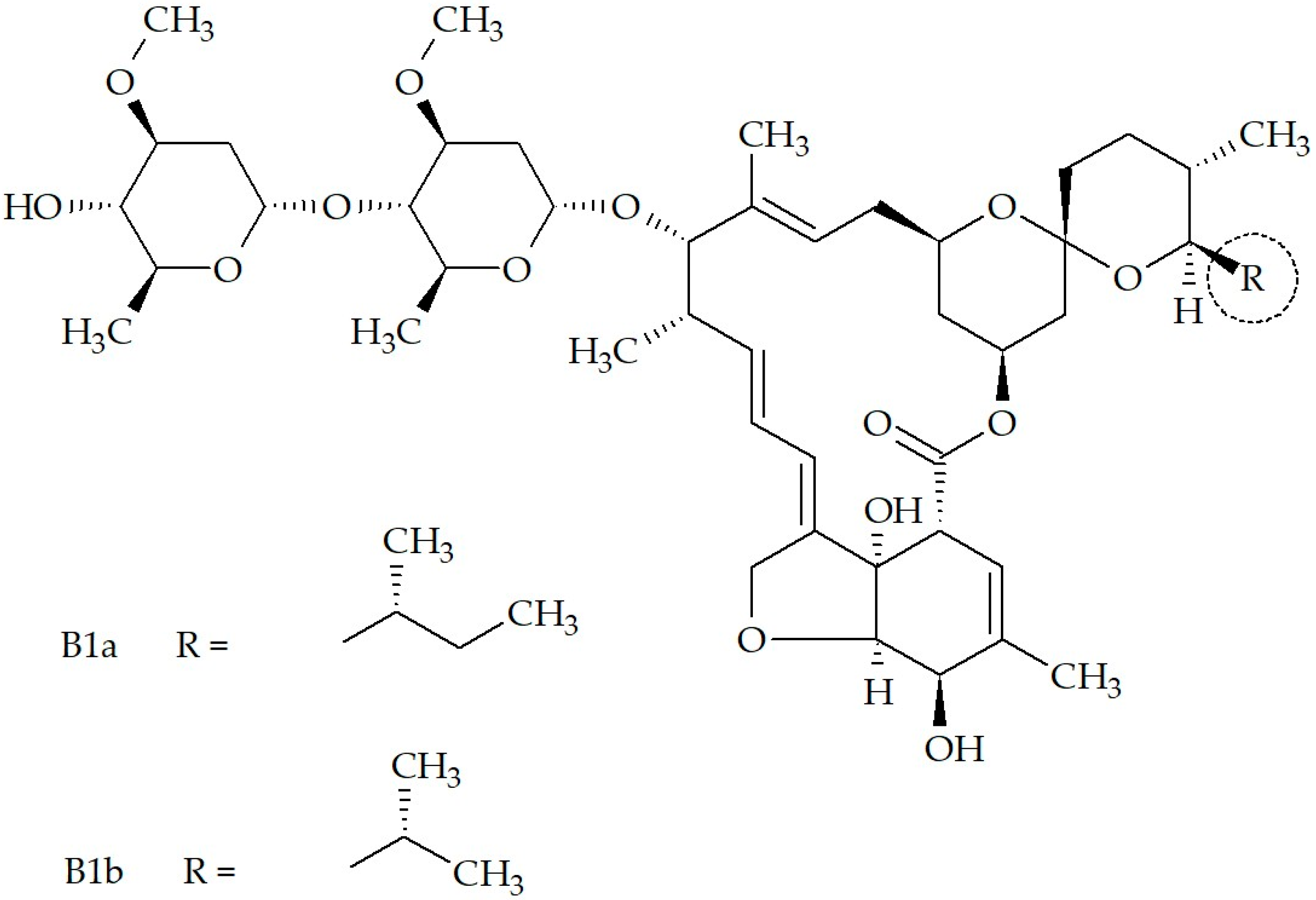
Therapeutic and Formulation Advances of Ivermectin in Veterinary and Human Medicine
Abstract
1. Introduction
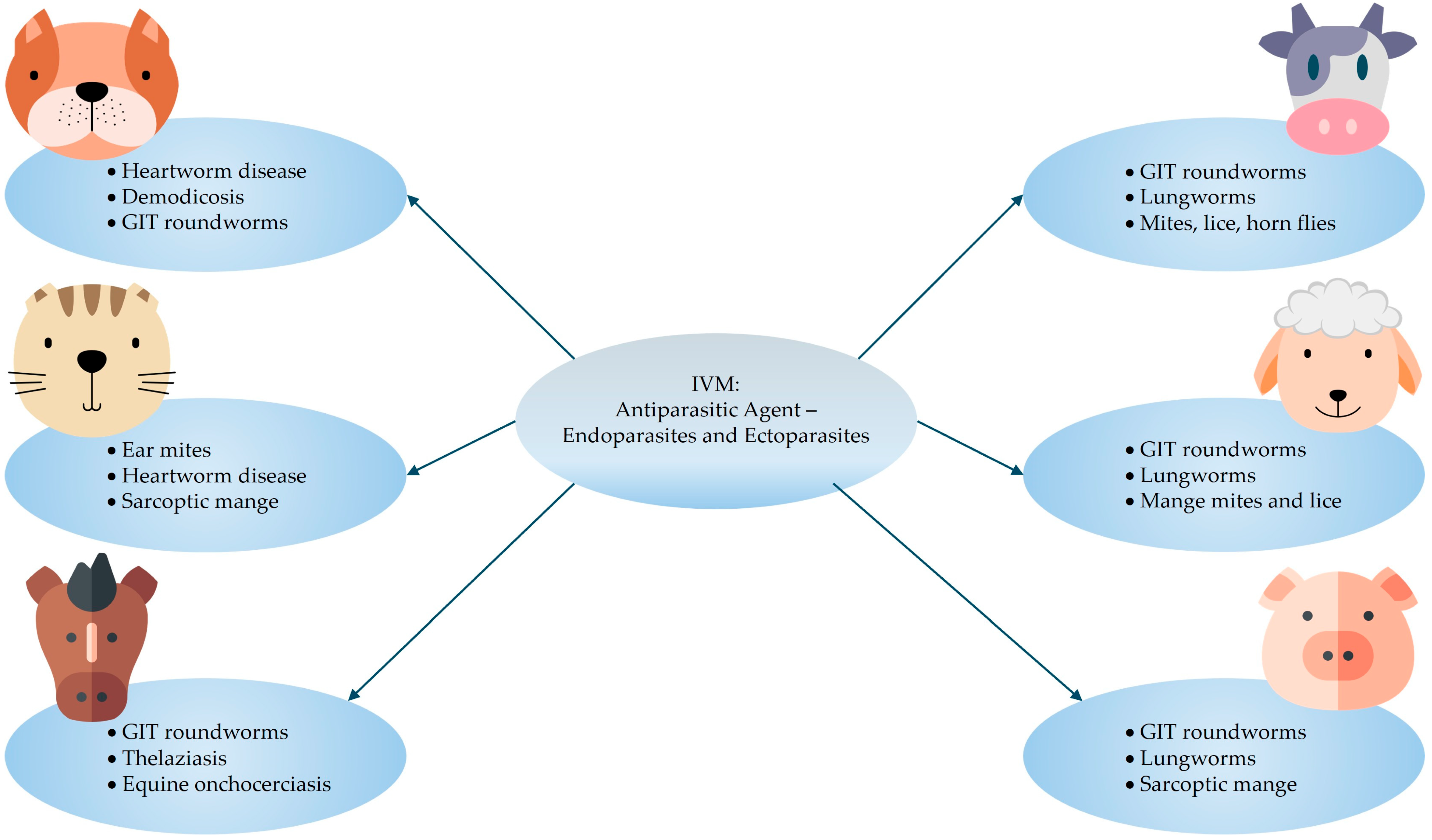
2. Veterinary Uses
2.1. Established
- Small animals: 0.300–0.600 mg/kg (oral dose, once daily, until two respective negative skin scrapings are obtained one month apart).
- Cattle and sheep: 0.200 mg/kg (single dose subcutaneous injection).
- Horses: 0.200 mg/kg (oral dose, repeated as necessary for adequate parasite management).
- Swine: 0.300 mg/kg (subcutaneous injection, repeated two-weekly) or 0.100–0.200 mg/kg (oral dose in feed for seven days).
