Targeted Drug Delivery Strategies in Overcoming Antimicrobial Resistance: Advances and Future Directions
Abstract
1. Introduction
2. Mechanisms of AMR
2.1. Genetic and Biochemical Mechanisms
2.2. Challenges Posed by AMR to Conventional Therapies
3. Role of Targeted Drug Delivery in AMR
3.1. Benefits of Targeted Drug Delivery
3.2. Comparison with Conventional Delivery Methods
| Factors | Conventional Delivery Methods | Limitations of Non-Targeted Antibiotics | Targeted Drug Delivery |
|---|---|---|---|
| Mechanism of Action | Broad-spectrum actions target multiple bacterial species [89,90]. | Random targeting impacts beneficial microbiota and enables AMR [14]. | Targeting antimicrobial agents directly at the site of infection or the pathogen reduces their contact with non-target regions [80]. |
| Specificity | Non-targeted methods impact both pathogenic and non-pathogenic bacteria [10]. | Inadequate selectivity disturbs beneficial microbiota and decreases colonization resistance [14,116]. | Specifically, it focuses on areas of infection or harmful microorganisms [79]. |
| Efficacy Against Biofilms | Efficacy is limited due to insufficient penetration and activity in biofilms [21]. | Conventional treatments are ineffective against biofilm-associated infections [21]. | It is effective against biofilms [122]. |
| Accumulation and Penetration | Penetration into cells is poor [87]. | Low-penetration efficiency results in a high dose being required [87]. | It targets intracellular pathogens by facilitating drug entry into cells [102]. |
| Side Effects and Toxicity | Frequent systemic side effects result from non-specific actions [14]. | Excessive dosage and fluctuating medication levels above the therapeutic range provide a toxicity risk [82]. | Drugs can be encapsulated in carriers like liposomes and nanoparticles to preserve healthy tissues, increase localized dosages at infection sites, and decrease systemic toxicity overall [116]. |
| Bioavailability | There is low bioavailability and quick clearance [102]. | Higher dosages are necessary due to inefficiencies at infection sites caused by limited medication uptake through biological membranes [102]. | Nanocarriers and delivery methods are beneficial since they can combat early degradation and extend the half-life of antibiotics, improving medication stability and bioavailability [118]. |
| Emergence of Antibiotic Resistance | Overuse and misuse make resistance possible [128]. | The overall emergence of antibiotic-resistant pathogens is increasing, with a special threat coming from those that are associated with the formation of biofilms [22]. | Targeted systems produce greater localized levels of antibiotics at the site of infection, effectively addressing drug resistance mechanisms [114]. |
| Impact on the Economy and Healthcare | Costs associated with long-term therapy and equipment replacement are considerable [17]. | Long-term infections are a cause of increased duration in hospitals and increased costs to the patients [17]. | Targeted drug delivery systems pose the potential to positively affect the economy and the healthcare industry by improving the precision of the treatments [127]. |
4. Innovative Strategies in Targeted Drug Delivery
4.1. Nanoparticle-Based Systems
4.2. Stimuli-Responsive Systems
4.3. Bacteriophage-Based Delivery
4.4. Antibody–Drug Conjugates (ADCs)
4.5. Peptide- and Protein-Based Delivery Systems
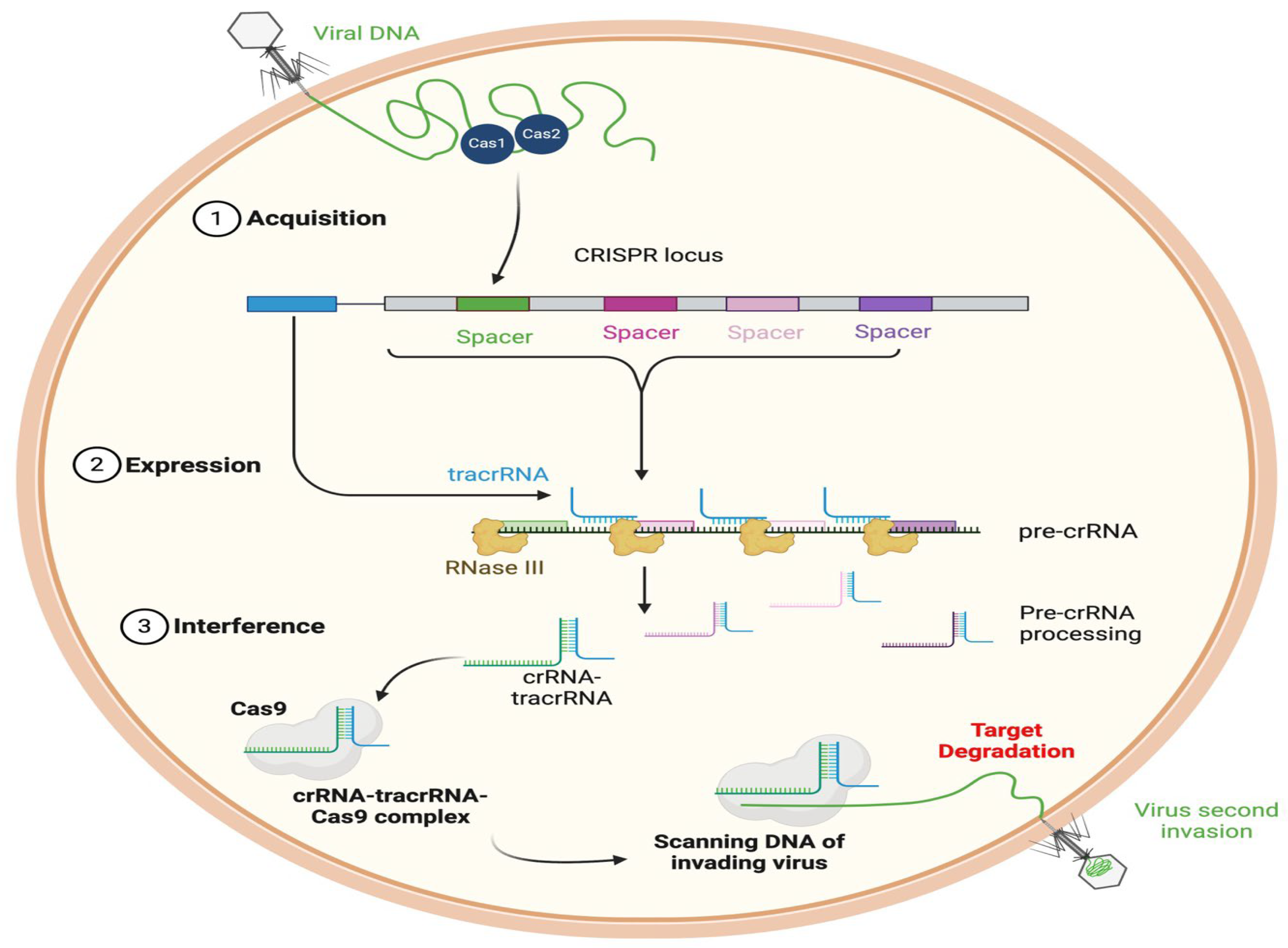
4.6. CRISPR-Cas Systems
5. Challenges and Limitations
5.1. Biological Barriers
5.2. Stability and Scalability
5.3. Regulatory and Ethical Challenges
6. Future Directions
6.1. Emerging Materials and Technologies
6.1.1. Biomaterials
6.1.2. Advanced Multifunctional Hybrid System
6.2. Personalized Medicine Approaches
6.3. Interdisciplinary Collaboration
Microbiology, Materials, and Pharmacology Come Together
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240062702. [Google Scholar]
- Mittal, A.K.; Bhardwaj, R.; Mishra, P.; Rajput, S.K. Antimicrobials Misuse/Overuse: Adverse Effect, Mechanism, Challenges and Strategies to Combat Resistance. Open Biotechnol. J. 2020, 14, 107–112. [Google Scholar] [CrossRef]
- Haley, V.W. Antibiotic Resistance: Review. SA Pharm. J. 2015, 82, 20–23. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Loewy, Z.G. Antimicrobial Resistance and Novel Alternative Approaches to Conventional Antibiotics. Bacteria 2024, 3, 171–182. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Radzevičienė, A. Antimicrobial Resistance: What Lies Beneath This Complex Phenomenon? Diagnostics 2024, 14, 2319. [Google Scholar] [CrossRef]
- Nwankwo, E.I.; Emeihe, E.V.; Ajegbile, M.D.; Olaboye, J.A. Chukwudi Cosmos Maha Innovative Drug Delivery Methods for Combating Antimicrobial Resistance. Int. Med. Sci. Res. J. 2024, 4, 834–858. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic Resistance Is Ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, E.; Wang, Y.; Miao, S.; Liu, Y.; Hu, Y.; Liu, J.; Xu, B.; Chen, D.; Shen, Y. Emerging Antibacterial Strategies with Application of Targeting Drug Delivery System and Combined Treatment. Int. J. Nanomed. 2021, 16, 6141–6156. [Google Scholar] [CrossRef]
- Aruković, E.; Fetahović, D.; Pehlivanović, B. Impact of Antibiotic Misuse on Genetics Alterations of Bacteria. In CMBEBIH 2019; Springer: Cham, Switzerland, 2020; pp. 617–621. [Google Scholar]
- Sundaramoorthy, N.S.; Shankaran, P.; Gopalan, V.; Nagarajan, S. New Tools to Mitigate Drug Resistance in Enterobacteriaceae—Escherichia coli and Klebsiella pneumoniae. Crit. Rev. Microbiol. 2023, 49, 435–454. [Google Scholar] [CrossRef]
- Padmini, N.; Ajilda, A.A.K.; Sivakumar, N.; Selvakumar, G. Extended Spectrum Β-lactamase Producing Escherichia coli and Klebsiella Pneumoniae: Critical Tools for Antibiotic Resistance Pattern. J. Basic. Microbiol. 2017, 57, 460–470. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Targeted Antimicrobial Therapies: A Solution to Overcoming Antimicrobial Resistance in Humans. BioMed 2024, 4, 318–337. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Akova, M. Epidemiology of Antimicrobial Resistance in Bloodstream Infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Vishwakarma, N.; Lehr, C.-M.; Prestidge, C.A.; Thomas, N.; Roberts, R.J.; Thorn, C.R.; Melero, A. Antibiotic Resistance and Tolerance: What Can Drug Delivery Do against This Global Threat? Drug Deliv. Transl. Res. 2024, 14, 1725–1734. [Google Scholar] [CrossRef]
- Rao, T.S. Bacterial Biofilms and Implant Infections: A Perspective. Arch. Orthop. 2020, 1, 98–105. [Google Scholar] [CrossRef]
- Lynch, A.S.; Robertson, G.T. Bacterial and Fungal Biofilm Infections. Annu. Rev. Med. 2008, 59, 415–428. [Google Scholar] [CrossRef]
- Wang, H.; Tang, C.; Xiang, Y.; Zou, C.; Hu, J.; Yang, G.; Zhou, W. Tea Polyphenol-Derived Nanomedicine for Targeted Photothermal Thrombolysis and Inflammation Suppression. J. Nanobiotechnol. 2024, 22, 146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cheng, H.; Jiang, S.; Tai, W. Fc Multisite Conjugation and Prolonged Delivery of the Folate-Targeted Drug Conjugate EC140. Bioconjug Chem. 2025, 36, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P. Helicobacter Pylori Efflux Pumps: A Double-Edged Sword in Antibiotic Resistance and Biofilm Formation. Int. J. Mol. Sci. 2024, 25, 12222. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Aggarwal, S.; Singh, D.V. Efflux Pumps: Gatekeepers of Antibiotic Resistance in Staphylococcus aureus Biofilms. Microb. Cell 2024, 11, 368–377. [Google Scholar] [CrossRef]
- McGowen, K.; Funck, T.; Wang, X.; Zinga, S.; Wolf, I.D.; Akusobi, C.; Denkinger, C.M.; Rubin, E.J.; Sullivan, M.R. Efflux Pumps and Membrane Permeability Contribute to Intrinsic Antibiotic Resistance in Mycobacterium abscessus. PLoS Pathog. 2025, 21, e1013027. [Google Scholar] [CrossRef]
- Novelli, M.; Bolla, J.-M. RND Efflux Pump Induction: A Crucial Network Unveiling Adaptive Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Antibiotics 2024, 13, 501. [Google Scholar] [CrossRef]
- Whittle, E.E.; Orababa, O.; Osgerby, A.; Siasat, P.; Element, S.J.; Blair, J.M.A.; Overton, T.W. Efflux Pumps Mediate Changes to Fundamental Bacterial Physiology via Membrane Potential. mBio 2024, 15, e0237024. [Google Scholar] [CrossRef]
- de Souza, J.; Vieira, A.Z.; dos Santos, H.G.; Faoro, H. Potential Involvement of Beta-Lactamase Homologous Proteins in Resistance to Beta-Lactam Antibiotics in Gram-Negative Bacteria of the ESKAPEE Group. BMC Genom. 2024, 25, 508. [Google Scholar] [CrossRef]
