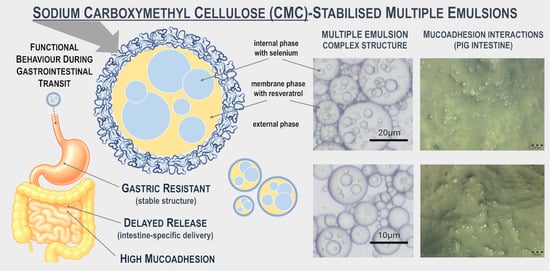
Sodium Carboxymethyl Cellulose-Stabilised Multiple Emulsions with pH-Sensitive Behaviour, Enhanced Stability and Mucoadhesion for Oral Delivery of Chemopreventive Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
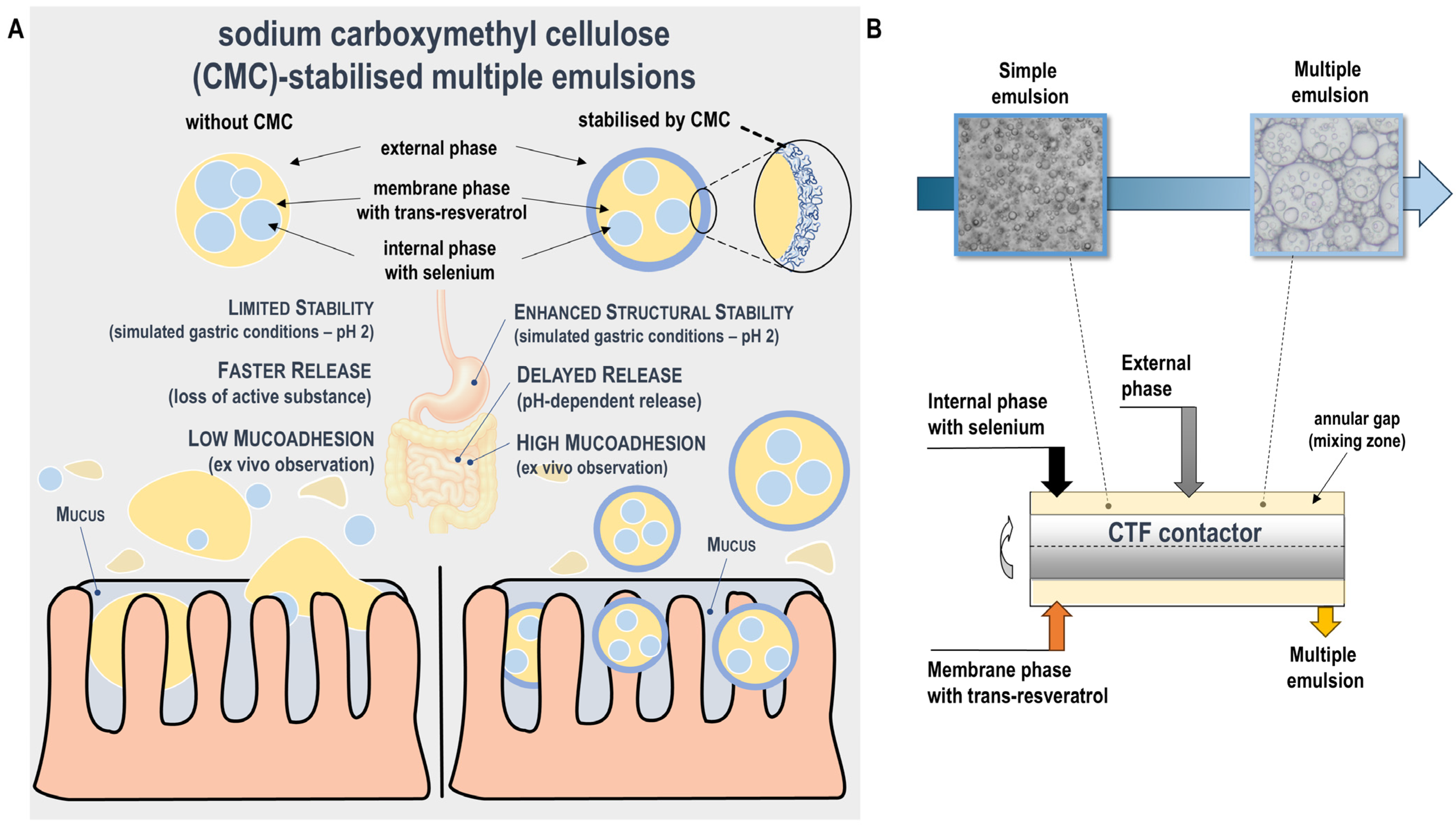
2.2. Preparation of Multiple Emulsions
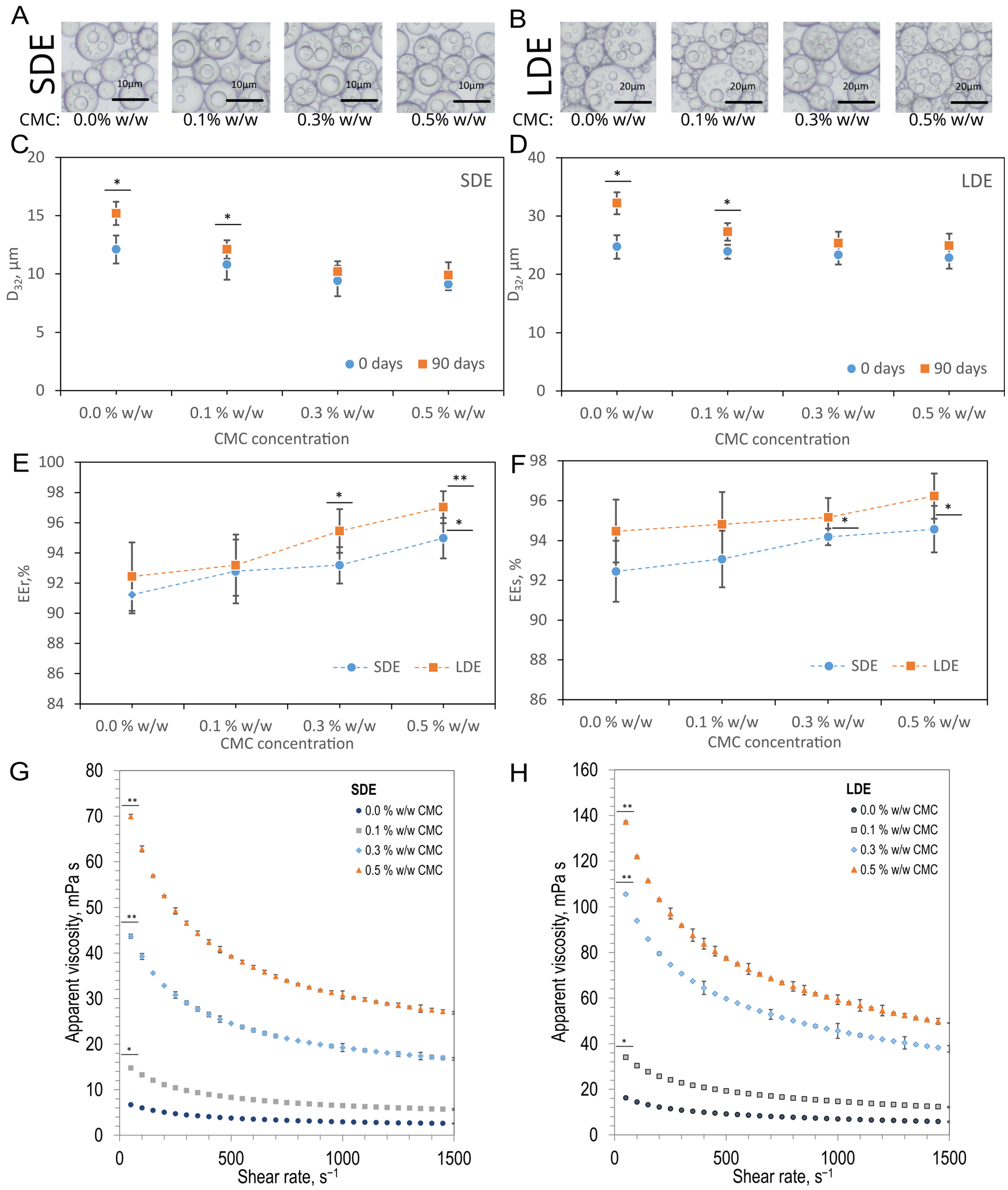