2.2. Investigational
2.3. Animal Toxicity Investigations and Applications
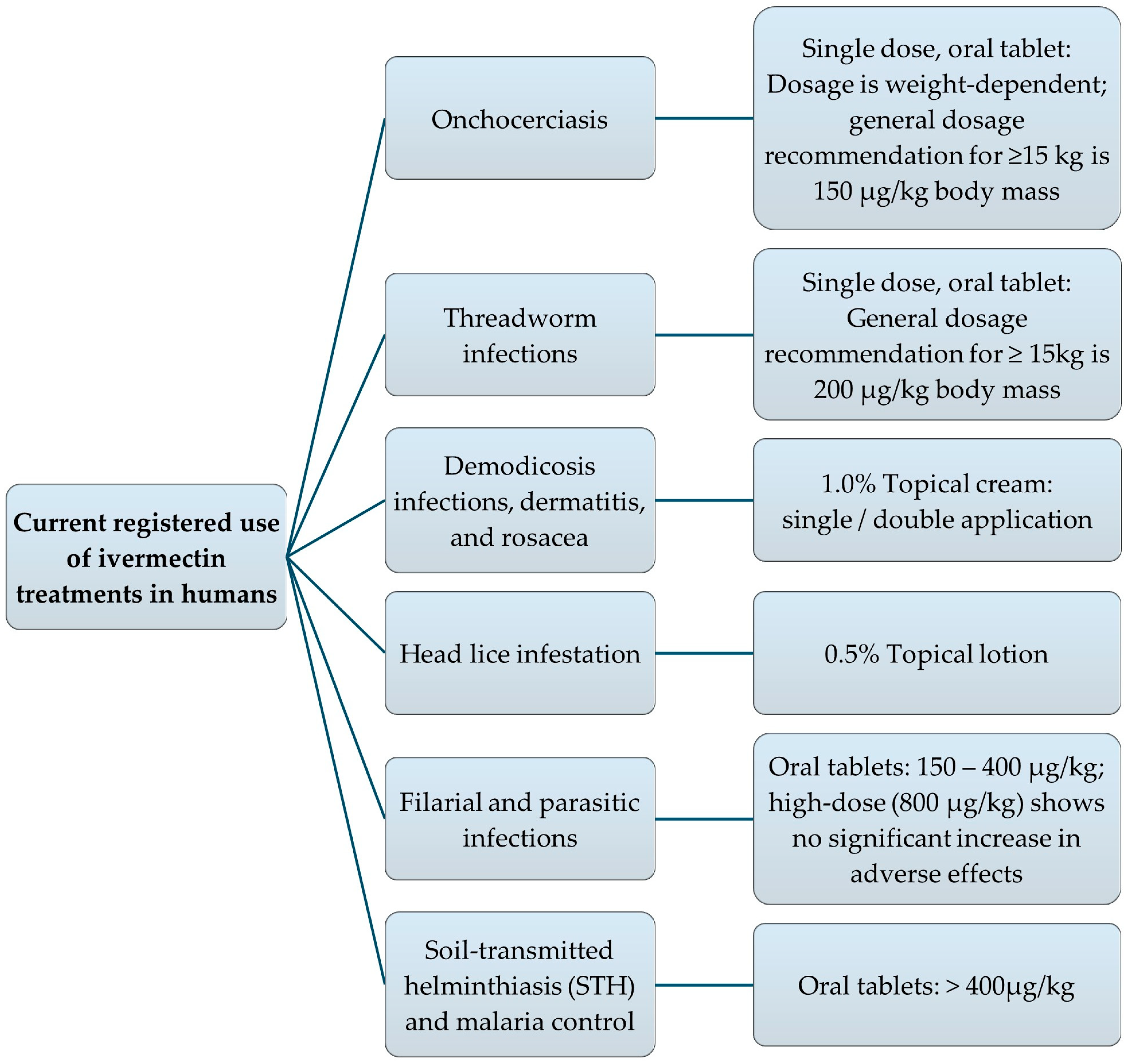
3. Human Uses
3.1. Established
3.2. Investigational
3.2.1. Antiviral
3.2.2. Antibacterial
3.2.3. Anticancer
3.2.4. Anti-Inflammatory
3.2.5. Other
3.3. Ivermectin Toxicity
3.3.1. Ivermectin Side-Effects and Overdose Toxicity in Humans
3.3.2. Safety Considerations for Humans Using IVM-Treated Animal Products
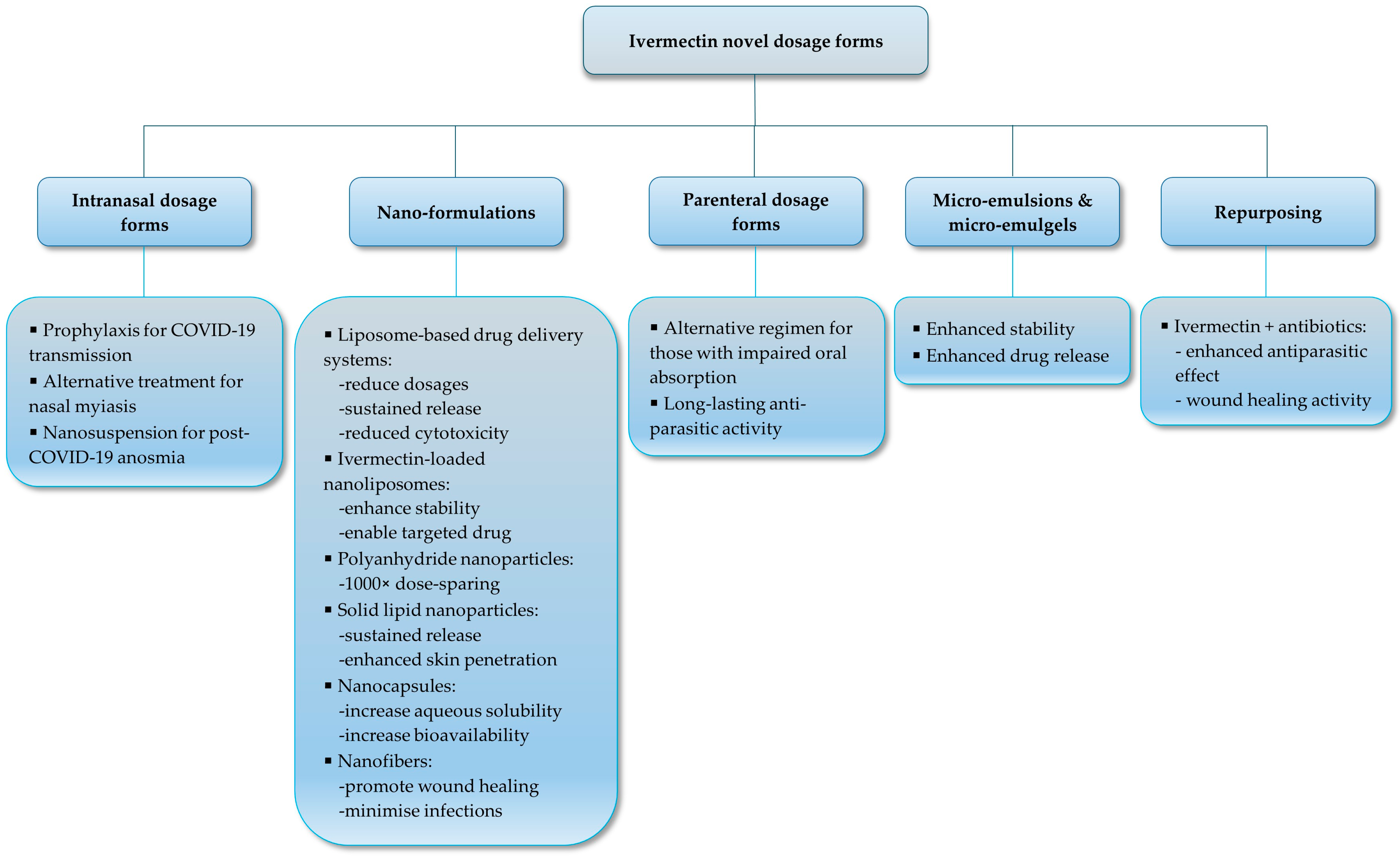
4. Novel Dosage Form Development for Human Use
4.1. Liquid-Based Dosage Forms
4.2. Solid Oral Dosage Forms
4.3. Powder Dosage Forms
4.4. Topical Semi-Solid Dosage Forms
4.5. Nanoformulations and Nanostructured Carriers
4.6. Lipid-Based Formulations
5. Status of Clinical Trials with Novel Dosage Forms
- Physicochemical issues: Achieving uniform particle sizes post-processing and complete drug release (often <60%) without agglomeration [232].
- Safety and biocompatibility: Potential immunogenicity from components like polyethylene glycol (PEG); narrow therapeutic window risking neurotoxicity at high doses [234].
- Regulatory and scalability: Good Manufacturing Practice (GMP)-compliant production, demonstrating superiority to approved forms, and navigating intellectual property (e.g., patents for nebulized nano-IVM) [231].
- Efficacy concerns: Addressing resistance in parasites and lack of human in vivo data for novel indications [227].