- Kang, S.-J.; Kim, D.-H.; Lee, B.-J. Metallo-β-Lactamase Inhibitors: A Continuing Challenge for Combating Antibiotic Resistance. Biophys. Chem. 2024, 309, 107228. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, J.; Zhao, Z.; Chan, A.W.E.; Mojica, M.F.; Hujer, A.M.; Bonomo, R.A.; Haider, S. Deciphering the Coevolutionary Dynamics of L2 β-Lactamases via Deep Learning. J. Chem. Inf. Model. 2024, 64, 3706–3717. [Google Scholar] [CrossRef]
- Arer, V.; Kar, D. Biochemical Exploration of β-Lactamase Inhibitors. Front. Genet. 2023, 13, 1060736. [Google Scholar] [CrossRef] [PubMed]
- Bertonha, A.F.; Silva, C.C.L.; Shirakawa, K.T.; Trindade, D.M.; Dessen, A. Penicillin-Binding Protein (PBP) Inhibitor Development: A 10-Year Chemical Perspective. Exp. Biol. Med. 2023, 248, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Grabrijan, K.; Benedik, N.S.; Krajnc, A.; Bozovičar, K.; Knez, D.; Proj, M.; Zdovc, I.; Sosič, I.; Contreras-Martel, C.; Dessen, A.; et al. Synthesis and Biochemical Evaluation of New 3-Amido-4-Substituted Monocyclic ß-Lactams as Inhibitors of Penicillin-Binding Protein(s). Acta Pharm. 2024, 74, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Puls, J.-S.; Brajtenbach, D.; Schneider, T.; Kubitscheck, U.; Grein, F. Inhibition of Peptidoglycan Synthesis Is Sufficient for Total Arrest of Staphylococcal Cell Division. Sci. Adv. 2023, 9, eade9023. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial Persisters: Molecular Mechanisms and Therapeutic Development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, M.; Li, D.; Yu, P.; Ma, H.; Lu, H. Protective Effects of Antibiotic Resistant Bacteria on Susceptibles in Biofilm: Influential Factors, Mechanism, and Modeling. Sci. Total Environ. 2024, 930, 172668. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic Control of Bacterial Biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef]
- Samanta, A.; Roy, D.; Lahiri, D.; Ray, R.R.; Nag, M. Genetics of Microbial Biofilm Development. In Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2023; pp. 19–38. [Google Scholar]
- Holden, E.R.; Yasir, M.; Turner, A.K.; Wain, J.; Charles, I.G.; Webber, M.A. Massively Parallel Transposon Mutagenesis Identifies Temporally Essential Genes for Biofilm Formation in Escherichia coli. Microb. Genom. 2021, 7, 000673. [Google Scholar] [CrossRef]
- Yang, Y.; Thomas, J.; Li, Y.; Vilchèze, C.; Derbyshire, K.M.; Jacobs, W.R.; Ojha, A.K. Defining a Temporal Order of Genetic Requirements for Development of Mycobacterial Biofilms. Mol. Microbiol. 2017, 105, 794–809. [Google Scholar] [CrossRef]
- Awoonor-Williams, E.; Abu-Saleh, A.A.-A.A. Molecular Insights into the Impact of Mutations on the Binding Affinity of Targeted Covalent Inhibitors of BTK. J. Phys. Chem. B 2024, 128, 2874–2884. [Google Scholar] [CrossRef]
- Friedman, R. Computational Studies of Protein–Drug Binding Affinity Changes upon Mutations in the Drug Target. WIREs Comput. Mol. Sci. 2022, 12, e1563. [Google Scholar] [CrossRef]
- Ammar, A.; Cavill, R.; Evelo, C.; Willighagen, E. PSnpBind-ML: Predicting the Effect of Binding Site Mutations on Protein-Ligand Binding Affinity. J. Cheminform 2023, 15, 31. [Google Scholar] [CrossRef]
- Kieseier, B.C. Assessing Long-Term Effects of Disease-Modifying Drugs. J. Neurol. 2006, 253, vi23–vi30. [Google Scholar] [CrossRef]
- Zhou, S.; Chan, E.; Duan, W.; Huang, M.; Chen, Y.-Z. Drug Bioactivation Covalent Binding to Target Proteins and Toxicity Relevance. Drug Metab. Rev. 2005, 37, 41–213. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Long-Term Consequences of Anesthetic Management. Anesthesiology 2009, 111, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Novel Therapeutics for Bacterial Infections. Emerg. Top. Life Sci. 2017, 1, 85–92. [Google Scholar] [CrossRef]
- Hughes, D.; Karlén, A. Discovery and Preclinical Development of New Antibiotics. Ups. J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef]
- Meimetis, N.; Lauffenburger, D.A.; Nilsson, A. Inference of Drug Off-Target Effects on Cellular Signaling Using Interactome-Based Deep Learning. iScience 2024, 27, 109509. [Google Scholar] [CrossRef]
- Bereczki, Z.; Benczik, B.; Balogh, O.M.; Marton, S.; Puhl, E.; Pétervári, M.; Váczy-Földi, M.; Papp, Z.T.; Makkos, A.; Glass, K.; et al. Mitigating Off-target Effects of Small RNAs: Conventional Approaches, Network Theory and Artificial Intelligence. Br. J. Pharmacol. 2025, 182, 340–379. [Google Scholar] [CrossRef]
- Lin, J.; Nguyen, M.A.; Lin, L.Y.; Zeng, J.; Verma, A.; Neri, N.R.; da Silva, L.F.; Mucci, A.; Wolfe, S.; Shaw, K.L.; et al. Scalable Assessment of Genome Editing Off-Targets Associated with Genetic Variants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Pham, T.; Loupias, P.; Dassonville-Klimpt, A.; Sonnet, P. Drug Delivery Systems Designed to Overcome Antimicrobial Resistance. Med. Res. Rev. 2019, 39, 2343–2396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sharma, V. Targeted Drug Delivery System: A Review. Res. J. Chem. Sci. 2011, 1, 135–138. [Google Scholar]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Huang, T.-H.; Yang, S.-C.; Chen, C.-C.; Fang, J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286. [Google Scholar] [CrossRef]
- Devarajan, P.V.; Dawre, S.M.; Dutta, R. Infectious Diseases: Need for Targeted Drug Delivery. In Targeted Drug Delivery: Concepts and Design; Springer: Berlin/Heidelberg, Germany, 2015; pp. 113–148. [Google Scholar]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- He, X.; Li, J.; An, S.; Jiang, C. PH-Sensitive Drug-Delivery Systems for Tumor Targeting. Ther. Deliv. 2013, 4, 1499–1510. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, Y.; He, Y.; Chen, F.; Gong, Y.; Chen, S.; Xu, Y.; Su, Y.; Wang, C.; Wang, J. Succinylated Casein-Coated Peptide-Mesoporous Silica Nanoparticles as an Antibiotic against Intestinal Bacterial Infection. Biomater. Sci. 2019, 7, 2440–2451. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Thorn, C.R.; Howell, P.L.; Wozniak, D.J.; Prestidge, C.A.; Thomas, N. Enhancing the Therapeutic Use of Biofilm-Dispersing Enzymes with Smart Drug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 179, 113916. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Biofilms and Antibiotic Therapy: Is There a Role for Combating Bacterial Resistance by the Use of Novel Drug Delivery Systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Sambhy, V.; MacBride, M.M.; Peterson, B.R.; Sen, A. Silver Bromide Nanoparticle/Polymer Composites: Dual Action Tunable Antimicrobial Materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, W.; Song, L.-J.; Luan, S.-F. Stimuli-Responsive Nanocarriers for Bacterial Biofilm Treatment. Rare Met. 2022, 41, 482–498. [Google Scholar] [CrossRef]
- Alzahrani, N.M.; Booq, R.Y.; Aldossary, A.M.; Bakr, A.A.; Almughem, F.A.; Alfahad, A.J.; Alsharif, W.K.; Jarallah, S.J.; Alharbi, W.S.; Alsudir, S.A.; et al. Liposome-Encapsulated Tobramycin and IDR-1018 Peptide Mediated Biofilm Disruption and Enhanced Antimicrobial Activity against Pseudomonas aeruginosa. Pharmaceutics 2022, 14, 960. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Lu, H.; Wang, X.; Wang, X.; Su, J.; Xia, G. Strategies to Promote the Journey of Nanoparticles Against Biofilm-Associated Infections. Small 2024, 20, e2305988. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.A.; Rodgers, A.M.; O’Brien, S.C.; Donnelly, R.F.; Gilmore, B.F. Gut Check Time: Antibiotic Delivery Strategies to Reduce Antimicrobial Resistance. Trends Biotechnol. 2020, 38, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; He, D.L.; Liao, D.; Khan, M.Z.I.; Huang, C.; He, Y. Strategies and Progresses for Enhancing Targeted Antibiotic Delivery. Adv. Drug Deliv. Rev. 2022, 189, 114502. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sinha, S.; Kumar, A.; Arya, V.; Kumar, D.; Dhansekhran, M. Nanoparticles as Drug Delivery Systems: Advances and Challenges. In Nanotechnology; Springer Nature: Singapore, 2024; pp. 245–288. [Google Scholar]
- Nazir, F.; Tabish, T.A.; Tariq, F.; Iftikhar, S.; Wasim, R.; Shahnaz, G. Stimuli-Sensitive Drug Delivery Systems for Site-Specific Antibiotic Release. Drug Discov. Today 2022, 27, 1698–1705. [Google Scholar] [CrossRef]
