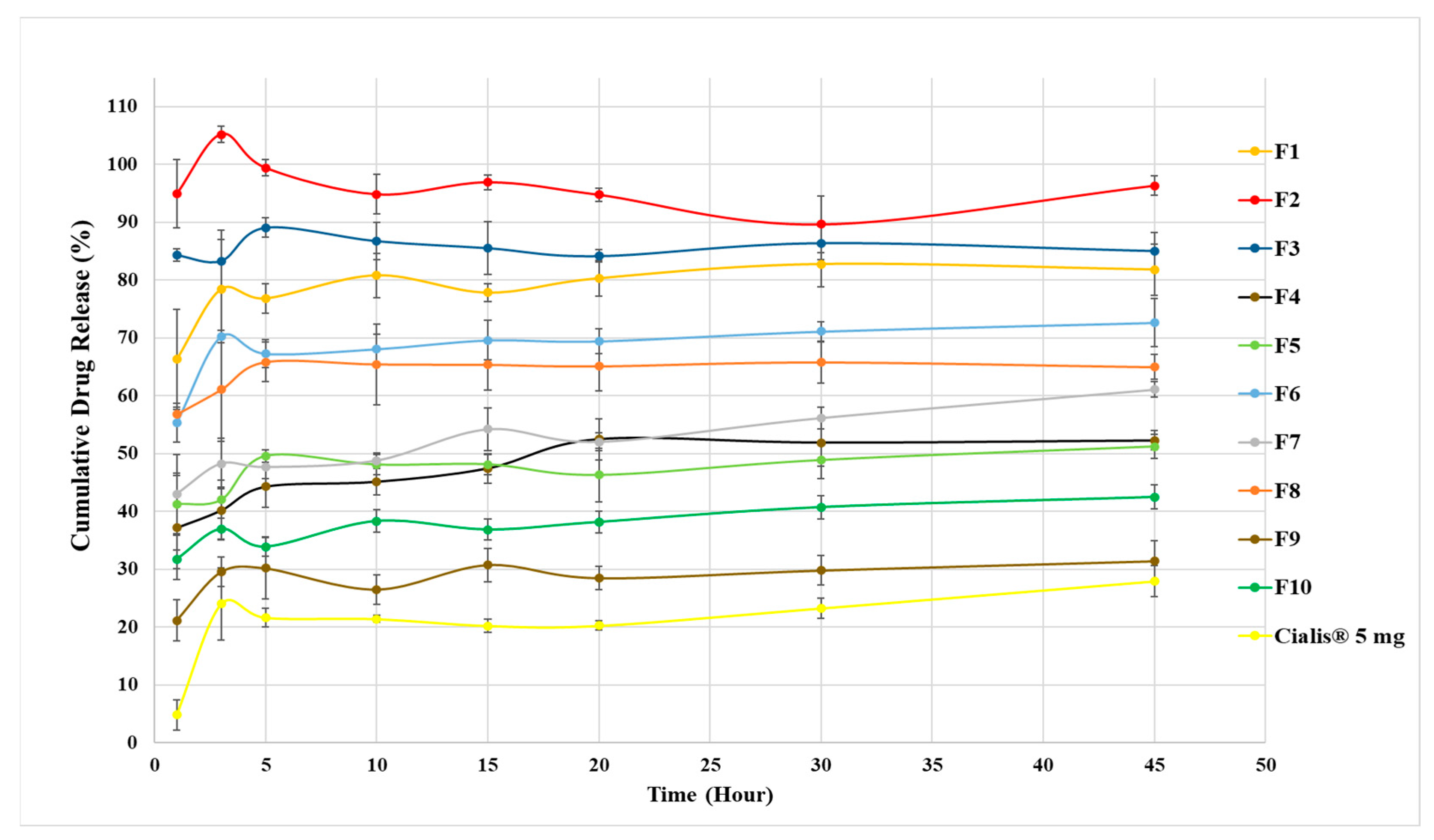
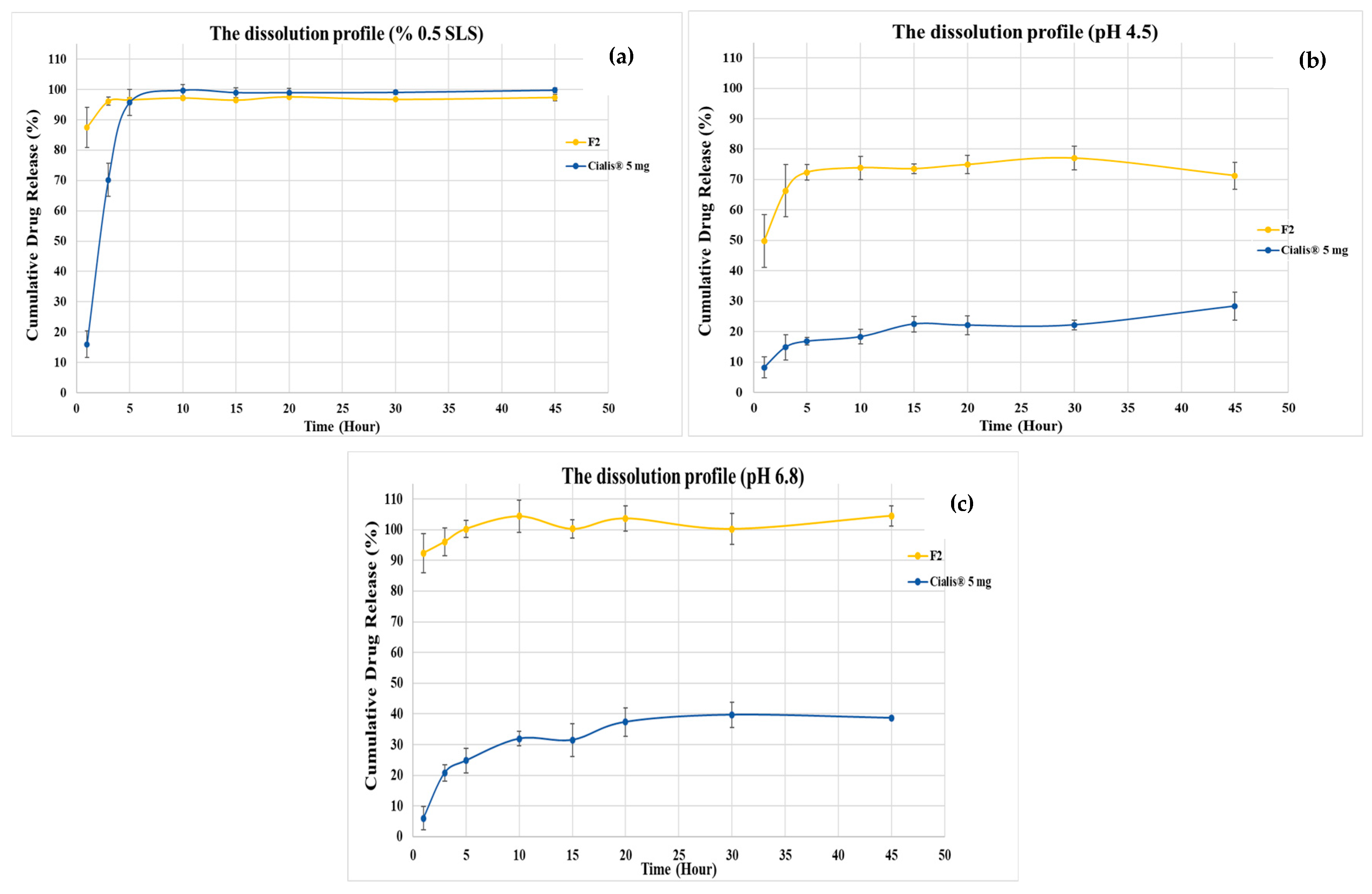
3.5. Dissolution Studies
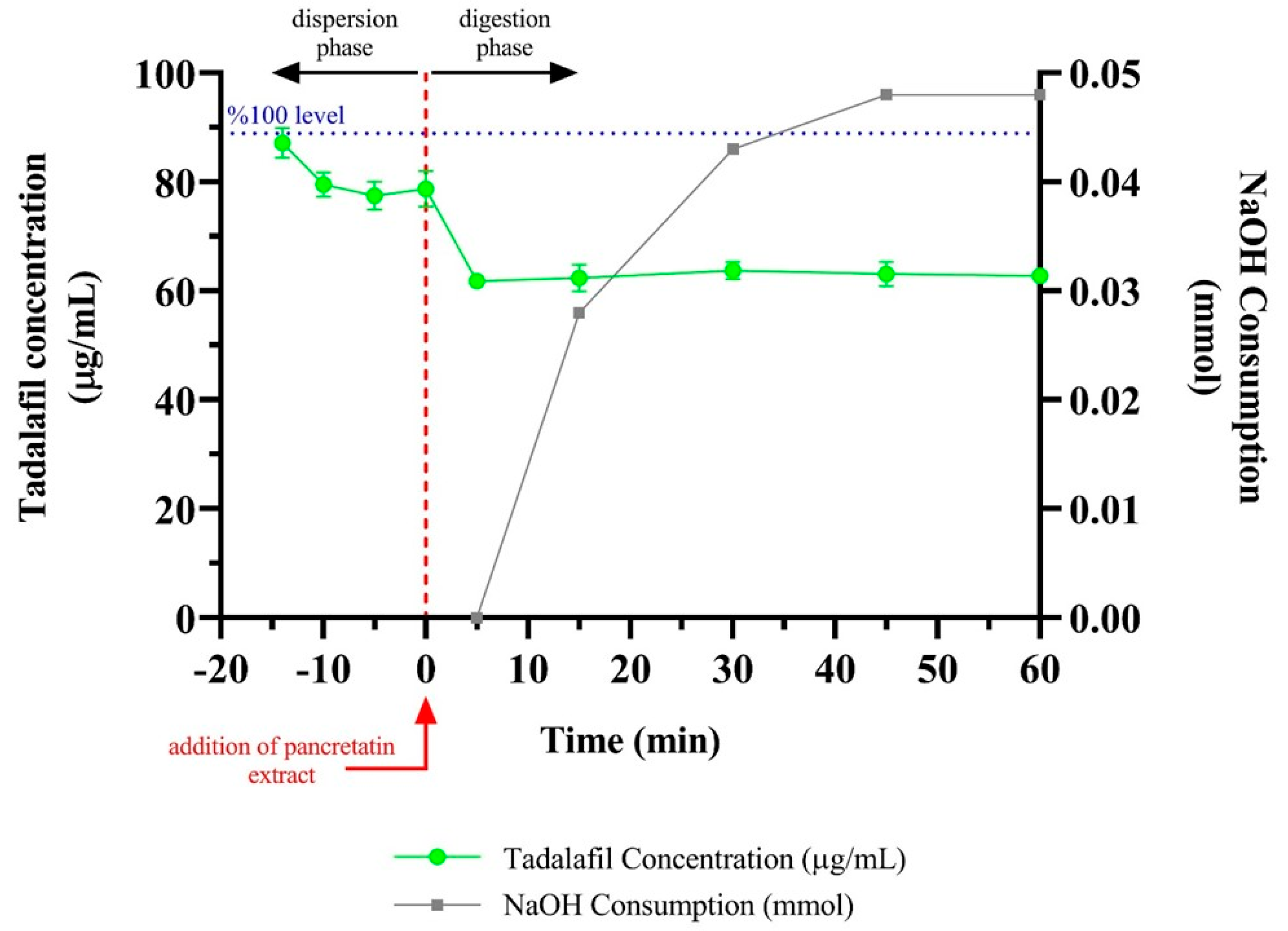
3.6. In Vitro Lipolysis Test Result
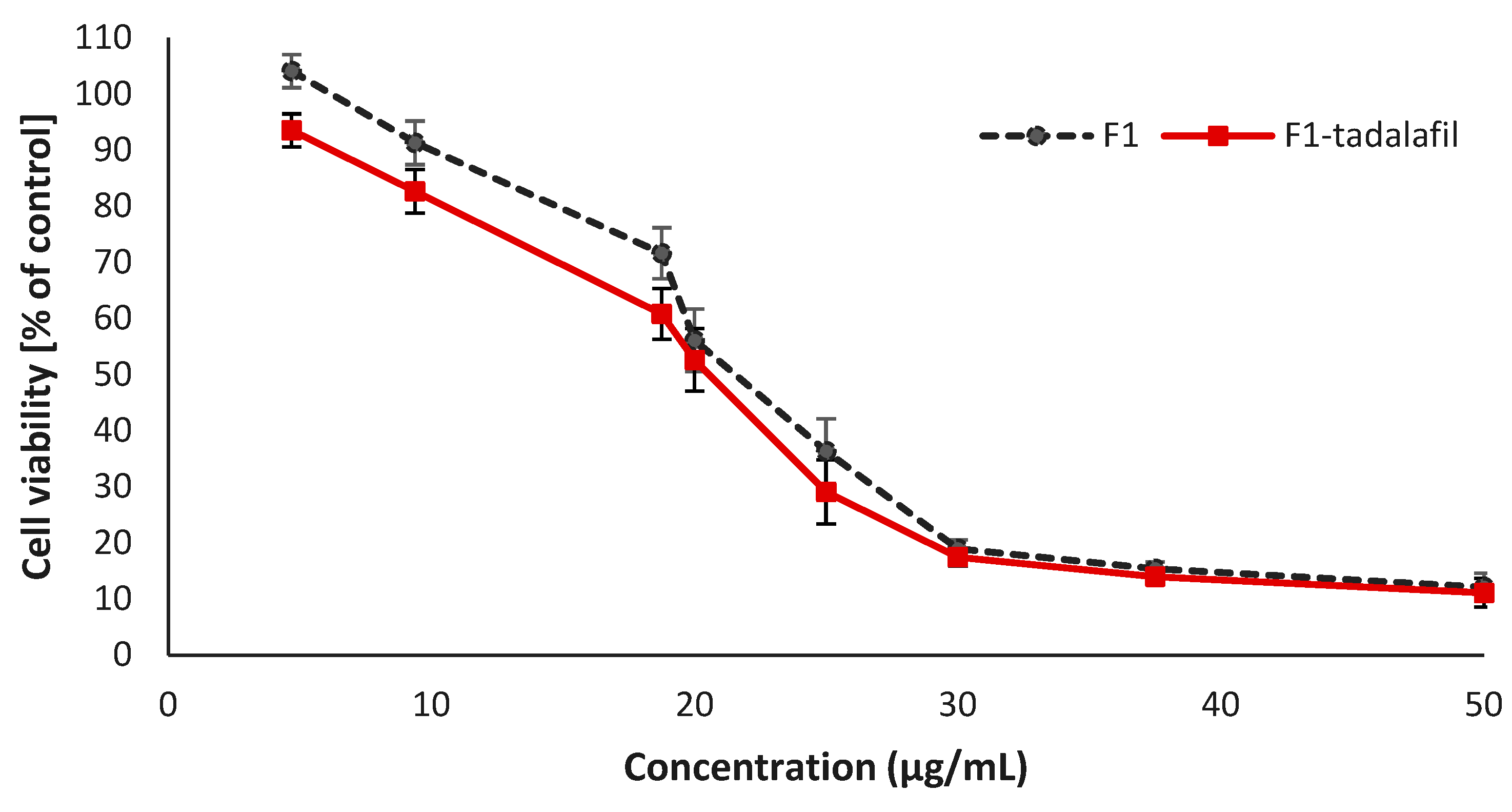
3.7. Cell Culture and Cytotoxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coward, R.M.; Carson, C.C. Tadalafil in the Treatment of Erectile Dysfunction. Ther. Clin. Risk Manag. 2008, 4, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Zanaty, F.; Badawy, A.; Kotb, H.; Elsarfy, F.; Salman, B. Efficacy and Safety of Low-Intensity Extracorporeal Shock Wave Therapy versus on-Demand Tadalafil for Erectile Dysfunction. Arab J. Urol. 2022, 20, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Won, J.E.J.; Sorsaburu, S.; Rivera, P.D.; Lee, S.W. Urinary Tract Symptoms (LUTS) Secondary to Benign Prostatic Hyperplasia (BPH) and LUTS/BPH with Erectile Dysfunction in Asian Men: A Systematic Review Focusing on Tadalafil. World J. Men’s Health 2013, 31, 193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, W.; Kang, H.; Lee, B.B.J.; Kim, T.S.; Hwang, S.J. Effect of Operating Parameters on PVP/Tadalafil Solid Dispersions Prepared Using Supercritical Anti-Solvent Process. J. Supercrit. Fluids 2014, 90, 126–133. [Google Scholar] [CrossRef]
- Strindberg, S.; Plum, J.; Stie, M.B.; Christiansen, M.L.; Hagner Nielsen, L.; Rades, T.; Müllertz, A. Effect of Supersaturation on Absorption of Indomethacin and Tadalafil in a Single Pass Intestinal Perfusion Rat Model, in the Absence and Presence of a Precipitation Inhibitor. Eur. J. Pharm. Biopharm. 2020, 151, 108–115. [Google Scholar] [CrossRef]
- Mobarak, D.; Salah, S.; Ghorab, M. Improvement of Dissolution of a Class II Poorly Water-Soluble Drug, by Developing a Five-Component Self-Nanoemulsifying Drug Delivery System. J. Drug Deliv. Sci. Technol. 2019, 50, 99–106. [Google Scholar] [CrossRef]
- Rao, Q.; Qiu, Z.; Huang, D.; Lu, T.; Zhang, Z.J.; Luo, D.; Pan, P.; Zhang, L.; Liu, Y.; Guan, S.; et al. Enhancement of the apparent solubility and bioavailability of Tadalafil nanoparticles via antisolvent precipitation. Eur. J. Pharm. Sci. 2019, 128, 222–231. [Google Scholar] [CrossRef]