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer/Funding
Abbreviations
| IVM | Ivermectin |
| COVID-19 | Coronavirus disease of 2019 |
| FDA | United States Food and Drug Administration |
| WHO | World Health Organization |
| NIH | National Institutes of Health |
| GIT | Gastrointestinal tract |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| ACE2 | Angiotensin-converting enzyme 2 |
| TMPRSS2 | Transmembrane protease, serine 2 |
| ROS | Reactive oxygen species |
| 3CLpro | 3-Chymotrypsin-like Protease |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| Vero/hSLAM cells | Vero cells strongly expressing human signaling lymphocyte activation molecules |
| HDA | Host-directed agent |
| RNA | Ribonucleic acid |
| IMP | Importin |
| HIV-1 | Human immunodeficiency virus type 1 |
| HAdV | Human adenovirus |
| BoAHV-1 | Varicellovirus bovinealpha 1 |
| MDBK | Madin-Darby Bovine Kidney |
| BT | Bovine turbinate |
| STD | Sexually transmitted disease |
| MIC | Minimum inhibitory concentration |
| DNA | Deoxyribonucleic acid |
| M. ulcerans | Mycobacterium ulcerans |
| S. aureus | Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| CSCs | Cancer stem-like cells |
| WNT | Wingless signaling |
| TCF | T-cell factor |
| mTOR | Mammalian target of rapamycin |
| PAK1 | p21-activated kinase 1 |
| SID | Surface-induced dissociation |
| MDR | Multi-drug resistance |
| Bax | Bcl-2 associated X protein |
| HIF | Hypoxia-inducible factor |
| JNK | c-Jun N-terminal kinase |
| ERK 1/2 | Extracellular signal-regulated kinase 1 and 2 |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| NOS | Nitric oxide synthase |
| COX2 | Cyclooxygenase-2 |
| TGF-β1 | Transforming growth factor-beta 1 |
| VEGF | Vascular endothelial growth factor |
| FXR | Farnesoid X receptor |
| LD50 | Lethal dose 50% |
| w/w | Weight per weight |
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| P-gp | P-glycoprotein |
| ATP | Adenosine triphosphate |
| CNS | Central nervous system |
| ADME | Absorption, distribution, metabolism, and excretion |
| SLNs | Solid lipid nanoparticles |
| Hb | Hemoglobin |
| TLC | Total leucocyte count |
| DLC | Differential leucocyte count |
| BUN | Blood urea nitrogen |
| ALT | Alanine transaminase |
| AST | Aspartate transferase |
| Cmax | Peak plasma concentration |
| t½ | Elimination half-life |
| IV | Intravenous |
| Tmax | Duration/time to reach Cmax |
| SAHPRA | South African Health Product Regulatory Authority |
| HPLC | High-performance liquid chromatography |
| AUC | Analytical peak area |
| ODT(s) | Oral disintegrating tablet(s) |
| EC50 | Half maximal effective concentration |
| ED | Epidermis-dermis |
| SCE | Stratum corneum-epidermis |
| CYP | Cytochrome P |
| PNPs | Polymeric nanoparticles |
| NLCs | Nanostructured lipid carriers |
| SNEDDS | Self-nano-emulsifying drug delivery systems |
| PLGA | Poly(lactide-co-glycolide) |
| PEG | Polyethylene glycol |
| GMP | Good Manufacturing Practice |
| MSNs | Mesoporous silica nanoparticles |
| IVM-MCM | Ivermectin mesoporous silica particles |
| IVM-NC | Ivermectin poly(ε-caprolactone) nanocapsules |
| PEDV | Porcine epidemic diarrhea virus |
| C6 | Coumarin 6 |
| SEDDS | Self-emulsifying drug delivery systems |
References
- Sulik, M.; Antoszczak, M.; Huczyński, A.; Steverding, D. Antiparasitic activity of ivermectin: Four decades of research into a “wonder drug”. Eur. J. Med. Chem. 2023, 261, 115838. [Google Scholar] [CrossRef]
- Nathan, C. Cooperative development of antimicrobials: Looking back to look ahead. Nat. Rev. Microbiol. 2015, 13, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Kubo, Y. Ivermectin and its target molecules: Shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J. Physiol. 2018, 596, 1833–1845. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Errouissi, F.; Floate, K.; Rombke, J.; Wardhaugh, K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef]
- Chabala, J.C.; Mrozik, H.; Tolman, R.L.; Eskola, P.; Lusi, A.; Peterson, L.H.; Woods, M.F.; Fisher, M.H.; Campbell, W.C. Ivermectin, a new broad-spectrum antiparasitic agent. J. Med. Chem. 1980, 23, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Crump, A. Ivermectin: Enigmatic multifaceted “wonder” drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Rizzo, E. Ivermectin, antiviral properties and COVID-19: A possible new mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacology 2020, 393, 1153–1156. [Google Scholar] [CrossRef]
- Miyajima, A.; Kigure, A.; Anata, T.; Hirota, T. Mechanism for transport of ivermectin to the stratum corneum in rats. Drug Metab. Pharmacokinet. 2015, 30, 385–390. [Google Scholar] [CrossRef]
- Sia, D.K.; Mensah, K.B.; Opoku-Agyemang, T.; Folitse, R.D.; Darko, D.O. Mechanisms of ivermectin-induced wound healing. BMC Vet. Res. 2020, 16, 397. [Google Scholar] [CrossRef]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.L.; Monaghan, R.L.; Olson, G.; Putter, I.; et al. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob. Agents Chemother. 1979, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharm. Biotechnol. 2012, 13, 853–865. [Google Scholar] [CrossRef]
- Hotson, I.K. The avermectins: A new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 1982, 53, 87–90. Available online: https://journals.co.za/doi/epdf/10.10520/AJA00382809_2579 (accessed on 19 May 2025).
- Htay, M.N.N.; Swed, S.; Elsayed, M.G.; Arafat, S.Y.; Marthoenis, M.; Marzo, R.R.; El-Abasiri, R.A.A.; Naing, Z.Y.; San, L.P.P.; Thantry, A.D.K.; et al. Knowledge and awareness of neglected tropical diseases and control strategies among healthcare students in five Asian countries: A cross-sectional study. Clin. Epidemiol. Glob. Health 2024, 27, 101576. [Google Scholar] [CrossRef]
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Boussinesq, M.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834. [Google Scholar] [CrossRef]
- Fimbo, A.M.; Mnkugwe, R.H.; Mlugu, E.M.; Kunambi, P.P.; Malishee, A.; Minzi, O.M.; Kamuhabwa, A.A.; Aklillu, E. Efficacy of ivermectin and albendazole combination in suppressing transmission of lymphatic filariasis following mass administration in Tanzania: A prospective cohort study. Infect. Dis. Poverty 2024, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef]
- Ashour, D.S. Ivermectin: From theory to clinical application. Int. J. Antimicrob. Agents 2019, 54, 134–142. [Google Scholar] [CrossRef]
- De Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kornobis, E.; Levallois, S.; Marchio, A.; Kergoat, L.; Hardy, D.; Cokelaer, T.; et al. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol. Med. 2021, 13, e14122. [Google Scholar] [CrossRef] [PubMed]
- Tiberti, N.; Buonfrate, D.; Carbone, C.; Piro, G.; Bisoffi, Z.; Piubelli, C. Systemic profile of immune factors in an elderly Italian population affected by chronic strongyloidiasis. Parasites Vectors 2020, 13, 515. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, C.; Sang, H.; Liu, W.; Wang, J. Ivermectin protects against experimental autoimmune encephalomyelitis in mice by modulating the Th17/Treg balance involved in the IL-2/STAT5 pathway. Inflammation 2023, 46, 1626–1638. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Xu, W.; Cheng, J.; Zhang, C.; Gao, J.; Li, Z.; Tao, L.; Zhang, Y. Immunotoxicity induced by Ivermectin is associated with NF-κB signaling pathway on macrophages. Chemosphere 2022, 289, 133087. [Google Scholar] [CrossRef]
- Coles, G.C. Anthelmintic resistance–looking to the future: A UK perspective. Res. Vet. Sci. 2005, 78, 99–108. [Google Scholar] [CrossRef]
- Erez, M.S.; Kozan, E. Anthelmintic resistance in farm animals. Kocatepe Vet. J. 2018, 11, 322–330. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef] [PubMed]
- Furnival-Adams, J.; Kiuru, C.; Sagna, A.B.; Mouline, K.; Maia, M.; Chaccour, C. Ivermectin resistance mechanisms in ectoparasites: A scoping review. Parasitol. Res. 2024, 123, 221. [Google Scholar] [CrossRef]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Kornele, M.L.; McLean, M.J.; O’Brien, A.E.; Phillippi-Taylor, A.M. Antiparasitic resistance and grazing livestock in the United States. J. Am. Vet. Med. Assoc. 2014, 244, 1020–1022. [Google Scholar] [CrossRef]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin—Old drug, new tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef]
- Mohammedsalih, K.M.; Ibrahim, A.I.; Juma, F.R.; Abdalmalaik, A.A.; Bashar, A.; Coles, G.; von Samson-Himmelstjerna, G.; Krücken, J. First evaluation and detection of ivermectin resistance in gastrointestinal nematodes of sheep and goats in South Darfur, Sudan. PLoS ONE 2024, 19, e0301554. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.; Molefe, N.; Tsotetsi-Khambule, A.; Oriel, T. Anthelmintic resistance in livestock. In Helminthiasis; Okwa, O.O., Ed.; InTechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Prichard, R.K. Anthelmintic resistance. Vet. Parasitol. 1994, 54, 259–268. [Google Scholar] [CrossRef]
- Prichard, R.K. Ivermectin resistance and overview of the Consortium for Anthelmintic Resistance SNPs’. Expert Opin. Drug Discov. 2007, 2, S41–S52. [Google Scholar] [CrossRef]
- Waller, P.J. Anthelmintic resistance. Vet. Parasitol. 1997, 72, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, J.C. Drug resistance in parasites: A review of mechanisms, drivers, and mitigation strategies. Microbes Infect. Dis. 2024, 6, 6152–6160. [Google Scholar] [CrossRef]
- White, N.J.; Pongtavornpinyo, W.; Maude, R.J.; Saralamba, S.; Aguas, R.; Stepniewska, K.; Lee, S.J.; Dondorp, A.M.; White, L.J.; Day, N.P. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar. J. 2009, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Gao, X.; Gao, Y.; Peng, L.; Ji, H.; Guo, D.; Jiang, S. Preparation and in vitro release kinetics of ivermectin sustained-release bolus optimized by response surface methodology. PeerJ 2018, 6, e5418. [Google Scholar] [CrossRef]
- Gnesotto, L.; Cutrone, M.; Mazzatenta, C.; Bassi, A.; Piccolo, V.; Sechi, A. Topical Ivermectin for Permethrin-Resistant Scabies: A Useful Application. Dermatol. Pract. Concept. 2024, 14, e2024029. [Google Scholar] [CrossRef]
- Algorta, J.; Krolewiecki, A.; Pinto, F.; Gold, S.; Muñoz, J. Pharmacokinetic characterization and comparative bioavailability of an innovative orodispersible fixed-dose combination of ivermectin and albendazole: A single dose, open label, sequence randomized, crossover clinical trial in healthy volunteers. Front. Pharmacol. 2022, 13, 914886. [Google Scholar] [CrossRef] [PubMed]
- Algorta, J.; Kepha, S.; Krolewiecki, A.; Li, H.; Giang, J.; Fleitas, P.; Mwandawiro, C.; Muñoz, J.; STOP Consortium. Population Pharmacokinetics and Exposure–Response Analysis of a Fixed-Dose Combination of Ivermectin and Albendazole in Children, Adolescents, and Adults. Clin. Pharmacol. Ther. 2025, 117, 203–213. [Google Scholar] [CrossRef]
- Monedero, I.; Caminero, J.A. Evidence for promoting fixed-dose combination drugs in tuberculosis treatment and control: A review [Unresolved issues]. Int. J. Tuberc. Lung Dis. 2011, 15, 433–439. [Google Scholar] [CrossRef]
- Konopka, J.K.; Chatterjee, P.; LaMontagne, C.; Brown, J. Environmental impacts of mass drug administration programs: Exposures, risks, and mitigation of antimicrobial resistance. Infect. Dis. Poverty 2022, 11, 78. [Google Scholar] [CrossRef]
- Mancini, L.; Lacchetti, I.; Chiudioni, F.; Cristiano, W.; Kevin, D.D.; Marcheggiani, S.; Carere, M.; Bindi, L.; Borrello, S. Need for a sustainable use of medicinal products: Environmental impacts of ivermectin. Ann. Ist. Super. Sanita 2020, 56, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Rawas-Qalaji, M.; El Hosary, R.; Jagal, J.; Ahmed, I.S. Formulation and optimization of ivermectin nanocrystals for enhanced topical delivery. Int. J. Pharm. X 2023, 6, 100210. [Google Scholar] [CrossRef]
- Chaccour, C.; Barrio, Á.I.; Royo, A.G.G.; Urbistondo, D.M.; Slater, H.; Hammann, F.; Del Pozo, J.L. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar. J. 2015, 14, 102. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef]
- Hennessy, D.R. Modifying the formulation or delivery mechanism to increase the activity of anthelmintic compounds. Vet. Parasitol. 1997, 72, 367–390. [Google Scholar] [CrossRef]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Ponmurugesan, K.; Murthannagari, V.R.; Devaraj, H.; Nagarajan, J.R.; Gnk, G. A Comparative Review Of Human And Veterinary Generic Drug Approval Systems. AJPCR 2025, 18, 47–53. [Google Scholar] [CrossRef]
- Wentzel, C.; Gernandt, N.; Gerber, M.; Van Der Kooy, F. Quantification of ivermectin in veterinary products consumed off-label as a treatment for COVID-19. Die Pharm. 2024, 79, 216–219. [Google Scholar] [CrossRef]
- Martin, R.J.; Robertson, A.P.; Choudhary, S. Ivermectin: An anthelmintic, an insecticide, and much more. Trends Parasitol. 2021, 37, 48–64. [Google Scholar] [CrossRef]
- Shipstone, M. Antiparasitic Drugs for Integumentary Disease in Animals. MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/pharmacology/systemic-pharmacotherapeutics-of-the-integumentary-system/antiparasitic-drugs-for-integumentary-disease-in-animals#Ivermectin_v3331036 (accessed on 17 April 2025).
