1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system, driven by a breakdown in immune tolerance that leads to inflammation, demyelination, and neurodegeneration. This dysregulation is marked by an expansion of autoreactive Th1 and Th17 cells and a concurrent reduction in regulatory T and B cell subsets [
1,
2,
3]. While current disease-modifying therapies (DMTs) effectively suppress inflammation, they target downstream immune responses and often cause broad immunosuppression, increasing the risk of infections and other serious adverse events [
4,
5].
The clinical management of MS typically follows one of two paradigms: an escalation approach starting with lower-risk therapies and progressing to more potent treatments as needed, or an induction/maintenance approach using high-efficacy therapies early to establish disease control before transitioning to a maintenance regimen [
6]. Approved DMTs span multiple mechanisms, including immunomodulators (e.g., glatiramer acetate, dimethyl fumarate), immune cell-depleting agents (e.g., alemtuzumab, ocrelizumab), anti-proliferative agents (e.g., teriflunomide), and migration inhibitors (e.g., natalizumab, fingolimod) [
7]. Despite their diversity, these therapies share a limitation: they act downstream of the root immunological defect, loss of tolerance and their use is often limited by tolerability and safety concerns, including risks of infections and other serious adverse events often leading to discontinuation and switching between different therapeutic classes [
4,
5].
Given these limitations, there is an unmet medical need in the clinical management of MS and other autoimmune diseases to address the root cause of autoimmunity, the loss of immune tolerance, without inducing broad immunosuppression. One promising approach is the induction of immune tolerance.
Antigen-specific immune tolerance strategies have shown promise in both preclinical and clinical settings [
8,
9]. However, its practical application has been hampered by the requirement to present disease-relevant antigens, which in many autoimmune diseases are often multiple and poorly defined, thus complicating the implementation of antigen-specific therapies.
The Tim family of receptors, including Tim-3 and Tim-4, plays a central role in maintaining immune homeostasis by regulating inflammation and promoting tolerance across autoimmune, allergic, and transplant settings. Tim-3, in particular, engages ligands such as galectin-9 and phosphatidylserine to suppress pathogenic Th1/Th17 responses and enhance regulatory T cell (Treg) function. Blockade of Tim-3 exacerbates disease in models like EAE, underscoring its role as a negative regulator of immune activation. Both Tim-3 and Tim-4 contribute to the expansion of regulatory T and B cells, supporting the use of Tim receptor agonism as a non-antigen-specific strategy for inducing immune tolerance in autoimmune diseases such as multiple sclerosis (MS) [
10,
11,
12,
13].
We have previously reported on the use of LPX3, a T cell immunoglobulin and mucin domain (Tim) family receptors agonist, to induce antigen-specific tolerance toward AAV capsid proteins in the context of gene therapy [
14] and to promote antigen-agnostic immune tolerance in MOG
35–55 induced EAE mouse models [
15].
Here, we present LPX-TI641, a novel, orally bioavailable, clinical-stage small-molecule agonist of Tim-3 and Tim-4 that is more potent than LPX3. We evaluated LPX-TI641 in vitro and in vivo, assessing its ability to expand CD4+Foxp3+ regulatory T cells (Tregs), restore Tim-3+ Tregs in peripheral blood mononuclear cells (PBMCs) from people with MS (PwMS), and improve suppressive Treg function. In EAE models, LPX-TI641 significantly reduced clinical scores and prevented relapses. These findings support the therapeutic potential of LPX-TI641 as a non-antigen-specific immune tolerance strategy for MS and related autoimmune conditions.
2. Materials and Methods
2.1. Docking Simulations
2.1.1. Preparation for Docking
The co-crystal structure of the Tim protein bound to its endogenous ligand, dicaproyl-phosphatidylserine (PS) was used for docking simulations. The ligand (PS) was removed from the binding site to allow docking of the proposed molecules. All water molecules were removed except for one, which was essential for forming a hydrogen bond bridge between PS and the protein. Charges for all atoms in the protein were calculated at a physiological pH of 7.4, matching the conditions used in the in vitro experiments. Similarly, charges for all docked molecules were also computed at the same physiological pH. Docking was performed using rigid binding, and the top 20 binding poses for each molecule were identified for further analysis.
2.1.2. Validation of the Docking Method
To validate the docking approach, the endogenous ligand (PS) was re-docked into the protein’s binding site after its removal. The root mean- square deviation (RMSD) of the pose that matched the native configuration was calculated and ranked to demonstrate the reliability of the docking procedure.