- Ali, H.S.M.; Ahmed, S.A.; Alqurshi, A.A.; Alalawi, A.M.; Shehata, A.M.; Alahmadi, Y.M. Boosting Tadalafil Bioavailability via Sono-Assisted Nano-Emulsion-Based Oral Jellies: Box–Behnken Optimization and Assessment. Pharmaceutics 2022, 14, 2592. [Google Scholar] [CrossRef]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 Years of Oral Lipid-Based Formulations: Provenance, Progress and Future Perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef]
- Choi, J.S.; Kwon, S.H.; Lee, S.E.; Jang, W.S.; Byeon, J.C.; Jeong, H.M.; Park, J.S. Use of Acidifier and Solubilizer in Tadalafil Solid Dispersion to Enhance the in Vitro Dissolution and Oral Bioavailability in Rats. Int. J. Pharm. 2017, 526, 77–87. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Mullertz, A. In Vitro Lipid Digestion Models in Design of Drug Delivery Systems for Enhancing Oral Bioavailability. Expert Opin. Drug Metab. Toxicol. 2008, 4, 65–76. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of Poorly Water-Soluble Drugs for Oral Administration: Physicochemical and Physiological Issues and the Lipid Formulation Classification System. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Kilic, M.; Avzoti, A.; Dressman, J.; Reppas, C. Lipid-Based Oral Formulations. In In Vitro Drug Release Testing of Special Dosage Forms; Wiley: Hoboken, NJ, USA, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Jannin, V.; Chevrier, S.; Michenaud, M.; Dumont, C.; Belotti, S.; Chavant, Y.; Demarne, F. Development of Self Emulsifying Lipid Formulations of BCS Class II Drugs with Low to Medium Lipophilicity. Int. J. Pharm. 2015, 495, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Narayana, L.; Chella, N.; Kumar, D.; Shastri, N.R. Design of a Novel Type IV Lipid-Based Delivery System for Improved Delivery of Drugs with Low Partition Coefficient. J. Liposome Res. 2015, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Jannin, V.; Pouton, C.W.; Boyd, B.J. Colloidal Aspects of Dispersion and Digestion of Self-Dispersing Lipid-Based Formulations for Poorly Water-Soluble Drugs. Adv. Drug Deliv. Rev. 2019, 142, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Holm, R. Bridging