- Marzaman, A.N.F.; Roska, T.P.; Sartini, S.; Utami, R.N.; Sulistiawati, S.; Enggi, C.K.; Manggau, M.A.; Rahman, L.; Shastri, V.P.; Permana, A.D. Recent Advances in Pharmaceutical Approaches of Antimicrobial Agents for Selective Delivery in Various Administration Routes. Antibiotics 2023, 12, 822. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, E.; Fakhar-ud-Din. Bioavailability of Antibiotics and Their Toxicity. In Antibiotics and Antimicrobial Resistance Genes. Emerging Contaminants and Associated Treatment Technologies; Springer: Cham, Switzerland,, 2020; pp. 211–238. [Google Scholar]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Ecological Impacts of Antibiotic Administration on the Human Intestinal Microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial Resistance Worldwide: Causes, Challenges and Responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Pereira, J.G.; Fernandes, J.; Duarte, A.R.; Fernandes, S.M. β-Lactam Dosing in Critical Patients: A Narrative Review of Optimal Efficacy and the Prevention of Resistance and Toxicity. Antibiotics 2022, 11, 1839. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, A.A.; Jones, C.E.; Read, R.C.; Bogaert, D. Microbiotoxicity: Antibiotic Usage and Its Unintended Harm to the Microbiome. Curr. Opin. Infect. Dis. 2023, 36, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the Impact of Antibiotic Perturbation on the Human Microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Huang, C.; Feng, S.; Huo, F.; Liu, H. Effects of Four Antibiotics on the Diversity of the Intestinal Microbiota. Microbiol. Spectr. 2022, 10, e0190421. [Google Scholar] [CrossRef]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Müller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the Collateral Damage of Antibiotics on Gut Bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The Effects of Antibiotics on the Microbiome throughout Development and Alternative Approaches for Therapeutic Modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Pamer, E.G. Resurrecting the Intestinal Microbiota to Combat Antibiotic-Resistant Pathogens. Science 2016, 352, 535–538. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal Colonization Resistance. Immunology 2013, 138, 1–11. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-Analysis of Fecal Metagenomes Reveals Global Microbial Signatures That Are Specific for Colorectal Cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-Analysis of Gut Microbiome Studies Identifies Disease-Specific and Shared Responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.M.; Kamboj, M.; et al. Vancomycin-Resistant Enterococcus Domination of Intestinal Microbiota Is Enabled by Antibiotic Treatment in Mice and Precedes Bloodstream Invasion in Humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Francino, M. Early Development of the Gut Microbiota and Immune Health. Pathogens 2014, 3, 769–790. [Google Scholar] [CrossRef]
- Subramaniam, S.; Joyce, P.; Thomas, N.; Prestidge, C.A. Bioinspired Drug Delivery Strategies for Repurposing Conventional Antibiotics against Intracellular Infections. Adv. Drug Deliv. Rev. 2021, 177, 113948. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H.M. Risks Associated with the Therapeutic Use of Fluoroquinolones. Expert. Opin. Drug Saf. 2013, 12, 497–505. [Google Scholar] [CrossRef]
- Haddad, N.; Carr, M.; Balian, S.; Lannin, J.; Kim, Y.; Toth, C.; Jarvis, J. The Blood–Brain Barrier and Pharmacokinetic/Pharmacodynamic Optimization of Antibiotics for the Treatment of Central Nervous System Infections in Adults. Antibiotics 2022, 11, 1843. [Google Scholar] [CrossRef]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting Systemic Treatment of Bacterial Endophthalmitis: A Review of Intravitreal Penetration of Systemic Antibiotics. Clin. Microbiol. Infect. 2019, 25, 1364–1369. [Google Scholar] [CrossRef]
- Yılmaz, Ç.; Özcengiz, G. Antibiotics: Pharmacokinetics, Toxicity, Resistance and Multidrug Efflux Pumps. Biochem. Pharmacol. 2017, 133, 43–62. [Google Scholar] [CrossRef]
- Wildermuth, A.; Holmes, M. A Preventable, Life-Altering Case of Fluoroquinolone-Associated Tendonitis. JAAPA 2022, 35, 33–36. [Google Scholar] [CrossRef]
- DeLaney, M.C. Risks Associated with the Use of Fluoroquinolones. Br. J. Hosp. Med. 2018, 79, 552–555. [Google Scholar] [CrossRef]
- Le, T.A.; Hiba, T.; Chaudhari, D.; Preston, A.N.; Palowsky, Z.R.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Aminoglycoside-Related Nephrotoxicity and Ototoxicity in Clinical Practice: A Review of Pathophysiological Mechanism and Treatment Options. Adv. Ther. 2023, 40, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Venugopalan, V.; Vouri, S.M.; Diaby, V.; Iovine, N.M.; Park, H. Oral Fluoroquinolones and Risk of Aortic Aneurysm or Dissection: A Nationwide Population-Based Propensity Score-matched Cohort Study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Maideen, N.M.P.; Narayanaswamy, H. Overview of Tendinopathy, Peripheral Neuropathy, Aortic Aneurysm, and Hypoglycemia Caused by Fluoroquinolones. Ibnosina J. Med. Biomed. Sci. 2024, 16, 29–37. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic Allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef]
- Ahmed, F.; Shamim, N.J.; Das, A.; Sharma, H.K.; Grewal, A.S.; Pandita, D.; Lather, V. Combating Antimicrobial Resistance: A Paradigm Shift from General to Precision Medicine. Chem. Biol. Lett. 2024, 11, 662. [Google Scholar] [CrossRef]
- Emeihe, E.V.; Nwankwo, E.I.; Ajegbile, M.D.; Olaboye, J.A.; Maha, C.C. Revolutionizing Drug Delivery Systems: Nanotechnology-Based Approaches for Targeted Therapy. Int. J. Life Sci. Res. Arch. 2024, 7, 40–58. [Google Scholar] [CrossRef]
- Zulfiqar, H. Nature of Nanoparticles and Their Applications in Targeted Drug Delivery. Pak. J. Sci. 2022, 75, 30. [Google Scholar] [CrossRef]
- Cavaco, M.; Castanho, M.A.R.B.; Neves, V. The Use of Antibody-Antibiotic Conjugates to Fight Bacterial Infections. Front. Microbiol. 2022, 13, 835677. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Huang, Y.; Jin, Q.; Ji, J. Inhibiting Quorum Sensing by Active Targeted PH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections. ACS Nano 2023, 17, 10019–10032. [Google Scholar] [CrossRef]
- Durgapal, S.; Joshi, B.C.; Pandey, B.S.; Kukreti, G.; Dhyani, A.; Jain, A.; Mukhopadhyay, S.; Mukhija, M.; Bajwa, P.S. Precision Drug Delivery to Tackle Antibiotic Resistance. In Frontiers in Combating Antibacterial Resistance: Current Perspectives and Future Horizons; IGI Global Scientific Publishing: Palmdale, PA, USA, 2024; pp. 1–32. [Google Scholar]
- Chen, Y.; Huang, Y.; Jin, Q. Polymeric Nanoplatforms for the Delivery of Antibacterial Agents. Macromol. Chem. Phys. 2022, 223, 2100440. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.B.; Gamarra, L.F. Multifunctional Nanoparticles for Successful Targeted Drug Delivery across the Blood-Brain Barrier. In Molecular Insight of Drug Design; InTech: Nappanee, IN, USA, 2018. [Google Scholar]
- Cheng, T.; Tai, Z.; Shen, M.; Li, Y.; Yu, J.; Wang, J.; Zhu, Q.; Chen, Z. Advance and Challenges in the Treatment of Skin Diseases with the Transdermal Drug Delivery System. Pharmaceutics 2023, 15, 2165. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, C.; Zhu, Y.; Zheng, L.; Zhang, L.; Hu, Y.; Yu, B.; Wang, Y.; Xu, F. Biofilm-Sensitive Photodynamic Nanoparticles for Enhanced Penetration and Antibacterial Efficiency. Adv. Funct. Mater. 2021, 31, 2103591. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Mariathasan, S.; Tan, M.-W. Antibody–Antibiotic Conjugates: A Novel Therapeutic Platform against Bacterial Infections. Trends Mol. Med. 2017, 23, 135–149. [Google Scholar] [CrossRef]
- Kharga, K.; Jha, S.; Vishwakarma, T.; Kumar, L. Current Developments and Prospects of the Antibiotic Delivery Systems. Crit. Rev. Microbiol. 2025, 51, 44–83. [Google Scholar] [CrossRef]
- Murage, M.W.; Amuhaya, E.K.; Mbatia, B.N.; Muge, E.K.; Derese, S. Drug Delivery Strategies for Porphyrin-Based Photosensitizers in Photodynamic Antimicrobial Chemotherapy. J. Porphyr. Phthalocyanines 2024, 28, 391–417. [Google Scholar] [CrossRef]
- Ranjbar, R.; Alam, M. Antimicrobial Resistance Collaborators (2022). Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Evid. Based Nurs. 2024, 27, 16. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Ioannou, P.; Baliou, S.; Samonis, G. Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections. Antibiotics 2024, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Nanotechnology: What It Can Do for Drug Delivery. J. Control. Release 2007, 120, 1–3. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.E.; Ali, M.; Nibbering, P.H.; Kłodzińska, S.N. Current Advances in Lipid and Polymeric Antimicrobial Peptide Delivery Systems and Coatings for the Prevention and Treatment of Bacterial Infections. Pharmaceutics 2021, 13, 1840. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The Role of Liposomes in Clinical Nanomedicine Development. What Now? Now What? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Muppidi, K.; Wang, J.; Betageri, G.; Pumerantz, A.S. PEGylated Liposome Encapsulation Increases the Lung Tissue Concentration of Vancomycin. Antimicrob. Agents Chemother. 2011, 55, 4537–4542. [Google Scholar] [CrossRef]