2.1.3. Docking of Experimentally Tested and Proposed Structures
After validating the docking method, several compounds (PS, LPX-TI641 and LPX3) were docked into the Tim receptor’s binding site using rigid docking methods. The binding affinity of the best-scoring pose (ΔG of binding in kcal/mol) was recorded. Based on the ΔG values, theoretical dissociation constants (Kd) were calculated at 298 K (250C).
2.2. Institutional Animal Care and Use Committee
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at CRADL Charles River, Boston, MA (protocol number EB17-029-303). Mice were housed under a 12-h light/dark cycle with ad libitum access to food and water.
2.3. Animals
Female C57BL/6, C57BL/6 Foxp3GFP, SJL, or CD1 mice (7–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA) and maintained under specific pathogen-free (SPF) conditions at the LAPIX Therapeutics animal facility, managed by CRADL Charles River.
2.4. Phosphatidylserine (PS) Liposome Preparation
PS liposomes were prepared using the thin-film hydration method. PS was dissolved in chloroform and mixed with DMPC (Avanti Polar Lipids, Alabaster, AL, USA) at a 30:70 molar ratio in a round-bottom flask. The solvent was evaporated under vacuum using a rotary evaporator to form a lipid film. The film was hydrated with PBS, followed by extrusion through a 0.1 µm filter to generate uniform liposomes.
2.5. In Vitro Pharmacology
2.5.1. Dose–Response Relationship
Splenocytes from C57BL/6 mice were cultured in complete DMEM supplemented with 10% FBS, 1% penicillin–streptomycin, 1% L-glutamine, and 0.1% 2-mercaptoethanol (ThermoFisher Scientific, Waltham, MA, USA). Cells were seeded in round-bottom 96-well plates (1 × 106 cells/mL). Cells were treated with increasing concentrations of LPX-TI641 (1–600 nM) or PS liposomes (1–600 nM). Cells were cultured for five days at 37 °C, 5% CO2, and analyzed for immune responses.
2.5.2. Tim-3 and Tim-4 Blocking Studies
Splenocytes from C57BL/6 mice were pre-incubated with 20 µg/mL α-Tim-3 (clone 8B.2C12, ThermoFisher Scientific, Waltham, MA, USA) or 20 µg/mL α-Tim-4 (clone RMT4-54, ThermoFisher Scientific) before treatment with increasing concentration of LPX-TI641 as above. Cells were cultured for five days at 37 °C, 5% CO2, and analyzed for immune responses.
2.5.3. T-Reg Suppressive Assay
T-regs and CD8+ T cells were isolated using magnetic bead-based kits from StemCell Technologies (Vancouver, BC, USA). T-regs were obtained from splenic C57Bl/6 CD45.1 mice treated with either a single 10 µg subcutaneous (SC) injection of LPX-TI641, from mice administered 500 ng/day of IL-2 intraperitoneally (IP) for 5 consecutive days, or mice administered PBS as control. CD8+ T cells were isolated from spleens of C57Bl/6 CD45.2 mice and labeled with CellTrace Violet, following the manufacturer’s protocol. For co-culture experiments, T-regs and CD8+ T cells were combined at ratios of 1:1, 1:2, 1:4, 1:8, 1:16 and 1:32 of T-reg: CD8 in the presence of 1 µg/mL α-CD3 and 10 µg/mL α-CD28 antibodies. Co-cultures were maintained for 3 days in complete DMEM supplemented with 10% FBS, 2 mM L-glutamine, and 100 U/mL penicillin–streptomycin. At the end of the culture period, cells were washed and stained with CD45.2, CD8, and a viability dye. CD8+ T cell proliferation was evaluated by measuring the dilution of CellTrace Violet using flow cytometry.