the Gaps between Academic Research and Industrial Product Developments of Lipid-Based Formulations. Adv. Drug Deliv. Rev. 2019, 142, 118–127. [Google Scholar] [CrossRef]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-Based Oral Formulation Strategies for Lipophilic Drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of Lipid-Based Delivery Systems for Oral Administration: Materials, Methods and Strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Charman, W.N. Intestinal Lymphatic Drug Transport: An Update. Adv. Drug Deliv. Rev. 2001, 50, 61–80. [Google Scholar] [CrossRef]
- Almeida, S.R.D.; Tippavajhala, V.K. A Rundown Through Various Methods Used in the Formulation of Solid Self-Emulsifying Drug Delivery Systems (S-SEDDS). AAPS PharmSciTech 2019, 20, 323. [Google Scholar] [CrossRef]
- Chauhan, G.; Shaik, A.A.; Kulkarni, N.S.; Gupta, V. The Preparation of Lipid-Based Drug Delivery System Using Melt Extrusion. Drug Discov. Today 2020, 25, 1930–1943. [Google Scholar] [CrossRef] [PubMed]
- Arabia, S. Dissolution Improvement of Solid Self-Emulsifying Drug Delivery Systems of Fenofi Brate Using an Inorganic High Surface Adsorption Material. Acta Pharm. 2015, 65, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Rao, S.; Prestidge, C.A. Transforming Lipid-Based Oral Drug Delivery Systems into Solid Dosage Forms: An Overview of Solid Carriers, Physicochemical Properties, and Biopharmaceutical Performance. Pharm. Res. 2013, 30, 2993–3017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Garg, V.; Singh, S.; Pandey, N.K.; Bhatia, A.; Prakash, T.; Gulati, M.; Singh, S.K. Impact of Spray Drying over Conventional Surface Adsorption Technique for Improvement in Micromeritic and Biopharmaceutical Characteristics of Self-Nanoemulsifying Powder Loaded with Two Lipophilic as Well as Gastrointestinal Labile Drugs. Powder Technol. 2018, 326, 425–442. [Google Scholar] [CrossRef]
- Joyce, P.; Dening, T.J.; Meola, T.R.; Schultz, H.B.; Holm, R.; Thomas, N.; Prestidge, C.A. Solidification to Improve the Biopharmaceutical Performance of SEDDS: Opportunities and Challenges. Adv. Drug Deliv. Rev. 2019, 142, 102–117. [Google Scholar] [CrossRef]