- Khan, O.; Chaudary, N. The Use of Amikacin Liposome Inhalation Suspension (Arikayce) in the Treatment of Refractory Nontuberculous Mycobacterial Lung Disease in Adults. Drug Des. Devel Ther. 2020, 14, 2287–2294. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; Zhang, E.; Yang, L. Electrostatically Entrapped Colistin Liposomes for the Treatment of Pseudomonas aeruginosa Infection. Pharm. Dev. Technol. 2017, 22, 436–444. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Wong, E.H. Nanoprobes for Advanced Nanotheranostic Applications. In Advanced Nanoformulations; Elsevier: Amsterdam, The Netherlands, 2023; pp. 557–586. [Google Scholar]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef]
- Ryan, A.; Patel, P.; O’Connor, P.M.; Ross, R.P.; Hill, C.; Hudson, S.P. Pharmaceutical Design of a Delivery System for the Bacteriocin Lacticin 3147. Drug Deliv. Transl. Res. 2021, 11, 1735–1751. [Google Scholar] [CrossRef]
- Fumakia, M.; Ho, E.A. Nanoparticles Encapsulated with LL37 and Serpin A1 Promotes Wound Healing and Synergistically Enhances Antibacterial Activity. Mol. Pharm. 2016, 13, 2318–2331. [Google Scholar] [CrossRef]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial Activity of Polymyxin-Loaded Solid Lipid Nanoparticles (PLX-SLN): Characterization of Physicochemical Properties and in Vitro Efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef]
- Durham, O.Z.; Poetz, K.L.; Shipp, D.A. Polyanhydride Nanoparticles: Thiol–Ene ‘Click’ Polymerizations Provide Functionalized and Cross-Linkable Nanoparticles with Tuneable Degradation Times. Aust. J. Chem. 2017, 70, 735. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle Adhesion to the Cell Membrane and Its Effect on Nanoparticle Uptake Efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective Sequential Modification of Chitosan via Azide-Alkyne Click Reaction: Synthesis, Characterization, and Antimicrobial Activity of Chitosan Derivatives and Nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, M.-T.; Ou-Yang, H.D.; Walker, S.G.; Wang, H.Z.; Gordon, C.R.; Guterman, S.; Zawacki, E.; Applebaum, E.; Brink, P.R.; et al. Exposure to TiO2 Nanoparticles Increases Staphylococcus aureus Infection of HeLa Cells. J. Nanobiotechnol. 2016, 14, 34. [Google Scholar] [CrossRef]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food Storage Material Silver Nanoparticles Interfere with DNA Replication Fidelity and Bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Pan, F.; Xu, A.; Xia, D.; Yu, Y.; Chen, G.; Meyer, M.; Zhao, D.; Huang, C.-H.; Wu, Q.; Fu, J. Effects of Octahedral Molecular Sieve on Treatment Performance, Microbial Metabolism, and Microbial Community in Expanded Granular Sludge Bed Reactor. Water Res. 2015, 87, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Zheng, X.; Wei, Y.; Zhou, X.; Zhang, K.; Wang, S.; Cheng, L.; Li, Y.; Ren, B.; Xu, X.; et al. D-Alanine Metabolism Is Essential for Growth and Biofilm Formation of Streptococcus Mutans. Mol. Oral Microbiol. 2016, 31, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-L.; Chou, C.-C.; Hung, D.-J.; Lin, S.-H.; Pao, I.-C.; Lin, J.-H.; Huang, F.-L.; Dong, R.-X.; Lin, J.-J. The Disruption of Bacterial Membrane Integrity through ROS Generation Induced by Nanohybrids of Silver and Clay. Biomaterials 2009, 30, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Meile, S.; Du, J.; Dunne, M.; Kilcher, S.; Loessner, M.J. Engineering Therapeutic Phages for Enhanced Antibacterial Efficacy. Curr. Opin. Virol. 2022, 52, 182–191. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Rather, M.A.; Khan, F.; Tabassum, N.; Mandal, M.; Kim, Y.-M. PH-Responsive Polymeric Nanomaterials for the Treatment of Oral Biofilm Infections. Colloids Surf. B Biointerfaces 2024, 234, 113727. [Google Scholar] [CrossRef]
- Gui, S.; Li, X.; Feng, M.; Liu, H.; Huang, L.; Niu, X. A Fresh PH-Responsive Imipenem-Loaded Nanocarrier against Acinetobacter Baumannii with a Synergetic Effect. Front. Bioeng. Biotechnol. 2023, 11, 1166790. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, X. Temperature Responsive Nanoparticles Based on PEGylated Polyaspartamide Derivatives for Drug Delivery. Polymers 2019, 11, 316. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Drulis-Kawa, Z.; Cater, K.; Knežević, P.; Winogradow, C.; Amaro, K.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Rękas, J.; et al. Bacteriophages and Antibiotic Interactions in Clinical Practice: What We Have Learned so Far. J. Biomed. Sci. 2022, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Delaney, J.C.; Guillard, T.; Reffuveille, F.; Varin-Simon, J.; Li, K.; Wollacott, A.; Frapy, E.; Mong, S.; Tissire, H.; et al. Development of an Antibody Fused with an Antimicrobial Peptide Targeting Pseudomonas Aeruginosa: A New Approach to Prevent and Treat Bacterial Infections. PLoS Pathog. 2023, 19, e1011612. [Google Scholar] [CrossRef] [PubMed]
- Babb, R.; Pirofski, L. Help Is on the Way: Monoclonal Antibody Therapy for Multi-Drug Resistant Bacteria. Virulence 2017, 8, 1055–1058. [Google Scholar] [CrossRef]
- Irani, V.; Guy, A.J.; Andrew, D.; Beeson, J.G.; Ramsland, P.A.; Richards, J.S. Molecular Properties of Human IgG Subclasses and Their Implications for Designing Therapeutic Monoclonal Antibodies against Infectious Diseases. Mol. Immunol. 2015, 67, 171–182. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A Multifunctional Bispecific Antibody Protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6, 262ra155. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Sellman, B.R. Antibacterial Monoclonal Antibodies: The next Generation? Curr. Opin. Microbiol. 2015, 27, 78–85. [Google Scholar] [CrossRef]
- Lovey, A.; Krel, M.; Borchardt, A.; Brady, T.; Cole, J.N.; Do, Q.-Q.; Fortier, J.; Hough, G.; Jiang, W.; Noncovich, A.; et al. Development of Novel Immunoprophylactic Agents against Multidrug-Resistant Gram-Negative Bacterial Infections. Antimicrob. Agents Chemother. 2021, 65, e0098521. [Google Scholar] [CrossRef]
- Hussack, G.; Tanha, J. Toxin-Specific Antibodies for the Treatment of Clostridium difficile: Current Status and Future Perspectives. Toxins 2010, 2, 998–1018. [Google Scholar] [CrossRef]
- Bregenholt, S.; Haurum, J. Pathogen-Specific Recombinant Human Polyclonal Antibodies: Biodefence Applications. Expert. Opin. Biol. Ther. 2004, 4, 387–396. [Google Scholar] [CrossRef]
- Salazar, G.; Zhang, N.; Fu, T.-M.; An, Z. Antibody Therapies for the Prevention and Treatment of Viral Infections. NPJ Vaccines 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Stefano, J.E.; Manning, C.; Kyazike, J.; Chen, B.; Gianolio, D.A.; Park, A.; Busch, M.; Bird, J.; Zheng, X.; et al. Site-Specific Antibody–Drug Conjugation through Glycoengineering. Bioconjug. Chem. 2014, 25, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel Antibody–Antibiotic Conjugate Eliminates Intracellular S. aureus. Nature 2015, 527, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mokhtary, P.; Pourhashem, Z.; Mehrizi, A.A.; Sala, C.; Rappuoli, R. Recent Progress in the Discovery and Development of Monoclonal Antibodies against Viral Infections. Biomedicines 2022, 10, 1861. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to Combat Viral Infections: Development Strategies and Progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Pecetta, S.; Finco, O.; Seubert, A. Quantum Leap of Monoclonal Antibody (MAb) Discovery and Development in the COVID-19 Era. Semin. Immunol. 2020, 50, 101427. [Google Scholar] [CrossRef]
- Pelfrene, E.; Mura, M.; Cavaleiro Sanches, A.; Cavaleri, M. Monoclonal Antibodies as Anti-Infective Products: A Promising Future? Clin. Microbiol. Infect. 2019, 25, 60–64. [Google Scholar] [CrossRef]
- Whaley, K.J.; Zeitlin, L. Emerging Antibody-Based Products for Infectious Diseases: Planning for Metric Ton Manufacturing. Hum. Vaccin. Immunother. 2022, 18, 1930847. [Google Scholar] [CrossRef]