- Lifschitz, A.; Nava, S.; Miró, V.; Canton, C.; Alvarez, L.; Lanusse, C. Macrocyclic lactones and ectoparasites control in livestock: Efficacy, drug resistance and therapeutic challenges. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 100559. [Google Scholar] [CrossRef]
- Suderman, M.T.; Craig, T.M. Efficacy of ivermectin against Dirofilaria immitis microfilariae in naturally infected dogs. Am. J. Vet. Res. 1984, 45, 1031–1032. [Google Scholar] [CrossRef]
- Yazwinski, T.A. Use of febantel or ivermectin for treatment of calves with experimentally induced Bunostomum phlebotomum infection. Am. J. Vet. Res. 1988, 49, 1407–1408. [Google Scholar] [CrossRef]
- Koch, S.N.; Torres, S.M.; Plumb, D.C. Section 1: Systemic Drugs. In Canine And Feline Dermatology Drug Handbook; Koch, S.N., Torres, S.M., Plumb, D.C., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2012; pp. 1–218. [Google Scholar]
- Rehbein, S.; Batty, A.F.; Barth, D.; Visser, M.; Timms, B.J.; Barrick, R.A.; Eagleson, S. Efficacy of an ivermectin controlled-release capsule against nematode and arthropod endoparasites in sheep. Vet. Rec. 1998, 142, 331–334. [Google Scholar] [CrossRef]
- Forbes, A.B.; Pitt, S.R.; Baggott, D.G.; Rehbein, S.; Barth, D.; Bridi, A.A.; Carvalho, L.A.; O’Brien, D.J. A review of the use of a controlled-release formulation of ivermectin in the treatment and prophylaxis of Psoroptes ovis infestations in sheep. Vet. Parasitol. 1999, 83, 319–326. [Google Scholar] [CrossRef]
- Alva-Valdes, R.; Wallace, D.H.; Benz, G.W.; Foster, A.G.; Holste, J.E. Efficacy of ivermectin against the mange mite Sarcoptes scabiei var suis in pigs. Am. J. Vet. Res. 1984, 45, 2113–2114. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.A.; Carvalho, L.A.; Cramer, L.G.; Barrick, R.A. Efficacy of a long-acting formulation of ivermectin against Psoroptes ovis (Hering, 1838) on cattle. Vet. Parasitol. 2001, 97, 277–283. [Google Scholar] [CrossRef]
- Ohba, S.; Toriumi, H.; Takeishi, M.; Noda, R. Efficacy of ivermectin against live mites and eggs of Sarcoptes scabiei in pigs. Jpn. J. Vet. Sci. 1989, 51, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Soll, M.D.; D’Assonville, J.A.; Smith, C.J.Z. Efficacy of topically applied ivermectin against sarcoptic mange (Sarcoptes scabiei var. bovis) of cattle. Parasitol. Res. 1992, 78, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.D.; Coulibaly, J.T.; Schindler, C.; Wimmersberger, D.; Keiser, J. Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2–12 years. J. Antimicrob. Chemother. 2019, 74, 1642–1647. [Google Scholar] [CrossRef]
- Lu, M.; Xiong, D.; Sun, W.; Yu, T.; Hu, Z.; Ding, J.; Cai, Y.; Yang, S.; Pan, B. Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 622–631. [Google Scholar] [CrossRef]
- Sharun, K.; Shyamkumar, T.S.; Aneesha, V.A.; Dhama, K.; Pawde, A.M.; Pal, A. Current therapeutic applications and pharmacokinetic modulations of ivermectin. Vet. World 2019, 12, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-loaded solid lipid nanoparticles: Preparation, characterisation, stability and transdermal behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Steenekamp, E.M.; Liebenberg, W.; Lemmer, H.J.R.; Gerber, M. Formulation and Ex Vivo Evaluation of Ivermectin Within Different Nano-Drug Delivery Vehicles for Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1466. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Luciani, P. Injectable lipid-based depot formulations: Where do we stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
- Kennedy, M.J. The efficacy of ivermectin against the eyeworm, Thelazia skrjabini, in experimentally infected cattle. Vet. Parasitol. 1992, 45, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J.; Holste, J.E.; Jacobsen, J.A. The efficacy of ivermectin (pour-on) against the eyeworms, Thelazia gulosa and Thelazia skrjabini in naturally infected cattle. Vet. Parasitol. 1994, 55, 263–266. [Google Scholar] [CrossRef]
- MSD Veterinary Manual. Onchocerciasis in Animals. Available online: https://www.msdvetmanual.com/integumentary-system/helminths-of-the-skin/onchocerciasis-in-animals (accessed on 18 August 2023).
- Britt, D.P.; Preston, J.M. Efficacy of ivermectin against Dictyocaulus arnfieldi in ponies. Vet. Rec. 1985, 116, 343–345. [Google Scholar] [CrossRef]
- Herd, R.P.; Donham, J.C. Efficacy of ivermectin against Onchocerca cervicalis microfilarial dermatitis in horses. Am. J. Vet. Res. 1983, 44, 1102–1105. [Google Scholar] [CrossRef]
- French, D.D.; Klei, T.M.; Foil, C.S.; Miller, R.I.; Foil, L.D.; Chapman, M.R.; McClure, J.J. Efficacy of ivermectin in paste and injectable formulations against microfilariae of Onchocerca cervicalis and resolution of associated dermatitis in horses. Am. J. Vet. Res. 1988, 49, 1550–1554. [Google Scholar] [CrossRef]
- Schröder, J.; Swan, G.E.; Soll, M.D.; Hotson, I.K. Efficacy of ivermectin against ectoparasites of cattle in South Africa. J. S. Afr. Vet. Assoc. 1985, 56, 31–35. Available online: https://pubmed.ncbi.nlm.nih.gov/3839021/ (accessed on 19 May 2025).
- Da Silva, C.F.; Almeida, T.; de Melo Barbosa, R.; Cardoso, J.C.; Morsink, M.; Souto, E.B.; Severino, P. New trends in drug delivery systems for veterinary applications. Pharm. Nanotechnol. 2021, 9, 15–25. [Google Scholar] [CrossRef]
- Alabaster, V. The fall and rise of in vivo pharmacology. Trends Pharmacol. Sci. 2002, 23, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Madrid, R.R.; Mathews, P.D.; Patta, A.C.; Gonzales-Flores, A.P.; Ramirez, C.A.; Rigoni, V.L.; Tavares-Dias, M.; Mertins, O. Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation. Heliyon 2021, 7, e05820. [Google Scholar] [CrossRef] [PubMed]
- Archana, N.K.; Gond, V.; Kumar, S.; Singh, S.; Jayachandran, C. Clinico-haematobiochemical profile after repeated subcutaneous administration of ivermectin in goats. J. Vet. Pharmacol. Ther. 2013, 12, 79–81. [Google Scholar] [CrossRef]
- Al-Azzam, S.I.; Fleckenstein, L.; Cheng, K.J.; Dzimianski, M.T.; McCall, J.W. Comparison of the pharmacokinetics of moxidectin and ivermectin after oral administration to beagle dogs. Biopharm. Drug Dispos. 2007, 28, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sartini, I.; Łebkowska-Wieruszewska, B.; Krupa, M.; Lisowski, A.; Poapolathep, A.; Giorgi, M. Pharmacokinetics of ivermectin after oral and intravenous administration in Biłgorajska geese (Anser anser domesticus). N. Z. Vet. J. 2022, 70, 313–318. [Google Scholar] [CrossRef]
- Shu, E.N.; Okonkwo, P.O. The Pharmacokinetics of Ivermectin in Rabbit. Orient J. Med. 2003, 15, 42–45. [Google Scholar] [CrossRef]
- Vanachayangkul, P.; Im-Erbsin, R.; Tungtaeng, A.; Kodchakorn, C.; Roth, A.; Adams, J.; Chaisatit, C.; Saingam, P.; Sciotti, R.J.; Reichard, G.A.; et al. Safety, pharmacokinetics, and activity of high-dose ivermectin and chloroquine against the liver stage of Plasmodium cynomolgi infection in rhesus macaques. Antimicrob. Agents Chemother. 2020, 64, 10–11. [Google Scholar] [CrossRef]
- Buckley, S.T.; Fischer, S.M.; Fricker, G.; Brandl, M. In vitro models to evaluate the permeability of poorly soluble drug entities: Challenges and perspectives. Eur. J. Pharm. Sci. 2012, 45, 235–250. [Google Scholar] [CrossRef]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Ponte, M.; Liebenberg, W.; Gerber, M. Formulation and in vitro skin diffusion of colchicine using different drug delivery vehicles. J. Drug Delivery Sci. Technol. 2023, 88, 104898. [Google Scholar] [CrossRef]
- Steyn, J.D.; Haasbroek-Pheiffer, A.; Pheiffer, W.; Weyers, M.; van Niekerk, S.E.; Hamman, J.H.; van Staden, D. Evaluation of Drug Permeation Enhancement by Using In Vitro and Ex Vivo Models. Pharmaceuticals 2025, 18, 195. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Approved Drug Products with Therapeutic Equivalence Evaluations|Orange Book: Ivermectin. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/search_product.cfm (accessed on 1 October 2025).