2.3. Microscopic Observation and Droplet Size Analysis
2.4. Encapsulation Efficiency Measurements
2.5. Rheological Measurements
2.6. ζ-Potential Measurements
2.7. Chemopreventive Agents Quantification
2.8. Simulated Gastrointestinal Transit (Git) Conditions of Emulsion
2.9. Emulsion Morphology Under Simulated Gastrointestinal Transit Conditions
2.10. Determination of Interfacial CMC Concentration Under Simulated GIT Conditions
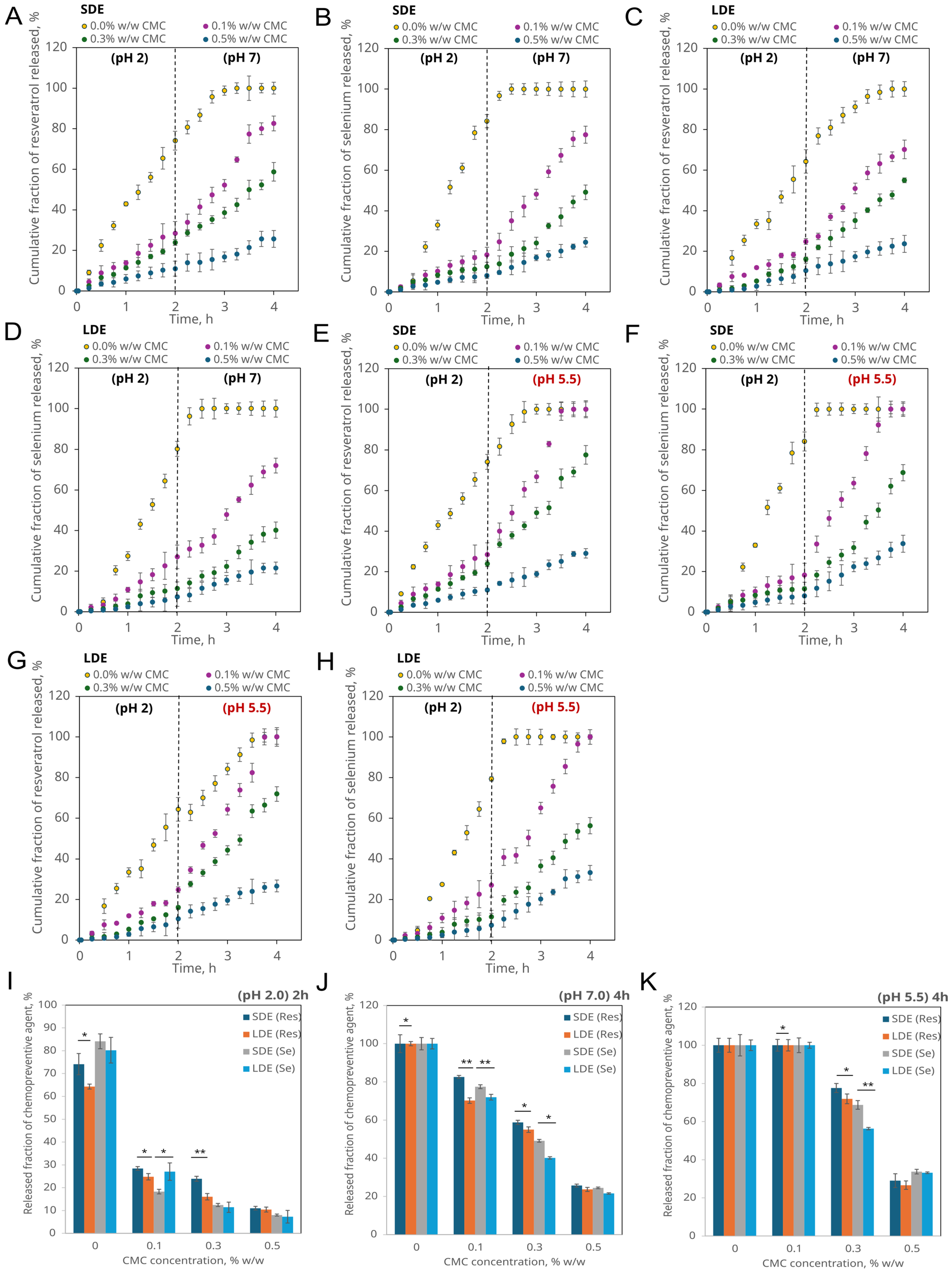
2.11. In Vitro Release Study of Chemopreventive Agents in Simulated Gastrointestinal Transit
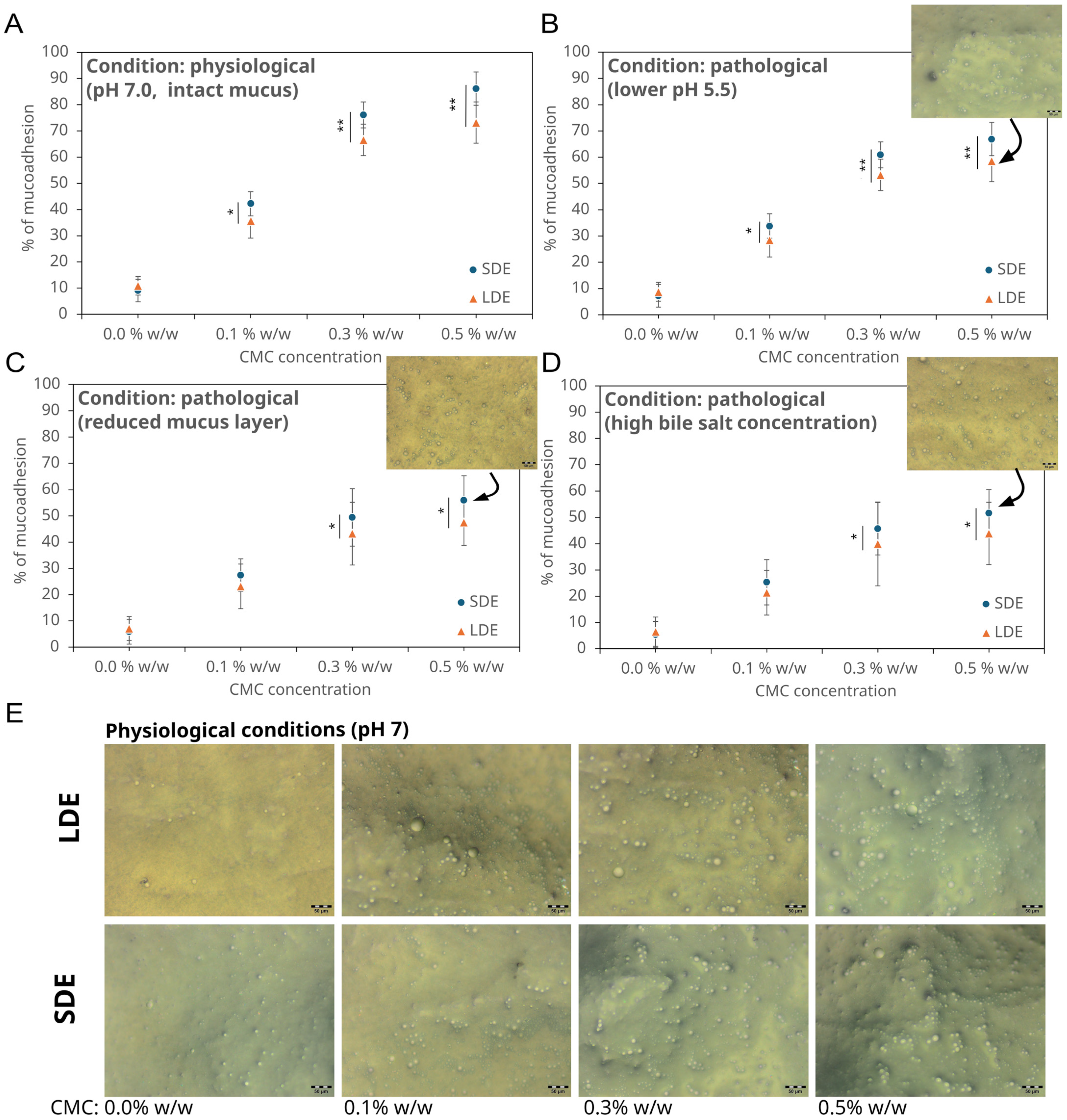
2.12. Ex Vivo Investigation of Intestinal Mucoadhesion Under Physiological and Pathological Conditions
- (1)
- Acidic pH environment (pH 5.5): To reproduce the mildly acidic microenvironment associated with intestinal inflammation, which alters mucin conformation and reduces CMC ionisation, the intestinal segments were preincubated for 15 min in PBS adjusted to pH 5.5 using 0.1 M HCl [32]. During washing, PBS (pH 5.5) was used instead of neutral buffer to maintain consistent conditions throughout the assay.
