Improved Intestinal Permeation of Cyclosporin a by FCIGRL-Modified Tight Junction Modulator in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of CsA Solutions for the Animal Study
2.4. Intraduodenal Administration of CsA Solutions to Rats
2.5. HPLC-Mass Spectrometer Conditions
2.6. Data Analysis
3. Results
3.1. Structures of FCIGRL-Modified Peptides
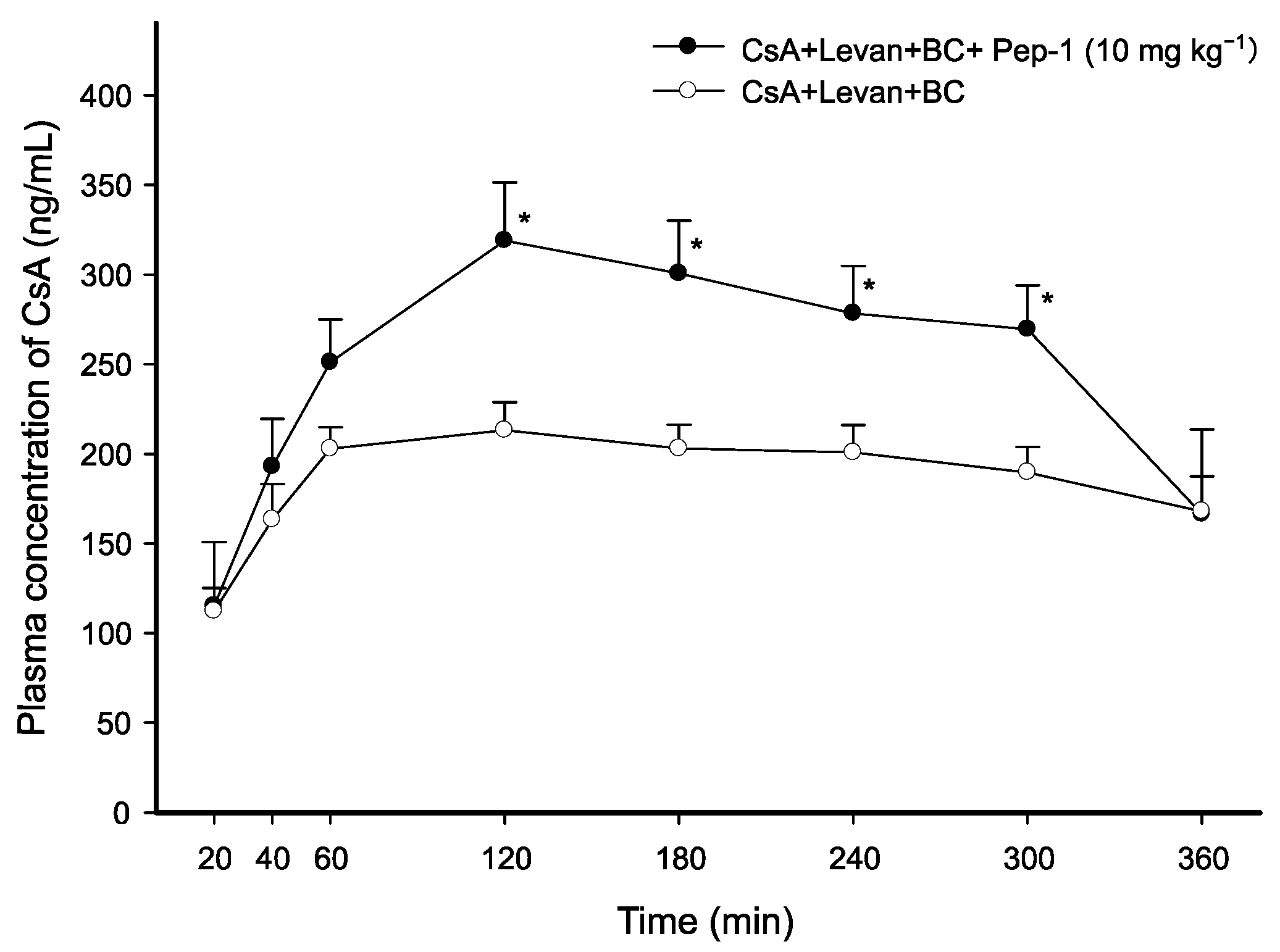
3.2. Intraduodenal Administration of CsA with FCIGRL-NH2, C-Terminal Amidated FCIGRL
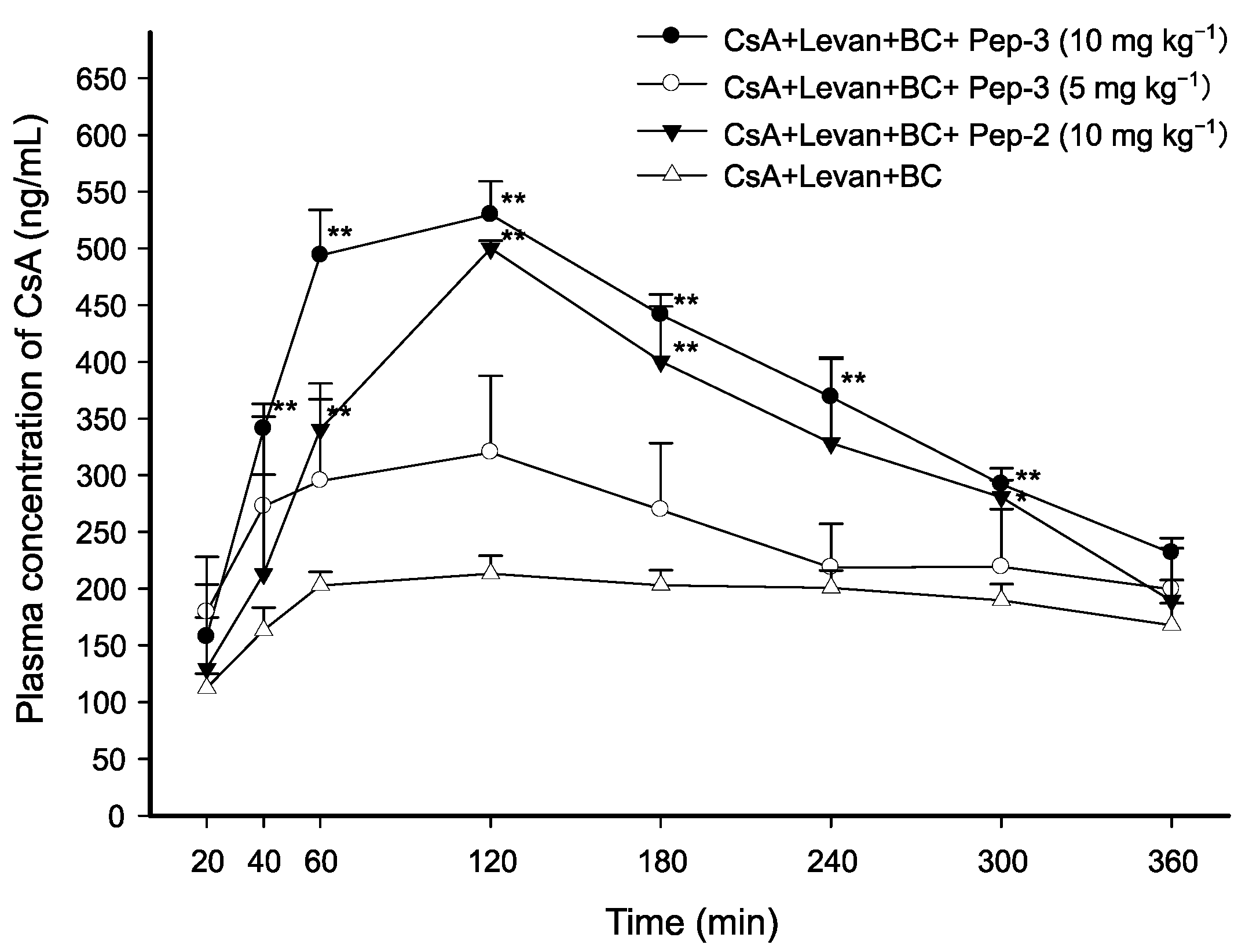
3.3. Effect of FCIGRL Homo-Dimers on Intraduodenal Absorption of CsA
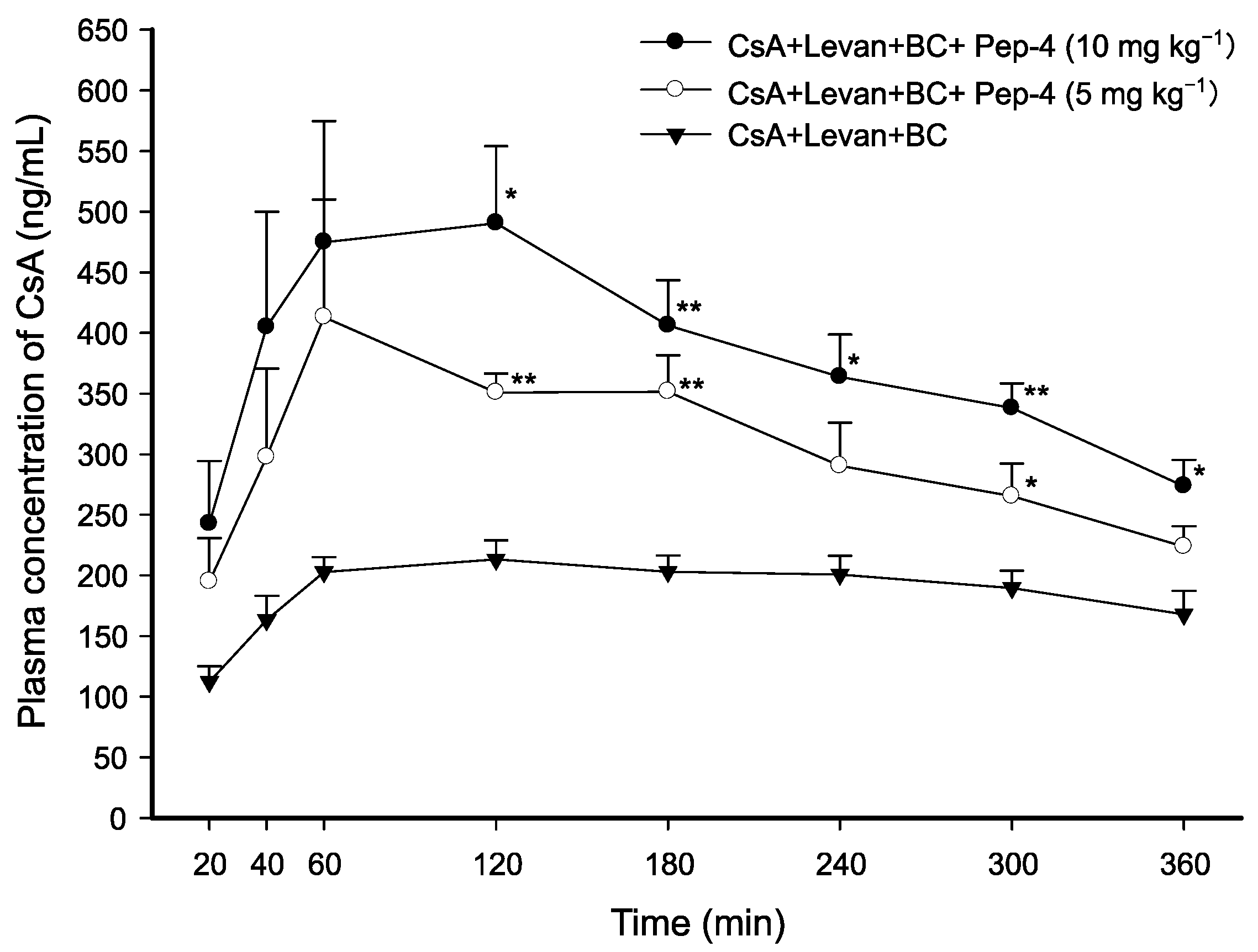
3.4. Effect of Cysteine-Substituted FCIGRL on Intraduodenal Absorption of CsA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guada, M.; Beloqui, A.; Kumar, M.N.; Préat, V.; Dios-Viéitez Mdel, C.; Blanco-Prieto, M.J. Reformulating cyclosporine A (CsA): More than just a life cycle management strategy. J. Control. Release 2016, 225, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, A. Oral cyclosporine A—The current picture of its liposomal and other delivery systems. Cell. Mol. Biol. Lett. 2009, 14, 139–152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, S.; Kambam, S.; Thankia, K.; Jain, A.K. Cyclosporine A loaded self-nanoemulsifying drug delivery system (SNEDDS): Implication of a functional excipient based co-encapsulation strategy on oral bioavailability and nephrotoxicity. RSC Adv. 2015, 5, 49633–49642. [Google Scholar] [CrossRef]