- World Health Organization (WHO). Lists of Essential Medicine: Ivermectin. Available online: https://list.essentialmeds.org/medicines/58 (accessed on 2 October 2025).
- Health Canada. Drug Product Database: Ivermectin. Available online: https://health-products.canada.ca/dpd-bdpp/dispatch-repartition (accessed on 2 October 2025).
- Australian Government Department of Health, Disability and Ageing. Australian Register of Therapeutic Goods (ARTG): Ivermectin. Available online: https://www.tga.gov.au/resources/australian-register-therapeutic-goods-artg?keywords=ivermectin&submit=Search (accessed on 2 October 2025).
- South African Health Products Regulatory Authority (SAHPRA). SAHPRA Registers Soolantra 10 mg/g Cream—An Ivermectin Formulation. Available online: https://www.sahpra.org.za/press-releases/sahpra-registers-soolantra-10mg-g-cream-an-ivermectin-formulation/ (accessed on 28 May 2024).
- Jermain, B.; Hanafin, P.O.; Cao, Y.; Lifschitz, A.; Lanusse, C.; Rao, G.G. Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing. J. Pharm. Sci. 2020, 109, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S.; Bhasin, B. Drug repurposing: An emerging tool for drug reuse, recycling and discovery. Curr. Drug Res. Rev. 2021, 13, 101–119. [Google Scholar] [CrossRef]
- Su, C.; Saha, T.; Sinha, S.; Hird, C.P.; Smith, S.X.; Quiñones-Mateu, M.E.; Das, S.C. Inhalable spray-dried dry powders combining ivermectin and niclosamide to inhibit SARS-CoV-2 infection in vitro. Int. J. Pharm. 2025, 671, 125302. [Google Scholar] [CrossRef]
- Wehbe, Z.; Wehbe, M.; Iratni, R.; Pintus, G.; Zaraket, H.; Yassine, H.M.; Eid, A.H. Repurposing ivermectin for COVID-19: Molecular aspects and therapeutic possibilities. Front. Immunol. 2021, 12, 663586. [Google Scholar] [CrossRef]
- Porubcin, S.; Rovnakova, A.; Zahornacky, O.; Jarcuska, P. Intravenous veterinary ivermectin in a COVID-19 patient causing neurotoxicity. IDCases 2022, 27, e01446. [Google Scholar] [CrossRef]
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Castillejos-López, M.; Torres-Espíndola, L.M.; Huerta-Cruz, J.C.; Flores-Soto, E.; Romero-Martinez, B.S.; Velázquez-Cruz, R.; Higuera-Iglesias, A.; Camarena, Á.; Torres-Soria, A.K.; Salinas-Lara, C.; et al. Ivermectin: A controversial focal point during the COVID-19 pandemic. Life 2022, 12, 1384. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Lal, S.K. Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166294. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The mechanisms of action of ivermectin against SARS-CoV-2—An extensive review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Motavallihaghi, S.; Nikfar, B.; Chaichian, S.; Momtazi-Borojeni, A.A. Potential therapeutic effects of ivermectin in COVID-19. Exp. Biol. Med. 2022, 247, 1388–1396. [Google Scholar] [CrossRef]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef]
- Zaheer, T.; Pal, K.; Abbas, R.Z.; Torres, M.D.P. COVID-19 and Ivermectin: Potential threats associated with human use. J. Mol. Struct. 2021, 1243, 130808. [Google Scholar] [CrossRef]
- Ceballos, L.; Alvarez, L.; Lifschitz, A.; Lanusse, C. Ivermectin systemic availability in adult volunteers treated with different oral pharmaceutical formulations. Biomed. Pharmacother. 2023, 160, 114391. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Bray, M.; Rayner, C.; Noël, F.; Jans, D.; Wagstaff, K. Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antivir. Res. 2020, 178, 104805. [Google Scholar] [CrossRef]
- Kaur, B.; Blavo, C.; Parmar, M.S. Ivermectin: A Multifaceted Drug With a Potential Beyond Antiparasitic Therapy. Cureus 2024, 16, e56025. [Google Scholar] [CrossRef]
- Pérez, S.; Miró, M.V.; Verna, A.; Altamiranda, E.G.; Barcos, O.; Lanusse, C.; Lifschitz, A. Ivermectin antiviral activity against Varicellovirus bovine alpha 1: Assessment of intracellular drug accumulation in virus-infected cells. Arch. Microbiol. 2024, 206, 78. [Google Scholar] [CrossRef]
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob. Resist. Infect. Control 2018, 7, 27. [Google Scholar] [CrossRef]
- Bazzano, M.; Di Salvo, A.; Diaferia, M.; Veronesi, F.; Galarini, R.; Paoletti, F.; Tesei, B.; McLean, A.; Veneziano, V.; Laus, F. Anthelmintic efficacy and pharmacokinetics of ivermectin paste after oral administration in mules infected by cyathostomins. Animals 2020, 10, 934. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy Beshbishy, A. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Piras, C.; Gugliandolo, E.; Castagna, F.; Palma, E.; Britti, D. Ivermectin (IVM) possible side activities and implications in antimicrobial resistance and animal welfare: The authors’ perspective. Vet. Sci. 2022, 9, 24. [Google Scholar] [CrossRef]
- Omansen, T.F.; Porter, J.L.; Johnson, P.D.R.; van der Werf, T.S.; Stienstra, Y.; Stinear, T.P. In-vitro Activity of Avermectins against Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2015, 9, e0003549. [Google Scholar] [CrossRef] [PubMed]
- Stetkevich, S.A.; Anzelc, M.J.; Burkhart, C.G. Intranasal ivermectin spray, the sunscreen to COVID-19. Open Dermatol. J. 2022, 16, e187437222205190. [Google Scholar] [CrossRef]
- Tan, X.; Xie, H.; Zhang, B.; Zhou, J.; Dou, Z.; Wang, X.; Wang, N. A novel ivermectin-derived compound D4 and its antimicrobial/biofilm properties against MRSA. Antibiotics 2021, 10, 208. [Google Scholar] [CrossRef]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. Available online: https://pubmed.ncbi.nlm.nih.gov/29511601/ (accessed on 25 May 2024).
- Sharmeen, S.; Skrtic, M.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Fonseca, S.B.; Sun, H.; Wood, T.E.; Ward, R.; et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood 2010, 116, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Melotti, A.; Mas, C.; Kuciak, M.; Lorente-Trigos, A.; Borges, I.; Ruiz i Altaba, A. The river blindness drug I vermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 2014, 6, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Cheng, N.; Zhao, Y.; Xu, H.; Dong, H.; Thamm, D.H.; Zhang, D.; Lin, D. Ivermectin inhibits canine mammary tumor growth by regulating cell cycle progression and WNT signaling. BMC Vet. Res. 2019, 15, 276. Available online: https://pubmed.ncbi.nlm.nih.gov/31375107/ (accessed on 19 May 2025). [CrossRef] [PubMed]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, K.; Cheng, L.; Zhu, H.; Xu, T. Progress in understanding the molecular mechanisms underlying the antitumour effects of ivermectin. Drug Des. Devel. Ther. 2020, 14, 285–296. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, T.; Zhu, Z.; Hong, F.; Xu, Y.; Zhang, X.; Xu, X.; Ma, A. Ivermectin Augments the In Vitro and In Vivo Efficacy of Cisplatin in Epithelial Ovarian Cancer by Suppressing Akt/mTOR Signaling. Am. J. Med. Sci. 2020, 359, 123–129. [Google Scholar] [CrossRef]
- Gallardo, F.; Teiti, I.; Rochaix, P.; Demilly, E.; Jullien, D.; Mariamé, B.; Tilkin-Mariamé, A.F. Macrocyclic lactones block melanoma growth, metastases development and potentiate activity of anti–BRAF V600 inhibitors. Clin. Skin Cancer 2016, 1, 4–14. [Google Scholar] [CrossRef]
- Gallardo, F.; Mariamé, B.; Gence, R.; Tilkin-Mariamé, A.F. Macrocyclic lactones inhibit nasopharyngeal carcinoma cells proliferation through PAK1 inhibition and reduce in vivo tumor growth. Drug Des. Devel. Ther. 2018, 12, 2805–2814. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, H.S.; Kim, S.L.; Lee, D.S. The PAK1-Stat3 Signaling Pathway Activates IL-6 Gene Transcription and Human Breast Cancer Stem Cell Formation. Cancers 2019, 11, 1527. [Google Scholar] [CrossRef]