2.6. Pharmacokinetics and In Vivo Dose Response Studies
2.6.1. Pharmacokinetics Studies of LPX-TI641
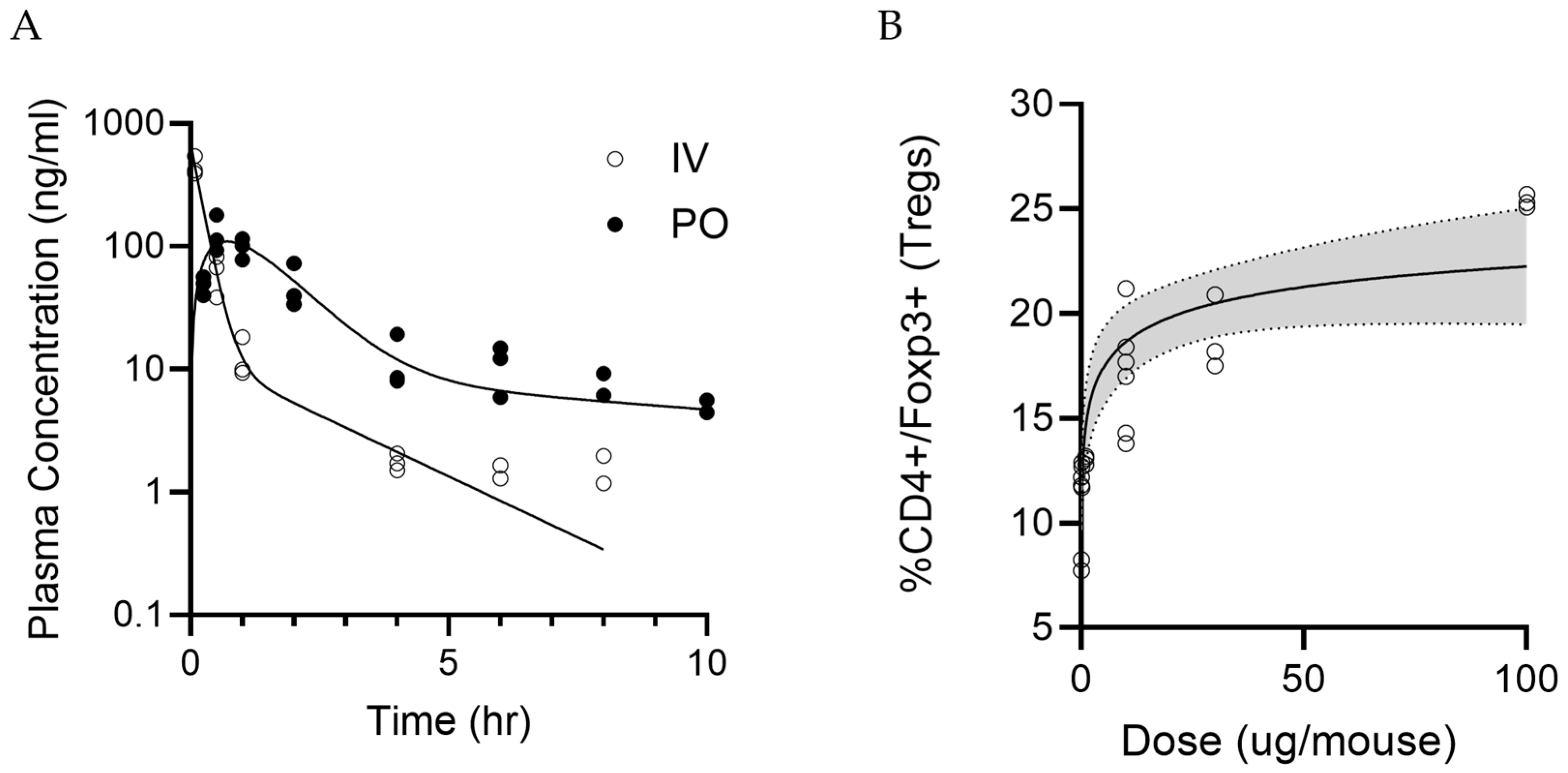
CD1 mice were divided into two groups and dosed with 0.15 mg/kg of LPX-TI641 intravenously (IV, n = 27) or 0.5 mg/kg orally (PO, n = 24). PK samples were collected at predetermined time points: 0.085, 0.25, 0.5, 1, 2, 4, 6, 8, and 10 h post-dose for the IV group, and 0.25, 0.5, 1, 2, 4, 6, 8, and 10 h post-dose for the PO group (n = 3 per time point).
Plasma concentrations were measured using a qualified LC-MS/MS method. Non-compartmental analysis (NCA) was performed using Phoenix (version 8.3.4.295) to obtain AUClast and AUC∞. The dosing option was set to “Extravascular” for PO and “IV Bolus” for IV. Linear trapezoidal interpolation and uniform weighting were applied. Bioavailability was calculated as the dose-corrected ratio of AUCs for PO versus IV.