- Gonçalves, A.; Nikmaram, N.; Roohinejad, S.; Estevinho, B.N.; Rocha, F.; Greiner, R.; McClements, D.J. Production, Properties, and Applications of Solid Self-Emulsifying Delivery Systems (S-SEDS) in the Food and Pharmaceutical Industries. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 108–126. [Google Scholar] [CrossRef]
- Williams, H.D.; Van Speybroeck, M.; Augustijns, P.; Porter, C.J.H. Lipid-Based Formulations Solidified via Adsorption onto the Mesoporous Carrier Neusilin® US2: Effect of Drug Type and Formulation Composition on in Vitro Pharmaceutical Performance. J. Pharm. Sci. 2014, 103, 1734–1746. [Google Scholar] [CrossRef]
- Turkish Pharmacopoeia. Tadalafil; Republic of Turkey Ministery of Health Medicines and Medical Devices Agency: Ankara, Turkey, 2017; Volume 2. [Google Scholar]
- Dening, T.J.; Rao, S.; Thomas, N.; Prestidge, C.A. Novel Nanostructured Solid Materials for Modulating Oral Drug Delivery from Solid-State Lipid-Based Drug Delivery Systems. AAPS J. 2016, 18, 23–40. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Campos, V.E.B.; Senna, J.P.; Coutinho, C.d.S.C.; Tebaldi, B.S.; Silva, K.G.d.H.E.; Mansur, C.R.E. Development and Characterization of Promising o/w Nanoemulsions Containing Sweet Fennel Essential Oil and Non-Ionic Sufactants. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 214–221. [Google Scholar] [CrossRef]
- Vu, G.T.T.; Phan, N.T.; Nguyen, H.T.; Nguyen, H.C.; Hai Tran, Y.T.; Pham, T.B.; Nguyen, L.T.; Nguyen, H.D. Application of the Artificial Neural Network to Optimize the Formulation of Self-Nanoemulsifying Drug Delivery System Containing Rosuvastatin. J. Appl. Pharm. Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaurasiya, A.; Awasthi, A.; Mishra, G.; Asati, D.; Khar, R.K.; Mukherjee, R. Oral Bioavailability Enhancement of Exemestane from Self-Microemulsifying Drug Delivery System (SMEDDS). AAPS PharmSciTech 2009, 10, 906–916. [Google Scholar] [CrossRef]