- Yin, J.; Park, G.; Lee, J.E.; Choi, E.Y.; Park, J.Y.; Kim, T.H.; Park, N.; Jin, X.; Jung, J.E.; Shin, D.; et al. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain 2015, 138, 2553–2570. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Petrie, K.; Leibovitch, B.A.; Zeng, L.; Mezei, M.; Howell, L.; Gil, V.; Christova, R.; Bansal, N.; Yang, S.; et al. Selective inhibition of SIN3 corepressor with avermectins as a novel therapeutic strategy in triple-negative breast cancer. Mol. Cancer Ther. 2015, 14, 1824–1836. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Wan, H.; Hu, J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2018, 497, 241–247. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Barroso-Arranda, J.; McCarty, M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart 2020, 7, e001350. [Google Scholar] [CrossRef] [PubMed]
- Dourmishev, A.L.; Dourmishev, L.A.; Schwartz, R.A. Ivermectin: Pharmacology and application in dermatology. Int. J. Dermatol. 2005, 44, 981–988. [Google Scholar] [CrossRef]
- Cairns, D.M.; Giordano, J.E.; Conte, S.; Levin, M.; Kaplan, D.L. Ivermectin promotes peripheral nerve regeneration during wound healing. ACS Omega 2018, 3, 12392–12402. [Google Scholar] [CrossRef]
- Tian, S.; Zheng, Y.; Xiao, S.; Luo, P.; Sun, R.; Liu, J.; Xia, Z. Ivermectin inhibits cell proliferation and the expression levels of type I collagen, α-SMA and CCN2 in hypertrophic scar fibroblasts. Mol. Med. Rep. 2021, 24, 488. [Google Scholar] [CrossRef]
- Kalangadan, N.; Mary, A.S.; Mani, K.; Nath, B.; Kondapalli, J.; Soni, S.; Raghavan, V.S.; Parsanathan, R.; Kannan, M.; Jenkins, D.; et al. Repurposing ivermectin and ciprofloxacin in nanofibers for enhanced wound healing and infection control against MDR wound pathogens. J. Drug Deliv. Sci. Technol. 2023, 90, 105166. [Google Scholar] [CrossRef]
- Green, J.A.; Stockton, R.A.; Johnson, C.; Jacobson, B.S. 5-Lipoxygenase and cyclooxygenase regulate wound closure in NIH/3T3 fibroblast monolayers. Am. J. Physiol. Cell Physiol. 2004, 287, C373–C383. [Google Scholar] [CrossRef]
- Morris-Schaffer, K.; McCoy, M.J. A review of the LD50 and its current role in hazard communication. ACS Chem. Health Saf. 2020, 28, 25–33. [Google Scholar] [CrossRef]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.K.; Lasseter, K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Rosenkrantz, W.; Bensignor, E.; Karaś-Tęcza, J.; Paterson, T.; Shipstone, M.A. Diagnosis and treatment of demodicosis in dogs and cats: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 4-e2. [Google Scholar] [CrossRef]
- Fobi, G.; Gardon, J.; Kamgno, J.; Aimard-Favennec, L.; Lafleur, C.; Gardon-Wendel, N.; Duke, B.O.; Boussinesq, M. A randomized, double-blind, controlled trial of the effects of ivermectin at normal and high doses, given annually or three-monthly, against Onchocerca volvulus: Ophthalmological results. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 279–289. [Google Scholar] [CrossRef]
- Burnham, G.M. Adverse reactions to ivermectin treatment for onchocerciasis. Results of a placebo-controlled, double-blind trial in Malawi. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 313–317. [Google Scholar] [CrossRef]
- Chandler, R.E. Serious neurological adverse events after ivermectin—Do they occur beyond the indication of onchocerciasis? Am. J. Trop. Med. Hyg. 2017, 98, 382. [Google Scholar] [CrossRef]
- Kipp, W.; Bamhuhiiga, J.; Rubaale, T.; Kabagambe, G. Adverse reactions to the ivermectin treatment of onchocerciasis patients: Does infection with the human immunodeficiency virus play a role? Ann. Trop. Med. Parasitol. 2005, 99, 395–402. [Google Scholar] [CrossRef]
- Zea-Flores, R.; Richards Jr, F.O.; González-Peralta, C.; Ramirez, J.C.; Zea-Flores, G.; Collins, R.C.; Cupp, E. Adverse reactions after community treatment of onchocerciasis with ivermectin in Guatemala. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 663–666. [Google Scholar] [CrossRef]
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature. PLoS Negl. Trop. Dis. 2018, 12, e0006454. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.P.; Sarver, J.G. Chapter 10: Pharmacokinetic modeling. In Pharmacology; Hacker, M., Messer, W., Bachmann, K., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 201–277. [Google Scholar] [CrossRef]
- Rowland Yeo, K.; Wesche, D. PBPK modeling of ivermectin—Considerations for the purpose of developing alternative routes to optimize its safety profile. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 598–609. [Google Scholar] [CrossRef]
- Löscher, W. Is the antiparasitic drug ivermectin a suitable candidate for the treatment of epilepsy? Epilepsia 2023, 64, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.P. Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects. Arch. Toxicol. 2021, 95, 1535–1546. [Google Scholar] [CrossRef]
- Udaykumar, P.; Shetty, B.; Kundapur, A. Drug interactions of ivermectin with a focus on COVID-19 treatment. Muller J. Med. Sci. Res. 2021, 12, 42–48. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Akgun, U. Characterizing the binding interactions between P-glycoprotein and eight known cardiovascular transport substrates. Pharmacol. Res. Perspect. 2015, 3, e00114. [Google Scholar] [CrossRef]
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jittamala, P.; Monteiro, W.; Smit, M.R.; Pedrique, B.; Specht, S.; Chaccour, C.J.; Dard, C.; Del Giudice, P.; Khieu, V.; Maruani, A.; et al. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 2021, 15, e0009144. [Google Scholar] [CrossRef]
- Wood, N.D.; Smith, D.; Kinrade, S.A.; Sullivan, M.T.; Rayner, C.R.; Wesche, D.; Patel, K.; Rowland-Yeo, K. The use of quantitative clinical pharmacology approaches to support moxidectin dosing recommendations in lactation. PLOS Negl. Trop. Dis. 2024, 18, e0012351. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasit. Vectors 2023, 16, 394. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Abd-Elsalam, S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective. World J. Gastroenterol. 2020, 26, 7272. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Amado, J.; Romero-Ortuno, R.; Carvajal, A. Hepatic disorders associated with the use of Ivermectin for SARS-CoV-2 infection in adults: A pharmacovigilance study in VigiBase. Gastroenterol. Hepatol. Bed Bench 2022, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.L.A.; Lima, T.D.M. Use of medicines for covid-19 treatment in patients with loss of kidney function: A narrative review. Braz. J. Nephrol. 2020, 43, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sonderup, M.W.; Mudini, W.; Spearman, C.W.N. Ivermectin drug-induced liver injury. S. Afr. Med. J. 2023, 113, 1203–1204. [Google Scholar] [CrossRef]
- Badia, B.D.M.L.; de Lima Serrano, P.; Barile, J.P.; Seneor, D.D.; Mendes, P.M.; Cavalheiro, R.B.R.; Peixoto, K.O.; Farias, I.B.; Machado, R.I.L.; de Rezende Pinto, W.B.V.; et al. Practical Recommendations in the Treatment of Acute and Chronic Life-Threatening Infectious Diseases in Patients with Acute Hepatic Porphyria. Metabolites 2025, 15, 99. [Google Scholar] [CrossRef]
- Abou El-Fetouh, M.S.; Elseddawy, N.M.; Abdelsamia, H.M. Insights on the therapeutic use of ivermectin: Mechanism of action and histopathological effects. JAVR 2024, 14, 339–341. Available online: https://www.advetresearch.com/index.php/AVR/article/view/1530 (accessed on 25 May 2024).
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Mercer, M.A.; Davis, J.L.; Wetzlich, S.E.; Clapham, M.O.; Tell, L.A. Residue depletion profile and withdrawal interval estimation of ivermectin in eggs following topical administration of injectable ivermectin to domestic chickens (Gallus domesticus): A pilot study. Front. Vet. Sci. 2025, 12, 1527808. [Google Scholar] [CrossRef]
- FAO/WHO. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods; World Health Organization—Technical Report Series; WHO: Geneva, Switzerland, 2023; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/hu/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXM%2B2%252FMRL2e.pdf (accessed on 2 October 2025).