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic Antibodies for Infectious Diseases. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Wagner, E.K.; Maynard, J.A. Engineering Therapeutic Antibodies to Combat Infectious Diseases. Curr. Opin. Chem. Eng. 2018, 19, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, K.H.; Ki, M.-R.; Pack, S.P. Antimicrobial Peptides and Their Biomedical Applications: A Review. Antibiotics 2024, 13, 794. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and Host-Defense Peptides as New Anti-Infective Therapeutic Strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial Peptides and Their Interaction with Biofilms of Medically Relevant Bacteria. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Hua, Y.; Qin, Z.; Gao, L.; Zhou, M.; Xue, Y.; Li, Y.; Xie, J. Protein Nanoparticles as Drug Delivery Systems for Cancer Theranostics. J. Control. Release 2024, 371, 429–444. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Patel, R.; Vafakish, B.; Yazdi, A.F.A.; Acharya, B. Nanocellulose in Targeted Drug Delivery: A Review of Modifications and Synergistic Applications. Int. J. Biol. Macromol. 2024, 278, 135200. [Google Scholar] [CrossRef] [PubMed]
- Incocciati, A.; Kubeš, J.; Piacentini, R.; Cappelletti, C.; Botta, S.; Bertuccini, L.; Šimůnek, T.; Boffi, A.; Macone, A.; Bonamore, A. Hydrophobicity-enhanced Ferritin Nanoparticles for Efficient Encapsulation and Targeted Delivery of Hydrophobic Drugs to Tumor Cells. Protein Sci. 2023, 32, e4819. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ramadan, W.; Rambhu, D.; Shakeel, F. Potential of Nanoemulsions for Intravenous Delivery of Rifampicin. Pharmazie 2008, 63, 806–811. [Google Scholar]
- Mistry, N.; Bandyopadhyaya, R.; Mehra, S. Enhancement of Antimycobacterial Activity of Rifampicin Using Mannose-Anchored Lipid Nanoparticles against Intramacrophage Mycobacteria. ACS Appl. Bio Mater. 2022, 5, 5779–5789. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Cacciafesta, M.; Mangoni, M.L. Promising Approaches to Optimize the Biological Properties of the Antimicrobial Peptide Esculentin-1a(1–21)NH2: Amino Acids Substitution and Conjugation to Nanoparticles. Front. Chem. 2017, 5, 26. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Fineran, P.C.; Charpentier, E. Memory of Viral Infections by CRISPR-Cas Adaptive Immune Systems: Acquisition of New Information. Virology 2012, 434, 202–209. [Google Scholar] [CrossRef]
- Abavisani, M.; Khayami, R.; Hoseinzadeh, M.; Kodori, M.; Kesharwani, P.; Sahebkar, A. CRISPR-Cas System as a Promising Player against Bacterial Infection and Antibiotic Resistance. Drug Resist. Updates 2023, 68, 100948. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Mukhopadhyay, P. Antimicrobial Resistance (AMR) Management Using CRISPR-Cas Based Genome Editing. Gene Genome Ed. 2024, 7, 100031. [Google Scholar] [CrossRef]
- Zhu, G.; Zhou, X.; Wen, M.; Qiao, J.; Li, G.; Yao, Y. CRISPR–Cas13: Pioneering RNA Editing for Nucleic Acid Therapeutics. BioDesign Res. 2024, 6, 0041. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shu, X.; Zhao, H.; Xue, Q.; Liu, C.; Wu, A.; Cheng, F.; Wang, L.; Zhang, Y.; Feng, J.; et al. Associate Toxin-Antitoxin with CRISPR-Cas to Kill Multidrug-Resistant Pathogens. Nat. Commun. 2023, 14, 2078. [Google Scholar] [CrossRef]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and Evolution of Bacterial Toxin–Antitoxin Systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, A.; Liu, C.; Cao, X.; Wang, R.; Shu, X.; Wang, L.; Zhang, Y.; Xiang, H.; Li, M. The Toxin–Antitoxin RNA Guards of CRISPR-Cas Evolved High Specificity through Repeat Degeneration. Nucleic Acids Res. 2022, 50, 9442–9452. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Kumariya, R.; Sood, S.K.; Rajput, Y.S.; Saini, N.; Garsa, A.K. Increased Membrane Surface Positive Charge and Altered Membrane Fluidity Leads to Cationic Antimicrobial Peptide Resistance in Enterococcus faecalis. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 1367–1375. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Anaya, N.M.; Astudillo-Castro, C.; Oyanedel-Craver, V. Kinetic, Metabolic and Macromolecular Response of Bacteria to Chronic Nanoparticle Exposure in Continuous Culture. Environ. Sci. Nano 2018, 5, 1386–1396. [Google Scholar] [CrossRef]
- Niño-Martínez, N.; Salas Orozco, M.F.; Martínez-Castañón, G.-A.; Torres Méndez, F.; Ruiz, F. Molecular Mechanisms of Bacterial Resistance to Metal and Metal Oxide Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef]
- Salusso, A.; Raimunda, D. Defining the Roles of the Cation Diffusion Facilitators in Fe2+/Zn2+ Homeostasis and Establishment of Their Participation in Virulence in Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Das, K.R.; Naik, M.M. Co-Selection of Multi-Antibiotic Resistance in Bacterial Pathogens in Metal and Microplastic Contaminated Environments: An Emerging Health Threat. Chemosphere 2019, 215, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, F.; Zhao, J.; Xu, Y.; Mao, D.; Zhu, X.; Luo, Y.; Alvarez, P.J.J. Bacterial Exposure to ZnO Nanoparticles Facilitates Horizontal Transfer of Antibiotic Resistance Genes. NanoImpact 2018, 10, 61–67. [Google Scholar] [CrossRef]
- Xiu, W.; Gan, S.; Wen, Q.; Qiu, Q.; Dai, S.; Dong, H.; Li, Q.; Yuwen, L.; Weng, L.; Teng, Z.; et al. Biofilm Microenvironment-Responsive Nanotheranostics for Dual-Mode Imaging and Hypoxia-Relief-Enhanced Photodynamic Therapy of Bacterial Infections. Research 2020, 2020, 9426453. [Google Scholar] [CrossRef]
- Hu, Y.; Ruan, X.; Lv, X.; Xu, Y.; Wang, W.; Cai, Y.; Ding, M.; Dong, H.; Shao, J.; Yang, D.; et al. Biofilm Microenvironment-Responsive Nanoparticles for the Treatment of Bacterial Infection. Nano Today 2022, 46, 101602. [Google Scholar] [CrossRef]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor Microenvironment-Responsive Intelligent Nanoplatforms for Cancer Theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, S.; Liu, Y.; Yin, W.; Gu, Z.; Zhao, Y. An Overview of the Use of Nanozymes in Antibacterial Applications. Chem. Eng. J. 2021, 418, 129431. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef]
- Dingman, R.; Balu-Iyer, S.V. Immunogenicity of Protein Pharmaceuticals. J. Pharm. Sci. 2019, 108, 1637–1654. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Beyssac, E.; Garrait, G.; Hsein, H.; Cury, B.S.F. Retrograded Starch/Pectin Coated Gellan Gum-Microparticles for Oral Administration of Insulin: A Technological Platform for Protection against Enzymatic Degradation and Improvement of Intestinal Permeability. Eur. J. Pharm. Biopharm. 2018, 123, 84–94. [Google Scholar] [CrossRef]
- Cornwell, S.E.; Okocha, S.O.; Ferrari, E. Multivariate Analysis of Protein–Nanoparticle Binding Data Reveals a Selective Effect of Nanoparticle Material on the Formation of Soft Corona. Nanomaterials 2023, 13, 2901. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wei, L.; Ye, J. Advancements in Mitochondrial-Targeted Nanotherapeutics: Overcoming Biological Obstacles and Optimizing Drug Delivery. Front. Immunol. 2024, 15, 1451989. [Google Scholar] [CrossRef]
- McCarthy, D.P.; Hunter, Z.N.; Chackerian, B.; Shea, L.D.; Miller, S.D. Targeted Immunomodulation Using Antigen-conjugated Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2014, 6, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Mugundhan, S.L.; Mohan, M. Nanoscale Strides: Exploring Innovative Therapies for Breast Cancer Treatment. RSC Adv. 2024, 14, 14017–14040. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ju, D.; Zeng, X. Mechanisms and Clinical Implications of Human Gut Microbiota-Drug Interactions in the Precision Medicine Era. Biomedicines 2024, 12, 194. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The Influence of the Gut Microbiota on the Bioavailability of Oral Drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Abou-el-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef]
- Chaudhary, S.; Ali, Z.; Pantoja-Angles, A.; Abdelrahman, S.; Juárez, C.O.B.; Rao, G.S.; Hong, P.; Hauser, C.; Mahfouz, M. High-yield, Plant-based Production of an Antimicrobial Peptide with Potent Activity in a Mouse Model. Plant Biotechnol. J. 2024, 22, 3392–3405. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant Molecular Farming—Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef]
- McNulty, M.J.; Gleba, Y.; Tusé, D.; Hahn-Löbmann, S.; Giritch, A.; Nandi, S.; McDonald, K.A. Techno-economic Analysis of a Plant-based Platform for Manufacturing Antimicrobial Proteins for Food Safety. Biotechnol. Prog. 2020, 36, e2896. [Google Scholar] [CrossRef]
- Özakar, E.; Özakar, R.S.; Adigüzel, M.C. Boron Nitride Nanoparticles: Preparation, Characterization, Stability and Evaluation of Antibacterial Activities. J. Res. Pharm. 2024, 28, 1188–1199. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 3 September 2025).
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner, Office of Policy, Legislation, and International Affairs, Office of Policy. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology Guidance for Industry; U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2014. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology (accessed on 10 August 2024).