- (2)
- Reduced mucus layer: To mimic the thinning of the mucus layer typical of inflammatory bowel disease, the mucosal surface was rinsed three times with PBS (pH 7.0) containing 0.5% w/v N-acetylcysteine (NAC), followed by a 10 min incubation at 37 °C to loosen and partially remove adherent mucins [33]. The tissue was washed thrice with PBS to remove residual NAC before applying the emulsion.
- (3)
- Increased bile salt concentration: Pathological bile reflux and malabsorption were simulated by supplementing the washing buffer with sodium deoxycholate (10 mM) during the 20 min peristaltic washing step. This concentration corresponds to the upper physiological range observed under pathological bile salt exposure [34].
2.13. Statistical Analysis
3. Results and Discussion
3.1. Emulsion Characterisation
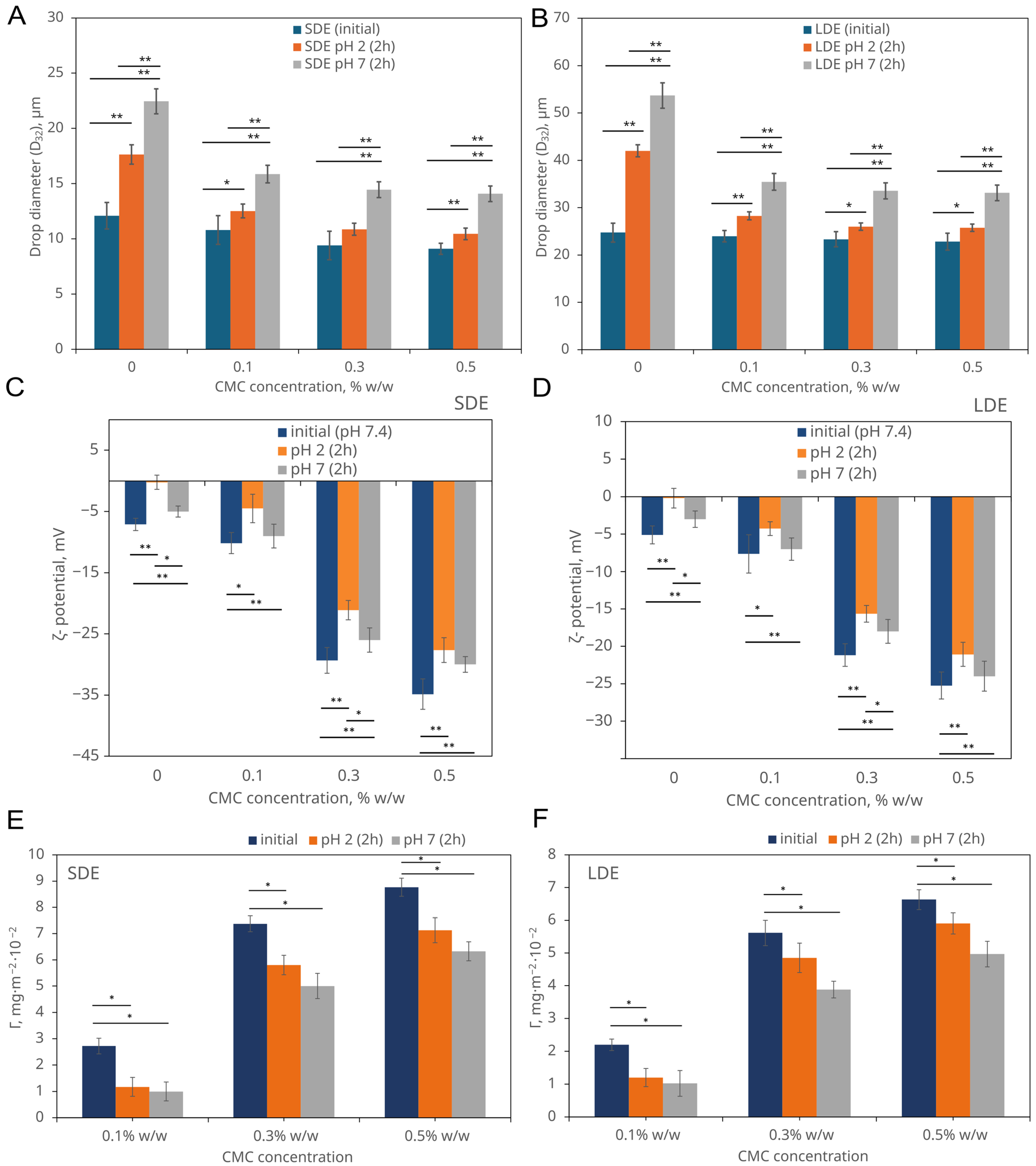
3.2. pH-Dependent Behaviour of Emulsions Under Simplified Simulated Gastrointestinal Conditions
3.2.1. Droplet Size Evolution
3.2.2. ζ-Potential Analysis of Emulsions
3.2.3. The Behaviour of CMC Adsorbed onto Emulsion Droplets
3.2.4. pH-Controlled Release of Encapsulated Compounds Under Simplified Gastric and Intestinal Physiological and Pathological Conditions
3.3. Mucoadhesive Properties of Chemopreventive Agents-Loaded Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katona, B.W.; Weiss, J.M. Chemoprevention of colorectal cancer. Gastroenterology 2020, 158, 368–388. [Google Scholar] [CrossRef] [PubMed]
- Lepore Signorile, M.; Grossi, V.; Fasano, C.; Simone, C. Colorectal Cancer Chemoprevention: A Dream Coming True? Int. J. Mol. Sci. 2023, 24, 7597. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Gonçalves, C.; Pastrana, L.M.; Coimbra, M.A.; Cerqueira, M.A. Recent advances in oral delivery systems of resveratrol: Foreseeing their use in functional foods. Food Funct. 2023, 14, 10286–10313. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2020, 379, 1256–1268. [Google Scholar] [CrossRef]
- Vancha, H.; Sharfuddin, M.; Tewari, D.; Pandey, N.K.; Vishwas, S.; Babu, M.R.; Salkini, M.A.; Rehman, Z.U.; Alotaibi, J.T.; Alotaibi, R.F.; et al. Unravelling the role of solid lipid nanoparticles in drug delivery: Journey from laboratory to clinical trial. J. Drug Deliv. Sci. Technol. 2023, 85, 104616. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-Based Hydrogels and Their Application as Drug Delivery Systems in Cancer Treatment: A Review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Xiao, T.; Ma, X.; Hu, H.; Xiang, F.; Zhang, X.; Zheng, Y.; Dong, H.; Adhikari, B.; Wang, Q.; Shi, A. Advances in emulsion stability: A review on mechanisms, role of emulsifiers, and applications in food. Food Chem. X 2025, 29, 102792. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zhang, R.; Zhou, R.; Zhu, J. A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double emulsions relevant to food systems: Preparation, stability, and applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, C.; Wang, H.; Chen, C.; Teng, H.; Xiao, J.; Chen, L. Emulsion and its application in the food field: An update review. eFood 2023, 4, e102. [Google Scholar] [CrossRef]
- Dluska, E.; Markowska-Radomska, A.; Metera, A.; Kosicki, K. Hierarchically structured emulsions for brain therapy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 201–212. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources. Foods 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Dluska, E.; Markowska-Radomska, A.; Metera, A.; Rudniak, L.; Kosicki, K. Mass transfer of anti-cancer drug delivery to brain tumours by a multiple emulsion-based implant. AIChE J. 2022, 68, e17501. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Legrand, G.; Baeza, G.P.; Peyla, M.; Porcar, L.; Fernández-de-Alba, C.; Manneville, S.; Divoux, T. Acid-induced gelation of carboxymethylcellulose solutions. arXiv 2023. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Mapile, A.N.; Scatena, L.F. Bulking up: The impact of polymer sterics on emulsion stability. Soft Matter 2024, 20, 7471–7483. [Google Scholar] [CrossRef]
- Harnsilawat, T.; Pongsawatmanit, R.; McClements, D.J. Influence of pH and Ionic Strength on Formation and Stability of Emulsions Containing Oil Droplets Coated by β-Lactoglobulin–Alginate Interfaces. Biomacromolecules 2006, 7, 2052–2058. [Google Scholar] [CrossRef]
- Dluska, E.; Markowska-Radomska, A. A novel strategy for brain cancer treatment through a multiple emulsion system for simultaneous therapeutics delivery. Phys. Sci. Rev. 2025, 10, 621–638. [Google Scholar] [CrossRef]
- Markowska-Radomska, A.; Dluska, E.; Metera, A.; Wojcieszak, M. Multiple emulsions for simultaneous active agents delivery in a skin topical application. Chem. Process Eng. 2021, 42, 263–273. [Google Scholar] [CrossRef]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-Inflammatory Effects of Resveratrol Occur via Inhibition of Lipopolysaccharide-Induced NF-κB Activation in Caco-2 and SW480 Human Colon Cancer Cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Cao, L.; Cui, J.; Ma, X.; Zhao, C.; Yin, S.; Hu, H. Glucose Limitation Sensitizes Cancer Cells to Selenite-Induced Cytotoxicity via SLC7A11-Mediated Redox Collapse. Cancers 2022, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Meng, Y.; Cai, W.; Luo, Q.; Gao, H.; Shen, Q.; Shi, D. Docosahexaenoic Acid Coordinating with Sodium Selenite Promotes Paraptosis in Colorectal Cancer Cells by Disrupting the Redox Homeostasis and Activating the MAPK Pathway. Nutrients 2024, 16, 1737. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Cottart, C.-H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.-L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta 2009, 634, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bera, B.C.; Chakrabartty, M.M. Spectrophotometric determination of selenium with 2-mercaptobenzothiazole. Analyst 1968, 93, 50–55. [Google Scholar] [CrossRef]
- Zam, W.; Alshahneh, M.; Hasan, A. Methods of Spectroscopy for Selenium Determination: A Review. Res. J. Pharm. Technol. 2019, 12, 6149–6152. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.-H. Characteristics and oxidative stability of soy protein-stabilised oil-in-water emulsions: Influence of ionic strength and heat pretreatment. Food Hydrocoll. 2014, 37, 149–158. [Google Scholar] [CrossRef]
- Winter, C.; Hartl, S.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigations to evaluate gastric mucoadhesion of an organic product to ameliorate gastritis. Pharmaceutics 2020, 12, 331. [Google Scholar] [CrossRef]
- Komati, S.; Swain, S.; Rao, M.E.B.; Jena, B.R.; Dasi, V. Mucoadhesive multiparticulate drug delivery systems: An extensive review of patents. Adv. Pharm. Bull. 2019, 9, 521–538. [Google Scholar] [CrossRef]
- Zhao, S.; Han, K.; Yuan, G.; Wang, L. Method for refractive index detection for emulsion concentration. Funct. Mater. 2018, 25, 401–405. [Google Scholar] [CrossRef]
- Cook, M.T.; Khutoryanskiy, V.V. Mucoadhesion and mucosa-mimetic materials—A mini-review. Int. J. Pharm. 2015, 495, 991–998. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Pham, S.Q.D.; Nöjd, S.; Edman, M.; Lindell, K.; Topgaard, D.; Wahlgren, M. Mucoadhesion: Mucin-polymer molecular interactions. Int. J. Pharm. 2021, 610, 121245. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ono, K.; Masuda, W.; Inagaki, T.; Yokota, M.; Inenaga, K. Rheological properties of human saliva and salivary mucins. J. Oral Biosci. 2008, 50, 134–141. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.A.; Almhöjd, U.; Çevik-Aras, H.; Fisic, A.; Olofsson, R.; Almståhl, A.; Kádár, R. The complex shear time response of saliva in healthy individuals. Phys. Fluids 2025, 37, 011911. [Google Scholar] [CrossRef]
- Kavishvar, D.; Ramachandran, A. The yielding behaviour of human mucus. Adv. Colloid Interface Sci. 2023, 322, 103049. [Google Scholar] [CrossRef]
- International Dysphagia Diet Standardisation Initiative. Complete IDDSI Framework (Detailed Definitions). 2019. Available online: https://www.iddsi.org/resources/framework-documents (accessed on 1 September 2025).