2.6.2. Dose–Response Data for LPX-TI641 in C57BL/6 Mice
Female C57BL/6 mice (n = 3 per group) were housed under standard conditions and received a single SC dose of 0.01, 0.1, 1, 10, 30 or 100 µg of LPX-TI641 per mouse. Five days post-dose, animals were euthanized, and spleens were collected for T-cell phenotyping. Spleens were prepared into a single-cell suspension and were stained for viability, TCR, CD4, and Foxp3. Cells were gated for TCR+CD4+ and percent CD4+Foxp3+ T-cells were evaluated using flow cytometry.
Dose–response data were analyzed using four-parameter logistic regression in R (version 4.5.0) with the drc package (version 3.0-1) to estimate ED50 and ED90 values.
2.7. Experimental Autoimmune Encephalomyelitis (EAE)
2.7.1. Dose-Ranging Studies in High Dose MOG35–55 EAE Model
EAE was induced in mice by subcutaneous immunization with 100 µg of MOG35–55 peptide (R&D Systems, Minneapolis, MN, USA, cat# 25681) emulsified in Complete Freund’s Adjuvant (CFA; ThermoScientific, cat# 77140). Pertussis toxin (200 ng/mouse; List Biological Labs, Campbell, CA, USA, cat# NC0830484) was administered intraperitoneally on day 0 and day 2.
Clinical scoring began on day 7 and continued daily until study completion using the following scoring system: 0: No clinical signs, 1: Tail paralysis, 2: Hind limb weakness or loss of righting reflex. Alternatively, the mouse appears normal (score 0) but exhibits obvious head tilting during walking, indicating poor balance, 2.5: One hind limb paralyzed, 3: Two hind limbs paralyzed, 3.5: Two hind limbs paralyzed and one front limb paralyzed, 4: Quadriplegia, 5: Found dead in cage.
Once mice reached a clinical score of 1, they were randomized into treatment groups (
n = 8/group) using a random number generator. Treatment groups included vehicle control, glatiramer acetate (100 µg SC daily; MedChem Express, Monmouth Junction, NJ, USA, cat# HY-109520), natalizumab (1.5 mg IV at disease onset and again on day 3; MedChem Express, cat# HY-108831), and several LPX-TI641 regimens as detailed in (
Figure 1).
Selected mice were sacrificed and perfused with PBS at the end of study. Spinal cords were processed for histological analysis using Luxol Fast Blue staining to assess myelin integrity and neuronal structure as described in [
16].
2.7.2. Dose–Response Studies in PLP139-151 EAE Model
Mice were immunized subcutaneously with 100 µg of PLP139–151 peptide (R&D Systems, cat# 2567/1) emulsified in Complete Freund’s Adjuvant (CFA; ThermoScientific, cat# 77140). Clinical signs developed between days 9 and 12. Daily monitoring and clinical scoring began on day 7 and continued throughout the study. On day 15, when the average clinical score reached ≤ 2.5, mice were randomized into five treatment groups: control (n = 9), natalizumab (n = 14; 0.1 mg IV on days 15, 18, and 21), natalizumab switch (n = 9; 0.1 mg IV on day 15 followed by 3 µg LPX-TI641 subcutaneously once daily from days 18 to 26), LPX-TI641 (n = 15; 3 µg SC daily from days 15 to 26), and LPX-TI641 (n = 10; 1 µg SC daily from days 15 to 26).
On day 21, one mouse per group (selected based on average clinical score) was sacrificed for brain and spleen collection. Brain tissues were processed into single-cell suspensions for flow cytometry analysis.