- Escribano, M.; I San Andres, M.; J de Lucas, J.; González-Canga, A. Ivermectin residue depletion in food producing species and its presence in animal foodstuffs with a view to human safety. Curr. Pharm. Biotechnol. 2012, 13, 987–998. [Google Scholar] [CrossRef]
- Paucar-Quishpe, V.; Cepeda-Bastidas, D.; Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Perez, C.; Enríquez, S.; Guzman, E.; Ulcuango, F.; Grijalva, J.; Vanwambeke, S.O.; et al. Evaluating the Human Risks of Consumption of Foods of Bovine Origin with Ivermectin Residues in Ecuador. Foods 2024, 13, 3470. [Google Scholar] [CrossRef]
- Errecalde, J.; Lifschitz, A.; Vecchioli, G.; Ceballos, L.; Errecalde, F.; Ballent, M.; Marín, G.; Daniele, M.; Turic, E.; Spitzer, E.; et al. Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model. J. Pharm. Sci. 2021, 110, 2501–2507. [Google Scholar] [CrossRef]
- Desu, H.R.; Narang, A.S.; Kumar, V.; Thoma, L.A.; Mahato, R.I. Chapter 10: Liquid dosage forms. In Pharmaceutics, 2nd ed.; Dash, A.K., Singh, S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 271–318. [Google Scholar] [CrossRef]
- Maggi, L.; Friuli, V.; Perugini, P.; Musitelli, G.; Venco, L. Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet. J. 2021, 11, 471–482. [Google Scholar] [CrossRef]
- Walsh, J.; Ranmal, S.R.; Ernest, T.B.; Liu, F. Patient acceptability, safety and access: A balancing act for selecting age-appropriate oral dosage forms for paediatric and geriatric populations. Int. J. Pharm. 2018, 536, 547–562. [Google Scholar] [CrossRef]
- Lin, T.Y.; Rodriguez Jr, C.O.; Li, Y. Nanomedicine in veterinary oncology. Vet. J. 2015, 205, 189–197. [Google Scholar] [CrossRef]
- Van Staden, D.; Gerber, M.; Lemmer, H.J.R. The Application of Nano Drug Delivery Systems in Female Upper Genital Tract Disorders. Pharmaceutics 2024, 16, 1475. [Google Scholar] [CrossRef]
- Mahato, R.I.; Narang, A.S. Chapter 18: Pharmaceutical Solutions. In Pharmaceutical Dosage Forms and Drug Delivery: Revised and Expanded, 3rd ed.; Mahato, R.I., Narang, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 425–426. [Google Scholar]
- Martinez, M.N.; Papich, M.G.; Fahmy, R. Impact of gastrointestinal differences in veterinary species on the oral drug solubility, in vivo dissolution, and formulation of veterinary therapeutics. ADMET DMPK 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Mestorino, N.; Turic, E.; Pesoa, J.; Echeverría, J.; Errecalde, J.O. Pharmacokinetics in plasma of ivermectin after its oral (solution and tablets) administration to sheep. J. Vet. Pharmacol. Ther. 2003, 26, 307–309. [Google Scholar] [CrossRef]
- Barker, S.A. Chapter 26: Suspensions. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 407–423. [Google Scholar]
- Murdan, S. Chapter 24: Solutions. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 386–396. [Google Scholar]
- Brako, F.; Boateng, J. Transmucosal drug delivery: Prospects, challenges, advances, and future directions. Expert. Opin. Drug Deliv. 2025, 22, 525–553. [Google Scholar] [CrossRef]
- Alderborn, G.; Frenning, G. Chapter 31: Tablets and compaction. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 501–541. [Google Scholar]
- Jones, B.E. Chapter 35: Hard capsules. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 586–598. [Google Scholar]
- Muñoz, J.; Ballester, M.R.; Antonijoan, R.M.; Gich, I.; Rodríguez, M.; Colli, E.; Gold, S.; Krolewiecki, A.J. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis. 2018, 12, e0006020. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Wang, X.; Sandler, N. Compounding tailored veterinary chewable tablets close to the point-of-care by means of 3D printing. Pharmaceutics 2022, 14, 1339. [Google Scholar] [CrossRef]
- Thombre, A.G. Oral delivery of medications to companion animals: Palatability considerations. Adv. Drug Deliv. Rev. 2004, 56, 1399–1413. [Google Scholar] [CrossRef]
- Paul, A.J.; Todd, K.S.; Acre, K.E.; Plue, R.E.; Wallace, D.H.; French, R.A.; Wallig, M.A. Efficacy of ivermectin chewable tablets and two new ivermectin tablet formulations against Dirofilaria immitis larvae in dogs. Am. J. Vet. Res. 1991, 52, 1922–1923. [Google Scholar] [CrossRef]
- Canga, A.G.; Prieto, A.M.S.; Liébana, M.J.D.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J.G. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 2009, 179, 25–37. [Google Scholar] [CrossRef]
- Gogolewski, R.P.; Allerton, G.R.; Langholff, W.K.; Cramer, L.G.; Eagleson, J.S. An ivermectin tablet for sheep: Efficacy against gastro-intestinal nematodes and a bioavailability comparison with a liquid ivermectin formulation. Vet. Parasitol. 1995, 60, 297–302. [Google Scholar] [CrossRef]
- Al-Obaidi, I.; Krome, A.K.; Wagner, K.G.; Pfarr, K.; Kuesel, A.C.; Batchelor, H.K. Drugs for neglected tropical diseases: Availability of age-appropriate oral formulations for young children. Parasit. Vectors 2022, 15, 462. [Google Scholar] [CrossRef]
- Del Moral Sanchez, J.M.; Gonzalez-Alvarez, I.; Cerda-Revert, A.; Gonzalez-Alvarez, M.; Navarro-Ruiz, A.; Amidon, G.L.; Bermejo, M. Biopharmaceutical optimization in neglected diseases for paediatric patients by applying the provisional paediatric biopharmaceutical classification system. Br. J. Clin. Pharmacol. 2018, 84, 2231–2241. [Google Scholar] [CrossRef]
- Juan, C.; Rodriguez, D.; Ceballos, L.; Lanusse, C.; Gallo, L.; Gonzalez Vidal, N. Development of ivermectin orally disintegrating tablets using factorial design: In-vitro evaluation and in vivo absorption pattern in rats. J. Drug Deliv. Sci. Technol. 2023, 87, 104757. [Google Scholar] [CrossRef]
- Wei, S.; Yue, H.; Li, G.; Sang, N. Particulate matter induces airway epithelial barrier dysfunction in vivo and in vitro: From a more realistic inhalation scenario. Environ. Sci. Nano 2022, 9, 2665–2677. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Cheung, C.C.K.; Chow, M.Y.T.; Harrop, E.; Lapwood, S.; Barclay, S.I.G.; Wong, I.C.K. Transmucosal drug administration as an alternative route in palliative and end-of-life care during the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2020, 160, 234–243. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Morton, D.A.V.; Barling, D. Developing Dry Powder Inhaler Formulations. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 90–99. [Google Scholar] [CrossRef]
- Oldham, J.; Sahota, A.; O’Toole, E.; Cunningham, M. P095 Ten cases of scabies successfully treated with topical ivermectin. Br. J. Dermatol. 2024, 191, i60. [Google Scholar] [CrossRef]
- Paradis, M.; de Jaham, C.; Pagé, N. Topical (pour-on) ivermectin in the treatment of canine scabies. Can. Vet. J. 1997, 38, 379–382. [Google Scholar]
- Smith, M.; Wolffsohn, J.S.; Chiang, J.C.B. Topical ivermectin 1.0% cream in the treatment of ocular demodicosis. Cont. Lens Anterior Eye 2023, 47, 102099. [Google Scholar] [CrossRef]
- Zargari, O.; Aghazadeh, N.; Moeineddin, F. Clinical applications of topical ivermectin in dermatology. Dermatol. Online J. 2016, 22, 9. [Google Scholar] [CrossRef]
- Aucamp, Z.; Liebenberg, W.; Lemmer, H.J.; Gerber, M. Formulation of semi-solid dosage forms intended for transdermal delivery of ivermectin. J. Drug Deliv. Sci. Technol. 2024, 101, 106174. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Cheng, J.Y.; Butcher, A.; Shafiq, F.; Osuoji, O.; Gallo, R.L.; Hata, T.R. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J. Investig. Dermatol. 2025, 145, 1226–1228. [Google Scholar] [CrossRef]
- Victoria, J.; Trujillo, R. Topical ivermectin: A new successful treatment for scabies. Pediatr. Dermatol. 2001, 18, 63–65. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef]
- Morad, R. Coating of Remdesivir and Ivermectin on silver nanoparticles: A density functional theory and molecular dynamics study. Results Surf. Interfaces 2025, 19, 100540. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Goharshadi, K.; Moghayedi, M. The use of nanotechnology in the fight against viruses: A critical review. Coord. Chem. Rev. 2022, 464, 214559. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation strategies for improving intestinal permeability of drugs: A more precise look at permeability assessment methods and pharmacokinetic properties changes. J. Control. Release 2020, 321, 669–709. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Gamboa, G.U.; Palma, S.D.; Lifschitz, A.; Ballent, M.; Lanusse, C.; Passirani, C.; Benoit, J.P.; Allemandi, D.A. Ivermectin-loaded lipid nanocapsules: Toward the development of a new antiparasitic delivery system for veterinary applications. Parasitol. Res. 2016, 115, 1945–1953. [Google Scholar] [CrossRef]
- Qamar, W.; Alkheraije, K.A. Anthelmintic Resistance in Haemonchus contortus of Sheep and Goats from Asia—A Review of In Vitro and In Vivo Studies. Pak. Vet. J. 2023, 43, 376–387. Available online: https://www.pvj.com.pk/pdf-files/23-292.pdf (accessed on 5 October 2025).
- Xu, X.; Gao, S.; Zuo, Q.; Gong, J.; Song, X.; Liu, Y.; Xiao, J.; Zhai, X.; Sun, H.; Zhang, M.; et al. Enhanced in vitro antiviral activity of ivermectin-loaded nanostructured lipid carriers against porcine epidemic diarrhea virus via improved intracellular delivery. Pharmaceutics 2024, 16, 601. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Dave, K.; Krishna Venuganti, V.V. Dendritic polymers for dermal drug delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef]
- Tanner, T.; Marks, R.J.S.R. Delivering drugs by the transdermal route: Review and comment. Skin Res. Tech. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Mohite, P.; Singh, S.; Pawar, A.; Sangale, A.; Prajapati, B.G. Lipid-based oral formulation in capsules to improve the delivery of poorly water-soluble drugs. Front. Drug Deliv. 2023, 3, 1232012. [Google Scholar] [CrossRef]
- Rajput, A.; Pingale, P.; Telange, D.; Chalikwar, S.; Borse, V. Lymphatic transport system to circumvent hepatic metabolism for oral delivery of lipid-based nanocarriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102934. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, Y.; Li, L.; Zhong, C.; Chen, C.; Gao, X. Lipid-based formulations: A promising approach for poorly soluble drug delivery via the intestinal lymphatic system. J. Drug Deliv. Sci. Technol. 2023, 87, 104770. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef]
- Cholakova, D.; Vinarov, Z.; Tcholakova, S.; Denkov, N.D. Self-emulsification in chemical and pharmaceutical technologies. Curr. Opin. Colloid. Interface Sci. 2022, 59, 101576. [Google Scholar] [CrossRef]
- Patel, V.; Lakkad, H.; Ashara, K. Formulation studies of solid self-emulsifying drug de-livery system of ivermectin. Folia Med. 2018, 60, 580–593. [Google Scholar] [CrossRef]
- Bennuru, S.; Nutman, T.B. Lymphatics in Human Lymphatic Filariasis: In Vitro Models of Parasite-Induced Lymphatic Remodeling. Lymphat. Res. Biol. 2009, 7, 215–219. [Google Scholar] [CrossRef]
- Brown, K.R.; Ricci, F.M.; Ottesen, E.A. Ivermectin: Effectiveness in lymphatic filariasis. Parasitology 2000, 121, S133–S146. [Google Scholar] [CrossRef]
- Failoc-Rojas, V.E.; Silva-Díaz, H.; Maguiña, J.L.; Rodriguez-Morales, A.J.; Díaz-Velez, C.; Apolaya-Segura, M.; Valladares-Garrido, M.J. Evidence-based indications for ivermectin in parasitic diseases: An integrated approach to context and challenges in Peru. Parasite Epidemiol. Control 2023, 23, e00320. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Library of Medicine (NIH). Efficacy of Nano-Ivermectin Impregnated Masks in Prevention of COVID-19 Among Healthy Contacts and Medical Staff. Available online: https://www.clinicaltrials.gov/study/NCT04723459?intr=ivermectin&viewType=Card&page=10&rank=92 (accessed on 14 October 2025).