- Verma, R.; Kaushik, D. Design and Optimization of Candesartan Loaded Self-Nanoemulsifying Drug Delivery System for Improving Its Dissolution Rate and Pharmacodynamic Potential. Drug Deliv. 2020, 27, 756–771. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-Nanoemulsifying Drug Delivery System (SNEDDS) of the Poorly Water-Soluble Grapefruit Flavonoid Naringenin: Design, Characterization, in Vitro and in Vivo Evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-Nanoemulsifying Drug Delivery System (Snedds) for Improved Oral Bioavailability of Chlorpromazine: In Vitro and in Vivo Evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef] [PubMed]
- Alghananim, A.; Özalp, Y.; Mesut, B.; Serakinci, N.; Özsoy, Y.; Güngör, S. A Solid Ultra Fine Self-Nanoemulsifying Drug Delivery System (S-Snedds) of Deferasirox for Improved Solubility: Optimization, Characterization, and in Vitro Cytotoxicity Studies. Pharmaceuticals 2020, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Nikolakakis, I.; Partheniadis, I. Self-Emulsifying Granules and Pellets: Composition and Formation Mechanisms for Instant or Controlled Release. Pharmaceutics 2017, 9, 50. [Google Scholar] [CrossRef]
- Niu, J.; Xu, Z.; Li, X.; Wang, Z.; Li, J.; Yang, Z.; Khattak, S.U.; Liu, Y.; Shi, Y. Development and Evaluation of Rhubarb Free Anthraquinones Loaded Self-Nanoemulsifying Tablets. J. Drug Deliv. Sci. Technol. 2020, 57, 101737. [Google Scholar] [CrossRef]
- Ghosh, D.; Singh, S.K.; Khursheed, R.; Pandey, N.K.; Kumar, B.; Kumar, R.; Kumari, Y.; Kaur, G.; Clarisse, A.; Awasthi, A.; et al. Impact of Solidification on Micromeritic Properties and Dissolution Rate of Self-Nanoemulsifying Delivery System Loaded with Docosahexaenoic Acid. Drug Dev. Ind. Pharm. 2020, 46, 597–605. [Google Scholar] [CrossRef]
- FDA. Dissolution Methods. FDA-Drug Database. Available online: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_SearchResults.cfm (accessed on 25 January 2023).
- Mu, H.; Holm, R.; Mullertz, A. Lipid-Based Formulations for Oral Administration of Poorly Water-Soluble Drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Ee, S.L.; Duan, X.; Liew, J.; Nguyen, Q.D. Droplet Size and Stability of Nano-Emulsions Produced by the Temperature Phase Inversion Method. Chem. Eng. J. 2008, 140, 626–631. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Huang, W.C.; Xue, C.; Mao, X. Formulation of Vitamin C Encapsulation in Marine Phospholipids Nanoliposomes: Characterization and Stability Evaluation during Long Term Storage. LWT 2020, 127, 109439. [Google Scholar] [CrossRef]