2.7.3. Efficacy in an Escalation Treatment Paradigm vs. Natalizumab and DMF
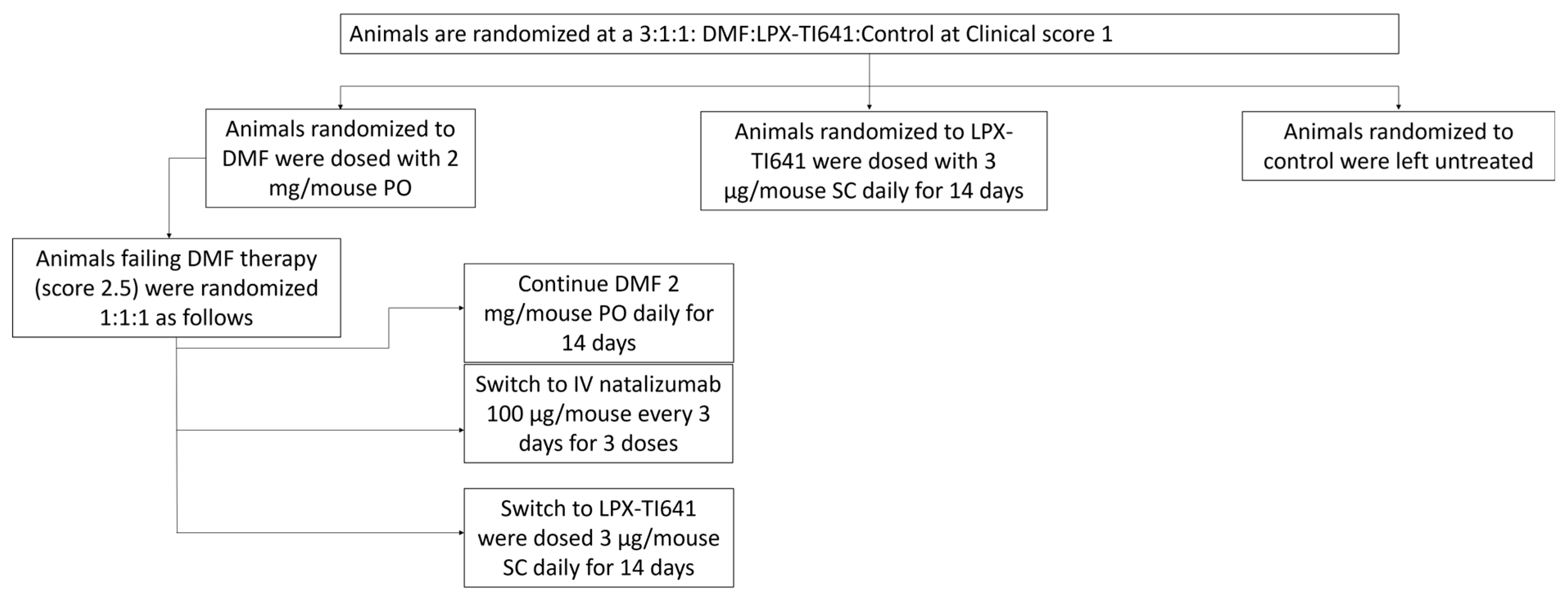
EAE was induced in mice as previously described. Daily clinical scoring began on day 7 and continued throughout the study, with symptom onset occurring between days 9 and 14. Once mice reached a clinical score of 1, they were randomized at a 3:1:1 ratio into: DMF (2 mg PO QD, Sigma, cat# 64625-100G-F), LPX-TI641 (sustained-use treatment groups, 3 µg SC QD for 14 days), or control as shown in
Figure 2.
Within the DMF group, animals that failed treatment—defined as receiving a clinical score of 2.5—were further randomized at a 1:1:1 ratio into an escalation cohort. These mice either continued DMF (2 mg/mouse PO QD for 14 days), switched to natalizumab (100 µg/mouse IV every three days for three doses; MedChem Express, cat# HY-108831), or switched to LPX-TI641 (3 µg/mouse SC QD for 14 days) (
Figure 2).
Survival analysis included animals from all cohorts. EAE-related death (defined as a clinical score of 5) was recorded as an event (“1”), while deaths due to ulceration, sacrifice for ex vivo analysis, or survival to day 27 were censored (“0”). Survival data were analyzed using GraphPad Prism 9.