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Uskoković, V. Nanomedicine for The Poor: A Lost Cause or An Idea Whose Time Has Yet to Come? Nanomedicine 2021, 16, 1203–1218. [Google Scholar] [CrossRef]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled Liposomal Ciprofloxacin in Patients with Non-Cystic Fibrosis Bronchiectasis and Chronic Lung Infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two Phase 3, Randomised Controlled Trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Goteri, G.; Lucarini, G.; Kamysz, E.; Kamysz, W.; Orlando, F.; Rizzetto, G.; Molinelli, E.; Morroni, G.; et al. Efficacy of Cathelicidin LL-37 in an MRSA Wound Infection Mouse Model. Antibiotics 2021, 10, 1210. [Google Scholar] [CrossRef]
- Johansson, L.; Thulin, P.; Sendi, P.; Hertzén, E.; Linder, A.; Åkesson, P.; Low, D.E.; Agerberth, B.; Norrby-Teglund, A. Cathelicidin LL-37 in Severe Streptococcus Pyogenes Soft Tissue Infections in Humans. Infect. Immun. 2008, 76, 3399–3404. [Google Scholar] [CrossRef]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin Reduces the Risk of Respiratory Tract Infections: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin Is a Peptide Antibiotic with Therapeutic Potential from a Saprophytic Fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sharma, G.; Dang, S.; Gupta, S.; Gabrani, R. Antimicrobial Peptides as Anti-Infectives against Staphylococcus epidermidis. Med. Princ. Pract. 2016, 25, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Euler, C.W.; Jiang, W.; Nussenzweig, P.M.; Goldberg, G.W.; Duportet, X.; Fischetti, V.A.; Marraffini, L.A. Exploiting CRISPR-Cas Nucleases to Produce Sequence-Specific Antimicrobials. Nat. Biotechnol. 2014, 32, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Ates, A.; Tastan, C.; Ermertcan, S. CRISPR-Cas9-Mediated Targeting of Multidrug Resistance Genes in Methicillin-Resistant Staphylococcus aureus. Cris. J. 2024, 7, 374–384. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Klumpe, H.E.; Luo, M.L.; Selle, K.; Barrangou, R.; Beisel, C.L. Programmable Removal of Bacterial Strains by Use of Genome-Targeting CRISPR-Cas Systems. mBio 2014, 5. [Google Scholar] [CrossRef]
- Kiga, K.; Tan, X.-E.; Ibarra-Chávez, R.; Watanabe, S.; Aiba, Y.; Sato’o, Y.; Li, F.-Y.; Sasahara, T.; Cui, B.; Kawauchi, M.; et al. Development of CRISPR-Cas13a-Based Antimicrobials Capable of Sequence-Specific Killing of Target Bacteria. Nat. Commun. 2020, 11, 2934. [Google Scholar] [CrossRef]
- Kaminski, R.; Bella, R.; Yin, C.; Otte, J.; Ferrante, P.; Gendelman, H.E.; Li, H.; Booze, R.; Gordon, J.; Hu, W.; et al. Excision of HIV-1 DNA by Gene Editing: A Proof-of-Concept in Vivo Study. Gene Ther. 2016, 23, 690–695. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, L.; Zhu, D.; Ding, W.; Wang, X.; Zhang, C.; Wang, L.; Jiang, X.; Shen, H.; He, D.; et al. Disruption of HPV16-E7 by CRISPR/Cas System Induces Apoptosis and Growth Inhibition in HPV16 Positive Human Cervical Cancer Cells. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Buratto, D.; Han, P.; Yang, Z.; Zhou, R. A PH-responsive Nanoparticle Delivery System Containing Dihydralazine and Doxorubicin-based Prodrug for Enhancing Antitumor Efficacy. Aggregate 2024, 5, e434. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Mi, P.; Dewi, N.; Yanagie, H.; Kokuryo, D.; Suzuki, M.; Sakurai, Y.; Li, Y.; Aoki, I.; Ono, K.; Takahashi, H.; et al. Hybrid Calcium Phosphate-Polymeric Micelles Incorporating Gadolinium Chelates for Imaging-Guided Gadolinium Neutron Capture Tumor Therapy. ACS Nano 2015, 9, 5913–5921. [Google Scholar] [CrossRef]
- Cao, M.; Lu, S.; Wang, N.; Xu, H.; Cox, H.; Li, R.; Waigh, T.; Han, Y.; Wang, Y.; Lu, J.R. Enzyme-Triggered Morphological Transition of Peptide Nanostructures for Tumor-Targeted Drug Delivery and Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 16357–16366. [Google Scholar] [CrossRef]
- Wan, D.; Zhu, Q.; Zhang, J.; Chen, X.; Li, F.; Liu, Y.; Pan, J. Intracellular and Extracellular Enzymatic Responsive Micelle for Intelligent Therapy of Cancer. Nano Res. 2023, 16, 2851–2858. [Google Scholar] [CrossRef]
- Shakya, A.K.; Al-Sulaibi, M.; Naik, R.R.; Nsairat, H.; Suboh, S.; Abulaila, A. Review on PLGA Polymer Based Nanoparticles with Antimicrobial Properties and Their Application in Various Medical Conditions or Infections. Polymers 2023, 15, 3597. [Google Scholar] [CrossRef]
- Sivadasan, D.; Sultan, M.H.; Madkhali, O.; Almoshari, Y.; Thangavel, N. Polymeric Lipid Hybrid Nanoparticles (PLNs) as Emerging Drug Delivery Platform—A Comprehensive Review of Their Properties, Preparation Methods, and Therapeutic Applications. Pharmaceutics 2021, 13, 1291. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Rivera_Gil, P.; Montenegro, J.-M.; Braeckmans, K.; Müllen, K.; et al. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef]
- Hwang, R.; Mirshafiee, V.; Zhu, Y.; Xia, T. Current Approaches for Safer Design of Engineered Nanomaterials. Ecotoxicol. Environ. Saf. 2018, 166, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Shinde, A.K.; Tchounwou, P.B. A Comparison of Poly-Ethylene-Glycol-Coated and Uncoated Gold Nanoparticle-Mediated Hepatotoxicity and Oxidative Stress in Sprague Dawley Rats. Int. J. Nanomed. 2019, 14, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Luanda, A.; Mahadev, M.; Charyulu, R.N.; Badalamoole, V. Locust Bean Gum-Based Silver Nanocomposite Hydrogel as a Drug Delivery System and an Antibacterial Agent. Int. J. Biol. Macromol. 2024, 282, 137097. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yu, C.-H.; Yeh, Y.-C. Engineering Nanocomposite Hydrogels Using Dynamic Bonds. Acta Biomater. 2021, 130, 66–79. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-Engineered Dendrimers in Gene Delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef]
- Sharma, A.; Mejía, D.; Maysinger, D.; Kakkar, A. Design and Synthesis of Multifunctional Traceable Dendrimers for Visualizing Drug Delivery. RSC Adv. 2014, 4, 19242–19245. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle–Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Q.; Ahmad, M.; Usman Minhas, M. Hydroxypropyl-β-cyclodextrin Hybrid Nanogels as Nano-drug Delivery Carriers to Enhance the Solubility of Dexibuprofen: Characterization, in Vitro Release, and Acute Oral Toxicity Studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting Cancer Cells with Nanotherapeutics and Nanodiagnostics: Current Status and Future Perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef]
- Liang, H.; Liu, H.; Tian, B.; Ma, R.; Wang, Y. Carbon Quantum Dot@Silver Nanocomposite–Based Fluorescent Imaging of Intracellular Superoxide Anion. Microchim. Acta 2020, 187, 484. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chen, Y.-J.; Kang, C.-H.; Lin, H.-Y.; Huang, C.-C.; Hsu, P.-H.; Lin, H.-J. Toxic or Not Toxic, That Is the Carbon Quantum Dot’s Question: A Comprehensive Evaluation with Zebrafish Embryo, Eleutheroembryo, and Adult Models. Polymers 2021, 13, 1598. [Google Scholar] [CrossRef]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced Nanoparticles in Combating Antibiotic Resistance: Current Innovations and Future Directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Ellington, M.J.; Ekelund, O.; Aarestrup, F.M.; Canton, R.; Doumith, M.; Giske, C.; Grundman, H.; Hasman, H.; Holden, M.T.G.; Hopkins, K.L.; et al. The Role of Whole Genome Sequencing in Antimicrobial Susceptibility Testing of Bacteria: Report from the EUCAST Subcommittee. Clin. Microbiol. Infect. 2017, 23, 2–22. [Google Scholar] [CrossRef]
- Hasman, H.; Saputra, D.; Sicheritz-Ponten, T.; Lund, O.; Svendsen, C.A.; Frimodt-Møller, N.; Aarestrup, F.M. Rapid Whole-Genome Sequencing for Detection and Characterization of Microorganisms Directly from Clinical Samples. J. Clin. Microbiol. 2014, 52, 139–146. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- The 100,000 Genomes Project Pilot Investigators. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care—Preliminary Report. N. Engl. J. Med. 2021, 385, 1868–1880. [Google Scholar] [CrossRef]
- Miotto, P.; Cirillo, D.M.; Migliori, G.B. Drug Resistance in Mycobacterium Tuberculosis. Chest 2015, 147, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-Chip Platforms for Studying Drug Delivery Systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, R.; Mashhadi Kashtiban, M.; Taghvaei, H.; Zolfagharian, A.; Bodaghi, M. 3D-Printed Microfluidic Droplet Generation Systems for Drug Delivery Applications. Mater. Today Proc. 2022, 70, 443–446. [Google Scholar] [CrossRef]
- Arango-Argoty, G.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A Deep Learning Approach for Predicting Antibiotic Resistance Genes from Metagenomic Data. Microbiome 2018, 6, 23. [Google Scholar] [CrossRef]
- de Nies, L.; Lopes, S.; Busi, S.B.; Galata, V.; Heintz-Buschart, A.; Laczny, C.C.; May, P.; Wilmes, P. PathoFact: A Pipeline for the Prediction of Virulence Factors and Antimicrobial Resistance Genes in Metagenomic Data. Microbiome 2021, 9, 49. [Google Scholar] [CrossRef]
- Anunobi, O.O. Pharmacogenomics as a Tool in Addressing Genetic VariationDependent Adverse Drug Reactions. Dutse J. Pure Appl. Sci. 2024, 10, 37–54. [Google Scholar] [CrossRef]
- Torres, M.D.T.; de la Fuente-Núñez, C.; Silva, G.G.O.; Franco, O.L. Antimicrobial Peptides. U.S. Patent 12,116,387, 31 December 2024. [Google Scholar]
- Zheng, J.; Knolhoff, A.M.; Brown, E.W.; Croley, T.R. Antimicrobial Peptides, Pharmaceutical Compositions, and Methods of Use Thereof. U.S. Patent 10,906,940, 2 February 2021. [Google Scholar]
- Bikard, D.; Marraffini, L. Sequence Specific Antimicrobials. U.S. Patent 11,491,210, 22 November 2022. [Google Scholar]
- Harper, D. Beneficial Effects of Bacteriophage Treatments. U.S. Patent 8,475,787, 2 July 2013. [Google Scholar]
- ClinicalTrials.gov. U.S. National Library of Medicine: Clinical Trials Database. 2024. Available online: https://clinicaltrials.gov (accessed on 16 September 2025).