- Guo, J.; Ping, Q.; Chen, Y. Pharmacokinetic behavior of cyclosporin A in rabbits by oral administration of lecithin vesicle and Sandimmun Neoral. Int. J. Pharm. 2001, 216, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, D.; Sjöblom, M.; Lennernäs, H. Intestinal absorption-modifying excipients: A current update on preclinical in vivo evaluations. Eur. J. Pharm. Biopharm. 2019, 142, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106 Pt B, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Fiorentini, C.; Donelli, G.; Uzzau, S.; Kaper, J.B.; Margaretten, K.; Ding, X.; Guandalini, S.; Comstock, L.; Goldblum, S.E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Investig. 1995, 96, 710–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasano, A.; Uzzau, S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Investig. 1997, 99, 1158–1164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fasano, A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann. N. Y. Acad. Sci. USA 2000, 915, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J.B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, D.S.; Gao, H.; Raje, S.; Scott, K.R.; Eddington, N.D. Enhancing the permeation of marker compounds and enaminone anticonvulsants across Caco-2 monolayers by modulating tight junctions using zonula occludens toxin. Eur. J. Pharm. Biopharm. 2001, 52, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.S.; Raje, S.; Gao, H.; Salama, N.N.; Eddington, N.D. Enhanced permeability of molecular weight markers and poorly bioavailable compounds across Caco-2 cell monolayers using the absorption enhancer, zonula occludens toxin. Pharm. Res. 2002, 19, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Fasano, A.; Eddington, N.D. Enhanced nasal absorption of hydrophilic markers after dosing with AT1002, a tight junction modulator. Eur. J. Pharm. Biopharm. 