- ClinicalTrials.gov. National Library of Medicine (NIH). Evaluation of Ivermectin Mucoadhesive Nanosuspension as Nasal Spray in Management of Early COVID-19. Available online: https://www.clinicaltrials.gov/study/NCT04716569?intr=ivermectin&viewType=Card&page=10&rank=98 (accessed on 14 October 2025).
- ClinicalTrials.gov. National Library of Medicine (NIH). Role of Ivermectin Nanosuspension as Nasal Spray in Treatment of Persistant Post COVID-19 Anosmia. Available online: https://www.clinicaltrials.gov/study/NCT04951362?intr=ivermectin&viewType=Card&page=11&rank=107 (accessed on 14 October 2025).
- Surnar, B.; Kamran, M.Z.; Shah, A.S.; Dhar, S. Clinically approved antiviral drug in an orally administrable nanoparticle for COVID-19. ACS Pharmacol. Transl. Sci. 2020, 3, 1371–1380. [Google Scholar] [CrossRef]
- Mohammed, S.W.; El-Megrab, N.A.; Hasan, A.A.; Gomaa, E. A remodeled ivermectin polycaprolactone-based nanoparticles for inhalation as a promising treatment of pulmonary inflammatory diseases. Eur. J. Pharm. Sci. 2024, 195, 106714. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Godrati-Azar, Z.; Spotin, A.; Norouzi, R.; Hamishehkar, H.; Nami, S.; Heydarian, P.; Rajabi, S.; Mohammadi, M.; Perez-Cordon, G. Nanostructured lipid carriers of ivermectin as a novel drug delivery system in hydatidosis. Parasit. Vectors 2019, 12, 469. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiu, R.; Wang, C.; Wang, J.; Guo, D.; Luo, W.; Jiang, S.; Ge, Z.; Gao, X. Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases. Pharmaceutics 2025, 17, 1216. [Google Scholar] [CrossRef]
- Hammad, S.K.; Almotayam, M.H.; Mohamed, A.S.N.; Farag, T.I. The impact of ivermectin-loaded solid lipid nanoparticles on the enteric phase of experimental trichinellosis. J. Helminthol. 2025, 99, e53. [Google Scholar] [CrossRef]
- Rodovalho, A.I.C.; Bedogni, G.; da Fonsêca Xavier, M.E.L.; de Andrade Picanço, G.; de Souza, J.Y.; de Campos, G.B.; da Costa, T.L.; Vinaud, M.C.; Salomon, C.J. Amorphous ivermectin nanoparticles: In vitro and in vivo studies supporting their potential in neurocysticercosis therapy. J. Drug Deliv. Sci. Technol. 2025, 113, 107398. [Google Scholar] [CrossRef]
- Velho, M.C.; Winck, V.L.; Mariot, C.D.S.; Scholl, J.N.; Weber, A.F.; Souza, R.D.K.; Visioli, F.; Figueiró, F.; Deon, M.; Pilger, D.A.; et al. Intranasal Delivery of Ivermectin Nanosystems as an Antitumor Agent: Focusing on Glioma Suppression. ACS Biomater. Sci. Eng. 2025, 11, 4231–4244. [Google Scholar] [CrossRef]
- Savrun, N.; Soydan, M.O. Nano-Ivermectin Compositions. United States Patent Application Publication US20240074971A1, 7 March 2024. Available online: https://patents.google.com/patent/US20240074971A1/en (accessed on 13 October 2025).
- Kassaee, S.N.; Ayoko, G.A.; Richard, D.; Wang, T.; Islam, N. Inhaled ivermectin-loaded lipid polymer hybrid nanoparticles: Development and characterization. Pharmaceutics 2024, 16, 1061. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. National Library of Medicine (NIH). Sars-CoV-2/COVID-19 Ivermectin Navarra-ISGlobal Trial (SAINT). Available online: https://clinicaltrials.gov/study/NCT04390022 (accessed on 13 October 2025).
- Velho, M.C.; de Andrade, D.F.; Beck, R.C.R. Ivermectin: Recent approaches in the design of novel veterinary and human medicines. Pharm. Dev. Technol. 2022, 27, 865–880. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Louisthelmy, R.; Maisel, K. Mechanisms of nanoparticle transport across intestinal tissue: An oral delivery perspective. ACS Nano. 2023, 17, 13044–13061. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Sun, Y.; Li, J.; An, Y.; Feng, N.; Liu, Y. Intestinal nanoparticle delivery and cellular response: A review of the bidirectional nanoparticle-cell interplay in mucosa based on physiochemical properties. J. Nanobiotechnol. 2024, 22, 669. [Google Scholar] [CrossRef]
- Zheng, Y.; Luo, S.; Xu, M.; He, Q.; Xie, J.; Wu, J.; Huang, Y. Transepithelial transport of nanoparticles in oral drug delivery: From the perspective of surface and holistic property modulation. Acta Pharm. Sin. B 2024, 14, 3876–3900. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, S.; Karthikeyan, K. Wonder drug for worms: A review of three decades of ivermectin use in dermatology. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Dy, J.H.; Juangco, D.N. Identifying the Toxidrome of Ivermectin Toxicity. Cureus 2023, 15, e42603. [Google Scholar] [CrossRef] [PubMed]
- Hoang, R.; Temple, C.; Correia, M.S.; Clemons, J.; Hendrickson, R.G. Characteristics of ivermectin toxicity in patients taking veterinary and human formulations for the prevention and treatment of COVID-19. Clin. Toxicol. 2022, 60, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Gerber, M.; Plessis, J.D.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Dhondale, M.R.; Nambiar, A.G.; Singh, M.; Mali, A.R.; Agrawal, A.K.; Shastri, N.R.; Kumar, P.; Kumar, D. Current trends in API co-processing: Spherical crystallization and co-precipitation techniques. J. Pharm. Sci. 2023, 112, 2010–2028. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, G. Development of Modified-Release Solid Oral Dosage Forms. In Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G.Z., Liu, L., Porter, W.R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 501–517. [Google Scholar]
- Van Staden, D.; du Plessis, J.; Viljoen, J. Development of topical/transdermal self-emulsifying drug delivery systems, not as simple as expected. Sci. Pharm. 2020, 88, 17. [Google Scholar] [CrossRef]
- Albert Lo, P.K.; Fink, D.W.; Williams, J.B.; Blodinger, J. Pharmacokinetic studies of ivermectin: Effects of formulation. Vet. Res. Commun. 1985, 9, 251–268. [Google Scholar] [CrossRef]
- King, T.A. The One Medicine concept: Its emergence from history as a systematic approach to re-integrate human and veterinary medicine. Emerg. Top. Life Sci. 2021, 5, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.; Pis, A.; Alvarez, L.; Virkel, G.; Sanchez Bruni, S.F.; Sallovitz, J.M.; Kujanek, R.; Lanusse, C.E. Bioequivalence of ivermectin formulations in pigs and cattle. J. Vet. Pharmacol. Ther. 1999, 22, 27–34. [Google Scholar] [CrossRef] [PubMed]

| Country | Company | Registered IVM Trade Product(s) | Dosage Form and Strength | Indication(s) |
|---|---|---|---|---|
| Australia | Galderma Australia Pty Ltd. | Vastreka | Topical cream, 1% | Rosacea |
| Canada | Pharmascience Inc. | PMS-Ivermectin | Topical cream, 1% | Rosacea |
| Canada | Galderma Canada Inc. | Rosiver | Topical cream, 1% | Rosacea |
| Canada | Merck Canada Inc. | Stromectol | Oral tablet, 3 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| Canada, India, United States of America | Rubicon Research Ltd. | Ivermectin | Oral tablet, 3 mg, 6 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| India | Zydus Lifesciences Global Fze | Ivermectin | Topical cream, 1% | Rosacea |
| India | Senores Pharmaceuticals Inc. | Ivermectin | Oral tablet, 3 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| Israel | Padagis Israel Pharmaceuticals Ltd. | Ivermectin | Topical cream, 1% | Rosacea |
| Israel | Taro Pharmaceutical Industries Ltd. | Ivermectin | Topical lotion, 0.5% | Head lice infestations |
| South Africa, United States of America | Galderma Laboratories Lp | Soolantra * | Topical cream, 1% | Rosacea |
| United States of America | Teva Pharmaceuticals USA Inc. | Ivermectin | Topical cream, 1% | Rosacea |
| United States of America | Edenbridge Pharmaceuticals Llc | Ivermectin | Oral tablet, 3 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| United States of America | Epic Pharma Llc | Ivermectin | Oral tablet, 3 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| United States of America | Merck Sharp And Dohme Corp. | Stromectol | Oral tablet, 3 mg | Intestinal strongyloidiasis, onchocerciasis, ascariasis, trichuriasis, ancylostomiasis, hookworm diseases, lymphatic filariasis, scabies |
| United States of America, India, Ireland, Switzerland | Arbor Pharmaceuticals Llc | Sklice | Topical lotion, 0.5% | Head lice infestations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gernandt, N.; Wentzel, C.; van Staden, D.; Liebenberg, W.; Lemmer, H.J.R.; Gerber, M. Therapeutic and Formulation Advances of Ivermectin in Veterinary and Human Medicine. Pharmaceutics 2025, 17, 1384. https://doi.org/10.3390/pharmaceutics17111384
Gernandt N, Wentzel C, van Staden D, Liebenberg W, Lemmer HJR, Gerber M. Therapeutic and Formulation Advances of Ivermectin in Veterinary and Human Medicine. Pharmaceutics. 2025; 17(11):1384. https://doi.org/10.3390/pharmaceutics17111384
Chicago/Turabian StyleGernandt, Nicezelle, Chanri Wentzel, Daniélle van Staden, Wilna Liebenberg, Hendrik J. R. Lemmer, and Minja Gerber. 2025. "Therapeutic and Formulation Advances of Ivermectin in Veterinary and Human Medicine" Pharmaceutics 17, no. 11: 1384. https://doi.org/10.3390/pharmaceutics17111384
APA StyleGernandt, N., Wentzel, C., van Staden, D., Liebenberg, W., Lemmer, H. J. R., & Gerber, M. (2025). Therapeutic and Formulation Advances of Ivermectin in Veterinary and Human Medicine. Pharmaceutics, 17(11), 1384. https://doi.org/10.3390/pharmaceutics17111384