- Thakur, G.S.; Misra, C.; Thotakura, N.; Al Saqr, A.; Almawash, S.; Preet, S.; Raza, K. Chitosan-Based Nanoconjugate for Safe and Effective Delivery of Docetaxel to Cancer Cells: An Explorative Study. J. Drug Deliv. Sci. Technol. 2021, 64, 102653. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(Ethylene Oxide)-Poly(Propylene Oxide) Block Copolymer Micelles as Drug Delivery Agents: Improved Hydrosolubility, Stability and Bioavailability of Drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, F.; Zhang, H.; Wang, Y.; Cheng, C.; Li, X.; Gu, C.-H.; Yang, Q.; Wu, H.; Zhang, S. Co-Delivery of PDTC and Doxorubicin by Multifunctional Micellar Nanoparticles to Achieve Active Targeted Drug Delivery and Overcome Multidrug Resistance. Biomaterials 2010, 31, 5634–5642. [Google Scholar] [CrossRef]
- Sakai, T.; Ikoshi, R.; Toshida, N.; Kagaya, M. Thermodynamically Stable Vesicle Formation and Vesicle-to-Micelle Transition of Single-Tailed Anionic Surfactant in Water. J. Phys. Chem. B 2013, 117, 5081–5089. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, Y.T.; Youn, Y.S.; Nam, M.; Park, B.; Yun, J.; Kim, J.H.; Song, H.T.; Oh, K.T. Binary Mixing of Micelles Using Pluronics for a Nano-Sized Drug Delivery System. Colloids Surfaces B Biointerfaces 2011, 82, 190–195. [Google Scholar] [CrossRef]
- Mandić, J.; Zvonar Pobirk, A.; Vrečer, F.; Gašperlin, M. Overview of Solidification Techniques for Self-Emulsifying Drug Delivery Systems from Industrial Perspective. Int. J. Pharm. 2017, 533, 335–345. [Google Scholar] [CrossRef]
- Wang, D.; Xing, H.; Jiang, J.; Chen, X.; Yang, T.; Wang, D.; Ding, P. Liquisolid Technique and Its Applications in Pharmaceutics. Asian J. Pharm. Sci. Shenyang Pharm. Univ. 2017, 12, 115–123. [Google Scholar] [CrossRef]
- Tomita Pharmaceutical. Florite; Tomita Pharmaceutical: Naruto, Japan, 2018; Available online: https://www.pharmaexcipients.com/product/florite-r/?attachment_id=298274&download_file=yslbz3t51diar (accessed on 25 January 2023).