2008, 69, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Eddington, N.D. The influence of AT1002 on the nasal absorption of molecular weight markers and therapeutic agents when co-administered with bioadhesive polymers and an AT1002 antagonist, AT1001. J. Pharm. Pharmacol. 2012, 64, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Eddington, N.D. The influence of stabilizer and bioadhesive polymer on the permeation-enhancing effect of AT1002 in the nasal delivery of a paracellular marker. Arch. Pharmacal Res. 2012, 35, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Eddington, N.D. The impact of AT1002 on the delivery of ritonavir in the presence of bioadhesive polymer, carrageenan. Arch. Pharmacal Res. 2012, 35, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Fasano, A.; Eddington, N.D. Effect of the six-mer synthetic peptide (AT1002) fragment of zonula occludens toxin on the intestinal absorption of cyclosporin A. Int. J. Pharm. 2008, 351, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, S.B.; Shim, C.K.; Chung, S.J.; Kim, D.D.; Rhee, S.K.; Choi, G.J.; Kim, C.H.; Kim, K. Paracellular permeation-enhancing effect of AT1002 C-terminal amidation in nasal delivery. Drug Des. Dev. Ther. 2015, 9, 1815–1823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, K.H. Effect of C-terminal amidated tight junction modulator and fructose polymer on the nasal and intestinal permeation of atenolol. Yakhak Hoeji 2019, 63, 37–42. [Google Scholar] [CrossRef]