2.7.4. Efficacy in Induction/Maintenance Paradigm vs. Natalizumab and DMF
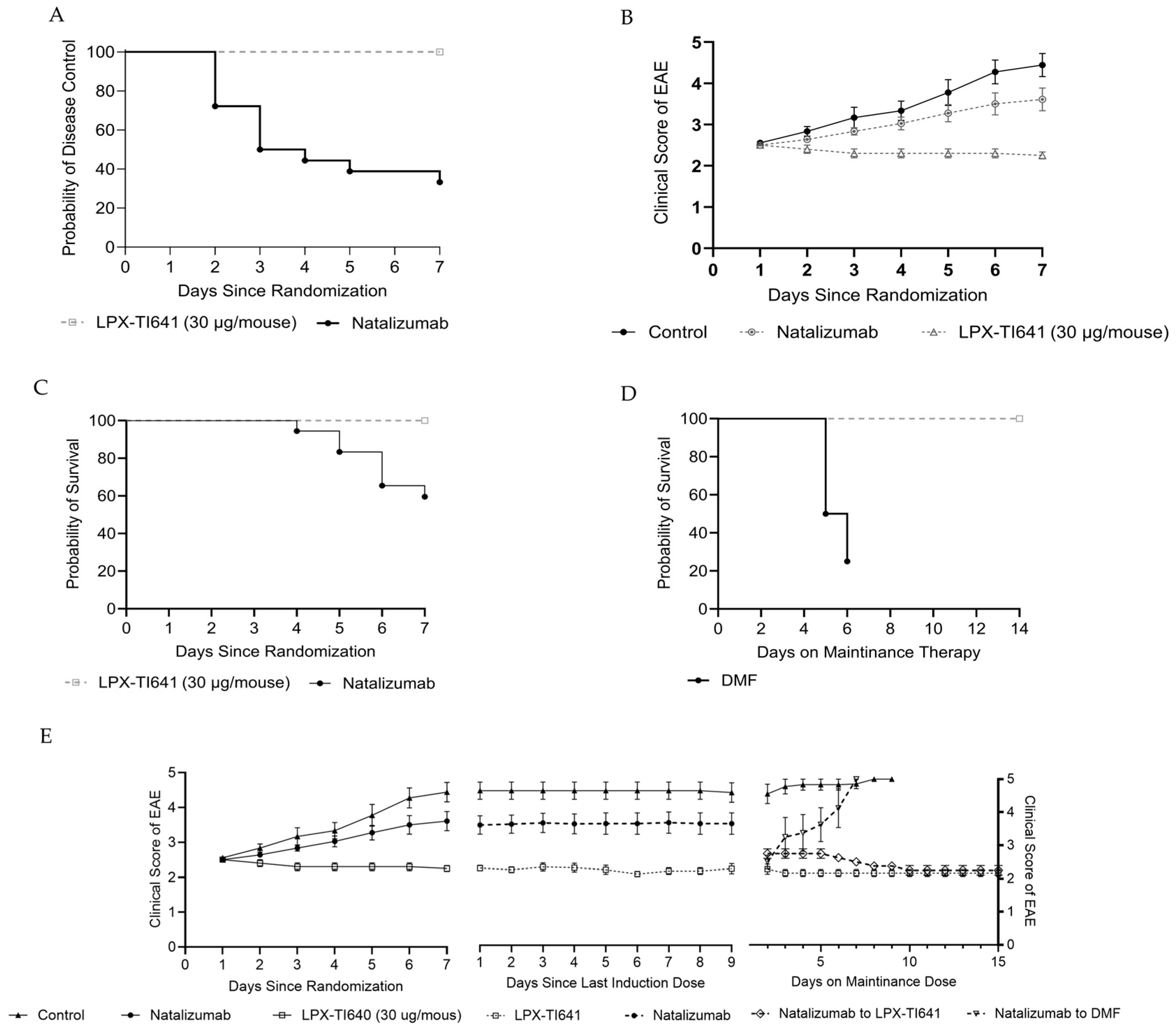
EAE was induced in mice as previously described in
Section 2.7.1. Daily clinical scoring began on day 7 and continued for the duration of the study. Symptoms typically developed between days 9 and 14. Once mice reached a clinical score of 2.5, they were randomized in a 2:1:1 ratio into natalizumab, LPX-TI641, or control groups, collectively referred to as the induction cohort (
Figure 3).
Mice in the LPX-TI641 group received 30 µg per mouse subcutaneously once daily for a maximum of 7 days or until disease control was achieved, defined as seven consecutive days without a change in clinical score. Upon achieving disease control, animals transitioned to a maintenance dose of 3 µg LPX-TI641 SC daily for 14 days. Natalizumab-treated animals received 100 µg IV every three days for a total of three doses. Once disease control was reached, they were randomized 1:1 to receive either dimethyl fumarate (2 mg/mouse orally once daily; Sigma Aldrich, cat# 242926-100G) or LPX-TI641 (3 µg/mouse SC once daily). All animals were monitored for an additional 14 days following their final dose to assess disease progression and survival outcomes (
Figure 3).
2.8. Single-Cell Preparation from Mouse Spleen and Brain
2.8.1. Spleen
Spleens were harvested under sterile conditions, crushed over a 70 µm cell strainer, and resuspended in DMEM with 10% FBS. Red blood cells were lysed with lysis buffer (Thermo Fisher Scientific) for 5 min at room temperature, and the remaining cells were washed and resuspended in complete DMEM.