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Ali Agha, A.S.A.; Al-Samydai, A.; Aburjai, T. New Frontiers in CRISPR: Addressing Antimicrobial Resistance with Cas9, Cas12, Cas13, and Cas14. Heliyon 2025, 11, e42013. [Google Scholar] [CrossRef]
- Hejabi, F.; Abbaszadeh, M.S.; Taji, S.; O’Neill, A.; Farjadian, F.; Doroudian, M. Nanocarriers: A Novel Strategy for the Delivery of CRISPR/Cas Systems. Front. Chem. 2022, 10, 957572. [Google Scholar] [CrossRef]
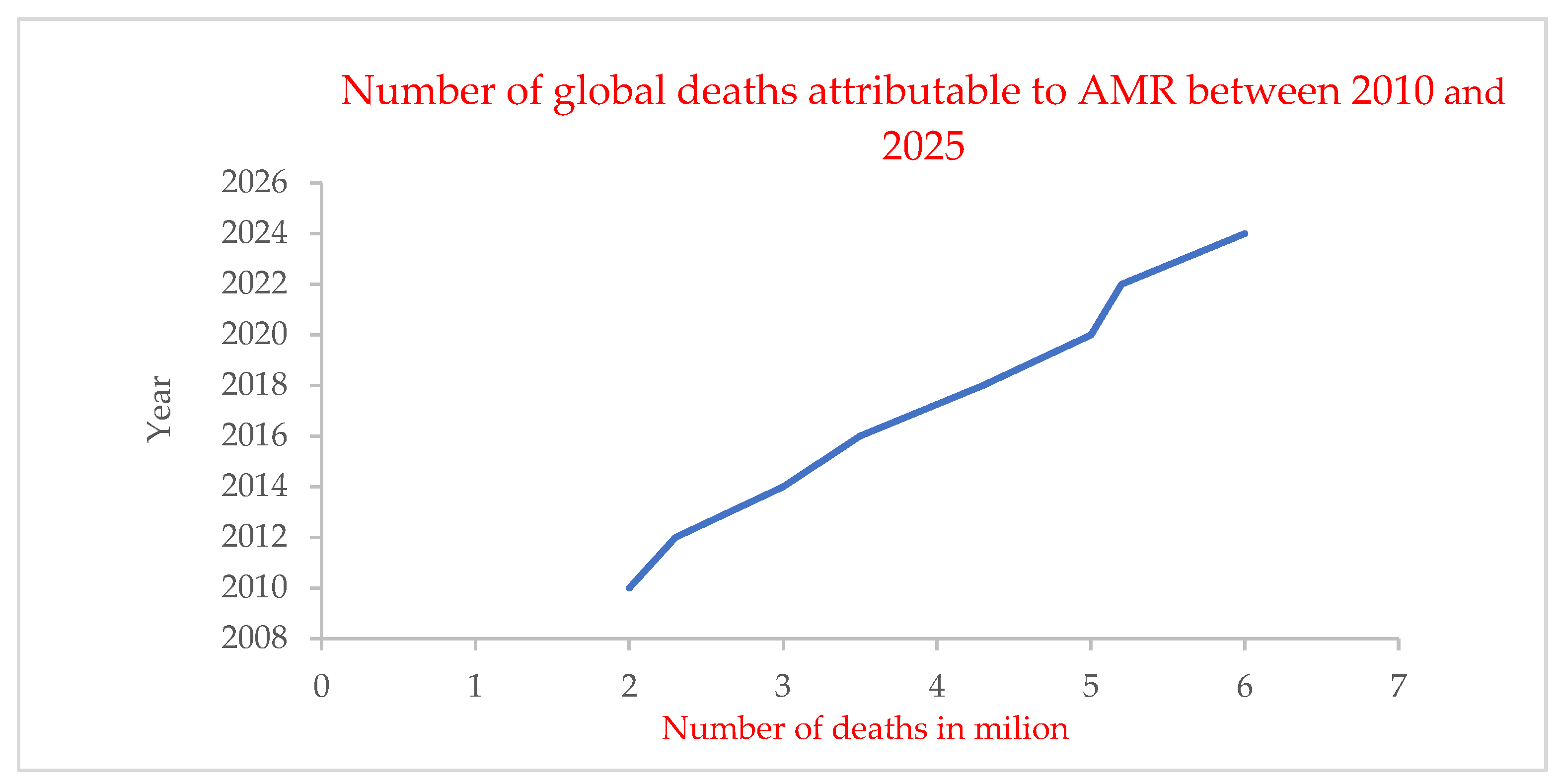
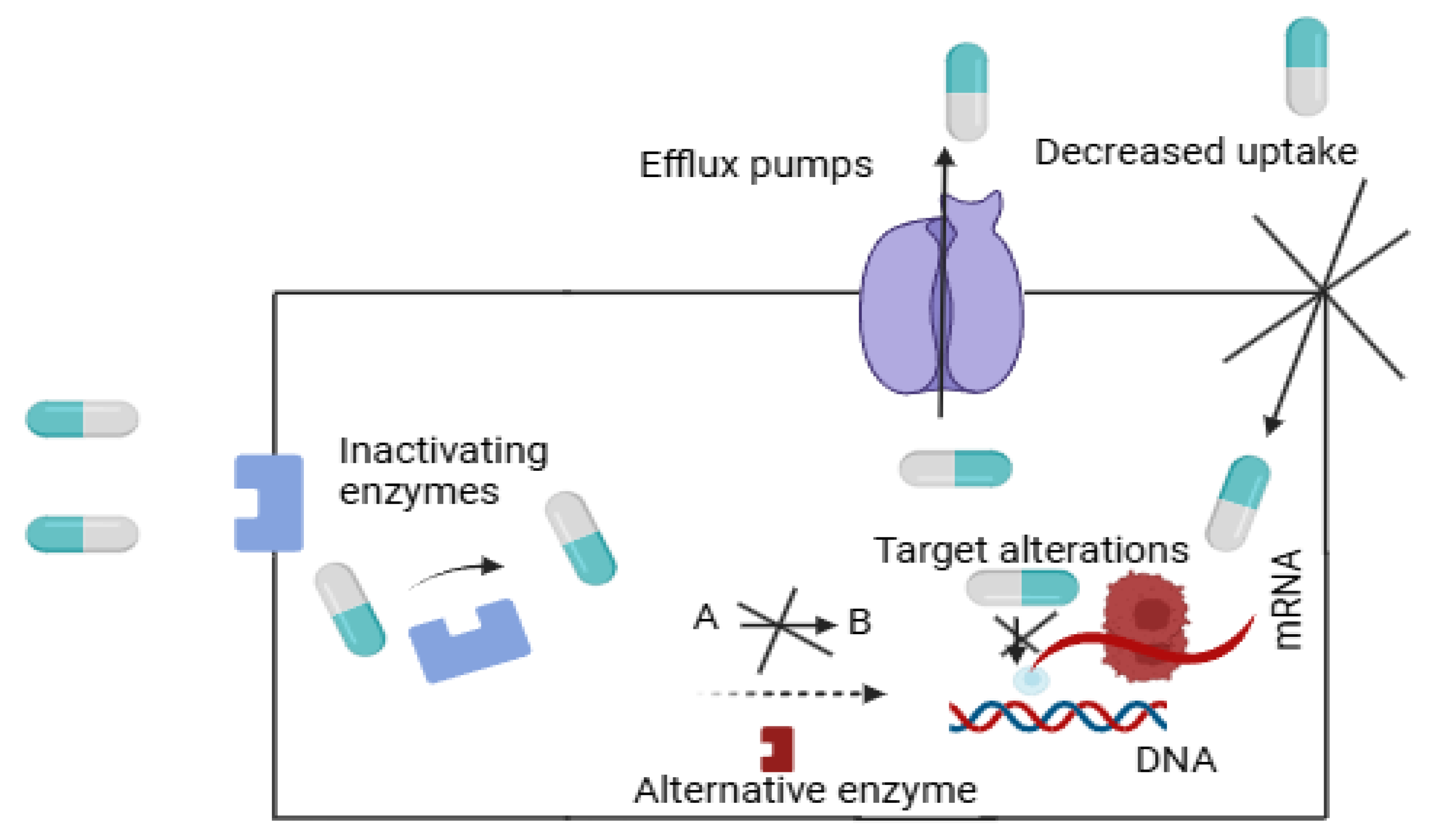
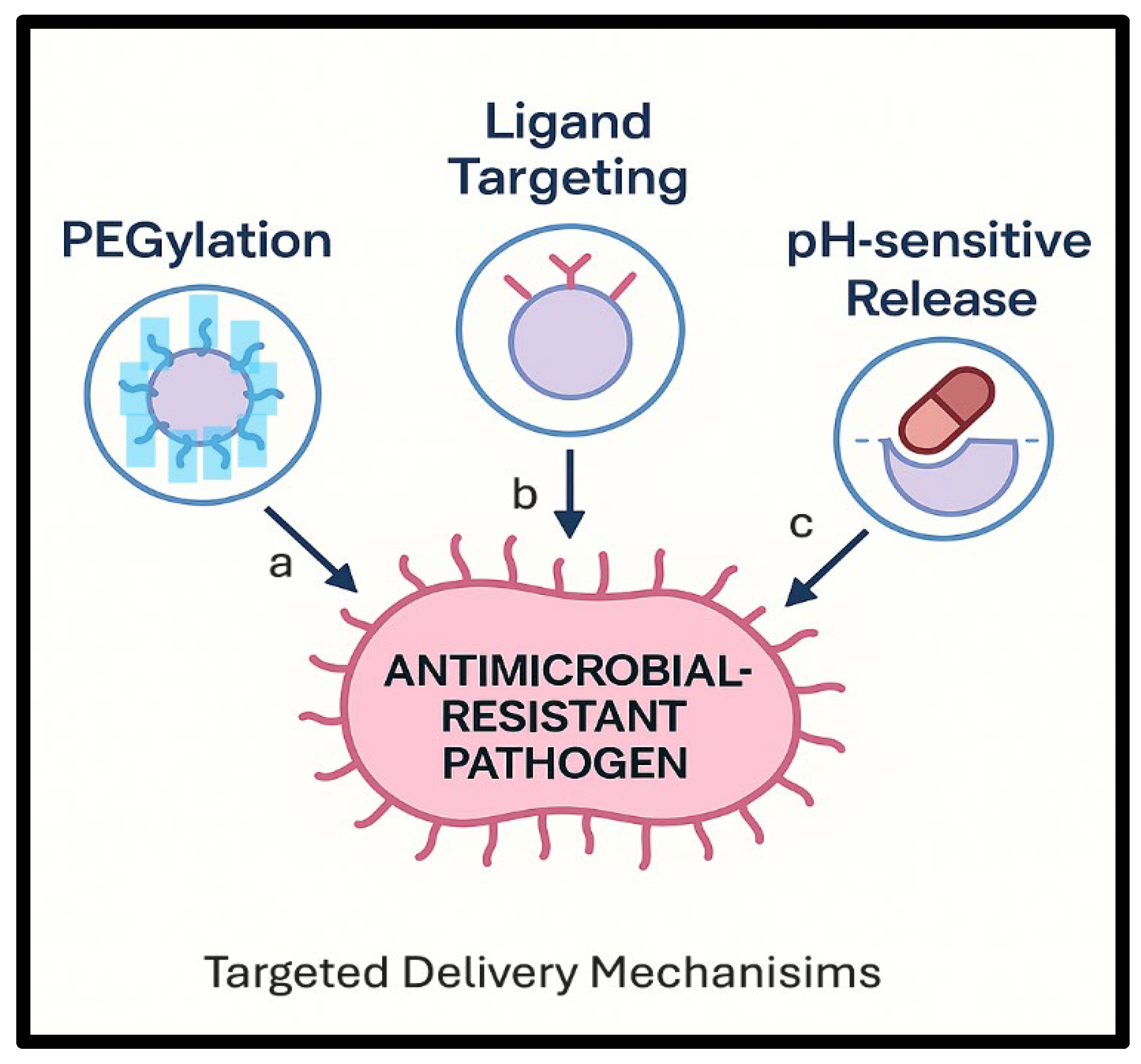
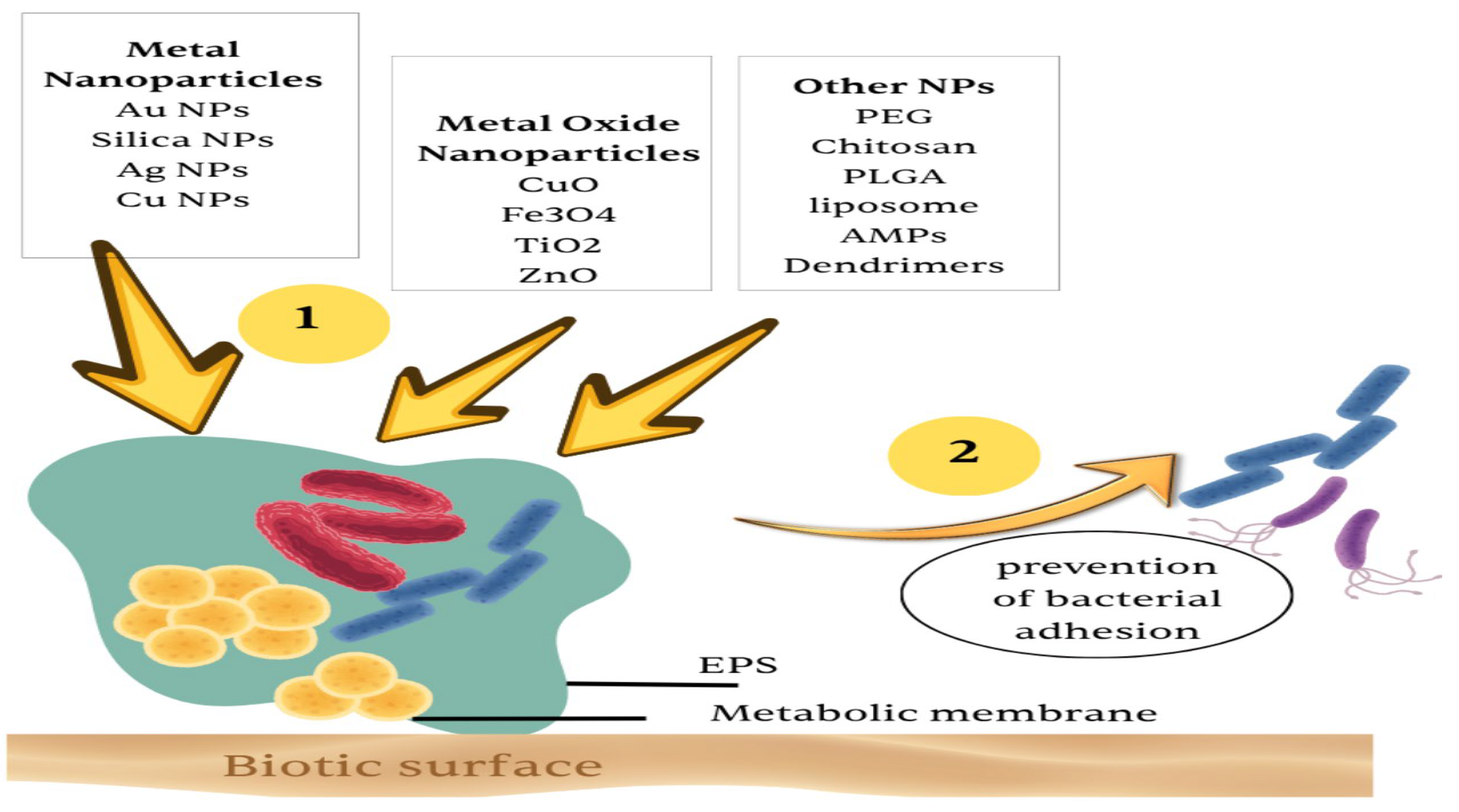

| Stimulus Type | Trigger Mechanism | Target Environment | Example/Application |
|---|---|---|---|
| pH-responsive | Structural change or degradation at lower pH | Acidic sites such as inflammatory zones, tumors, biofilms, and oral cavities | Treatment of A. baumannii infection; prevention of gingivitis and cavities [158,159]. |
| Temperature-responsive | Drug release triggered by local or external temperature increase | Inflammatory sites (local heat) or externally heated areas | Heat-induced drug release at specific sites [156]. |
| Enzyme-responsive | Activation via enzymes overexpressed in infection (e.g., bacterial lipases) | Infected tissues with high enzyme expression | Lipase-sensitive delivery for Gram-negative and Gram-positive bacterial infections [75]. |
| Targeted Drug Delivery (TDD) | Advantages | Disadvantages | TDD and Its AMR Mechanism | Examples of Treatment/Diagnosis of Infections |
|---|---|---|---|---|
| Nanoparticle-based systems | Site targeted delivery High loading stability Biocompatibility Rapid AMP release | Complex synthesis procedures. Rapid clearance by the immune system. Aggregation and degradation | Penetrate membrane and target DNA/enzymes/metabolism, alter permeability, alter adhesion and inhibit biofilm | Colistin-loaded liposomes (lower systemic toxicity and higher survival rate of mice infected with Pseudomonas aeruginosa [136,137] |
| Stimuli-responsive systems | Targeted release at acidic pH, elevated temperature, or enzyme-rich sites. Stable at physiological pH | Complex synthesis, poor reproducibility. The pH/temperature difference between tumor and normal tissue may be too small for precise control | pH-responsive carriers disintegrate and degrade in the acidic microenvironment (inflammation, tumors, biofilms) on site and release the enzyme-responsive (lipase-sensitive) drug where bacterial enzymes are present | pH-responsive carriers for A. baumannii infection control [159] |
| Bacteriophage-Based Delivery | Synergy with antibiotics Fewer phage/antibiotic resistance mutants Can carry drugs/NPs/genes. Can re-sensitize resistant bacteria | Needs further safety profiling and clinical standards | Direct bactericidal lysis phage-derived enzymes degrade bacterial polysaccharides, leading to improved antibiotic penetration. Combined thereby activates biofilm destruction and bacterial eradication | Phage + daptomycin markedly increase killing of E. faecium and decrease resistant mutants [161] Re-sensitization of colistin resistance after phage exposure [161]. Phages act as delivering tools for genes/antibiotic/NPs [77] |
| Antibody–Drug Conjugates | Pathogen-specific targeting (protecting host microbiota), reduced potential for resistance High antibacterial activity Extended half-lives Activity against Gram-positive bacteria and S. aureus | Poor efficiency for some infections, very costly manufacturing, large scale capacity needed | Antibody-guided delivery of chemotherapeutic/antibiotics In some cases DSTA4637 outperforms antibiotics in preclinical studies. Gene delivery vectors proposed for mAb production/delivery | DSTA4637 against S. aureus was better than vancomycin [163,173] Effective antibodies against Clostridium difficile [169] |