- Li, M.; Oliver, E.; Kitchens, K.M.; Vere, J.; Alkan, S.S.; Tamiz, A.P. Structure-activity relationship studies of permeability modulating peptide AT-1002. Bioorg. Med. Chem. Lett. 2008, 18, 4584–4586. [Google Scholar] [CrossRef]
- Sreenivasan, S.; Kandasamy, R. Levan: A Biocompatible Homopolysaccharide Excipient for Stabilization of Peptide Drugs. Int. J. Pept. Res. Ther. 2017, 23, 305–311. [Google Scholar] [CrossRef]
- Song, K.H.; An, H.M.; Kim, H.J.; Ahn, S.H.; Chung, S.J.; Shim, C.K. Simple liquid chromatography-electrospray ionization mass spectrometry method for the routine determination of salmon calcitonin in serum. J. Chromatogr. B 2002, 775, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Åmand, H.L.; Nordén, B.; Fant, K. Functionalization with C-terminal cysteine enhances transfection efficiency of cell-penetrating peptides through dimer formation. Biochem. Biophys. Res. Commun. 2012, 418, 469–474. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Li, T.; Zhang, M.; Liu, Y.; Gauthier, M.A.; Zhao, Y.; Wu, C. The Interplay of Disulfide Bonds, α-Helicity, and Hydrophobic Interactions Leads to Ultrahigh Proteolytic Stability of Peptides. Biomacromolecules 2015, 16, 2347–2355. [Google Scholar] [CrossRef]
- Goldblum, S.E.; Rai, U.; Tripathi, A.; Thakar, M.; De Leo, L.; Di Toro, N.; Not, T.; Ramachandran, R.; Puche, A.C.; Hollenberg, M.D.; et al. The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011, 25, 144–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang Ms, J.; Kang Ms, X.; Huang Ms, Z.Q.; Shen Ms, L.; Luo Md, Q.; Li Ms, M.Y.; Luo Ms, L.P.; Tu Ms, J.H.; Han Ms, M.; Ye, J. Protease-Activated Receptor-2 Decreased Zonula Occlidens-1 and Claudin-1 Expression and Induced Epithelial Barrier