2.8.2. Brain
Mice were euthanized following intracardiac perfusion with cold PBS under isoflurane anesthesia. Brains were rapidly harvested, minced, and digested with 30 U/mL collagenase B (Roche) and 1 mg/mL DNase I (Roche) at 37 °C for 30 min. The tissue was filtered through a 70 µm cell strainer, centrifuged, and processed with Percoll gradients (30%/70%) to isolate mononuclear cells for flow cytometry as described in [
17].
2.9. Immunostaining and Flow Cytometry
Cells prepared in vitro or isolated ex vivo from spleen or brain were washed and pre-incubated with anti-mouse CD16/CD32 (clone 93, ThermoFisher Scientific) for 10–20 min at 4 °C to block Fc receptors. Surface staining was performed using fluorochrome-conjugated antibodies against CD3 and CD4 (clone GK1.5) for 30 min at 4 °C. After washing with PBS, cells were incubated with Fixable Viability Dye (ThermoFisher Scientific) to assess viability. For intracellular staining, cells were fixed and permeabilized using the Transcription Factor Buffer Set (ThermoFisher Scientific) according to the manufacturer’s instructions, followed by staining for Foxp3 (clone FJK-16s). Cells were then washed, fixed, and analyzed on an Attune NxT Flow Cytometer (ThermoFisher Scientific). Data was processed using FlowJo v10 (TreeStar, Ashland, OR, USA).
2.10. PBMCs from Patient with MS (PwMS) Cultured with LPX-TI641
2.10.1. Clinical Samples
A hundred mL of whole blood from six PwMS was acquired via Sanguine Biosciences (Woburn, MA, USA) under their protocols and IRB approvals. PBMCs were collected from whole blood were isolated and drawn by Ficoll (GE Healthcare, Chicago, IL, USA) density gradient centrifugation before being used in subsequent in vitro studies.
2.10.2. PwMS-Derived PBMCs Culture with LPX-TI641
Isolated PBMCs were cultured for 5 days in the presence of increasing concentrations of LPX-TI641, with or without 100 µg/mL of myelin basic protein peptide MBP87–99. At the end of the incubation period, cells were harvested, washed, and stained for flow cytometric analysis.
2.11. Statistics
Unless otherwise specified, data are shown as either means ± standard deviation (SD) from a single representative experiment with triplicates. All statistical analyses were conducted using the GraphPad Prism program version 10. Comparisons between treatment and control groups were made using unpaired t tests. For 3 groups or more, 1- or 2-way ANOVA with multiple comparison to the control was used, correction for multiple comparison (Tukey) was used as appropriate. A p-value less than 0.05, 0.01, 0.001, or 0.0001 is shown as *, **, ***, or ****, respectively.
4. Discussion
Immune tolerance induction remains promising for the treatment of autoimmune conditions but faces challenges in practicality and scalability, especially for diseases with multiple antigens such as MS or those with undefined antigens. Most existing tolerance induction strategies rely on a priori identification of disease-relevant antigens or epitopes [
9] and subsequent administration of antigenic material in a tolerogenic context requiring complex nanoparticles or ex vivo manipulation of cells [
7,
20,
21].
In this study, we introduce LPX-TI641, an orally bioavailable, clinical stage, small-molecule agonist of Tim-3 and Tim-4 receptors that promotes immune tolerance by expanding Foxp3
+ regulatory T cells (Tregs), including the stable and highly suppressive Foxp3
+Tim3
+ subset. LPX-TI641 mimics phosphatidylserine (PS), a natural Tim-receptor ligand involved in apoptotic cell clearance and self-tolerance [
22], but exhibits markedly greater potency. In splenocyte assays, LPX-TI641 was 60-fold more potent than PS at inducing CD4
+Foxp3
+ Tregs; this effect was abrogated by Tim-3 or Tim-4 blockade, confirming receptor specificity. Moreover, Tregs from LPX-TI641-treated mice suppressed CD8
+ T-cell proliferation significantly at all tested Treg:CD8 ratios, whereas IL-2-derived Tregs were effective only at higher ratios. LPX-TI641–derived Tregs had a lower IC
50 compared to IL-2–derived or PBS-derived Tregs. These results demonstrate the superior capacity of LPX-TI641 to induce highly suppressive Tregs and support its potential as a therapeutic agent for promoting immune tolerance.