| Peptide- and Protein-Based Delivery Systems | Natural biocompatibility Low toxicity enables broad-spectrum activity High target specificity effectively combating biofilms and reducing resistance risk when used with antibiotics and nanocarriers. | Short half-life, environmental sensitivity, low bioavailability, potential immune reactions, high production costs, and the need for extensive safety testing | Antimicrobial peptides (AMPs) bypass bacterial defenses by targeting membranes, creating pores, inhibiting efflux pumps, disrupting biofilms, and remaining effective against enzyme alteration | LL-37 for skin and soft tissue infections caused by MRSA and S. pneumoniae [241,242]. Lactoferrin has a promising efficacy for respiratory tract infections caused by many viruses, including SARS-CoV-2 [243]. Plectasin as a treatment for abdominal infections caused by Streptococci [244]. CP10A is a derivative of Indolicidin and has antimicrobial activity against S. epidermidis for the prevention of prosthetic device infections and biofilms [245] |
| CRISPR-Cas Systems | The tool efficiently targets specific DNA or RNA in various pathogens, eliminates antibiotic resistance genes, minimizes microbiome disruption, and can be easily adapted for emerging threats | Off-target cleavage may occur due to design errors and delivering CRISPR to infection sites. Limited efficacy in systemic infections, potential immune reactions, microbial resistance development, and biosafety and ethical concerns | CRISPR gene editing can target resistance genes, disrupt mutated drug targets, reduce drug efflux, and enhance antibiotic entry | CRISPR-Cas9 antimicrobials can be potentially used for skin infections caused by S. aureus [246]. Very promising in treatment of MRSA by targeting resistant genes like mecA, aacA, and grlA and grlB [247]. Eliminating Gram-negative bacteria from mixed cultures like E. coli and S. enterica through targeting chromosomal genes essential for metabolism and cell division [248] Treatment and diagnosis of MDR E. coli by targeting carbapenem and colistin resistant genes [249]. Potential treatment of HIV virus by excision of HIV-1 DNA from the genomes of infected people [250]. Potential treatment of HPV virus by disrupting the HPV16-E7 gene with the which can trigger apoptosis and inhibiting the growth of HPV16-positive cervical cancer cells [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alidriss, O.M.; AlSudais, H.; Alhumaidan, O.S.; Altwaijry, H.D.; Bakhsh, A.; Almuhanna, Y.; Alkudmani, Z.S.; Alqarni, I.A.; Alenazi, D.; Aljasham, A.T.; et al. Targeted Drug Delivery Strategies in Overcoming Antimicrobial Resistance: Advances and Future Directions. Pharmaceutics 2025, 17, 1426. https://doi.org/10.3390/pharmaceutics17111426
Alidriss OM, AlSudais H, Alhumaidan OS, Altwaijry HD, Bakhsh A, Almuhanna Y, Alkudmani ZS, Alqarni IA, Alenazi D, Aljasham AT, et al. Targeted Drug Delivery Strategies in Overcoming Antimicrobial Resistance: Advances and Future Directions. Pharmaceutics. 2025; 17(11):1426. https://doi.org/10.3390/pharmaceutics17111426
Chicago/Turabian StyleAlidriss, Ohoud M., Hamood AlSudais, Ohoud S. Alhumaidan, Haifa D. Altwaijry, Afnan Bakhsh, Yasir Almuhanna, Zeina S. Alkudmani, Ibrahim A. Alqarni, Daheeya Alenazi, Alanoud T. Aljasham, and et al. 2025. "Targeted Drug Delivery Strategies in Overcoming Antimicrobial Resistance: Advances and Future Directions" Pharmaceutics 17, no. 11: 1426. https://doi.org/10.3390/pharmaceutics17111426
APA StyleAlidriss, O. M., AlSudais, H., Alhumaidan, O. S., Altwaijry, H. D., Bakhsh, A., Almuhanna, Y., Alkudmani, Z. S., Alqarni, I. A., Alenazi, D., Aljasham, A. T., & Jamous, Y. F. (2025). Targeted Drug Delivery Strategies in Overcoming Antimicrobial Resistance: Advances and Future Directions. Pharmaceutics, 17(11), 1426. https://doi.org/10.3390/pharmaceutics17111426