Dysfunction in Allergic Rhinitis. Am. J. Rhinol. Allergy 2021, 35, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, S.; Ohama, T.; Sato, K. Regulation of epithelial cell tight junctions by protease-activated receptor 2. J. Vet. Med. Sci. 2014, 76, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Pierro, M.; Lu, R.; Uzzau, S.; Wang, W.; Margaretten, K.; Pazzani, C.; Maimone, F.; Fasano, A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J. Biol. Chem. 2001, 276, 19160–19165. [Google Scholar] [CrossRef] [PubMed]
| Peptide | F-X-IGRL | Dimerization | |
|---|---|---|---|
| X | C-Terminus | ||
| Pep-1 | Cysteine | -CONH2 | - |
| Pep-2 | Cysteine | -COOH | Cysteine homo-dimer |
| Pep-3 | Cysteine | -CONH2 | Cysteine homo-dimer |
| Pep-4 | 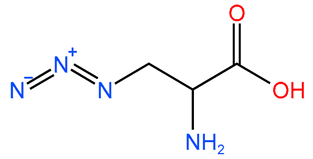 | -CONH2 | - |
| CsA + Levan + BC | AUC0–360min (min·ng·mL−1) | Cmax (ng·mL−1) | Tmax (min) | T1/2 (min) | Vd/F (L·kg−1) | Cl/F (mL·min−1·kg−1) |
|---|---|---|---|---|---|---|
| + Pep-4 (10 mg·kg−1) | 136,063.27 ± 16,010.40 * (2.03-fold) | 511.91 ± 76.37 * (2.37-fold) | 90.00 ± 17.32 (0.86-fold) | 280.61 ± 6.77 (0.96-fold) | 3.37 ± 0.31 ** (0.54-fold) | 8.34 ± 0.81 * (0.56-fold) |
| + Pep-4 (5 mg·kg−1) | 108,567.23 ± 8691.20 ** (1.62-fold) | 470.70 ± 41.30 ** (2.18-fold) | 100.00 ± 40.00 (0.95-fold) | 262.97 ± 36.95 (0.90-fold) | 3.90 ± 0.15 * (0.63-fold) | 10.63 ± 1.30 (0.71-fold) |
| + Pep-3 (10 mg·kg−1) | 134,627.65 ± 5928.82 ** (2.01-fold) | 529.73 ± 29.55 ** (2.46-fold) | 120.00 ± 0.00 (1.14-fold) | 289.85 ± 37.15 (1.00-fold) | 3.59 ± 0.34 * (0.58-fold) | 8.66 ± 0.34 * (0.58-fold) |