The therapeutic efficacy of LPX-TI641 was tested in two experimental autoimmune encephalomyelitis (EAE) models that mimic features of MS. In the MOG
35–55-induced EAE model, LPX-TI641 demonstrated robust symptom control in a dose-dependent manner. Animals treated with higher doses (10 µg oral QD or 3 µg SC twice weekly) showed better outcomes than those on the low-dose oral regimen. Notably, when animals were switched from a low-dose regimen to a higher dose during disease progression, disease scores improved, which may suggest either enhanced inflammation control or support for tissue repair by Tregs, which have been implicated in myelin restoration [
23]. When compared to glatiramer acetate (GA), a low-efficacy DMT, LPX-TI641 provided superior disease control post-onset, consistent with GA’s known limitations in therapeutic EAE settings [
19]. Importantly, LPX-TI641 matched the efficacy of natalizumab during active treatment but sustained symptom control even after drug washout—something not observed with natalizumab. Additionally, switching from natalizumab to LPX-TI641 after one or two doses preserved disease remission and improved myelin integrity histologically, with earlier switches correlating with better myelin preservation.
In the PLP139–151-induced EAE model, which recapitulates epitope spreading, a hallmark of MS progression, LPX-TI641 treatment initiated during the acute phase (day 15–26) effectively prevented both the first and second relapses. In contrast, natalizumab-treated animals experienced relapses after discontinuation, with more severe secondary relapses than untreated controls. LPX-TI641 not only prevented relapses but also maintained disease remission beyond the treatment window, despite the drug’s short half-life in mice. This suggests a durable pharmacodynamic effect, likely mediated by the sustained presence of Tregs in circulation. Given that treatment ceased before the onset of the first relapse, these data support a mechanism of immune tolerance, rather than simple immunosuppression or T-cell trafficking blockade.
We also explored therapeutic positioning of LPX-TI641 using treatment escalation and induction/maintenance paradigms. In the treatment escalation model, mice were initially treated with dimethyl fumarate (DMF), a moderate-efficacy DMT. Animals that failed DMF treatment were switched to either natalizumab or LPX-TI641. While both agents improved disease control, LPX-TI641 demonstrated higher survival and better clinical scores post-switch. The most effective group in the study consisted of animals started on LPX-TI641 from disease onset, further underscoring the benefit of early intervention.
In the induction/maintenance paradigm, animals were treated at a late disease stage (clinical score of 2.5). LPX-TI641 was superior to natalizumab in both disease control and survival during induction. Furthermore, animals switched to LPX-TI641 for maintenance therapy showed continued clinical improvement, while those switched to DMF for maintenance therapy relapsed and experienced high mortality. These findings suggest that LPX-TI641 is not only effective as an induction agent but may also support long-term immune homeostasis when used for maintenance.
Importantly, the immune tolerance–inducing potential of LPX-TI641 was validated ex vivo using PBMCs derived from PwMS. Compared to healthy controls, PwMS had lower baseline levels of Foxp3
+Tim3
+ Tregs. LPX-TI641 treatment of patient-derived PBMCs significantly expanded both total Foxp3
+ Tregs and the Foxp3
+Tim3
+ subset in a dose-dependent manner. This is notable because the Tim3
+ Treg population is believed to play a key role in durable immune regulation and is impaired in MS [
11,
24,
25,
26]. In a recall response study using the myelin basic protein (MBP
87–99) peptide, LPX-TI641 reversed antigen-induced suppression of Tregs in responsive patient samples, further supporting its ability to restore tolerance even in the context of autoreactive T-cell memory.
Collectively, our data demonstrates that LPX-TI641, a Tim-3/Tim-4 receptor agonist, promotes immune tolerance and restores immune balance by expanding regulatory T-cell populations. It exhibited robust efficacy across multiple therapeutic EAE models in an antigen-independent manner. Importantly, effects of LPX-TI641persisted after treatment cessation and effectively expanded Tim-3
+ Tregs derived from patient immune cells. Unlike tolerance strategies that require rapamycin or DMT’s such as dexamethasone, Tim agonists does not broadly suppress TLR signaling pathways [
14]. Taken together, these findings support that Tim-agonism, and LPX-TI641 in particular, holds promise as a non-immune suppressant, durable, and practical immunomodulatory therapy for autoimmune diseases such as multiple sclerosis. LPX-TI641 is currently being evaluated clinically in a series of phase I and phase Ib studies to evaluate its safety, tolerability and pharmacokinetics.