- Subbotin, A.V.; Semenov, A.N. Adsorption of flexible polyelectrolytes on charged surfaces. Soft Matter 2016, 12, 6771–6787. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nosal-Wiercińska, A.; Ostolska, I.; Sternik, D.; Nowicki, P.; Pietrzak, R.; Bazan-Wozniak, A.; Goncharuk, O. Nanostructure of Poly(Acrylic Acid) Adsorption Layer on the Surface of Activated Carbon Obtained from Residue After Supercritical Extraction of Hops. Nanoscale Res. Lett. 2017, 12, 2. [Google Scholar] [CrossRef]
- Ma, H.; Cameron, A. Dual-responsive polymers synthesized via RAFT polymerization for controlled demulsification and desorption. J. Polym. Res. 2023, 30, 278. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, S. Recent development in mucosal drug delivery system. In Novel Formulations and Future Trends; Nayak, A.K., Sen, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 61–84. [Google Scholar] [CrossRef]
| Phase | Composition |
|---|---|
| Internal | Gelatin (0.2% w/w), Pluronic P-123 (0.25% w/w), Milli-Q water (to 100% w/w) |
| Membrane | Sesame oil (98% w/w), Span 83 (2% w/w) |
| External | Sodium carboxymethyl cellulose—CMC (0.0–0.5% w/w), Pluronic P-123 (0.25% w/w), Tween 80 (0.25% w/w), Milli-Q water (to 100% w/w) |
| Emulsion Series | CMC Content % w/w | Rotational Frequency rpm | Flow Rate of the Emulsion Phases | ||
|---|---|---|---|---|---|
| Internal cm3 min−1 | Membrane cm3 min−1 | External cm3 min−1 | |||
| SDE 1 | 0.0 | 900 | 30 | 30 | 60 |
| 0.1 | 1200 | ||||
| 0.3 | 1400 | ||||
| 0.5 | 1800 | ||||
| LDE 2 | 0.0 | 1300 | 60 | ||
| 0.1 | 1800 | ||||
| 0.3 | 2100 | ||||
| 0.5 | 2450 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska-Radomska, A.; Kosicki, K.; Dluska, E. Sodium Carboxymethyl Cellulose-Stabilised Multiple Emulsions with pH-Sensitive Behaviour, Enhanced Stability and Mucoadhesion for Oral Delivery of Chemopreventive Agents. Pharmaceutics 2025, 17, 1401. https://doi.org/10.3390/pharmaceutics17111401
Markowska-Radomska A, Kosicki K, Dluska E. Sodium Carboxymethyl Cellulose-Stabilised Multiple Emulsions with pH-Sensitive Behaviour, Enhanced Stability and Mucoadhesion for Oral Delivery of Chemopreventive Agents. Pharmaceutics. 2025; 17(11):1401. https://doi.org/10.3390/pharmaceutics17111401
Chicago/Turabian StyleMarkowska-Radomska, Agnieszka, Konrad Kosicki, and Ewa Dluska. 2025. "Sodium Carboxymethyl Cellulose-Stabilised Multiple Emulsions with pH-Sensitive Behaviour, Enhanced Stability and Mucoadhesion for Oral Delivery of Chemopreventive Agents" Pharmaceutics 17, no. 11: 1401. https://doi.org/10.3390/pharmaceutics17111401
APA StyleMarkowska-Radomska, A., Kosicki, K., & Dluska, E. (2025). Sodium Carboxymethyl Cellulose-Stabilised Multiple Emulsions with pH-Sensitive Behaviour, Enhanced Stability and Mucoadhesion for Oral Delivery of Chemopreventive Agents. Pharmaceutics, 17(11), 1401. https://doi.org/10.3390/pharmaceutics17111401