| + Pep-3 (5 mg·kg−1) | 88,473.93 ± 15,317.75 (1.32-fold) | 384.55 ± 82.62 (1.78-fold) | 90.00 ± 17.32 (0.86-fold) | 299.42 ± 9.85 (1.03-fold) | 5.47 ± 0.99 (0.88-fold) | 12.73 ± 2.44 (0.85-fold) |
| + Pep-2 (10 mg·kg−1) | 116,743.61 ± 13,310.67 ** (1.74-fold) | 500.02 ± 6.78 ** (2.32-fold) | 120.00 ± 0.00 (1.14-fold) | 264.41 ± 5.44 (0.91-fold) | 4.08 ± 0.39 (0.66-fold) | 10.72 ± 1.23 (0.71-fold) |
| + Pep-1 (10 mg·kg−1) | 91,231.24 ± 9560.07 (1.36-fold) | 323.02 ± 29.21 * (1.50-fold) | 140.00 ± 20.00 (1.33-fold) | 263.44 ± 42.10 (0.90-fold) | 4.95 ± 0.44 (0.80-fold) | 13.91 ± 2.83 (0.93-fold) |
| CsA + Levan + BC (control) | 67,055.95 ± 4429.74 | 215.69 ± 14.38 | 105.00 ± 28.72 | 291.24 ± 24.81 | 6.21 ± 0.66 | 15.01 ± 1.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.-H.; Kim, J.-W.; Song, K.-H. Improved Intestinal Permeation of Cyclosporin a by FCIGRL-Modified Tight Junction Modulator in Rats. Pharmaceutics 2025, 17, 1395. https://doi.org/10.3390/pharmaceutics17111395
Jeong D-H, Kim J-W, Song K-H. Improved Intestinal Permeation of Cyclosporin a by FCIGRL-Modified Tight Junction Modulator in Rats. Pharmaceutics. 2025; 17(11):1395. https://doi.org/10.3390/pharmaceutics17111395
Chicago/Turabian StyleJeong, Dong-Ho, Jung-Woo Kim, and Keon-Hyoung Song. 2025. "Improved Intestinal Permeation of Cyclosporin a by FCIGRL-Modified Tight Junction Modulator in Rats" Pharmaceutics 17, no. 11: 1395. https://doi.org/10.3390/pharmaceutics17111395
APA StyleJeong, D.-H., Kim, J.-W., & Song, K.-H. (2025). Improved Intestinal Permeation of Cyclosporin a by FCIGRL-Modified Tight Junction Modulator in Rats. Pharmaceutics, 17(11), 1395. https://doi.org/10.3390/pharmaceutics17111395