- Fuji Chemical Industries. The Specialty Excipient; Fuji Chemical Industries: Nakaniikawa, Japan, 2015; Available online: http://www.fujichemical.co.jp/english/medical/medicine/neusilin/neusilin_brochure.pdf (accessed on 25 January 2023).
- Williams, H.D.; Sassene, P.; Kleberg, K.; Bakala-N’Goma, J.C.; Calderone, M.; Jannin, V.; Igonin, A.; Partheil, A.; Marchaud, D.; Jule, E.; et al. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: Method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J. Pharm. Sci. 2012, 101, 3360–3380. [Google Scholar] [CrossRef]
- Arnold, Y.E.; Imanidis, G.; Kuentz, M. In vitro digestion kinetics of excipients for lipid-based drug delivery and introduction of a relative lipolysis half life. Drug Dev. Ind. Pharm. 2012, 38, 1262–1269. [Google Scholar] [CrossRef]
- FDA. Cialis Label Data. 2007. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/021368s012lbl.pdf (accessed on 25 January 2023).

| Codes * | Surfactant/Co-Surfactant | Ratios (w/w) |
|---|---|---|
| Khs15T11 * | Kolliphor HS 15®/Transcutol HP® | 1:1 |
| Khs15T12 * | Kolliphor HS 15®/Transcutol HP® | 1:2 |
| Khs15T21 * | Kolliphor HS 15®/Transcutol HP® | 2:1 |
| KPS80T11 * | Kolliphor PS 80®/Transcutol HP® | 1:1 |
| KPS80T12 * | Kolliphor PS 80®/Transcutol HP® | 1:2 |
| KPS80T21 * | Kolliphor PS 80®/Transcutol HP® | 2:1 |
| KELT11 * | Kolliphor EL®/Transcutol HP® | 1:1 |
| KELT12 * | Kolliphor EL®/Transcutol HP® | 1:2 |
| KELT21 * | Kolliphor EL®/Transcutol HP® | 2:1 |
| Formulations (Smix) and Codes | Droplet Size | PDI ** |
|---|---|---|
| Kolliphor HS 15/Transcutol (1:1)—Khs15T11 | 37.74 ± 36.26 | 0.14 ± 0.04 |
| Kolliphor HS 15/Transcutol (1:2)—Khs15T12 | 91.24 ± 32.01 | 0.15 ± 0.04 |
| Kolliphor HS 15/Transcutol (2:1)—Khs15T21 | 12.91 ± 0.19 | 0.15 ± 0.00 |
| Kolliphor PS 80/Transcutol (1:1)—KPS80T11 | 62.02 ± 39.30 | 0.24 ± 0.11 |
| Kolliphor PS 80/Transcutol (1:2)—KPS80T12 | 60.27 ± 37.48 | 0.13 ± 0.04 |
| Kolliphor PS 80/Transcutol (2:1)—KPS80T21 | 48.06 ± 3.83 | 0.17 ± 0.05 |
| Kolliphor EL/Transcutol (1:1)—KELT11 | 15.77 ± 3.94 | 0.11 ± 0.05 |
| Kolliphor EL/Transcutol (1:2)—KELT12 | 13.71 ± 0.23 | 0.15 ± 0.05 |
| Kolliphor EL/Transcutol (2:1)—KELT21 | 26.56 ± 12.43 | 0.12 ± 0.04 |
| Formulations | pH = 1.2 | pH = 4.5 | pH = 6.8 | pH = 7.4 | ||||
|---|---|---|---|---|---|---|---|---|
| Droplet Size * | PDI ** | Droplet Size * | PDI ** | Droplet Size * | PDI ** | Droplet Size * | PDI ** | |
| Khs15T21 | 14.03 ± 1.69 | 0.17 ± 0.03 | 27.87 ± 8.40 | 0.11 ± 0.06 | 12.85 ± 0.15 | 0.07 ± 0.01 | 15.01 ± 2.48 | 0.17 ± 0.05 |
| KPS80T21 | 43.03 ± 4.62 | 0.16 ± 0.11 | 76.28 ± 34.54 | 0.25 ± 0.07 | 34.51± 40.03 | 0.18 ± 0.05 | 39.79 ± 44.34 | 0.24 ± 0.15 |
| KELT21 | 15.00 ± 1.83 | 0.17 ± 0.04 | 14.10 ± 0.03 | 0.08 ± 0.03 | 13.70 ± 0.21 | 0.16 ± 0.01 | 14.06 ± 0.17 | 0.16 ± 0.03 |
| 48 h *** | ||||||||
| Khs15T21 | 31.43 ± 30.14 | 0.19 ± 0.03 | 34.76 ± 35.99 | 0.23 ± 0.00 | 12.78 ± 0.13 | 0.16 ± 0.02 | 5504.00 ± 82.78 | 0.73 ± 0.23 |
| KPS80T21 | 17.89 ± 4.87 | 0.15 ± 0.07 | 190.90 ± 53.09 | 0.30 ± 0.17 | 69.27 ± 17.57 | 0.23 ± 0.08 | 177.60 ± 90.20 | 0.29 ± 0.17 |
| KELT21 | 14.69 ± 0.17 | 0.10 ± 0.02 | 71.23 ± 51.53 | 0.22 ± 0.07 | 99.22 ± 16.10 | 0.22 ± 0.10 | 47.38 ± 32.40 | 0.15 ± 0.06 |
| Formulations (Smix) | Droplet Size * | PDI ** |
|---|---|---|
| KPS80T21 | 32.99 ± 26.85 | 0.12 ± 0.04 |
| KELT21 | 13.71 ± 0.67 | 0.11 ± 0.01 |
| Formulations (Smix) | pH = 1.2 | pH = 4.5 | pH = 6.8 | pH = 7.4 | ||||
|---|---|---|---|---|---|---|---|---|
| Droplet Size * | PDI ** | Droplet Size * | PDI ** | Droplet Size * | PDI ** | Droplet Size * | PDI ** | |
| KPS80T21 | 12.61 ± 2.72 | 0.16 ± 0.05 | 28.97 ± 13.90 | 0.18 ± 0.03 | 140.50 ± 9.47 | 0.17 ± 0.01 | 29.50 ± 14.54 | 0.16 ± 0.03 |
| KELT21 | 13.80 ± 0.16 | 0.06 ± 0.01 | 22.17 ± 14.16 | 0.16 ± 0.04 | 14.59 ± 1.96 | 0.12 ± 0.02 | 14.03 ± 0.13 | 0.11 ± 0.06 |
| Formulations (Smix) | TDL Amount (mg) | Smix: Adsorbent Ratio (w/w) | Type of Carrier | Solid TDL-Loaded Type IV Formulations |
|---|---|---|---|---|
| KPS80T21 | 7.5 | 2:1 | Florite R | F1 |
| KELT21 | F6 | |||
| KPS80T21 | 7.5 | 2:1 | Neusilin US2 | F2 |
| KELT21 | F7 | |||
| KPS80T21 | 7.5 | 2:1 | Neusilin UFL2 | F3 |
| KELT21 | F8 | |||
| KPS80T21 | 7.5 | 1.5:1 | Syloid XDP 3050 | F4 |
| KELT21 | F9 | |||
| KPS80T21 | 7.5 | 1.5:1 | Syloid XDP 3150 | F5 |
| KELT21 | F10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husuzade, G.; Demiralp, B.; Nazlı, H.; Boran, T.; Güngör, S. Comprehensive Analytical Studies on the Solubility and Dissolution Rate Enhancement of Tadalafil with Type IV Lipid Formulations. Pharmaceutics 2025, 17, 1436. https://doi.org/10.3390/pharmaceutics17111436
Husuzade G, Demiralp B, Nazlı H, Boran T, Güngör S. Comprehensive Analytical Studies on the Solubility and Dissolution Rate Enhancement of Tadalafil with Type IV Lipid Formulations. Pharmaceutics. 2025; 17(11):1436. https://doi.org/10.3390/pharmaceutics17111436
Chicago/Turabian StyleHusuzade, Günay, Burcu Demiralp, Hakan Nazlı, Tuğçe Boran, and Sevgi Güngör. 2025. "Comprehensive Analytical Studies on the Solubility and Dissolution Rate Enhancement of Tadalafil with Type IV Lipid Formulations" Pharmaceutics 17, no. 11: 1436. https://doi.org/10.3390/pharmaceutics17111436
APA StyleHusuzade, G., Demiralp, B., Nazlı, H., Boran, T., & Güngör, S. (2025). Comprehensive Analytical Studies on the Solubility and Dissolution Rate Enhancement of Tadalafil with Type IV Lipid Formulations. Pharmaceutics, 17(11), 1436. https://doi.org/10.3390/pharmaceutics17111436








